SMARCB1 Acts as a Quiescent Gatekeeper for Cell Cycle and Immune Response in Human Cells
Abstract
1. Introduction
2. Results
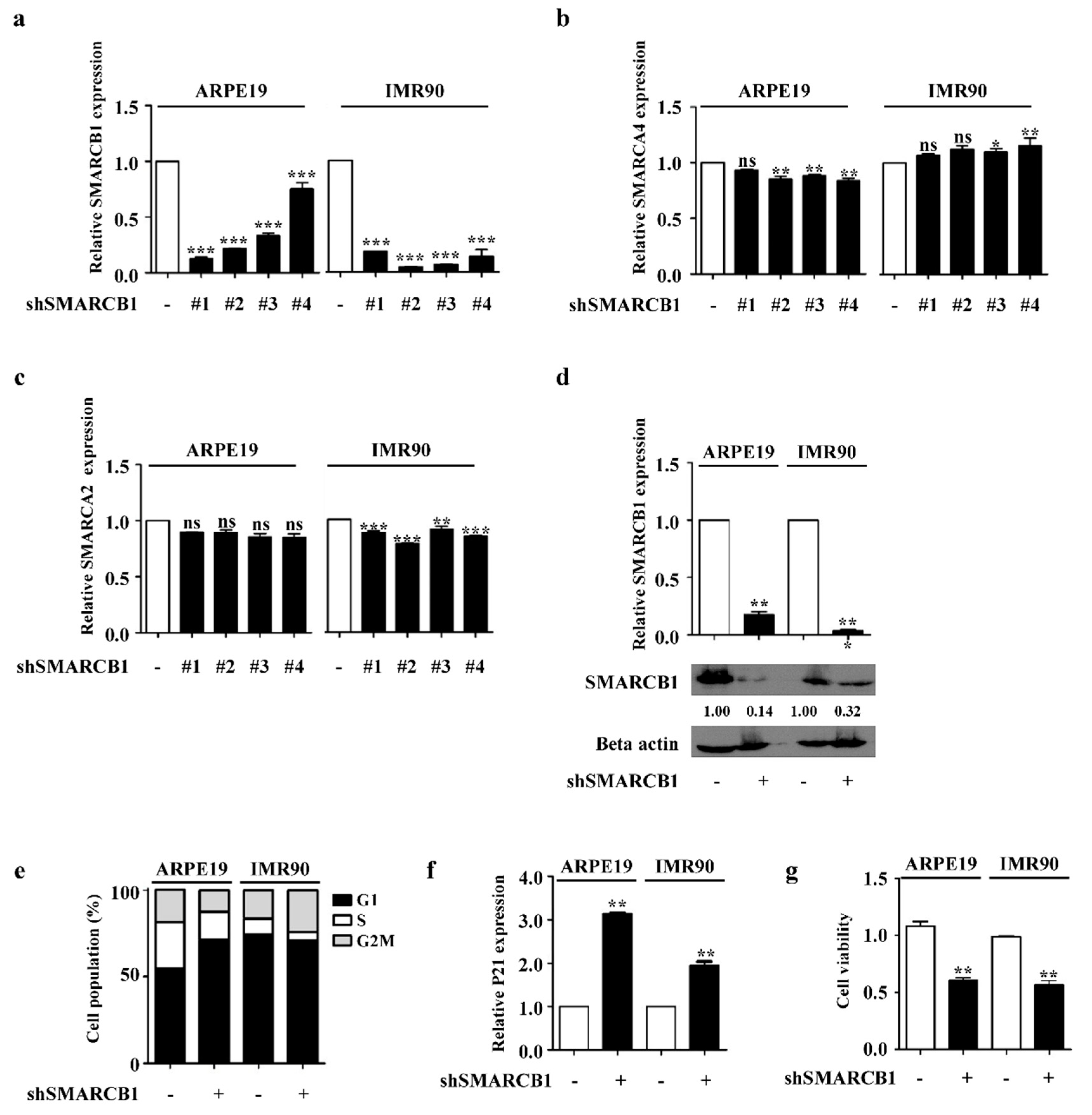
2.1. SMARCB1 Controls Cell Cycle
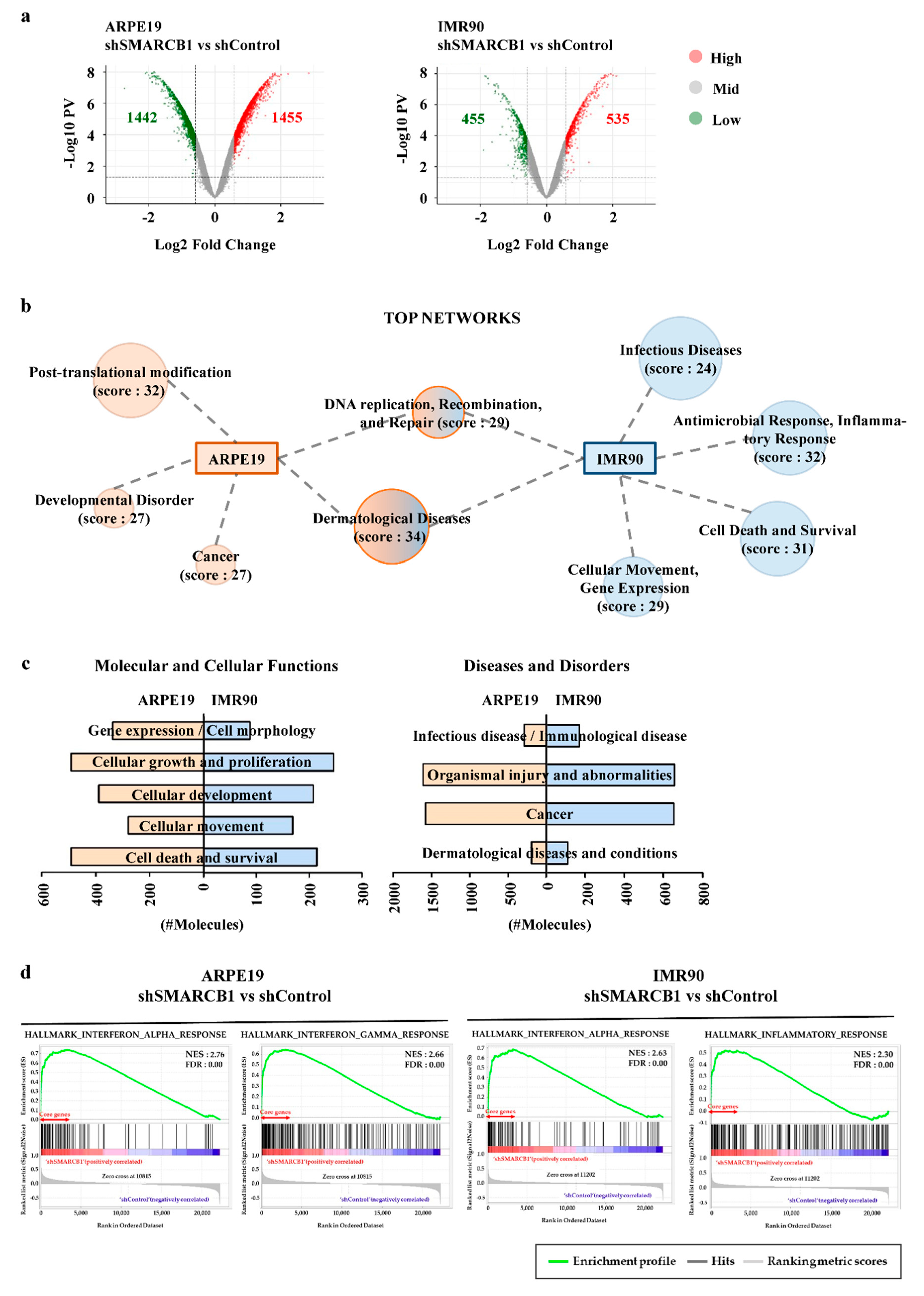
2.2. SMARCB1 Modulates the Transcriptome in Cellular Maintenance and Immune Response
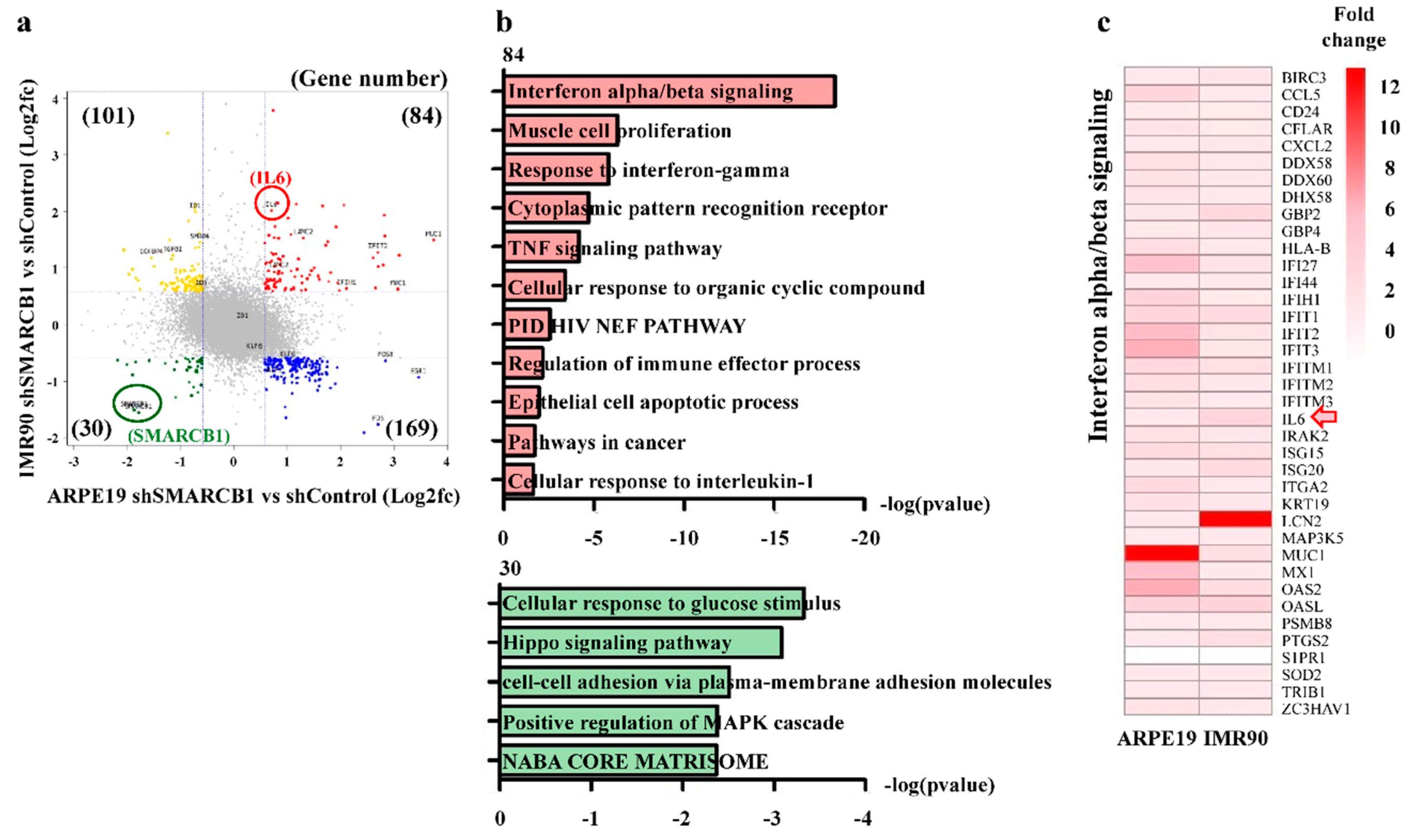
2.3. SMARCB1 Loss Activates the Immune Response Gene Set and Represses the Cell Maintenance Gene Set
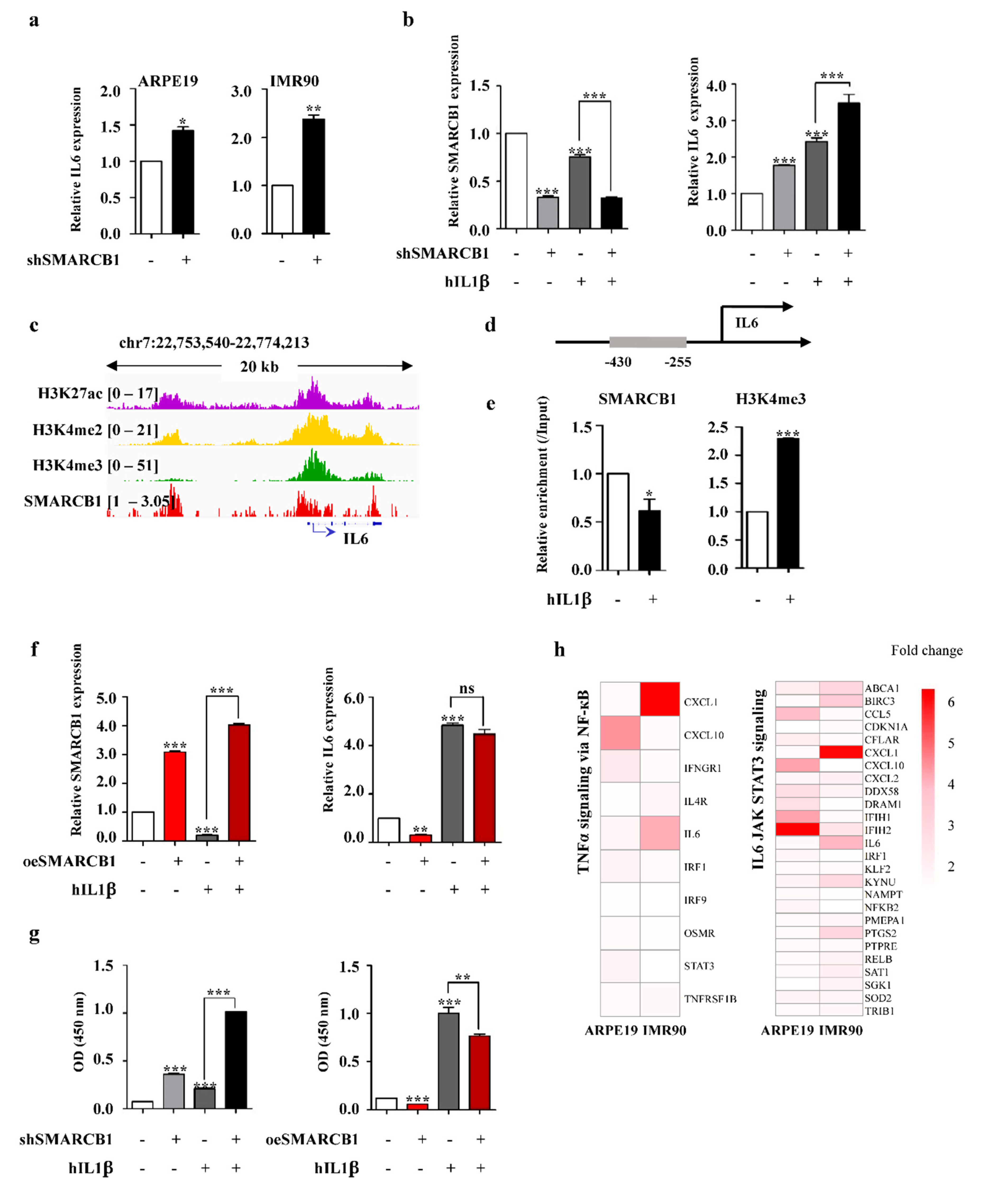
2.4. SMARCB1 Directly Regulates IL6 as a Transcriptional Repressor
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. shRNA Infection
4.3. RNA Extraction and Reverse Transcription PCR
4.4. Chromatin Immunoprecipitation Assay (ChIP)
4.5. ChIP Sequencing
4.6. Transcriptome Analysis
4.7. IPA (Ingenuity® Pathway Analysis)
4.8. GSEA (Gene Set Enrichment Analysis)
4.9. Gene Ontology Analysis
4.10. IL6 ELISA
4.11. Cell Cycle Analysis
4.12. Western Blot Analysis
4.13. MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide) Assay
4.14. Western Blot Quantitation
4.15. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| SMARC | SWI/SNF-related matrix-associated actin-dependent regulator of chromatin |
| SWI/SNF | switch/sucrose non-fermentable |
| IL | interleukin |
| ATP | adenosine triphosphate |
| BRM | Brahma homolog |
| BRG1 | brahma-related gene 1 |
| BAF | BRG1-associated factor |
| RB | retinoblastoma |
| E2F | E2 promoter binding factor |
| OCT4 | octamer-binding transcription factor 4 |
| RPE | retinal pigment epithelium |
| H-Tert | Human telomerase reverse transcriptase |
| PBRM1 | Protein polybromo 1 |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| Th2 | T-helper 2 |
| IFN | interferon |
| sh | short hairpin |
| RT | reverse transcription |
| PCR | polymerase chain reaction |
