WT and A53T α-Synuclein Systems: Melting Diagram and Its New Interpretation
Abstract
1. Introduction
2. Results
2.1. α-Synuclein Monomers
2.2. α-Synuclein Oligomers
2.3. α-Synuclein Amyloids
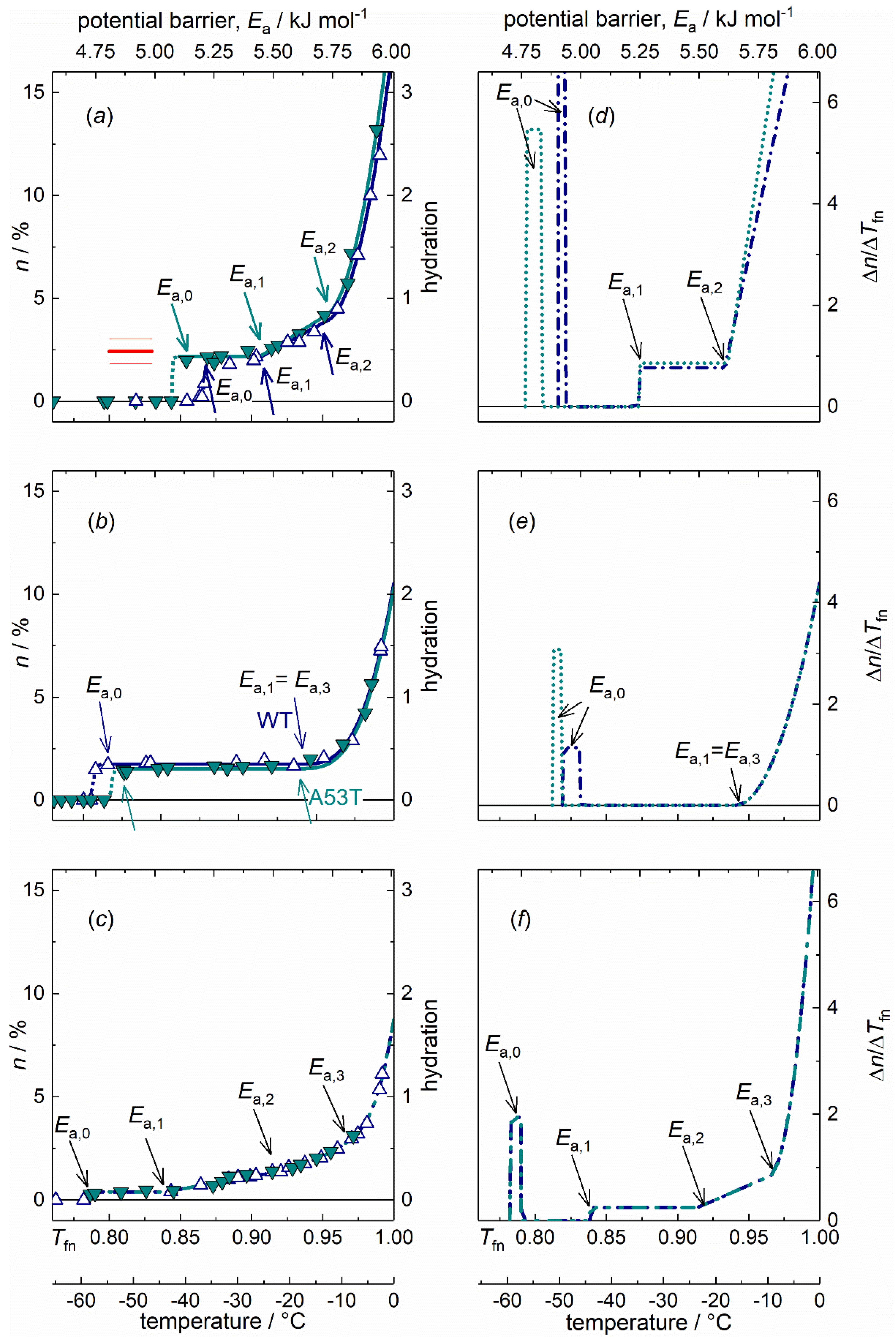
2.4. Dynamic Parameters and Comparisons
3. Discussion
4. Materials and Methods
4.1. Proteins
4.2. Wide-Line NMR Measurements
4.3. Interpretation Methods of the Melting Diagram
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| αS | α-synuclein |
| FID | Free induction decay |
| IDP | Intrinsically disordered protein |
| MD | Melting diagram |
| NMR | Nuclear magnetic resonance |
| PD | Parkinson’s disease |
| SAS | Solvent accessible surface |
| WT | Wild type |
Appendix A
Wide-line NMR Spectometry
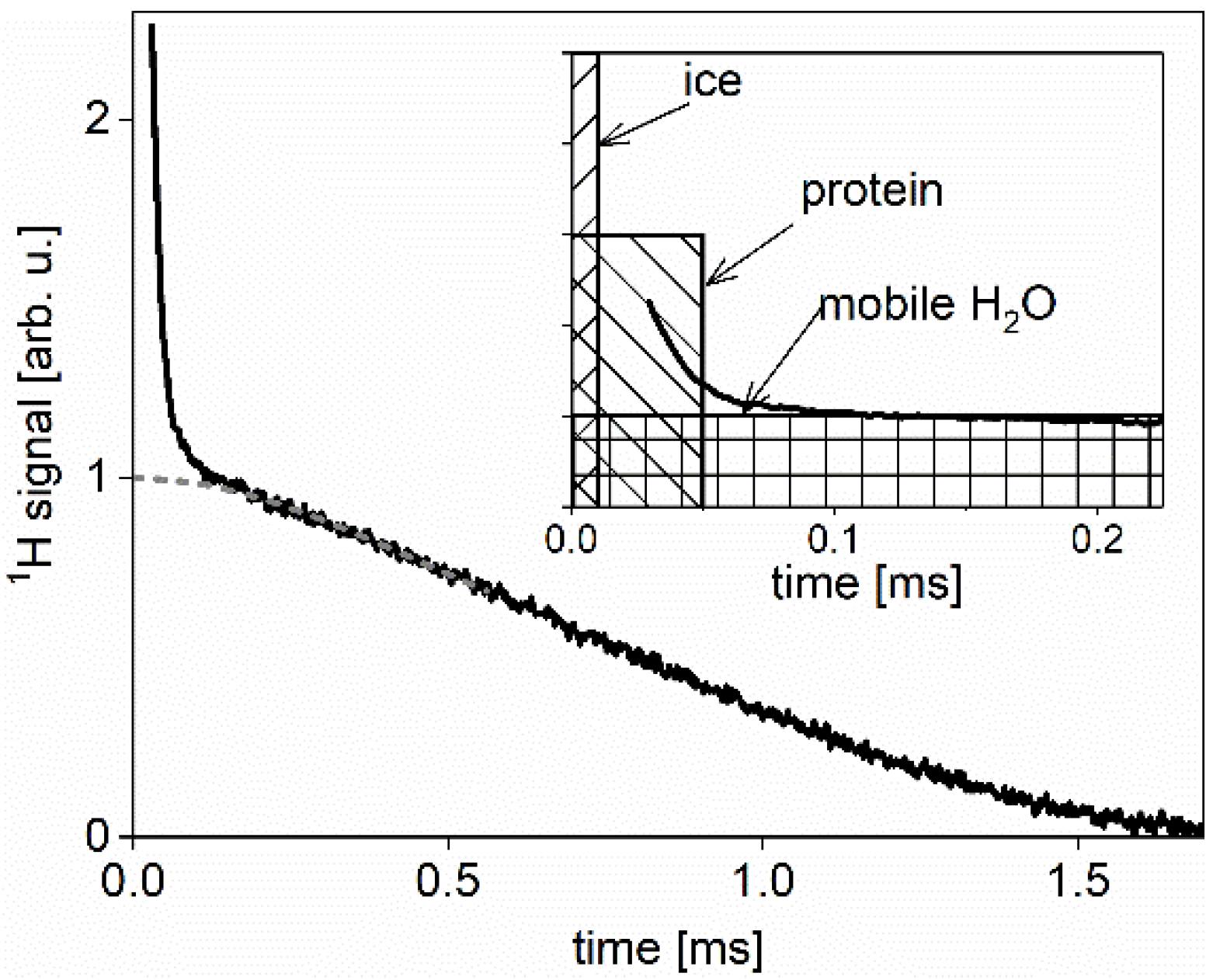
References
- Yu, H.; Han, W.; Ma, W.; Schulten, K.J. Transient β-hairpin formation in α-synuclein monomer revealed by coarse-grained molecular dynamics simulation. J. Chem. Phys. 2015, 143, 243142. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N.; Eliezer, D. Biophysics of Parkinson’s disease: Structure and aggregation of α-synuclein. Curr. Protein Pept. Sci. 2005, 10, 483–499. [Google Scholar] [CrossRef] [PubMed]
- Bendor, J.T.; Logan, T.P.; Edwards, R.H. The function of α-synuclein. Neuron 2013, 79, 1044–1066. [Google Scholar] [CrossRef] [PubMed]
- Házy, E.; Bokor, M.; Kalmár, L.; Gelencsér, A.; Kamasa, P.; Han, K.-H.; Tompa, K.; Tompa, P. Distinct hydration properties of wild-type and familial point mutant A53T of α-synuclein associated with Parkinson’s disease. Biophys. J. 2011, 101, 2260–2266. [Google Scholar] [CrossRef]
- Weinreb, P.H.; Zhen, W.; Poon, A.W.; Conway, K.A.; Lansbury, P.T. NACP, a protein implicated in Alzheimer’s disease and learning, is natively unfolded. Biochemistry 1996, 35, 13709–13715. [Google Scholar] [CrossRef]
- Uversky, V.N.; Gillespie, J.R.; Fink, A.L. Why are “natively unfolded” proteins unstructured under physiologic conditions? Proteins 2000, 41, 415–427. [Google Scholar] [CrossRef]
- Eliezer, D.; Kutluay, E.; Bussell, R., Jr.; Browne, G.J. Conformational properties of alpha-synuclein in its free and lipid-associated states. Mol. Biol. 2001, 307, 1061–1073. [Google Scholar] [CrossRef]
- Uversky, V.N.; Li, J.; Fink, A.L. Evidence for a partially folded intermediate in alpha-synuclein fibril formation. J. Biol. Chem. 2001, 276, 10737–10744. [Google Scholar] [CrossRef]
