Conversion of Isocyanide to Amine in The Presence of Water and Hg(II) Ions: Kinetics and Mechanism as Detected by Fluorescence Spectroscopy and Mass Spectrometry
Abstract
1. Introduction
2. Experimental Materials
2.1. General Procedure for the Reaction of 1,5-ICAN and/or 1,5-DIN with Water and HgCl2
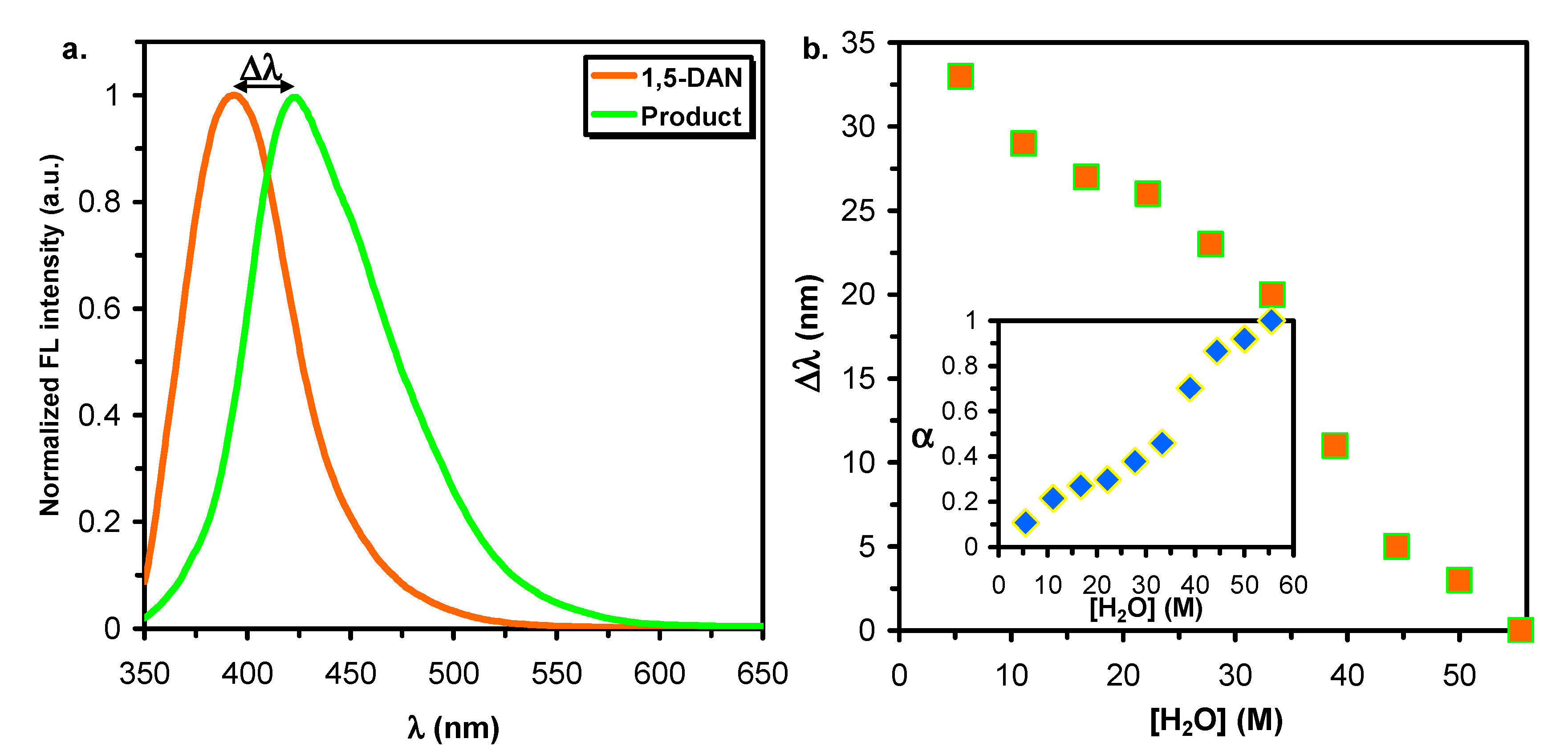
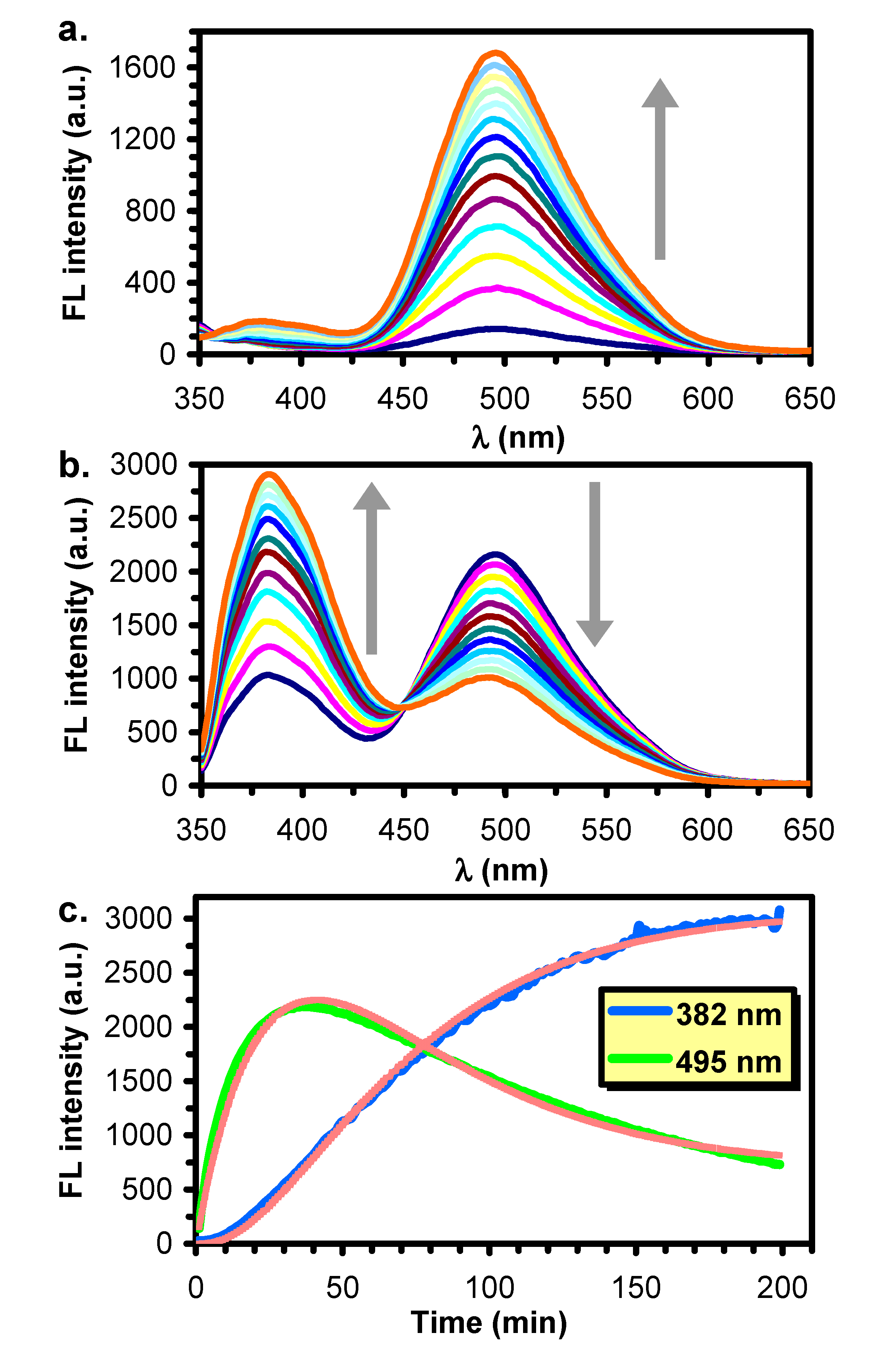
2.2. Monitoring the Reaction Using Steady State Fluorescence Spectroscopy
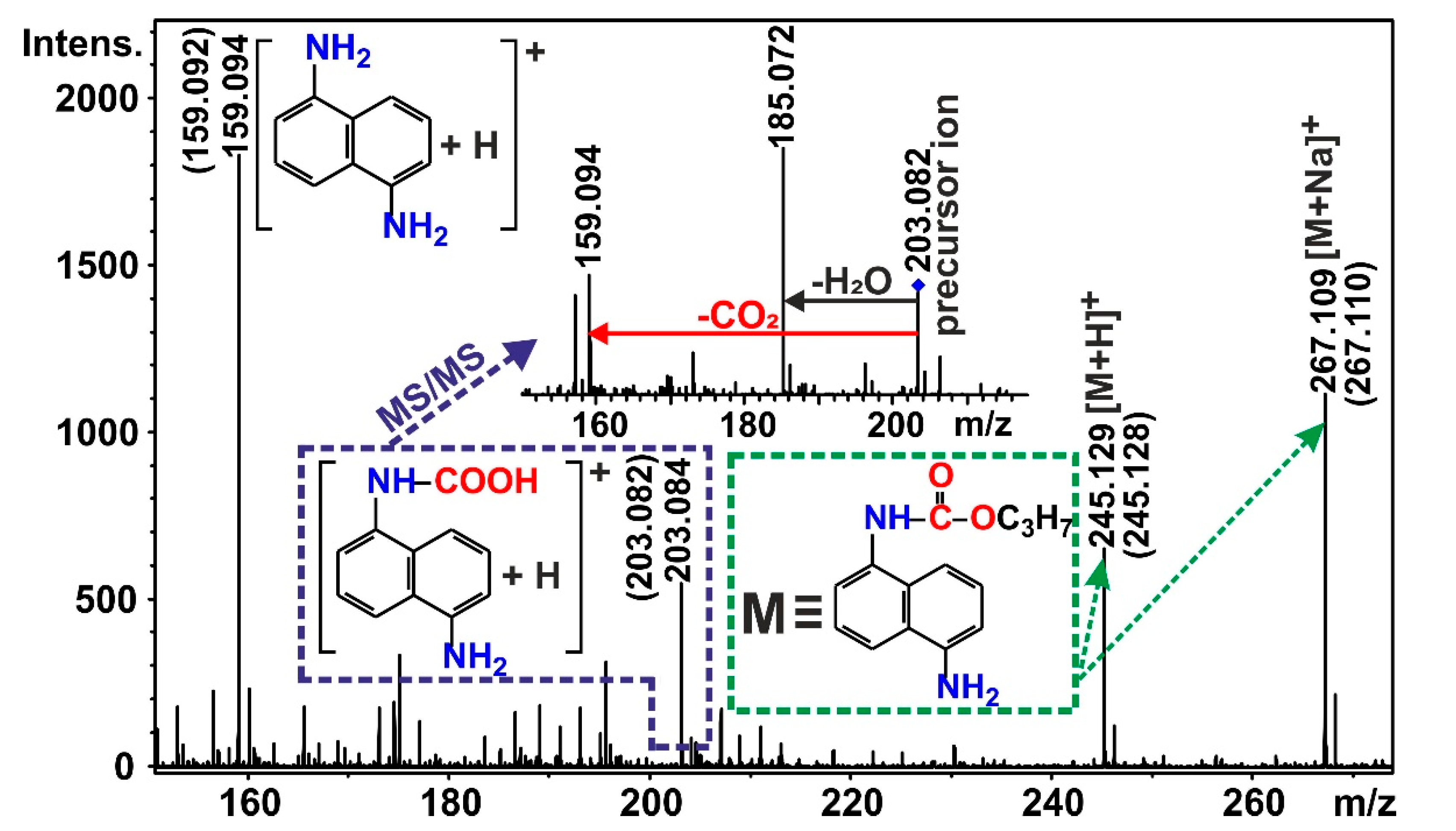
2.3. Electrospray Ionization Mass Spectrometry (ESI-MS)
2.4. Evaluation of the Kinetic Data
3. Results and Discussion
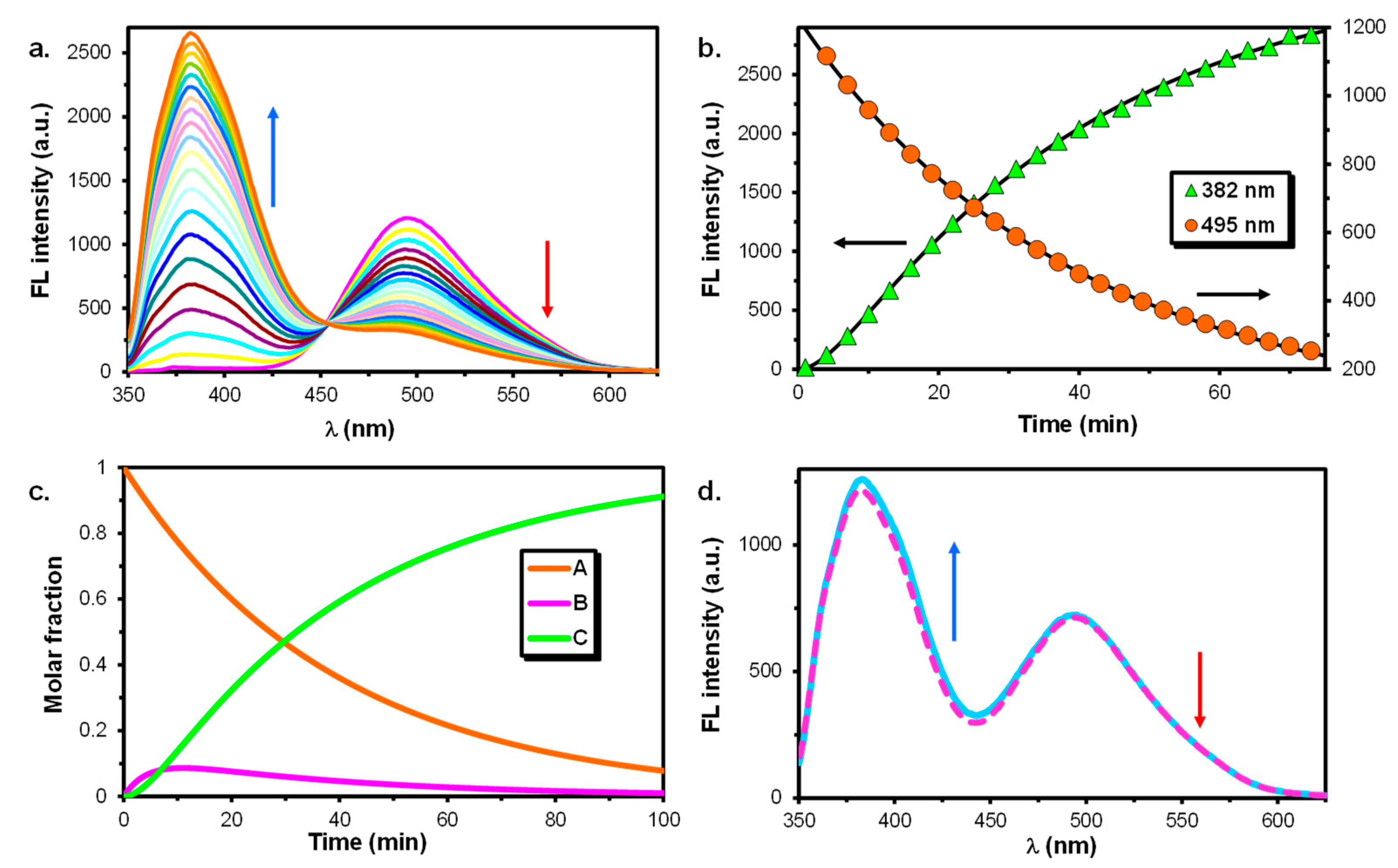
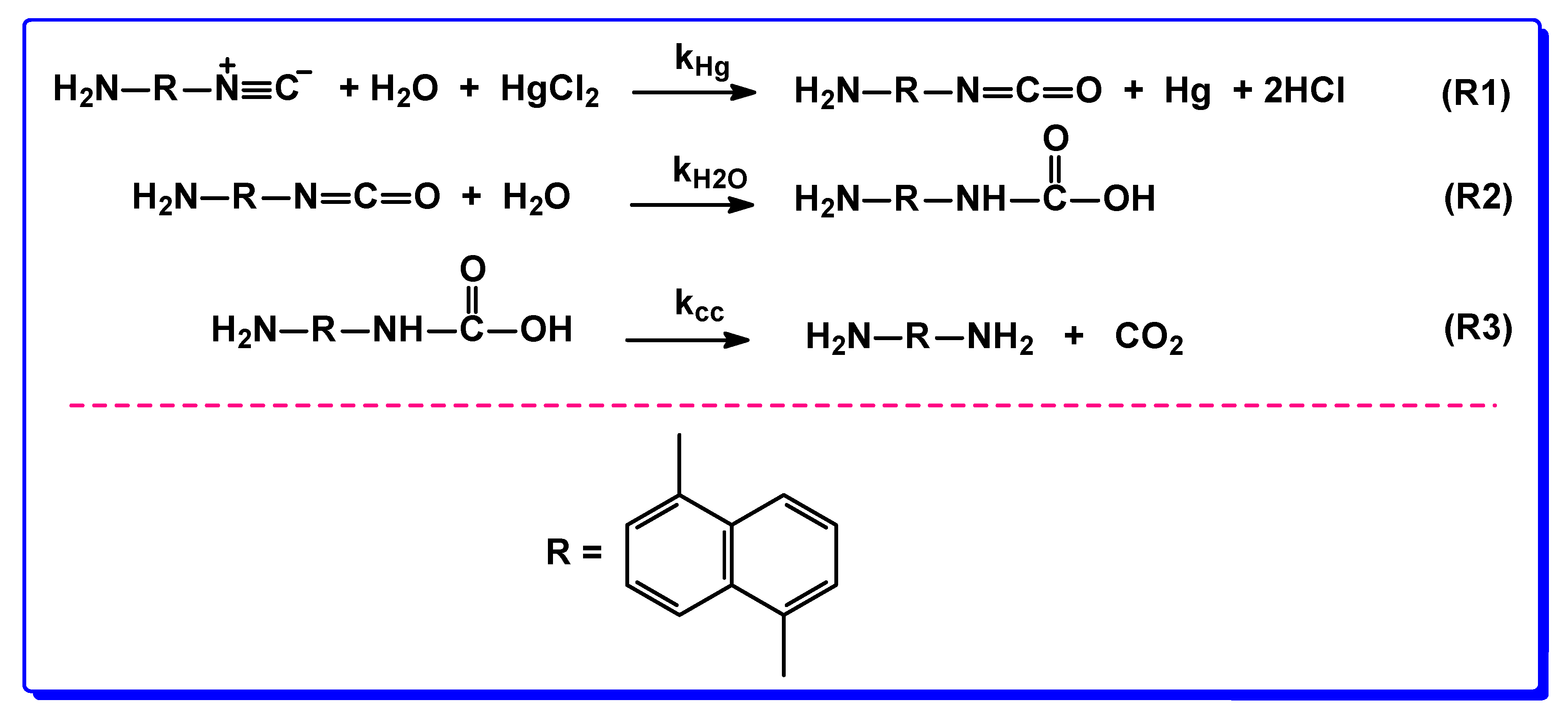
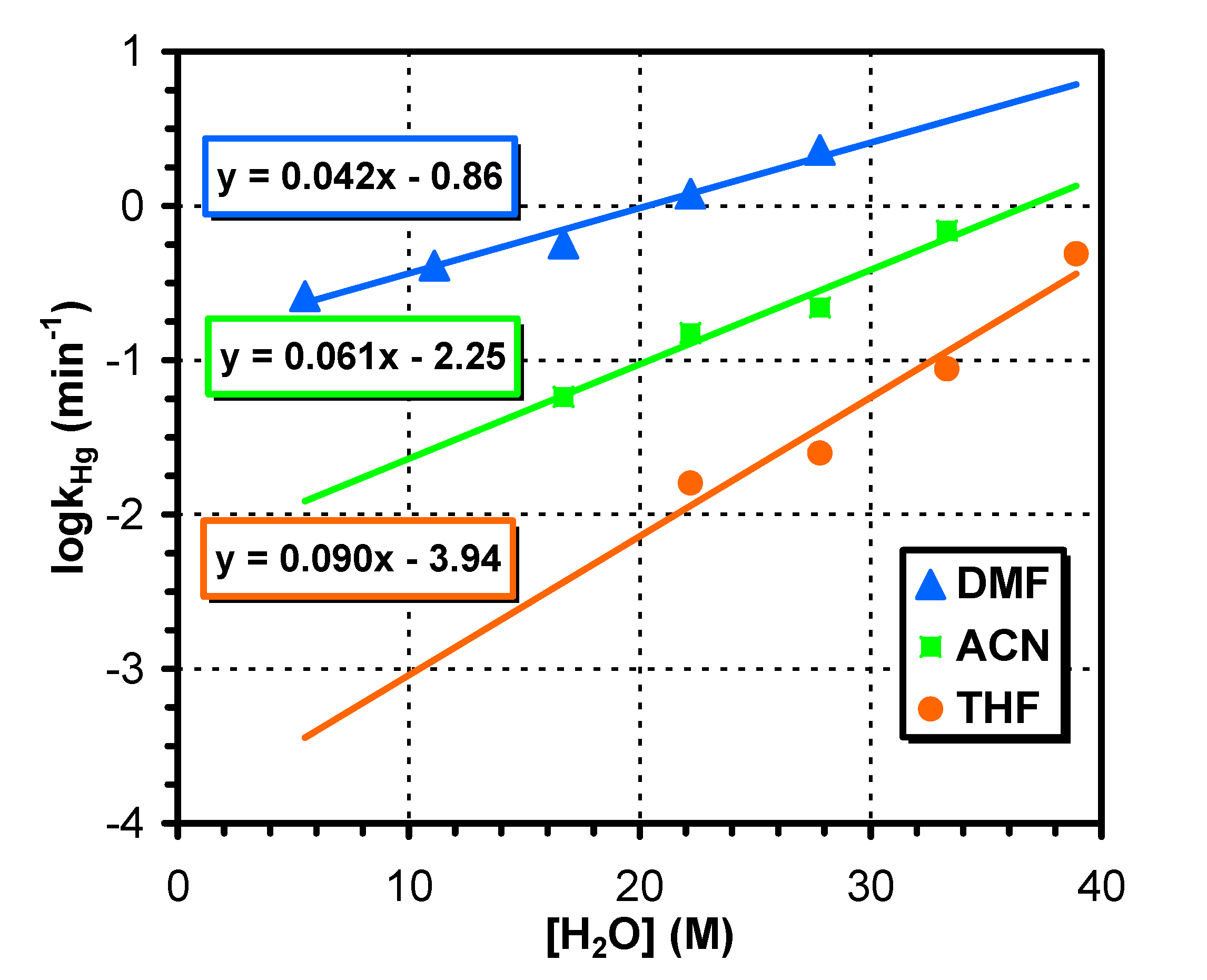
3.1. Reactions in Water/Aprotic Solvent Mixtures
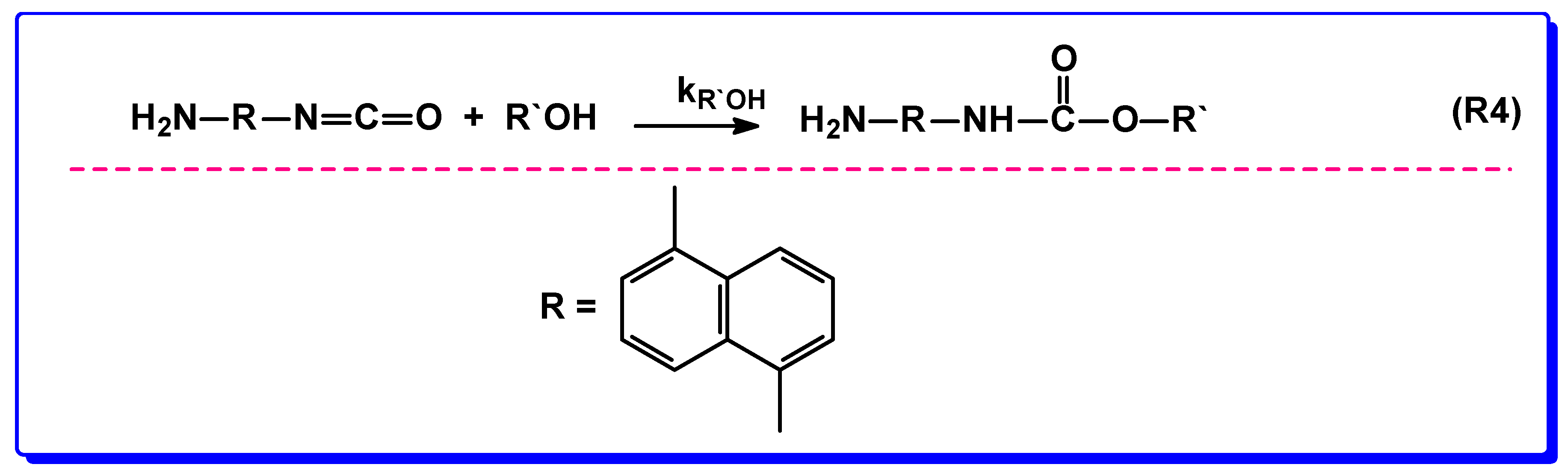
