Seed Germination in Oil Palm (Elaeis guineensis Jacq.): A Review of Metabolic Pathways and Control Mechanisms
Abstract
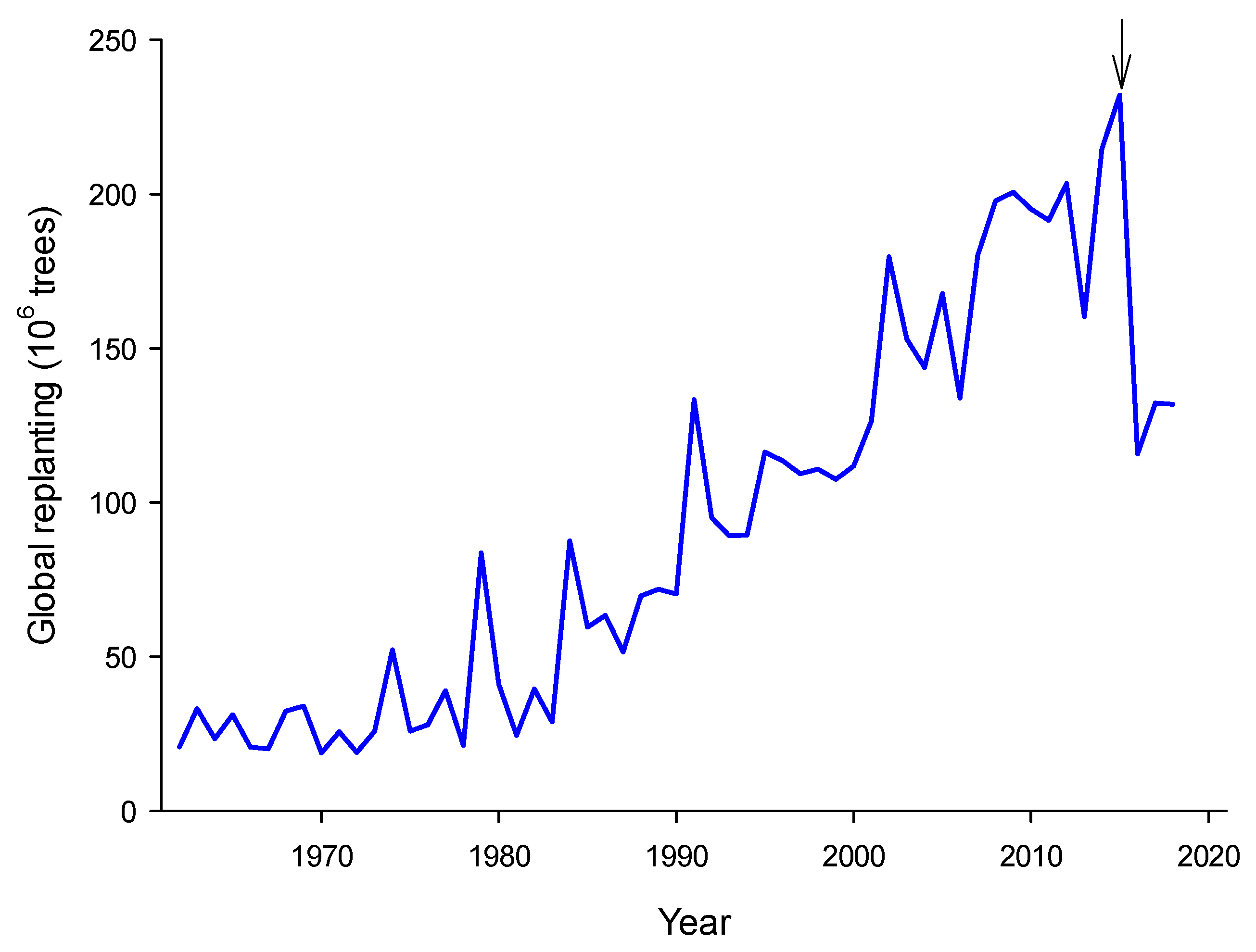
1. Introduction
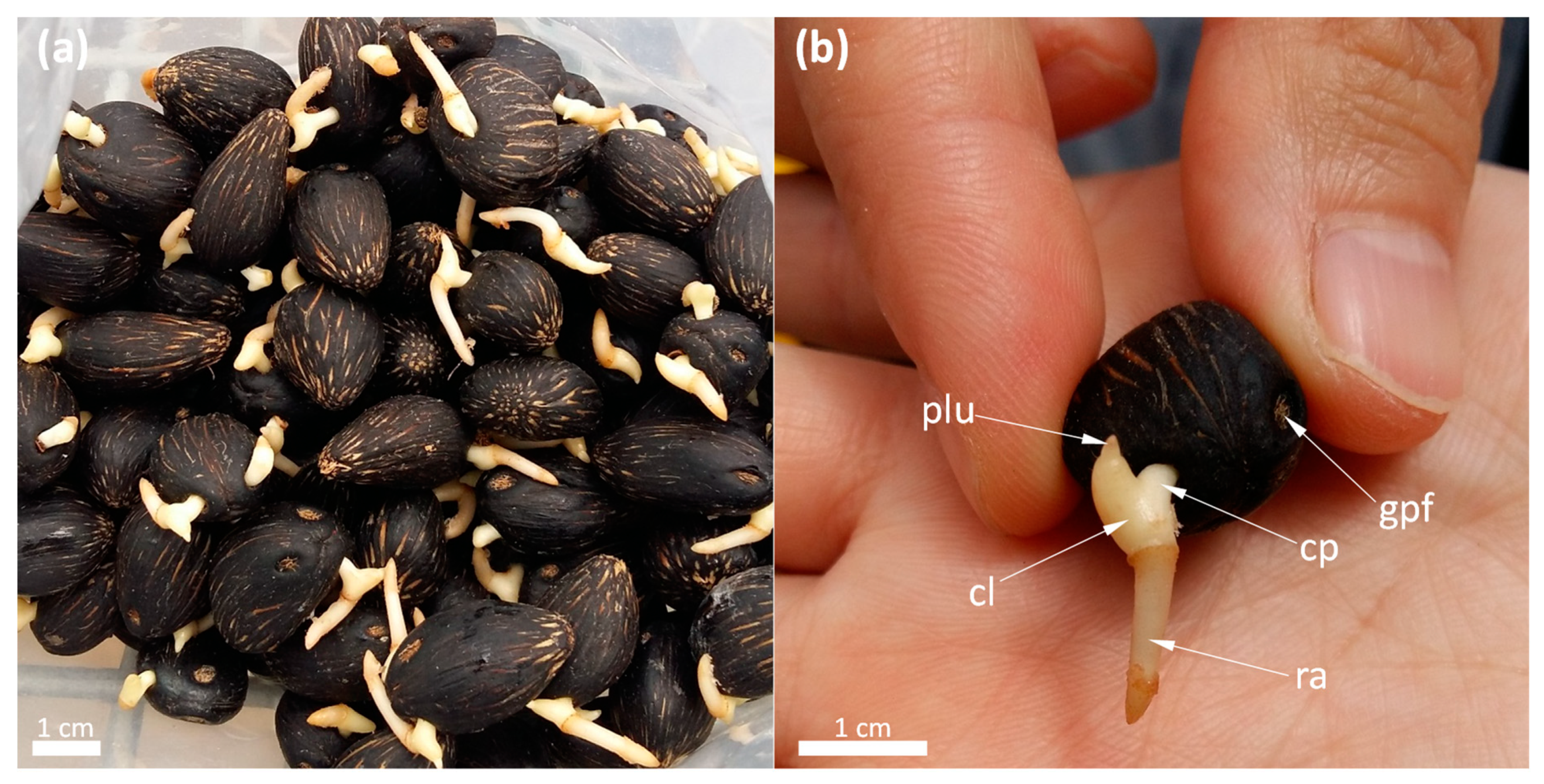
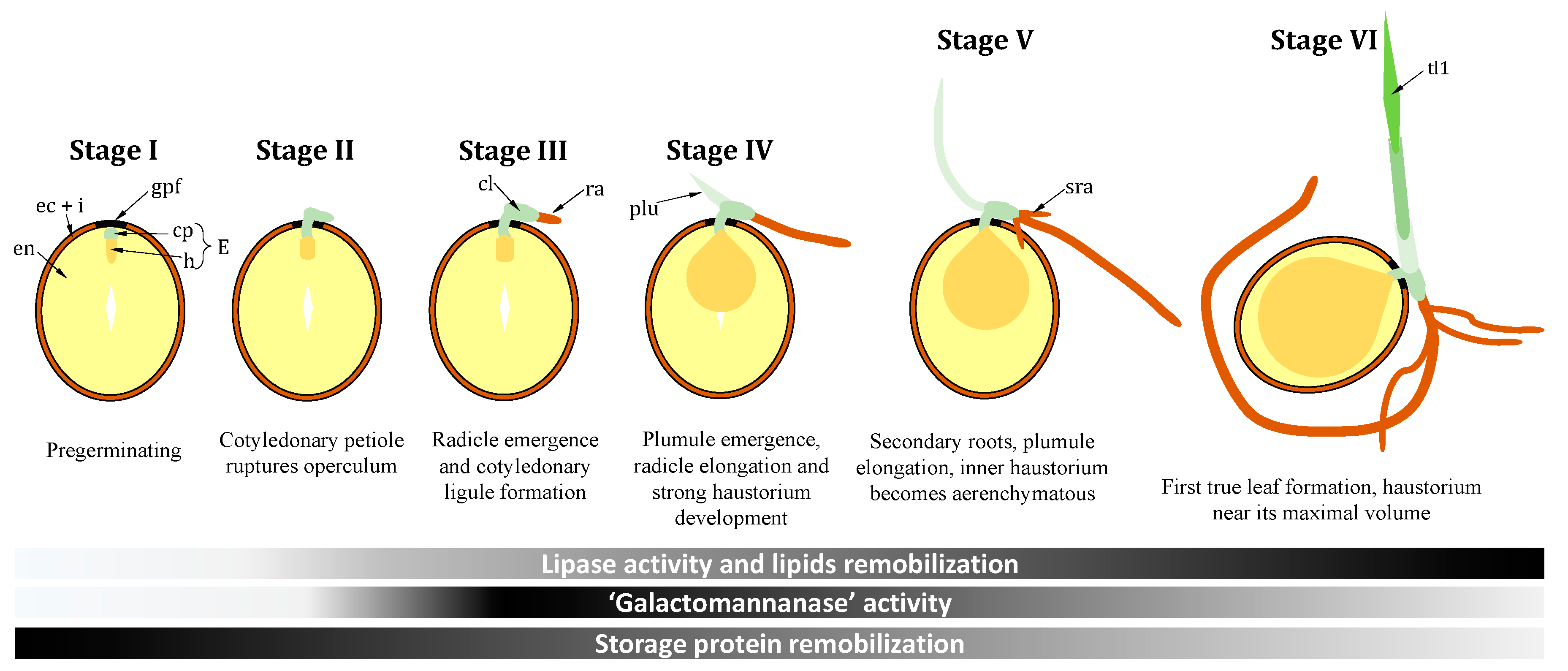
2. Setting the Scene: Specific Germination Stages
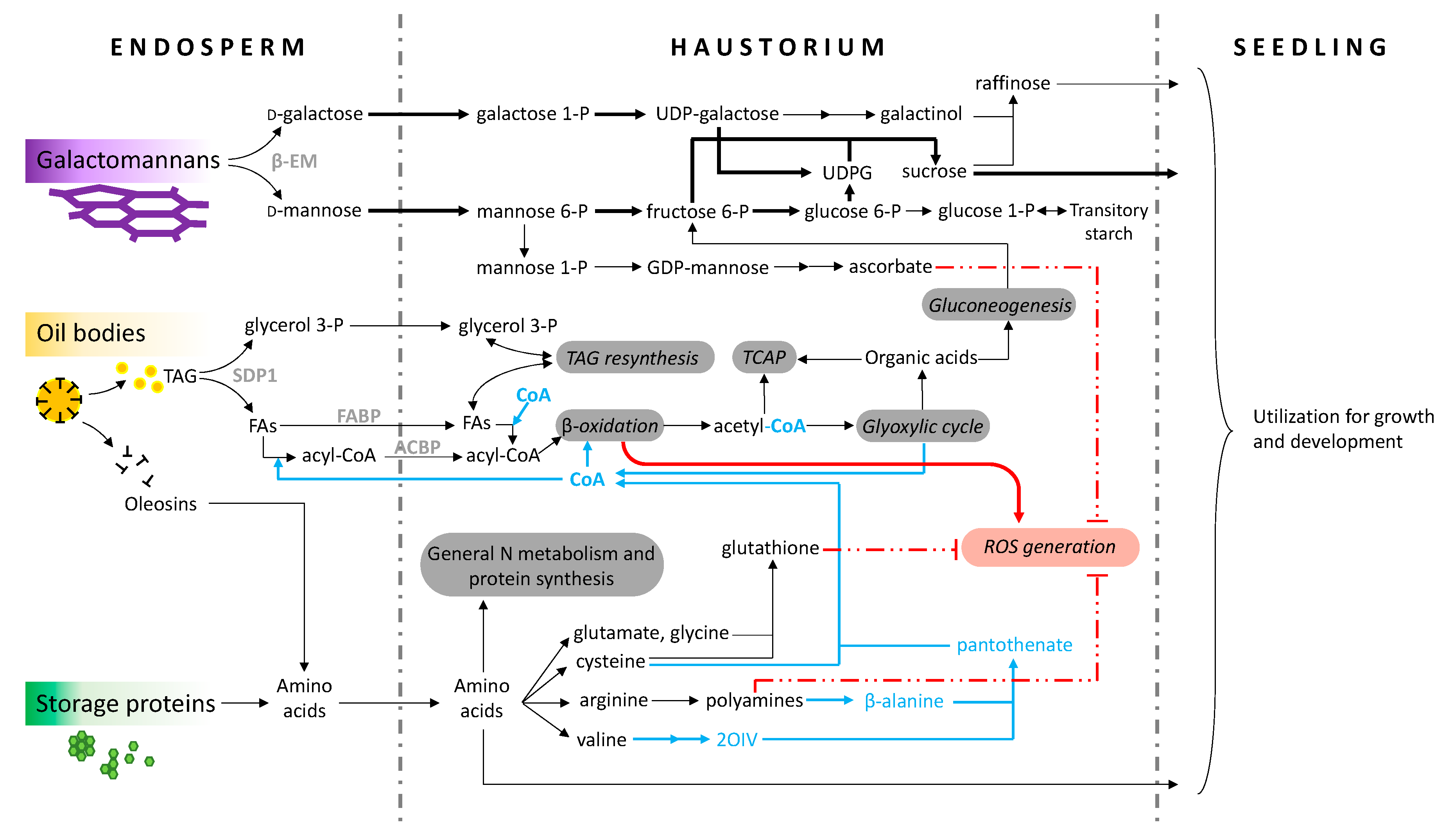
3. Non-lipid Reserve Remobilization
4. Lipid Remobilization
5. ROS Metabolism
6. Control of Germination
7. Perspectives
Funding
Conflicts of Interest
References
- FAO Database FAOSTAT. Available online: http://www.fao.org/faostat/en/#data. (accessed on 25 May 2020).
- Corley, R.H.V.; Tinker, P.B. The Oil Palm; Wiley Blackwell: Chichester, UK, 2016. [Google Scholar]
- Durand-Gasselin, T.; Cochard, B. Oil palm seed distribution. Oilseeds Fats Crops Lipids 2005, 12, 148–153. [Google Scholar] [CrossRef][Green Version]
- Alang, Z.C.; Moir, G.F.J.; Jones, L.H. Composition, degradation and utilization of endosperm during germination in the oil palm (Elaeis guineensis Jacq.). Ann. Bot. 1988, 61, 261–268. [Google Scholar] [CrossRef]
- Oo, K.C. Biochemistry of the germinated oil palm seedlings. PORIM Bull. 1986, 12, 36–41. [Google Scholar]
- Oo, K.C. Stumpf, P.K. The metabolism of the germinating oil palm (Elaeis guineensis) seedlings. Plant Physiol. 1983, 73, 1033–1037. [Google Scholar] [CrossRef] [PubMed]
- Oo, K.C.; Stumpf, P.K. Some enzymic activities in the germinating oil palm (Elaeis guineensis) seedling. Plant Physiol. 1983, 73, 1028–1032. [Google Scholar] [CrossRef]
- Domergue, J.B.; Abadie, C.; Limami, A.; Way, D.; Tcherkez, G. Seed quality and carbon primary metabolism. Plant Cell Environ. 2019, 42, 2776–2788. [Google Scholar] [CrossRef]
- Mazzottini-dos-Santos, H.C.; Ribeiro, L.M.; Oliveira, D.M.T. Roles of the haustorium and endosperm during the development of seedlings of Acrocomia aculeata (Arecaceae): Dynamics of reserve mobilization and accumulation. Protoplasma 2016, 254, 1563–1578. [Google Scholar] [CrossRef]
- Mazzottini-dos-Santos, H.C.; Ribeiro, L.M.; Oliveira, D.M.T. Structural changes in the micropylar region and overcoming dormancy in Cerrado palms seeds. Trees 2018, 32, 1415–1428. [Google Scholar] [CrossRef]
