Effects of Arbuscular Mycorrhization on Fruit Quality in Industrialized Tomato Production
Abstract
1. Introduction
2. Results
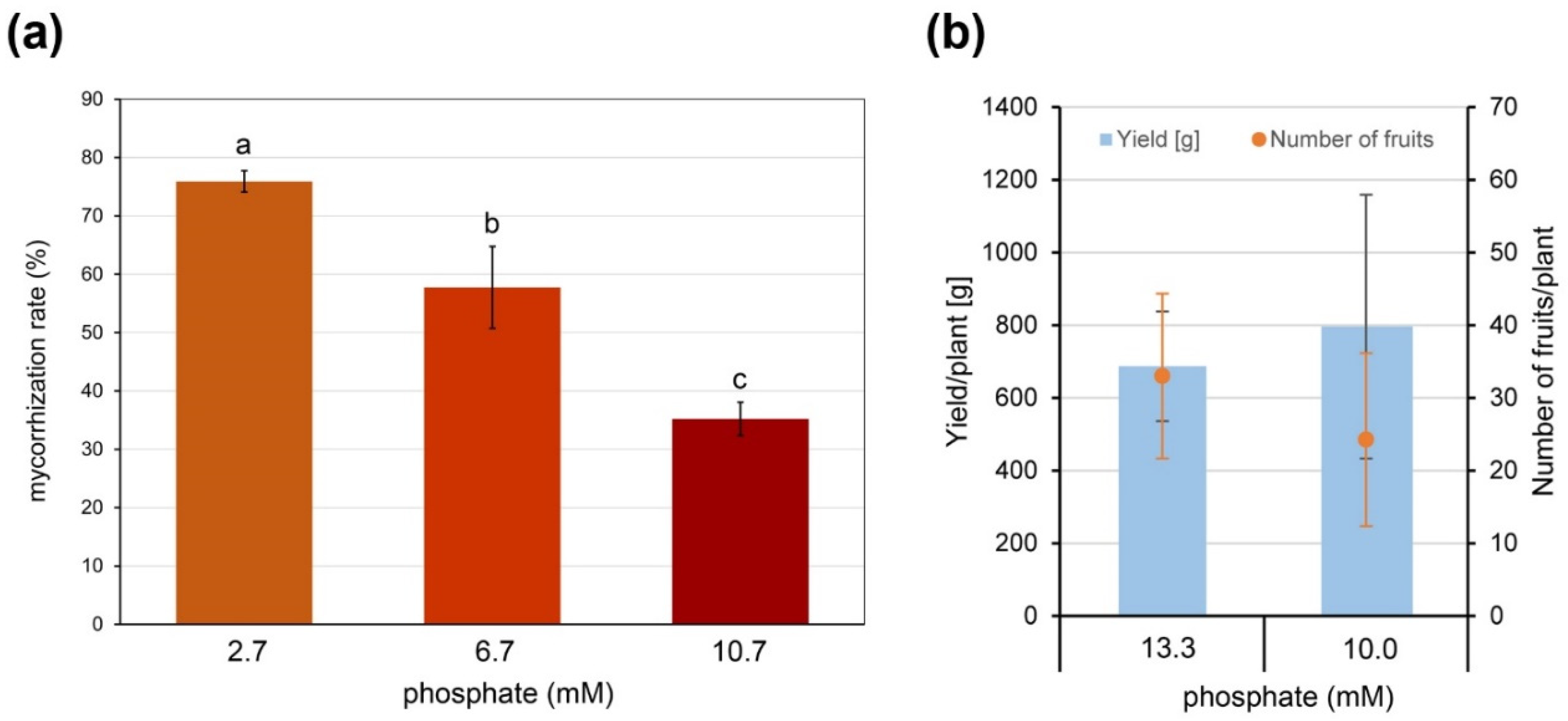
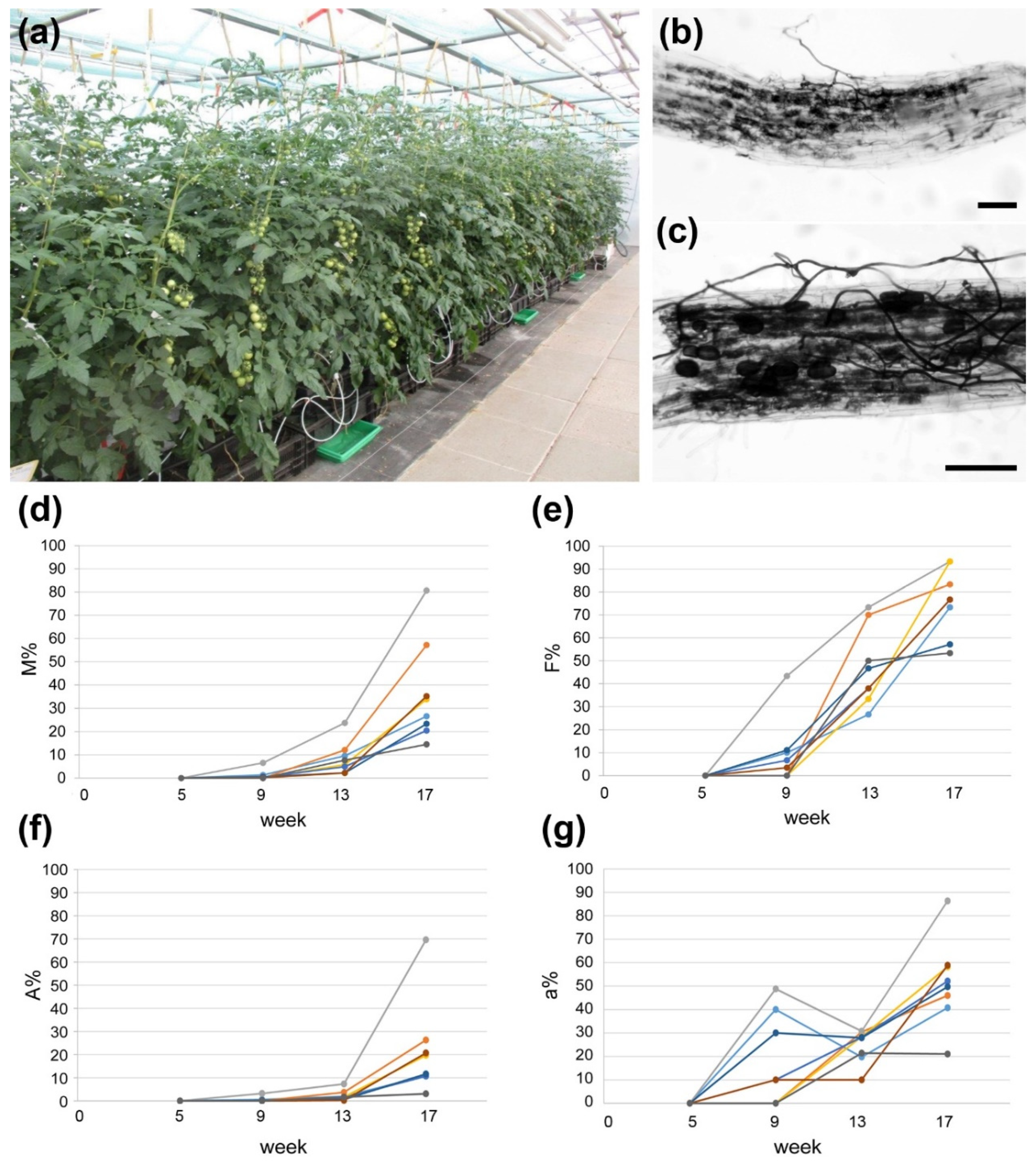
2.1. AM Establishment in Hydroponics Is Influenced by Growth Substrate and Fertilization
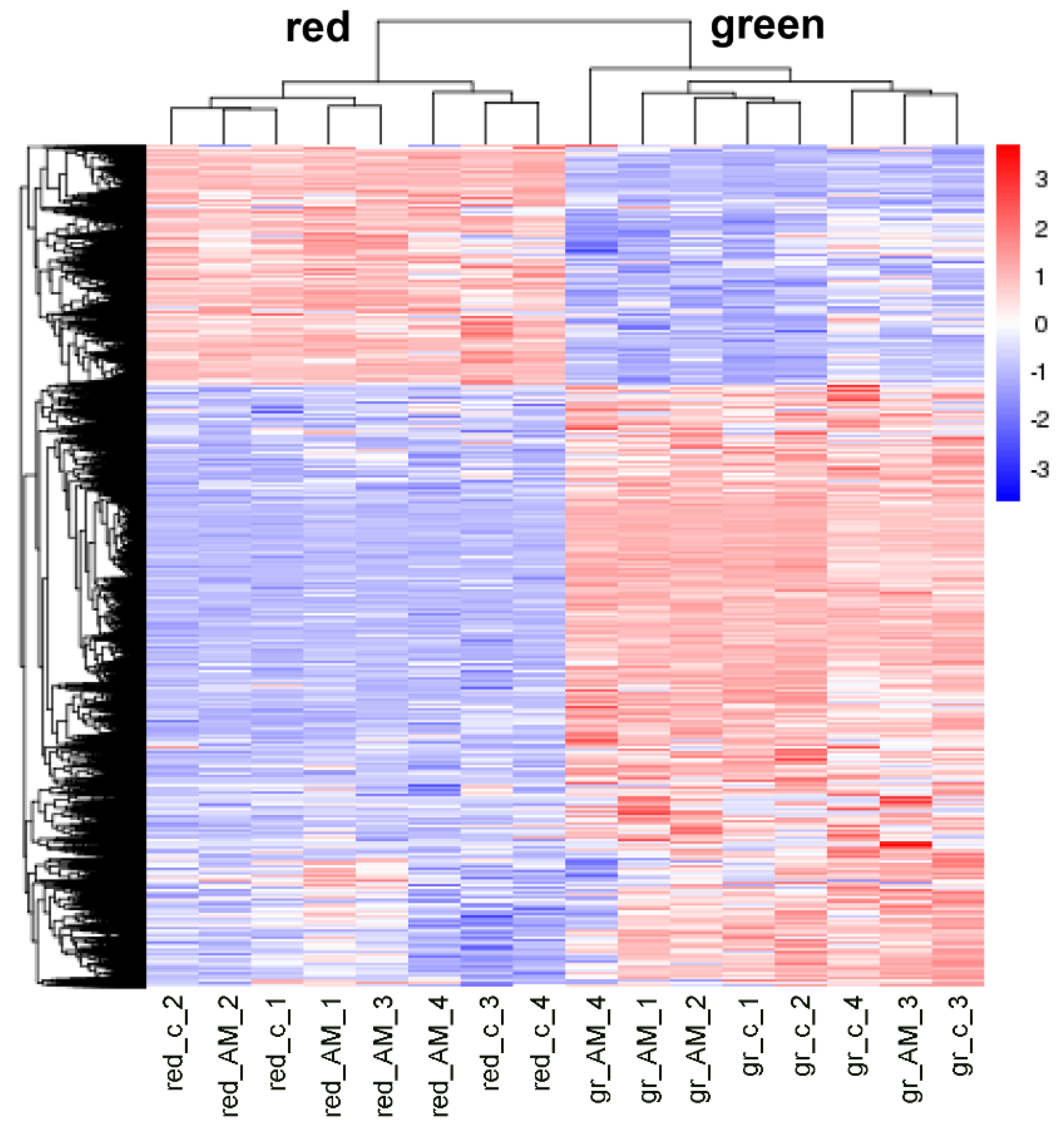
2.2. Mycorrhization of Plants Resulted in Minor Effects on Tomato Fruit Transcriptome
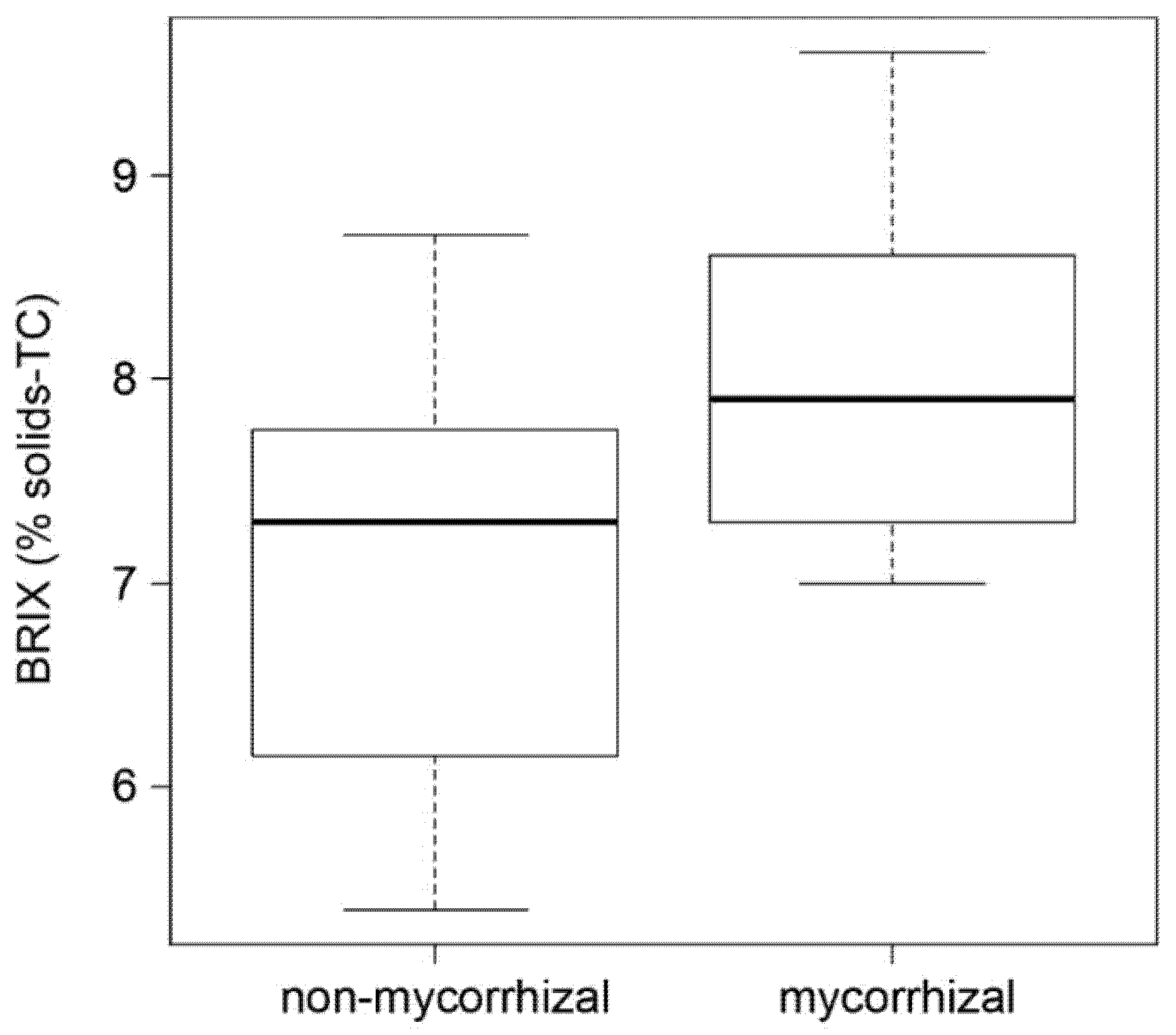
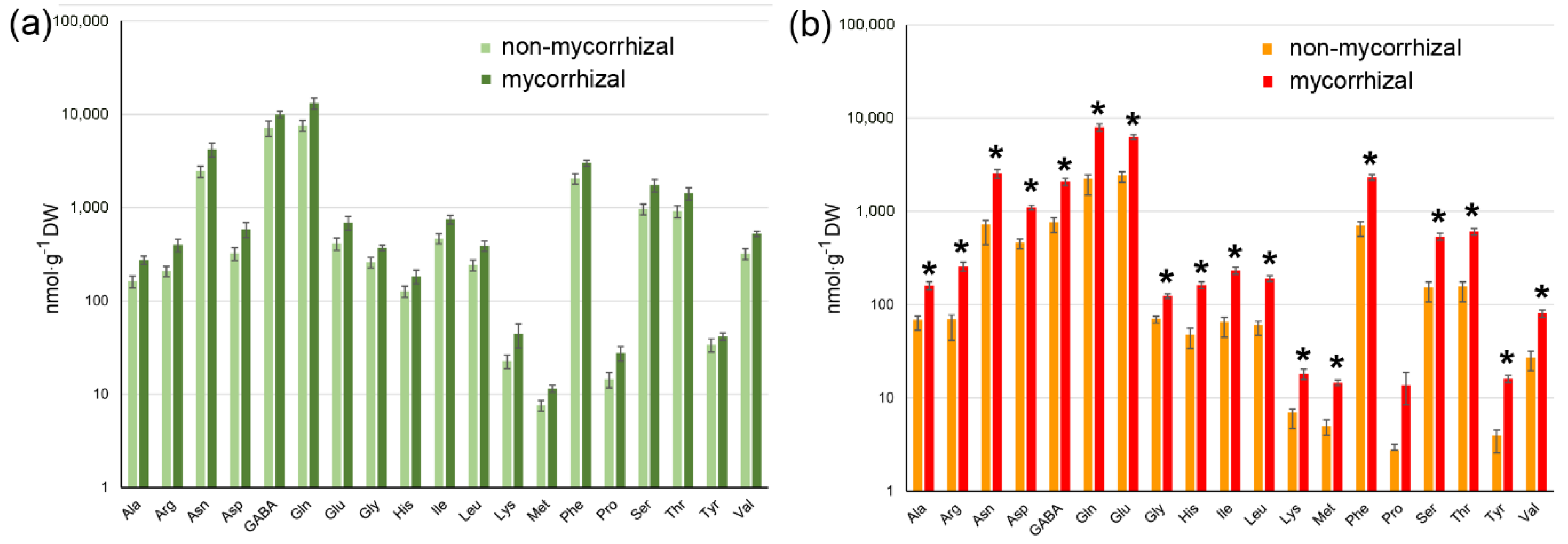
2.3. AM Effect on Tomato Fruit Quality
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Determination of Mycorrhization Rate
4.3. Transcriptomics and Quantitative Real-Time PCR (qPCR)
4.4. Determination of BRIX Values
4.5. Determination of Amino Acids, Carotenoids and Minerals
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AM | Arbuscular mycorrhiza |
| AMF | Arbuscular mycorrhizal fungus/fungi |
| DEGs | Differentially expressed genes |
| DW | Dry weight |
| FPKM | Fragments per kilobase of exon per million reads mapped |
| HPLC | High pressure liquid chromatography |
| Pi | Phosphate |
| qPCR | Quantitative polymerase chain reaction (real-time PCR) |
References
- Tieman, D.; Zhu, G.; Resende, M.F.R.; Lin, T.; Nguyen, C.; Bies, D.; Rambla, J.L.; Beltran, K.S.O.; Taylor, M.; Zhang, B.; et al. A chemical genetic roadmap to improved tomato flavor. Science 2017, 355, 391–394. [Google Scholar] [CrossRef]
- Li, B.; Boiarkina, I.; Young, B.; Yu, W.; Singhal, N. Prediction of Future Phosphate Rock: A Demand Based Model. J. Environ. Inform. 2018, 31, 41–53. [Google Scholar] [CrossRef]
- Spatafora, J.W.; Chang, Y.; Benny, G.L.; Lazarus, K.; Smith, M.E.; Berbee, M.L.; Bonito, G.; Corradi, N.; Grigoriev, I.; Gryganskyi, A.; et al. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 2016, 108, 1028–1046. [Google Scholar] [CrossRef] [PubMed]
- Brundrett, M.C. Coevolution of roots and mycorrhizas of land plants. New Phytol. 2002, 154, 275–304. [Google Scholar] [CrossRef]
