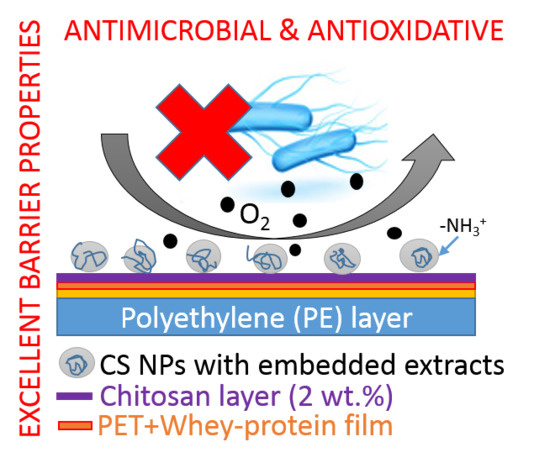
Development of Biodegradable Whey-Based Laminate Functionalised by Chitosan–Natural Extract Formulations
Abstract
1. Introduction
2. Results and Discussion
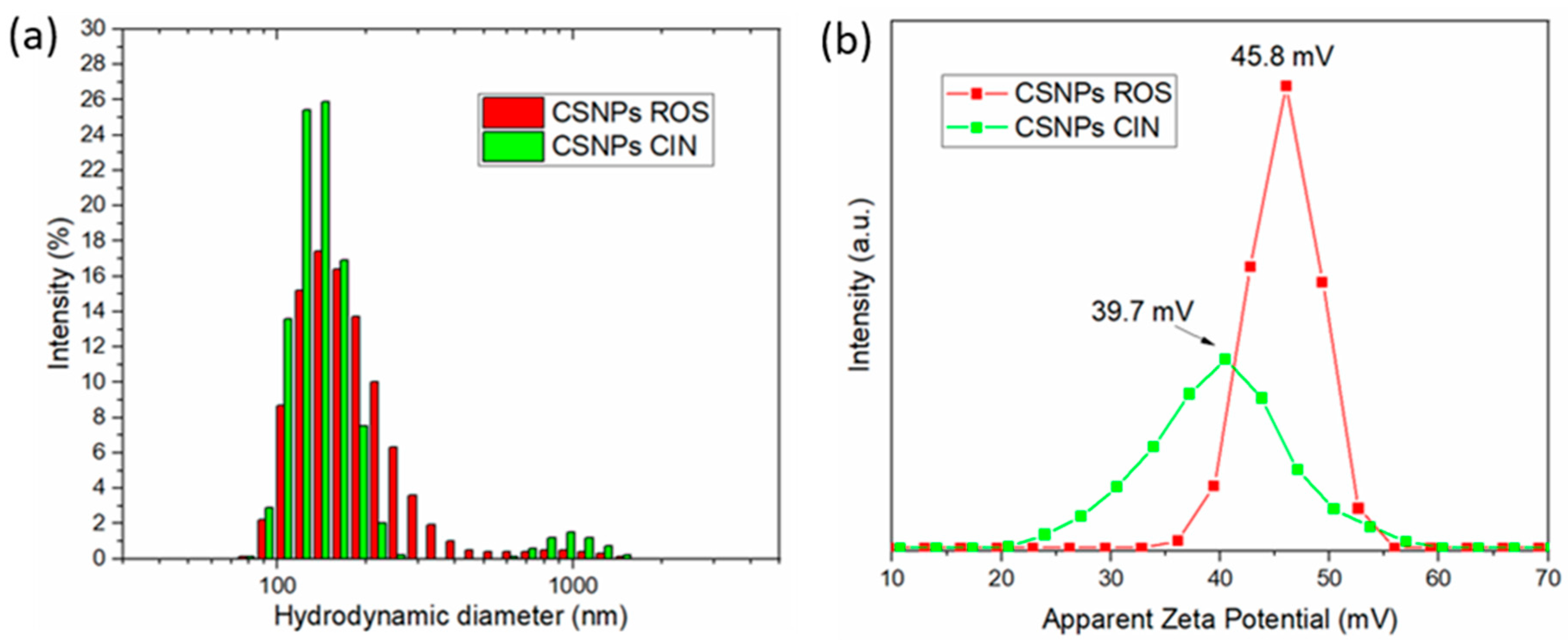
2.1. Characterization of CSNPs Colloidal Formulations with Embedded Extracts
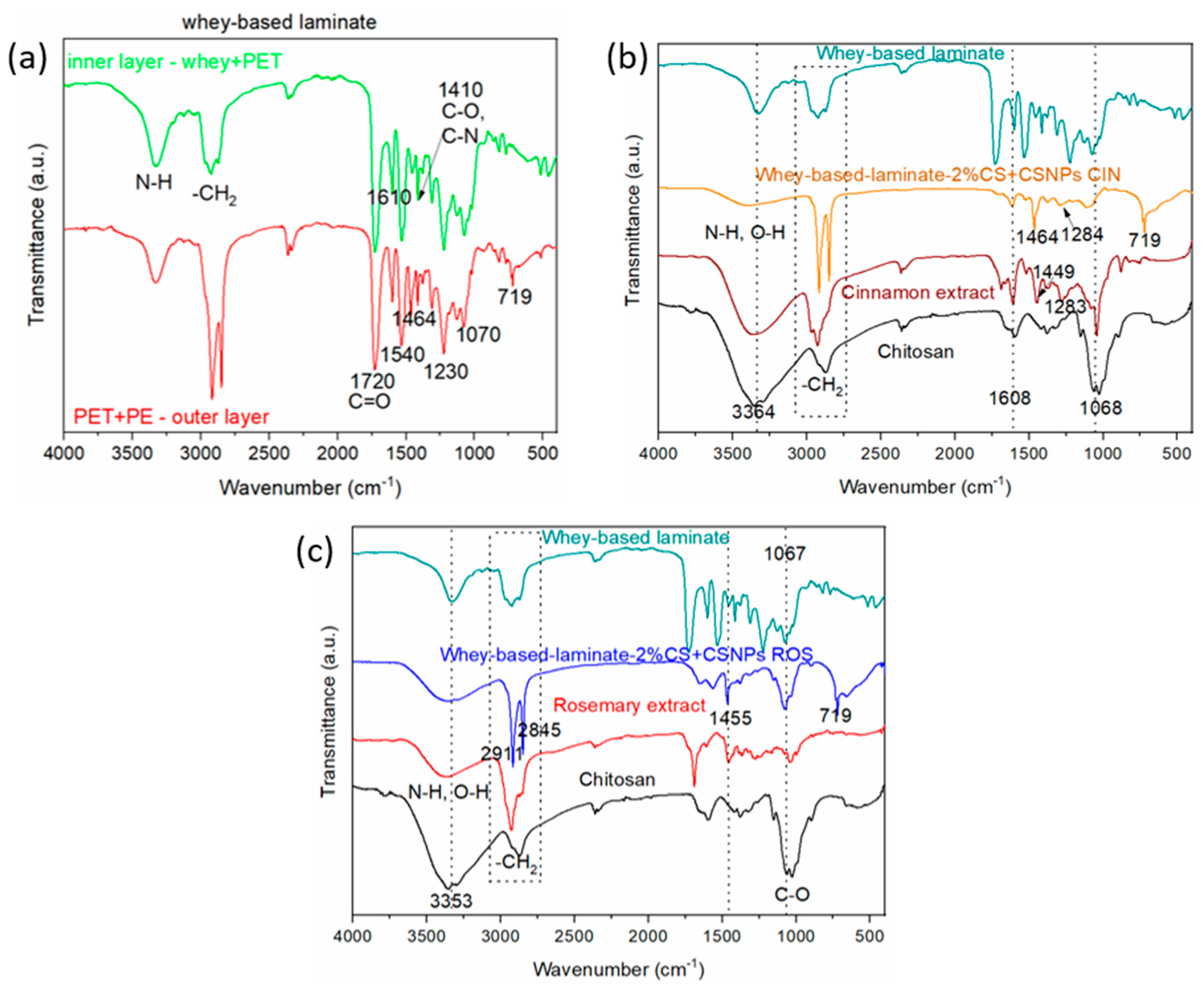
2.2. ATR-FTIR Spectroscopy
2.3. Goniometry
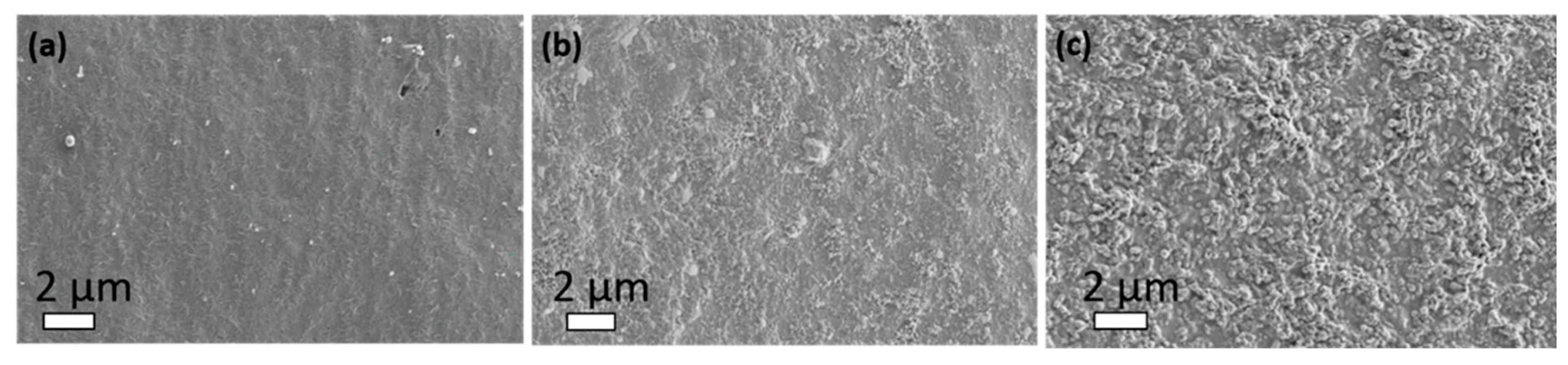
2.4. Morphology by SEM
2.5. Oxygen Permeability
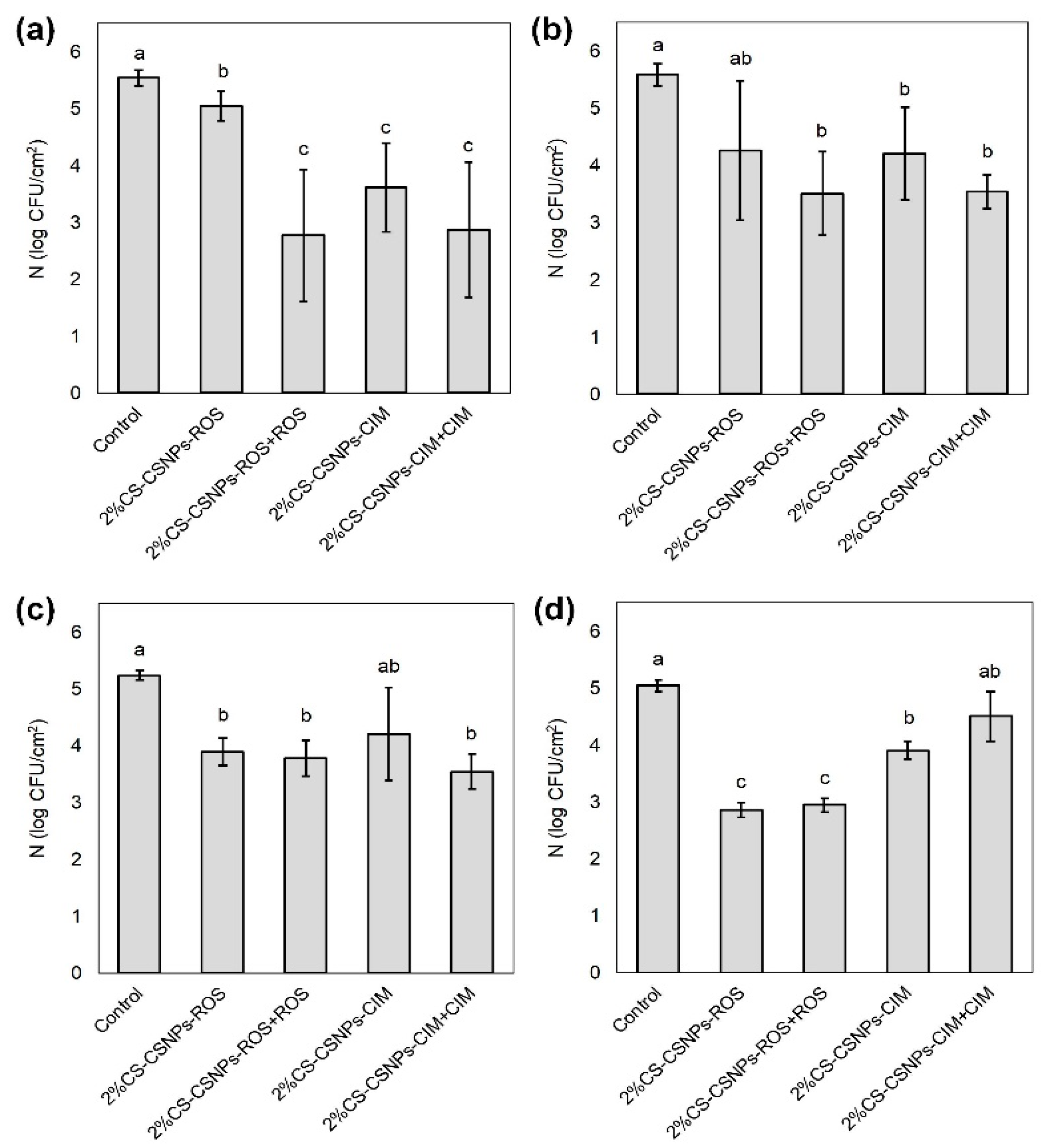
2.6. Antimicrobial Activity
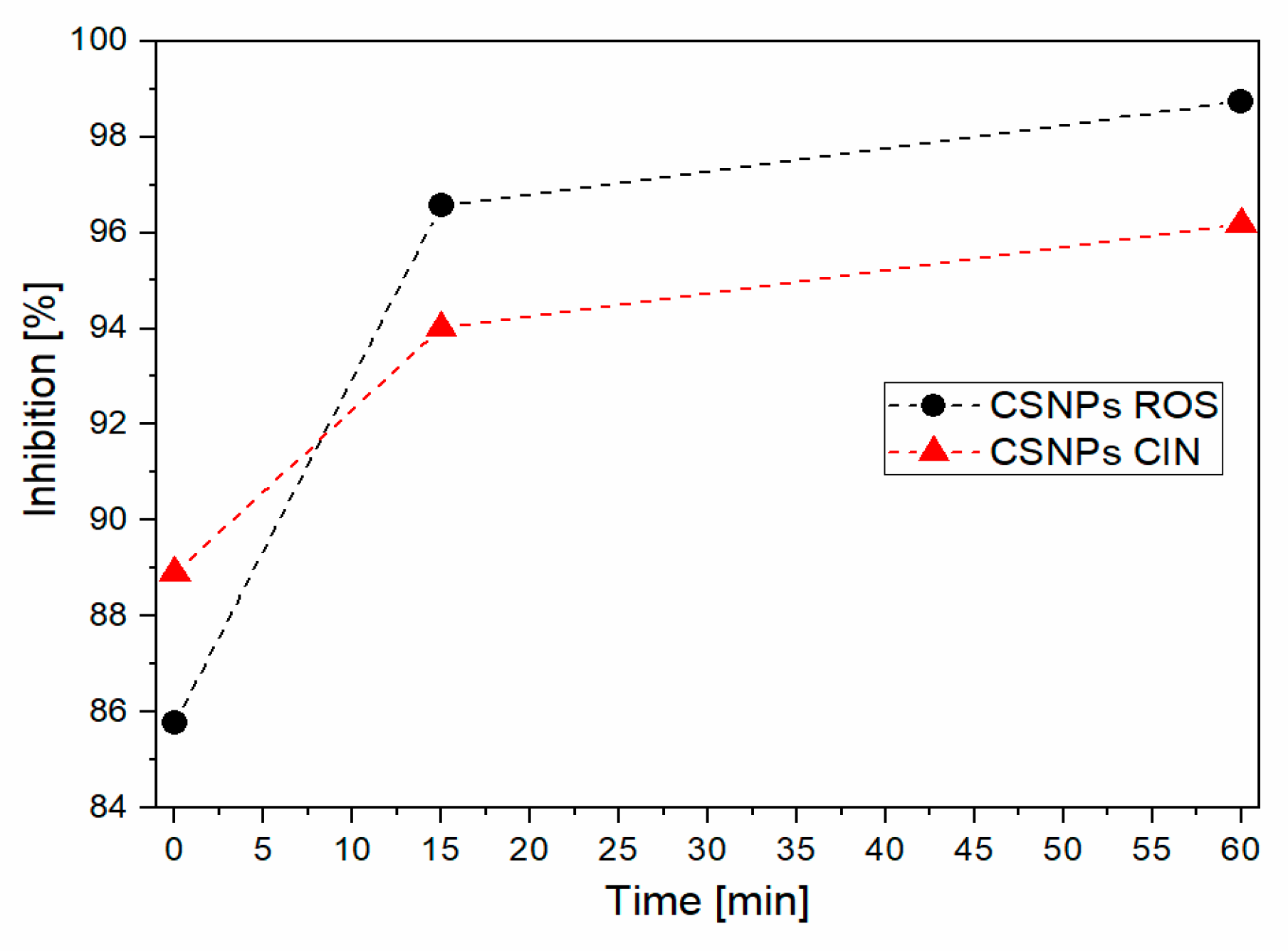
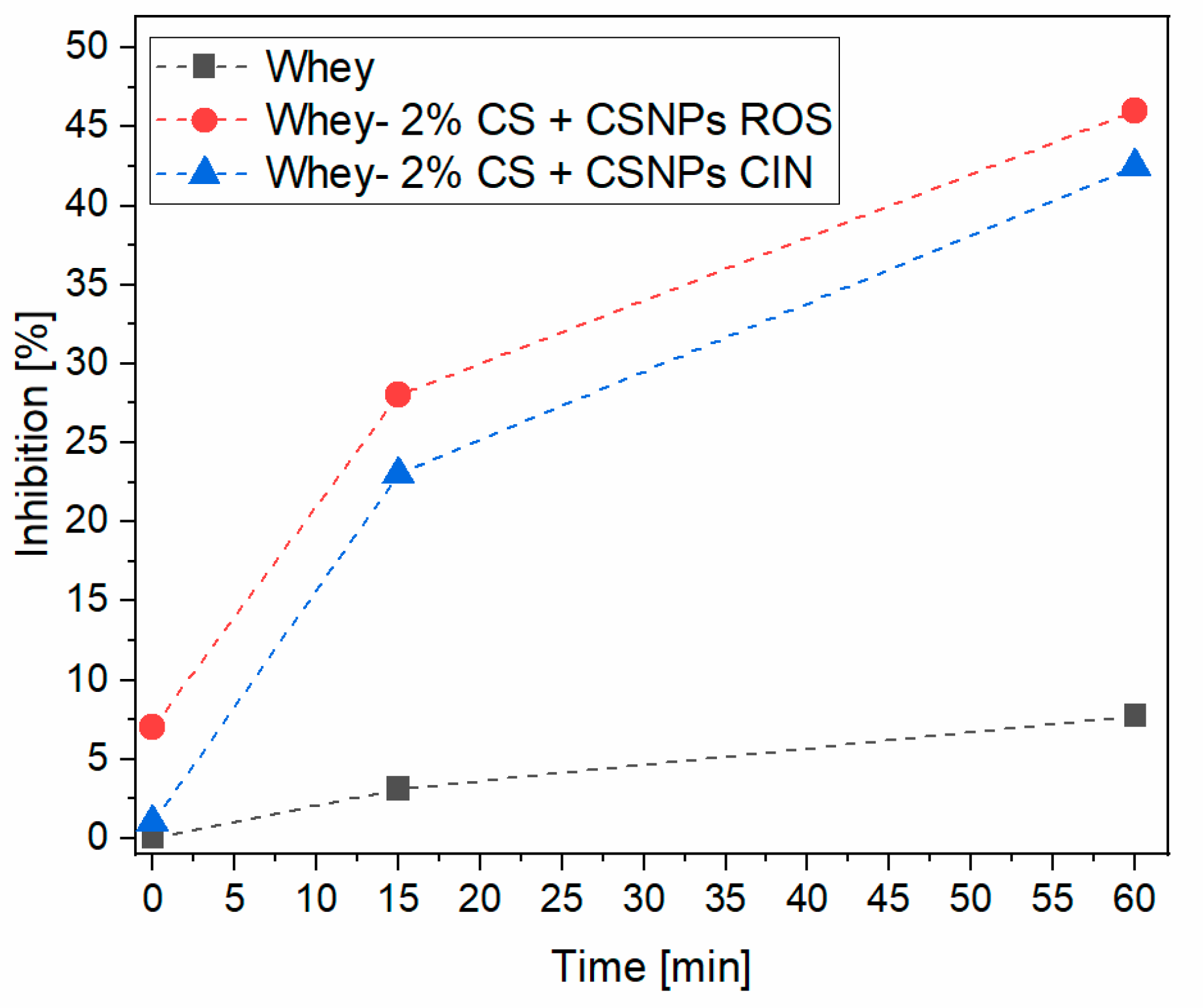
2.7. Antioxidative Activity
3. Materials and Methods
3.1. Materials
3.2. Extraction of the Plant Material
3.3. Preparation of Solutions
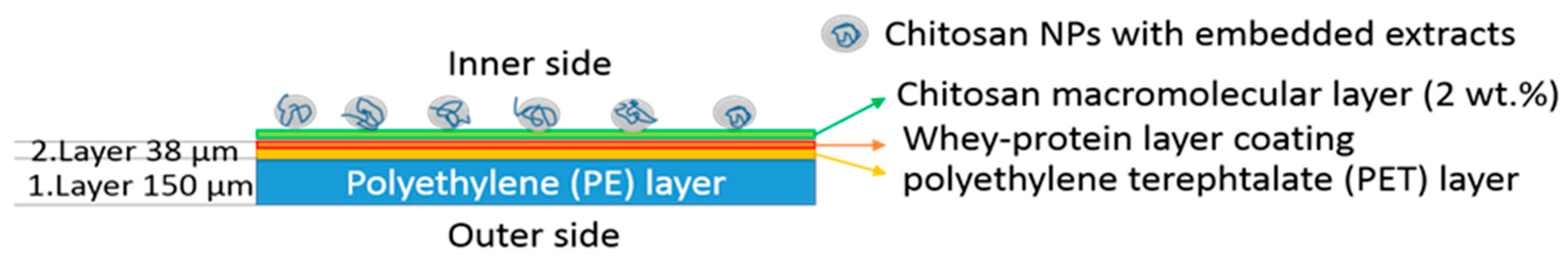
3.4. Functionalization of the Laminates