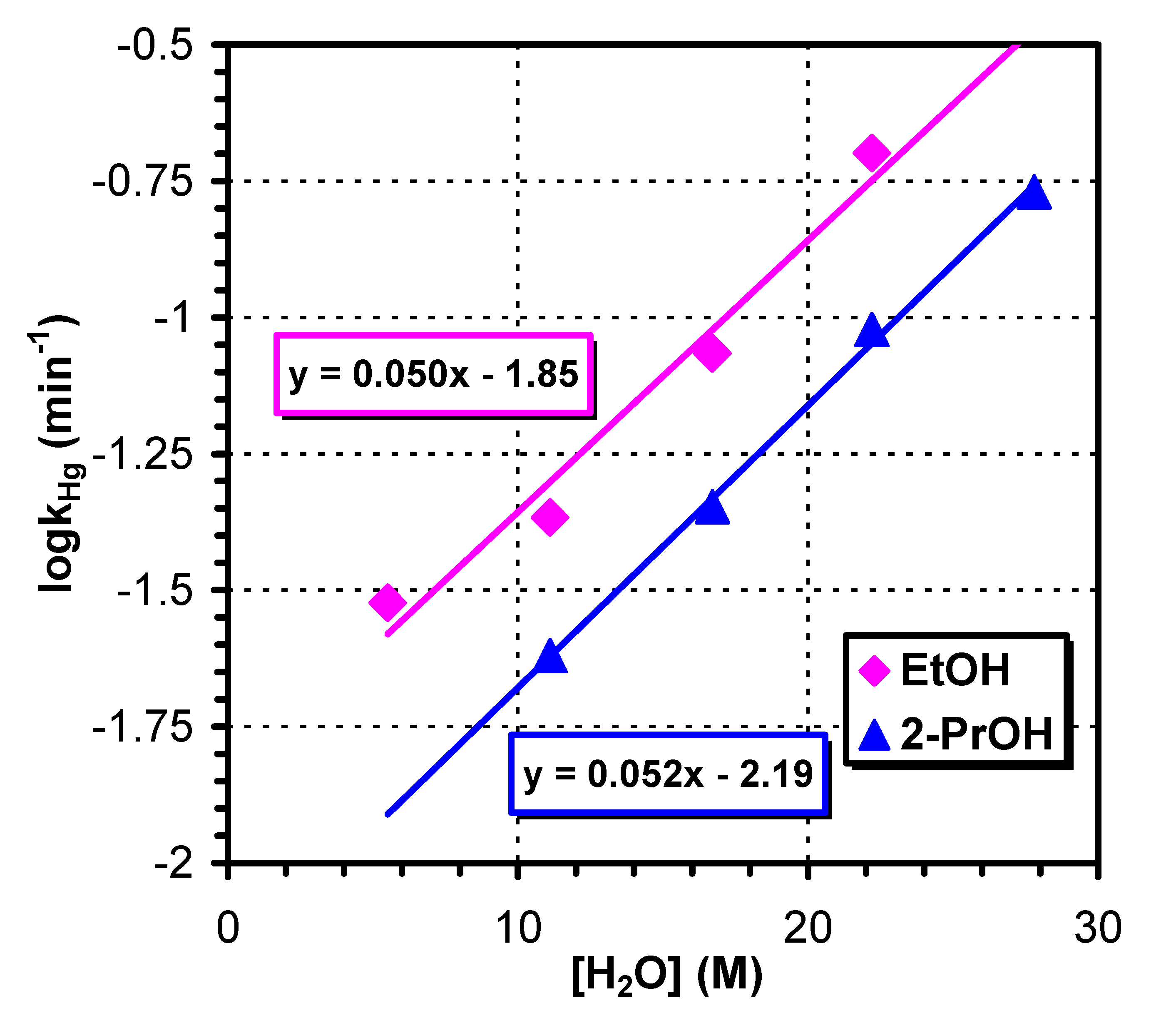
3.2. Reactions in Water/Protic Solvent Mixtures
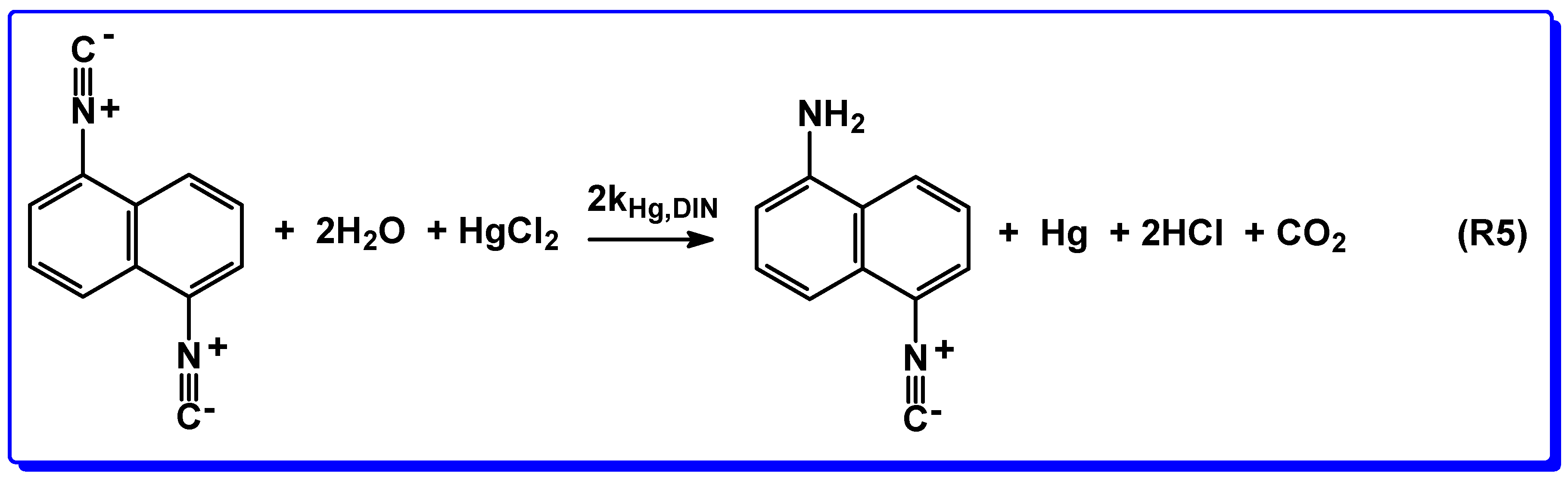
3.3. Reaction of 1,5-DIN with Water and HgCl2
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Morita, M.; Yoshinaga, J.; Edmonds, J.S. The determination of mercury species in environmental and biological samples (Technical report). Pure Appl. Chem. 1998, 70, 1585–1615. [Google Scholar] [CrossRef]
- Driscoll, C.T.; Mason, R.P.; Chan, H.M.; Jacob, D.J.; Pirrone, N. Mercury as a globalpollutant: Sources, pathways, and effects. Environ. Sci. Technol. 2013, 47, 4967–4983. [Google Scholar] [CrossRef] [PubMed]
- Li, X.G.; Dou, Q.; Huan, M.R. Titrimetric analysis of total mercury ions including mercury(I) ions. Monatsh. Chem. 2008, 139, 1157–1162. [Google Scholar] [CrossRef]