- Bonfante, P.; Genre, A. Arbuscular mycorrhizal dialogues: Do you speak ‘plantish’ or ‘fungish’? Trends Plant Sci. 2015, 20, 150–154. [Google Scholar] [CrossRef]
- Smith, F.; Smith, S. What is the significance of the arbuscular mycorrhizal colonisation of many economically important crop plants? Plant Soil 2011, 348, 63–79. [Google Scholar] [CrossRef]
- Ferrol, N.; Tamayo, E.; Vargas, P. The heavy metal paradox in arbuscular mycorrhizas: From mechanisms to biotechnological applications. J. Exp. Bot. 2016, 67, 6253–6265. [Google Scholar] [CrossRef]
- Smith, S.; Smith, F.; Jakobsen, I. Mycorrhizal Fungi Can Dominate Phosphate Supply to Plants Irrespective of Growth Responses. Plant Physiol. 2003, 133, 16–20. [Google Scholar] [CrossRef]
- Rich, M.K.; Nouri, E.; Courty, P.-E.; Reinhardt, D. Diet of Arbuscular Mycorrhizal Fungi: Bread and Butter? Trends Plant Sci. 2017, 22, 652–660. [Google Scholar] [CrossRef]
- Pozo, M.J.; Azcón-Aguilar, C. Unraveling mycorrhiza-induced resistance. Curr. Opin. Plant Biol. 2007, 10, 393–398. [Google Scholar] [CrossRef]
- Evelin, H.; Kapoor, R.; Giri, B. Arbuscular mycorrhizal fungi in alleviation of salt stress: A review. Ann. Bot. 2009, 104, 1263–1280. [Google Scholar] [CrossRef] [PubMed]
- Miransari, M. Contribution of arbuscular mycorrhizal symbiosis to plant growth under different types of soil stress. Plant Biol. 2010, 12, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Bona, E.; Cantamessa, S.; Massa, N.; Manassero, P.; Marsano, F.; Copetta, A.; Lingua, G.; D’Agostino, G.; Gamalero, E.; Berta, G. Arbuscular mycorrhizal fungi and plant growth-promoting pseudomonads improve yield, quality and nutritional value of tomato: A field study. Mycorrhiza 2017, 27, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Guo, H.; Zhang, Q.; Guo, H.; Zhang, L.; Zhang, C.; Gou, Z.; Liu, Y.; Wei, J.; Chen, A.; et al. Arbuscular mycorrhizal fungi (AMF) enhanced the growth, yield, fiber quality and phosphorus regulation in upland cotton (Gossypium hirsutum L.). Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kapulnik, Y.; Lahkim, L.T.; Zipori, I.; Hazanovsky, M.; Wininger, S.; Dag, A. Effect of AMF application on growth, productivity and susceptibility to Verticillium wilt of olives grown under desert conditions. Symbiosis 2010, 52, 103–111. [Google Scholar] [CrossRef]
