Silicon Regulates Source to Sink Metabolic Homeostasis and Promotes Growth of Rice Plants under Sulfur Deficiency
Abstract
1. Introduction
2. Results
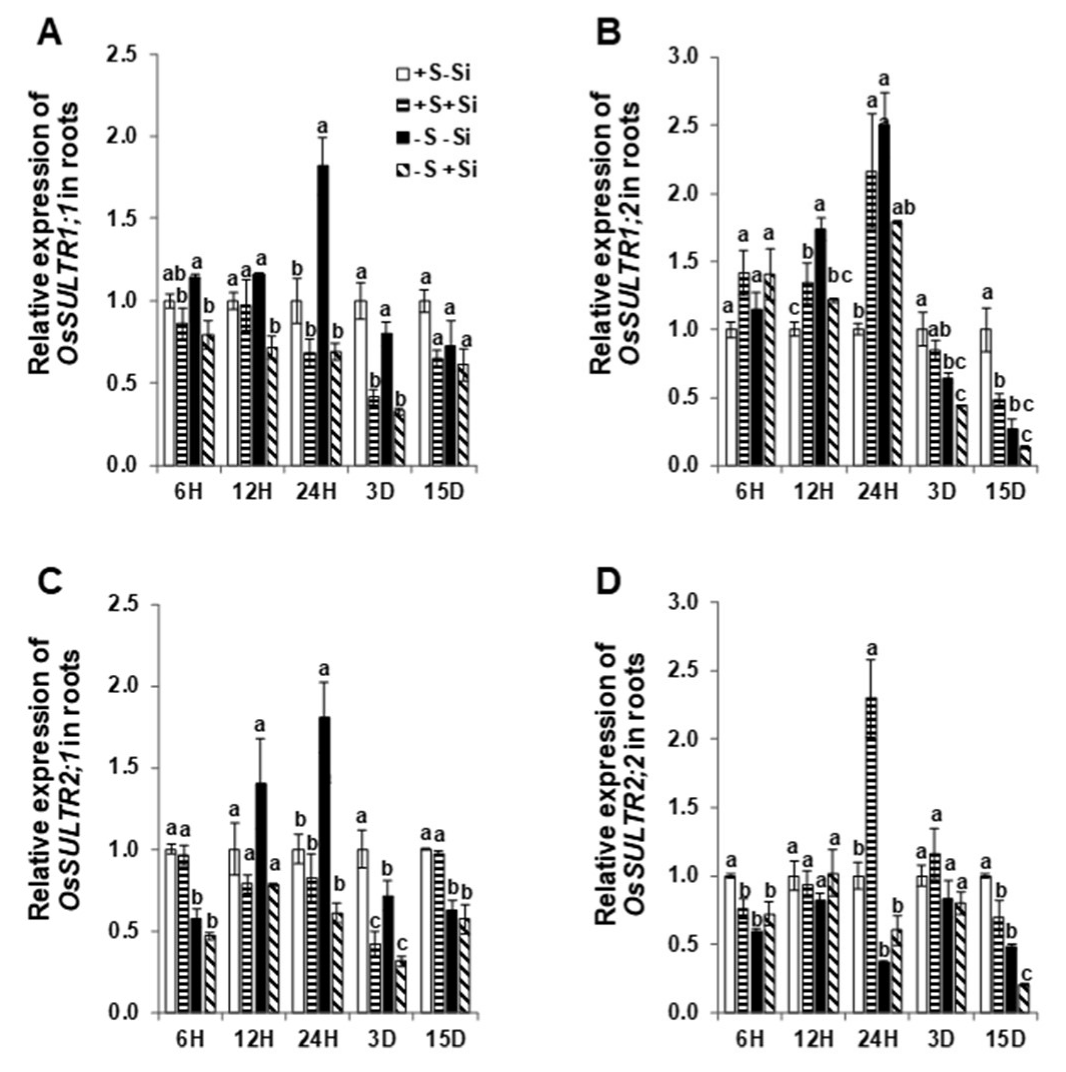
2.1. Silicon Transcriptionally Changed the Expression of S and Si Transporters under Sulfur Deficiency
2.2. Si Changed the Nutritional Homeostasis in Root and Shoot under Control and S Deficiency
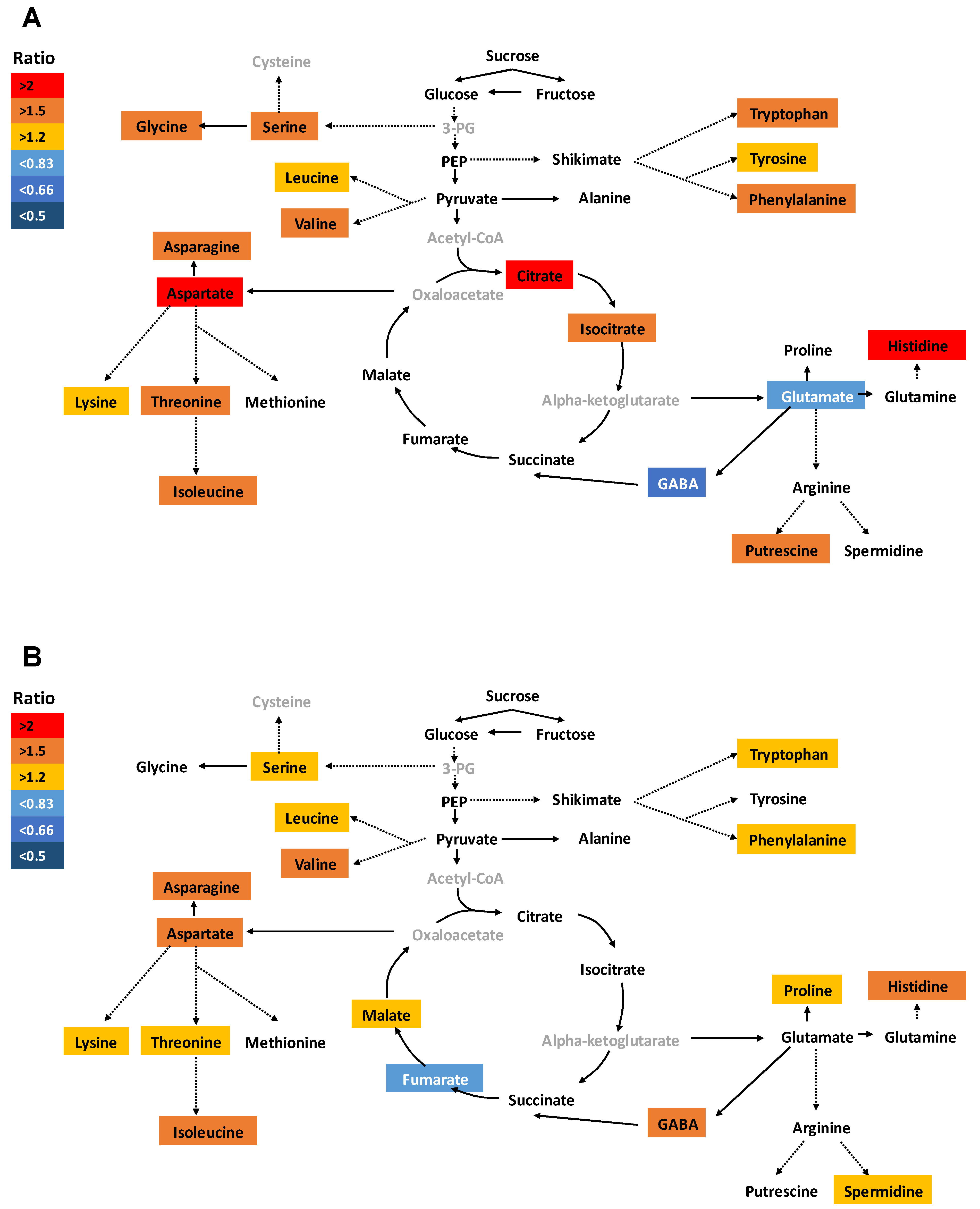
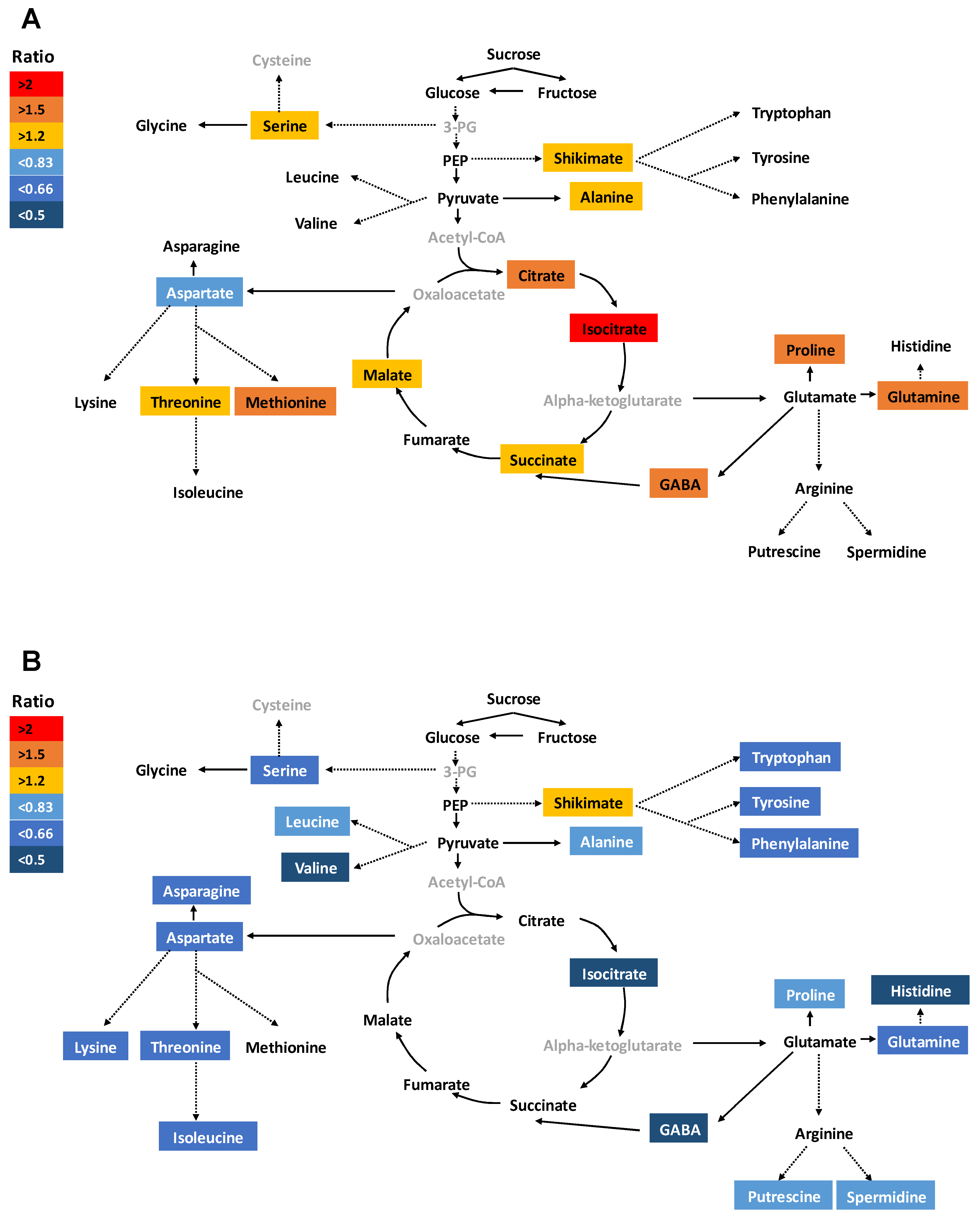
2.3. Application of Si Differentially Regulated Primary Metabolism in Root and Shoot under S Deficiency
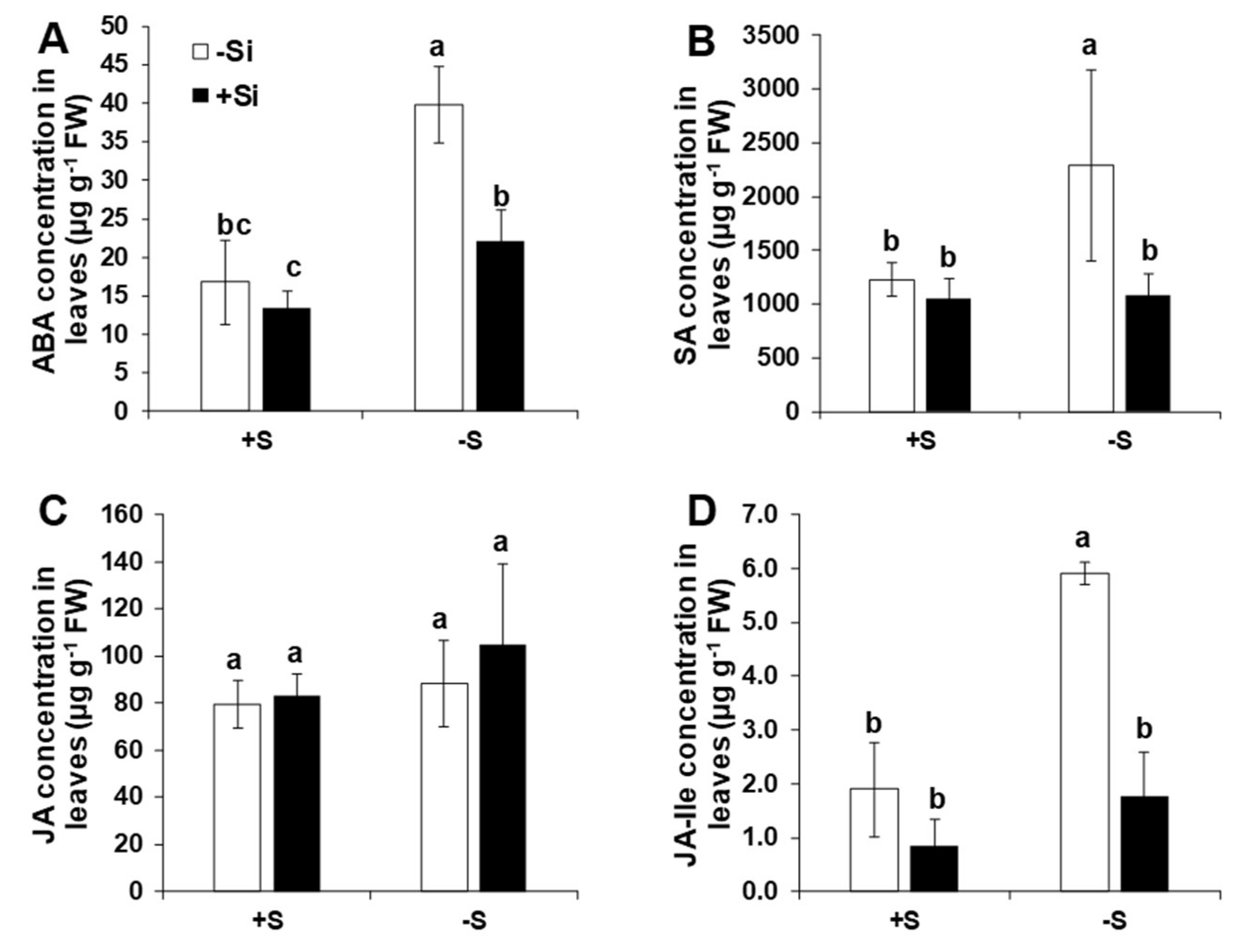
2.4. Application of Si Modulated Hormonal Responses in Shoots under S Deficiency
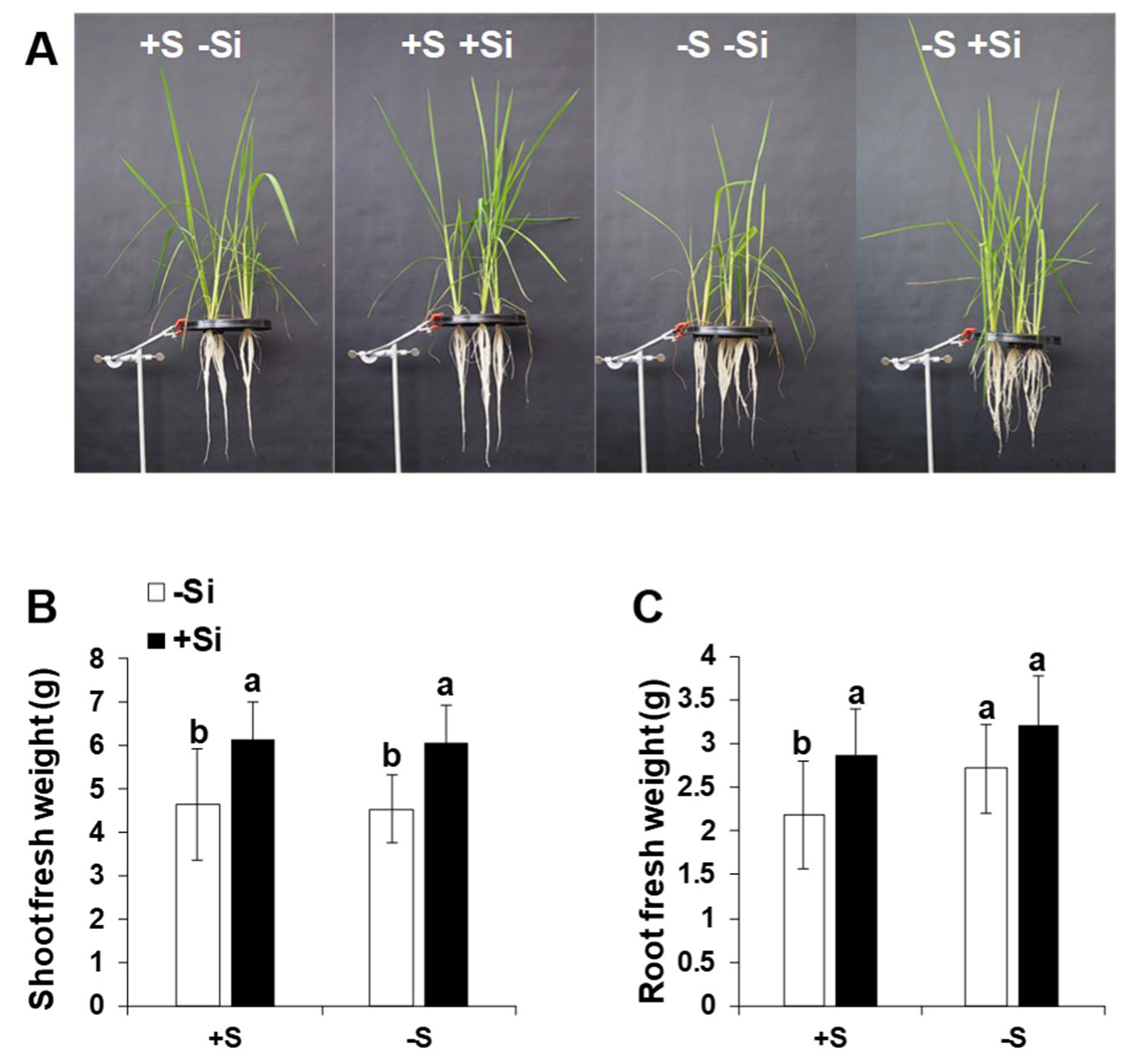
2.5. Application of Si Improved Plant Growth and Development under Control Condition and S Deficiency
3. Discussion
3.1. Si Supply Differentially Changes the Expression of S and Si Transporters in Response to Short and Long-Term S Deficiencies
3.2. Si Regulates Source to Sink Metabolic Homeostasis and Maintains the Growth of Rice under S Deficiency
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. RNA Extraction and Gene Expression Analysis
4.3. Determination of Mineral Elements
4.4. Determination of Primary Metabolites
4.5. Determination of Phytohormones
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABA | Abscisic acid |
| GABA | Gamma-aminobutyric acid |
| JA | Jasmonic acid |
| JA-Ile | Jasmonoyl-isoleucine |
| SA | Salicylic acid |
References
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants; Academic Press: San Diego, CA, USA, 2012; ISBN 9780123849052. [Google Scholar]
- Maruyama-Nakashita, A. Metabolic changes sustain the plant life in low-sulfur environments. Curr. Opin. Plant Biol. 2017, 39, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, M.; Sirko, A. Recent advances in understanding plant response to sulfur-deficiency stress. Acta Bochimica Pol. 2008, 55, 457–471. [Google Scholar] [CrossRef]
- Chan, K.X.; Wirtz, M.; Phua, S.Y.; Estavillo, G.M.; Pogson, B.J. Balancing metabolites in drought: The sulfur assimilation conundrum. Trends Plant Sci. 2013, 18, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, M.; Malagoli, M. Role of Sulfate and S-Rich Compounds in Heavy Metal Tolerance and Accumulation. In Sulfur Assimilation and Abiotic Stress in Plants; Khan, N.A., Singh, S., Umar, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 253–269. [Google Scholar]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Malagoli, M.; Wirtz, M.; Hell, R. Drought stress in maize causes differential acclimation responses of glutathione and sulfur metabolism in leaves and roots. BMC Plant Biol. 2016, 16, 247. [Google Scholar] [CrossRef]