- Holzbecher, J.; Ryan, D.E. The fluorimetric determination of mercury. Anal. Chim. Acta. 1973, 64, 333–336. [Google Scholar] [CrossRef]
- Palma, R.J.; Pearson, K.H. A spectropolarimetric titrimetric method for the determination of cadmium, mercury, lead and bismuth. Anal. Chim. Acta. 1970, 49, 497–504. [Google Scholar] [CrossRef]
- Kulomaki, S.; Lahtinen, E.; Peramaki, S.; Vaisanen, A. Determination of mercury at picogram level in natural waters with inductively coupled plasma mass spectrometry by using 3D printed metal scavengers. Anal. Chim. Acta. 2019, 1092, 24–31. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, W. Nitrogen-doped carbon quantum dots: Facile synthesis and application as a “turn-off’ fluorescent probe for detection of Hg2+ ions. Biosens. Bioelectron. 2014, 55, 83–90. [Google Scholar] [CrossRef]
- Guang, S.Y.; Tian, J.C.; We, G.; Yan, Z.Q.; Pan, H.F.; Feng, J.H.; Xu, H.Y. A modified fluorescein derivative with improved water-solubility for turn-on fluorescent determination of Hg2+ in aqueous and living cells. Talanta 2017, 89–170, 96. [Google Scholar]
- Liu, W.; Wang, X.Y.; Wang, Y.Q.; Li, J.H.; Shen, D.Z.; Kang, Q.; Chen, L.X. Ratiometric fluorescence sensor based on dithiothreitol modified carbon dots-gold nanoclusters for the sensitive detection of mercury ions in water samples. Sens. Actuators. B Chem. 2018, 262, 810–817. [Google Scholar] [CrossRef]
- Ma, F.; Sun, M.T.; Zhang, K.; Wang, S.H. A ratiometric fluorescence sensor for highly selective and sensitive detection of mercuric ion. Sens. Actuators. B Chem. 2015, 209, 377–383. [Google Scholar] [CrossRef]
