Specific Receptors for the Chemokines CXCR2 and CXCR4 in Pancreatic Cancer
Abstract
1. Introduction
2. Patients and Methods
Statistical Analysis
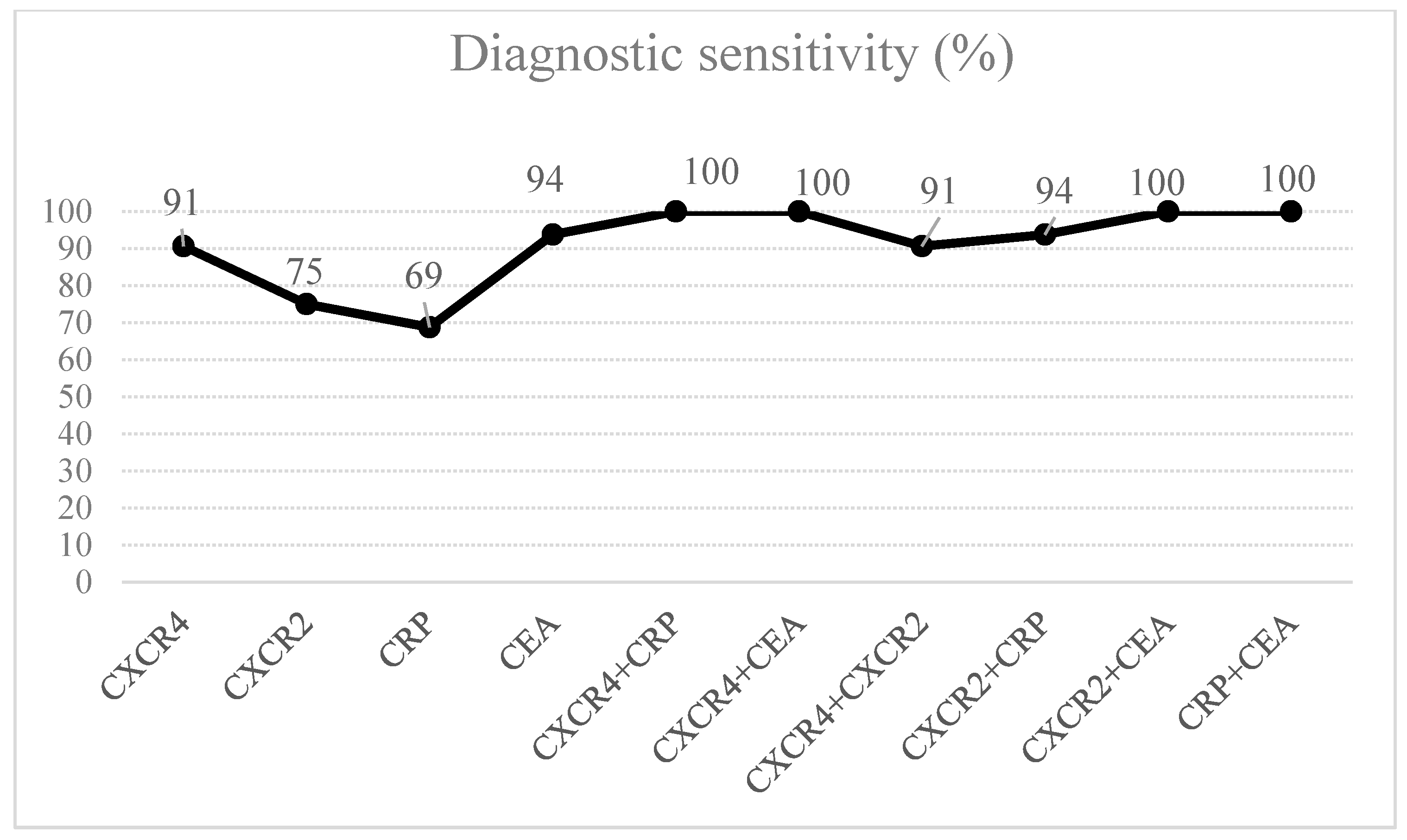
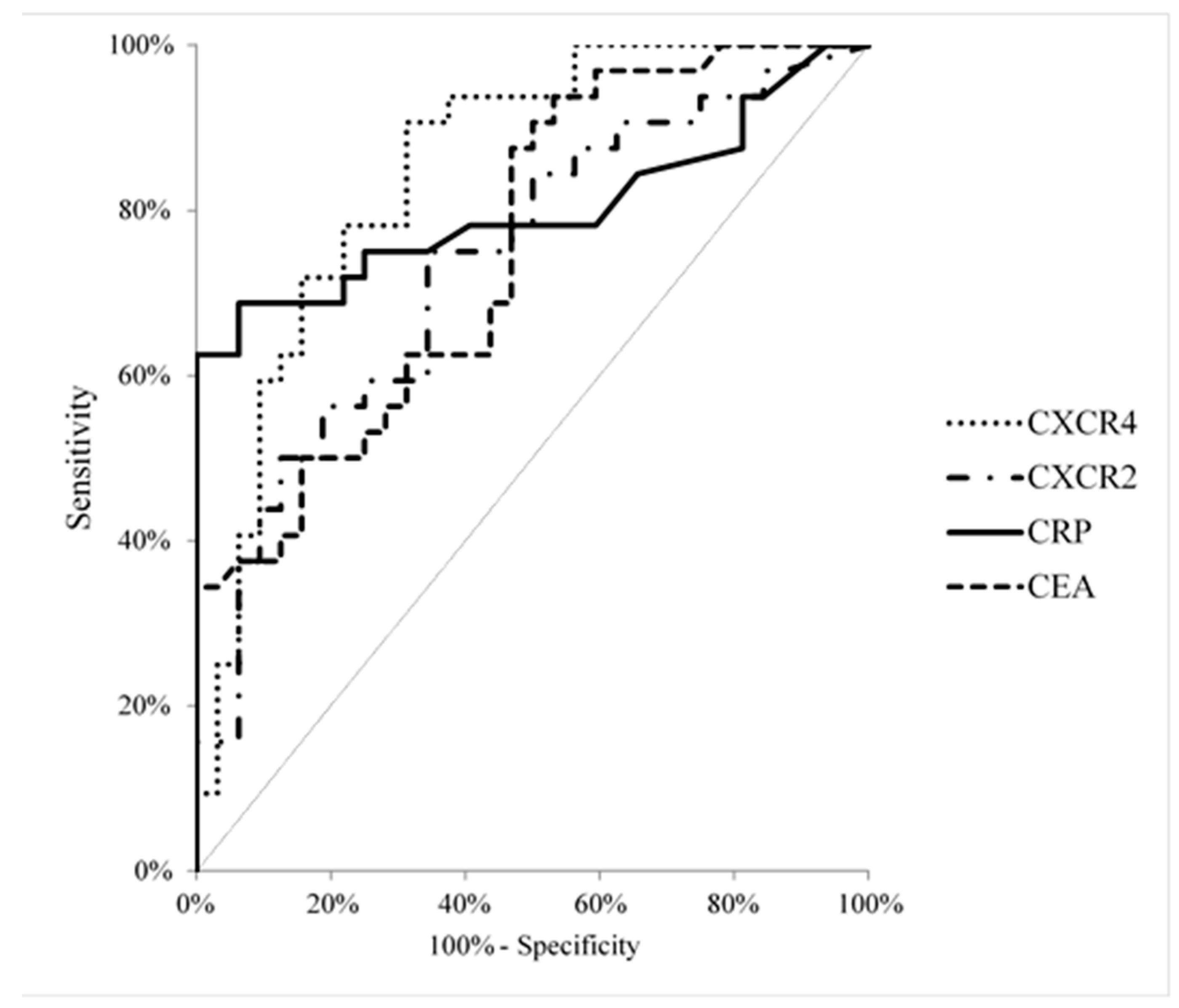
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Raman, D.; Baugher, P.J.; Thu, Y.M.; Richmond, A. Role of chemokines in tumor growth. Cancer Lett. 2007, 256, 137–165. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, M.S.; Hussain, Z.; Giricz, O.; Shenoy, N.; Polineni, R.; Maitra, A.; Verma, A.K. Targeting chemokine pathways in esophageal adenocarcinoma. Cell Cycle 2014, 13, 3320–3327. [Google Scholar] [CrossRef] [PubMed]
- Rot, A.; von Andrian, U.H. Chemokines in innate and adaptive host defense: Basic chemokinese grammar for immune cells. Annu. Rev. Immunol. 2004, 22, 891–928. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, C.; Mo, X.; Shi, H.; Li, S. Mechanisms by which CXCR4/CXCL12 cause metastatic behavior in pancreatic cancer. Oncol. Lett. 2018, 15, 1771–1776. [Google Scholar] [CrossRef]
- Vandercappellen, J.; Van Damme, J.; Struyf, S. The role of CXC chemokines and their receptors in cancer. Cancer Lett. 2008, 267, 226–244. [Google Scholar] [CrossRef]
- Chatterjee, S.; Behnam Azad, B.; Nimmagadda, S. The intricate role of CXCR4 in cancer. Adv. Cancer Res. 2017, 124, 31–82. [Google Scholar]
- Zhao, H.; Guo, L.; Zhao, J.; Weng, H.; Zhao, B.; Zhao, H. CXCR4 over-expression and survival in cancer: A system review and meta-analysis. Oncotarget 2015, 6, 5022–5040. [Google Scholar] [CrossRef]
