The Precious Few Grams of Glucose During Exercise
Abstract
:1. Introduction
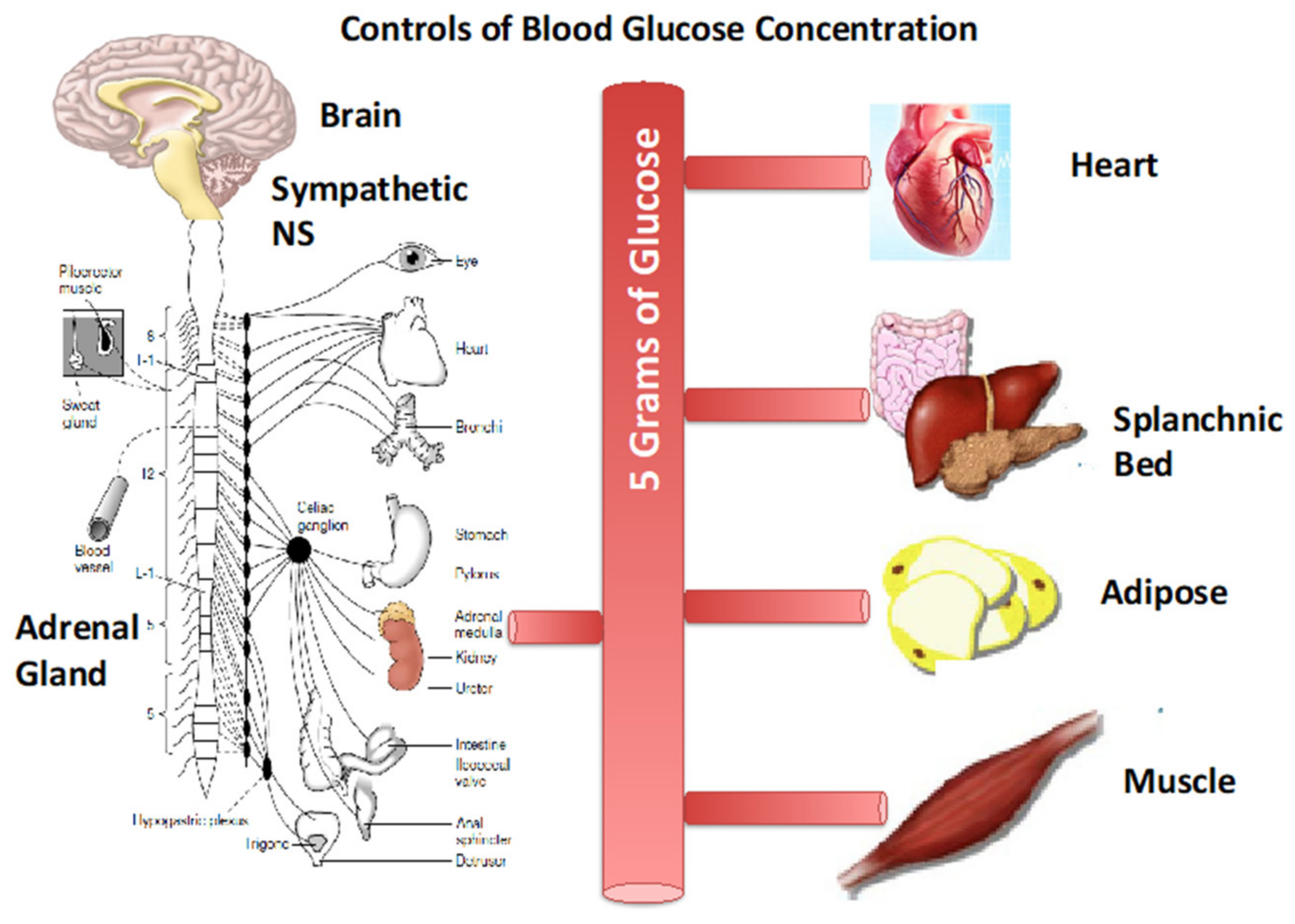
2. Glucose Blood Pool Size and Energy Content
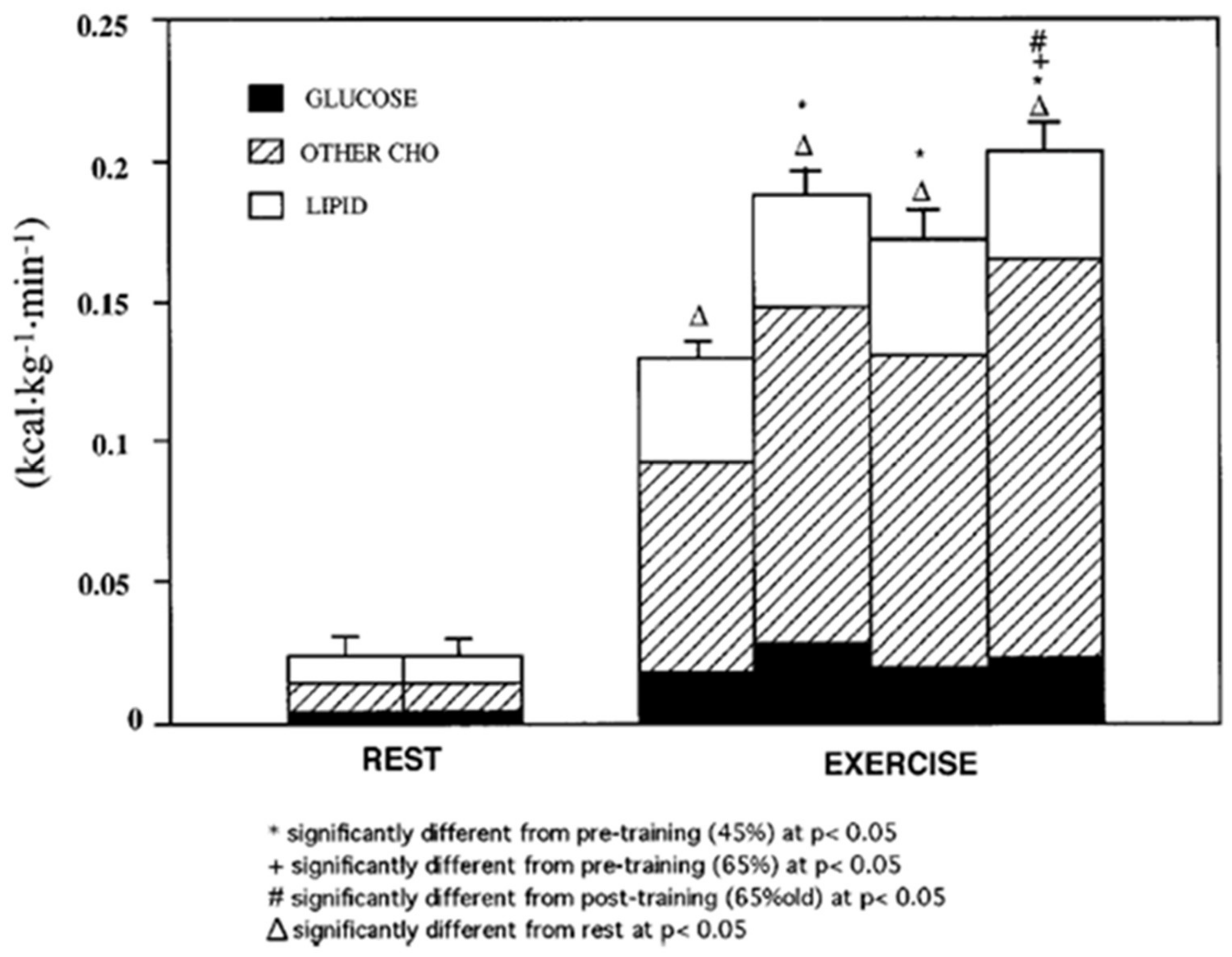
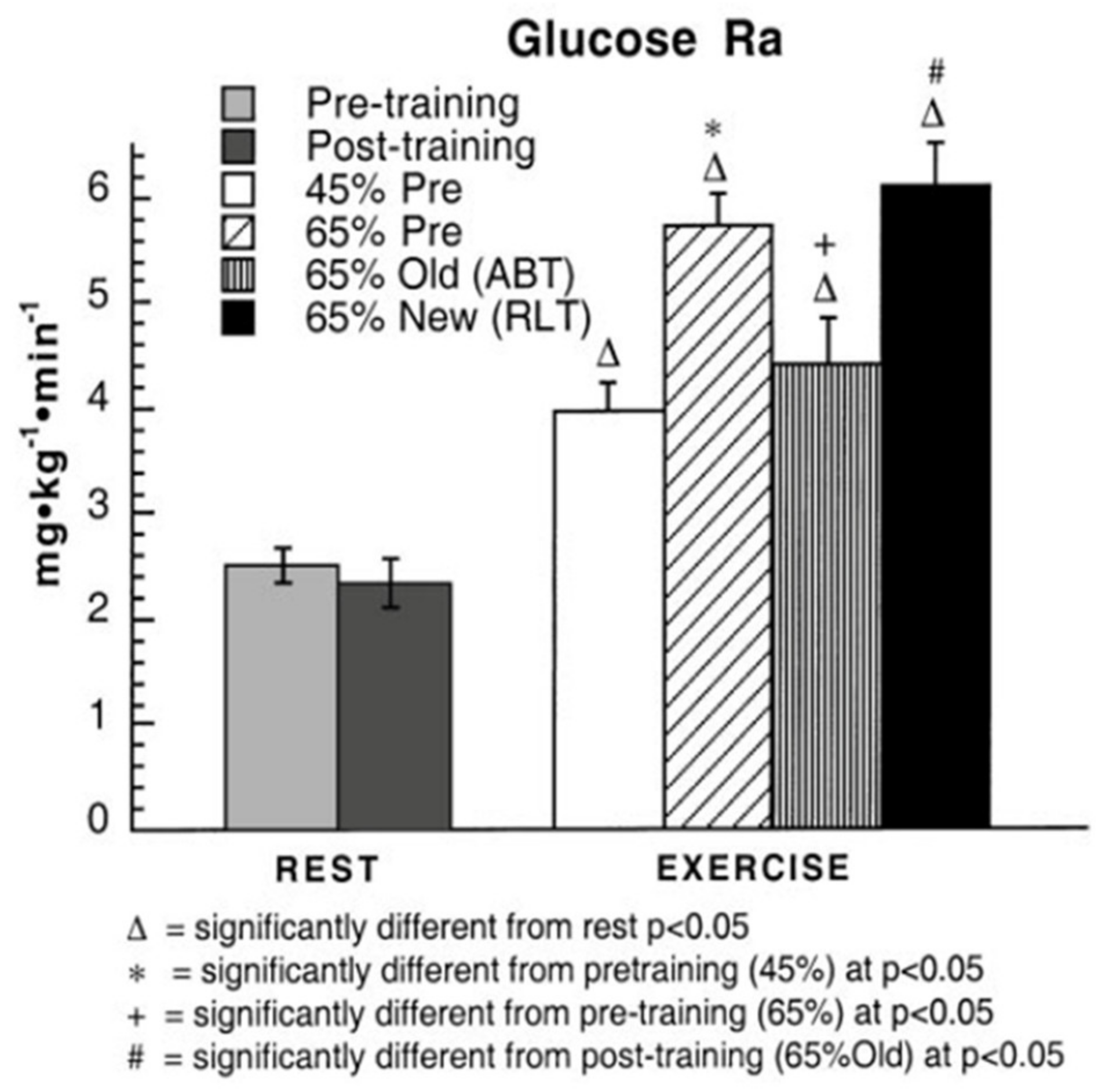
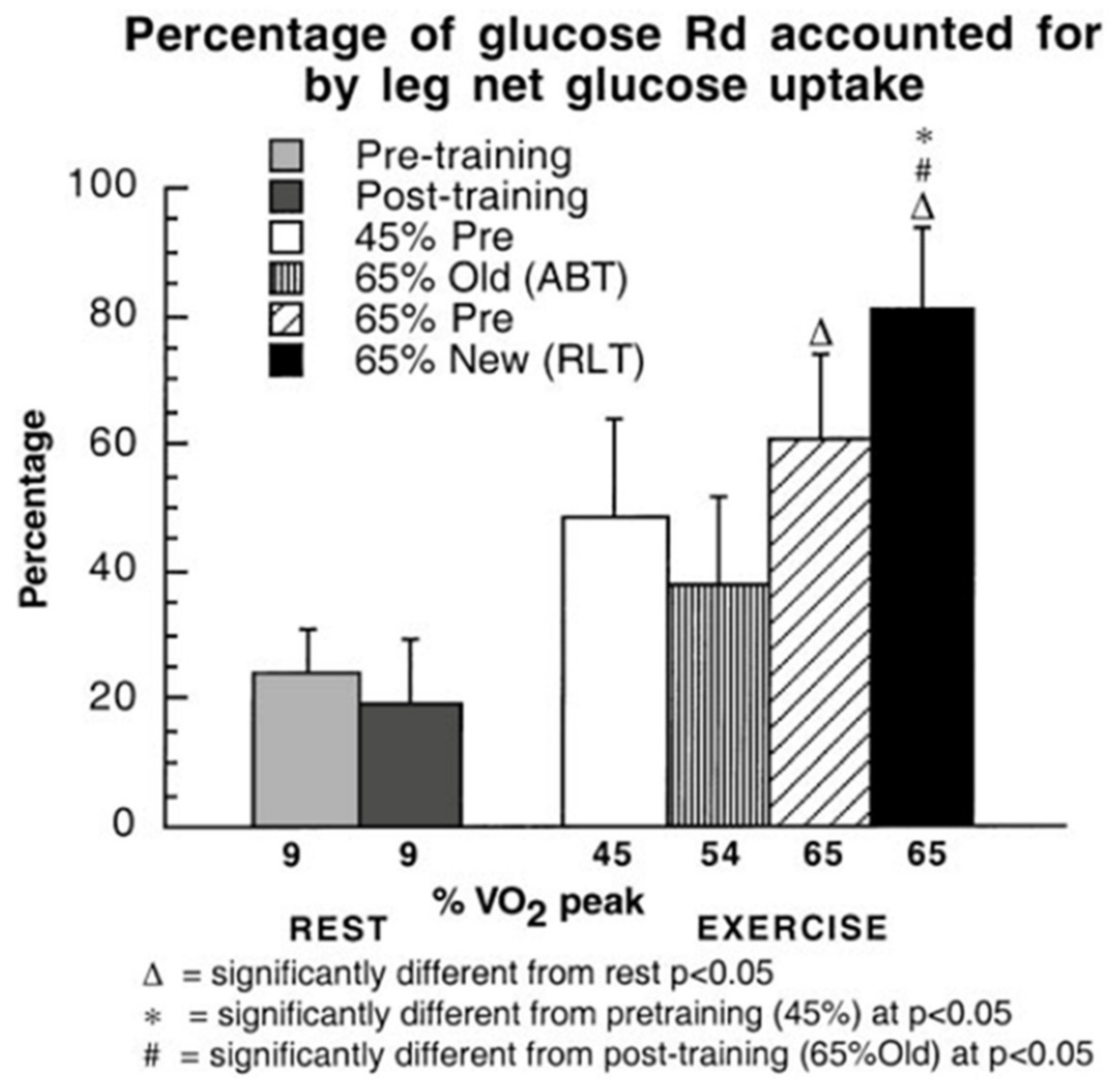
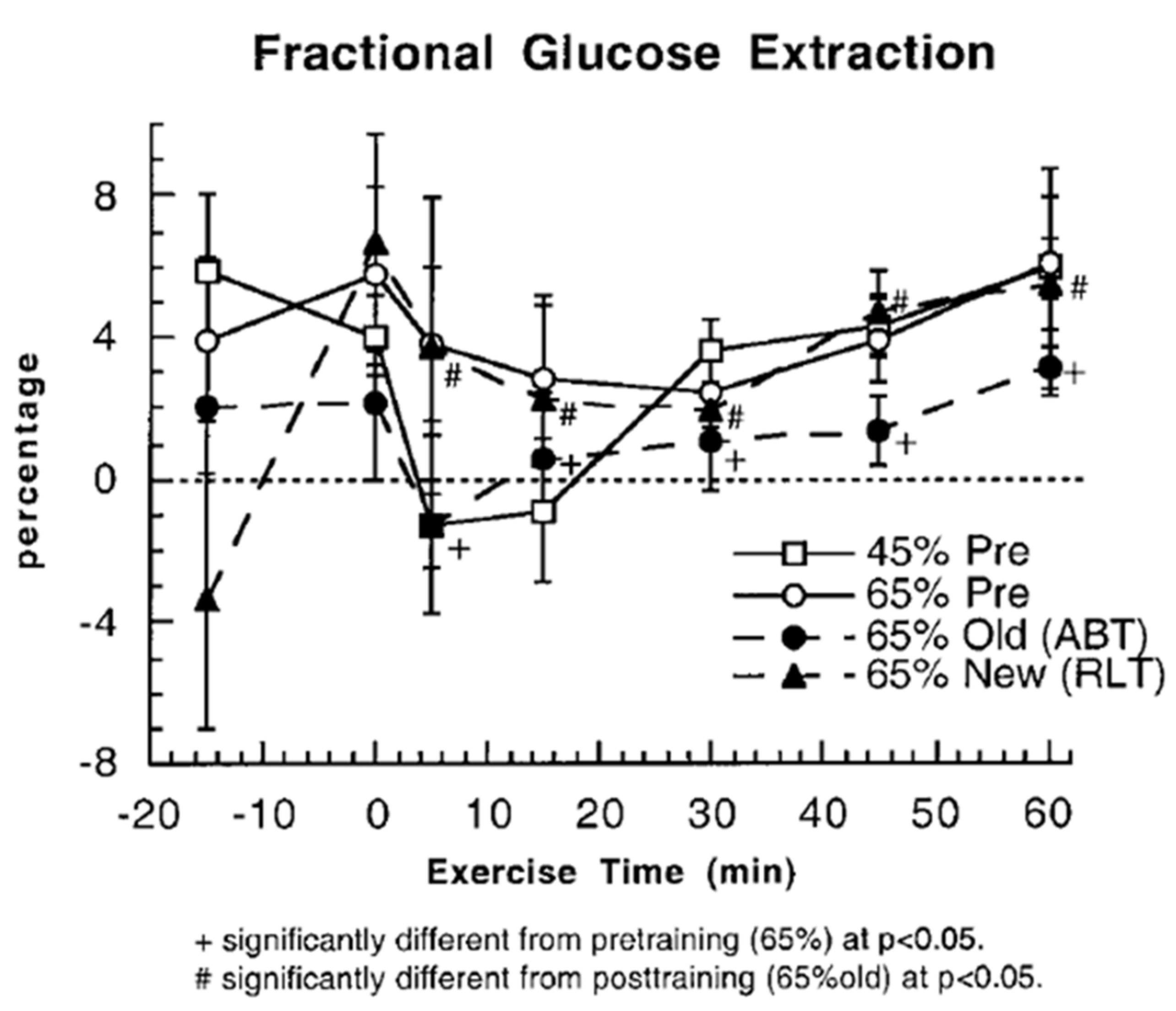
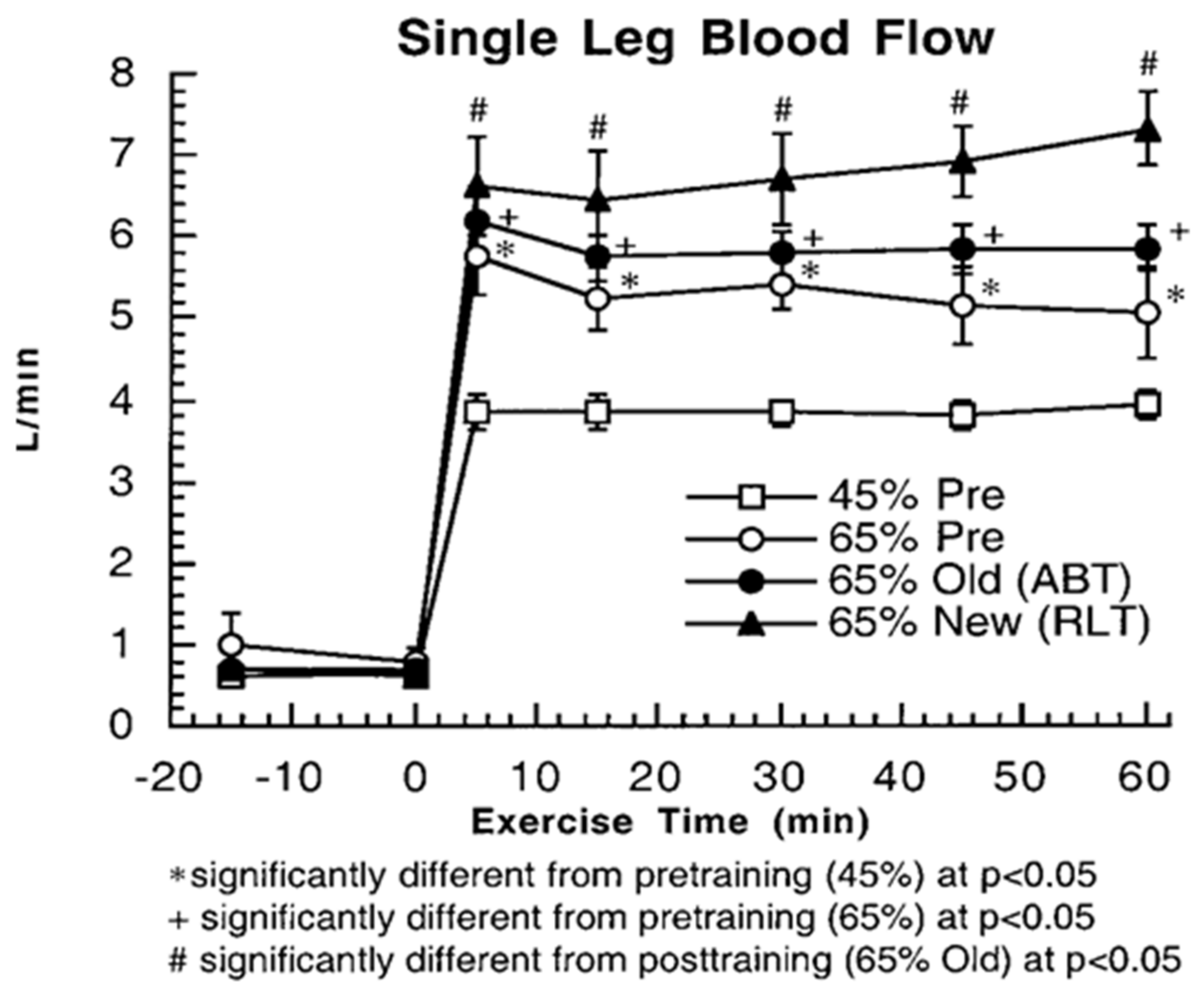
3. Does Muscle Glucose Use Rise During Exercise?
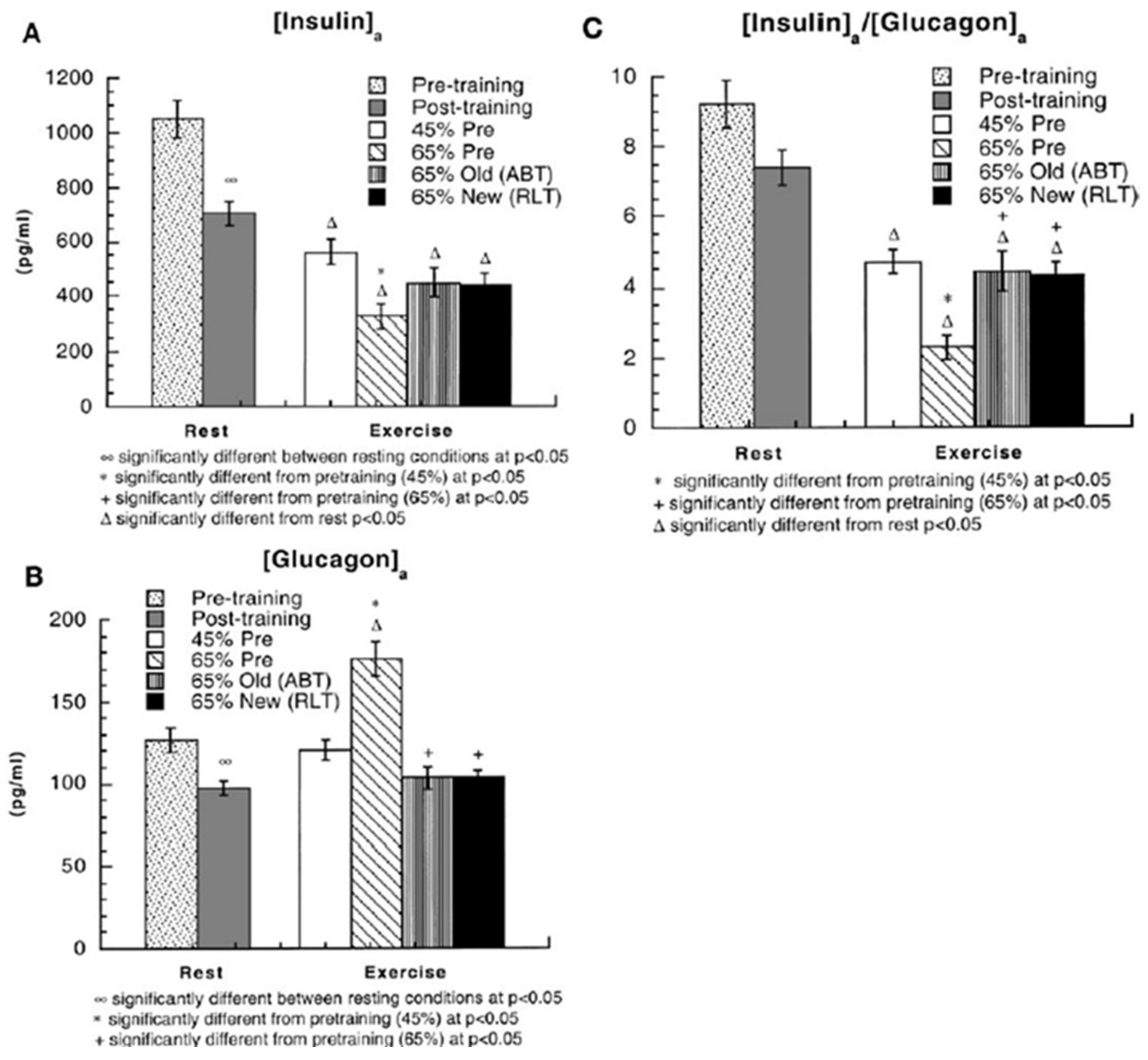
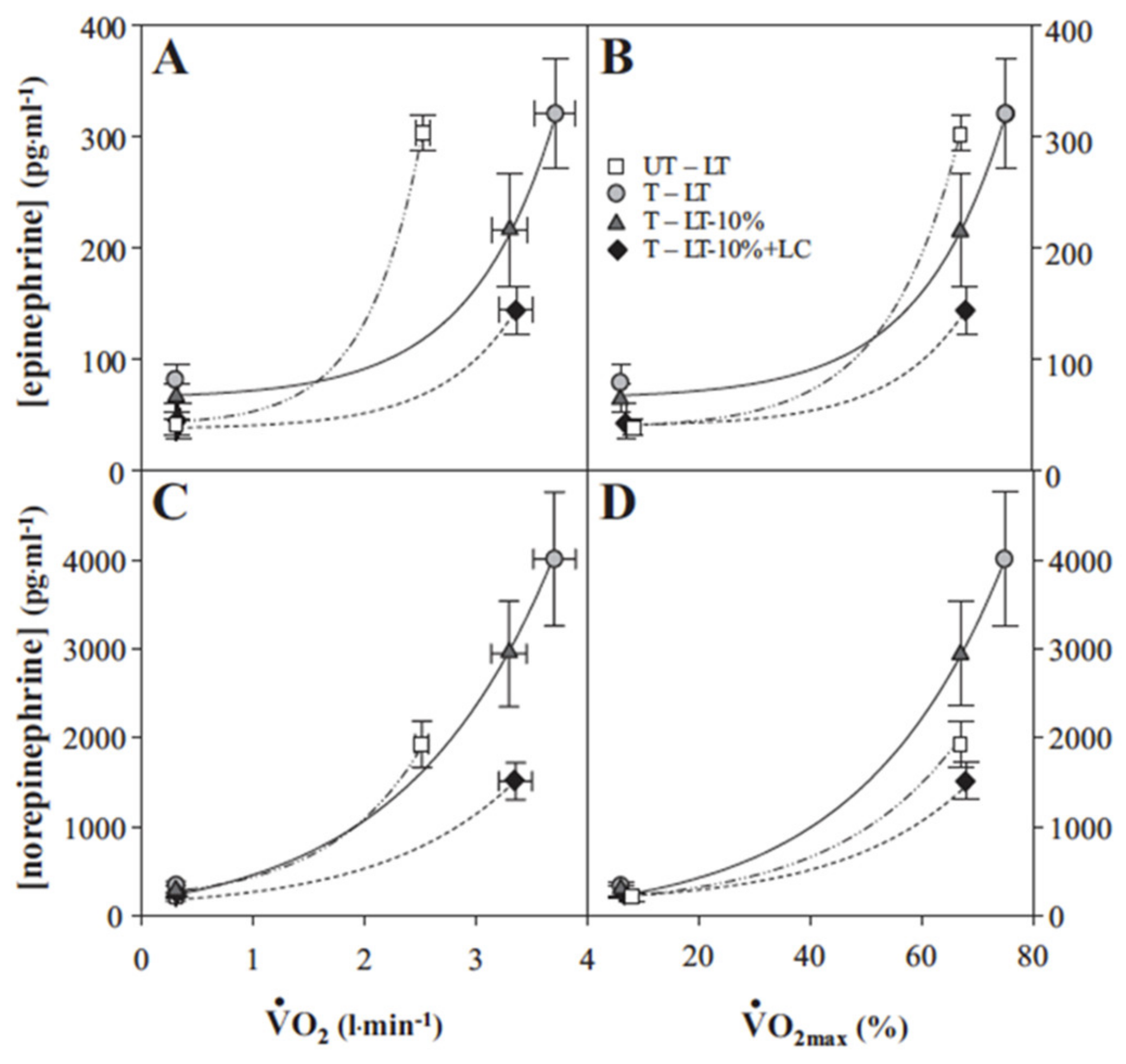
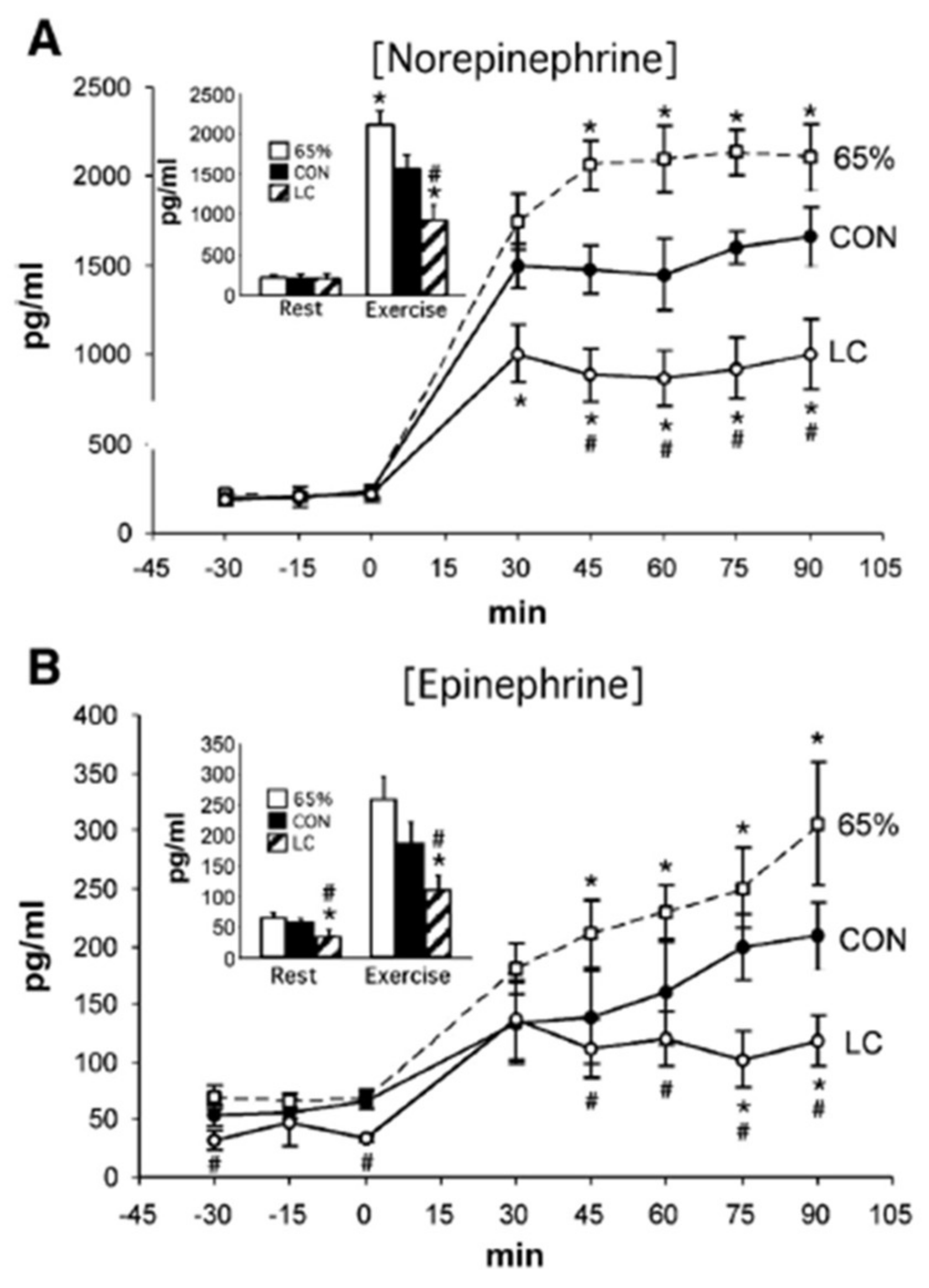
4. Counter Regulatory, Feed Forward, and Feedback Responses
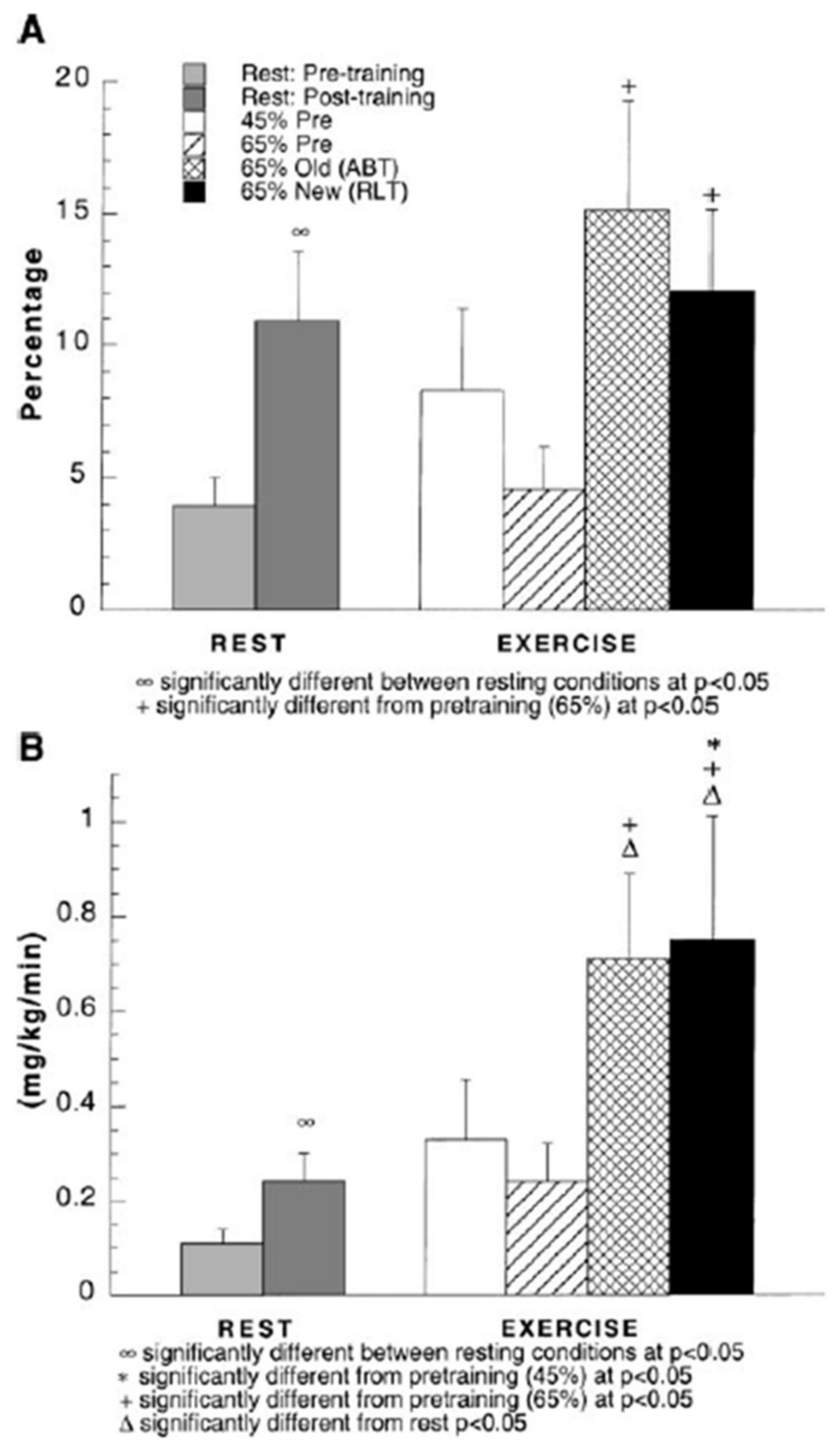
5. Glucose Regulation by Direct and Indirect Feedback—Something Was Missing
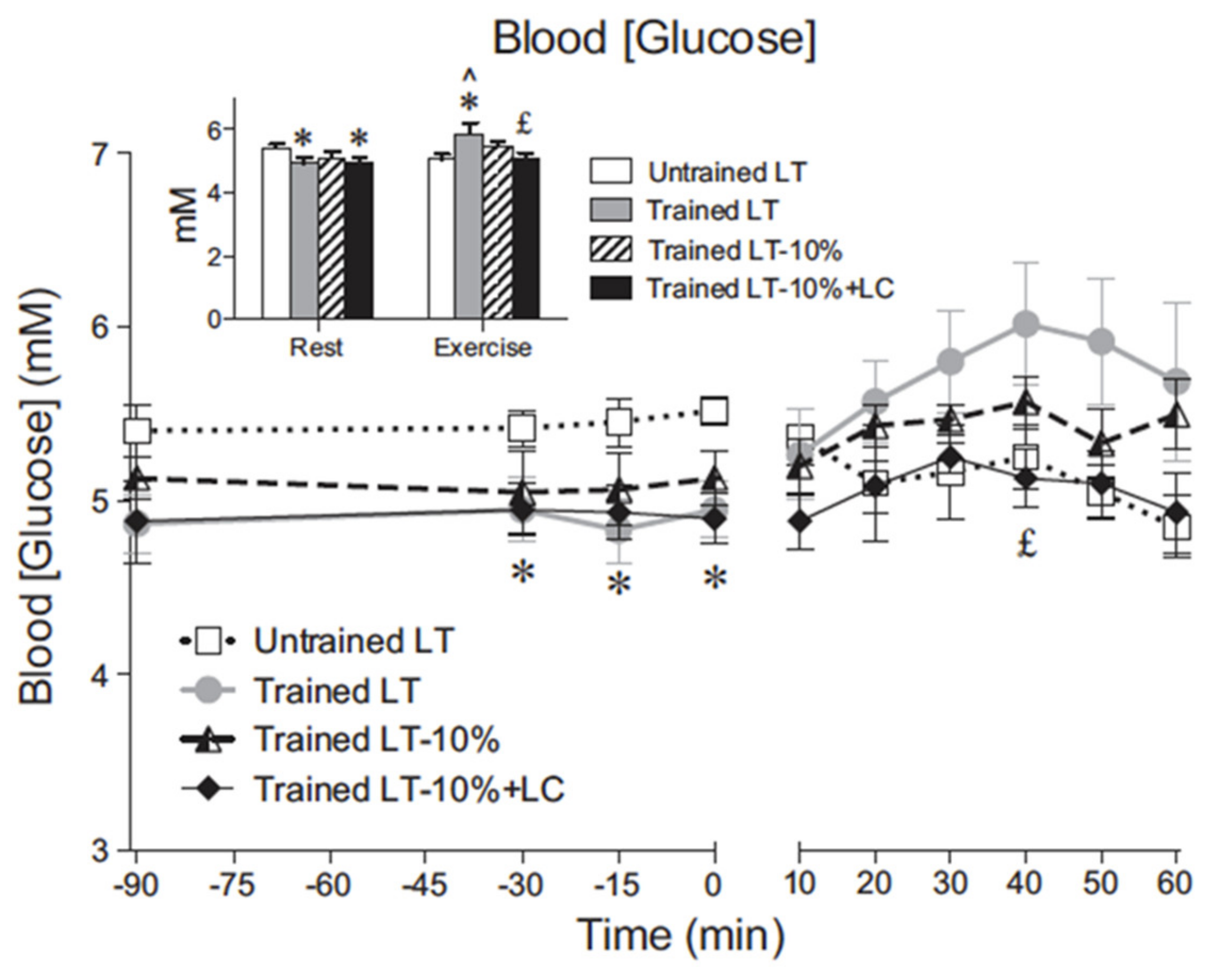
6. Glucose, Glycogen, and Lactate Interactions: The Lactate Shuttle and Glycemia
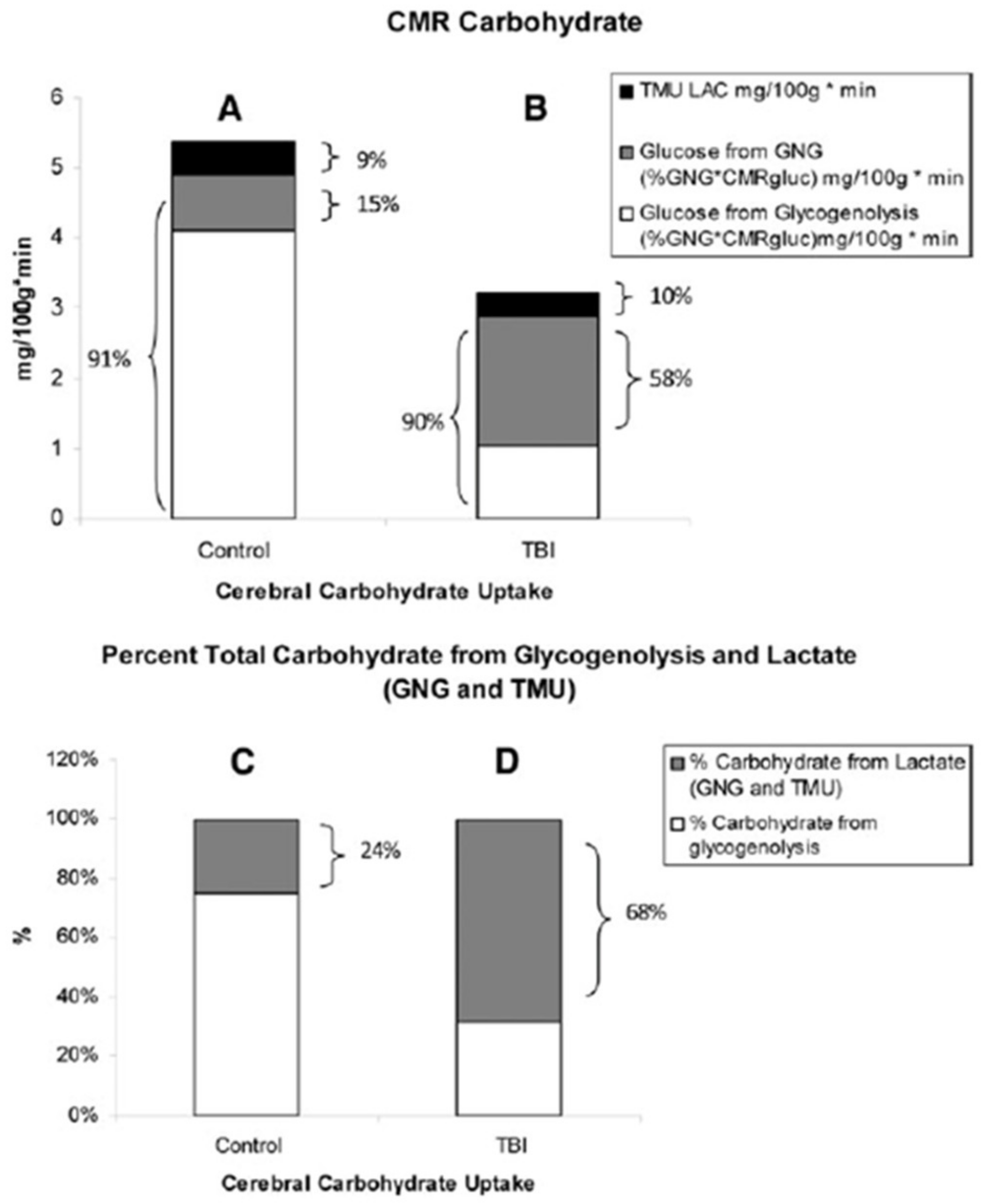
7. A Teaspoon of Goodness: Brain Glucose and Lactate Interactions
8. Glycemia and Nutrition: Splanchnic and Hypothalamic Interactions
9. Glycemia and Hydration in Exercise: Discoveries that Led to Founding of an Industry
10. Who’s the Big Boss? Autonomic Responses or Insulin?
11. Summary
Funding
Acknowledgments
Conflicts of Interest
References
- Brooks, G.A.; Fahey, T.D.; Baldwin, K.M. Exercise Physiology: Human Bioenergetics and Its Applications; Kindle Direct Publishing: Lexington, KY, USA, 1984. [Google Scholar]
- Ahlborg, G.; Felig, P. Lactate and glucose exchange across the forearm, legs, and splanchnic bed during and after prolonged leg exercise. J. Clin. Investig. 1982, 69, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Gerich, J.E.; Meyer, C.; Woerle, H.J.; Stumvoll, M. Renal gluconeogenesis: Its importance in human glucose homeostasis. Diabetes Care 2001, 24, 382–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lecavalier, L.; Bolli, G.; Cryer, P.; Gerich, J. Contributions of gluconeogenesis and glycogenolysis during glucose counterregulation in normal humans. Am. J. Physiol. 1989, 256, E844–E851. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.; Stumvoll, M.; Dostou, J.; Welle, S.; Haymond, M.; Gerich, J. Renal substrate exchange and gluconeogenesis in normal postabsorptive humans. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E428–E434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wasserman, D.H. Four grams of glucose. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E11–E21. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A.; Mercier, J. Balance of carbohydrate and lipid utilization during exercise: The “crossover” concept. J. Appl. Physiol. 1994, 76, 2253–2261. [Google Scholar] [CrossRef] [Green Version]
- Wahren, J.; Ekberg, K. Splanchnic regulation of glucose production. Annu. Rev. Nutr. 2007, 27, 329–345. [Google Scholar] [CrossRef]
- Bergman, B.C.; Brooks, G.A. Respiratory gas-exchange ratios during graded exercise in fed and fasted trained and untrained men. J. Appl. Physiol. 1999, 86, 479–487. [Google Scholar] [CrossRef]
- Bergman, B.C.; Butterfield, G.E.; Wolfel, E.E.; Lopaschuk, G.D.; Casazza, G.A.; Horning, M.A.; Brooks, G.A. Muscle net glucose uptake and glucose kinetics after endurance training in men. Am. J. Physiol. 1999, 277, E81–E92. [Google Scholar] [CrossRef]
- Emhoff, C.A.; Messonnier, L.A.; Horning, M.A.; Fattor, J.A.; Carlson, T.J.; Brooks, G.A. Gluconeogenesis and hepatic glycogenolysis during exercise at the lactate threshold. J. Appl. Physiol. 2013, 114, 297–306. [Google Scholar] [CrossRef] [Green Version]
- Friedlander, A.L.; Casazza, G.A.; Horning, M.A.; Huie, M.J.; Piacentini, M.F.; Trimmer, J.K.; Brooks, G.A. Training-induced alterations of carbohydrate metabolism in women: Women respond differently from men. J. Appl. Physiol. 1998, 85, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Zarins, Z.A.; Johnson, M.L.; Faghihnia, N.; Horning, M.A.; Wallis, G.A.; Fattor, J.A.; Brooks, G.A. Training improves the response in glucose flux to exercise in postmenopausal women. J. Appl. Physiol. 2009, 107, 90–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedlander, A.L.; Casazza, G.A.; Horning, M.A.; Huie, M.J.; Brooks, G.A. Training-induced alterations of glucose flux in men. J. Appl. Physiol. 1997, 82, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- San-Millan, I.; Brooks, G.A. Assessment of Metabolic Flexibility by Means of Measuring Blood Lactate, Fat, and Carbohydrate Oxidation Responses to Exercise in Professional Endurance Athletes and Less-Fit Individuals. Sports Med. 2018, 48, 467–479. [Google Scholar] [CrossRef] [Green Version]
