Abstract
The enzyme Cypridina luciferase (CLase) enables Cypridina luciferin to emit light efficiently through an oxidation reaction. The catalytic mechanism on the substrate of CLase has been studied, but the details remain to be clarified. Here, we examined the luminescence of Cypridina luciferin in the presence of several proteins with drug-binding ability. Luminescence measurements showed that the mixture of human plasma alpha 1-acid glycoprotein (hAGP) and Cypridina luciferin produced light. The total value of the luminescence intensity over 60 s was over 12.6-fold higher than those in the presence of ovalbumin, human serum albumin, or bovine serum albumin. In the presence of heat-treated hAGP, the luminescence intensity of Cypridina luciferin was lower than in the presence of intact hAGP. Chlorpromazine, which binds to hAGP, showed an inhibitory effect on the luminescence of Cypridina luciferin, both in the presence of hAGP and a recombinant CLase. Furthermore, BlastP analysis showed that hAGP had partial amino acid sequence similarity to known CLases in the region including amino acid residues involved in the drug-binding ability of hAGP. These findings indicate enzymological similarity between hAGP and CLase and provide insights into both the enzymological understanding of CLase and development of a luminescence detection method for hAGP.
1. Introduction
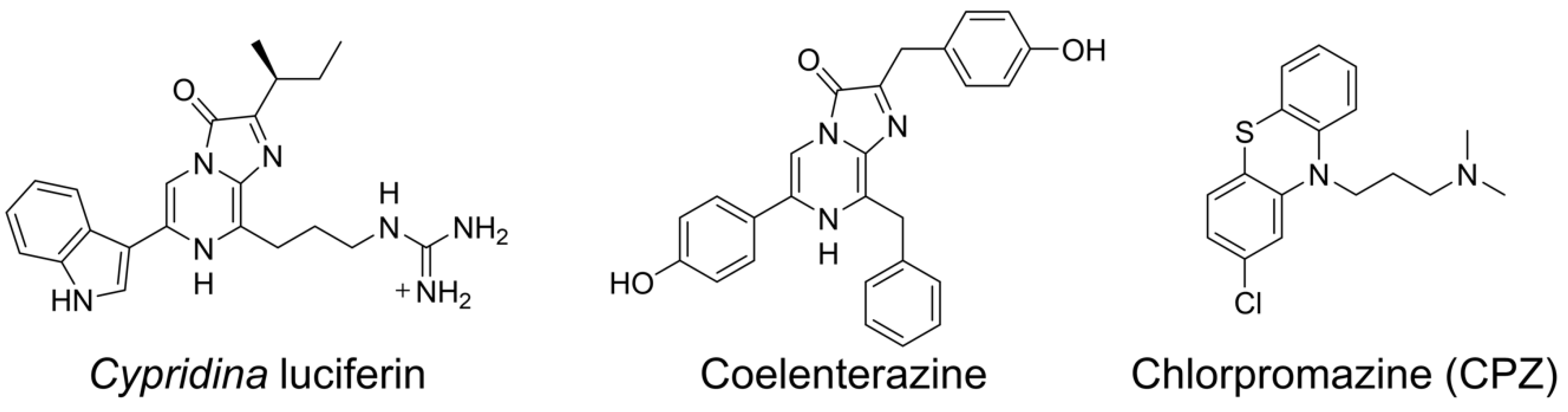
The luminous ostracods of the family Cypridinidae, commonly called sea fireflies, produce blue light (λmax = 448–463 nm depending on the buffer composition) by an enzyme-catalyzed chemical reaction with excellent quantum yield (ΦBL = ~0.30) [1,2,3]. In the bioluminescence reaction, Cypridina luciferin (Figure 1), recently called cypridinid luciferin, is oxidized in the presence of Cypridina luciferase (CLase), recently called cypridinid luciferase, and molecular oxygen (oxidation step), followed by generation of the oxyluciferin in the excited state (excitation step) and subsequent change to the ground state with light emission (light production step) [3,4,5]. The molecular mechanism of Cypridina bioluminescence has been studied [3] since Hirata’s group determined the structure of Cypridina luciferin after many great efforts to study Cypridina bioluminescence in the first half of the nineteenth century [6,7,8]. For understanding the mechanism in terms of the enzyme, CLase genes were cloned from Vargula (Cypridina) hilgendorfii and Cypridina noctiluca, and these recombinant CLases were characterized [9,10]. Recently, transcriptome analyses of cypridinid ostracods that inhabit the Caribbean Sea or the coast of California identified thirteen putative CLases [11], and an efficient expression system of a recombinant CLase was developed [12]. However, the protein crystal structure of CLase has yet to be solved, and the catalytic mechanisms, including knowledge of the active sites, remain to be clarified. To date, some applications of Cypridina bioluminescence have been reported [13], but the poor enzymological understanding of the bioluminescence is one obstacle hindering future applications.
Figure 1.
Chemical structures of compounds used in this study.
To understand how the reaction of Cypridina luciferin with CLase results in efficient luminescence, researchers have focused on the chemiluminescence features of Cypridina luciferin or its analogs under various chemical conditions [14,15,16,17,18,19,20]. In 1966, Johnson et al. reported spontaneous luminescence of Cypridina luciferin in dimethyl sulfoxide (DMSO) without CLase [21] while such luminescence was slightly observed in an aqueous solution in the absence of CLase [22]. After these findings, Goto et al. reported that Cypridina luciferin or its analogs emitted light more efficiently in diethylene glycol dimethyl ether containing acetate buffer (pH 5.6) or Tris buffer (pH 9.0) containing cetyltrimethylammonium bromide than in DMSO [23,24]. In addition, fluorometric titration analyses of the oxidized Cypridina luciferin–CLase complex indicated that Cypridina luciferin was surrounded by a hydrophobic environment in CLase [25]. From these observations, Goto et al. hypothesized that, for Cypridina bioluminescence, CLase plays an important role not only in promoting the reaction of Cypridina luciferin with molecular oxygen but also in providing Cypridina luciferin with a hydrophobic environment to emit light efficiently [24,26].
Supporting this hypothesis, previous papers have reported luminescence of Cypridina luciferin or its imidazopyrazinone-type analogs in the presence of molecules that have hydrophobic cavities responsible for their compound-binding abilities (e.g., cyclodextrins or serum albumins) [15,27,28,29,30,31,32,33]. The mechanism of luminescence in the presence of a serum albumin or a cyclodextrin is not well understood, but it is suggested that the hydrophobic cavities of these molecules are involved [15,30]. For further understanding of Cypridina bioluminescence through Goto’s hypothesis, we focused on human plasma alpha 1-acid glycoprotein (hAGP), also called orosomucoid. The glycoprotein hAGP is a plasma protein-like serum albumin and has one hydrophobic cavity responsible for its binding to basic drugs [34,35,36]. Chlorpromazine (CPZ) is one of the basic drugs that bind to hAGP (Figure 1), and the cocrystal structure analysis showed that CPZ bound to the hydrophobic cavity [35]. Given the basic guanidine moiety found in Cypridina luciferin and that the luminescence of Cypridina luciferin analogs depended on fitting to cyclodextrin’s hydrophobic cavity [15,27], we conjectured that Cypridina luciferin would bind to hAGP with high affinity and would emit light more efficiently than in the presence of a serum albumin.
In this study, we examined the luminescence of Cypridina luciferin in the presence of hAGP. We showed that the luminescence intensity of Cypridina luciferin in the presence of hAGP was higher than in the presence of ovalbumin (OVA), human serum albumin (HSA), or bovine serum albumin (BSA). In addition, we found that hAGP had partial amino acid sequence similarity to CLases from C. noctiluca and V. hilgendorfii in the region including amino acid residues responsible for the drug-binding ability of hAGP.
2. Results
2.1. Luminescence of Cypridina Luciferin in the Presence of hAGP
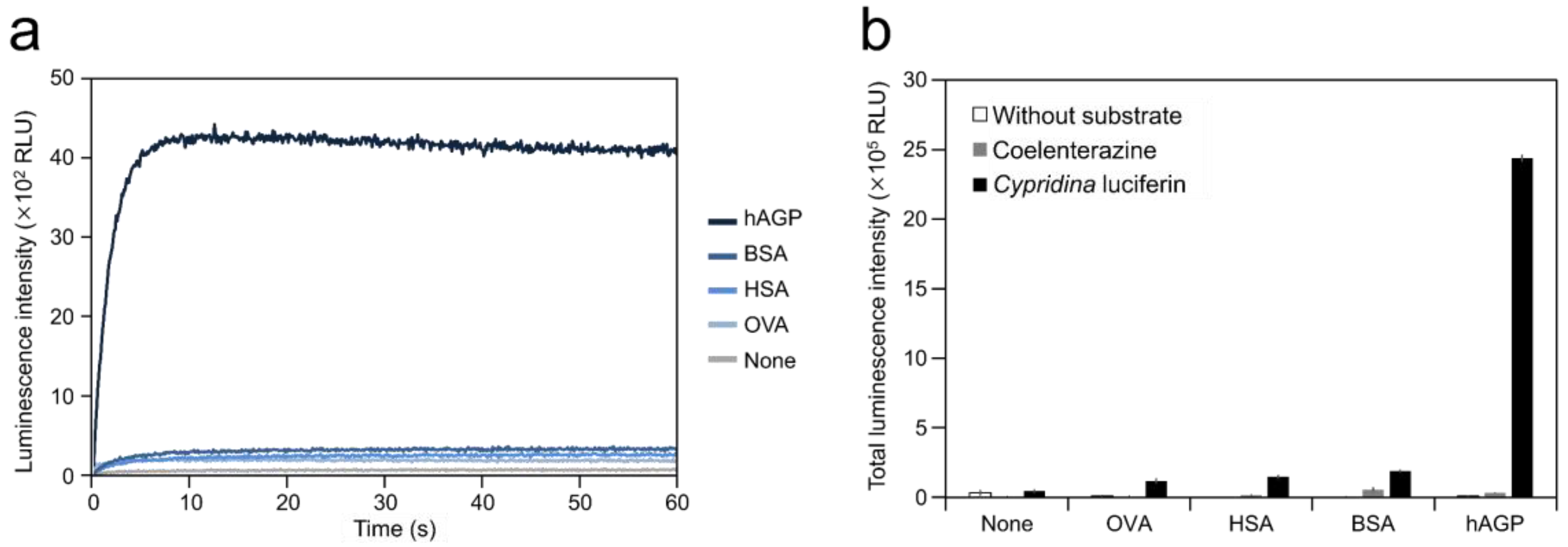
To test the ability of hAGP to cause luminescence in Cypridina luciferin, we measured luminescence from a mixture of Cypridina luciferin with hAGP. Luminescence measurement showed that the mixture of Cypridina luciferin and hAGP in Tris-HCl (pH 7.5) exhibited glow-type light emission (Figure 2a). The total value of the luminescence intensity over 60 s was 19.9, 16.1, and 12.6-fold higher than those for the mixtures of Cypridina luciferin with OVA, HSA, and BSA (Figure 2b and Table 1). In contrast, no luminescence was observed from the mixture of coelenterazine, another imidazopyrazinone-type luciferin (Figure 1), with hAGP in Tris-HCl (pH 7.5) (Figure 2b). A comparison of hAGP and CLase showed that the total value of luminescence intensity of Cypridina luciferin over 60 s in the presence of 2.5 μg (approximately 106 pmol) of hAGP was 122-fold lower than that in the presence of 0.25 ng (approximately 4 fmol) of a recombinant CLase from C. noctiluca (Table 1).
