Long-Term Impact of Chemical and Alternative Fungicides Applied to Grapevine cv Nebbiolo on Berry Transcriptome
Abstract
1. Introduction
2. Results
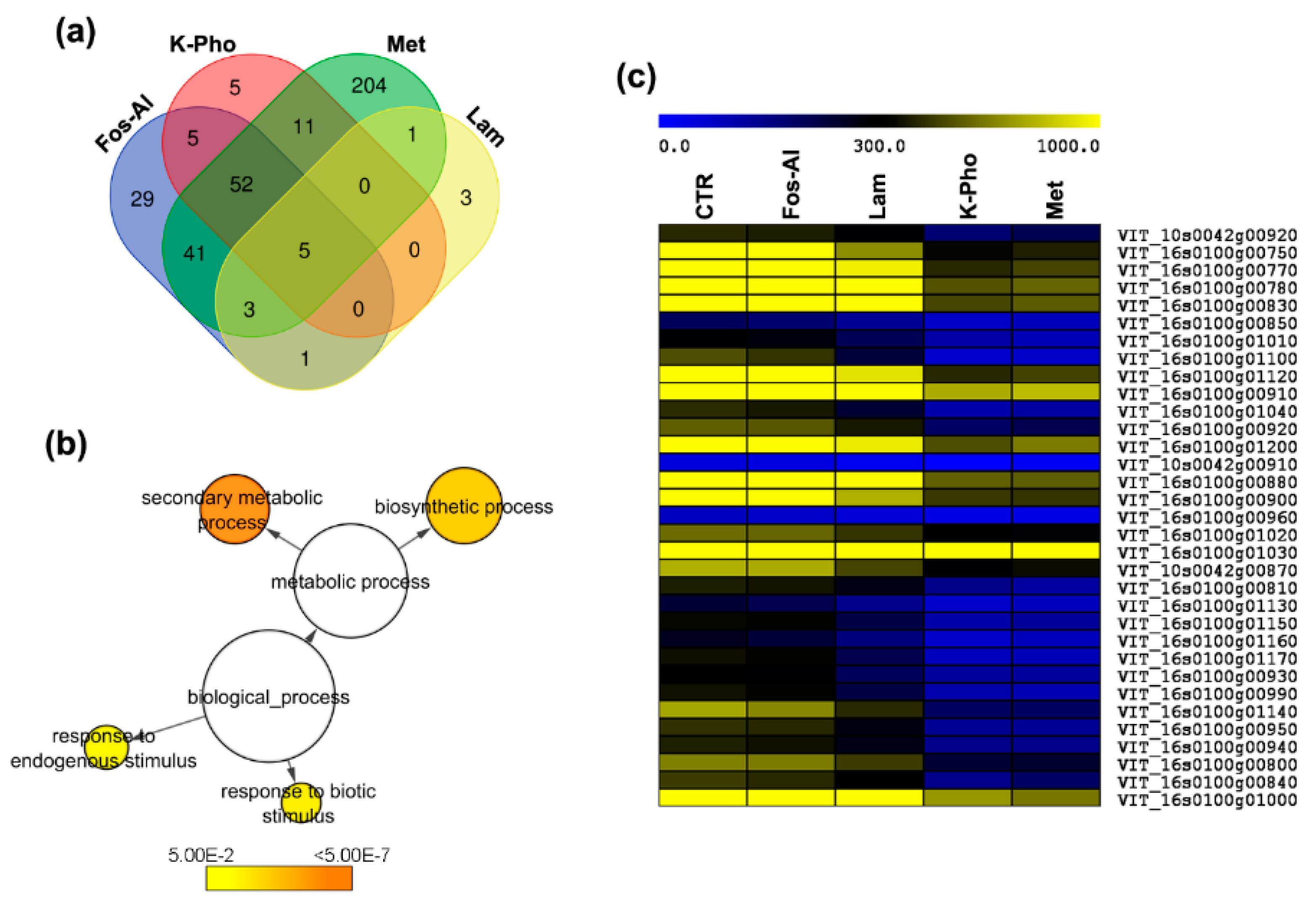
2.1. Transcriptional Modifications Induced by Antifungal Treatments in Berry
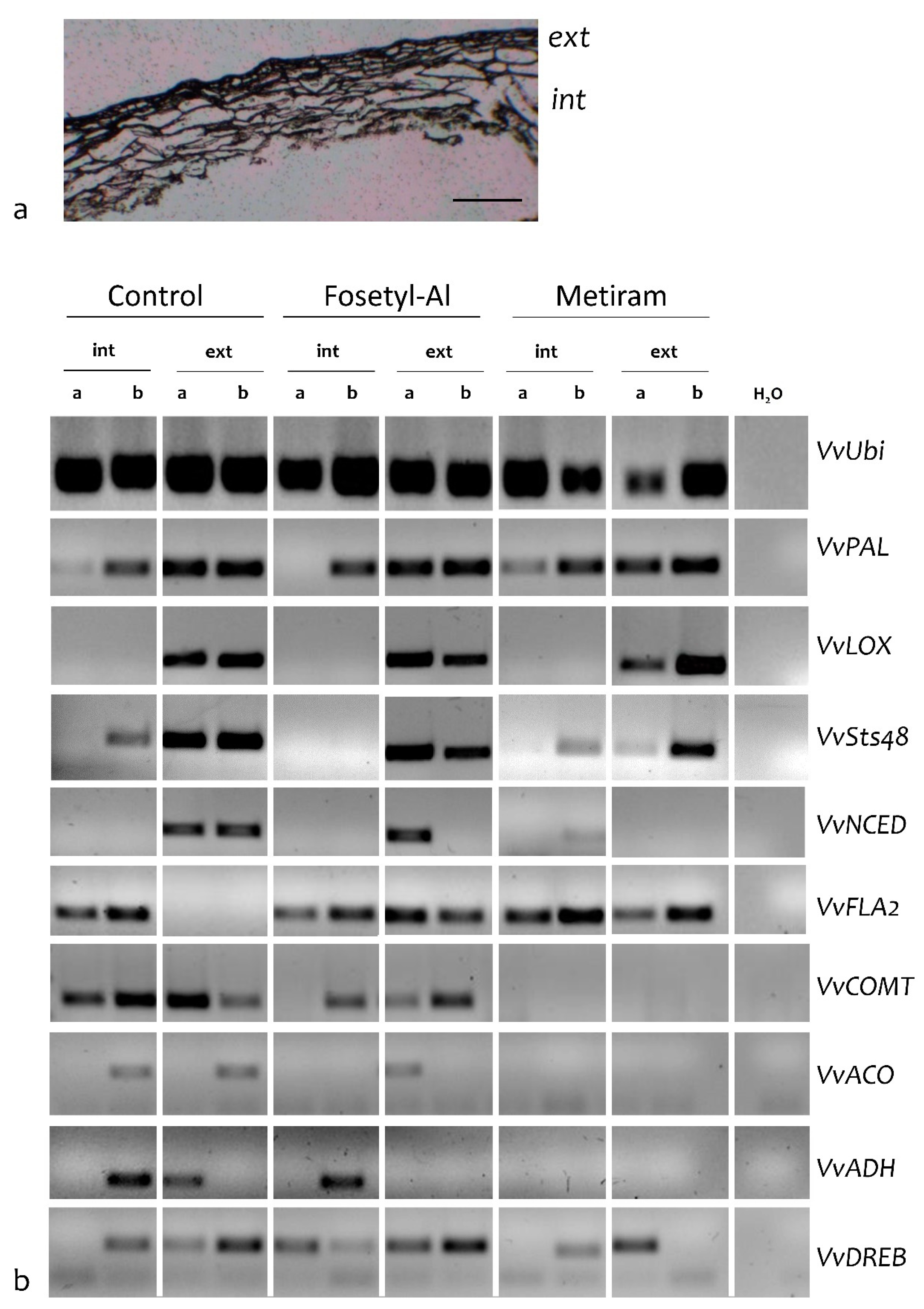
2.2. Laser Microdissection
3. Discussion
3.1. A snapshot on the Berry Transcriptome
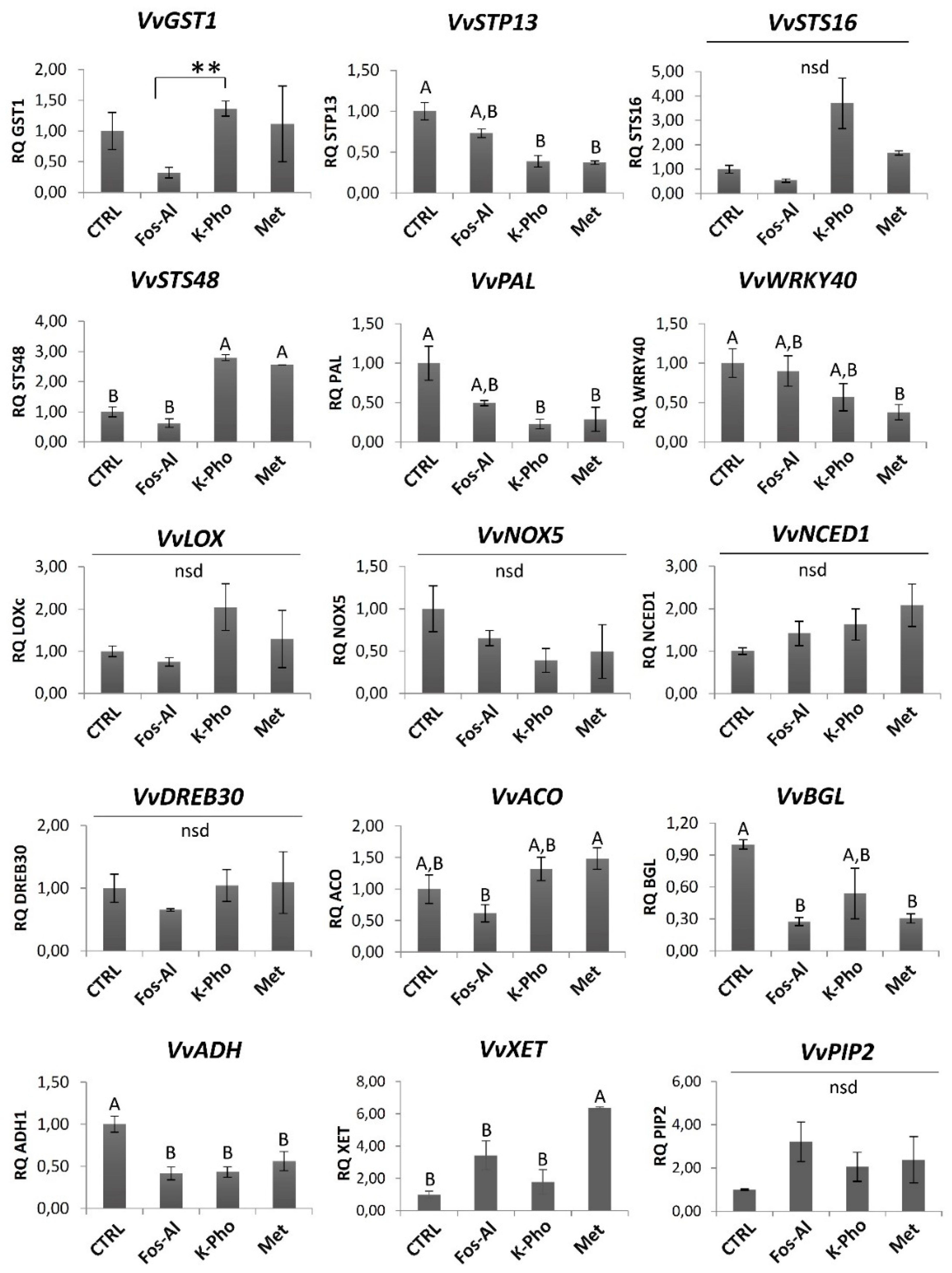
3.2. DEGs Associated to Secondary Metabolism and Cell-Wall Modifications
3.3. Cell-Specificity in Two Different Cell-Type Populations in Grape Berry Skin
4. Materials and Methods
4.1. Field Trials
4.2. Grape Sampling
4.3. RNA Extraction and Illumina Sequencing
4.4. RNA-Seq Analysis
4.5. Real-Time Quantitative PCR Analysis Validation
4.6. Laser Microdissection
4.6.1. Tissue Preparation for LMD
4.6.2. Collection of Specific Cell-Type at Laser Microdissection
4.6.3. RNA Extraction and RT-PCR from Microdissected Cells
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Fos-Al | Fosetyl-Al |
| K-Pho | potassium phosphonate |
| Lam | Laminarin |
| LMD | Laser microdissection |
| Met | Metiram |
References
- Carbonell-Bejerano, P.; Santa María, E.; Torres-Pérez, R.; Royo, C.; Lijavetzky, D.; Bravo, G.; Aguirreolea, J.; Sánchez-Díaz, M.; Antolín, M.C.; Martínez-Zapater, J.M. Thermotolerance responses in ripening berries of Vitis vinifera L. cv muscat hamburg. Plant Cell Physiol. 2013, 54, 1200–1216. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, N.; Guan, L.; Dai, Z.W.; Wu, B.H.; Lauvergeat, V.; Gomès, E.; Li, S.H.; Godoy, F.; Arce-Johnson, P.; Delrot, S. Berry ripening recently heard through the grapevine. J. Exp. Bot. 2014, 65, 4543–4559. [Google Scholar] [CrossRef] [PubMed]
- Ghan, R.; Van Sluyter, S.C.; Hochberg, U.; Degu, A.; Hopper, D.W.; Tillet, R.L.; Schlauch, K.A.; Haynes, P.A.; Fait, A.; Cramer, G.R. Five omic technologies are concordant in differentiating the biochemical characteristics of the berries of five grapevine Vitis vinifera L. cultivars. BMC Genomics. 2015, 16, 946. [Google Scholar] [CrossRef] [PubMed]
- Young, P.R.; Eyeghe-Bickong, H.A.; du Plessis, K.; Alexandersson, E.; Jacobson, D.A.; Coetzee, Z.; Deloire, A.; Vivier, M.A. Grapevine plasticity in response to an altered microclimate, Sauvignon Blanc modulates specific metabolites in response to increased berry exposure. Plant Physiol. 2016, 170, 1235–1254. [Google Scholar] [CrossRef]