- Wahren, J.; Felig, P.; Ahlborg, G.; Jorfeldt, L. Glucose metabolism during leg exercise in man. J. Clin. Investig. 1971, 50, 2715–2725. [Google Scholar] [CrossRef] [Green Version]
- Ploug, T.; Galbo, H.; Richter, E.A. Increased muscle glucose uptake during contractions: No need for insulin. Am. J. Physiol. 1984, 247, E726–E731. [Google Scholar] [CrossRef]
- Richter, E.A.; Ploug, T.; Galbo, H. Increased muscle glucose uptake after exercise: No need for insulin during exercise. Diabetes 1985, 34, 1041–1048. [Google Scholar] [CrossRef]
- Wasserman, D.H.; Lickley, H.L.; Vranic, M. Interactions between glucagon and other counterregulatory hormones during normoglycemic and hypoglycemic exercise in dogs. J. Clin. Investig. 1984, 74, 1404–1413. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, I.B.; Marker, J.C.; Smith, L.J.; Spina, R.J.; Parvin, C.A.; Holloszy, J.O.; Cryer, P.E. Insulin and glucagon in prevention of hypoglycemia during exercise in humans. Am. J. Physiol. 1991, 260, E695–E704. [Google Scholar] [CrossRef]
- Messonnier, A.L.; Emhoff, C.W.; Fattor, J.A.; Horning, M.A.; Carlson, T.J.; Brooks, G.A. Lactate kinetics at the lactate threshold in trained and untrained men. J. Appl. Physiol. 2013, 114, 1593–1602. [Google Scholar] [CrossRef]
- Kjaer, M.; Farrell, P.A.; Christensen, N.J.; Galbo, H. Increased epinephrine response and inaccurate glucoregulation in exercising athletes. J. Appl. Physiol. 1986, 61, 1693–1700. [Google Scholar] [CrossRef] [PubMed]
- Kjaer, M.; Kiens, B.; Hargreaves, M.; Richter, E.A. Influence of active muscle mass on glucose homeostasis during exercise in humans. J. Appl. Physiol. 1991, 71, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.F.; Fattor, J.A.; Jacobs, K.A.; Horning, M.A.; Navazio, F.; Lindinger, M.I.; Brooks, G.A. Lactate and glucose interactions during rest and exercise in men: Effect of exogenous lactate infusion. J. Physiol. 2002, 544, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.F.; Fattor, J.A.; Jacobs, K.A.; Horning, M.A.; Suh, S.H.; Navazio, F.; Brooks, G.A. Metabolic and cardiorespiratory responses to “the lactate clamp”. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E889–E898. [Google Scholar] [CrossRef]
