Reduced Fecal Calprotectin and Inflammation in a Murine Model of Atopic Dermatitis Following Probiotic Treatment
Abstract
1. Introduction
2. Results
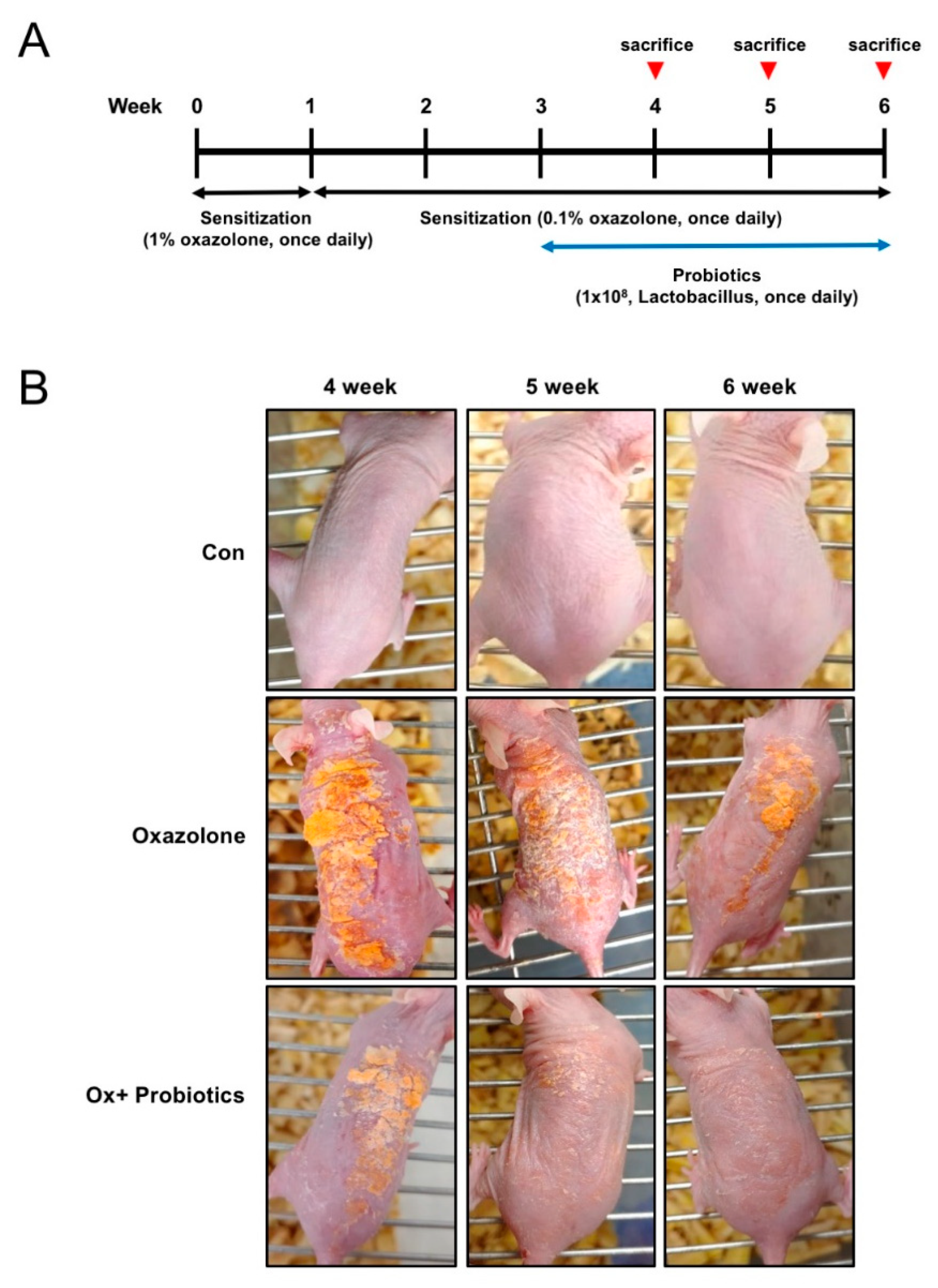
2.1. Probiotics Can Improve AD-Like Skin Lesions in Oxazolone (Ox)-Induced AD
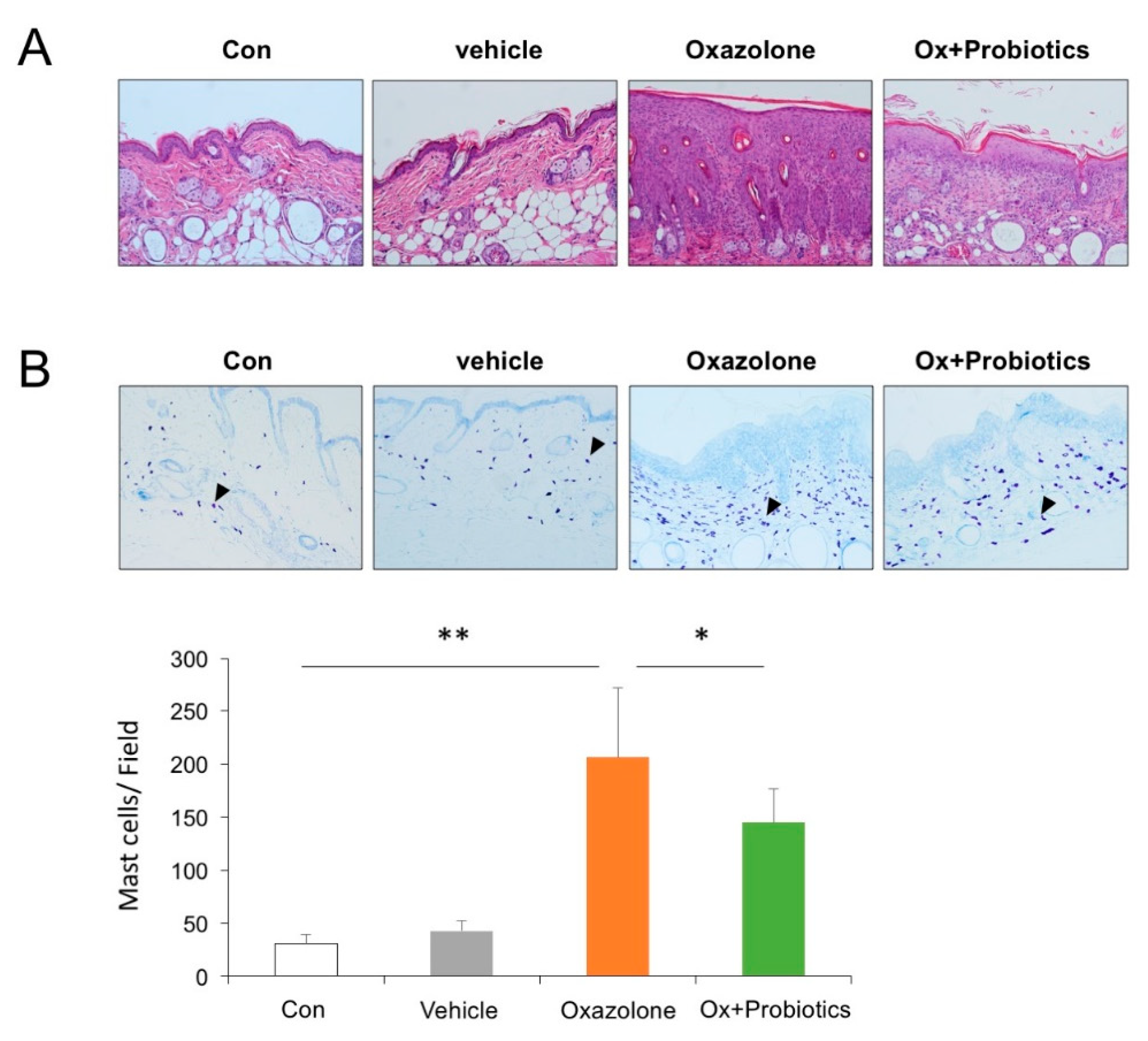
2.2. Probiotics Can Attenuate Epidermal Skin Lesions in Ox-Induced AD Mice
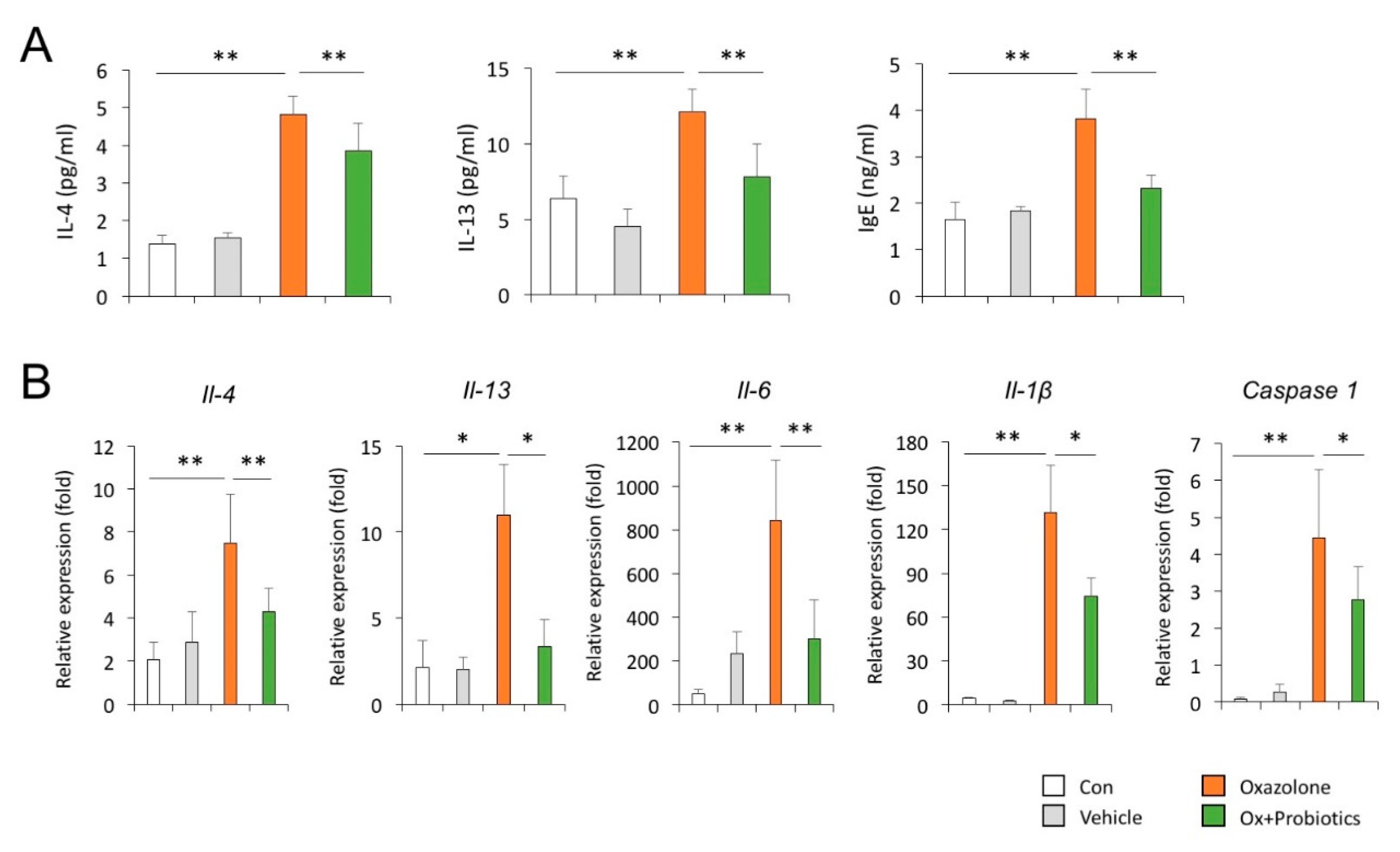
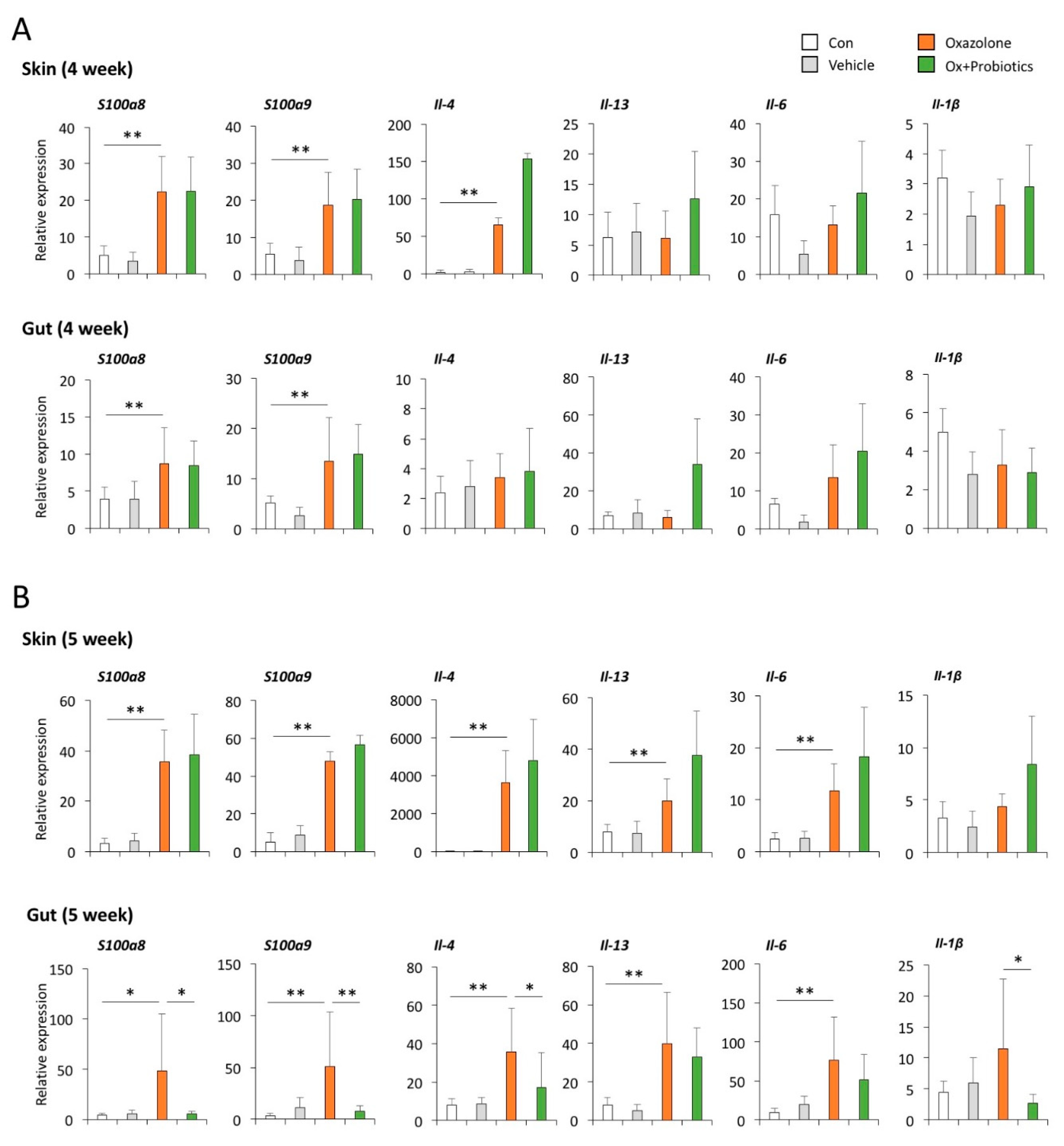
2.3. Probiotics Can Reduce Th2-Associated Cytokines and Pro-Inflammatory Cytokines
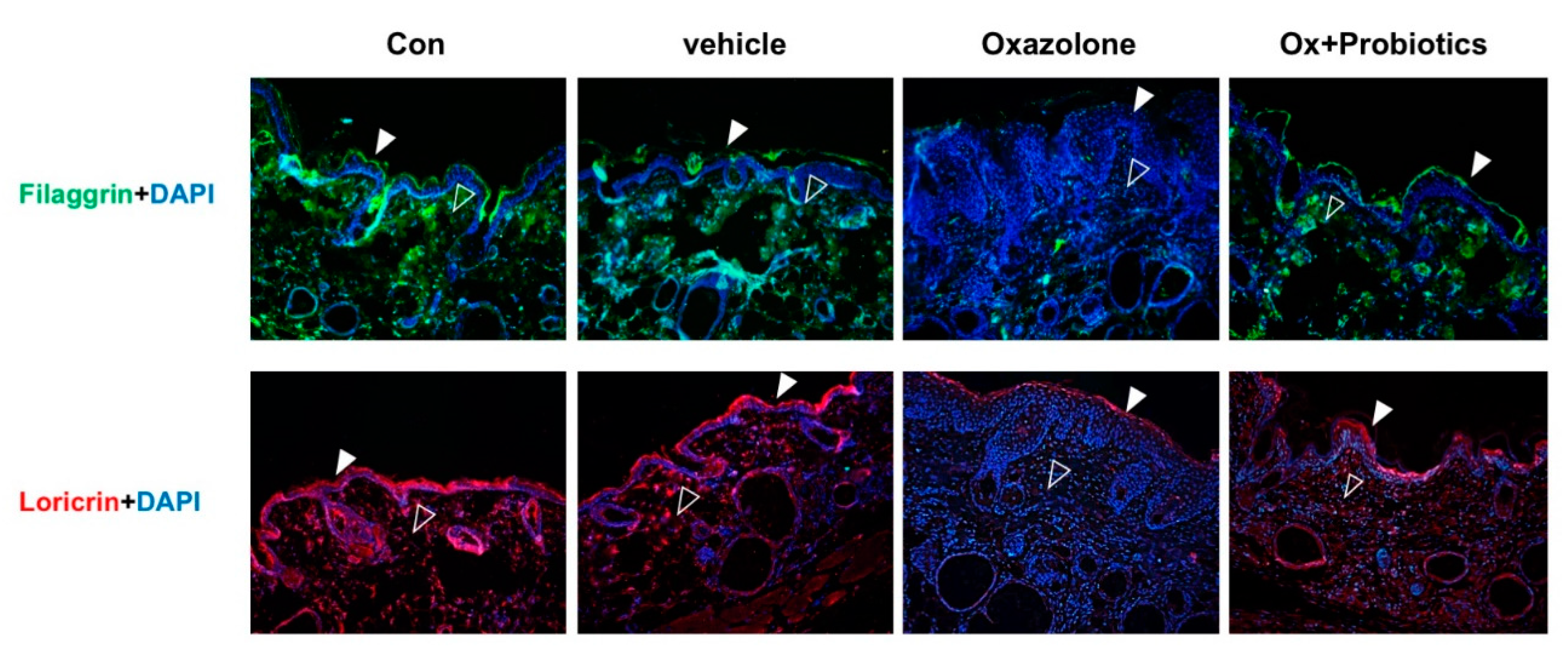
2.4. Probiotics Can Effectively Restore Impaired Skin Barrier Formation
2.5. Probiotics Can Decrease the Level of Calprotectin Increased in the Feces of AD Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. ELISA
4.3. Immunohistochemistry
4.4. Immunofluorescence Staining
4.5. Quantitative Real-Time PCR (qPCR)
4.6. Western Blotting
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hahm, M.I.; Kim, J.; Kwon, H.J.; Chae, Y.; Ahn, K.; Lee, H.Y. Exposure to mould allergens and rhinoconjunctivitis in Korean children. Pediatr. Allergy Immunol. 2016, 27, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Oh, I.H.; Choi, S.H.; Rha, Y.H. Analysis of Epidemiology and Risk Factors of Atopic Dermatitis in Korean Children and Adolescents from the 2010 Korean National Health and Nutrition Examination Survey. Biomed. Res. Int. 2017, 2017, 5142754. [Google Scholar] [CrossRef] [PubMed]