- Massonnet, M.; Fasoli, M.; Tornielli, G.B.; Altieri, M.; Sandri, M.; Zuccolotto, P.; Paci, P.; Gardiman, M.; Zenoni, S.; Pezzotti, M. Ripening transcriptomic program in red and white grapevine varieties correlates with berry skin anthocyanin accumulation. Plant Physiol. 2017, 174, 2376–2396. [Google Scholar] [CrossRef]
- Dal Santo, S.; Tornielli, G.B.; Zenoni, S.; Fasoli, M.; Farina, L.; Anesi, A.; Guzzo, F.; Delledonne, M.; Pezzotti, M. The plasticity of the grapevine berry transcriptome. Genome Biol. 2013, 14, R54. [Google Scholar] [CrossRef]
- Dal Santo, S.; Fasoli, M.; Negri, S.; D’Incà, E.; Vicenzi, N.; Guzzo, F.; Tornielli, G.B.; Pezzotti, M.; Zenoni, S. Plasticity of the Berry Ripening Program in a White Grape Variety. Front. Plant Sci. 2016, 7, 970. [Google Scholar] [CrossRef]
- Dal Santo, S.; Zenoni, S.; Sandri, M.; De Lorenzis, G.; Magris, G.; De Paoli, E.; Di Gaspero, G.; Del Fabbro, C.; Morgante, M.; Brancadoro, L.; et al. Grapevine field experiments reveal the contribution of genotype, the influence of environment and the effect of their interaction G×E on the berry transcriptome. Plant J. 2018, 93, 1143–1159. [Google Scholar] [CrossRef]
- Balic, I.; Vizoso, P.; Nilo-Poyanco, R.; Sanhueza, D.; Olmedo, P.; Sepúlveda, P.; Arriagada, C.; Defilippi, B.G.; Meneses, C.; Campos-Vargas, R. Transcriptome analysis during ripening of table grape berry cv. Thompson Seedless. PLoS ONE 2018, 13, e0190087. [Google Scholar] [CrossRef]
- Pagliarani, C.; Boccacci, P.; Chitarra, W.; Cosentino, E.; Sandri, M.; Perrone, I.; Mori, A.; Cuozzo, D.; Nerva, L.; Rossato, M.; et al. Distinct metabolic signals underlie clone by environment interplay in “Nebbiolo” grapes over ripening. Front. Plant Sci. 2019, 10, 1575. [Google Scholar] [CrossRef]
- Trouvelot, S.; Bonneau, L.; Redecker, D.; van Tuinen, D.; Adrian, M.; Wipf, D. Arbuscular mycorrhiza symbiosis in viticulture: A review. Agron. Sustain. Dev. 2015, 35, 1449–1467. [Google Scholar] [CrossRef]
- Provost, C.; Pedneault, K. The organic vineyard as a balanced ecosystem: Improved organic grape management and impacts on wine quality. Sci. Hortic. 2016, 208, 43–56. [Google Scholar] [CrossRef]
- Nesler, A.; Perazzolli, M.; Puopolo, G.; Giovannini, O.; Pertot, I. A complex protein hydrolysate acts as biogenic elicitor of plant resistance against powdery mildew. Front. Plant Sci. 2015, 6, 715. [Google Scholar] [CrossRef] [PubMed]