- Mor, D.E.; Ugras, S.E.; Daniels, M.J.; Ischiropoulos, H. Dynamic structural flexibility of α-synuclein. Neurobiol. Dis. 2016, 88, 66–74. [Google Scholar] [CrossRef]
- Uversky, V.N.; Li, J.; Souillac, P.; Millett, I.S.; Doniach, S.; Jakes, R.; Goedert, M.; Fink, A.L. Biophysical properties of the synucleins and their propensities to fibrillate: Inhibition of alpha-synuclein assembly by beta- and gamma-synucleins. J. Biol. Chem. 2002, 277, 11970–11978. [Google Scholar] [CrossRef]
- Tompa, K.; Bokor, M.; Verebélyi, T.; Tompa, P. Water rotation barriers on protein molecular surfaces. Chem. Phys. 2015, 448, 15–25. [Google Scholar] [CrossRef]
- Tompa, K.; Bokor, M.; Han, K.-H.; Tompa, P. Hydrogen skeleton, mobility and protein architecture. Intrinsically Disord. Proteins 2013, 1, e25767. [Google Scholar] [CrossRef] [PubMed]
- Schiro, G.; Fichou, Y.; Gallat, F.-X.; Wood, K.; Gabel, F.; Moulin, M.; Härtlein, M.; Heyden, M.; Colletier, J.-P.; Orecchini, A.; et al. Translational diffusion of hydration water correlates with functional motions in folded and intrinsically disordered proteins. Nat. Commun. 2015, 6, 6490. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, W. The dynamics of water in heterogeneous systems with emphasis on subzero temperatures. In Water a Comprehensive Treatise; Franks, F., Ed.; Springer: Boston, MA, USA, 1982; Volume 7, pp. 339–430. [Google Scholar] [CrossRef]
- Tompa, K.; Bokor, M.; Tompa, P. The melting diagram of protein solutions and its thermodynamic interpretation. Int. J. Mol. Sci. 2018, 19, 3571. [Google Scholar] [CrossRef] [PubMed]
- Tompa, K.; Bokor, M.; Tompa, P. Hydration of intrinsically disordered proteins from wide-line NMR. In Instrumental Analysis of Intrinsically Disordered Proteins: Assessing Structure and Conformation; Wiley Series in Protein and Peptide Science; Uversky, V.N., Longhi, S., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 345–368. [Google Scholar] [CrossRef]
- Tompa, K.; Bokor, M.; Ágner, D.; Iván, D.; Kovács, D.; Verebélyi, T.; Tompa, P. Hydrogen Mobility and Protein—Water Interactions in Proteins in the Solid State. Chem. Phys. Chem. 2017, 18, 677–682. [Google Scholar] [CrossRef]
- Bokor, M.; Tantos, Á.; Mészáros, A.; Jenei, B.; Haminda, R.; Tompa, P.; Tompa, K. Molecular motions and interactions in aqueous solutions of thymosin-β4, stabilin c-terminal domain (CTD) and their 1:1 complex studied by 1H NMR spectroscopy. Chem. Phys. Chem. 2018, 19, 848–856. [Google Scholar] [CrossRef]