| IPA | ingenuity pathway analysis |
| GSEA | gene set enrichment analysis |
| TNF | Tumor necrosis factor |
| MAPK | mitogen activated protein kinase |
| DC | Dendritic cells |
| BMM | Bone marrow derived macrophages |
| hIL1β | human IL1β |
| NF-κB | nuclear factor-κB subunit |
| ARID5A | AT-rich interactive domain-containing protein 5A |
| TP53 | tumor protein p53 |
| ChIP | chromatin immunoprecipitation |
| ELISA | enzyme-linked immunosorbent assay |
| MRT | Malignant rhabdoid tumor |
References
- Tyagi, M.; Imam, N.; Verma, K.; Patel, A.K. Chromatin remodelers: We are the drivers!! Nucleus 2016, 7, 388–404. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Torres, K.; Liu, X.; Liu, C.G.; Pollock, R.E. An overview of chromatin-regulating proteins in cells. Curr. Protein Pept. Sci. 2016, 17, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, V.R.; Jarmasz, J.S.; Murugeshan, N.; Del Bigio, M.R.; Rastegar, M.; Davie, J.R. DNA modifications: Function and applications in normal and disease states. Biology 2014, 3, 670–723. [Google Scholar] [CrossRef] [PubMed]
- Vignali, M.; Hassan, A.H.; Neely, K.E.; Workman, J.L. Atp-dependent chromatin-remodeling complexes. Mol. Cell Biol. 2000, 20, 1899–1910. [Google Scholar] [CrossRef] [PubMed]
- Hota, S.K.; Bruneau, B.G. Atp-dependent chromatin remodeling during mammalian development. Development 2016, 143, 2882–2897. [Google Scholar] [CrossRef] [PubMed]
- Gangaraju, V.K.; Bartholomew, B. Mechanisms of atp dependent chromatin remodeling. Mutat. Res. 2007, 618, 3–17. [Google Scholar] [CrossRef]
- Alver, B.H.; Kim, K.H.; Lu, P.; Wang, X.; Manchester, H.E.; Wang, W.; Haswell, J.R.; Park, P.J.; Roberts, C.W. The swi/snf chromatin remodelling complex is required for maintenance of lineage specific enhancers. Nat. Commun. 2017, 8, 14648. [Google Scholar] [CrossRef]