- Hawkesford, M.J. Plant responses to sulphur deficiency and the genetic manipulation of sulphate transporters to improve S-utilization efficiency. J. Exp. Bot. 2000, 51, 131–138. [Google Scholar] [CrossRef]
- Kopriva, S.; Calderwood, A.; Weckopp, S.C.; Koprivova, A. Plant sulfur and Big Data. Plant Sci. 2015, 241, 1–10. [Google Scholar] [CrossRef]
- Hawkesford, M.J.; De Kok, L.J. Managing sulphur metabolism in plants. Plant Cell Environ. 2006, 29, 382–395. [Google Scholar] [CrossRef]
- Smith, F.W.; Ealing, P.M.; Hawkesfordt, M.J.; Clarkson, D.T. Plant members of a family of sulfate transporters reveal functional subtypes. Proc. Natl. Acad. Sci. USA 1995, 92, 9373–9377. [Google Scholar] [CrossRef]
- Yoshimoto, N.; Takahashi, H.; Smith, F.W.; Yamaya, T.; Saito, K. Two distinct high-affinity sulfate transporters with different inducibilities mediate uptake of sulfate in Arabidopsis roots. Plant J. 2002, 29, 465–473. [Google Scholar] [CrossRef]
- Yoshimoto, N.; Inoue, E.; Watanabe-Takahashi, A.; Saito, K.; Takahashi, H. Posttranscriptional Regulation of High-Affinity Sulfate Transporters in Arabidopsis by Sulfur Nutrition. Plant Physiol. 2007, 145, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Gigolashvili, T.; Kopriva, S. Transporters in plant sulfur metabolism. Front. Plant Sci. 2014, 5, 442. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Asif, M.H.; Chakrabarty, D.; Tripathi, R.D.; Trivedi, P.K. Differential expression and alternative splicing of rice sulphate transporter family members regulate sulphur status during plant growth, development and stress conditions. Funct. Integr. Genom. 2011, 11, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Godwin, R.M.; Rae, A.L.; Carroll, B.J.; Smith, F.W. Cloning and characterization of two genes encoding sulfate transporters from rice (Oryza sativa L.). Plant Soil 2003, 257, 113–123. [Google Scholar] [CrossRef]
- Lunde, C.; Zygadlo, A.; Simonsen, H.T.; Nielsen, P.L.; Blennow, A.; Haldrup, A. Sulfur starvation in rice: The effect on photosynthesis, carbohydrate metabolism, and oxidative stress protective pathways. Physiol. Plant. 2008, 134, 508–521. [Google Scholar] [CrossRef] [PubMed]
- Hoefgen, R.; Nikiforova, V.J. Metabolomics integrated with transcriptomics: Assessing systems response to sulfur-deficiency stress. Physiol. Plant. 2008, 132, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Yamaji, N. A cooperative system of silicon transport in plants. Trends Plant Sci. 2015, 20, 435–442. [Google Scholar] [CrossRef]
- Miao, B.H.; Han, X.G.; Zhang, W.H. The ameliorative effect of silicon on soybean seedlings grown in potassium-deficient medium. Ann. Bot. 2010, 105, 967–973. [Google Scholar] [CrossRef]
- Chen, D.; Cao, B.; Wang, S.; Liu, P.; Deng, X.; Yin, L.; Zhang, S. Silicon moderated the K deficiency by improving the plant-water status in sorghum. Sci. Rep. 2016, 6, 22882. [Google Scholar] [CrossRef]