- Highfill, S.L.; Cui, Y.; Giles, A.; Smith, J.P.; Zhang, H.; Morse, E.; Kaplan, R.N.; Mackall, C.L. Disruption of CXCR2-Mediated MDSC Tumor Trafficking Enhances Anti-PD1 Efficacy. Sci. Transl. Med. 2014, 6, 237ra67. [Google Scholar] [CrossRef]
- Steele, C.W.; Karim, S.A.; Leach, J.D.; Bailey, P.; Upstill-Goddard, R.; Rishi, L.; Foth, M.; Bryson, S.; McDaid, K.; Wilson, Z.; et al. CXCR2 Inhibition Profoundly Suppresses Metastases and Augments Immunotherapy in Pancreatic Ductal Adenocarcinoma. Cancer Cell 2016, 29, 832–845. [Google Scholar] [CrossRef]
- Müller, A.; Homey, B.; Soto, H. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001, 410, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Lazennec, G.; Richmond, A. Chemokines and chemokine receptors: New insights into cancer-related inflammation. Trends Mol. Med. 2010, 16, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Ma, X.L.; Wei, Y.Q.; Wei, X.W. Potential roles and targeted therapy of the CXCLs/CXCR2 axis in cancer and inflammatory diseases. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 289–312. [Google Scholar] [CrossRef]
- Ikeda, O.; Egami, H.; Ishiko, T.; Ishikawa, S.; Kamohara, H.; Hidaka, H.; Takahashi, M.; Ogawa, M. Signal of proteinase-activated receptor-2 contributes to highly malignant potential of human pancreatic cancer by up-regulation of interleukin-8 release. Int. J. Oncol. 