- Sabatino, L.; Iapichino, G.; Consentino, B.B.; D’Anna, F.; Rouphel, Y. Rootstock and Arbuscular Mycorrhiza Combinatorial Effects on Eggplant Crop Performance and Fruit Quality under Greenhouse Conditions. Agronomy 2020, 10, 693. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Zhou, J.; Wang, G.; Dong, Y. Effects of Arbuscular Mycorrhizal Fungi on Growth and Yield of Cucumber Plants. Commun. Soil Sci. Plant Anal. 2008, 39, 499–509. [Google Scholar] [CrossRef]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial Phosphorus Solubilization and Its Potential for Use in Sustainable Agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef]
- Gianinazzi, S.; Gollotte, A.; Binet, M.-N.; Van Tuinen, D.; Redecker, D.; Wipf, D. Agroecology: The key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza 2010, 20, 519–530. [Google Scholar] [CrossRef]
- Rodriguez, A.; Sanders, I.R. The role of community and population ecology in applying mycorrhizal fungi for improved food security. ISME J. 2015, 9, 1053–1061. [Google Scholar] [CrossRef]
- Chialva, M.; Zouari, I.; Salvioli, A.; Novero, M.; Vrebalov, J.; Giovannoni, J.J.; Bonfante, P. Gr and hp-1 tomato mutants unveil unprecedented interactions between arbuscular mycorrhizal symbiosis and fruit ripening. Planta 2016, 244, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Miranda, H.; Ehret, D.L.; Krumbein, A.; Leung, C.; Murch, S.; Turi, C.E.; Franken, P. Inoculation with arbuscular mycorrhizal fungi improves the nutritional value of tomatoes. Mycorrhiza 2015, 25, 359–376. [Google Scholar] [CrossRef]