- Chen, D.; Cao, B.; Qi, L.; Yin, L.; Wang, S.; Deng, X. Silicon-moderated K-deficiency-induced leaf chlorosis by decreasing putrescine accumulation in sorghum. Ann. Bot. 2016, 118, 305–315. [Google Scholar] [CrossRef]
- Maillard, A.; Ali, N.; Schwarzenberg, A.; Jamois, F.; Yvin, J.C.; Hosseini, S.A. Silicon transcriptionally regulates sulfur and ABA metabolism and delays leaf senescence in barley under combined sulfur deficiency and osmotic stress. Environ. Exp. Bot. 2018, 155, 394–410. [Google Scholar] [CrossRef]
- Haddad, C.; Arkoun, M.; Jamois, F.; Schwarzenberg, A.; Yvin, J.C.; Etienne, P.; Laîné, P. Silicon promotes growth of Brassica napus L. And delays leaf senescence induced by nitrogen starvation. Front. Plant Sci. 2018, 9, 516. [Google Scholar] [CrossRef] [PubMed]
- Kostic, L.; Nikolic, N.; Bosnic, D.; Samardzic, J.; Nikolic, M. Silicon increases phosphorus (P) uptake by wheat under low P acid soil conditions. Plant Soil 2017, 419, 447–455. [Google Scholar] [CrossRef]
- Agostinho, F.B.; Tubana, B.S.; Martins, M.S.; Datnoff, L.E. Effect of Different Silicon Sources on Yield and Silicon Uptake of Rice Grown under Varying Phosphorus Rates. Plants 2017, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.A.; Rad, S.N.; Ali, N.; Yvin, J.-C. The Ameliorative Effect of Silicon on Maize Plants Grown in Mg-Deficient Conditions. Int. J. Mol. Sci. 2019, 20, 969. [Google Scholar] [CrossRef] [PubMed]
- Montpetit, J.; Vivancos, J.; Mitani-Ueno, N.; Yamaji, N.; Rémus-Borel, W.; Belzile, F.; Ma, J.F.; Bélanger, R.R. Cloning, functional characterization and heterologous expression of TaLsi1, a wheat silicon transporter gene. Plant Mol. Biol. 2012, 79, 35–46. [Google Scholar] [CrossRef]
- Chiba, Y.; Mitani, N.; Yamaji, N.; Ma, J.F. HvLsi1 is a silicon influx transporter in barley. Plant J. 2009, 57, 810–818. [Google Scholar] [CrossRef]
- Mitani, N.; Chiba, Y.; Yamaji, N.; Ma, J.F. Identification and Characterization of Maize and Barley Lsi2-Like Silicon Efflux Transporters Reveals a Distinct Silicon Uptake System from That in Rice. Plant Cell 2009, 21, 2133–2142. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N.; Mitani-Ueno, N. Transport of silicon from roots to panicles in plants. Proc. Jpn. Acad. Ser. B 2011, 87, 377–385. [Google Scholar] [CrossRef]
- Yamaji, N.; Mitatni, N.; Ma, J.F. A Transporter Regulating Silicon Distribution in Rice Shoots. Plant Cell 2008, 20, 1381–1389. [Google Scholar] [CrossRef]
- Yamaji, N.; Ma, J.F. Spatial Distribution and Temporal Variation of the Rice Silicon Transporter Lsi1. Plant Physiol. 2007, 143, 1306–1313. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, N.; Ma, J.F. Further characterization of a rice silicon efflux transporter, Lsi2. Soil Sci. Plant Nutr. 2011, 57, 259–264. [Google Scholar] [CrossRef]