3.5. Electrokinetic Properties and Particle Size
3.6. ATR-FTIR Spectroscopy
3.7. Goniometry
3.8. Morphology
3.9. Oxygen Permeability
3.10. Testing for Antimcirobial Activity
3.11. Antioxidant Activity (ABTS Assay)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Lavender Law, K. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Nordin, N.; Selke, S. Social aspects of sustainable packaging. Packag. Technol. Sci. 2010, 23, 317–326. [Google Scholar] [CrossRef]
- Boz, Z.; Korhonen, V.; Koelsch Sand, K. Consumer considerations for the implementation of sustainable packaging: A review. Sustainability 2020, 12, 2192. [Google Scholar] [CrossRef]
- Scott, L.; Vigar-Ellis, D. Consumer understanding, perception and behaviours with regard to environmentally friendly packaging in a developing nation. Int. J. Consum. Stud. 2014, 38, 642–649. [Google Scholar] [CrossRef]
- Davis, G.; Song, J.H. Biodegradable packaging based on raw materials from crops and their impact on waste management. Ind. Crops Prod. 2006, 23, 147–161. [Google Scholar] [CrossRef]
- Lambert, S.; Wagner, M. Environmental performance of bio-based and biodegradable plastics: The road ahead. Chem. Soc. Rev. 2017, 46, 6855–6871. [Google Scholar] [CrossRef]
- Ivanković, A.; Zeljko, K.; Talić, S.; Maritnović Bevanda, A.; Lasić, M. Biodegradable packaging in the food industry. Arch. Lebensmittelhyg. 2017, 68, 26–38. [Google Scholar] [CrossRef]
- Silvestre, C.; Duraccio, D.; Cimmino, S. Food packaging based on polymer nanomaterials. Prog. Polym. Sci. 2011, 36, 1766–1782. [Google Scholar] [CrossRef]
- Guillard, V.; Gaucel, S.; Fornaciari, C.; Angellier-Coussy, H.; Buche, P.; Gontard, N. The next generation of sustainable food packaging to preserve our environment in a circular economy context. Front. Nutr. 2018, 5, 121. [Google Scholar] [CrossRef]