- Boatman, S.G.; Crombie, M. Fat metabolism in the West African oil palm (Elaeis guineensis). J. Exp. Bot. 1958, 9, 52–74. [Google Scholar] [CrossRef]
- Bicalho, E.M.; Motoike, S.Y.; Lima e Borges, E.E.d.; Ataíde, G.d.M.; Guimarães, V.M. Enzyme activity and reserve mobilization during Macaw palm (Acrocomia aculeata) seed germination. Acta Botanica Brasilica 2016, 30, 438–444. [Google Scholar] [CrossRef]
- Alimon, A. The nutritive value of palm kernel cake for animal feed. Palm Oil Dev 2004, 40, 12–14. [Google Scholar]
- Akpanabiatu, M.I.; Ekpa, O.D.; Mauro, A.; Rizzo, R. Nutrient composition of Nigerian palm kernel from the dura and tenera varieties of the oil palm (Elaeis guineensis). Food Chem. 2001, 72, 173–177. [Google Scholar] [CrossRef]
- Fournié, F. Etude de la maturation des embryons de palmier à huile (Elaeis guineensis Jacq.): Protéines de réserve et facteurs intervenant dans la tolérance à la dessication. Master’s Thesis, Université des sciences et techniques du Languedoc, Montpellier, France, 1994. [Google Scholar]
- Morcillo, F.; Aberlenc-Bertossi, F.; Trouslot, P.; Hamon, S.; Duval, Y. Characterization of 2S and 7S storage proteins in embryos of oil palm. Plant Sci. 1997, 122, 141–151. [Google Scholar] [CrossRef]
- Aberlenc-Bertossi, F.; Chabrillange, N.; Duval, Y.; Tregear, J. Contrasting globulin and cysteine proteinase gene expression patterns reveal fundamental developmental differences between zygotic and somatic embryos of oil palm. Tree Physiol. 2008, 28, 1157–1167. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chang, S.K.; Ismail, A.; Yanagita, T.; Esa, N.M.; Baharuldin, M.T.H. Biochemical characterisation of the soluble proteins, protein isolates and hydrolysates from oil palm (Elaeis guineensis) kernel. Food Biosci. 2014, 7, 1–10. [Google Scholar] [CrossRef]
- Cha, T.S.; Habib Shah, F. Kernel-specific cDNA clones encoding three different isoforms of seed storage protein glutelin from oil palm Elaeis guineensis. Plant Sci. 2001, 160, 913–923. [Google Scholar] [CrossRef]
- Masclaux-Daubresse, C.; d’Andrea, S.; Bouchez, I.; Cacas, J.-L. Reserve lipids and plant autophagy. J. Exp. Bot. 2020. In press. [Google Scholar] [CrossRef]
- Daud, M.J.; Jarvis, M.C. Mannan of oil palm kernel. Phytochemistry 1992, 31, 463–464. [Google Scholar] [CrossRef]