- Giovannetti, M.; Avio, L.; Barale, R.; Ceccarelli, N.; Cristofani, R.; Iezzi, A.; Mignolli, F.; Picciarelli, P.; Pinto, B.; Reali, D.; et al. Nutraceutical value and safety of tomato fruits produced by mycorrhizal plants. Br. J. Nutr. 2012, 107, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Di Fossalunga, A.S.; Zouari, I.; Chalot, M.; Bonfante, P. The arbuscular mycorrhizal status has an impact on the transcriptome profile and amino acid composition of tomato fruit. BMC Plant Biol. 2012, 12, 44. [Google Scholar] [CrossRef]
- Schwarz, D.; Welter, S.; George, E.; Franken, P.; Lehmann, K.; Weckwerth, W.; Dölle, S.; Worm, M. Impact of arbuscular mycorrhizal fungi on the allergenic potential of tomato. Mycorrhiza 2011, 21, 341–349. [Google Scholar] [CrossRef]
- Igiehon, N.O.; Babalola, O.O. Biofertilizers and sustainable agriculture: Exploring arbuscular mycorrhizal fungi. Appl. Microbiol. Biotechnol. 2017, 101, 4871–4881. [Google Scholar] [CrossRef]
- Tisserant, E.; Malbreil, M.; Kuo, A.; Kohler, A.; Symeonidi, A.; Balestrini, R.; Charron, P.; Duensing, N.; Frey, N.F.D.; Gianinazzi-Pearson, V.; et al. Genome of an arbuscular mycorrhizal fungus provides insight into the oldest plant symbiosis. Proc. Natl. Acad. Sci. USA 2013, 110, 20117–20122. [Google Scholar] [CrossRef]
- Subramanian, K.; Santhanakrishnan, P.; Balasubramanian, P. Responses of field grown tomato plants to arbuscular mycorrhizal fungal colonization under varying intensities of drought stress. Sci. Hortic. 2006, 107, 245–253. [Google Scholar] [CrossRef]
- Menge, J.A.; Steirle, D.; Bagyaraj, D.J.; Johnson, E.L.V.; Leonard, R.T. Phosphorus Concentrations in Plants Responsible for Inhibition of Mycorrhizal Infection. New Phytol. 1978, 80, 575–578. [Google Scholar] [CrossRef]
- Thomson, B.D.; Robson, A.D.; Abbott, L.K. Effects of Phosphorus on the Formation of Mycorrhizas by Gigaspora Calospora and Glomus Fasciculatum in Relation to Root Carbohydrates. New Phytol. 1986, 103, 751–765. [Google Scholar] [CrossRef]
- Amijee, F.; Tinker, P.B.; Stribley, D.P. The development of endomycorrhizal root systems. VII. A detailed study of effects of soil phosphorus on colonization. New Phytol. 1989, 111, 435–446. [Google Scholar] [CrossRef]