- Fantke, P.; Friedrich, R.; Jolliet, O. Health impact and damage cost assessment of pesticides in Europe. Environ Int. 2012, 49, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Pertot, I.; Caffi, T.; Rossi, V.; Mugnai, L.; Hoffmann, C.; Grando, M.S.; Gary, C.; Lafond, D.; Duso, C.; Thiery, D.; et al. A critical review of plant protection tools for reducing pesticide use on grapevine and new perspectives for the implementation of IPM in viticulture. Crop Protect. 2017, 97, 70–84. [Google Scholar] [CrossRef]
- Flores, S.S. What is sustainability in the wine world? A cross-country analysis of wine sustainability frameworks. J. Clean. Prod. 2018, 172, 2301–2312. [Google Scholar] [CrossRef]
- Cappelletti, M.; Perazzolli, M.; Antonielli, L.; Nesler, A.; Torboli, E.; Bianchedi, P.L.; Pindo, M.; Puopolo, G.; Pertot, I. Leaf treatments with a protein-based resistance inducer partially modify phyllosphere microbial communities of grapevine. Front. Plant Sci. 2016, 7, 1053. [Google Scholar] [CrossRef]
- Nerva, L.; Pagliarani, C.; Pugliese, M.; Monchiero, M.; Gonthier, S.; Gullino, M.L.; Gambino, G.; Chitarra, W. Grapevine phyllosphere community analysis in response to elicitor application against powdery mildew. Microorganisms 2019, 7, 662. [Google Scholar] [CrossRef]
- Gadoury, D.M.; Cadle-Davidson, L.; Wilcox, W.F.; Dry, I.B.; Seem, R.C.; Milgroom, M.G. Grapevine powdery mildew (Erysiphe necator): A fascinating system for the study of the biology, ecology and epidemiology of an obligate biotroph. Mol. Plant Pathol. 2012, 13, 1–16. [Google Scholar] [CrossRef]
- Thind, T.S. Role of Fungicides in Crop Health Management: Prospects and Challenges. In Developments in Fungal Biology and Applied Mycology; Satyanarayana, T., Deshmukh, S., Johri, B., Eds.; Springer: Singapore, 2017; pp. 433–447. [Google Scholar]
- Gutiérrez-Gamba, G.; Romanazzi, G.; Garde-Cerdàn, T.; Pérez-Alvarez, E.P. A review of the use of biostimulants in the vineyard for improved grape and wine quality: Effects on prevention of grapevine diseases. J. Sci. Food Agric. 2019, 99, 1001–1009. [Google Scholar] [CrossRef]
- Rantsiou, K.; Giacosa, S.; Pugliese, M.; Englezos, V.; Ferrocino, I.; Río Segade, S.; Monchiero, M.; Gribaudo, I.; Gambino, G.; Gullino, M.L.; et al. Impact of chemical and alternative fungicides applied to grapevine cv Nebbiolo on microbial ecology and chemical-physical grape characteristics at harvest. Front. Plant Sci. 2020, 11, 700. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.; Poinssot, B.; Daire, X.; Adrian, M.; Bézier, A.; Lambert, B.; Joubert, J.M.; Pugin, A. Laminarin elicits defense responses in grapevine and induces protection against Botrytis cinerea and Plasmopara viticola. Mol. Plant Microbe Interact. 