- Flowers, S.; Nagl, N.G.; Beck, G.R.; Moran, E. Antagonistic roles for brm and brg1 swi/snf complexes in differentiation. J. Biol. Chem. 2009, 284, 10067–10075. [Google Scholar] [CrossRef]
- Raab, J.R.; Runge, J.S.; Spear, C.C.; Magnuson, T. Co-regulation of transcription by brg1 and brm, two mutually exclusive swi/snf atpase subunits. Epigenetics Chromatin 2017, 10, 62. [Google Scholar] [CrossRef]
- Ooi, L.; Belyaev, N.D.; Miyake, K.; Wood, I.C.; Buckley, N.J. Brg1 chromatin remodeling activity is required for efficient chromatin binding by repressor element 1-silencing transcription factor (rest) and facilitates rest-mediated repression. J. Biol. Chem. 2006, 281, 38974–38980. [Google Scholar] [CrossRef]
- Kalimuthu, S.N.; Chetty, R. Gene of the month: Smarcb1. J. Clin. Pathol. 2016, 69, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Kohashi, K.; Oda, Y. Oncogenic roles of smarcb1/ini1 and its deficient tumors. Cancer Sci. 2017, 108, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.W.; Biegel, J.A. The role of smarcb1/ini1 in development of rhabdoid tumor. Cancer Biol. Ther. 2009, 8, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Stockhammer, F.; Schmitz, U.; von Deimling, A. Mutational analysis of ini1 in sporadic human brain tumors. Acta Neuropathol. 2001, 101, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Hong, A.L.; Tseng, Y.Y.; Wala, J.A.; Kim, W.J.; Kynnap, B.D.; Doshi, M.B.; Kugener, G.; Sandoval, G.J.; Howard, T.P.; Li, J.; et al. Renal medullary carcinomas depend upon smarcb1 loss and are sensitive to proteasome inhibition. Elife 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Mora-Blanco, E.L.; Mishina, Y.; Tillman, E.J.; Cho, Y.J.; Thom, C.S.; Pomeroy, S.L.; Shao, W.; Roberts, C.W. Activation of beta-catenin/tcf targets following loss of the tumor suppressor snf5. Oncogene 2014, 33, 933–938. [Google Scholar] [CrossRef]