- Nutten, S. Atopic dermatitis: Global epidemiology and risk factors. Ann. Nutr. Metab. 2015, 66, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, A.; Nomura, T.; Rerknimitr, P.; Seidel, J.A.; Honda, T.; Kabashima, K. The interplay between genetic and environmental factors in the pathogenesis of atopic dermatitis. Immunol. Rev. 2017, 278, 246–262. [Google Scholar] [CrossRef]
- Salem, I.; Ramser, A.; Isham, N.; Ghannoum, M.A. The Gut Microbiome as a Major Regulator of the Gut-Skin Axis. Front. Microbiol. 2018, 9, 1459. [Google Scholar] [CrossRef]
- Makrgeorgou, A.; Leonardi-Bee, J.; Bath-Hextall, F.J.; Murrell, D.F.; Tang, M.L.; Roberts, A.; Boyle, R.J. Probiotics for treating eczema. Cochrane Database Syst. Rev. 2018, 11, CD006135. [Google Scholar] [CrossRef]
- Rodrigo, L. Fecal calprotectin. Rev. Esp. Enferm. Dig. 2007, 99, 683–688. [Google Scholar] [CrossRef]
- Kosiewicz, M.M.; Dryden, G.W.; Chhabra, A.; Alard, P. Relationship between gut microbiota and development of T cell associated disease. FEBS Lett. 2014, 588, 4195–4206. [Google Scholar] [CrossRef]
- Maslowski, K.; Vieira, A.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef]