2003, 16, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, A.; Trouvelot, S.; Kelloniemi, J.; Frettinger, P.; Wendehenne, D.; Daire, X.; Joubert, J.M.; Ferrarini, A.; Delledonne, M.; Flors, V.; et al. The sulfated laminarin triggers a stress transcriptome before priming the SA- and ROS-dependent defenses during grapevine’s Induced Resistance against Plasmopara viticola. PLoS ONE 2014, 9, e88145. [Google Scholar] [CrossRef] [PubMed]
- Chalal, M.; Winkler, J.B.; Gourrat, K.; Trouvelot, S.; Adrian, M.; Schnitzler, J.P.; Jamois, F.; Daire, X. Sesquiterpene volatile organic compounds (VOCs) are markers of elicitation by sulfated laminarin in grapevine. Front. Plant Sci. 2015, 6, 1–9. [Google Scholar] [CrossRef]
- Romanazzi, G.; Mancini, V.; Feliziani, E.; Servili, A.; Endeshaw, S.; Neri, D. Impact of alternative fungicides on grape downy mildew control and vine growth and development. Plant Dis. 2016, 100, 739–748. [Google Scholar] [CrossRef]
- Pugliese, M.; Monchiero, M.; Gullino, M.L.; Garibaldi, A. Application of laminarin and calcium oxide for the control of grape powdery mildew on Vitis vinifera cv. Moscato. J. Plant Dis. Prot. 2018, 125, 477–482. [Google Scholar] [CrossRef]
- Savoi, S.; Herrera, J.C.; Forneck, A.; Griesser, M. Transcriptomics of the grape berry shrivel ripening disorder. Plant Mol. Biol. 2019, 100, 285–301. [Google Scholar] [CrossRef]
- Young, P.R.; Lashbrooke, J.G.; Alexandersson, E.; Jacobson, D.; Moser, C.; Velasco, R.; Vivier, M.A. The genes and enzymes of the carotenoid metabolic pathway in Vitis vinifera L. BMC Genom. 2012, 13, 243. [Google Scholar] [CrossRef]
- Fasoli, M.; Dell’Anna, R.; Dal Santo, S.; Balestrini, R.; Sanson, A.; Pezzotti, M.; Monti, F.; Zenoni, S. Pectins, hemicelluloses and celluloses show specific dynamics in the internal and external surfaces of grape berry skin during ripening. Plant Cell Physiol. 2016, 57, 1332–1349. [Google Scholar] [CrossRef]
- Fasoli, M.; Dal Santo, S.; Zenoni, S.; Tornielli, G.B.; Farina, L.; Zamboni, A.; Porceddu, A.; Venturini, L.; Bicego, M.; Murino, V.; et al. The grapevine expression atlas reveals a deep transcriptome shift driving the entire plant into a maturation program. Plant Cell 2012, 24, 3489–3505. [Google Scholar] [CrossRef]
- Tornielli, G.B.; Zamboni, A.; Zenoni, S.; Delledonne, M.; Pezzotti, M. Transcriptomics and metabolomics for the analysis of grape berry development. Biochem. Grape Berry 2012, 1, 218–240. [Google Scholar]