- Kumar, K.V.; Suneetha, W.J.; Kumari, B.A. Active packaging in food packaging for enhanced shelf life. J. Pharmacogn. Phytocehm. 2018, 7, 2044–2046. [Google Scholar]
- Fuertes, G.; Soto, I.; Carrasco, R.; Vargas, M.; Sabattin, J.; Lagos, C. Intelligent packaging systems: Sensors and nanosensors to monitor food quality and safety. J. Sens. 2016, 2016, 4046061. [Google Scholar] [CrossRef]
- Realini, C.E.; Marcos, B. Active and intelligent packaging systems for a modern society. Meat Sci. 2014, 98, 404–419. [Google Scholar] [CrossRef]
- Nychas, G.J.; Skandamis, P.N.; Tassou, C.C.; Koutsoumanis, K.P. Meat spoilage during distribution. Meat Sci. 2008, 78, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Pal, M. Nanotechnology: A new approach in food packaging. J. Food Microbiol. Saf. Hyg. 2017, 2, 1000121. [Google Scholar] [CrossRef]
- Marsh, K.; Bugusu, B. Food packaging–roles, materials, and environmental issues. J. Food Sci. 2007, 72, R39–R55. [Google Scholar] [CrossRef]
- Fang, J.M.; Fowler, P.A.; Escrig, C.; Gonzalez, R.; Costa, J.A.; Chamudis, L. Development of biodegradable laminate films derived from naturally occurring carbohydrate polymers. Carbohydr. Polym. 2005, 60, 39–42. [Google Scholar] [CrossRef]
- Kaiser, K.; Schmid, M.; Schlummer, M. Recycling of polymer-based multilayer packaging: A review. Recycling 2018, 3, 1. [Google Scholar] [CrossRef]
- Langhe, D.; Ponting, M. Novel multilayers structures and applications. In Manufacturing and Novel Applications of Multilayer Polymer Films; Langhe, D., Ponting, M., Eds.; Wiliam Andrew: Norwich, NY, USA, 2016; pp. 190–220. [Google Scholar] [CrossRef]