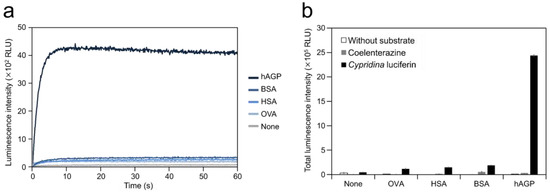
Figure 2.
Luminescence of Cypridina luciferin in the presence of human plasma alpha 1-acid glycoprotein (hAGP): (a) luminescence kinetics of Cypridina luciferin in the presence of various proteins and (b) luminescence intensity of Cypridina luciferin or coelenterazine in the presence of various proteins. None, in the absence of any proteins; OVA, ovalbumin; HSA, human serum albumin; BSA, bovine serum albumin; hAGP, human plasma alpha 1-acid glycoprotein; and CLase, a recombinant Cypridina luciferase from C. noctiluca. The bars represent the mean values ± SD for n = 3.

Table 1.
Luminescence of Cypridina luciferin in the presence of various proteins.
2.2. Luminescence Property of Cypridina Luciferin in the Presence of hAGP
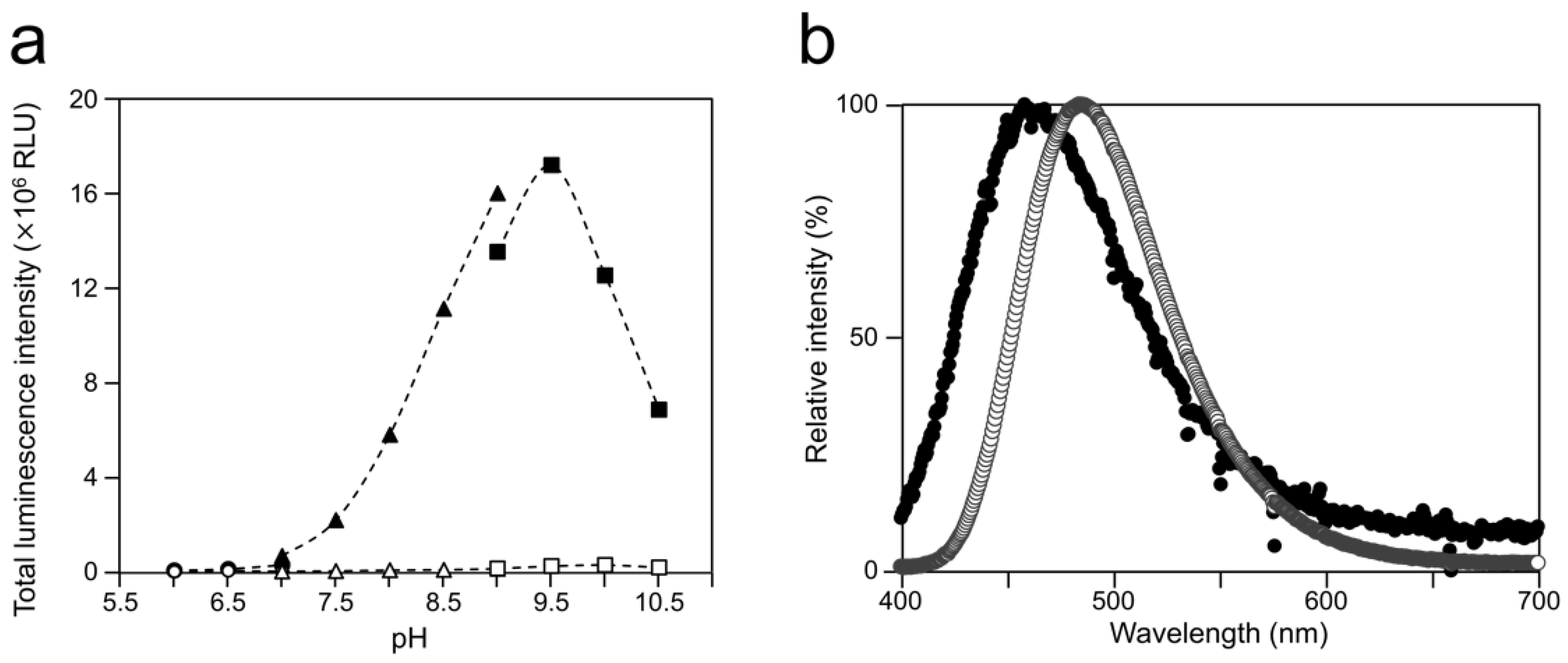
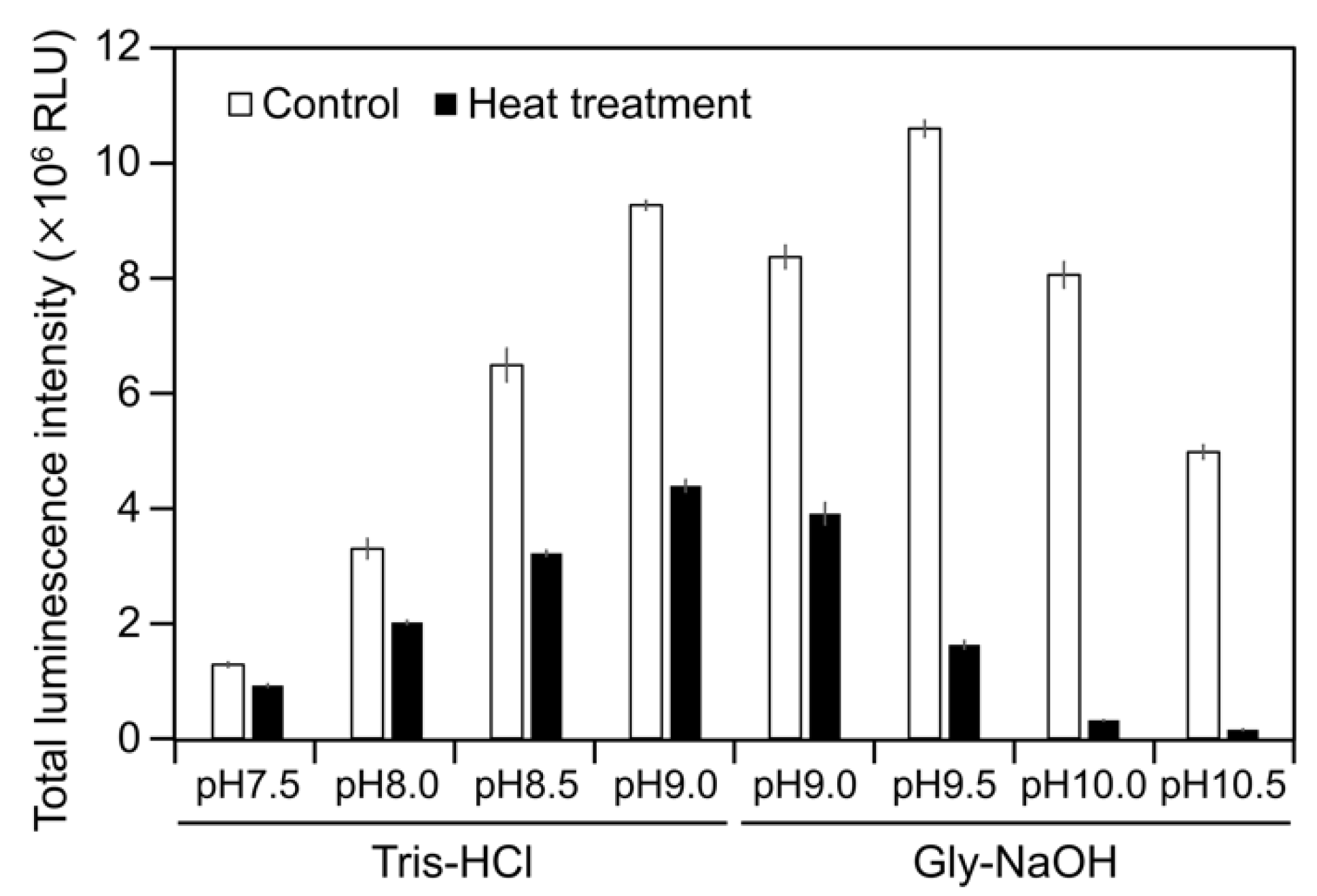
To examine the luminescence properties of the Cypridina luciferin-hAGP mixture, we measured luminescence intensity under various conditions. We observed luminescence in the range from pH 7.0 to 10.5 (Figure 3a). The maximum total value of the luminescence intensity over 60 s was obtained with Gly-NaOH (pH 9.5) buffer condition (Figure 3a). Under pH 7.5, 8.5, 9.5, and 10.5 buffer conditions, the maximum emission wavelength was 457 or 458 nm (Figure 3b, Figure A1 and Table A1). Although Cypridina luciferin showed chemiluminescence in the absence of hAGP at pH 9.0, 9.5, 10.0, and 10.5, the maximum total value of the luminescence intensity over 60 s was 60-fold lower than that in the presence of hAGP (Figure 3a). By heat treatment of hAGP at 95 °C for 30 min under pH 7.5–10.5 buffer conditions, the luminescence intensity of Cypridina luciferin decreased compared to that in the presence of intact hAGP (Figure 4). Under Gly-NaOH (pH 9.5) buffering conditions, as the concentration of Cypridina luciferin increased, luminescence intensity in the presence of hAGP increased and reached a plateau (Figure A2). Assuming that hAGP acted as a luciferase for Cypridina luciferin, the analysis of Michaelis–Menten kinetics of hAGP for Cypridina luciferin was performed and showed that the Km value was 0.46 μM (Figure A2). Luminescence measurements of the mixtures of Cypridina luciferin with various amounts of hAGP showed that the values of the total luminescence intensity over 60 s were proportional to the concentration of hAGP from 400 ng mL−1 to 10 μg mL−1 with a correlation efficient of 0.9993 (Figure A3).