- Zenoni, S.; Ferrarini, A.; Giacomelli, E.; Xumerle, L.; Fasoli, M.; Malerba, G.; Bellin, D.; Pezzotti, M.; Delledonne, M. Characterization of transcriptional complexity during berry development in Vitis vinifera using RNA-Seq. Plant Physiol. 2010, 152, 1787–1795. [Google Scholar] [CrossRef] [PubMed]
- Grimplet, J.; Tello, J.; Laguna, N.; Ibáñez, J. Differences in flower transcriptome between grapevine clones are related to their cluster compactness, fruitfulness and berry size. Front. Plant Sci. 2017, 8, 632. [Google Scholar] [CrossRef] [PubMed]
- Haile, Z.M.; Malacarne, G.; Pilati, S.; Sonego, P.; Moretto, M.; Masuero, D.; Vrhovsek, U.; Engelen, K.; Baraldi, E.; Moser, C. Dual transcriptome and metabolic analysis of Vitis vinifera cv. Pinot Noir berry and Botrytis cinerea during quiescence and egressed Infection. Front. Plant Sci. 2020, 10, 1704. [Google Scholar] [CrossRef] [PubMed]
- Pilati, S.; Bagagli, G.; Sonego, P.; Moretto, M.; Brazzale, D.; Castorina, G.; Simoni, L.; Tonelli, C.; Guella, G.; Engelen, K.; et al. Abscisic acid is a major regulator of grape berry ripening onset: New insights into ABA signaling network. Front. Plant Sci. 2017, 8, 1093. [Google Scholar] [CrossRef] [PubMed]
- Asselbergh, B.; De Vleesschauwer, D.; Höfte, M. Global switches and fine-tuning-ABA modulates plant pathogen defense. Mol. Plant Microbe Interact. 2008, 21, 709–719. [Google Scholar] [CrossRef]
- Spence, C.; Bais, H. Role of plant growth regulators as chemical signals in plant–microbe interactions: A double edged sword. Curr. Op. Plant Biol. 2015, 27, 52–58. [Google Scholar] [CrossRef]
- He, R.; Zhuang, Y.; Cai, Y.; Agüero, C.B.; Liu, S.; Wu, J.; Deng, S.; Walker, M.A.; Lu, J.; Zhang, Y. Overexpression of 9-cis-epoxycarotenoid dioxygenase cisgene in grapevine increases drought tolerance and results in pleiotropic effects. Front. Plant Sci. 2018, 9, 970. [Google Scholar] [CrossRef]
- Daou, M.; Faulds, C.B. Glyoxal oxidases: Their nature and properties. World J. Microbiol. Biotech. 2017, 33, 87. [Google Scholar] [CrossRef]
- Wang, Y.-Z.; Dai, M.-S.; Zhang, S.J.; Shi, Z.-B. Exploring candidate genes for pericarp russet pigmentation of sand pear (Pyrus pyrifolia) via RNA-Seq data in two genotypes contrasting for pericarp color. PLoS ONE 2014, 9, 83675. [Google Scholar] [CrossRef]
- Williamson, J.D.; Desai, A.; Krasnyanski, S.F.; Ding, F.; Guo, W.W.; Nguyen, T.T.; Olson, H.A.; Dole, J.M.; Allen, G.C. Overexpression of mannitol dehydrogenase in zonal geranium confers increased resistance to the mannitol secreting fungal pathogen Botrytis cinerea. Plant Cell Tissue Organ Cult. 2013, 115, 367–375. [Google Scholar] [CrossRef]