- Fattor, J.A.; Miller, B.F.; Jacobs, K.A.; Brooks, G.A. Catecholamine response is attenuated during moderate-intensity exercise in response to the “lactate clamp”. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E143–E147. [Google Scholar] [CrossRef]
- Greenhaff, P.L.; Ren, J.M.; Soderlund, K.; Hultman, E. Energy metabolism in single human muscle fibers during contraction without and with epinephrine infusion. Am. J. Physiol. 1991, 260, E713–E718. [Google Scholar] [CrossRef]
- Spriet, L.L.; Ren, J.M.; Hultman, E.R.I.C. Epinephrine infusion enhances muscle glycogenolysis during prolonged electrical stimulation. J. Appl. Physiol. 1988, 64, 1439–1444. [Google Scholar] [CrossRef]
- Febbraio, M.A.; Ott, P.; Nielsen, H.B.; Steensberg, A.; Keller, C.; Krustrup, P.; Secher, N.H.; Pedersen, B.K. Hepatosplanchnic clearance of interleukin-6 in humans during exercise. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E397–E402. [Google Scholar] [CrossRef] [Green Version]
- Hojman, P.; Brolin, C.; Norgaard-Christensen, N.; Dethlefsen, C.; Lauenborg, B.; Olsen, C.K.; Abom, M.M.; Krag, T.; Gehl, J.; Pedersen, B.K. IL-6 release from muscles during exercise is stimulated by lactate-dependent protease activity. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E940–E947. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef] [Green Version]
- Steensberg, A.; van Hall, G.; Osada, T.; Sacchetti, M.; Saltin, B.; Klarlund Pedersen, B. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J. Physiol. 2000, 529, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, M.P. Metaboreflex control of the heart. J. Physiol. 2010, 588, 1037–1038. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A. The Science and Translation of Lactate Shuttle Theory. Cell Metab. 2018, 27, 757–785. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A.; Martin, N.A. Cerebral metabolism following traumatic brain injury: New discoveries with implications for treatment. Front. Neurosci. 2014, 8, 408. [Google Scholar] [CrossRef] [PubMed]
- Bergman, B.C.; Horning, M.A.; Casazza, G.A.; Wolfel, E.E.; Butterfield, G.E.; Brooks, G.A. Endurance training increases gluconeogenesis during rest and exercise in men. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E244–E251. [Google Scholar] [CrossRef] [Green Version]