- Song, H.; Yoo, Y.; Hwang, J.; Na, Y.C.; Kim, H.S. Faecalibacterium prausnitzii subspecies-level dysbiosis in the human gut microbiome underlying atopic dermatitis. J. Allergy Clin. Immunol. 2016, 137, 852–860. [Google Scholar] [CrossRef]
- Wang, S.; Song, R.; Wang, Z.; Jing, Z.; Ma, J. S100A8/A9 in Inflammation. Front. Immunol. 2018, 9, 1298. [Google Scholar] [CrossRef] [PubMed]
- Beser, O.F.; Sancak, S.; Erkan, T.; Kutlu, T.; Cokugras, H.; Cokugras, F.C. Can Fecal Calprotectin Level Be Used as a Markers of Inflammation in the Diagnosis and Follow-Up of Cow’s Milk Protein Allergy? Allergy Asthma Immunol. Res. 2014, 6, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Orivuori, L.; Mustonen, K.; De Goffau, M.C.; Hakala, S.; Paasela, M.; Roduit, C.; Genuneit, J.; Lauener, R.; Riedler, J.; Weber, J.; et al. High level of fecal calprotectin at age 2 months as a marker of intestinal inflammation predicts atopic dermatitis and asthma by age 6. Clin. Exp. Allergy 2015, 45, 928–939. [Google Scholar] [CrossRef] [PubMed]
- Levkovich, T.; Poutahidis, T.; Smillie, C.; Varian, B.J.; Ibrahim, Y.M.; Lakritz, J.R.; Alm, E.J.; Erdman, S.E. Probiotic bacteria induce a ‘glow of health’. PLoS ONE 2013, 8, e53867. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, C.A.; Monteleone, G.; McLaughlin, J.T.; Paus, R. The gut-skin axis in health and disease: A paradigm with therapeutic implications. Bioessays 2016, 38, 1167–1176. [Google Scholar] [CrossRef]