- Hu, A.Y.; Che, J.; Shao, J.F.; Yokosho, K.; Zhao, X.Q.; Shen, R.F.; Ma, J.F. Silicon accumulated in the shoots results in down-regulation of phosphorus transporter gene expression and decrease of phosphorus uptake in rice. Plant Soil 2018, 423, 317–325. [Google Scholar] [CrossRef]
- Jang, S.W.; Kim, Y.; Khan, A.L.; Na, C.-I.; Lee, I.-J. Exogenous short-term silicon application regulates macro-nutrients, endogenous phytohormones, and protein expression in Oryza sativa L. BMC Plant Biol. 2018, 18, 4. [Google Scholar] [CrossRef]
- Coskun, D.; Deshmukh, R.; Sonah, H.; Menzies, J.G.; Reynolds, O.; Ma, J.F.; Kronzucker, H.J.; Bélanger, R.R. The controversies of silicon’s role in plant biology. New Phytol. 2019, 221, 67–85. [Google Scholar] [CrossRef]
- Rubio, V.; Bustos, R.; Irigoyen, M.L.; Cardona-López, X.; Rojas-Triana, M.; Paz-Ares, J. Plant hormones and nutrient signaling. Plant Mol. Biol. 2009, 69, 361–373. [Google Scholar] [CrossRef]
- Barberon, M.; Berthomieu, P.; Clairotte, M.; Shibagaki, N.; Davidian, J.-C.; Gosti, F. Unequal functional redundancy between the two Arabidopsis thaliana high-affinity sulphate transporters. New Phytol. 2008, 180, 608–619. [Google Scholar] [CrossRef]
- Maruyama-Nakashita, A.; Inoue, E.; Watanabe-Takahashi, A.; Yamaya, T.; Takahashi, H. Transcriptome Profiling of Sulfur-Responsive Genes in Arabidopsis Reveals Global Effects of Sulfur Nutrition on Multiple Metabolic Pathways. Plant Physiol. 2003, 132, 597–605. [Google Scholar] [CrossRef]
- Rouached, H.; Wirtz, M.; Alary, R.; Hell, R.; Arpat, A.B.; Davidian, J.-C.; Fourcroy, P.; Berthomieu, P. Differential Regulation of the Expression of Two High-Affinity Sulfate Transporters, SULTR1.1 and SULTR1.2, in Arabidopsis. Plant Physiol. 2008, 147, 897–911. [Google Scholar] [CrossRef]
- Takahashi, H.; Watanabe-Takahashi, A.; Smith, F.W.; Blake-Kalff, M.; Hawkesford, M.J.; Saito, K. The roles of three functional sulphate transporters involved in uptake and translocation of sulphate in Arabidopsis thaliana. Plant J. 2000, 23, 171–182. [Google Scholar] [CrossRef]
- Zhang, B.; Pasini, R.; Dan, H.; Joshi, N.; Zhao, Y.; Leustek, T.; Zheng, Z.L. Aberrant gene expression in the Arabidopsis SULTR1;2 mutants suggests a possible regulatory role for this sulfate transporter in response to sulfur nutrient status. Plant J. 2014, 77, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Buchner, P.; Parmar, S.; Kriegel, A.; Carpentier, M.; Hawkesford, M.J. The Sulfate Transporter Family in Wheat: Tissue-Specific Gene Expression in Relation to Nutrition. Mol. Plant 2010, 3, 374–389. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, A.; Yamaji, N.; Ma, J.F. Transporters involved in mineral nutrient uptake in rice. J. Exp. Bot. 2016, 67, 3645–3653. [Google Scholar] [CrossRef] [PubMed]