- Bender, K.; Newsholme, P.; Brennan, L.; Maechler, P. The importance of redox shuttles to pancreatic beta-cell energy metabolism and function. Biochem. Soc. Trans. 2006, 34, 811–814. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, H.; Alves, C.R.R.; Stanford, K.I.; Middelbeek, R.J.W.; Pasquale, N.; Ryan, R.E.; Xue, R.; Sakaguchi, M.; Lynes, M.D.; So, K.; et al. TGF-beta2 is an exercise-induced adipokine that regulates glucose and fatty acid metabolism. Nat. Metab. 2019, 1, 291–303. [Google Scholar] [CrossRef]
- Hultman, E.A. Physiological role of muscle glycogen in man. Physiol. Muscular Exerc. 1967, I99–I112. [Google Scholar]
- Nilsson, L.H.; Hultman, E. Liver and muscle glycogen in man after glucose and fructose infusion. Scand. J. Clin. Lab. Investig. 1974, 33, 5–10. [Google Scholar] [CrossRef]
- Cahill, G.J., Jr.; Owen, O.E.; Morgan, A.P. The consumption of fuels during prolonged starvation. Adv. Enzym. Regul. 1968, 6, 143–150. [Google Scholar] [CrossRef]
- Emhoff, C.A.; Messonnier, L.A.; Horning, M.A.; Fattor, J.A.; Carlson, T.J.; Brooks, G.A. Direct and indirect lactate oxidation in trained and untrained men. J. Appl. Physiol. 2013, 115, 829–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanley, W.C.; Wisneski, J.A.; Gertz, E.W.; Neese, R.A.; Brooks, G.A. Glucose and lactate interrelations during moderate-intensity exercise in humans. Metab. Clin. Exp. 1988, 37, 850–858. [Google Scholar] [CrossRef]
- Bergman, B.C.; Tsvetkova, T.; Lowes, B.; Wolfel, E.E. Myocardial glucose and lactate metabolism during rest and atrial pacing in humans. J. Physiol. 2009, 587, 2087–2099. [Google Scholar] [CrossRef] [PubMed]
- Bergman, B.C.; Wolfel, E.E.; Butterfield, G.E.; Lopaschuk, G.D.; Casazza, G.A.; Horning, M.A.; Brooks, G.A. Active muscle and whole body lactate kinetics after endurance training in men. J. Appl. Physiol. 1999, 87, 1684–1696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gertz, E.W.; Wisneski, J.A.; Stanley, W.C.; Neese, R.A. Myocardial substrate utilization during exercise in humans. Dual Carbon-Labeled Carbohydrate Isotope Experiments. J. Clin. Investig. 1988, 82, 2017–2025. [Google Scholar] [CrossRef]