2006, 28, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Lesina, M.; Kurkowski, M.U.; Ludes, K.; Rose-John, S.; Treiber, M.; Klöppel, G.; Yoshimura, A.; Reindl, W.; Sipos, B.; Akira, S.; et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell 2011, 19, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, A.; Wang, S.C.; Morris, J.P.; Folias, A.E.; Liou, A.; Kim, G.E.; Akira, S.; Boucher, K.M.; Firpo, M.A.; Mulvihill, S.J.; et al. Stat3 and MMP7 contribute to pancreatic ductal adenocarcinoma initiation and progression. Cancer Cell 2011, 19, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Kuwada, Y.; Sasaki, T.; Morinaka, K.; Kitadai, Y.; Mukaida, N.; Chayama, K. Potential involvement of IL-8 and its receptors in the invasiveness of pancreatic cancer cells. Int. J. Oncol. 2003, 22, 765–771. [Google Scholar] [CrossRef]
- Rajarathnam, K.; Desai, U.R. Structural Insights Into How Proteoglycans Determine Chemokine-CXCR1/CXCR2 Interactions: Progress and Challenges. Front. Immunol. 2020, 11, 660. [Google Scholar] [CrossRef]
- Ali, S.; Lazennec, G. Chemokines: Novel targets for breast cancer metastasis. Cancer Metastasis Rev. 2007, 26, 401–420. [Google Scholar] [CrossRef]
- Strieter, R.M.; Burdick, M.D.; Gomperts, B.N.; Belperio, J.A.; Keane, M.P. CXC chemokines in angiogenesis. Cytokine Growth Factor Rev. 2005, 16, 593–609. [Google Scholar] [CrossRef]