- Tompa, K.; Bokor, M.; Tompa, P. Globuláris szerkezetû fehérjék vizes oldatainak olvadási diagramja és termodinamikai értelmezésük. Globular proteins—melting diagrams of aqueous solutions, thermodynamic interpretation. Magyar Kémiai Folyóirat 2019, 125, 147–155. [Google Scholar] [CrossRef]
- Li, J.; Uversky, V.N.; Fink, A.L. Conformational behavior of human alpha-synuclein is modulated by familial Parkinson’s disease point mutations A30P and A53T. Neurotoxicology 2002, 23, 553–567. [Google Scholar] [CrossRef]
- Coskuner, O.; Wise-Scira, O. Structures and free energy landscapes of the A53T mutant-type α-synuclein protein and impact of A53T mutation on the structures of the wild-type α-synuclein protein with dynamics. ACS Chem. Neurosci. 2013, 4, 1101–1113. [Google Scholar] [CrossRef]
- Fujiwara, S.; Araki, K.; Matsuo, T.; Yagi, H.; Yamada, T.; Shibata, K.; Mochizuki, H. Dynamical behavior of human α-synuclein studied by quasielastic neutron scattering. PLoS ONE 2016, 11, e0151447. [Google Scholar] [CrossRef]
- Conway, K.A.; Harper, J.D.; Lansbury, P.T. Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat. Med. 1998, 4, 1318–1320. [Google Scholar] [CrossRef]
- Li, J.; Uversky, V.N.; Fink, A.L. Effect of familial Parkinson’s disease point mutations A30P and A53T on the structural properties, aggregation, and fibrillation of human α-synuclein. Biochemistry 2001, 40, 11604–110613. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.W.; Drakulic, S.; Deas, E.; Ouberai, M.; Aprile, F.A.; Arranz, R.; Ness, S.; Roodveldt, C.; Guilliams, T.; De-Genst, E.J.; et al. Structural characterization of toxic oligomers that are kinetically trapped during α-synuclein fibril formation. Proc. Natl. Acad. Sci. USA 2015, 112, E1994–E2003. [Google Scholar] [CrossRef] [PubMed]
- Miake, H.; Mizusawa, H.; Iwatsubo, T.; Hasegawa, M. Biochemical Characterization of the Core Structure of α-Synuclein Filaments. J. Biol. Chem. 2002, 277, 19213–19219. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.-P.; Fink, A.L.; Uversky, V.N. Structural characteristics of alpha-synuclein oligomers stabilized by the flavonoid baicalein. J. Mol. Biol. 2008, 383, 214–223. [Google Scholar] [CrossRef]
- Brotzakis, Z.F.; Groot, C.C.M.; Brandeburgo, W.H.; Bakker, H.J.; Bolhuis, P.G. Dynamics of hydration water around native and misfolded α-lactalbumin. J. Phys. Chem. B 2016, 120, 4756–4766. [Google Scholar] [CrossRef]
