Characterization of the Heavy-Metal-Associated Isoprenylated Plant Protein (HIPP) Gene Family from Triticeae Species
Abstract
1. Introduction
2. Results
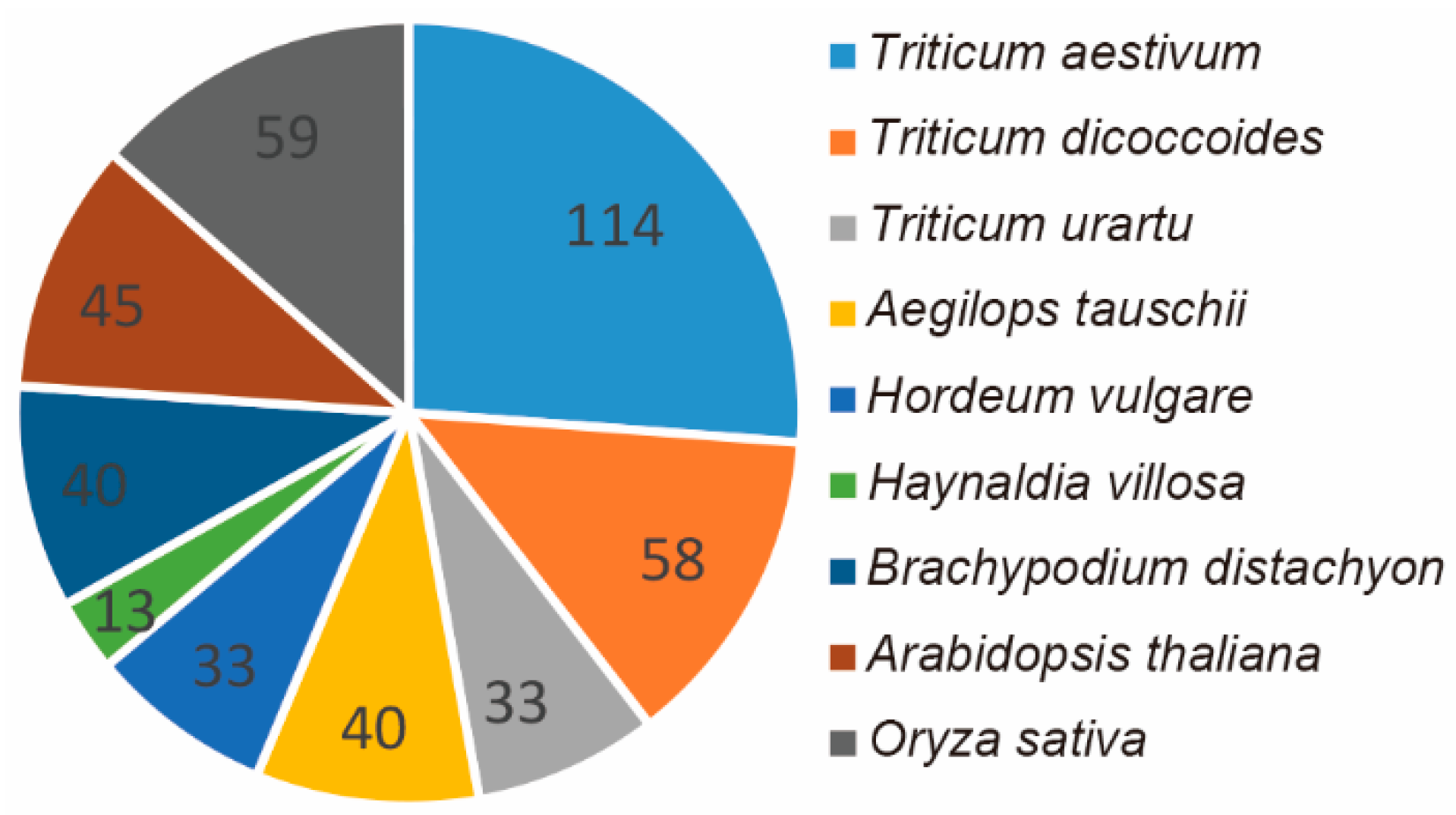
2.1. Genome-Wide Identification, Phylogenetic Analysis, and Protein Structure Analysis of the HIPP Gene Family in Triticeae Species
2.2. Chromosomal Distribution of HIPPs
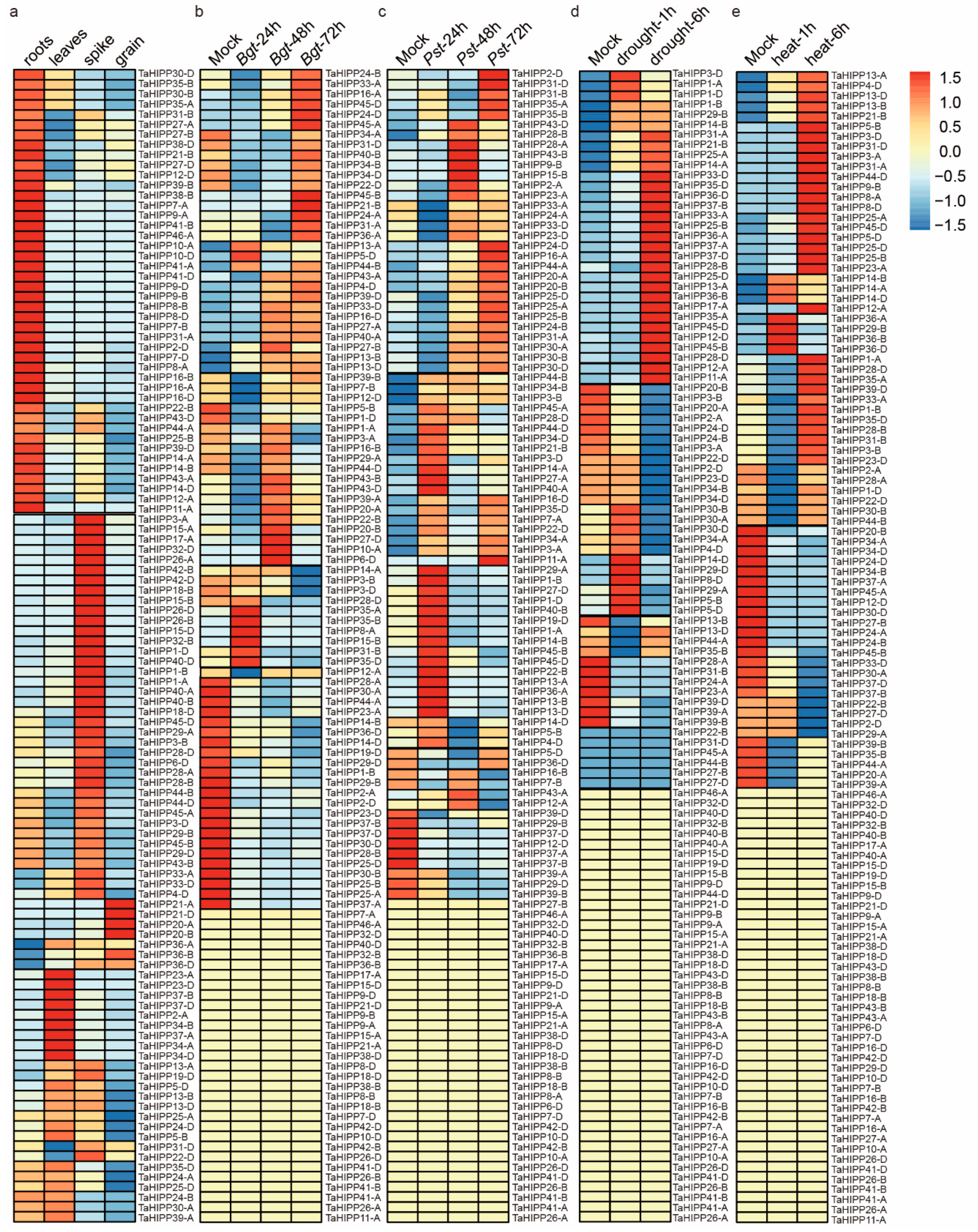
2.3. The Expression Pattern of the HIPP Gene Family in Common Wheat
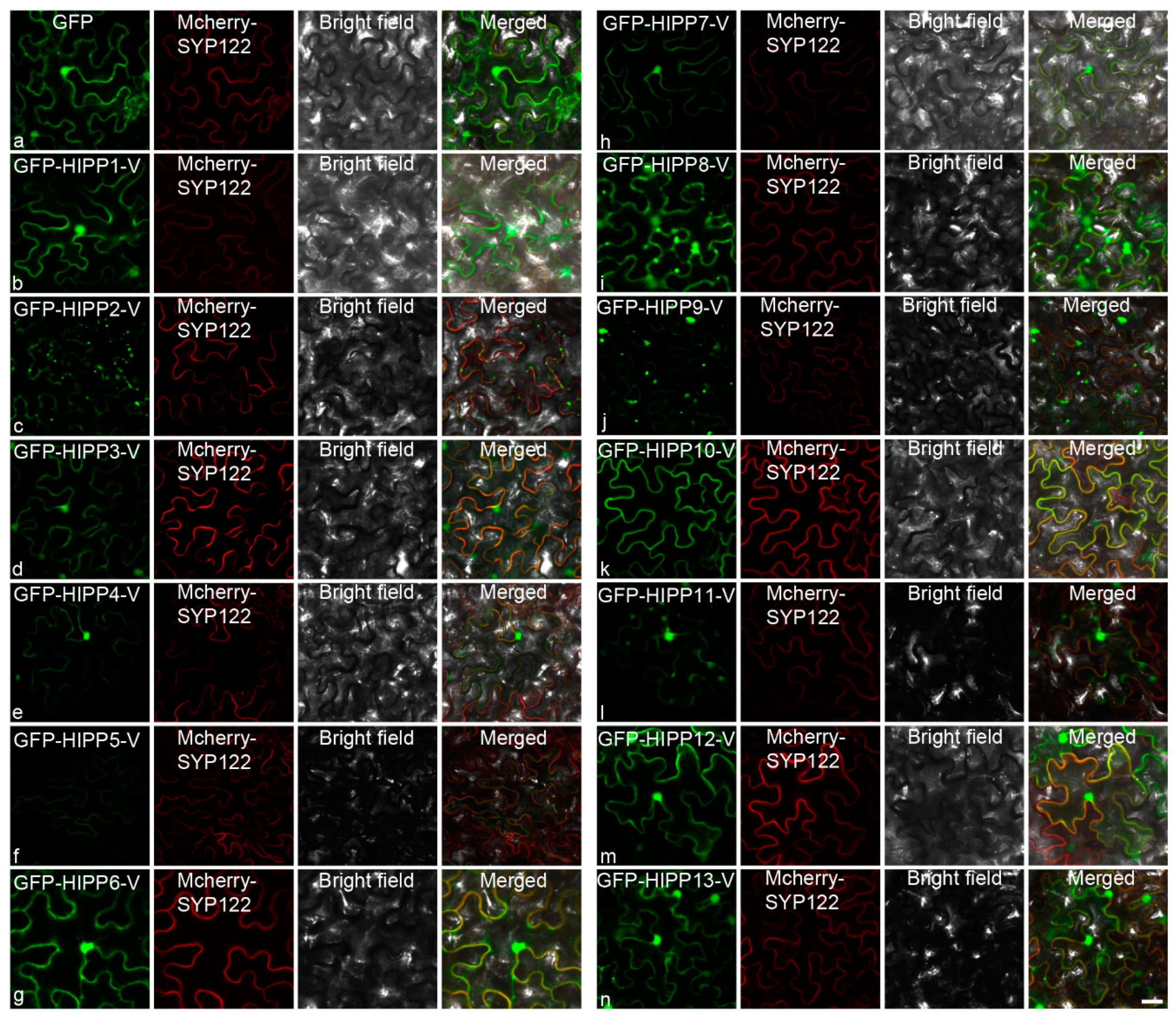
2.4. Cloning and Subcellular Localization Analysis of the HIPPs from H. villosa
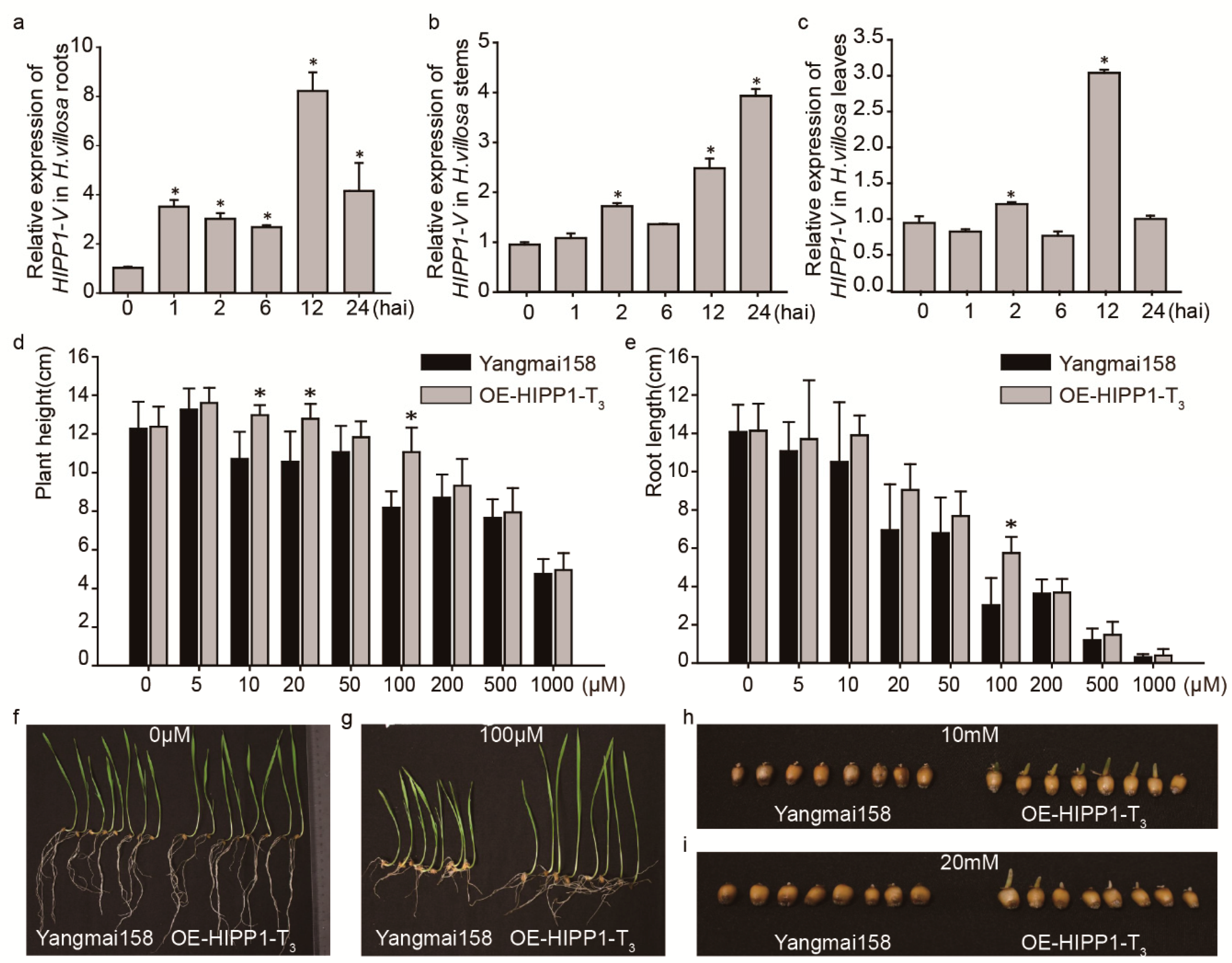
2.5. HIPP1-V Positively Regulates Cd Tolerance in Common Wheat
3. Discussion
3.1. The Cd Tolerance of HIPP1-V Could Expand Wheat Breeding Resources
3.2. Identification and Characterization of the HIPP Gene Family Can Help to Better Understand the Evolutionary Relationships in Triticeae Species
3.3. The HIPP Gene Family Might Regulate Plant Development and Various Responses to Stress in Common Wheat
3.4. Diversification of the Subcellular Localization Pattern of HIPP in H. villosa
4. Materials and Methods
4.1. Plant Materials
4.2. Identification of the HIPP Gene Family in Triticeae Species
4.3. Cloning of HIPP Genes from Haynaldia villosa
4.4. Subcellular Localization
4.5. Phylogenetic Analysis of the HIPP Gene Family
4.6. Chromosome Distribution and Protein Structure Analysis of the HIPP Gene Family
4.7. RNA-seq Expression Analysis
4.8. Transcription Abundance Analysis
4.9. Genetic Transformation
4.10. Cd-Tolerance Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| GFP | Green Fluorescent Protein |
| CDS | Coding Sequence |
| aa | Amino Acid |
| qRT-PCR | Quantitative Reverse Transcription PCR |
| ORF | Open Reading Frame |
References
- Eapen, S.; D’Souza, S.F. Prospects of genetic engineering of plants for phytoremediation of toxic metals. Biotechnol. Adv. 2005, 23, 97–114. [Google Scholar] [CrossRef] [PubMed]
- Miransari, M. Hyperaccumulators, arbuscular mycorrhizal fungi and stress of heavy metals. Biotechnol. Adv. 2011, 29, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Wu, P. Elevated selenium and other mineral element concentrations in soil and plant tissue in bone coal sites in Haoping area, Ziyang County, China. Plant Soil 2004, 261, 135–146. [Google Scholar] [CrossRef]
- Macnair, M.R. The genetics of metal tolerance in vascular plants. New Phytol. 1993, 124, 541–559. [Google Scholar] [CrossRef]
- Garzon, T.; Gunse, B.; Moreno, A.R.; Tomos, A.D.; Barcelo, J.; Poschenrieder, C. Aluminium-induced alteration of ion homeostasis in root tip vacuoles of two maize varieties differing in Al tolerance. Plant Sci. Int. J. Exp. Plant Biol. 2011, 180, 709–715. [Google Scholar] [CrossRef]