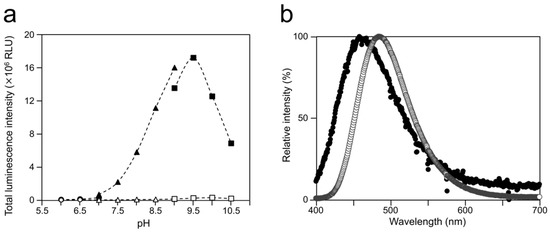
Figure 3.
Optimum pH for luminescence of Cypridina luciferin with hAGP and spectra at the optimum pH: (a) luminescence of Cypridina luciferin in the presence of hAGP under various pH buffer conditions and (b) emission spectra in the presence of hAGP or a recombinant CLase from C. noctiluca in 50 mM Gly-NaOH (pH 9.5). In panel (a), closed circles represent 50 mM BisTris-HCl containing hAGP, open circles represent 50 mM BisTris-HCl, closed triangles represent 50 mM Tris-HCl containing hAGP, open triangles represent 50 mM Tris-HCl, closed squares represent 50 mM Gly-NaOH containing hAGP, and open squares represent 50 mM Gly-NaOH. The bars represent the mean values ± SD for n = 3. In panel (b), closed circles represent the presence of hAGP and open circles represent the presence of CLase.
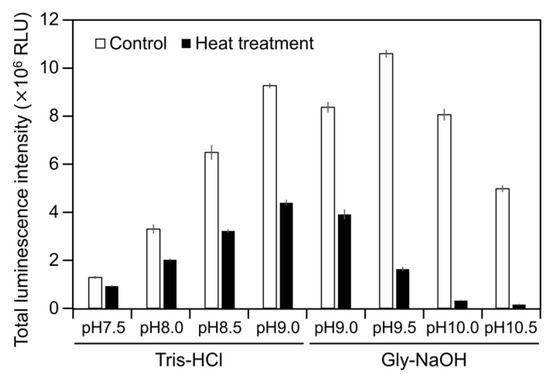
Figure 4.
Luminescence intensity of Cypridina luciferin in the presence of intact or heat-treated hAGP under various pH buffer conditions. Control, without heat treatment; heat treatment, heated at 95 °C for 30 min. The bars represent the mean values ± SD for n = 3.
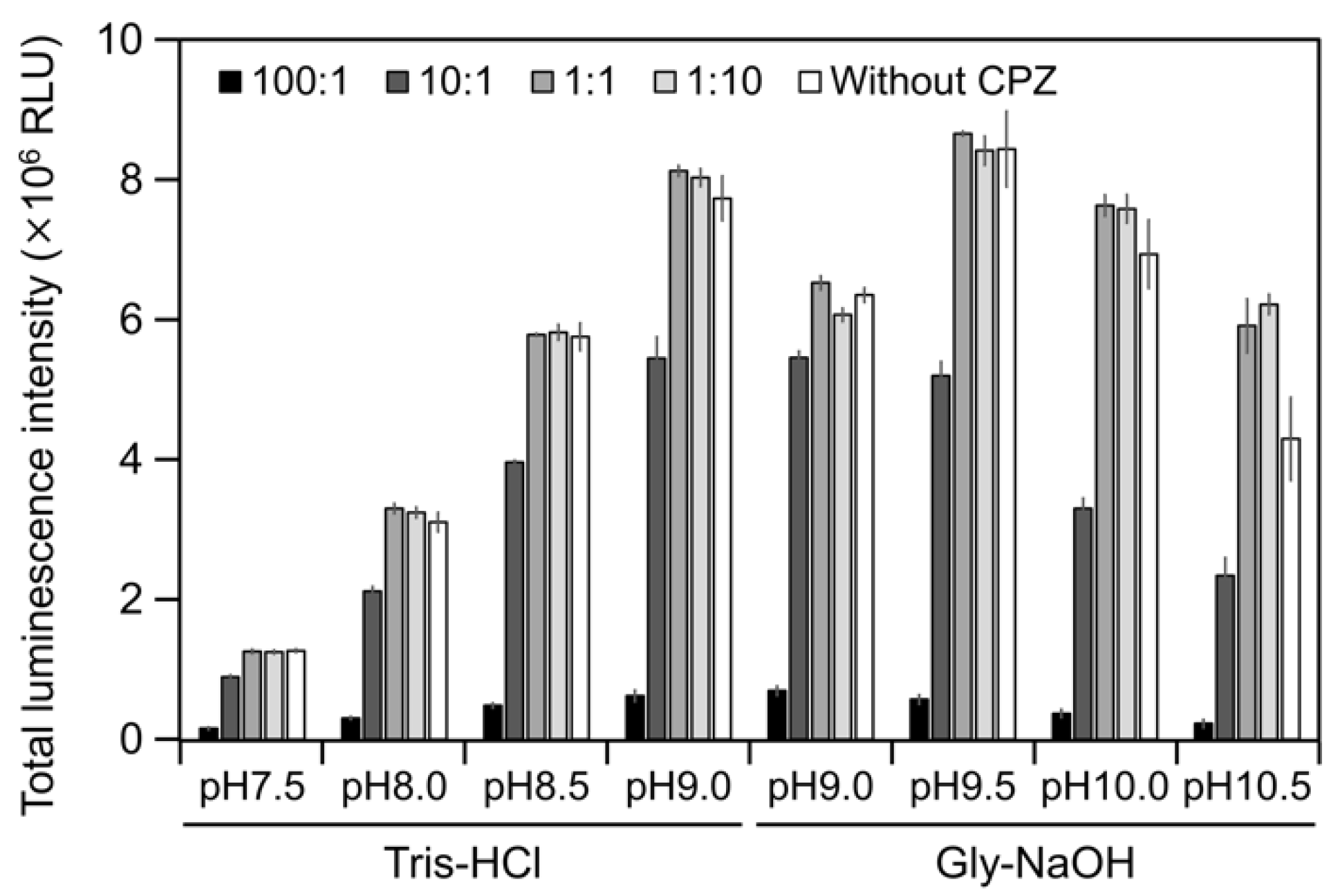
2.3. CPZ Effect on Luminescence of Cypridina Luciferin in the Presence of hAGP
To estimate the interaction of Cypridina luciferin with the drug-binding pocket in hAGP, we measured luminescence in the presence of hAGP with CPZ (Figure 1) under various pH conditions. When CPZ was present in the molar ratio of 100:1 or 10:1 (CPZ–Cypridina luciferin), the total values of the luminescence intensity over 60 s were lower than in the absence of CPZ under each buffer condition (Figure 5). Under the condition using a recombinant CLase from C. noctiluca instead of hAGP, the presence of CPZ in molar ratios of 100:1, 10:1, 1:1, or 1:10 (CPZ–Cypridina luciferin) also decreased the total values of luminescence intensity over 60 s compared to the absence of CPZ (Figure A4). BlastP analysis between hAGP (GenBank accession numer: CAA26397) and two CLases from V. hilgendorfii and C. noctiluca (AAA30332 and BAD08210) indicated that hAGP had a partial amino acid sequence similarity to these CLases in the region including amino acid residues involved in the drug-binding ability of hAGP, with E-values of 0.07 and 0.72, respectively (Figure A5 and Figure S1).
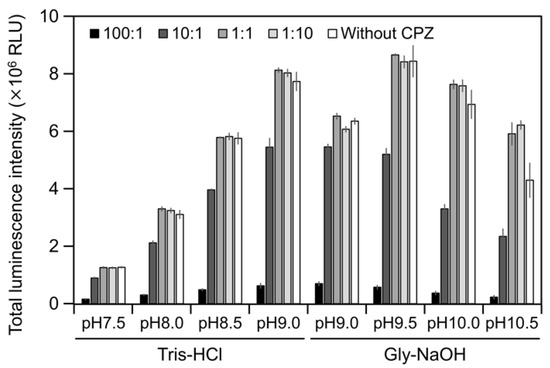
Figure 5.