- Nonogaki, H.; Chen, F.; Bradford, K.J. Mechanisms and genes involved in germination sensu stricto; Annual Plant Reviews Online; Wiley: London, UK, 2018; pp. 264–304. [Google Scholar]
- Smith, A.G.; Croft, M.T.; Moulin, M.; Webb, M.E. Plants need their vitamins too. Curr. Opin. Plant Biol. 2007, 10, 266–275. [Google Scholar] [CrossRef]
- Cui, J.; Davanture, M.; Zivy, M.; Lamade, E.; Tcherkez, G. Metabolic responses to potassium availability and waterlogging reshape respiration and carbon use efficiency in oil palm. New Phytologist 2019, 223, 310–322. [Google Scholar] [CrossRef]
- Tan, C.-P.; Nehdi, I.A. The physicochemical properties of palm oil and its components. In Palm Oil: Production, Characterization and Uses; Lai, O.-M., Tan, C.-P., Akoh, C.C., Eds.; AOCS Press: Berlin, Germany, 2012; pp. 377–391. [Google Scholar]
- Guerin, C.; Serret, J.; Montúfar, R.; Vaissayre, V.; Bastos-Siqueira, A.; Durand-Gasselin, T.; Tregear, J.; Morcillo, F.; Dussert, S. Palm seed and fruit lipid composition: Phylogenetic and ecological perspectives. Ann. Bot. 2020, 125, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, K.; Huang, A.H.C. Endoplasmic reticulum, oleosins, and oils in seeds and tapetum cells. Plant Physiol. 2004, 136, 3427–3434. [Google Scholar] [CrossRef] [PubMed]
- Theodoulou, F.L.; Eastmond, P.J. Seed storage oil catabolism: A story of give and take. Curr. Opin. Plant Biol. 2012, 15, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Hayashi, Y.; Otomo, M.; Mano, S.; Oikawa, K.; Hayashi, M.; Nishimura, M. Sucrose production mediated by lipid metabolism suppresses the physical interaction of peroxisomes and oil bodies during germination of Arabidopsis thaliana. J. Biol. Chem. 2016, 291, 19734–19745. [Google Scholar] [CrossRef] [PubMed]
- Nik Shazana, N.; Rosli, R.; Ab Halim, M.; Chan, K.; Nagappan, J.; Azizi, N.; Amiruddin, N.; Tatarimova, T.; Low, E. PalmXplore: Oil palm gene database. Database 2018, 1, 9. [Google Scholar]
- Baker, A.; Graham, I.A.; Holdsworth, M.; Smith, S.M.; Theodoulou, F.L. Chewing the fat: β-oxidation in signalling and development. Trends Plant Sci. 2006, 11, 124–132. [Google Scholar] [CrossRef]