- Hayat, S.; Khalique, G.; Irfan, M.; Wani, A.S.; Tripathi, B.N.; Ahmad, A. Physiological changes induced by chromium stress in plants: An overview. Protoplasma 2012, 249, 599–611. [Google Scholar] [CrossRef]
- Shahid, M.; Pinelli, E.; Dumat, C. Review of Pb availability and toxicity to plants in relation with metal speciation; role of synthetic and natural organic ligands. J. Hazard. Mater. 2012, 219–220, 1–12. [Google Scholar] [CrossRef]
- Gill, S.S.; Hasanuzzaman, M.; Nahar, K.; Macovei, A.; Tuteja, N. Importance of nitric oxide in cadmium stress tolerance in crop plants. Plant Physiol. Biochem. 2013, 63, 254–261. [Google Scholar] [CrossRef]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy Metal Tolerance in Plants: Role of Transcriptomics, Proteomics, Metabolomics, and Ionomics. Front. Plant Sci. 2015, 6, 1143. [Google Scholar] [CrossRef]
- Morel, F.M. The co-evolution of phytoplankton and trace element cycles in the oceans. Geobiology 2008, 6, 318–324. [Google Scholar] [CrossRef]
- Chen, F.; Wang, F.; Wu, F.; Mao, W.; Zhang, G.; Zhou, M. Modulation of exogenous glutathione in antioxidant defense system against Cd stress in the two barley genotypes differing in Cd tolerance. Plant Physiol. Biochem. 2010, 48, 663–672. [Google Scholar] [CrossRef]
- Yu, C.; Sun, C.; Shen, C.; Wang, S.; Liu, F.; Liu, Y.; Chen, Y.; Li, C.; Qian, Q.; Aryal, B.; et al. The auxin transporter, OsAUX1, is involved in primary root and root hair elongation and in Cd stress responses in rice (Oryza sativa L.). Plant J. Cell Mol. Biol. 2015, 83, 818–830. [Google Scholar] [CrossRef]
- Rizwan, M.; Meunier, J.D.; Davidian, J.C.; Pokrovsky, O.S.; Bovet, N.; Keller, C. Silicon alleviates Cd stress of wheat seedlings (Triticum turgidum L. cv. Claudio) grown in hydroponics. Environ. Sci. Pollut. Res. Int. 2016, 23, 1414–1427. [Google Scholar] [CrossRef]
- Dong, J.; Wu, F.B.; Zhang, G.P. Effect of cadmium on growth and photosynthesis of tomato seedlings. J. Zhejiang Univ. Sci. B 2005, 6, 974–980. [Google Scholar] [CrossRef]
- Martinoia, E.; Klein, M.; Geisler, M.; Bovet, L.; Forestier, C.; Kolukisaoglu, U.; Muller-Rober, B.; Schulz, B. Multifunctionality of plant ABC transporters-more than just detoxifiers. Planta 2002, 214, 345–355. [Google Scholar] [CrossRef]
- Lin, Y.F.; Liang, H.M.; Yang, S.Y.; Boch, A.; Clemens, S.; Chen, C.C.; Wu, J.F.; Huang, J.L.; Yeh, K.C. Arabidopsis IRT3 is a zinc-regulated and plasma membrane localized zinc/iron transporter. New Phytol. 2009, 182, 392–404. [Google Scholar] [CrossRef]