- Kim, Y.G.; Udayanga, K.G.; Totsuka, N.; Weinberg, J.B.; Nunez, G.; Shibuya, A. Gut dysbiosis promotes M2 macrophage polarization and allergic airway inflammation via fungi-induced PGE(2). Cell Host Microbe 2014, 15, 95–102. [Google Scholar] [CrossRef]
- Purchiaroni, F.; Tortora, A.; Gabrielli, M.; Bertucci, F.; Gigante, G.; Ianiro, G.; Ojetti, V.; Scarpellini, E.; Gasbarrini, A. The role of intestinal microbiota and the immune system. Eur. Rev. Med. Pharm. Sci. 2013, 17, 323–333. [Google Scholar]
- Seite, S.; Bieber, T. Barrier function and microbiotic dysbiosis in atopic dermatitis. Clin. Cosmet. Investig. Dermatol. 2015, 8, 479–483. [Google Scholar] [CrossRef]
- Johnson, C.C.; Ownby, D.R. The infant gut bacterial microbiota and risk of pediatric asthma and allergic diseases. Transl. Res. 2017, 179, 60–70. [Google Scholar] [CrossRef]
- Huang, R.; Ning, H.; Shen, M.; Li, J.; Zhang, J.; Chen, X. Probiotics for the Treatment of Atopic Dermatitis in Children: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Cell Infect. Microbiol. 2017, 7, 392. [Google Scholar] [CrossRef]
- Wang, I.J.; Wang, J.Y. Children with atopic dermatitis show clinical improvement after Lactobacillus exposure. Clin. Exp. Allergy 2015, 45, 779–787. [Google Scholar] [CrossRef]