- Laîné, P.; Haddad, C.; Arkoun, M.; Yvin, J.-C.; Etienne, P. Silicon Promotes Agronomic Performance in Brassica napus Cultivated under Field Conditions with Two Nitrogen Fertilizer Inputs. Plants 2019, 8, 137. [Google Scholar] [CrossRef]
- Yamaji, N.; Chiba, Y.; Mitani-Ueno, N.; Ma, J.F. Functional Characterization of a Silicon Transporter Gene Implicated in Silicon Distribution in Barley. Plant Physiol. 2012, 160, 1491–1497. [Google Scholar] [CrossRef]
- Wang, H.-S.; Yu, C.; Fan, P.-P.; Bao, B.-F.; Li, T.; Zhu, Z.-J. Identification of Two Cucumber Putative Silicon Transporter Genes in Cucumis sativus. J. Plant Growth Regul. 2015, 34, 332–338. [Google Scholar] [CrossRef]
- Mitani-Ueno, N.; Yamaji, N.; Ma, J.F. High silicon accumulation in the shoot is required for down-regulating the expression of Si transporter genes in rice. Plant Cell Physiol. 2016, 57, 2510–2518. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Khan, A.L.; Kim, D.-H.; Lee, S.-Y.; Kim, K.-M.; Waqas, M.; Jung, H.-Y.; Shin, J.-H.; Kim, J.-G.; Lee, I.-J. Silicon mitigates heavy metal stress by regulating P-type heavy metal ATPases, Oryza sativa low silicon genes, and endogenous phytohormones. BMC Plant Biol. 2014, 14, 13. [Google Scholar] [CrossRef]
- Wu, X.; Yu, Y.; Baerson, S.R.; Song, Y.; Liang, G.; Ding, C.; Niu, J.; Pan, Z.; Zeng, R. Interactions between nitrogen and silicon in rice and their effects on resistance toward the brown planthopper Nilaparvata lugens. Front. Plant Sci. 2017, 8, 28. [Google Scholar] [CrossRef]
- Pavlovic, J.; Samardzic, J.; Kostic, L.; Laursen, K.H.; Natic, M.; Timotijevic, G.; Schjoerring, J.K.; Nikolic, M. Silicon enhances leaf remobilization of iron in cucumber under limited iron conditions. Ann. Bot. 2016, 118, 271–280. [Google Scholar] [CrossRef]
- Greger, M.; Landberg, T.; Vaculík, M. Silicon Influences Soil Availability and Accumulation of Mineral Nutrients in Various Plant Species. Plants 2018, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Nikiforova, V.J.; Bieleck, M.; Gakière, B.; Krueger, S.; Rinder, J.; Kempa, S.; Morcuende, R.; Scheible, W.-R.; Hesse, H.; Hoefgen, R. Effect of sulfur availability on the integrity of amino acid biosynthesis in plants. Amino Acids 2006, 30, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Nikiforova, V.J.; Kopka, J.; Tolstikov, V.; Fiehn, O.; Hopkins, L.; Hawkesford, M.J.; Hesse, H.; Hoefgen, R. Systems Rebalancing of Metabolism in Response to Sulfur Deprivation, as Revealed by Metabolome Analysis of Arabidopsis Plants. Plant Physiol. 2005, 138, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Prosser, I.M.; Purves, J.V.; Saker, L.R.; Clarkson, D.T. Rapid disruption of nitrogen metabolism and nitrate transport in spinach plants deprived of sulphate. J. Exp. Bot. 2001, 52, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Chandna, R.; Pandey, R.; Abrol, Y.P.; Iqbal, M.; Ahmad, A. Sulfur starvation and restoration affect nitrate uptake and assimilation in rapeseed. Protoplasma 2011, 248, 299–311. [Google Scholar] [CrossRef]