- Kuntz, I.D., Jr. Hydration of macromolecules. III. Hydration of polypeptides. J. Am. Chem. Soc. 1971, 93, 514–516. [Google Scholar] [CrossRef]
- Matsuoka, D.; Nakasako, M. Application of empirical hydration distribution functions around polar atoms for assessing hydration structures of proteins. Chem. Phys. 2013, 419, 59–64. [Google Scholar] [CrossRef]
- Tompa, K.; Bokor, M.; Tompa, P. Wide-Line NMR and Protein Hydration. In Intrinsically Disordered Protein Analysis; Uversky, V.N., Dunker, A.K., Eds.; Springer: New York, NY, USA; Springer: Heidelberg, Germany; Springer: Dodrecht, The Netherlands; Springer: London, UK, 2012; Volume 2, pp. 167–196. [Google Scholar] [CrossRef]
- Bizzarri, A.R.; Wang, C.X.; Chen, W.Z.; Cannistraro, S. Hydrogen bond analysis by MD simulation of copper plastocyanin at different hydration levels. Chem. Phys. 1995, 201, 463–472. [Google Scholar] [CrossRef]
- Kim, D.H.; Han, K.H. Transient secondary structures as general target-binding motifs in intrinsically disordered proteins. Int. Mol. Sci. 2018, 19, 3614. [Google Scholar] [CrossRef]
- Bussell, R., Jr.; Eliezer, D. Residual structure and dynamics in Parkinson’s disease-associated mutants of α-synuclein. J. Biol. Chem. 2001, 276, 45996–46003. [Google Scholar] [CrossRef] [PubMed]
- Bertoncini, C.W.; Fernandez, C.O.; Griesinger, C.; Jovin, T.M.; Zweckstetter, M. Familial mutants of α-synuclein with increased neurotoxicity have a destabilized conformation. J. Biol. Chem. 2005, 280, 30649–30652. [Google Scholar] [CrossRef] [PubMed]
- Sunde, M.; Serpell, L.C.; Bartlam, M.; Fraser, P.E.; Pepys, M.B.; Blake, C.C. Common core structure of amyloid fibrils by synchrotron X-ray diffraction. J. Mol. Biol. 1997, 273, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Sahay, S.; Ranjan, P.; Salot, S.; Mohite, G.M.; Singh, P.K.; Dwivedi, S.; Carvalho, E.; Banerjee, R.; Kumar, A.; et al. The newly discovered Parkinson’s disease associated Finnish mutation (A53E) attenuates α-synuclein aggregation and membrane binding. Biochemistry 2014, 53, 6419–6421. [Google Scholar] [CrossRef] [PubMed]
- Giasson, B.I.; Uryu, K.; Trojanowski, J.Q.; Lee, V.M.-Y. Mutant and wild type human α-synucleins assemble into elongated filaments with distinct morphologies in vitro. J. Biol. Chem. 1999, 274, 7619–7622. [Google Scholar] [CrossRef] [PubMed]