Luminescence intensity of Cypridina luciferin in the presence of hAGP with or without Chlorpromazine (CPZ) under various pH buffer conditions: the ratios indicate the molar ratio of CPZ to 6 pmol of Cypridina luciferin; without CPZ represents in the presence of only 6 pmol of Cypridina luciferin. The bars represent the mean values ± SD for n = 3.
3. Discussion
In this study, we showed the luminescence of Cypridina luciferin in the presence of hAGP, a human plasma protein, under pH 7.5–10.5 buffer conditions (Figure 2 and Figure 3a). Luminescence intensity was highest at pH 9.5 (Figure 3a) and decreased in the presence of heat-treated hAGP compared to intact hAGP (Figure 4). These results did not reveal the role of hAGP in the luminescence process of Cypridina luciferin but suggested that hAGP exhibited enzymatic activity similar to that of CLase. When hAGP was considered an enzyme, its Km value for Cypridina luciferin was 0.46 μM, comparable to that of CLase (0.45 μM; Figure A2) [12], indicating that hAGP had a high affinity for Cypridina luciferin. This affinity probably enabled a low concentration of hAGP to interact with Cypridina luciferin to emit light (Figure 2 and Table 1). In contrast, insignificant luminescence was observed in the presence of the same concentrations of BSA or HSA (Figure 2 and Table 1), which were reported to cause luminescence of Cypridina luciferin analogs in previous studies [29,30,31,32]. This insignificant luminescence could be due to the lower affinity of both serum albumins to Cypridina luciferin, although efficiencies for each step of oxidation, excitation, and light production in the luminescence reaction of Cypridina luciferin in the presence of BSA, HSA, and hAGP must be clarified through an exact calculation of the quantum yield. Despite the high affinity of hAGP to Cypridina luciferin, the luminescence intensity in the presence of hAGP was much lower than in the presence of a recombinant CLase (Table 1). This result indicated that hAGP was not a specialized protein like luciferase for Cypridina luciferin, but the reason for the weaker luminescence is still unclear.
The luminescence spectra obtained from Cypridina luciferin in the presence of hAGP under various pH buffer conditions showed that λmax was in the range of 457–458 nm (Figure 3b and Figure A1 and Table A1), while that in the presence of a recombinant CLase was in the range of 478–483 nm (Figure 3b and Figure A1 and Table A1). Considering a previous report that λmax in luminescence of Cypridina luciferin depended on the surrounding environment [17,22], the difference implied that the environment inside each protein was different. However, we assumed that the character of the binding site of Cypridina luciferin in hAGP was similar to that of the catalytic site in CLase. This is because one possible explanation for the inhibitory effect by CPZ (Figure 5 and Figure A4) is that both catalytic sites for Cypridina luciferin inside hAGP and CLase are suitable for binding CPZ and therefore blocking the binding of Cypridina luciferin. Alternatively, a direct interaction of CPZ with Cypridina luciferin or electron-transfer quenching of the oxyluciferin in the excited state could reduce luminescence. Although the inhibitory effect on hAGP was observed only under excess CPZ conditions (CPZ–Cypridina luciferin = 100:1 or 10:1) (Figure 5), we cannot rule out the possibility that the CPZ-binding pocket of hAGP interacted with Cypridina luciferin considering the reported association constant of CPZ to hAGP, 232 ± 57 μM [37]. To clarify the inhibitory mechanism by CPZ, further investigation of the inhibitory effect by a CPZ analog that does not bind to hAGP or by a hAGP-binding compound structurally dissimilar to CPZ is required.
As shown in Figure A5 and Figure S1, we found that hAGP had partial amino acid sequence similarity to CLases from C. noctiluca and V. hilgendorfii mainly in the region that includes amino acid residues involved in the drug-binding ability of hAGP [34]. When we focused on CLase, we noticed that the partial amino acid sequences probably included amino acid residues responsible for the luciferase activity based on the previous study of truncated CLases [38,39]. Therefore, the alignment analysis of hAGP with CLase also allowed us to assume that the character of the drug-binding pocket of hAGP was similar to that of the catalytic pocket of CLase. It was noteworthy that the similarity between hAGP and CLases was found only when searching for amino acid sequence alignment between hAGP and CLase on a one-to-one basis, while an exhaustive homology search using CLase as a query did not reveal significant similarity between hAGP and CLase. This suggests that a one-to-one homology search is an important approach to find proteins with latent luciferase activity.
In future work based on our findings, we expect to develop a clinical examination method to quantify hAGP. In addition, we hope to conduct a study in terms of molecular evolution. Recently, hAGP has attracted attention as a clinical biomarker, and thus, a more practical method for detecting hAGP in a clinical setting will be required [36,40]. As shown in Figure A3, the luminescence intensity of Cypridina luciferin was proportional to the concentration of hAGP, at least in the range from 400 ng mL−1 to 10 μg mL−1, and to the quantitative linearity range reliable to detect the concentration of hAGP in a disease state [36]. Xu et al. reported that luminescence of a Cypridina luciferin analog in the presence of HSA can be used for clinical application to quantify HSA [31]. Therefore, luminescence detection of hAGP using Cypridina luciferin would seem to be another promising method, although further experiments using clinical specimens will be necessary to confirm this. When we considered hAGP as a luciferase to be used for a reporter assay or in vivo imaging, the luminescence intensity from the combination of hAGP with Cypridina luciferin was much lower than that from known luciferin-luciferase systems (Table 1). However, because molecular evolutionary studies have reported better bioluminescence systems using a non-natural luciferase or a synthetic luciferin analog [41,42,43], molecular engineering of hAGP or synthetic improvement of Cypridina luciferin could produce a practical Cypridina luciferin-based hAGP detection system to observe biological processes. Possibly, since known luciferases do not originate from mammalian proteins, the hAGP-based system could allow us to observe an intact biological event in mammalian systems. In addition, the study of proteins homologous to luciferases has contributed to understanding the origin of luciferases [32,44,45,46]. Although we do not consider hAGP to be directly related to the origin of CLase, our finding of the partial similarity between hAGP and CLase could provide insight into the substrate recognition sites related to its critical function and could pave a way to seek the origin of CLase.
4. Materials and Methods
4.1. Materials
The commercially available materials used in this study were obtained from the following commercial suppliers. Human alpha 1-acid glycoprotein (alpha 1-acid glycoprotein from human plasma; lot number: SLBJ6840V) was from Sigma-Aldrich (St. Louis, MO, USA). OVA (albumin from egg; lot number: CAL1231), BSA (albumin from bovine serum, fatty acid/IgG/protease free; lot number: CAQ4454), coelenterazine, chlorpromazine hydrochloride, Tris-HCl buffers, glycine, sodium hydroxide, and hydrochloric acid were from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). HSA (albumin, human serum, F-V; lot number: M8H7013) was from Nacalai Tesque (Kyoto, Japan). Cypridina luciferin was from ATTO Corporation (Tokyo, Japan). BisTris was from Dojindo Laboratories. All materials were used without further purification. A recombinant CLase from C. noctiluca was prepared according to the method reported previously [12].
4.2. Measurement of Luminescence Intensity
The luminescence intensity of the reaction mixtures in white 96-well plates (Eppendorf microplate 96/F-PP; Eppendorf, Hamburg, Germany) was measured using a luminometer (Phelios AB-2350; ATTO, Tokyo, Japan) and recorded in relative light units (RLU) in 0.1-s intervals over 60 s at room temperature.
4.3. Measurement of Luminescence Intensity of Cypridina Luciferin in the Presence of Proteins
To 50 μL of a 50 ug mL−1 solution of OVA, HSA, BSA, or hAGP in 100 mM Tris-HCl (pH 7.5) or 50 μL of a 5 ng mL−1 solution of a recombinant CLase in 100 mM Tris-HCl (pH 7.5) in 96-well plates was added 50 μL of a 0.2 μM aqueous solution of Cypridina luciferin or coelenterazine using the auto injector in the Phelios luminometer, followed by immediate measurement of luminescence intensity at room temperature. Each reaction was performed in triplicate. The aqueous solutions of Cypridina luciferin and coelenterazine were prepared by diluting concentrated solutions with distilled water. Concentrations of Cypridina luciferin or coelenterazine in the concentrated solutions were determined spectrophotometrically using the reported molar absorption coefficients [3,47]. Concentrations of OVA, HSA, BSA, hAGP, and CLase in the buffer solutions were determined using the corresponding molar extinction coefficients at 280 nm, calculated with a peptide property calculator [48]. These solutions were prepared immediately prior to their use in this experiment.
4.4. Measurement of Luminescence Intensity of Cypridina Luciferin with hAGP under Various pH Conditions
To 50 μL of a 50 μg mL−1 solution of hAGP in 100 mM BisTris-HCl (pH 6.0, 6.5, and 7.0), Tris-HCl (pH 7.0, 7.5, 8.0, 8.5, and 9.0), or Gly-NaOH (pH 9.0, 9.5, 10.0, and 10.5) in 96-well plates was added 50 μL of a 0.2 μM aqueous solution of Cypridina luciferin using the auto injector in the luminometer, followed by immediate measurement of luminescence intensity at room temperature. Each reaction was performed in triplicate. The aqueous solution of Cypridina luciferin and the buffer solutions of hAGP were prepared as described in Section 4.3.
4.5. Measurement of Luminescence Emission Spectrum
The luminescence emission spectra of Cypridina luciferin in the presence of hAGP or CLase under various pH buffer conditions were measured using a high sensitivity charge coupled device (CCD) spectrophotometer, LumiFLspectrocapture (AB-1850C; ATTO), with the following settings: measurement mode, single; measurement time, 1 min for CLase or 5 min for hAGP; slit width, 0.5 mm; camera gain, high; diffraction grating, 150 lines/mm; and shutter for measurement, automatic. To 50 μL of a 50 ug mL−1 solution of hAGP or a 50 ng mL−1 solution of a recombinant CLase in 100 mM Tris-HCl (pH 7.5 or pH 8.5) or Gly-NaOH (pH 9.5 or pH 10.5) in a 0.2 mL micro-tube (0.2 mL thin-walled tube; Thermo Fisher Scientific, MA, USA) was added 50 μL of a 0.2 μM (for CLase) or a 2 μM (for hAGP) aqueous solution of Cypridina luciferin manually, followed by immediate measurement of luminescence emission spectrum at room temperature.