- Sarda, X.; Diquelou, S.; Abdallah, M.; Nesi, N.; Cantat, O.; Le Gouee, P.; Avice, J.C.; Ourry, A. Assessment of sulphur deficiency in commercial oilseed rape crops from plant analysis. J. Agric. Sci. 2014, 152, 616–633. [Google Scholar] [CrossRef]
- Sorin, E.; Etienne, P.; Maillard, A.; Zamarreño, A.-M.; Garcia-Mina, J.-M.; Arkoun, M.; Jamois, F.; Cruz, F.; Yvin, J.; Ourry, A. Effect of sulphur deprivation on osmotic potential components and nitrogen metabolism in oilseed rape leaves: Identification of a new early indicator. J. Exp. Bot. 2015, 66, 6175–6189. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Seifikalhor, M.; Aliniaeifard, S.; Hassani, B.; Niknam, V.; Lastochkina, O. Diverse role of γ-aminobutyric acid in dynamic plant cell responses. Plant Cell Rep. 2019, 38, 847–867. [Google Scholar] [CrossRef]
- Wutipraditkul, N.; Wongwean, P.; Buaboocha, T. Alleviation of salt-induced oxidative stress in rice seedlings by proline and/or glycinebetaine. Biol. Plant. 2015, 59, 547–553. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Shao, H.; Qi, W.; Hamoud, Y.A.; Shaghaleh, H.; Khan, N.U.; Yang, R.; Tang, B. GABA-alleviated oxidative injury induced by salinity, osmotic stress and their combination by regulating cellular and molecular signals in rice. Int. J. Mol. Sci. 2019, 20, 5709. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Schwarzenberg, A.; Yvin, J.-C.; Hosseini, S.A. Regulatory Role of Silicon in Mediating Differential Stress Tolerance Responses in Two Contrasting Tomato Genotypes Under Osmotic Stress. Front. Plant Sci. 2018, 9, 1475. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.S.; Baek, K.H. Jasmonic acid signaling pathway in response to abiotic stresses in plants. Int. J. Mol. Sci. 2020, 21, 621. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Schachtman, D.P. The Role of Ethylene in Plant Responses to K+ Deficiency. Front. Plant Sci. 2015, 6, 1153. [Google Scholar] [CrossRef]
- Lynch, J.; Brown, K.M. Ethylene and plant responses to nutritional stress. Physiol. Plant. 1997, 100, 613–619. [Google Scholar] [CrossRef]
- Werner, T.; Nehnevajova, E.; Köllmer, I.; Novák, O.; Strnad, M.; Krämer, U.; Schmülling, T. Root-specific reduction of cytokinin causes enhanced root growth, drought tolerance, and leaf mineral enrichment in Arabidopsis and tobacco. Plant Cell 2010, 22, 3905–3920. [Google Scholar] [CrossRef]
- Ohkama, N.; Takei, K.; Sakakibara, H.; Hayashi, H.; Yoneyama, T.; Fujiwara, T. Regulation of Sulfur-Responsive Gene Expression by Exogenously Applied Cytokinins in Arabidopsis thaliana. Plant Cell Physiol. 2002, 43, 1493–1501. [Google Scholar] [CrossRef]