- Nagy, R.; Drissner, D.; Amrhein, N.; Jakobsen, I.; Bucher, M. Mycorrhizal phosphate uptake pathway in tomato is phosphorus-repressible and transcriptionally regulated. New Phytol. 2009, 181, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Breuillin, F.; Schramm, J.; Hajirezaei, M.; Ahkami, A.H.; Favre, P.; Druege, U.; Hause, B.; Bucher, M.; Kretzschmar, T.; Bossolini, E.; et al. Phosphate systemically inhibits development of arbuscular mycorrhiza in Petunia hybrida and represses genes involved in mycorrhizal functioning. Plant J. 2010, 64, 1002–1017. [Google Scholar] [CrossRef] [PubMed]
- Bucher, M.; Hause, B.; Krajinski, F.; Küster, H. Through the doors of perception to function in arbuscular mycorrhizal symbioses. New Phytol. 2014, 204, 833–840. [Google Scholar] [CrossRef]
- Bonneau, L.; Huguet, S.; Wipf, D.; Pauly, N.; Truong, H.-N. Combined phosphate and nitrogen limitation generates a nutrient stress transcriptome favorable for arbuscular mycorrhizal symbiosis in Medicago truncatula. New Phytol. 2013, 199, 188–202. [Google Scholar] [CrossRef]
- Boldt, K.; Pörs, Y.; Haupt, B.; Bitterlich, M.; Kuhn, C.; Grimm, B.; Franken, P. Photochemical processes, carbon assimilation and RNA accumulation of sucrose transporter genes in tomato arbuscular mycorrhiza. J. Plant Physiol. 2011, 168, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Ceballos, I.; Ruiz, M.; Fernández, C.; Peña, R.; Rodríguez, A.; Sanders, I.R. The In Vitro Mass-Produced Model Mycorrhizal Fungus, Rhizophagus irregularis, Significantly Increases Yields of the Globally Important Food Security Crop Cassava. PLoS ONE 2013, 8, e70633. [Google Scholar] [CrossRef]
- Dasgan, H.; Kusvuran, S.; Ortas, I. Responses of soilless grown tomato plants to arbuscular mycorrhizal fungal (Glomus fasciculatum) colonization in re-cycling and open systems. Afr. J. Biotechnol. 2008, 7, 3606–3613. [Google Scholar]
- Conversa, G.; Lazzizera, C.; Bonasia, A.; Elia, A. Yield and phosphorus uptake of a processing tomato crop grown at different phosphorus levels in a calcareous soil as affected by mycorrhizal inoculation under field conditions. Biol. Fertil. Soils 2013, 49, 691–703. [Google Scholar] [CrossRef]