- Hooks, M.A. Molecular biology, enzymology, and physiology of β-oxidation. In Plant Peroxisomes: Biochemistry, Cell Biology and Biotechnological Applications; Baker, A., Graham, I.A., Eds.; Springer: Dordrecht, The Netherland, 2002; pp. 19–55. [Google Scholar]
- Opute, F.I. Lipid composition and the role of the haustorium in the young seedling of the West African oil palm, Elaeis guineensis Jacq. Ann. Bot. 1975, 39, 1057–1061. [Google Scholar] [CrossRef]
- Sun, C.; Cao, Y.-z.; Huang, A.H.C. Acyl coenzyme A preference of the glycerol phosphate pathway in the microsomes from the maturing seeds of palm, maize, and rapeseed. Plant Physiol. 1988, 88, 56–60. [Google Scholar] [CrossRef]
- Souza Dias, D.; Monteiro Ribeiro, L.; Sérgio Nascimento Lopes, P.; Aclécio Melo, G.; Müller, M.; Munné-Bosch, S. Haustorium-endosperm relationships and the integration between developmental pathways during reserve mobilization in Butia capitata (Arecaceae) seeds. Ann. Bot. 2018, 122, 267–277. [Google Scholar] [CrossRef]
- Sugimura, Y.; Murakami, T. Structure and function of the haustorium in germinating coconut palm seed. J.A.R.Q. 1990, 24, 1–14. [Google Scholar]
- Webb, M.E.; Smith, A.G. Pantothenate biosynthesis in higher plants. Adv. Bot. Res. 2011, 58, 203–255. [Google Scholar]
- Nakabayashi, K.; Okamoto, M.; Koshiba, T.; Kamiya, Y.; Nambara, E. Genome-wide profiling of stored mRNA in Arabidopsis thaliana seed germination: Epigenetic and genetic regulation of transcription in seed. Plant J. 2005, 41, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Kerr, E.W.; Shumar, S.A.; Leonardi, R. Nudt8 is a novel CoA diphosphohydrolase that resides in the mitochondria. FEBS Lett. 2019, 593, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Neuburger, M.; Day, D.A.; Douce, R. Transport of coenzyme A in plant mitochondria. Arch. Biochem. Biophys. 1984, 229, 253–258. [Google Scholar] [CrossRef]
- Agrimi, G.; Russo, A.; Pierri, C.L.; Palmieri, F. The peroxisomal NAD+ carrier of Arabidopsis thaliana transports coenzyme A and its derivatives. J. Bioenerg. Biomembr. 2012, 44, 333–340. [Google Scholar] [CrossRef]
- Galland, M.; Huguet, R.; Arc, E.; Cueff, G.; Job, D.; Rajjou, L. Dynamic Proteomics Emphasizes the Importance of Selective mRNA Translation and Protein Turnover during Arabidopsis Seed Germination. Mol. Cell. Proteomics 2014, 13, 252–268. [Google Scholar] [CrossRef]