- Filatov, V.; Dowdle, J.; Smirnoff, N.; Ford-Lloyd, B.; Newbury, H.J.; Macnair, M.R. Comparison of gene expression in segregating families identifies genes and genomic regions involved in a novel adaptation, zinc hyperaccumulation. Mol. Ecol. 2006, 15, 3045–3059. [Google Scholar] [CrossRef]
- Lei, G.J.; Fujii-Kashino, M.; Wu, D.Z.; Hisano, H.; Saisho, D.; Deng, F.; Yamaji, N.; Sato, K.; Zhao, F.-J.; Ma, J.F. Breeding for low cadmium barley by introgression of a Sukkula-like transposable element. Nat. Food 2020, 1, 489–499. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, C.; Chen, C.; Zhang, W.; Huang, X.-Y.; Zhao, F.-J. Overexpression of Rice OsHMA3 in Wheat Greatly Decreases Cadmium Accumulation in Wheat Grains. Environ. Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Gustin, J.L.; Loureiro, M.E.; Kim, D.; Na, G.; Tikhonova, M.; Salt, D.E. MTP1-dependent Zn sequestration into shoot vacuoles suggests dual roles in Zn tolerance and accumulation in Zn-hyperaccumulating plants. Plant J. Cell Mol. Biol. 2009, 57, 1116–1127. [Google Scholar] [CrossRef]
- Lanquar, V.; Lelievre, F.; Bolte, S.; Hames, C.; Alcon, C.; Neumann, D.; Vansuyt, G.; Curie, C.; Schroder, A.; Kramer, U.; et al. Mobilization of vacuolar iron by AtNRAMP3 and AtNRAMP4 is essential for seed germination on low iron. EMBO J. 2005, 24, 4041–4051. [Google Scholar] [CrossRef]
- Gao, W.; Xiao, S.; Li, H.Y.; Tsao, S.W.; Chye, M.L. Arabidopsis thaliana acyl-CoA-binding protein ACBP2 interacts with heavy-metal-binding farnesylated protein AtFP6. New Phytol. 2009, 181, 89–102. [Google Scholar] [CrossRef]
- de Abreu-Neto, J.B.; Turchetto-Zolet, A.C.; de Oliveira, L.F.; Zanettini, M.H.; Margis-Pinheiro, M. Heavy metal-associated isoprenylated plant protein (HIPP): Characterization of a family of proteins exclusive to plants. FEBS J. 2013, 280, 1604–1616. [Google Scholar] [CrossRef]
- Tehseen, M.; Cairns, N.; Sherson, S.; Cobbett, C.S. Metallochaperone-like genes in Arabidopsis thaliana. Metallomics 2010, 2, 556–564. [Google Scholar] [CrossRef]
- Zschiesche, W.; Barth, O.; Daniel, K.; Bohme, S.; Rausche, J.; Humbeck, K. The zinc-binding nuclear protein HIPP3 acts as an upstream regulator of the salicylate-dependent plant immunity pathway and of flowering time in Arabidopsis thaliana. New Phytol. 2015, 207, 1084–1096. [Google Scholar] [CrossRef]
- Khan, I.U.; Rono, J.K.; Zhang, B.Q.; Liu, X.S.; Wang, M.Q.; Wang, L.L.; Wu, X.C.; Chen, X.; Cao, H.W.; Yang, Z.M. Identification of novel rice (Oryza sativa) HPP and HIPP genes tolerant to heavy metal toxicity. Ecotoxicol. Environ. Saf. 2019, 175, 8–18. [Google Scholar] [CrossRef]