- Gruber, C.; Wendt, M.; Sulser, C.; Lau, S.; Kulig, M.; Wahn, U.; Werfel, T.; Niggemann, B. Randomized, placebo-controlled trial of Lactobacillus rhamnosus GG as treatment of atopic dermatitis in infancy. Allergy 2007, 62, 1270–1276. [Google Scholar] [CrossRef]
- Folster-Holst, R.; Müller, F.; Schnopp, N.; Abeck, D.; Kreiselmaier, I.; Lenz, T.; Von Rüden, U.; Schrezenmeir, J.; Christophers, E.; Weichenthal, M. Prospective, randomized controlled trial on Lactobacillus rhamnosus in infants with moderate to severe atopic dermatitis. Br. J. Dermatol. 2006, 155, 1256–12561. [Google Scholar] [CrossRef]
- Tibble, J.A.; Sigthorsson, G.; Foster, R.; Forgacs, I.; Bjarnason, I. Use of surrogate markers of inflammation and Rome criteria to distinguish organic from nonorganic intestinal disease. Gastroenterology 2002, 123, 450–460. [Google Scholar] [CrossRef]
- Takashima, S.; Kato, J.; Hiraoka, S.; Nakarai, A.; Takei, D.; Inokuchi, T.; Sugihara, Y.; Takahara, M.; Harada, K.; Okada, H.; et al. Evaluation of Mucosal Healing in Ulcerative Colitis by Fecal Calprotectin Vs. Fecal Immunochemical Test. Am. J. Gastroenterol. 2015, 110, 873–880. [Google Scholar] [CrossRef]
- Seo, S.C.; Ahn, S.H.; Ri, S.; Yoon, Y.; Byeon, J.H.; Kim, S.H.; Yoon, W.; Yoo, Y. Elevated fecal calprotectin levels are associated with severity of atopic dermatitis in children. Asian Pac. J. Allergy Immunol. 2018, 36, 82–87. [Google Scholar]