- Bicalho, E.M.; Santos, T.R.S.d.; Garcia, Q.S. Abscisic acid and the antioxidant system are involved in germination of Butia capitata seeds. Acta Botanica Brasilica 2019, 33, 174–178. [Google Scholar] [CrossRef]
- Logan, D.C.; Millar, A.H.; Sweetlove, L.J.; Hill, S.A.; Leaver, C.J. Mitochondrial biogenesis during germination in maize embryos. Plant Physiol. 2001, 125, 662–672. [Google Scholar] [CrossRef]
- Paszkiewicz, G.; Gualberto, J.M.; Benamar, A.; Macherel, D.; Logan, D.C. Arabidopsis seed mitochondria are bioenergetically active immediately upon imbibition and specialize via biogenesis in preparation for autotrophic growth. Plant Cell 2017, 29, 109–128. [Google Scholar] [CrossRef]
- Rajesh, M.K.; Radha, E.; Karun, A.; Parthasarathy, V.A. Plant regeneration from embryo-derived callus of oil palm – the effect of exogenous polyamines. Plant Cell, Tissue Organ Cult. 2003, 75, 41–47. [Google Scholar] [CrossRef]
- Cui, J.; Pottosin, I.; Lamade, E.; Tcherkez, G. What is the role of putrescine accumulated under potassium deficiency? Plant, Cell Environ. 2020. In press. [Google Scholar] [CrossRef] [PubMed]
- Siles, L.; Alegre, L.; González-Solís, A.; Cahoon, E.B.; Munné-Bosch, S. Transcriptional regulation of vitamin E biosynthesis during germination of dwarf fan palm seeds. Plant, Cell Physiol. 2018, 59, 2490–2501. [Google Scholar] [CrossRef] [PubMed]
- Munné-Bosch, S.; Alegre, L. The function of tocopherols and tocotrienols in plants. Crit. Rev. Plant Sci. 2002, 21, 31–57. [Google Scholar] [CrossRef]
- Barreto, L.C.; Garcia, Q.S. Accelerated ageing and subsequent imbibition affect seed viability and the efficiency of antioxidant system in macaw palm seeds. Acta Physiologiae Plantarum 2017, 39, 72. [Google Scholar] [CrossRef]
- Bicalho, E.M.; Pintó-Marijuan, M.; Morales, M.; Müller, M.; Munné-Bosch, S.; Garcia, Q.S. Control of macaw palm seed germination by the gibberellin/abscisic acid balance. Plant Biol. 2015, 17, 990–996. [Google Scholar] [CrossRef]
- Wang, Y.; Htwe, Y.M.; Li, J.; Shi, P.; Zhang, D.; Zhao, Z.; Ihase, L.O. Integrative omics analysis on phytohormones involved in oil palm seed germination. BMC Plant Biol. 2019, 19, 363. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. What kind of seed dormancy might palms have? Seed Sci. Res. 2013, 24, 17–22. [Google Scholar] [CrossRef]