4.6. Measurement of Luminescence Intensity of Cypridina Luciferin with Heat-Treated hAGP
To 30 μL of a 50 ug mL−1 solution of intact or heat-treated hAGP in 100 mM Tris-HCl (pH 7.5, 8.0, 8.5, and 9.0) or Gly-NaOH (pH 9.0, 9.5, 10.0, and 10.5) in 96-well plates was added 30 μL of a 0.2 μM aqueous solution of Cypridina luciferin using the auto injector in the luminometer, followed by immediate measurement of luminescence intensity at room temperature. Each reaction was performed in triplicate. The aqueous solution of Cypridina luciferin and the buffer solutions of hAGP were prepared as described in Section 4.3. Heat denaturation of hAGP (heat-treated hAGP) was performed in 100 mM Tris-HCl (pH 7.5, 8.0, 8.5, and 9.0) or Gly-NaOH (pH 9.0, 9.5, 10.0, and 10.5) at 95 °C for 30 min using a Block Bath Shaker (MyBL-100CS; AS ONE Corporation, Osaka, Japan).
4.7. Measurement of Luminescence Intensity of Cypridina Luciferin and CPZ with hAGP or CLase
To 30 μL of a 50 ug mL−1 solution of hAGP or a 25 pg mL−1 solution of a recombinant CLase in 100 mM Tris-HCl (pH 7.5, 8.0, 8.5, and 9.0) or Gly-NaOH (pH 9.0, 9.5, 10.0, and 10.5) containing 20, 2, 0.2, or 0.02 μM CPZ or without CPZ in 96-well plates was added 30 μL of a 0.2 μM aqueous solution of Cypridina luciferin using the auto injector in the luminometer, followed by immediate measurement of luminescence intensity at room temperature. Each reaction was performed in triplicate. The aqueous solution of Cypridina luciferin and the buffer solutions of hAGP and CLase were prepared as described in Section 4.3. Before the measurement of luminescence intensity, the solutions containing CPZ were incubated for 10 min at room temperature.
4.8. Linear Regression Analysis between Luminescence Intensity of Cypridina Luciferin and Amount of hAGP
To 50 μL of a 6.4, 16, 32, 80, 160, 400, 800, 2000, 4000, 10,000, or 20,000 ng mL−1 solution of hAGP in 100 mM Gly-NaOH (pH 9.5) in 96-well plates was added 50 μL of a 1.6 μM aqueous solution of Cypridina luciferin by the auto injector in the luminometer, followed by immediate measurement of luminescence intensity at room temperature. Each reaction was performed in triplicate. The aqueous solution of Cypridina luciferin and the buffer solution of hAGP were prepared as described in Section 4.3. The obtained data were subjected to linear regression analysis using Excel (2016, Microsoft, Redmond, WA, USA).
4.9. Kinetic Analysis of hAGP with Cypridina Luciferin
To 50 μL of a 50 μg mL−1 solution of hAGP in 100 mM Gly-NaOH (pH 9.5) in 96-well plates was added 50 μL of a 0, 0.08, 0.16, 0.4, 0.8, 1.6, or 3.2 μM aqueous solution of Cypridina luciferin using the auto injector in the luminometer, followed by immediate measurement of luminescence intensity at room temperature. Each reaction was performed in triplicate. The aqueous solution of Cypridina luciferin and the buffer solutions of hAGP and CLase were prepared as described in Section 4.3. The obtained data were subjected to kinetic analysis using the R program [49] to fit the Michaelis–Menten equation.
4.10. Comparison of Amino Acid Sequences between hAGP and CLases
Protein similarity between hAGP and CLases was estimated using the BlastP program (National Center for Biotechnology Information) [50]. Alignment of hAGP with CLases was performed using ClustalW (DNA Data Bank of Japan) [51]. These analyses used the information of hAGP (CAA26397) and CLases from C. noctiluca and V. hilgendorfii (BAD08210 and AAA30332).
5. Conclusions
We showed for the first time that hAGP enabled Cypridina luciferin to emit light and that hAGP had a partial amino acid sequence similarity to CLase in the region including amino acid residues involved in the drug-binding pocket of hAGP. These findings could help to break the current impasse in both fundamental and applied research on the Cypridina bioluminescence system, although further enzymological understanding of not only CLase but also hAGP in the presence of Cypridina luciferin is required.
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/1422-0067/21/20/7516/s1. Figure S1: Overall alignment of hAGP with Clases.
Author Contributions
Conceptualization, S.K. and Y.M.; early set up of this work and preparation of CLase, S.K. and M.K.; formal analysis, S.K. and Y.M.; investigation, S.K.; resources, S.K. and Y.M.; data curation, S.K. and Y.M.; writing—original draft preparation, S.K.; writing—review and editing, S.K. and Y.M.; visualization, S.K.; funding acquisition, S.K. and Y.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research and the APC were partially funded by JSPS KAKENHI grant numbers JP19K20205 (to S.K.) and JP18KK0199 (to S.K. and Y.M.).
Acknowledgments
We are grateful to Nicholai M. Hensley (University of California, Santa Barbara, CA, USA) for reviewing the manuscript, and we thank Tsutomu Kanda (AIST, Hokkaido, Japan) and the Atsubetsu Library for their assistance in obtaining literature related with this work.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
| CLase | Cypridina luciferase |
| hAGP | Human alpha 1-acid glycoprotein |
| λmax | Maximum emission wavelength |
| ΦBL | Quantum yield of bioluminescence |
| DMSO | Dimethyl sulfoxide |
| OVA | Ovalbumin |
| BSA | Bovine serum albumin |
| HSA | Human serum albumin |
| Tris | Tris(hydroxymethyl)aminomethane |
| Gly | Glycine |
| CPZ | Chlorpromazine |
| BlastP | Protein-protein basic local alignment search tool |
| RLU | Relative light unit |
| E-value | Expect value |
| SD | Standard deviation |
| HCl | Hydrochloric acid |
| BisTris | Bis(2-hydroxyethyl)iminotris(hydroxymethyl)methane |
| NaOH | Sodium hydroxide |
| CCD | Charge coupled device |
Appendix A
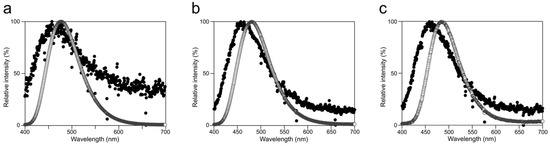
Figure A1.
Luminescence emission spectra of Cypridina luciferin in the presence of hAGP or a recombinant CLase from C. noctiluca: spectra (a) in 50 mM Tris-HCl (pH 7.5), (b) in 50 mM Tris-HCl (pH 8.5), and (c) in 50 mM Gly-NaOH (pH 10.5). Closed circles, with hAGP; open circles, with CLase.
Figure A1.
Luminescence emission spectra of Cypridina luciferin in the presence of hAGP or a recombinant CLase from C. noctiluca: spectra (a) in 50 mM Tris-HCl (pH 7.5), (b) in 50 mM Tris-HCl (pH 8.5), and (c) in 50 mM Gly-NaOH (pH 10.5). Closed circles, with hAGP; open circles, with CLase.

Table A1.
Maximum emission wavelength with hAGP or CLase under various pH buffer conditions.
Table A1.
Maximum emission wavelength with hAGP or CLase under various pH buffer conditions.
| Protein | Buffer Conditions | λmax (nm) |
|---|---|---|
| hAGP | Tris-HCl buffer (pH 7.5) | 458 |
| Tris-HCl buffer (pH 8.5) | 457 | |
| Gly-NaOH buffer (pH 9.5) | 458 | |
| Gly-NaOH buffer (pH 10.5) | 458 | |
| CLase | Tris-HCl buffer (pH 7.5) | 478 |
| Tris-HCl buffer (pH 8.5) | 480 | |
| Gly-NaOH buffer (pH 9.5) | 484 | |
| Gly-NaOH buffer (pH 10.5) | 483 |
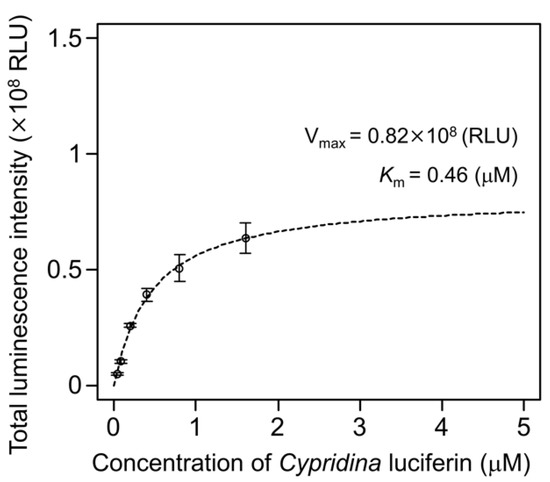
Figure A2.
Luminescence intensity of various concentrations of Cypridina luciferin with hAGP under a Gly-NaOH (pH 9.5) buffer condition: the bars represent the mean values ± SD for n = 3.
Figure A2.
Luminescence intensity of various concentrations of Cypridina luciferin with hAGP under a Gly-NaOH (pH 9.5) buffer condition: the bars represent the mean values ± SD for n = 3.