- Greenbaum, E.A.; Graves, C.L.; Mishizen-Eberz, A.J.; Lupoli, M.A.; Lynch, D.R.; Englander, S.W.; Axelsen, P.H.; Giasson, B.I. The E46K mutation in α-synuclein increases amyloid fibril formation. J. Biol. Chem. 2005, 280, 7800–7807. [Google Scholar] [CrossRef]
- Mahul-Mellier, A.-L.; Vercruysse, F.; Maco, B.; Ait-Bouziad, N.; De Roo, M.; Muller, D.; Lashuel, H.A. Fibril growth and seeding capacity play key roles in α-synuclein-mediated apoptotic cell death. Cell Death Differ. 2015, 22, 2107–2122. [Google Scholar] [CrossRef]
- Narhi, L.; Wood, S.J.; Steavenson, S.; Jiang, Y.; Wu, G.M.; Anafi, D.; Kaufman, S.A.; Martin, F.; Sitney, K.; Denis, P.; et al. Both familial Parkinson’s disease mutations accelerate α-synuclein aggregation. J. Biol. Chem. 1999, 274, 9843–9846. [Google Scholar] [CrossRef]
- Dobson, C.M. Protein misfolding, evolution and disease. Trends Biochem. Sci. 1999, 24, 329–332. [Google Scholar] [CrossRef]
- Dobson, C.M. Protein folding and misfolding. Nature 2003, 426, 884–890. [Google Scholar] [CrossRef]
- Vilar, M.; Chou, H.-T.; Lührs, T.; Maji, S.K.; Riek-Loher, D.; Verel, R.; Manning, G.; Stahlberg, H.; Riek, R. The fold of α-synuclein fibrils. Proc. Natl. Acad. Sci. USA 2008, 105, 8637–8642. [Google Scholar] [CrossRef] [PubMed]
- Atsmon-Raz, Y.; Miller, Y. A proposed atomic structure of the self-assembly of the non-amyloid-β component of human α-synuclein as derived by computational tools. J. Phys. Chem. B 2015, 119, 10005–10015. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.A.; Ivanova, M.I.; Sawaya, M.R.; Cascio, D.; Reyes, F.E.; Shi, D.; Sangwan, S.; Guenther, E.L.; Johnson, L.M.; Zhang, M.; et al. Structure of the toxic core of α-synuclein from invisible crystals. Nature 2015, 525, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Serpell, L.C.; Berriman, J.; Jakes, R.; Goedert, M.; Crowther, R.A. Fiber diffraction of synthetic α-synuclein filaments shows amyloid-like cross-β conformation. Proc. Natl. Acad. Sci. USA 2000, 97, 4897–4902. [Google Scholar] [CrossRef]
- Roeters, S.J.; Iyer, A.; Pletikapić, G.; Kogan, V.; Subramaniam, V.; Woutersen, S. Evidence for intramolecular antiparallel beta-sheet structure in alpha-synuclein fibrils from a combination of two-dimensional infrared spectroscopy and atomic force microscopy. Sci. Rep. 2017, 7, 41051. [Google Scholar] [CrossRef]