- Manara, A.; Fasani, E.; Molesini, B.; DalCorso, G.; Pennisi, F.; Pandolfini, T.; Furini, A. The Tomato Metallocarboxypeptidase Inhibitor I, which Interacts with a Heavy Metal-Associated Isoprenylated Protein, Is Implicated in Plant Response to Cadmium. Molecules 2020, 25. [Google Scholar] [CrossRef]
- Barth, O.; Zschiesche, W.; Siersleben, S.; Humbeck, K. Isolation of a novel barley cDNA encoding a nuclear protein involved in stress response and leaf senescence. Physiol. Plant. 2004, 121, 282–293. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, H.; Feng, C.; Xu, H.; Huang, X.; Wang, Q.; Duan, X.; Wang, X.; Wei, G.; Huang, L.; et al. Isolation and characterisation of cDNA encoding a wheat heavy metal-associated isoprenylated protein involved in stress responses. Plant Biol. 2015, 17, 1176–1186. [Google Scholar] [CrossRef]
- Suzuki, N.; Yamaguchi, Y.; Koizumi, N.; Sano, H. Functional characterization of a heavy metal binding protein CdI19 from Arabidopsis. Plant J. Cell Mol. Biol. 2002, 32, 165–173. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, R.; Ju, Q.; Li, W.; Tran, L.P.; Xu, J. The R2R3-MYB Transcription Factor MYB49 Regulates Cadmium Accumulation. Plant Physiol. 2019, 180, 529–542. [Google Scholar] [CrossRef]
- Zhang, B.Q.; Liu, X.S.; Feng, S.J.; Zhao, Y.N.; Wang, L.L.; Rono, J.K.; Li, H.; Yang, Z.M. Developing a cadmium resistant rice genotype with OsHIPP29 locus for limiting cadmium accumulation in the paddy crop. Chemosphere 2020, 247, 125958. [Google Scholar] [CrossRef]
- Barth, O.; Vogt, S.; Uhlemann, R.; Zschiesche, W.; Humbeck, K. Stress induced and nuclear localized HIPP26 from Arabidopsis thaliana interacts via its heavy metal associated domain with the drought stress related zinc finger transcription factor ATHB29. Plant Mol. Biol. 2009, 69, 213–226. [Google Scholar] [CrossRef]
- Radakovic, Z.S.; Anjam, M.S.; Escobar, E.; Chopra, D.; Cabrera, J.; Silva, A.C.; Escobar, C.; Sobczak, M.; Grundler, F.M.W.; Siddique, S. Arabidopsis HIPP27 is a host susceptibility gene for the beet cyst nematode Heterodera schachtii. Mol. Plant Pathol. 2018. [Google Scholar] [CrossRef]
- Cowan, G.H.; Roberts, A.G.; Jones, S.; Kumar, P.; Kalyandurg, P.B.; Gil, J.F.; Savenkov, E.I.; Hemsley, P.A.; Torrance, L. Potato Mop-Top Virus Co-Opts the Stress Sensor HIPP26 for Long-Distance Movement. Plant Physiol. 2018, 176, 2052–2070. [Google Scholar] [CrossRef]
- Liu, J.L.; Zhang, D.X.; Hong, L. Isolation, characterization and functional annotation of the salt tolerance genes through screening the high-quality cDNA library of the halophytic green alga Dunaliella salina (Chlorophyta). Ann. Microbiol. 2015, 65, 1293–1302. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Wang, S.; Li, H.; Xin, Q. Overexpression of a Common Wheat Gene GALACTINOL SYNTHASE3 Enhances Tolerance to Zinc in Arabidopsis and Rice Through the Modulation of Reactive Oxygen Species Production. Plant Mol. Biol. Rep. 2016, 34, 794–806. [Google Scholar] [CrossRef]