- Kutz, A.; Müller, A.; Hennig, P.; Kaiser, W.M.; Piotrowski, M.; Weiler, E.W. A role for nitrilase 3 in the regulation of root morphology in sulphur-starving Arabidopsis thaliana. Plant J. 2002, 30, 95–106. [Google Scholar] [CrossRef]
- Koprivova, A.; Kopriva, S. Hormonal control of sulfate uptake and assimilation. Plant Mol. Biol. 2016, 91, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Ernst, L.; Goodger, J.Q.D.; Alvarez, S.; Marsh, E.L.; Berla, B.; Lockhart, E.; Jung, J.; Li, P.; Bohnert, H.J.; Schachtman, D.P. Sulphate as a xylem-borne chemical signal precedes the expression of ABA biosynthetic genes in maize roots. J. Exp. Bot. 2010, 61, 3395–3405. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.A.; Maillard, A.; Hajirezaei, M.R.; Ali, N.; Schwarzenberg, A.; Jamois, F.; Yvin, J.-C. Induction of Barley Silicon Transporter HvLsi1 and HvLsi2, increased silicon concentration in the shoot and regulated Starch and ABA Homeostasis under Osmotic stress and Concomitant Potassium Deficiency. Front. Plant Sci. 2017, 8, 1359. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowska, N.; Wilmowicz, E.; Marzec-Schmidt, K.; Ludwików, A.; Bagniewska-Zadworna, A. Abscisic Acid and Jasmonate Metabolisms Are Jointly Regulated During Senescence in Roots and Leaves of Populus trichocarpa. Int. J. Mol. Sci. 2020, 21, 2042. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, C. Signal transduction in leaf senescence. Plant Mol. Biol. 2013, 82, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Armengaud, P.; Breitling, R.; Antmann, A. The Potassium-Dependent Transcriptome of Arabidopsis Reveals a Prominent Role of Jasmonic Acid in Nutrient Signaling. Plant Physiol. 2004, 136, 2556–2576. [Google Scholar] [CrossRef]
- Yin, L.; Wang, S.; Li, J.; Tanaka, K.; Oka, M. Application of silicon improves salt tolerance through ameliorating osmotic and ionic stresses in the seedling of Sorghum bicolor. Acta Physiol. Plant. 2013, 35, 3099–3107. [Google Scholar] [CrossRef]
- Maillard, A.; Etienne, P.; Diquélou, S.; Trouverie, J.; Billard, V.; Yvin, J.-C.; Ourry, A. Nutrient deficiencies in Brassica napus modify the ionomic composition of plant tissues: A focus on cross-talk between molybdenum and other nutrients. J. Exp. Bot. 2016, 67, 5631–5641. [Google Scholar] [CrossRef]
- Abdallah, M.; Dubousset, L.; Meuriot, F.; Etienne, P.; Avice, J.-C.; Ourry, A. Effect of mineral sulphur availability on nitrogen and sulphur uptake and remobilization during the vegetative growth of Brassica napus L. J. Exp. Bot. 2010, 61, 2635–2646. [Google Scholar] [CrossRef]
- Kim, Y.M.; Heinzel, N.; Giese, J.O.; Koeber, J.; Melzer, M.; Rutten, T.; Von Wirén, N.; Sonnewald, U.; Hajirezaei, M.R. A dual role of tobacco hexokinase 1 in primary metabolism and sugar sensing. Plant Cell Environ. 2013, 36, 1311–1327. [Google Scholar] [CrossRef]