- Isakoff, M.S.; Sansam, C.G.; Tamayo, P.; Subramanian, A.; Evans, J.A.; Fillmore, C.M.; Wang, X.; Biegel, J.A.; Pomeroy, S.L.; Mesirov, J.P.; et al. Inactivation of the snf5 tumor suppressor stimulates cell cycle progression and cooperates with p53 loss in oncogenic transformation. Proc. Natl Acad. Sci. USA 2005, 102, 17745–17750. [Google Scholar] [CrossRef]
- Gadd, S.; Sredni, S.T.; Huang, C.C.; Perlman, E.J.; Renal Tumor Committee of the Children’s Oncology Group. Rhabdoid tumor: Gene expression clues to pathogenesis and potential therapeutic targets. Lab. Investig. 2010, 90, 724–738. [Google Scholar] [CrossRef]
- You, J.S.; De Carvalho, D.D.; Dai, C.; Liu, M.; Pandiyan, K.; Zhou, X.J.; Liang, G.; Jones, P.A. Snf5 is an essential executor of epigenetic regulation during differentiation. PLoS Genet. 2013, 9, e1003459. [Google Scholar] [CrossRef]
- Yeager, T.R.; Reddel, R.R. Constructing immortalized human cell lines. Curr. Opin. Biotechnol. 1999, 10, 465–469. [Google Scholar] [CrossRef]
- Shay, J.W.; Wright, W.E. Senescence and immortalization: Role of telomeres and telomerase. Carcinogenesis 2005, 26, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Del Priore, L.V. Gene expression profile of cultured adult compared to immortalized human rpe. Mol. Vis. 2006, 12, 1–14. [Google Scholar] [PubMed]
- Dunn, K.C.; Aotaki-Keen, A.E.; Putkey, F.R.; Hjelmeland, L.M. Arpe-19, a human retinal pigment epithelial cell line with differentiated properties. Exp. Eye Res. 1996, 62, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Ehler, E.; Babiychuk, E.; Draeger, A. Human foetal lung (imr-90) cells: Myofibroblasts with smooth muscle-like contractile properties. Cell Motil. Cytoskelet. 1996, 34, 288–298. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- A current view on inflammation. Nat. Immunol. 2017, 18, 825. [CrossRef]