- Zhou, Z.; Xia, G.-K.; Xiang, Z.; Liu, M.; Wei, Z.-W.; Yan, J.; Chen, W.; Zhu, J.-T.; Awasthi, N.; Sun, X.; et al. C-X-C chemokine receptor type 2-dominated crosstalk between tumor cells and macrophages drives gastric cancer metastasis. Clin. Cancer Res. 2019. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, M.; Yu, G.-Z.; Qin, X.-R.; Jin, G.; Chen, P.; Zhu, M.-H. Interleukin-8, a promising predictor for prognosis of pancreatic cancer. World J. Gastroenterol. 2012, 18, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Sleightholm, R.L.; Neilsen, B.K.; Li, J. Emerging roles of the CXCL12/CXCR4 axis in pancreatic cancer progression and therapy. Pharmacol. Ther. 2017, 179, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Wehler, T.; Wolfert, F.; Schimanski, C.C.; Gockel, I.; Herr, W.; Biesterfeld, S.; Seifert, J.K.; Adwan, H.; Berger, M.R.; Junginger, T.; et al. Strong expression of chemokine receptor CXCR4 by pancreatic cancer correlates with advanced disease. Oncol. Rep. 2006, 16, 1159–1164. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saur, D.; Seidler, B.; Schneider, G.; Algül, H.; Beck, R.; Senekowitsch–Schmidtke, R.; Schwaiger, M.; Schmid, R.M. CXCR4 expression increases liver and lung metastasis in a mouse model of pancreatic cancer. Gastroenterology 2005, 129, 1344–1347. [Google Scholar] [CrossRef]
- Chu, L.C.; Goggins, M.G.; Fishman, E.K. Diagnosis and Detection of Pancreatic Cancer. Cancer J. 2017, 23, 333–342. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef]
- National Cancer Institute. SEER Cancer Statistics Review, 1975–2014. 2017. Available online: https://seer.cancer.gov/csr/1975_2014/ (accessed on 27 March 2019).