- Zouari, I.; Di Fossalunga, A.S.; Chialva, M.; Novero, M.; Miozzi, L.; Tenore, G.C.; Bagnaresi, P.; Bonfante, P. From root to fruit: RNA-Seq analysis shows that arbuscular mycorrhizal symbiosis may affect tomato fruit metabolism. BMC Genom. 2014, 15, 221. [Google Scholar] [CrossRef]
- Rosati, C.; Aquilani, R.; Dharmapuri, S.; Pallara, P.; Marusic, C.; Tavazza, R.; Bouvier, F.; Camara, B.; Giuliano, G. Metabolic engineering of beta-carotene and lycopene content in tomato fruit. Plant J. 2000, 24, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Tierney, A.C.; Rumble, C.E.; Billings, L.M.; George, E.S. Effect of dietary and supplemental lycopene on cardiovascular risk factors: A systematic review and meta-analysis. Adv. Nutr. 2020, nmaa069. [Google Scholar] [CrossRef] [PubMed]
- Bona, E.; Lingua, G.; Manassero, P.; Cantamessa, S.; Marsano, F.; Todeschini, V.; Copetta, A.; D’Agostino, G.; Massa, N.; Avidano, L.; et al. AM fungi and PGP pseudomonads increase flowering, fruit production, and vitamin content in strawberry grown at low nitrogen and phosphorus levels. Mycorrhiza 2015, 25, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Guo, S.-R.; Chaoxing, H.; Yan, Y.; Yu, X.-C. Effects of arbuscular mycorrhiza fungi (AMF) on the plant growth, fruit yield, and fruit quality of cucumber under salt stress. Yingyong Shengtai Xuebao 2012, 23, 154–158. [Google Scholar] [PubMed]
- Hewitt, E. Sand and Water Culture Methods Used in the Study of Plant Nutrition; Commonwealth Agricultural Bureau: London, UK, 1966; Volume 187–237, pp. 430–434. [Google Scholar]
- Schaarschmidt, S.; Kopka, J.; Ludwig-Müller, J.; Hause, B. Regulation of arbuscular mycorrhization by apoplastic invertases: Enhanced invertase activity in the leaf apoplast affects the symbiotic interaction. Plant J. 2007, 51, 390–405. [Google Scholar] [CrossRef]