- Khoudi, H.; Maatar, Y.; Gouiaa, S.; Masmoudi, K. Transgenic tobacco plants expressing ectopically wheat H(+)-pyrophosphatase (H(+)-PPase) gene TaVP1 show enhanced accumulation and tolerance to cadmium. J. Plant Physiol. 2012, 169, 98–103. [Google Scholar] [CrossRef]
- Ren, Y.; Chen, Y.; An, J.; Zhao, Z.; Zhang, G.; Wang, Y.; Wang, W. Wheat expansin gene TaEXPA2 is involved in conferring plant tolerance to Cd toxicity. Plant Sci. Int. J. Exp. Plant Biol. 2018, 270, 245–256. [Google Scholar] [CrossRef]
- Qiao, K.; Gong, L.; Tian, Y.; Wang, H.; Chai, T. The metal-binding domain of wheat heavy metal ATPase 2 (TaHMA2) is involved in zinc/cadmium tolerance and translocation in Arabidopsis. Plant Cell Rep. 2018, 37, 1343–1352. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Z.; Zhu, C. Heteroexpression of the wheat phytochelatin synthase gene (TaPCS1) in rice enhances cadmium sensitivity. Acta Biochim. Biophys. Sin. 2012, 44, 886–893. [Google Scholar] [CrossRef]
- Qiao, K.; Wang, F.; Liang, S.; Wang, H.; Hu, Z.; Chai, T. Improved Cd, Zn and Mn tolerance and reduced Cd accumulation in grains with wheat-based cell number regulator TaCNR2. Sci. Rep. 2019, 9, 870. [Google Scholar] [CrossRef]
- Clemens, S.; Kim, E.J.; Neumann, D.; Schroeder, J.I. Tolerance to toxic metals by a gene family of phytochelatin synthases from plants and yeast. EMBO J. 1999, 18, 3325–3333. [Google Scholar] [CrossRef]
- Sun, H.; Song, J.; Lei, J.; Song, X.; Dai, K.; Xiao, J.; Yuan, C.; An, S.; Wang, H.; Wang, X. Construction and application of oligo-based FISH karyotype of Haynaldia villosa. J. Genet. Genom. Yi Chuan Xue Bao 2018, 45, 463–466. [Google Scholar] [CrossRef]
- Cao, A.; Xing, L.; Wang, X.; Yang, X.; Wang, W.; Sun, Y.; Qian, C.; Ni, J.; Chen, Y.; Liu, D.; et al. Serine/threonine kinase gene Stpk-V, a key member of powdery mildew resistance gene Pm21, confers powdery mildew resistance in wheat. Proc. Natl. Acad. Sci. USA 2011, 108, 7727–7732. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Y.; Fei, F.; Wang, Z.; Wang, W.; Cao, A.; Liu, Y.; Han, S.; Xing, L.; Wang, H.; et al. E3 ubiquitin ligase gene CMPG1-V from Haynaldia villosa L. contributes to powdery mildew resistance in common wheat (Triticum aestivum L.). Plant J. Cell Mol. Biol. 2015, 84, 154–168. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Moon, C.S.; Watanabe, T.; Shimbo, S.; Ikeda, M. Contents of pollutant and nutrient elements in rice and wheat grown on the neighboring fields. Biol. Trace Elem. Res. 1997, 57, 39–50. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, K.; Zhao, F.J.; Sun, C.; Jin, C.; Shi, Y.; Sun, Y.; Li, Y.; Yang, M.; Jing, X.; et al. OsATX1 Interacts with Heavy Metal P1B-Type ATPases and Affects Copper Transport and Distribution. Plant Physiol. 2018, 178, 329–344. [Google Scholar] [CrossRef]