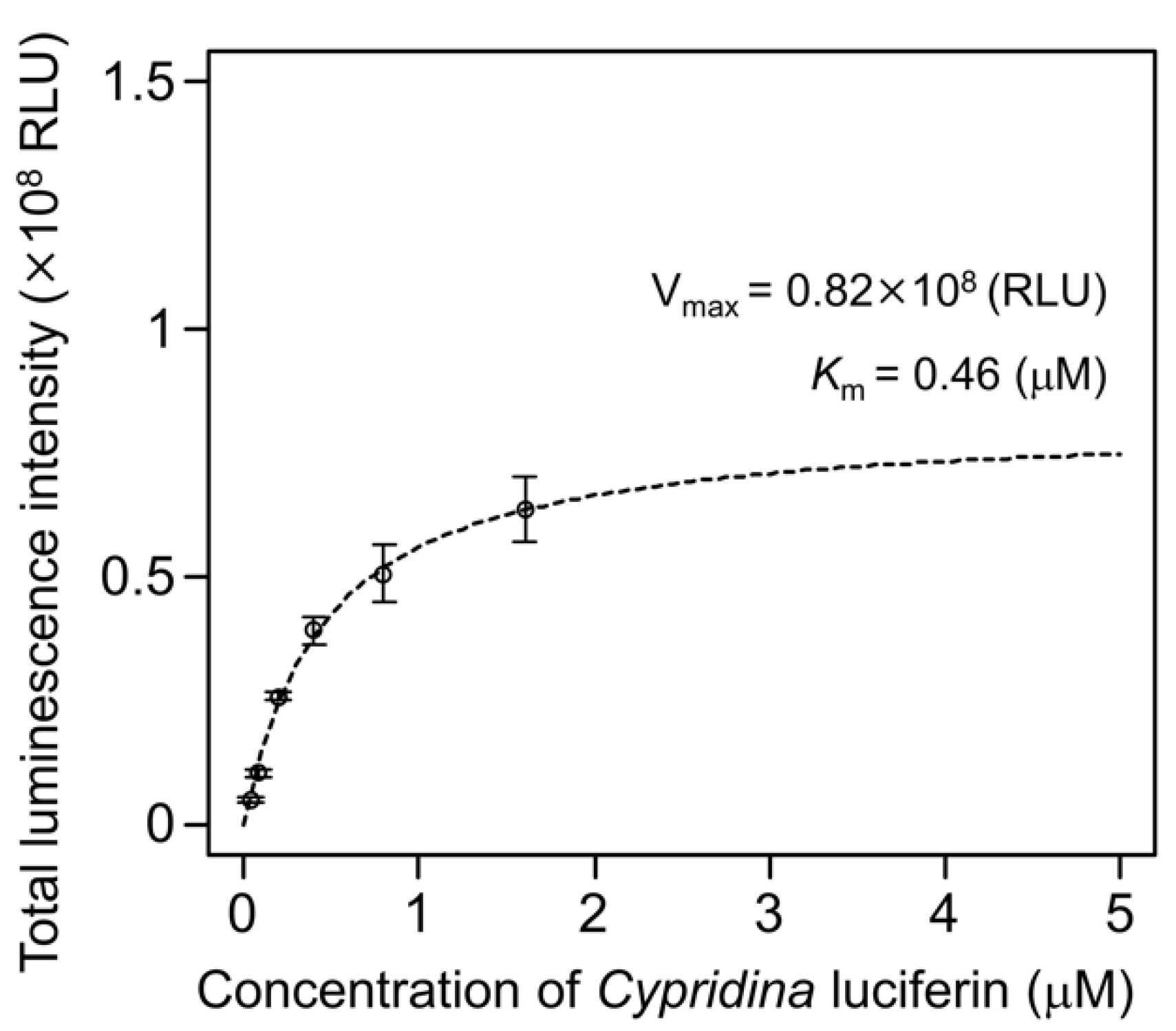
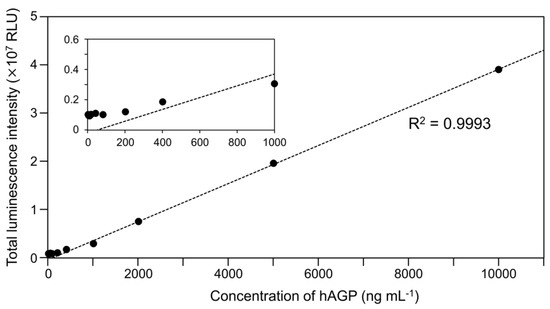
Figure A3.
Luminescence intensity of Cypridina luciferin with various concentrations of hAGP under a Gly-NaOH (pH 9.5) buffer condition: quantitative linearity (correlation efficient R2 = 0.9993) was observed from 400 ng mL−1 to 10 mg mL−1. The bars represent the mean values ± SD for n = 3. The inset shows an enlarged view of the range from 0–1000 ng mL−1.
Figure A3.
Luminescence intensity of Cypridina luciferin with various concentrations of hAGP under a Gly-NaOH (pH 9.5) buffer condition: quantitative linearity (correlation efficient R2 = 0.9993) was observed from 400 ng mL−1 to 10 mg mL−1. The bars represent the mean values ± SD for n = 3. The inset shows an enlarged view of the range from 0–1000 ng mL−1.
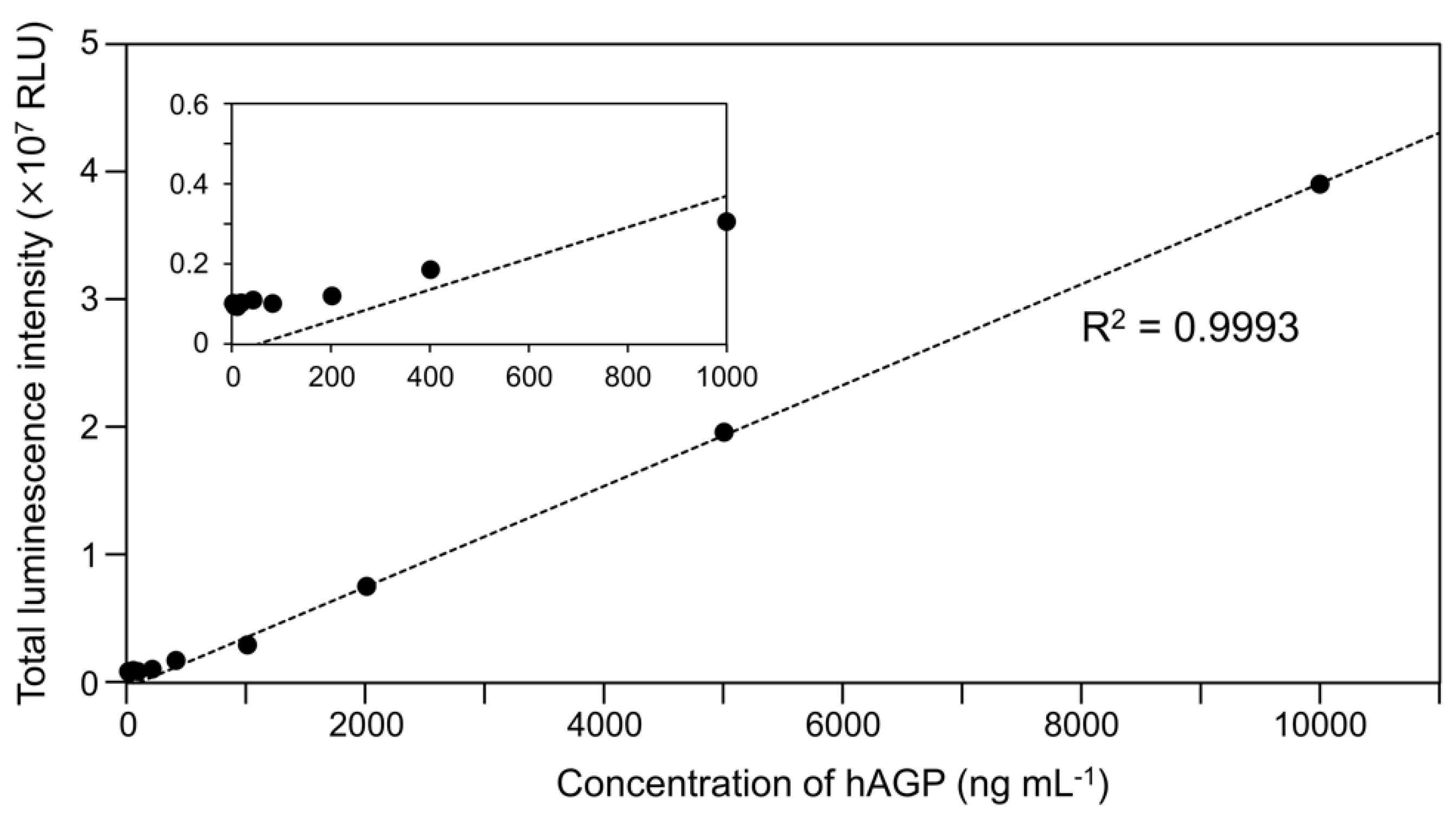
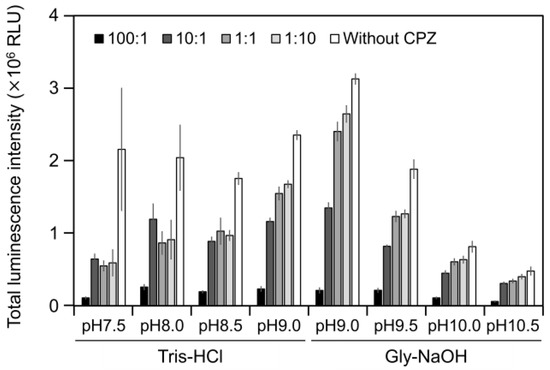
Figure A4.
Luminescence intensity of Cypridina luciferin in the presence of a recombinant CLase from C. noctiluca with or without CPZ under various pH buffer conditions: the ratios indicate the molar ratio of CPZ to 6 pmol of Cypridina luciferin; without CPZ represents in the presence of only 6 pmol of Cypridina luciferin. The bars represent the mean values ± SD for n = 3.