- Ngororabanga, J.M.V.; Tshentu, Z.R.; Mama, N. A highly selective and sensitive ESIPT-based coumarin–triazole polymer for the ratiometric, detection of Hg2+. New J. Chem. 2019, 43, 12168–12177. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Chen, D.; Wu, D.; Chen, Z.; Zhang, J.; Chen, X.; Liu, S.; Yin, J. A colorimetric and ratiometric fluorescent probe for mercury (II) in lysosome. Sens. Actuators. B Chem. 2016, 224, 907–914. [Google Scholar] [CrossRef]
- Ngororabanga, J.M.V.; Tshentu, Z.R.; Mama, N. A New Highly Selective Colorimetric and Fluorometric Coumarin-based Chemosensor for Hg2+. J. Fluoresc. 2020. [Google Scholar] [CrossRef] [PubMed]
- Nagy, M.; Kovács, S.L.; Nagy, T.; RáCZ, D.; Zsuga, M.; Kéki, S. Isocyanonaphthalenes as extremely low molecular weight, selective, ratiometric fluorescent probes for Mercury(II). Talanta 2019, 201, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shi, W.; Chen, X.; Xie, Z.F. Isocyano-functionalized, 1,8-naphthalimide-based chromophore as efficient ratiometric fluorescence probe for Hg2+ in aqueous medium. Sens. Actuators. B Chem. 2018, 255, 3074–3084. [Google Scholar] [CrossRef]
- Tian, M.; Wang, C.; Ma, Q.; Bai, Y.; Sun, J.; Ding, C. A Highly Selective Fluorescent Probe for Hg2+ Based on a 1,8-Naphthalimide Derivative. ACS Omega 2020. [Google Scholar] [CrossRef]
- Rácz, D.; Nagy, M.; Mándi, A.; Zsuga, M.; Kéki, S. Solvatochromic properties of a new isocyanonaphthalene based fluorophore. J. Photochem. Photobiol. A Chem. 2013, 270, 19–37. [Google Scholar]
- Marquardt, D.J. An algorithm for least-squares estimation of nonlinear parameters. Appl. Math. 1963, 11, 431–441. [Google Scholar] [CrossRef]
- Sawai, H.; Takizawa, T. The reaction of isocyanide-mercuric chloride complexes, with amines. Preparation of guanidines. J. Organomet. Chem. 1975, 94, 333–343. [Google Scholar] [CrossRef]
- Sawai, H.; Takizawa, T. Oxidative D-addition of isonitrile by use of mercuric salts synthesis of urea and urethane. Tetrahedron Lett. 1972, 42, 4263–4266. [Google Scholar] [CrossRef]
- Sawai, H.; Takizawa, T. Reaction of isocyanides with mercuric-chloride. Bull. Chem. Soc. Jpn. 1976, 49, 1906–1908. [Google Scholar] [CrossRef]
- Sonnenschein, M.F. Polyurethanes-Science. Technology, Markets, and Trends; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; Available online: https://www.wiley.com/en-hu/Polyurethanes%3A+Science%2C+Technology%2C+Markets%2C+and+Trends-p-9781118737910 (accessed on 15 May 2020).