- Glenn, T.C.; Martin, N.A.; McArthur, D.L.; Hovda, D.A.; Vespa, P.; Johnson, M.L.; Horning, M.A.; Brooks, G.A. Endogenous Nutritive Support after Traumatic Brain Injury: Peripheral Lactate Production for Glucose Supply via Gluconeogenesis. J. Neurotrauma 2015, 32, 811–819. [Google Scholar] [CrossRef] [Green Version]
- Gertz, E.W.; Wisneski, J.A.; Neese, R.; Bristow, J.D.; Searle, G.L.; Hanlon, J.T. Myocardial lactate metabolism: Evidence of lactate release during net chemical extraction in man. Circulation 1981, 63, 1273–1279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokoloff, L. Metabolism of ketone bodies by the brain. Annu. Rev. Med. 1973, 24, 271–280. [Google Scholar] [CrossRef]
- Sokoloff, L. Relation between physiological function and energy metabolism in the central nervous system. J. Neurochem. 1977, 29, 13–26. [Google Scholar] [CrossRef]
- Glenn, T.C.; Martin, N.A.; Horning, M.A.; McArthur, D.L.; Hovda, D.; Vespa, P.M.; Brooks, G.A. Lactate: Brain Fuel in Human Traumatic Brain Injury. A Comparison to Normal Healthy Control Subjects. J. Neurotrauma 2015, 32, 820–832. [Google Scholar] [CrossRef]
- Van Hall, G.; Stromstad, M.; Rasmussen, P.; Jans, O.; Zaar, M.; Gam, C.; Quistorff, B.; Secher, N.H.; Nielsen, H.B. Blood lactate is an important energy source for the human brain. J. Cereb. Blood Flow Metab. 2009, 29, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Schurr, A. Lactate: A major and crucial player in normal function of both muscle and brain. J. Physiol. 2008, 586, 2665–2666. [Google Scholar] [CrossRef] [PubMed]
- Schurr, A. Lactate: The ultimate cerebral oxidative energy substrate? J. Cereb. Blood Flow Metab. 2006, 26, 142–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooks, G.A.; Butte, N.F.; Rand, W.M.; Flatt, J.P.; Caballero, B. Chronicle of the Institute of Medicine physical activity recommendation: How a physical activity recommendation came to be among dietary recommendations. Am. J. Clin. Nutr. 2004, 79, 921S–930S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallagher, C.N.; Carpenter, K.L.; Grice, P.; Howe, D.J.; Mason, A.; Timofeev, I.; Menon, D.K.; Kirkpatrick, P.J.; Pickard, J.D.; Sutherland, G.R.; et al. The human brain utilizes lactate via the tricarboxylic acid cycle: A 13C-labelled microdialysis and high-resolution nuclear magnetic resonance study. Brain 2009, 132, 2839–2849. [Google Scholar] [CrossRef] [Green Version]