Figure A4.
Luminescence intensity of Cypridina luciferin in the presence of a recombinant CLase from C. noctiluca with or without CPZ under various pH buffer conditions: the ratios indicate the molar ratio of CPZ to 6 pmol of Cypridina luciferin; without CPZ represents in the presence of only 6 pmol of Cypridina luciferin. The bars represent the mean values ± SD for n = 3.
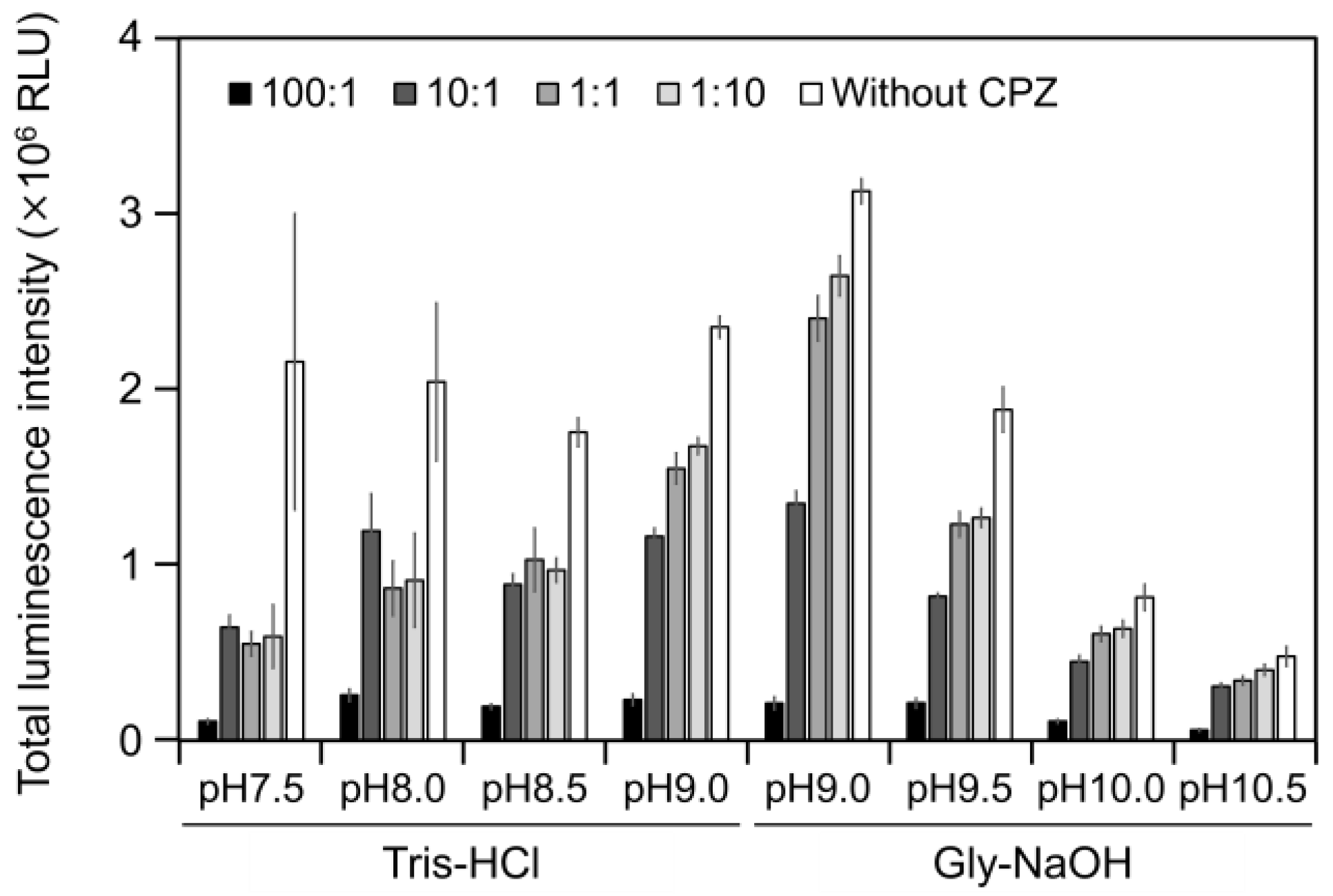

Figure A5.
Partial alignment of hAGP with CLases in the region where the amino acid sequence similarity was found in BlastP analysis: CLase1 and CLase2 indicate CLase from C. noctiluca and CLase from V. hilgendorfii, respectively. Gaps are represented as dashes (-). Numbers in parentheses indicate the positions of initial and last amino acid residues. Red characters indicate amino acids involved in the drug-binding ability of hAGP [34]. Navy-shaded characters marked with an asterisk (*) indicate identical amino acid residues. Steel blue-shaded characters marked with a colon (:) indicate sites belonging to group exhibiting strong similarity. Light blue-shaded characters marked with a period (.) indicate sites belonging to a group exhibiting weak similarity. The site similarity followed the criteria of ClustalW [51].
Figure A5.
Partial alignment of hAGP with CLases in the region where the amino acid sequence similarity was found in BlastP analysis: CLase1 and CLase2 indicate CLase from C. noctiluca and CLase from V. hilgendorfii, respectively. Gaps are represented as dashes (-). Numbers in parentheses indicate the positions of initial and last amino acid residues. Red characters indicate amino acids involved in the drug-binding ability of hAGP [34]. Navy-shaded characters marked with an asterisk (*) indicate identical amino acid residues. Steel blue-shaded characters marked with a colon (:) indicate sites belonging to group exhibiting strong similarity. Light blue-shaded characters marked with a period (.) indicate sites belonging to a group exhibiting weak similarity. The site similarity followed the criteria of ClustalW [51].

References
- Johnson, F.H.; Shimomura, O.; Saiga, Y.; Gershman, L.C.; Reynolds, G.T.; Waters, J.R. Quantum efficiency of Cypridina luminescence, with a note on that of Aequorea. J. Cell. Comp. Physiol. 1962, 60, 85–103. [Google Scholar] [CrossRef]
- Shimomura, O.; Johnson, F.H. Mechanisms in Quantum Yield of Cypridina Bioluminescence. Photochem. Photobiol. 1970, 12, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, O.; Yampolsky, I.V. Bioluminescence: Chemical Principles and Methods, 3rd ed.; World Scientific: Hackensack, NJ, USA, 2019. [Google Scholar]
- Johnson, F.H.; Shimomura, O. Introduction to the Cypridina System. Methods Enzymol. 1978, 57, 331–364. [Google Scholar]
- Morin, J.G. Based on a review of the data, use of the term ‘cypridinid’ solves the Cypridina/Vargula dilemma for naming the constituents of the luminescent system of ostracods in the family Cypridinidae. Luminescence 2011, 26, 1–4. [Google Scholar] [CrossRef]
- Harvey, E.N. Bioluminescence; Academic Press: New York, NY, USA, 1952. [Google Scholar]
- Shimomura, O.; Goto, T.; Hirata, Y. Crystalline Cypridina Luciferin. Bull. Chem. Soc. Jpn. 1957, 30, 929–933. [Google Scholar] [CrossRef]
- Hoffmann, R.W. Classical Methods in Structure Elucidation of Natural Products; Wiley-VHCA: Zürich, Switzerland, 2018. [Google Scholar]
- Thompson, E.M.; Nagata, S.; Tsuji, F.I. Cloning and expression of cDNA for the luciferase from the marine ostracod Vargula hilgendorfii. Proc. Natl. Acad. Sci. USA 1989, 86, 6567–6571. [Google Scholar] [CrossRef]
- Nakajima, Y.; Kobayashi, K.; Yamagishi, K.; Enomoto, T.; Ohmiya, Y. cDNA cloning and characterization of a secreted luciferase from the luminous Japanese ostracod, Cypridina noctiluca. Biosci. Biotechnol. Biochem. 2004, 68, 565–570. [Google Scholar] [CrossRef]
- Hensley, N.M.; Ellis, E.A.; Leung, N.Y.; Coupart, J.; Mikhailovsky, A.; Taketa, D.A.; Tessler, M.; Gruber, D.F.; De Tomaso, A.W.; Mitani, Y.; et al. Molecular evolution of luciferase diversified bioluminescent signals in sea fireflies. bioRxiv 2020. [Google Scholar] [CrossRef]
- Mitani, Y.; Oshima, Y.; Mitsuda, N.; Tomioka, A.; Sukegawa, M.; Fujita, M.; Kaji, H.; Ohmiya, Y. Efficient production of glycosylated Cypridina luciferase using plant cells. Protein Expr. Purif. 2017, 133, 102–109. [Google Scholar] [CrossRef]
- Kaskova, Z.M.; Tsarkova, A.S.; Yampolsky, I.V. 1001 lights: Luciferins, luciferases, their mechanisms of action and applications in chemical analysis, biology and medicine. Chem. Soc. Rev. 2016, 45, 6048–6077. [Google Scholar] [CrossRef]
- Takahashi, Y.; Kondo, H.; Maki, S.; Niwa, H.; Ikeda, H.; Hirano, T. Chemiluminescence of 6-aryl-2-methylimidazo 1,2-a pyrazin-3(7H)-ones in DMSO/TMG and in diglyme/acetate buffer: Support for the chemiexcitation process to generate the singlet-excited state of neutral oxyluciferin in a high quantum yield in the Cypridina (Vargula) bioluminescence mechanism. Tetrahedron Lett. 2006, 47, 6057–6061. [Google Scholar]
- Teranishi, K. Luminescence of imidazo 1,2-a pyrazin-3(7H)-one compounds. Bioorg. Chem. 2007, 35, 82–111. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Takahashi, Y.; Kondo, H.; Maki, S.; Kojima, S.; Ikeda, H.; Niwa, H. The reaction mechanism for the high quantum yield of Cypridina (Vargula) bioluminescence supported by the chemiluminescence of 6-aryl-2-methylimidazo 1,2-a pyrazin-3(7H)-ones (Cypridina luciferin analogues). Photochem. Photobiol. Sci. 2008, 7, 197–207. [Google Scholar] [PubMed]
- Naumov, P.; Wu, C.; Liu, Y.J.; Ohmiya, Y. Spectrochemistry and artificial color modulation of Cypridina luminescence: Indirect evidence for chemiexcitation of a neutral dioxetanone and emission from a neutral amide. Photochem. Photobiol. Sci. 2012, 11, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.W.; Naumov, P.; Liu, Y.J. Mechanistic Insight into Marine Bioluminescence: Photochemistry of the Chemiexcited Cypridina (Sea Firefly) Lumophore. J. Chem. Theory Comput. 2015, 11, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, C.M.; da Silva, J.; da Silva, L.P. Comparative study of the chemiluminescence of coelenterazine, coelenterazine-e and Cypridina luciferin with an experimental and theoretical approach. J. Photochem. Photobiol. B Biol. 2019, 190, 21–31. [Google Scholar]
- Min, C.G.; Liu, Q.B.; Leng, Y.; Magalhaes, C.M.; Huang, S.J.; Liu, C.X.; Yang, X.K.; da Silva, L.P. Mechanistic Insight into the Chemiluminescent Decomposition of Cypridina Dioxetanone and the Chemiluminescent, Fluorescent Properties of the Light Emitter of Cypridina Bioluminescence. J. Chem. Inf. Model. 2019, 59, 4393–4401. [Google Scholar]