- Zhu, Q.; Li, F.; Wang, J.; Ma, J.; Sheng, X. Upregulation of calprotectin in mild IgE-mediated ovalbumin hypersensitivity. Oncotarget 2017, 8, 37342–37354. [Google Scholar] [CrossRef][Green Version]
- Conte, M.P.; Schippa, S.; Zamboni, I.; Penta, M.; Chiarini, F.; Seganti, L.; Osborn, J.; Falconieri, P.; Borrelli, O.; Cucchiara, S. Gut-associated bacterial microbiota in paediatric patients with inflammatory bowel disease. Gut 2006, 55, 1760–1767. [Google Scholar] [CrossRef]
- Mylonaki, M.; Rayment, N.B.; Rampton, D.S.; Hudspith, B.N.; Brostoff, J. Molecular characterization of rectal mucosa-associated bacterial flora in inflammatory bowel disease. Inflamm. Bowel Dis. 2005, 11, 481–487. [Google Scholar] [CrossRef]
- Ott, S.J.; Musfeldt, M.; Wenderoth, D.F.; Hampe, J.; Brant, O.; Folsch, U.R.; Timmis, K.N.; Schreiber, S. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut 2004, 53, 685–693. [Google Scholar] [CrossRef]
- Savino, F.; Cordisco, L.; Tarasco, V.; Calabrese, R.; Palumeri, E.; Matteuzzi, D. Molecular identification of coliform bacteria from colicky breastfed infants. Acta Paediatr. 2009, 98, 1582–1588. [Google Scholar] [CrossRef]
- Savino, F.; Cresi, F.; Pautasso, S.; Palumeri, E.; Tullio, V.; Roana, J.; Silvestro, L.; Oggero, R. Intestinal microflora in breastfed colicky and non-colicky infants. Acta Paediatr. 2004, 93, 825–829. [Google Scholar] [CrossRef]