- Ciaffi, M.; Paolacci, A.R.; Paolocci, M.; Alicandri, E.; Bigini, V.; Badiani, M.; Muganu, M. Transcriptional regulation of stilbene synthases in grapevine germplasm differentially susceptible to downy mildew. BMC Plant Biol. 2019, 19, 404. [Google Scholar] [CrossRef] [PubMed]
- Vannozzi, A.; Dry, I.B.; Fasoli, M.; Zenoni, S.; Lucchin, M. Genome-wide analysis of the grapevine stilbene synthase multigenic family: Genomic organization and expression profiles upon biotic and abiotic stresses. BMC Plant Biol. 2012, 12, 130. [Google Scholar] [CrossRef] [PubMed]
- Pensec, F.; Pączkowski, C.; Grabarczyk, M.; Woźniak, A.; Bénard-Gellon, M.; Bertsch, C.; Chong, J.; Szakiel, A. Changes in the triterpenoid content of cuticular waxes during fruit ripening of eight grape (Vitis vinifera) cultivars grown in the Upper Rhine Valley. J. Agric. Food Chem. 2014, 62, 7998–8007. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.P.; Fangel, J.U.; Willats, W.G.T.; Vivier, M.A. Pectic-β (1,4)-galactan, extensin and arabinogalactan–protein epitopes differentiate ripening stages in wine and table grape cell walls. Ann. Bot. 2014, 114, 1279–1294. [Google Scholar] [CrossRef]
- Giribaldi, M.; Gény, L.; Delrot, S.; Schubert, A. Proteomic analysis of the effects of ABA treatments on ripening Vitis vinifera berries. J. Exp. Bot. 2010, 61, 2447–2458. [Google Scholar] [CrossRef]
- Fasoli, M.; Amato, A.; Dal Santo, S.; Monti, F.; Zenoni, S. Active rearrangements in the cell wall follow polymer concentration during postharvest withering in the berry skin of Vitis vinifera cv. Corvina. Plant Physiol. Bioch. 2018, 135, 411–422. [Google Scholar] [CrossRef]
- Deluc, L.G.; Grimplet, J.; Wheatley, M.D.; Tillett, R.L.; Quilici, D.R.; Osborne, C.; Schooley, D.A.; Schlauch, K.A.; Cushman, J.C.; Cramer, G.R. Transcriptomic and metabolite analyses of Cabernet Sauvignon grape berry development. BMC Genom. 2007, 8, 429–471. [Google Scholar] [CrossRef]
- Rolle, L.; Torchio, F.; Zeppa, G.; Gerbi, V. Anthocyanin extractability assessment of grape skins by texture analysis. J. Int. Sci. Vigne Vin. 2008, 42, 157–162. [Google Scholar]
- Matas, A.J.; Agustí, J.; Tadeo, F.R.; Talón, M.; Rose, J.K. Tissue-specific transcriptome profiling of the citrus fruit epidermis and subepidermis using laser capture microdissection. J. Exp. Bot. 2010, 61, 3321–3330. [Google Scholar] [CrossRef]