- Johnson, F.H.; Stachel, H.D.; Taylor, E.C.; Shimomura, O. Chemiluminescence and Fluorescence of Cypridina Luciferin and of Some New Indole Compounds in Dimethylsulfoxide. In Bioluminescence in Progress; Johnson, F.H., Haneda, Y., Eds.; Princeton University Press: Princeton, NJ, USA, 1966; pp. 67–82. [Google Scholar]
- Goto, T. Chemistry of bioluminescence. Pure Appl. Chem. 1968, 17, 421–441. [Google Scholar]
- Goto, T.; Inoue, S.; Sugiura, S. Cypridina bioluminescence IV. Synthesis and chemiluminescence of 3,7-dihydroimidazo[1,2-a]pyrazin-3-one and its 2-methyl derivative. Tetrahedron Lett. 1968, 9, 3873–3876. [Google Scholar] [CrossRef]
- Goto, T.; Fukatsu, H. Cypridina bioluminescence VII. chemiluminescence in micelle solutions—A model system for cypridina bioluminescence. Tetrahedron Lett. 1969, 10, 4299–4302. [Google Scholar] [CrossRef]
- Shimomura, O.; Johnson, F.H.; Masugi, T. Cypridina Bioluminescence: Light-Emitting Oxyluciferin-Luciferase Complex. Science 1969, 164, 1299–1300. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Kubota, I.; Suzuki, N.; Kishi, Y.; Inoue, S. Aspects of the Mechanism of Bioluminescence. In Chemiluminescence and Bioluminescence; Cormier, M.J., Hercules, D.M., Lee, J., Eds.; Springer US: Boston, MA, USA, 1973; pp. 325–335. [Google Scholar]
- Toya, Y. Chemistry of Vargula (formerly Cypridina) BIOLUMINESCENCE. Nippon Nogeikagaku Kaishi J. Jpn. Soc. Biosci. Biotechnol. Agrochem. 1992, 66, 742–747. (In Japanese) [Google Scholar]
- Mitani, M.; Sakaki, S.; Koinuma, Y.; Toya, Y.; Kosugi, M. Enhancement Effect of 2, 6-O-Dimethyl-β-cyclodextrin on the Chemiluminescent Detection of β-D-Galactosidase Using a Cypridina Luciferin Analog. Anal. Sci. 1995, 11, 1013–1015. [Google Scholar] [CrossRef]
- Wang, J.; Xing, D.; He, Y.H.; Hu, X.J. Experimental study on photodynamic diagnosis of cancer mediated by chemiluminescence probe. FEBS Lett. 2002, 523, 128–132. [Google Scholar] [CrossRef]
- Zhou, J.; Zing, D.; Chen, Q. Enhancement of Fluoresceinyl Cypridina Luciferin Analog Chemiluminescence by Human Serum Albumin for Singlet Oxygen Detection. Photochem. Photobiol. 2006, 82, 1058–1064. [Google Scholar] [CrossRef]
- Xu, W.; Wei, Y.C.; Xing, D.; Chen, Q. A novel chemiluminescence technique for quantitative measurement of low concentration human serum albumin. Anal. Sci. 2008, 24, 115–119. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vassel, N.; Cox, C.D.; Naseem, R.; Morse, V.; Evans, R.T.; Power, R.L.; Brancale, A.; Wann, K.T.; Campbell, A.K. Enzymatic activity of albumin shown by coelenterazine chemiluminescence. Luminescence 2012, 27, 234–241. [Google Scholar] [CrossRef]
- Inouye, S.; Sahara-Miura, Y. A Novel Catalytic Function of Synthetic IgG-Binding Domain (Z Domain) from Staphylococcal Protein A: Light Emission with Coelenterazine. Photochem. Photobiol. 2014, 90, 137–144. [Google Scholar] [CrossRef]
- Bteich, M. An overview of albumin and alpha-1-acid glycoprotein main characteristics: Highlighting the roles of amino acids in binding kinetics and molecular interactions. Heliyon 2019, 5, e02879. [Google Scholar] [CrossRef]
- Nishi, K.; Ono, T.; Nakamura, T.; Fukunaga, N.; Izumi, M.; Watanabe, H.; Suenaga, A.; Maruyama, T.; Yamagata, Y.; Curry, S.; et al. Structural Insights into Differences in Drug-binding Selectivity between Two Forms of Human Alpha(1)-Acid Glycoprotein Genetic Variants, the A and F1*S Forms. J. Biol. Chem. 2011, 286, 14427–14434. [Google Scholar] [CrossRef]
- Smith, S.A.; Waters, N.J. Pharmacokinetic and Pharmacodynamic Considerations for Drugs Binding to Alpha-1-Acid Glycoprotein. Pharm. Res. 2019, 36, 30. [Google Scholar] [CrossRef]
- Matsumoto, K.; Sukimoto, K.; Nishi, K.; Maruyama, T.; Suenaga, A.; Otagiri, M. Characterization of Ligand Binding Sites on the α1-Acid Glycoprotein in Humans, Bovines and Dogs. Drug Metab. Pharmacokinet. 2002, 17, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Hunt, E.A.; Moutsiopoulou, A.; Broyles, D.; Head, T.; Dikici, E.; Daunert, S.; Deo, S.K. Expression of a soluble truncated Vargula luciferase in Escherichia coli. Protein Expr. Purif. 2017, 132, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Ueda, H.; Kazami, J.; Kawano, G.; Suzuki, E.; Nagamune, T. Truncation of Vargula luciferase still results in retention of luminescence. J. Biochem. 1996, 119, 601–603. [Google Scholar] [CrossRef] [PubMed]
- Gannon, B.M.; Glesby, M.J.; Finkelstein, J.L.; Raj, T.; Erickson, D.; Mehta, S. A point-of-care assay for alpha-1-acid glycoprotein as a diagnostic tool for rapid, mobile-based determination of inflammation. Curr. Res. Biotechnol. 2019, 1, 41–48. [Google Scholar] [CrossRef]
- Mofford, D.M.; Reddy, G.R.; Miller, S.C. Latent luciferase activity in the fruit fly revealed by a synthetic luciferin. Proc. Natl. Acad. Sci. USA 2014, 111, 4443–4448. [Google Scholar] [CrossRef]
- Iwano, S.; Sugiyama, M.; Hama, H.; Watakabe, A.; Hasegawa, N.; Kuchimaru, T.; Tanaka, K.Z.; Takahashi, M.; Ishida, Y.; Hata, J.; et al. Single-cell bioluminescence imaging of deep tissue in freely moving animals. Science 2018, 359, 935–939. [Google Scholar] [CrossRef]
- Adams, S.T.; Miller, S.C. Enzymatic promiscuity and the evolution of bioluminescence. FEBS J. 2020, 287, 1369–1380. [Google Scholar] [CrossRef]
- Chaloupkova, R.; Liskova, V.; Toul, M.; Markova, K.; Sebestova, E.; Hernychova, L.; Marek, M.; Pinto, G.P.; Pluskal, D.; Waterman, J.; et al. Light-Emitting Dehalogenases: Reconstruction of Multifunctional Biocatalysts. ACS Catal. 2019, 9, 4810–4823. [Google Scholar] [CrossRef]
- Fallon, T.R.; Lower, S.E.; Chang, C.H.; Bessho-Uehara, M.; Martin, G.J.; Bewick, A.J.; Behringer, M.; Debat, H.J.; Wong, I.; Day, J.C.; et al. Firefly genomes illuminate parallel origins of bioluminescence in beetles. Elife 2018, 7, e36495. [Google Scholar] [CrossRef]
- Campbell, A.K. Darwin shines light on the evolution of bioluminescence. Luminescence 2012, 27, 447–449. [Google Scholar]
- Kishi, Y.; Goto, T.; Hirata, Y.; Shimomura, O.; Johnson, F.H. Cypridina bioluminescence I Sructure of Cypridina luciferin. Tetrahedron Lett. 1966, 7, 3427–3436. [Google Scholar]
- Peptide Property Calculator. Available online: http://biotools.nubic.northwestern.edu/proteincalc.html (accessed on 7 August 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- BlastP. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi?PAGE=Proteins (accessed on 7 August 2020).
- ClustalW. Available online: https://clustalw.ddbj.nig.ac.jp/ (accessed on 7 August 2020).
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).