- Vierheilig, H.; Coughlan, A.P.; Wyss, U.; Pich, Y. Ink and Vinegar, a Simple Staining Technique for Arbuscular-Mycorrhizal Fungi. Appl. Environ. Microbiol. 1998, 64, 5004–5007. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An Evaluation of Techniques for Measuring Vesicular Arbuscular Mycorrhizal Infection in Roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Trouvelot, A.; Kough, J.; Gianinazzi-Pearson, V. Mesure du taux de mycorhization VA d’un système radiculaire. Recherche des méthodes d’estimation ayant une signification fonctionnelle. In The Mycorrhizae: Physiology and Genetic; Gianinazzi-Pearson, V., Gianinazzi, S., Eds.; INRA Presse: Paris, France, 1986; pp. 217–221. [Google Scholar]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.; Hellemans, J.; Huggett, J.F.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
| Gene Name 1 | Solyc No | −AM | +AM | Log2FC | −AM | +AM | Log2FC | ||
|---|---|---|---|---|---|---|---|---|---|
| FPKM | FPKM | rEx 2 | SE | rEx 2 | SE | ||||
| Dehydrin1 | Solyc02g084840.3 | 0.081 | 41.328 | 9.0 | 0.0019 | 0.0018 | 0.6899 | 0.6234 | 8.5 |
| malic enzyme | Solyc12g008430.2 | 0.038 | 5.033 | 7.0 | 0.0371 | 0.0075 | 0.1357 | 0.1027 | 1.9 |
| 2S albumin seed storage | Solyc07g064210.2 | 0.138 | 16.656 | 6.9 | 0.0011 | 0.0011 | 0.1695 | 0.0890 | 7.2 |
| LEA 4 | Solyc10g078780.2 | 0.054 | 5.674 | 6.7 | 0.0008 | 0.0008 | 0.1121 | 0.1007 | 7.2 |
| LEA | Solyc02g062770.2 | 0.127 | 11.423 | 6.5 | 0.0015 | 0.0015 | 0.2108 | 0.1842 | 7.2 |
| MADS-box TF 1 | Solyc04g078300.3 | 0.019 | 1.173 | 5.9 | 0.0000 | 0.0000 | 0.0147 | 0.0112 | ∞ |
| Aminotrans-ferase | Solyc01g007940.3 | 0.051 | 2.819 | 5.8 | 0.0009 | 0.0009 | 0.0571 | 0.0389 | 6.0 |
| Vicilin | Solyc02g085590.3 | 0.177 | 6.867 | 5.3 | 0.0059 | 0.0037 | 0.2590 | 0.1765 | 5.5 |
| bZIP TF | Solyc10g080410.2 | 0.007 | 0.278 | 5.3 | 0.0000 | 0.0000 | 0.0112 | 0.0057 | ∞ |
| Zinc finger TF 50 | Solyc07g053750.1 | 0.139 | 4.944 | 5.2 | 0.0017 | 0.0007 | 0.0548 | 0.0402 | 5.0 |
| Desiccation-related | Solyc05g053350.3 | 0.467 | 13.981 | 4.9 | 0.0060 | 0.0024 | 0.2046 | 0.1108 | 5.1 |