- Tan, J.; Wang, J.; Chai, T.; Zhang, Y.; Feng, S.; Li, Y.; Zhao, H.; Liu, H.; Chai, X. Functional analyses of TaHMA2, a P(1B)-type ATPase in wheat. Plant Biotechnol. J. 2013, 11, 420–431. [Google Scholar] [CrossRef]
- Ci, D.; Jiang, D.; Wollenweber, B.; Dai, T.; Jing, Q.; Cao, W. Genetic Variance in Cadmium Tolerance and Accumulation in Wheat Materials Differing in Ploidy and Genome at Seedling Stage. J. Agron. Crop Sci. 2010, 196, 302–310. [Google Scholar] [CrossRef]
- Dykema, P.E.; Sipes, P.R.; Marie, A.; Biermann, B.J.; Crowell, D.N.; Randall, S.K. A new class of proteins capable of binding transition metals. Plant Mol. Biol. 1999, 41, 139–150. [Google Scholar] [CrossRef]
- Lynch, M.; Conery, J.S. The evolutionary fate and consequences of duplicate genes. Science 2000, 290, 1151–1155. [Google Scholar] [CrossRef]
- Himelblau, E.; Mira, H.; Lin, S.J.; Culotta, V.C.; Penarrubia, L.; Amasino, R.M. Identification of a functional homolog of the yeast copper homeostasis gene ATX1 from Arabidopsis. Plant Physiol. 1998, 117, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.J.; Liu, X.S.; Ma, L.Y.; Khan, I.U.; Rono, J.K.; Yang, Z.M. Identification of epigenetic mechanisms in paddy crop associated with lowering environmentally related cadmium risks to food safety. Environ. Pollut. 2020, 256, 113464. [Google Scholar] [CrossRef] [PubMed]
- Novelli, G.; D’Apice, M.R. Protein farnesylation and disease. J. Inherit. Metab. Dis. 2012, 35, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Galichet, A.; Hoyerova, K.; Kaminek, M.; Gruissem, W. Farnesylation directs AtIPT3 subcellular localization and modulates cytokinin biosynthesis in Arabidopsis. Plant Physiol. 2008, 146, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Galichet, A.; Gruissem, W. Developmentally controlled farnesylation modulates AtNAP1;1 function in cell proliferation and cell expansion during Arabidopsis leaf development. Plant Physiol. 2006, 142, 1412–1426. [Google Scholar] [CrossRef]
- Chu, C.C.; Lee, W.C.; Guo, W.Y.; Pan, S.M.; Chen, L.J.; Li, H.M.; Jinn, T.L. A copper chaperone for superoxide dismutase that confers three types of copper/zinc superoxide dismutase activity in Arabidopsis. Plant Physiol. 2005, 139, 425–436. [Google Scholar] [CrossRef]