- van Raaij, M.E.; Segers-Nolten, I.M.; Subramaniam, V. Quantitative morphological analysis reveals ultrastructural diversity of amyloid fibrils from alpha-synuclein mutants. Biophys. J. 2006, 91, L96–L98. [Google Scholar] [CrossRef]
- Bertoncini, C.W.; Jung, Y.S.; Fernandez, C.O.; Hoyer, W.; Griesinger, C.; Jovin, T.M.; Zweckstetter, M. Release of long-range tertiary interactions potentiates aggregation of natively unstructured α-synuclein. Proc. Natl. Acad. Sci. USA 2005, 102, 1430–1435. [Google Scholar] [CrossRef]
- Morar, A.S.; Olteanu, A.; Young, G.B.; Pielak, G.J. Solvent-induced collapse of alpha-synuclein and acid-denatured cytochrome c. Protein Sci. 2001, 10, 2195–2199. [Google Scholar] [CrossRef]
- Waudby, C.A.; Camilloni, C.; Fitzpatrick, A.W.P.; Cabrita, L.D.; Dobson, C.M.; Vendruscolo, M.; Christodoulou, J. In-cell NMR characterization of the secondary structure populations of a disordered conformation of α-synuclein within E. coli cells. PLoS ONE 2013, 8, e72286. [Google Scholar] [CrossRef]
- Cremades, N.; Chen, S.W.; Dobson, C.M. Structural Characteristics of α-Synuclein Oligomers. Int. Rev. Cell Mol. Biol. 2017, 329, 79–143. [Google Scholar] [CrossRef]
- Tuttle, M.D.; Comellas, G.; Nieuwkoop, A.J.; Covell, D.J.; Berthold, D.A.; Kloepper, K.D.; Courtney, J.M.; Kim, J.K.; Barclay, A.M.; Kendall, A.; et al. Solid-state NMR structure of a pathogenic fibril of full-length human α-synuclein. Nat. Struct. Mol. Biol. 2016, 23, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, S. Dynamic aspects of amyloid fibrils of α-synuclein related to the pathogenesis of Parkinson’s disease. J. Alzheimers Dis. Parkinsonism 2017, 7, 310. [Google Scholar] [CrossRef]
- Waugh, J.S.; Fedin, E.J. Determination of hindered rotation barriers in solids. Sov. Phys. Solid State 1963, 4, 1633–1636. [Google Scholar]
| Polymerization | Monomer | Oligomer | Amyloid | ||
|---|---|---|---|---|---|
| αS variant | WT 1 | A53T | WT | A53T | WT, A53T |
| A = n(Tfno) 2 = n(Tfn1) | 0.022(4) | 0.022(4) | 0.0174(6) | 0.0153(4) | 0.0039(3) |
| h(Ea,o) 3 = h(Ea,1) | 0.44(8) | 0.44(8) | 0.35(1) | 0.306(8) | 0.077(6) |
| B | 0.38(5) | 0.43(3) | 0 | 0 | 0.12(1) |
| C | 45(7) | 0.6(1)·102 | 0 | 0 | 2.9(4) |
| D | 0 | 0 | 4(1)·102 | 4(1)·102 | 10(2)·102 |
| Tfno | 0.8662(9) | 0.854(5) | 0.816(4) | 0.8257(2) | 0.784(2) |
| Ea,0/kJ mol−1 | 5.206(6) | 5.13(3) | 4.90(2) | 4.963(1) | 4.71(1) |
| t0/°C 4 | −36.5(2) | −40(1) | −50(1) | −47.60(5) | −58.9(6) |