- Lima, W.A.A.; Lopes, R.; Green, M.; Cunha, R.N.V.; Abreu, S.C.; Cysne, A.Q. Heat treatment and germination of seeds of interspecific hybrid between American oil palm (Elaeis oleifera (H.B.K) Cortes) and African oil palm (Elaeis guineensis Jacq.). J. Seed Sci. 2014, 36, 451–457. [Google Scholar] [CrossRef]
- Comont, G.; Jacquemard, J.C. Germination of oil palm seeds (E. guineensis) in polyethylene bags “dry heat” method. Oléagineux 1977, 32, 149–154. [Google Scholar]
- Corrado, E.; Wuidart, W. Germination of oil palm (E. guineensis) seeds in polyethylene bags. The dry heat method. Oléagineux 1990, 45, 511–518. [Google Scholar]
- Fondom, N.; Etta, C.; Mih, A. Breaking seed dormancy: Revisiting heat-treatment duration on germination and subsequent seedling growth of oil palm (Elaeis guineensis Jacq.) progenies. J. Agric. Sci. 2010, 2, 101–110. [Google Scholar] [CrossRef]
- Beugré-Manéhonon, M.; Kouakou-Kouakou, L.; Bognonkpé, J.P.; Konan, K.; Kouakou-Tanoh, H.; Kouadio-Yatty, J. Effect of storage and heat treatments on the germination of oil palm (Elaeis guineensis Jacq.) seed. African J. Agric. Res. 2009, 4, 931–937. [Google Scholar]
- Norsazwan, M.; Sinniah, U.R.; Puteh, A.; Namasivayam, P.; Mohaimi, M.; Aminuddin, I.A. Temperature fluctuation improves oil palm (Elaeis guineensis) Dura x Pisifera seed germination. Seed Sci. Technol. 2020, 48, 49–55. [Google Scholar] [CrossRef]
- Hussey, G. An analysis of the factors controlling the germination of the seed of the oil palm, Elaeis guineensis (Jacq.). Ann. Bot. 1958, 22, 259–284. [Google Scholar] [CrossRef]
- Alang, Z.C. Some physiological effects of the commercial heat treatment of oil palm (Elaeis guineensis) seeds. In Proceedings of the International Conference on Oil Palm in Agriculture in the Eighties. Session-Physiology and Propagation, Kuala Lumpur, Malaysia, 17–20 June 1981; pp. 17–20. [Google Scholar]
- Kaewtaphan, P.; Chanprasert, W.; Sayasoonthorn, S.; Wongsri, O.; Petchrrun, T. Germination of de-operculated oil palm (Elaeis guineensis) seed as affected by gibberellic acid (GA3). Seed Sci. Technol. 2016, 44, 298–309. [Google Scholar] [CrossRef]