- Chang, J.C.; Kundranda, M. Novel Diagnostic and Predictive Biomarkers in Pancreatic Adenocarcinoma. Int. J. Mol. Sci. 2017, 18, 667. [Google Scholar] [CrossRef]
- Heaney, M.L.; Golde, D.W. Soluble receptor in human disease. J. Leukoc. Biol. 1998, 64, 135–146. [Google Scholar] [CrossRef]
- Levine, S.J. Mechanism of soluble cytokine receptor generation. J. Immunol. 2004, 173, 5343–5348. [Google Scholar] [CrossRef]
- Tsimanis, T.; Kalinkovich, A.; Bentwich, Z. Soluble chemokine CCR5 receptor is present in human plasma. Immunol. Lett. 2005, 96, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Katlinski, K.; Akalovich, S.; Katlinskaya, Y. Immunoregulation SLBAW2-C Soluble human CXCR2: Structure, properties, bioactivity. Cytokine 2009, 48, 81. [Google Scholar] [CrossRef]
- Malvoisin, E.; Livrozet, J.-M.; Makloufi, D.; Vincent, N. Soluble chemokine receptor CXCR4 is present in human sera. Anal. Biochem. 2011, 414, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Łukaszewicz-Zając, M.; Mroczko, B.; Kozłowski, M.; Szmitkowski, M. Serum Concentrations of Chemokine CXCL12 and Its Specific Receptor CXCR4 in Patients with Esophageal Cancer. Dis. Markers 2016, 2016, 7963895. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Łukaszewicz-Zając, M.; Muszyński, P.; Kozłowski, M.; Kulczyńska-Przybik, A.; Szmitkowski, M.; Mroczko, B. Serum concentrations of receptor for interleukin 8 in patients with esophageal cancer. Pol. Arch. Med. Wewn. 2016, 126, 854–861. [Google Scholar] [CrossRef]
- Łukaszewicz-Zając, M.; Pączek, S.; Muszyński, P.; Kozłowski, M.; Mroczko, B. Comparison between clinical significance of serum CXCL-8 and classical tumor markers in oesophageal cancer (OC) patients. Clin. Exp. Med. 2019. [Google Scholar] [CrossRef]
- Litman-Zawadzka, A.; Łukaszewicz-Zając, M.; Gryko, M.; Kulczyńska-Przybik, A.; Mroczko, B. Serum chemokine CXCL8 as a better biomarker for diagnosis and prediction of pancreatic cancer than its specific receptor CXCR2, C-reactive protein, and classic tumor markers CA 19-9 and CEA. Pol. Arch. Med. Wewn. 2018, 128, 524–531. [Google Scholar]
- Jass, J.R.; Sobin, L.H. WHO International Histological Classification of Tumors. Histological Typing of Intestinal Tumors; Springer: New York, NY, USA, 1989. [Google Scholar]
- Hollander, M.; Wolfe, D.A. Nonparametric Statistical Methods; John Wiley & Sons: New York, NY, USA, 1999; pp. 240–249. [Google Scholar]
- Evans, U.B.; Lee, J.E.; Pisters, P.W.T.; Charnsangavej, C.; Ellis, L.M.; Chiao, P.J.; Lenzi, R.; Abbruzzese, J.L. Advances in the diagnosis and treatment of adenocarcinoma of the pancreas. Cancer Treat. Res. 1997, 90, 109–125. [Google Scholar]
- Wanebo, H.J.; Vezeridis, M.P. Pancreatic carcinoma in perspective. A continuing challenge. Cancer 1996, 78, 580–591. [Google Scholar] [CrossRef]