| Tfn1 | 0.908(4) | 0.906(2) | 0.941(5) | 0.940(5) | 0.838(5) |
| Ea,1/kJ mol−1 | 5.46(2) | 5.44(1) | 5.65(3) | 5.65(3) | 5.04(3) |
| t1/°C | −25.2(1) | −25.7(5) | −16(1) | −16(1) | −44(1) |
| Tfn2 | 0.951(3) | 0.953(4) | — | — | 0.914(3) |
| Ea,2/kJ mol−1 | 5.72(1) | 5.73(2) | 5.49(2) | ||
| t2/°C | −13.3(8) | −13(1) | −23.5(9) | ||
| Tfn3 | — | — | 0.940(5) | 0.940(5) | 0.965(2) |
| Ea,3/kJ mol−1 | 5.65(3) | 5.65(3) | 5.80(1) | ||
| t3/°C | −16(1) | −16 (1) | −9.6(7) | ||
| n(Tfn2) | 0.039(3) | 0.042(8) | — | — | 0.013(2) |
| h(Ea,2) | 0.77(6) | 0.77(3) | 0.26(4) | ||
| n(Tfn3) | — | — | 0.0174(6) | 0.0153(4) | 0.027(1) |
| h(Ea,3) | 0.35(1) | 0.306(8) | 0.54(3) | ||
| n(Tfn = 1) | 0.164(5) | 0.20(6) | 0.105(5) | 0.103(5) | 0.088(4) |
| h(Ea = 6.01 kJ mol−1) | 3.3(1) | 4(1) | 2.1(1) | 2.1(1) | 1.75(9) |
| Protein | Variant | nho2 | nhe4 | HeR | HeRn | HeM |
|---|---|---|---|---|---|---|
| hho3 | hhe5 | |||||
| monomer | WT 1 | 0.22(4) | 0.142(9) | 0.70(4) | 0.87(3) | 9.8(5)·102 |
| 0.44(8) | 2.8(2) | |||||
| A53T | 0.22(4) | 0.18(6) | 0.65(4) | 0.89(3) | 1.30(6)·103 | |
| 0.44(8) | 4(1) | |||||
| oligomer | WT | 0.0174(6) | 0.08790(7) | 0.32(2) | 0.83(4) | 2.1(1)·104 |
| 0.35(1) | 1.76(1) | |||||
| A53T | 0.0153(4) | 0.09(1) | 0.35(2) | 0.85(4) | 2.0(1)·104 | |
| 0.306(8) | 1.8(1) | |||||
| amyloid | WT, A53T | 0.0039(3) | 0.084(5) | 0.75(3) | 0.96(5) | 1.82(9)·104 |
| 0.077(6) | 1.68(9) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bokor, M.; Tantos, Á.; Tompa, P.; Han, K.-H.; Tompa, K. WT and A53T α-Synuclein Systems: Melting Diagram and Its New Interpretation. Int. J. Mol. Sci. 2020, 21, 3997. https://doi.org/10.3390/ijms21113997
Bokor M, Tantos Á, Tompa P, Han K-H, Tompa K. WT and A53T α-Synuclein Systems: Melting Diagram and Its New Interpretation. International Journal of Molecular Sciences. 2020; 21(11):3997. https://doi.org/10.3390/ijms21113997
Chicago/Turabian StyleBokor, Mónika, Ágnes Tantos, Péter Tompa, Kyou-Hoon Han, and Kálmán Tompa. 2020. "WT and A53T α-Synuclein Systems: Melting Diagram and Its New Interpretation" International Journal of Molecular Sciences 21, no. 11: 3997. https://doi.org/10.3390/ijms21113997
APA StyleBokor, M., Tantos, Á., Tompa, P., Han, K.-H., & Tompa, K. (2020). WT and A53T α-Synuclein Systems: Melting Diagram and Its New Interpretation. International Journal of Molecular Sciences, 21(11), 3997. https://doi.org/10.3390/ijms21113997