- Martin, L.B.; Nicolas, P.; Matas, A.J.; Shinozaki, Y.; Catalá, C.; Rose, J.K. Laser microdissection of tomato fruit cell and tissue types for transcriptome profiling. Nat. Protoc. 2016, 11, 2376–2388. [Google Scholar] [CrossRef] [PubMed]
- Lenzi, L.; Caruso, C.; Bianchedi, P.L.; Pertot, I.; Perazzolli, M. Laser microdissection of grapevine leaves reveals site-specific regulation of transcriptional response to Plasmopara viticola. Plant Cell Physiol. 2016, 57, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Santi, S.; Grisan, S.; Pierasco, A.; De Marco, F.; Musetti, R. Laser microdissection of grapevine leaf phloem infected by stolbur reveals site-specific gene responses associated to sucrose transport and metabolism. Plant Cell Environ. 2013, 36, 343–355. [Google Scholar] [CrossRef]
- Chitarra, W.; Balestrini, R.; Vitali, M.; Pagliarani, C.; Perrone, I.; Schubert, A.; Lovisolo, C. Gene expression in vessel-associated cells upon xylem embolism repair in Vitis vinifera L. petioles. Planta 2014, 239, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Puryear, J.; Cairney, J. Simple and efficient method for isolating RNA from pine trees. Plant Mol. Biol. Rep. 1993, 11, 113–116. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. The R package Rsubread is easier, faster, cheaper and better for alignment and quantification of RNA sequencing reads. Nucleic Acids Res. 2019, 47, e47. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Rau, A.; Gallopin, M.; Celeux, G.; Jaffrezic, F. Data-based filtering for replicated high-throughput transcriptome sequencing experiments. Bioinformatics 2013, 29, 2146–2152. [Google Scholar] [CrossRef]
- Balestrini, R.; Fiorilli, V. Laser Microdissection as a useful tool to study gene expression in plant and fungal partners in AM symbiosis. In Methods Molecular Biology, Arbuscular Mycorrhizal Fungi; Ferrol, N., Lanfranco, L., Eds.; Springer: Heidelberg, Germany, 2020; pp. 171–184. [Google Scholar]
| Gene ID | Putative Function * | Fold Change in Each Treatment | ||
|---|---|---|---|---|
| Fos-Al | K-Pho | Met | ||
| VIT_00s0207g00010 | ω-hydroxypalmitate O-feruloyl transferase | 1.6 | 1.7 | 1.8 |
| VIT_02s0025g02600 | Aldehyde oxidase GLOX | 1.8 | 1.7 | 1.7 |
| VIT_07s0031g02200 | Auxin efflux carrier-like protein (PIN-LIKE) | 0.7 | 0.8 | 0.7 |
| VIT_07s0031g03220 | Patellin protein | 1.2 | 1.1 | 1.2 |
| VIT_12s0028g03480 | O-acyltransferase WSD1 | 1.7 | 1.9 | 1.9 |
| VIT_19s0093g00550 | 9-cis-epoxycarotenoid dioxygenase (VvNCED) | 1.0 | 0.9 | 1.0 |