| PIN5 | Solyc01g068410.3 | 0.042 | 0.766 | 4.2 | 0.0004 | 0.0004 | 0.0076 | 0.0046 | 4.3 |
| ERF13 | Solyc04g080910.1 | 0.050 | 0.896 | 4.2 | 0.0002 | 0.0002 | 0.0130 | 0.0068 | 5.8 |
| Oleosin | Solyc06g069260.1 | 0.162 | 2.715 | 4.1 | 0.0007 | 0.0007 | 0.0233 | 0.0109 | 5.0 |
| ACC-Oxidase5 | Solyc07g026650.3 | 10.087 | 4.424 | −1.2 | 0.0271 | 0.0077 | 0.0108 | 0.0023 | −1.3 |
| Carotenoid 1 | Green Fruits | Red Fruits | ||
|---|---|---|---|---|
| −AM | +AM | −AM | +AM | |
| Lutein | 3.08 ± 0.46 | 3.44 ± 0.48 | 1.27 ± 0.41 | 1.22 ± 0.24 |
| Zeaxanthin | 4.36 ± 1.41 | 5.86 ± 1.19 | n.d. | n.d. |
| Lycopene | n.d. | n.d. | 2.87 ± 0.71 | 4.22 ± 1.97 |
| ß-Carotene | 0.63 ± 0.14 | 0.62 ± 0.19 | 7.10 ± 1.09 | 9.23 ± 2.15 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schubert, R.; Werner, S.; Cirka, H.; Rödel, P.; Tandron Moya, Y.; Mock, H.-P.; Hutter, I.; Kunze, G.; Hause, B. Effects of Arbuscular Mycorrhization on Fruit Quality in Industrialized Tomato Production. Int. J. Mol. Sci. 2020, 21, 7029. https://doi.org/10.3390/ijms21197029
Schubert R, Werner S, Cirka H, Rödel P, Tandron Moya Y, Mock H-P, Hutter I, Kunze G, Hause B. Effects of Arbuscular Mycorrhization on Fruit Quality in Industrialized Tomato Production. International Journal of Molecular Sciences. 2020; 21(19):7029. https://doi.org/10.3390/ijms21197029
Chicago/Turabian StyleSchubert, Ramona, Stephanie Werner, Hillary Cirka, Philipp Rödel, Yudelsy Tandron Moya, Hans-Peter Mock, Imke Hutter, Gotthard Kunze, and Bettina Hause. 2020. "Effects of Arbuscular Mycorrhization on Fruit Quality in Industrialized Tomato Production" International Journal of Molecular Sciences 21, no. 19: 7029. https://doi.org/10.3390/ijms21197029
APA StyleSchubert, R., Werner, S., Cirka, H., Rödel, P., Tandron Moya, Y., Mock, H.-P., Hutter, I., Kunze, G., & Hause, B. (2020). Effects of Arbuscular Mycorrhization on Fruit Quality in Industrialized Tomato Production. International Journal of Molecular Sciences, 21(19), 7029. https://doi.org/10.3390/ijms21197029