- O′Shea, J.J.; Murray, P.J. Cytokine signaling modules in inflammatory responses. Immunity 2008, 28, 477–487. [Google Scholar] [CrossRef]
- Wan, M.; Zhang, J.; Lai, D.; Jani, A.; Prestone-Hurlburt, P.; Zhao, L.; Ramachandran, A.; Schnitzler, G.R.; Chi, T. Molecular basis of cd4 repression by the swi/snf-like baf chromatin remodeling complex. Eur. J. Immunol. 2009, 39, 580–588. [Google Scholar] [CrossRef]
- Wurster, A.L.; Precht, P.; Becker, K.G.; Wood, W.H., 3rd; Zhang, Y.; Wang, Z.; Pazin, M.J. Il-10 transcription is negatively regulated by baf180, a component of the swi/snf chromatin remodeling enzyme. BMC Immunol. 2012, 13, 9. [Google Scholar] [CrossRef]
- Morozov, A.; Lee, S.J.; Zhang, Z.K.; Cimica, V.; Zagzag, D.; Kalpana, G.V. Ini1 induces interferon signaling and spindle checkpoint in rhabdoid tumors. Clin. Cancer Res. 2007, 13, 4721–4730. [Google Scholar] [CrossRef]
- Liu, H.; Kang, H.; Liu, R.; Chen, X.; Zhao, K. Maximal induction of a subset of interferon target genes requires the chromatin-remodeling activity of the baf complex. Mol. Cell Biol. 2002, 22, 6471–6479. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.; Tailor, P.; Liu, H.; Chen, X.; Ozato, K.; Zhao, K. The chromatin-remodeling baf complex mediates cellular antiviral activities by promoter priming. Mol. Cell Biol. 2004, 24, 4476–4486. [Google Scholar] [CrossRef] [PubMed]
- Chi, T.H.; Wan, M.; Lee, P.P.; Akashi, K.; Metzger, D.; Chambon, P.; Wilson, C.B.; Crabtree, G.R. Sequential roles of brg, the atpase subunit of baf chromatin remodeling complexes, in thymocyte development. Immunity 2003, 19, 169–182. [Google Scholar] [CrossRef]