- Gale, S.M.; Castracane, V.D.; Mantzoros, C.S. Energy homeostasis, obesity and eating disorders: Recent advances in endocrinology. J. Nutr. 2004, 134, 295–298. [Google Scholar] [CrossRef] [Green Version]
- Murphy, K.G.; Bloom, S.R. Gut hormones and the regulation of energy homeostasis. Nature 2006, 444, 854–859. [Google Scholar] [CrossRef]
- Mayer, J.; Roy, P.; Mitra, K.P. Relation between caloric intake, body weight, and physical work: Studies in an industrial male population in West Bengal. Am. J. Clin. Nutr. 1956, 4, 169–175. [Google Scholar] [CrossRef]
- Miller, B.F.; Lindinger, M.I.; Fattor, J.A.; Jacobs, K.A.; Leblanc, P.J.; Duong, M.; Heigenhauser, G.J.; Brooks, G.A. Hematological and acid-base changes in men during prolonged exercise with and without sodium-lactate infusion. J. Appl. Physiol. 2005, 98, 856–865. [Google Scholar] [CrossRef] [Green Version]
- Schmid, S.M.; Jauch-Chara, K.; Hallschmid, M.; Oltmanns, K.M.; Peters, A.; Born, J.; Schultes, B. Lactate overrides central nervous but not beta-cell glucose sensing in humans. Metabolism 2008, 57, 1733–1739. [Google Scholar] [CrossRef]
- Schultes, B.; Schmid, S.M.; Wilms, B.; Jauch-Chara, K.; Oltmanns, K.M.; Hallschmid, M. Lactate infusion during euglycemia but not hypoglycemia reduces subsequent food intake in healthy men. Appetite 2012, 58, 818–821. [Google Scholar] [CrossRef] [PubMed]
- Islam, H.; Townsend, L.K.; McKie, G.L.; Medeiros, P.J.; Gurd, B.J.; Hazell, T.J. Potential involvement of lactate and interleukin-6 in the appetite-regulatory hormonal response to an acute exercise bout. J. Appl. Physiol. 2017, 123, 614–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanderheyden, L.W.; McKie, G.L.; Howe, G.J.; Hazell, T.J. Greater lactate accumulation following an acute bout of high-intensity exercise in males suppresses acylated ghrelin and appetite postexercise. J. Appl. Physiol. 2020, 128, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Torres-Fuentes, C.; Golubeva, A.V.; Zhdanov, A.V.; Wallace, S.; Arboleya, S.; Papkovsky, D.B.; El Aidy, S.; Ross, P.; Roy, B.L.; Stanton, C.; et al. Short-Chain Fatty Acids and Microbiota Metabolites Attenuate Ghrelin Receptor Signaling. FASEB J. 2019, 33, 13546–13559. [Google Scholar] [CrossRef] [Green Version]