- Norsazwan, M.; Puteh, A.; Rafii, M.Y. Oil palm (Elaeis guineensis) seed dormancy type and germination pattern. Seed Sci. Technol. 2016, 44, 1–12. [Google Scholar]
- Yang, N.; Guo, X.; Wu, Y.; Hu, X.; Ma, Y.; Zhang, Y.; Wang, H.; Tang, Z. The inhibited seed germination by ABA and MeJA is associated with the disturbance of reserve utilizations in Astragalus membranaceus. J. Plant Interact. 2018, 13, 388–397. [Google Scholar] [CrossRef]
- Jiménez, V.M.; Guevara, E.; Herrera, J.; Alizaga, R.; Bangerth, F. Changes in hormone concentrations during dormancy release of oil palm (Elaeis guineensis) seeds. Seed Sci. Technol. 2008, 36, 575–587. [Google Scholar] [CrossRef]
- Yan, A.; Chen, Z. The pivotal role of abscisic acid signaling during transition from seed maturation to germination. Plant Cell Rep. 2017, 36, 689–703. [Google Scholar] [CrossRef]
- Holdsworth, M.J.; Bentsink, L.; Soppe, W.J.J. Molecular networks regulating Arabidopsis seed maturation, after-ripening, dormancy and germination. New Phytologist 2008, 179, 33–54. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Wu, M.; Li, B.; Bücker, B.; Keil, P.; Zhang, S.; Li, J.; Kang, D.; Liu, J.; Dong, J.; et al. The COP9 Signalosome regulates seed germination by facilitating protein degradation of RGL2 and ABI5. PLOS Genetics 2018, 14, e1007237. [Google Scholar] [CrossRef] [PubMed]
- Herrera, J.; Alizaga, R.; Guevara, E. Use of chemical treatments to induce seed germination in oil palm (Elaeis guineensis Jacq.). ASD Oil Palm Papers 1998, 18, 1–16. [Google Scholar]
- Duval, Y.; Durand-Gasselin, T.; Konan, K.; Pannetier, C. Multiplication végétative du palmier à huile par culture in vitro. Stratégies et résultats. Oléagineux 1988, 43, 39–44. [Google Scholar]
- Sun, J.; Jia, H.; Wang, P.; Zhou, T.; Wu, Y.; Liu, Z. Exogenous gibberellin weakens lipid breakdown by increasing soluble sugars levels in early germination of Zanthoxylum seeds. Plant Sci. 2019, 280, 155–163. [Google Scholar] [CrossRef]
- Raineri, J.; Hartman, M.D.; Chan, R.L.; Iglesias, A.A.; Ribichich, K.F. A sunflower WRKY transcription factor stimulates the mobilization of seed-stored reserves during germination and post-germination growth. Plant Cell Rep. 2016, 35, 1875–1890. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhou, L.; Lei, X.; Cao, H.; Wang, Y.; Dou, Y.; Tang, W.; Xia, W. Genome-wide identification of WRKY genes and their expression profiles under different abiotic stresses in Elaeis guineensis. PLoS ONE 2017. [Google Scholar] [CrossRef]