- Mascher, M.; Gundlach, H.; Himmelbach, A.; Beier, S.; Twardziok, S.O.; Wicker, T.; Radchuk, V.; Dockter, C.; Hedley, P.E.; Russell, J.; et al. A chromosome conformation capture ordered sequence of the barley genome. Nature 2017, 544, 427–433. [Google Scholar] [CrossRef]
- Maurer-Stroh, S.; Eisenhaber, F. Refinement and prediction of protein prenylation motifs. Genome Biol. 2005, 6, R55. [Google Scholar] [CrossRef]
- Ling, H.Q.; Ma, B.; Shi, X.; Liu, H.; Dong, L.; Sun, H.; Cao, Y.; Gao, Q.; Zheng, S.; Li, Y.; et al. Genome sequence of the progenitor of wheat A subgenome Triticum Urartu. Nature 2018, 557, 424–428. [Google Scholar] [CrossRef] [PubMed]
- International Wheat Genome Sequencing Consortium. A chromosome-based draft sequence of the hexaploid bread wheat (Triticum aestivum) genome. Science 2014, 345, 1251788. [Google Scholar] [CrossRef]
- International Brachypodium Initiative. Genome sequencing and analysis of the model grass Brachypodium distachyon. Nature 2010, 463, 763–768. [Google Scholar] [CrossRef]
- Avni, R.; Nave, M.; Barad, O.; Baruch, K.; Twardziok, S.O.; Gundlach, H.; Hale, I.; Mascher, M.; Spannagl, M.; Wiebe, K.; et al. Wild emmer genome architecture and diversity elucidate wheat evolution and domestication. Science 2017, 357, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.C.; Gu, Y.Q.; Puiu, D.; Wang, H.; Twardziok, S.O.; Deal, K.R.; Huo, N.; Zhu, T.; Wang, L.; Wang, Y.; et al. Genome sequence of the progenitor of the wheat D genome Aegilops tauschii. Nature 2017, 551, 498–502. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, X.; Wan, W.; Zhang, H.; Liu, J.; Li, M.; Wang, H.; Xiao, J.; Wang, X. Identification and Characterization of the EXO70 Gene Family in Polyploid Wheat and Related Species. Int. J. Mol. Sci. 2018, 20. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xia, R.; Chen, H.; He, Y. TBtools, a Toolkit for Biologists integrating various HTS-data handling tools with a user-friendly interface. BioRxiv 2018, 289660. [Google Scholar] [CrossRef]
- Borrill, P.; Ramirez-Gonzalez, R.; Uauy, C. expVIP: A Customizable RNA-seq Data Analysis and Visualization Platform. Plant Physiol. 2016, 170, 2172–2186. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Xing, L.P.; Wang, H.Z.; Jiang, Z.N.; Ni, J.L.; Cao, A.Z.; Yu, L.; Chen, P.D. Transformation of Wheat Thaumatin-Like Protein Gene and Analysis of Reactions to Powdery Mildew and Fusarium Head Blight in Transgenic Plants. Acta Agron. Sin. 2008, 34, 349–354. [Google Scholar] [CrossRef]

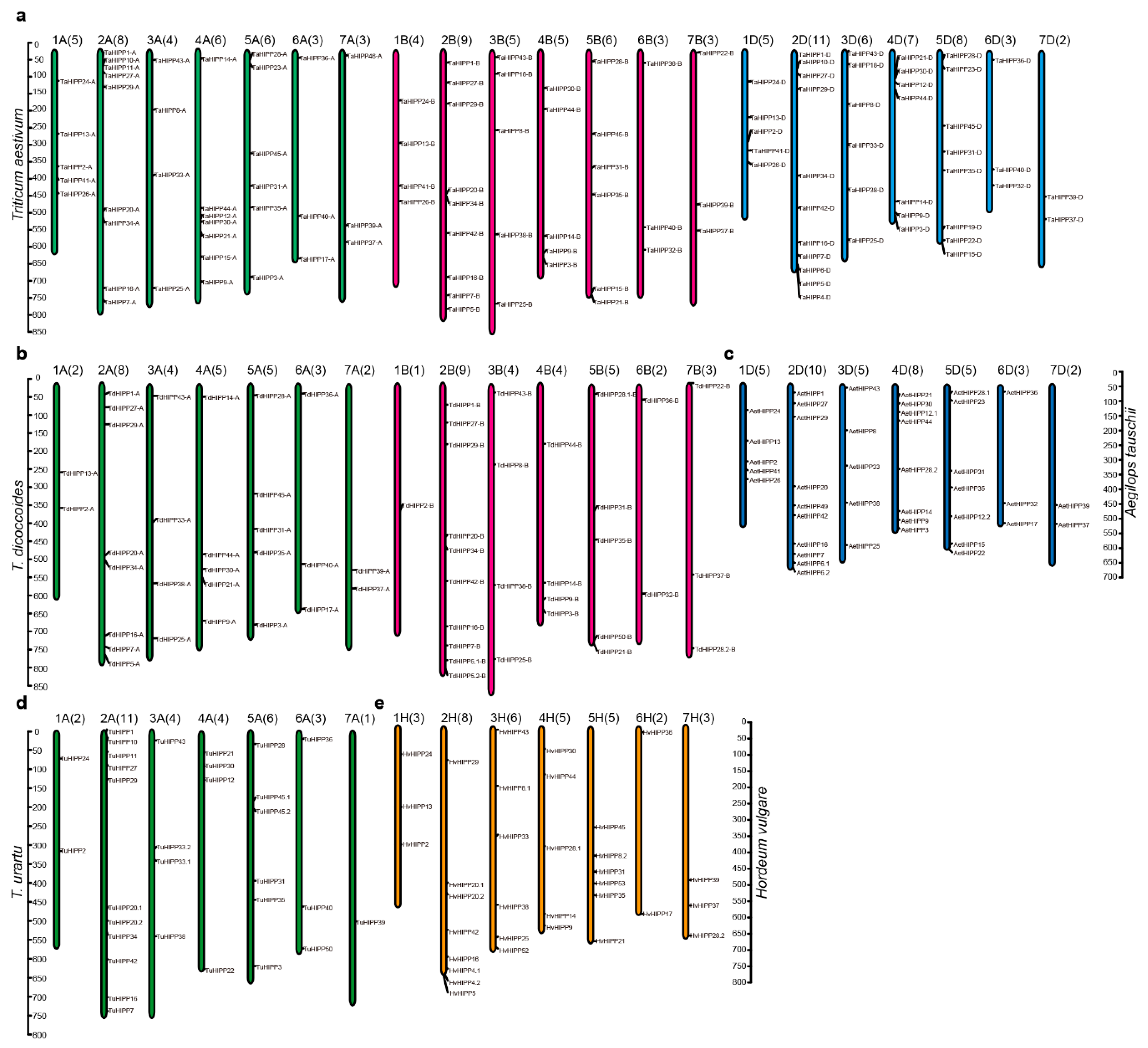
| Chromosome | T. aestivum | T. dicoccoides | T. urartu | Ae. tauschii | H. vulgare | Total | |||
|---|---|---|---|---|---|---|---|---|---|
| A | B | D | A | B | A | D | H | ||
| 1 | 5 | 4 | 5 | 2 | 1 | 2 | 5 | 3 | 27 |