- Chen, Z.R.; Yang, W.T.; Yin, H.; Yuan, S.F. Kinetics of water-isocyanate reaction in N,N-dimethylformamide. Chin. J. Chem. Eng. 2017, 25, 1435–1441. [Google Scholar] [CrossRef]
- Abushammala, H. A simple method for the quantification of free isocyanates on the surface of cellulose nanocrystals upon carbamation using toluene diisocyanate. Surfaces 2019, 2, 444–454. [Google Scholar] [CrossRef]
- Smallwood, I.M. Handbook of Organic Solvent Properties; Elsevier: Amsterdam, The Netherlands, 1996; ISBN 978-0-340-64578-9. Available online: https://www.directtextbook.com/isbn/9780340645789 (accessed on 18 May 2020).

| k (min−1) | [H2O] (mol/L) | |||||||
|---|---|---|---|---|---|---|---|---|
| 5.5 | 11.1 | 16.7 | 22.2 | 27.8 | 33.3 | 38.9 | ||
| THF | kHg (k1) | - | - | - | 0.016 | 0.025 | 0.088 | 0.49 |
| kH2O (k2) | - | - | - | 0.10 | 0.22 | 0.20 | 0.62 | |
| ACN | kHg (k1) | - | - | 0.058 | 0.15 | 0.22 | 0.69 | - |
| kH2O (k2) | - | - | 0.070 | 0.099 | 0.16 | 0.28 | - | |
| DMF | kHg (k1) | 0.26 | 0.41 | 0.57 | 1.2 | 2.3 | - | - |
| kH2O (k2) | 1.3 | 1.7 | 1.8 | 2.7 | 2.5 | - | - | |
| k (min−1) | [H2O] (mol/L) | |||||
|---|---|---|---|---|---|---|
| 5.5 | 11.1 | 16.7 | 22.2 | 27.8 | ||
| EtOH | kHg (k1) | 0.030 | 0.043 | 0.086 | 0.20 | - |
| kH2O + kR`OH (k2) | 0.43 | 0.40 | 0.63 | 0.68 | - | |
| iPrOH | kHg (k1) | - | 0.024 | 0.045 | 0.095 | 0.17 |
| kH2O + kR`OH (k2) | - | 0.091 | 0.14 | 0.12 | 0.18 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamoczky, A.; Nagy, L.; Nagy, M.; Zsuga, M.; Kéki, S. Conversion of Isocyanide to Amine in The Presence of Water and Hg(II) Ions: Kinetics and Mechanism as Detected by Fluorescence Spectroscopy and Mass Spectrometry. Int. J. Mol. Sci. 2020, 21, 5588. https://doi.org/10.3390/ijms21155588
Adamoczky A, Nagy L, Nagy M, Zsuga M, Kéki S. Conversion of Isocyanide to Amine in The Presence of Water and Hg(II) Ions: Kinetics and Mechanism as Detected by Fluorescence Spectroscopy and Mass Spectrometry. International Journal of Molecular Sciences. 2020; 21(15):5588. https://doi.org/10.3390/ijms21155588
Chicago/Turabian StyleAdamoczky, Anita, Lajos Nagy, Miklós Nagy, Miklós Zsuga, and Sándor Kéki. 2020. "Conversion of Isocyanide to Amine in The Presence of Water and Hg(II) Ions: Kinetics and Mechanism as Detected by Fluorescence Spectroscopy and Mass Spectrometry" International Journal of Molecular Sciences 21, no. 15: 5588. https://doi.org/10.3390/ijms21155588
APA StyleAdamoczky, A., Nagy, L., Nagy, M., Zsuga, M., & Kéki, S. (2020). Conversion of Isocyanide to Amine in The Presence of Water and Hg(II) Ions: Kinetics and Mechanism as Detected by Fluorescence Spectroscopy and Mass Spectrometry. International Journal of Molecular Sciences, 21(15), 5588. https://doi.org/10.3390/ijms21155588