- Gresh, L.; Bourachot, B.; Reimann, A.; Guigas, B.; Fiette, L.; Garbay, S.; Muchardt, C.; Hue, L.; Pontoglio, M.; Yaniv, M.; et al. The swi/snf chromatin-remodeling complex subunit snf5 is essential for hepatocyte differentiation. EMBO J. 2005, 24, 3313–3324. [Google Scholar] [CrossRef]
- Wilson, B.G.; Roberts, C.W. Swi/snf nucleosome remodellers and cancer. Nat. Rev. Cancer 2011, 11, 481–492. [Google Scholar] [CrossRef]
- Zhu, J.; Thompson, C.B. Metabolic regulation of cell growth and proliferation. Nat. Rev. Mol. Cell Biol. 2019, 20, 436–450. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.T. Mapk signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef]
- Su, H.; Lei, C.T.; Zhang, C. Interleukin-6 signaling pathway and its role in kidney disease: An update. Front. Immunol. 2017, 8, 405. [Google Scholar] [CrossRef]
- Confalone, E.; D’Alessio, G.; Furia, A. Il-6 induction by tnfalpha and il-1beta in an osteoblast-like cell line. Int. J. Biomed. Sci. 2010, 6, 135–140. [Google Scholar]
- Stavreva, D.A.; Hager, G.L. Chromatin structure and gene regulation: A dynamic view of enhancer function. Nucleus 2015, 6, 442–448. [Google Scholar] [CrossRef]
- Grossi, E.; Raimondi, I.; Goni, E.; Gonzalez, J.; Marchese, F.P.; Chapaprieta, V.; Martin-Subero, J.I.; Guo, S.; Huarte, M. A lncrna-swi/snf complex crosstalk controls transcriptional activation at specific promoter regions. Nat. Commun 2020, 11, 936. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. Il-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.; Rose-John, S.; Garbers, C. Interleukin-6 and its receptors: A highly regulated and dynamic system. Cytokine 2014, 70, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Choy, E.H.S.; Calabrese, L.H. Neuroendocrine and neurophysiological effects of interleukin 6 in rheumatoid arthritis. Rheumatology (Oxford) 2018, 57, 1885–1895. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhou, S.; Wang, C.; Huang, Y.; Li, H.; Wang, Y.; Zhu, Z.; Tang, J.; Yan, M. Epigenetic modifications of interleukin-6 in synovial fibroblasts from osteoarthritis patients. Sci. Rep. 2017, 7, 43592. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Yu, Y.; Huang, H.; Fan, H.; Hu, L.; Yin, C.; Li, K.; Fulton, D.J.; Chen, F. Epigenetic regulation of interleukin 6 by histone acetylation in macrophages and its role in paraquat-induced pulmonary fibrosis. Front. Immunol. 2016, 7, 696. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, Y.; Charboneau, A.; Knudsen, E.S.; Weissman, B.E. Reexpression of hsnf5 in malignant rhabdoid tumor cell lines causes cell cycle arrest through a p21(cip1/waf1)-dependent mechanism. Cancer Res. 2010, 70, 1854–1865. [Google Scholar] [CrossRef]
- Gunther, J.; Seyfert, H.M. The first line of defence: Insights into mechanisms and relevance of phagocytosis in epithelial cells. Semin. Immunopathol. 2018, 40, 555–565. [Google Scholar] [CrossRef]
- Hammer, A.M.; Morris, N.L.; Earley, Z.M.; Choudhry, M.A. The first line of defense: The effects of alcohol on post-burn intestinal barrier, immune cells, and microbiome. Alcohol. Res. 2015, 37, 209–222. [Google Scholar]
- Riera Romo, M.; Perez-Martinez, D.; Castillo Ferrer, C. Innate immunity in vertebrates: An overview. Immunology 2016, 148, 125–139. [Google Scholar] [CrossRef]
- Holtkamp, G.M.; Kijlstra, A.; Peek, R.; de Vos, A.F. Retinal pigment epithelium-immune system interactions: Cytokine production and cytokine-induced changes. Prog. Retin. Eye Res. 2001, 20, 29–48. [Google Scholar] [CrossRef]
- Jordana, M.; Sarnstrand, B.; Sime, P.J.; Ramis, I. Immune-inflammatory functions of fibroblasts. Eur. Respir J. 1994, 7, 2212–2222. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef]