| 2 | 9 | 9 | 11 | 8 | 10 | 11 | 10 | 8 | 76 |
| 3 | 4 | 5 | 6 | 4 | 4 | 4 | 5 | 6 | 38 |
| 4 | 7 | 5 | 7 | 5 | 4 | 4 | 8 | 5 | 45 |
| 5 | 6 | 6 | 8 | 5 | 5 | 6 | 7 | 5 | 48 |
| 6 | 3 | 3 | 3 | 3 | 2 | 3 | 3 | 3 | 23 |
| 7 | 3 | 3 | 2 | 2 | 3 | 1 | 2 | 3 | 19 |
| Unknown * | 2 | 2 | |||||||
| Total | 37 | 35 | 42 | 29 | 29 | 33 | 40 | 33 | 278 |
| Name | Homologs in H. vulgare | ORF | aa | Location of HMA Domain (aa) | No. of HMA Domain | PI | MW (kDa) |
|---|---|---|---|---|---|---|---|
| HIPP1-V | HvHIPP33 | 456 | 151 | 37–91 | 1 | 9.61 | 16.63 |
| HIPP2-V | HvHIPP43 | 1251 | 416 | 16–63 | 1 | 7.54 | 44.21 |
| HIPP3-V | HvHIPP13 | 1023 | 340 | 22–76, 162–204 | 2 | 9.63 | 36.24 |
| HIPP4-V | HvHIPP31 | 486 | 161 | 35–94 | 1 | 10.01 | 17.81 |
| HIPP5-V | HvHIPP31 | 489 | 162 | 36–95 | 1 | 10.3 | 17.64 |
| HIPP6-V | HvHIPP25 | 999 | 332 | 12–73 | 1 | 5.62 | 36.36 |
| HIPP7-V | HvHIPP33 | 456 | 151 | 37–91 | 1 | 9.78 | 16.59 |
| HIPP8-V | HvHIPP39 | 507 | 168 | 10–61 | 1 | 8.41 | 18.72 |
| HIPP9-V | HvHIPP28.1 | 921 | 306 | 12–59 | 1 | 4.86 | 33.35 |
| HIPP10-V | HvHIPP36 | 468 | 155 | 35–90 | 1 | 10.04 | 17.35 |
| HIPP11-V | HvHIPP29 | 489 | 162 | 39–98 | 1 | 9.97 | 17.64 |
| HIPP12-V | HvHIPP2 | 498 | 165 | 11–60 | 1 | 6.79 | 17.92 |
| HIPP13-V | HvHIPP20.2 | 1065 | 354 | 71–122, 167–227 | 2 | 5.21 | 39.23 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Zhang, X.; Liu, J.; Niu, Y.; Chen, Y.; Hao, Y.; Zhao, J.; Sun, L.; Wang, H.; Xiao, J.; et al. Characterization of the Heavy-Metal-Associated Isoprenylated Plant Protein (HIPP) Gene Family from Triticeae Species. Int. J. Mol. Sci. 2020, 21, 6191. https://doi.org/10.3390/ijms21176191
Zhang H, Zhang X, Liu J, Niu Y, Chen Y, Hao Y, Zhao J, Sun L, Wang H, Xiao J, et al. Characterization of the Heavy-Metal-Associated Isoprenylated Plant Protein (HIPP) Gene Family from Triticeae Species. International Journal of Molecular Sciences. 2020; 21(17):6191. https://doi.org/10.3390/ijms21176191
Chicago/Turabian StyleZhang, Heng, Xu Zhang, Jia Liu, Ying Niu, Yiming Chen, Yongli Hao, Jia Zhao, Li Sun, Haiyan Wang, Jin Xiao, and et al. 2020. "Characterization of the Heavy-Metal-Associated Isoprenylated Plant Protein (HIPP) Gene Family from Triticeae Species" International Journal of Molecular Sciences 21, no. 17: 6191. https://doi.org/10.3390/ijms21176191
APA StyleZhang, H., Zhang, X., Liu, J., Niu, Y., Chen, Y., Hao, Y., Zhao, J., Sun, L., Wang, H., Xiao, J., & Wang, X. (2020). Characterization of the Heavy-Metal-Associated Isoprenylated Plant Protein (HIPP) Gene Family from Triticeae Species. International Journal of Molecular Sciences, 21(17), 6191. https://doi.org/10.3390/ijms21176191