| Roots | Shoots | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| +S-Si | +S+Si | -S-Si | -S+Si | +S-Si | +S+Si | -S-Si | -S+Si | ||
| Macro-Elements | S | 3.79 ± 0.18 a | 3.90 ± 0.19 a | 2.68 ± 0.08 b | 2.59 ± 0.15 b | 4.37 ± 0.39 a | 3.29 ± 0.24 b | 3.08 ± 0.25 b | 2.11 ± 0.12 c |
| Si | 3.00 ± 0.00 b | 11.50 ± 1.00 a | 2.50 ± 0.58 b | 10.50 ± 1.00 a | 3.00 ± 0.82 c | 84.50 ± 3.32 a | 2.75 ± 0.50 c | 69.75 ± 5.97 b | |
| N | 28.83 ± 1.76 ns | 27.94 ± 0.98 ns | 28.61 ± 0.93 ns | 27.75 ± 0.44 ns | 36.99 ± 0.65 a | 31.43 ± 0.39 b | 36.03 ± 1.57 a | 28.54 ± 1.61 c | |
| P | 9.00 ± 0.17 a | 7.64 ± 0.38 b | 8.01 ± 0.35 b | 6.98 ± 0.35 c | 10.26 ± 0.53 a | 6.42 ± 0.37 c | 8.68 ± 0.96 b | 5.43 ± 0.15 d | |
| K | 29.42 ± 1.60 a | 28.00 ± 0.99 ab | 25.51 ± 2.01 b | 27.76 ± 0.35 ab | 37.67 ± 2.93 ns | 35.14 ± 1.93 ns | 34.12 ± 2.19 ns | 34.77 ± 3.62 ns | |
| Mg | 2.27 ± 0.20 b | 3.10 ± 0.77 a | 2.27 ± 0.13 b | 1.91 ± 0.18 b | 4.76 ± 0.26 a | 2.51 ± 0.16 b | 4.58 ± 0.52 a | 2.25 ± 0.19 b | |
| Ca | 1.51 ± 0.20 ns | 1.53 ± 0.19 ns | 1.51 ± 0.09 ns | 1.33 ± 0.09 ns | 5.09 ± 0.55 a | 2.88 ± 0.28 b | 5.36 ± 0.83 a | 2.73 ± 0.35 b | |
| Ions | NO3− | 12.84 ± 3.07 a | 15.01 ± 0.72 a | 8.74 ± 0.32 b | 8.20 ± 0.67 b | 5.18 ± 0.97 ab | 6.71 ± 0.61 a | 3.18 ± 2.12 bc | 2.04 ± 1.85 c |
| NH4+ | 0.19 ± 0.02 ab | 0.18 ± 0.01 b | 0.20 ± 0.02 ab | 0.22 ± 0.01a | 0.19 ± 0.02 a | 0.12 ± 0.01 b | 0.21 ± 0.02 a | 0.11 ± 0.02 b | |
| SO42− | 3.02 ± 0.38 b | 3.49 ± 0.25 a | 0.92 ± 0.17 c | 1.10 ± 0.24 c | 4.13 ± 0.92 a | 2.82 ± 0.49 b | 1.63 ± 0.24 c | 0.71 ± 0.13 d | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Réthoré, E.; Ali, N.; Yvin, J.-C.; Hosseini, S.A. Silicon Regulates Source to Sink Metabolic Homeostasis and Promotes Growth of Rice Plants under Sulfur Deficiency. Int. J. Mol. Sci. 2020, 21, 3677. https://doi.org/10.3390/ijms21103677
Réthoré E, Ali N, Yvin J-C, Hosseini SA. Silicon Regulates Source to Sink Metabolic Homeostasis and Promotes Growth of Rice Plants under Sulfur Deficiency. International Journal of Molecular Sciences. 2020; 21(10):3677. https://doi.org/10.3390/ijms21103677
Chicago/Turabian StyleRéthoré, Elise, Nusrat Ali, Jean-Claude Yvin, and Seyed Abdollah Hosseini. 2020. "Silicon Regulates Source to Sink Metabolic Homeostasis and Promotes Growth of Rice Plants under Sulfur Deficiency" International Journal of Molecular Sciences 21, no. 10: 3677. https://doi.org/10.3390/ijms21103677
APA StyleRéthoré, E., Ali, N., Yvin, J.-C., & Hosseini, S. A. (2020). Silicon Regulates Source to Sink Metabolic Homeostasis and Promotes Growth of Rice Plants under Sulfur Deficiency. International Journal of Molecular Sciences, 21(10), 3677. https://doi.org/10.3390/ijms21103677