- Sarvaiya, P.J.; Guo, D.; Ulasov, I.; Gabikian, P.; Lesniak, M.S. Chemokines in tumor progression and metastasis. Oncotarget 2013, 4, 2171–2185. [Google Scholar] [CrossRef]
- Groblewska, M.; Mroczko, B.; Wereszczynska-Siemiatkowska, U.; Mysliwiec, P.; Kędra, B.; Szmitkowski, M. Serum levels of granulocyte colony-stimulating factor (G-CSF) and macrophage colony-stimulating factor (M-CSF) in pancreatic cancer patients. Clin. Chem. Lab. Med. 2007, 45, 30–34. [Google Scholar] [CrossRef]
- Mroczko, B.; Lukaszewicz-Zajac, M.; Wereszczynska-Siemiatkowska, U.; Groblewska, M.; Gryko, M.; Kedra, B.; Jurkowska, G.; Szmitkowski, M. Clinical significance of the measurements of serum matrix metalloproteinase-9 and its inhibitor (tissue inhibitor of metalloproteinase-1) in patients with pancreatic cancer: Metalloproteinase-9 as an independent prognostic factor. Pancreas 2009, 38, 613–618. [Google Scholar] [CrossRef]
- Łukaszewicz-Zając, M.; Gryko, M.; Pączek, S.; Szmitkowski, M.; Kędra, B.; Mroczko, B. Matrix metalloproteinase 2 (MMP-2) and its tissue inhibitor 2 (TIMP-2) in pancreatic cancer (PC). Oncotarget 2019, 10, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zheng, J.; Ni, Q.; Zhu, H.; Lu, Y.; Qian, H.; Zhu, J. Prognostic significance of CXCR2 expression in pancreatic ductal carcinoma. Zhonghua Yi Xue Za Zhi 2014, 94, 3805–3808. [Google Scholar] [PubMed]
- Ding, Y.; Du, Y. Clinicopathological significance and prognostic role of chemokine receptor CXCR4 expression in pancreatic ductal adenocarcinoma, a meta-analysis and literature review. Int. J. Surg. 2019. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, F.; Monti, P.; Leone, B.E.; Zerbi, A.; Vecchi, A.; Piemonti, L.; Mantovani, A.; Allavena, P. Increased survival, proliferation, and migration in metastatic human pancreatic tumor cells expressing functional CXCR4. Cancer Res. 2004, 64, 8420–8427. [Google Scholar] [CrossRef]
| Variable Tested | Number of Patients | |
|---|---|---|
| Group | Pancreatic cancer (PC) | 32 |
| Gender | Male | 20 |
| Female | 12 | |
| TNM Stage | I + II | 8 |
| III | 10 | |
| IV | 14 | |
| Depth of Tumor Invasion (T Factor) | T1 + 2 + 3 | 10 |
| T4 | 22 | |
| Nodal Involvement (N Factor) | N0 | 13 |
| N1 | 19 | |
| Distant Metastases (M factor) | M0 | 18 |
| M1 | 14 | |
| Group Tested | CXCR-4 [ng/mL] | CXCR-2 [ng/mL] | CRP [mg/L] | CEA [ng/mL] | |
|---|---|---|---|---|---|
| Pancreatic Cancer (PC) | Min | 0.54 | 0.00 | 0.30 | 0.92 |
| Me | 6.48 | 1.02 | 10.30 | 2.67 | |
| Max | 45.03 | 2.26 | 269.40 | 319.21 | |
| Control Group (Healthy Subjects) | Min | 0.07 | 0.000 | 0.20 | 0.50 |
| Me | 0.89 | 0.63 | 0.95 | 1.34 | |
| Max | 25.05 | 1.72 | 5.00 | 4.54 | |
| p (PC vs. Healthy Controls) | <0.001 a | 0.001 a | <0.001 a | <0.001 a | |