- Robinson, M.M.; Dasari, S.; Konopka, A.R.; Johnson, M.L.; Manjunatha, S.; Esponda, R.R.; Carter, R.E.; Lanza, I.R.; Nair, K.S. Enhanced Protein Translation Underlies Improved Metabolic and Physical Adaptations to Different Exercise Training Modes in Young and Old Humans. Cell Metab. 2017, 25, 581–592. [Google Scholar] [CrossRef] [Green Version]
- Jeukendrup, A.E.; Moseley, L.; Mainwaring, G.I.; Samuels, S.; Perry, S.; Mann, C.H. Exogenous carbohydrate oxidation during ultraendurance exercise. J. Appl. Physiol. 2006, 100, 1134–1141. [Google Scholar] [CrossRef]
- Azevedo, J.L.; Tietz, E.; Two-Feathers, T.; Paull, J.; Chapman, K. Lactate, fructose and glucose oxidation profiles in sports drinks and the effect on exercise performance. PLoS ONE 2007, 2, e927. [Google Scholar] [CrossRef]
- Lecoultre, V.; Benoit, R.; Carrel, G.; Schutz, Y.; Millet, G.P.; Tappy, L.; Schneiter, P. Fructose and glucose co-ingestion during prolonged exercise increases lactate and glucose fluxes and oxidation compared with an equimolar intake of glucose. Am. J. Clin. Nutr. 2010, 92, 1071–1079. [Google Scholar] [CrossRef] [Green Version]
- Lins, P.E.; Wajngot, A.; Adamson, U.; Vranic, M.; Efendic, S. Minimal increases in glucagon levels enhance glucose production in man with partial hypoinsulinemia. Diabetes 1983, 32, 633–636. [Google Scholar] [CrossRef]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brooks, G.A. The Precious Few Grams of Glucose During Exercise. Int. J. Mol. Sci. 2020, 21, 5733. https://doi.org/10.3390/ijms21165733
Brooks GA. The Precious Few Grams of Glucose During Exercise. International Journal of Molecular Sciences. 2020; 21(16):5733. https://doi.org/10.3390/ijms21165733
Chicago/Turabian StyleBrooks, George A. 2020. "The Precious Few Grams of Glucose During Exercise" International Journal of Molecular Sciences 21, no. 16: 5733. https://doi.org/10.3390/ijms21165733
APA StyleBrooks, G. A. (2020). The Precious Few Grams of Glucose During Exercise. International Journal of Molecular Sciences, 21(16), 5733. https://doi.org/10.3390/ijms21165733