- Kok, S.-Y.; Namasivayam, P.; Ee, G.C.-L.; Ong-Abdullah, M. Biochemical characterisation during seed development of oil palm (Elaeis guineensis). J. Plant. Res. 2013, 126, 539–547. [Google Scholar] [CrossRef]
- Xu, L.; Yuan, Y.; Zhang, L.; Wan, L.; Zheng, Y.; Zhou, P.; Li, D. Identification and characterization of differential gene expression in the mesocarp and kernel of oil palm nuts using suppression subtractive hybridization. Tree Genet. Genomes 2011, 7, 999–1010. [Google Scholar] [CrossRef]
- DeMason, D.A.; Stillman, J.I.; Ellmore, G.S. Acid phosphatase localization in seedling tissues of the palms, Phoenix dactylifera and Washingtonia filifera, and its relevance to controls of germination. Can. J. Bot. 1989, 67, 1103–1110. [Google Scholar] [CrossRef]
- Feenstra, A.D.; Alexander, L.E.; Song, Z.; Korte, A.R.; Yandeau-Nelson, M.D.; Nikolau, B.J.; Lee, Y.J. Spatial mapping and profiling of metabolite distributions during germination. Plant. Physiol. 2017, 174, 2532–2548. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, J.; Lamade, E.; Tcherkez, G. Seed Germination in Oil Palm (Elaeis guineensis Jacq.): A Review of Metabolic Pathways and Control Mechanisms. Int. J. Mol. Sci. 2020, 21, 4227. https://doi.org/10.3390/ijms21124227
Cui J, Lamade E, Tcherkez G. Seed Germination in Oil Palm (Elaeis guineensis Jacq.): A Review of Metabolic Pathways and Control Mechanisms. International Journal of Molecular Sciences. 2020; 21(12):4227. https://doi.org/10.3390/ijms21124227
Chicago/Turabian StyleCui, Jing, Emmanuelle Lamade, and Guillaume Tcherkez. 2020. "Seed Germination in Oil Palm (Elaeis guineensis Jacq.): A Review of Metabolic Pathways and Control Mechanisms" International Journal of Molecular Sciences 21, no. 12: 4227. https://doi.org/10.3390/ijms21124227
APA StyleCui, J., Lamade, E., & Tcherkez, G. (2020). Seed Germination in Oil Palm (Elaeis guineensis Jacq.): A Review of Metabolic Pathways and Control Mechanisms. International Journal of Molecular Sciences, 21(12), 4227. https://doi.org/10.3390/ijms21124227