- Kim, J.; Kim, W.J.; Ha, K.S.; Han, E.T.; Park, W.S.; Yang, S.R.; Hong, S.H. Perivascular Stem Cells Suppress Inflammasome Activation during Inflammatory Responses in Macrophages. Int. J. Stem. Cells 2019, 12, 419–429. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, J.Y.; Ha, K.S.; Han, E.T.; Park, W.S.; Min, C.K.; Hong, S.H. Perivascular Cells and NADPH Oxidase Inhibition Partially Restore Hyperglycemia-Induced Alterations in Hematopoietic Stem Cell and Myeloid-Derived Suppressor Cell Populations in the Bone Marrow. Int. J. Stem. Cells 2018, 12, 63–72. [Google Scholar] [CrossRef]

| Genes | Sequences 5′ to 3′ | Product Size (bp) | |
|---|---|---|---|
| ll-6 | F | AGGATACCACTCCCAACAGACCT | 141 |
| R | CAAGTGCATCATCGTTGTTCATAC | ||
| ll-4 | F | AGATGGATGTGCCAAACGTCCTCA | 107 |
| R | AATATGCGAAGCACCTTGGAAGCC | ||
| ll-13 | F | TGAGGAGCTGAGCAACATCACACA | 176 |
| R | TGCGGTTACAGAGGCCATGCAAT | ||
| ll-10 | F | GCCAAGCCTTATCGGAAATG | 102 |
| R | CACCCAGGGAATTCAAATGC | ||
| ll-1α | F | CCGACCTCATTTTCTTCTGG | 104 |
| R | GTGCACCCGACTTTGTTCTT | ||
| ll-1β | F | CCCAAGCAATACCCAAAGAA | 133 |
| R | GCTTGTGCTCTGCTTGTGAG | ||
| Caspase 1 | F | AGATGGCACATTTCCAGGAC | 221 |
| R | GATCCTCCAGCAGCAACTTC | ||
| S100a8 | F | GCCCTCTACAAGAATGACTTCAAG | 151 |
| R | ATC ACC ATC GCA AGG AAC TCC | ||