| CXCR4 [ng/mL] | CXCR2 [ng/mL] | CRP [mg/L] | CEA [ng/mL] | ||
|---|---|---|---|---|---|
| I + II | Min | 1.70 | 0.00 | 0.30 | 1.20 |
| Me | 3.95 | 1.03 | 12.95 | 1.72 | |
| Max | 45.03 | 1.86 | 161.50 | 10.94 | |
| III | Min | 0.69 | 0.08 | 0.30 | 0.92 |
| Me | 6.39 | 1.02 | 2.80 | 2.69 | |
| Max | 22.36 | 2.17 | 39.20 | 17.35 | |
| IV | Min | 0.54 | 0.25 | 0.60 | 1.31 |
| Me | 7.08 | 1.02 | 26.15 | 2.91 | |
| Max | 25.62 | 2.26 | 269.40 | 319.21 | |
| Control Group (CG) | Min | 0.07 | 0.00 | 0.20 | 0.50 |
| Me | 0.89 | 0.63 | 0.95 | 1.34 | |
| Max | 25.05 | 1.72 | 5.00 | 4.54 | |
| p (Kruskal-Wallis Test) | <0.001 a | 0.01 a | <0.001 a | 0.02 a | |
| p (post hoc Dwass-Steele-Critchlow-Fligner test | I + II vs. III | 0.98 | 1.00 | 0.63 | 0.76 |
| I + II vs. IV | 0.99 | 1.00 | 0.76 | 0.29 | |
| I + II vs. CG | 0.01 a | 0.16 | 0.04 a | 0.49 | |
| III vs. IV | 0.90 | 1.00 | 0.10 | 0.90 | |
| III vs. CG | 0.01 a | 0.21 | 0.31 | 0.10 | |
| IV vs. CG | <0.001 a | 0.03 a | <0.001 a | 0.01 a | |
| Pancreatic Cancer (PC) | CXCR4 [ng/mL] | CXCR2 [ng/mL] | CRP [mg/L] | CEA [ng/mL] | ||
|---|---|---|---|---|---|---|
| Depth of tumor invasion (T factor) | T1 + 2 + 3 | Min | 1.70 | 0.00 | 0.70 | 1.20 |
| Me | 5.88 | 0.93 | 12.65 | 1.99 | ||
| Max | 45.03 | 1.86 | 161.50 | 10.94 | ||
| T4 | Min | 0.54 | 0.08 | 0.30 | 0.92 | |
| Me | 6.70 | 1.05 | 8.10 | 2.91 | ||
| Max | 25.62 | 2.26 | 269.40 | 319.21 | ||
| Control group (CG) | Min | 0.07 | 0.00 | 0.20 | 0.50 | |
| Me | 0.89 | 0.63 | 0.95 | 1.34 | ||
| Max | 25.05 | 1.72 | 5.00 | 4.54 | ||
| p (Kruskal-Wallis test) | <0.001 a | 0.01 a | <0.001 a | 0.01 a | ||
| p (post hoc Dwass-Steele-Critchlow-Fligner test) | 1 + 2 + 3 vs. 4 | 0.98 | 0.90 | 1.00 | 0.54 | |
| 1 + 2 + 3 vs. | 0.01 a | 0.12 | 0.01 a | 0.10 | ||
| 4 vs. CG | <0.001 a | 0.01 a | 0.01 a | 0.10 | ||
| Presence of lymph node metastasis (N factor) | N0 | Min | 0.69 | 0.08 | 0.30 | 0.92 |
| Me | 3.15 | 0.77 | 7.10 | 1.92 | ||
| Max | 45.03 | 1.86 | 161.50 | 7.22 | ||
| N1 | Min | 0.54 | 0.00 | 0.30 | 1.24 | |
| Me | 6.74 | 1.06 | 14.30 | 3.00 | ||
| Max | 25.62 | 2.26 | 269.40 | 319.21 | ||
| Control group (CG) | Min | 0.07 | 0.00 | 0.20 | 0.50 | |
| Me | 0.89 | 0.63 | 0.95 | 1.34 | ||
| Max | 25.05 | 1.72 | 5.00 | 4.54 | ||
| p (Kruskal-Wallis test) | <0.001 a | 0.01 a | <0.001 a | 0.01 a | ||
| p (post hoc Dwass-Steele-Critchlow-Fligner test) | 0 vs. 1 | 0.47 | 0.64 | 0.36 | 0.25 | |
| 0 vs. CG | 0.01 a | 0.11 | 0.01 a | 0.13 | ||
| 1 vs. CG | <0.001 a | 0.01 a | <0.001 a | 0.01 a | ||
| Presence of distant metastasis (M factor) | M0 | Min | 0.69 | 0.00 | 0.30 | 0.92 |
| Me | 5.78 | 1.03 | 6.75 | 1.99 | ||
| Max | 45.03 | 2.17 | 161.50 | 17.35 | ||
| M1 | Min | 0.54 | 0.25 | 0.60 | 1.31 | |
| Me | 7.08 | 1.02 | 26.15 | 2.91 | ||
| Max | 25.62 | 2.26 | 269.40 | 319.21 | ||