- Pandya, P.H.; Murray, M.E.; Pollok, K.E.; Renbarger, J.L. The immune system in cancer pathogenesis: Potential therapeutic approaches. J. Immunol. Res. 2016, 2016, 4273943. [Google Scholar] [CrossRef] [PubMed]
- Pandolfi, F.; Cianci, R.; Pagliari, D.; Casciano, F.; Bagala, C.; Astone, A.; Landolfi, R.; Barone, C. The immune response to tumors as a tool toward immunotherapy. Clin. Dev. Immunol. 2011, 2011, 894704. [Google Scholar] [CrossRef] [PubMed]
- Pulice, J.L.; Kadoch, C. Composition and function of mammalian swi/snf chromatin remodeling complexes in human disease. Cold Spring Harb. Symp. Quant. Biol. 2016, 81, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Kadoch, C.; Hargreaves, D.C.; Hodges, C.; Elias, L.; Ho, L.; Ranish, J.; Crabtree, G.R. Proteomic and bioinformatic analysis of mammalian swi/snf complexes identifies extensive roles in human malignancy. Nat. Genet. 2013, 45, 592–601. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.K.; Kim, M.J.; You, J.S. SMARCB1 Acts as a Quiescent Gatekeeper for Cell Cycle and Immune Response in Human Cells. Int. J. Mol. Sci. 2020, 21, 3969. https://doi.org/10.3390/ijms21113969
Choi SK, Kim MJ, You JS. SMARCB1 Acts as a Quiescent Gatekeeper for Cell Cycle and Immune Response in Human Cells. International Journal of Molecular Sciences. 2020; 21(11):3969. https://doi.org/10.3390/ijms21113969
Chicago/Turabian StyleChoi, Sung Kyung, Myoung Jun Kim, and Jueng Soo You. 2020. "SMARCB1 Acts as a Quiescent Gatekeeper for Cell Cycle and Immune Response in Human Cells" International Journal of Molecular Sciences 21, no. 11: 3969. https://doi.org/10.3390/ijms21113969
APA StyleChoi, S. K., Kim, M. J., & You, J. S. (2020). SMARCB1 Acts as a Quiescent Gatekeeper for Cell Cycle and Immune Response in Human Cells. International Journal of Molecular Sciences, 21(11), 3969. https://doi.org/10.3390/ijms21113969