| S100a9 | F | TGGTGGAAGCACAGTTGGCAAC | 165 |
| R | CAGCATCATACACTCCTCAAAGC | ||
| Gapdh | F | GTTGTCTCCTGCGACTTCA | 184 |
| R | GGTGGTCC GGGTTTCTTA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-J.; Kim, J.-Y.; Kang, M.; Won, M.-H.; Hong, S.-H.; Her, Y. Reduced Fecal Calprotectin and Inflammation in a Murine Model of Atopic Dermatitis Following Probiotic Treatment. Int. J. Mol. Sci. 2020, 21, 3968. https://doi.org/10.3390/ijms21113968
Kim M-J, Kim J-Y, Kang M, Won M-H, Hong S-H, Her Y. Reduced Fecal Calprotectin and Inflammation in a Murine Model of Atopic Dermatitis Following Probiotic Treatment. International Journal of Molecular Sciences. 2020; 21(11):3968. https://doi.org/10.3390/ijms21113968
Chicago/Turabian StyleKim, Myoung-Ju, Ji-Young Kim, Minje Kang, Moo-Ho Won, Seok-Ho Hong, and Young Her. 2020. "Reduced Fecal Calprotectin and Inflammation in a Murine Model of Atopic Dermatitis Following Probiotic Treatment" International Journal of Molecular Sciences 21, no. 11: 3968. https://doi.org/10.3390/ijms21113968
APA StyleKim, M.-J., Kim, J.-Y., Kang, M., Won, M.-H., Hong, S.-H., & Her, Y. (2020). Reduced Fecal Calprotectin and Inflammation in a Murine Model of Atopic Dermatitis Following Probiotic Treatment. International Journal of Molecular Sciences, 21(11), 3968. https://doi.org/10.3390/ijms21113968