| Control group (CG) | Min | 0.07 | 0.00 | 0.20 | 0.50 | |
| Me | 0.89 | 0.63 | 0.95 | 1.34 | ||
| Max | 25.05 | 1.72 | 5.00 | 4.54 | ||
| p (Kruskal-Wallis test) | <0.001 a | 0.01 a | <0.001 a | 0.01 a | ||
| p (post hoc Dwass-Steele-Critchlow-Fligner test) | 0 vs. 1 | 0.82 | 1.00 | 0.10 | 0.32 | |
| 0 vs. CG | <0.001 a | 0.03 a | 0.01 a | 0.04 a | ||
| 1 vs. CG | <0.001 a | 0.02 a | <0.001 a | 0.01 a | ||
| T | N | TNM | Age | CXCR4 | CXCR2 | CRP | CEA | ||
|---|---|---|---|---|---|---|---|---|---|
| T | r | 1.00 | 0.42 | 0.49 | 0.01 | −0.04 | 0.06 | 0.02 | 0.22 |
| p | 0.02 a | <0.001 a | 0.97 | 0.84 | 0.74 | 0.90 | 0.24 | ||
| N | r | 0.42 | 1.00 | 0.53 | 0.00 | 0.21 | 0.16 | 0.24 | 0.29 |
| p | 0.02 a | <0.001 a | 0.99 | 0.25 | 0.38 | 0.18 | 0.11 | ||
| TNM | r | 0.49 | 0.53 | 1.00 | 0.33 | 0.04 | −0.02 | 0.26 | 0.30 |
| p | <0.001 a | <0.001 a | 0.06 | 0.81 | 0.90 | 0.15 | 0.09 | ||
| Age | r | 0.01 | 0.00 | 0.33 | 1.00 | 0.41 | 0.44 | 0.57 | 0.31 |
| p | 0.97 | 0.99 | 0.06 | <0.001 a | <0.001 a | <0.00 a | <0.001 a | ||
| CXCR4 | R | −0.04 | 0.21 | 0.04 | 0.41 | 1.00 | 0.71 | 0.37 | 0.11 |
| p | 0.84 | 0.25 | 0.81 | <0.001 a | <0.001 a | <0.00 a | 0.39 | ||
| CXCR2 | R | 0.06 | 0.16 | −0.02 | 0.44 | 0.71 | 1.00 | 0.27 | 0.00 |
| p | 0.74 | 0.38 | 0.90 | <0.001 a | <0.00 a | 0.03 a | 0.98 | ||
| CRP | R | 0.02 | 0.24 | 0.26 | 0.57 | 0.37 | 0.27 | 1.00 | 0.44 |
| p | 0.90 | 0.18 | 0.15 | <0.001 a | <0.001 a | 0.03 a | <0.001 a | ||
| CEA | R | 0.22 | 0.29 | 0.30 | 0.31 | 0.11 | 0.00 | 0.44 | 1.00 |
| p | 0.24 | 0.11 | 0.09 | 0.01 a | 0.39 | 0.98 | <0.001 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Litman-Zawadzka, A.; Łukaszewicz-Zając, M.; Gryko, M.; Kulczyńska-Przybik, A.; Kędra, B.; Mroczko, B. Specific Receptors for the Chemokines CXCR2 and CXCR4 in Pancreatic Cancer. Int. J. Mol. Sci. 2020, 21, 6193. https://doi.org/10.3390/ijms21176193
Litman-Zawadzka A, Łukaszewicz-Zając M, Gryko M, Kulczyńska-Przybik A, Kędra B, Mroczko B. Specific Receptors for the Chemokines CXCR2 and CXCR4 in Pancreatic Cancer. International Journal of Molecular Sciences. 2020; 21(17):6193. https://doi.org/10.3390/ijms21176193
Chicago/Turabian StyleLitman-Zawadzka, Ala, Marta Łukaszewicz-Zając, Mariusz Gryko, Agnieszka Kulczyńska-Przybik, Bogusław Kędra, and Barbara Mroczko. 2020. "Specific Receptors for the Chemokines CXCR2 and CXCR4 in Pancreatic Cancer" International Journal of Molecular Sciences 21, no. 17: 6193. https://doi.org/10.3390/ijms21176193
APA StyleLitman-Zawadzka, A., Łukaszewicz-Zając, M., Gryko, M., Kulczyńska-Przybik, A., Kędra, B., & Mroczko, B. (2020). Specific Receptors for the Chemokines CXCR2 and CXCR4 in Pancreatic Cancer. International Journal of Molecular Sciences, 21(17), 6193. https://doi.org/10.3390/ijms21176193