| VIT_01s0150g00460 | Probable xyloglucan endotransglucosylase/hydrolase (VvXET) | 2.6 | 2.2 | |
| VIT_08s0007g01900 | Proton-dependent oligopeptide transport (POT) family protein | 1.6 | 1.5 | |
| VIT_13s0067g00110 | Cytochrome P450 family | 2.0 | 2.0 | |
| VIT_13s0074g00390 | Cytochrome P450 family | 1.4 | 1.3 | |
| VIT_15s0048g02480 | O-methyltransferase | 2.5 | 2.5 | |
| VIT_15s0048g02490 | O-methyltransferase (COMT type) | 2.5 | 2.5 | |
| VIT_17s0000g08070 | Aldehyde dehydrogenase family | 0.6 | ||
| VIT_18s0001g03910 | Nitrate reductase 2 (NR2) | 0.9 | ||
| VIT_18s0001g04470 | TGACG MOTIF-binding factor 4 (transcription factor TGA4) | 0.6 | ||
| VIT_18s0001g08100 | Zinc finger family protein | 0.9 | ||
| VIT_18s0001g11600 | Protein JINGUBANG | 1.1 | ||
| VIT_18s0001g12660 | TUBBY like protein | 1.3 | ||
| VIT_18s0001g15330 | Bidirectional sugar transporter SWEET (Nodulin MtN3) | 1.3 | ||
| VIT_18s0072g00970 | DegP protease | 0.8 | ||
| VIT_19s0015g01270 | Proteasome activator subunit 4 | 0.8 | ||
| VIT_18s0001g14910 | Mannitol dehydrogenase | 1.7 | ||
| VIT_18s0001g08090 | Auxin-responsive protein | 0.7 | ||
| VIT_19s0085g00920 | Organic cation transport protein OCT2 | 0.9 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balestrini, R.; Ghignone, S.; Quiroga, G.; Fiorilli, V.; Romano, I.; Gambino, G. Long-Term Impact of Chemical and Alternative Fungicides Applied to Grapevine cv Nebbiolo on Berry Transcriptome. Int. J. Mol. Sci. 2020, 21, 6067. https://doi.org/10.3390/ijms21176067
Balestrini R, Ghignone S, Quiroga G, Fiorilli V, Romano I, Gambino G. Long-Term Impact of Chemical and Alternative Fungicides Applied to Grapevine cv Nebbiolo on Berry Transcriptome. International Journal of Molecular Sciences. 2020; 21(17):6067. https://doi.org/10.3390/ijms21176067
Chicago/Turabian StyleBalestrini, Raffaella, Stefano Ghignone, Gabriela Quiroga, Valentina Fiorilli, Irene Romano, and Giorgio Gambino. 2020. "Long-Term Impact of Chemical and Alternative Fungicides Applied to Grapevine cv Nebbiolo on Berry Transcriptome" International Journal of Molecular Sciences 21, no. 17: 6067. https://doi.org/10.3390/ijms21176067
APA StyleBalestrini, R., Ghignone, S., Quiroga, G., Fiorilli, V., Romano, I., & Gambino, G. (2020). Long-Term Impact of Chemical and Alternative Fungicides Applied to Grapevine cv Nebbiolo on Berry Transcriptome. International Journal of Molecular Sciences, 21(17), 6067. https://doi.org/10.3390/ijms21176067