- Kreiger, M.; Anzalone, G.C.; Mulder, M.L.; Glover, A.; Pearce, J.M. Distributed recycling of post-consumer plastic waste in rural areas. Mater. Res. Soc. Symp. Proc. 2013, 1492, 91–96. [Google Scholar] [CrossRef]
- Surin, P.; Rakkwamsuk, P.; Wimolmala, E.; Sombatsompop, N. Effect of coir fiber and maleic anhydride modification on the properties of thermoplastic starch/PLA composite laminates. J. Nat. Fibers 2015, 12, 108–120. [Google Scholar] [CrossRef]
- Masmoudi, F.; Bessadok, A.; Dammak, M.; Jaziri, M.; Ammar, E. Biodegradable packaging materialy conception based on starch and polylactic acid (PLA) reinforced with cellulose. Environ. Sci. Pollut. Res. Int. 2016, 23, 20904–20914. [Google Scholar] [CrossRef]
- Bugnicourt, E.; Schmid, M.; Nerney, O.M.; Wildner, J.; Smykala, L.; Lazzeri, A.; Cinelli, P. Processing and validation of whey-protein-coated films and laminates at semi-industrial scale as novel recyclable food packaging materials with excellent barrier properties. Adv. Mater. Sci. Eng. 2013, 2013, 496207. [Google Scholar] [CrossRef]
- Mir, S.A.; Dar, B.N.; Wani, A.A.; Shah, M.A. Effect of plant extracts on the techno-functional properties of biodegradable packaging films. Trends Food Sci. Technol. 2018, 80, 141–154. [Google Scholar] [CrossRef]
- Shen, Z.; Kamdem, D.P. Development and characterization of biodegradable chitosan films containing two essential oils. Int. J. Biol. Macromol. 2015, 74, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Atarés, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Sánchez-Gonzalez, L.; Vargas, M.; Gónzalez-Martínez, C.; Chiralt, A.; Cháfer, M. Use of essential oils in bioactive edible coatings: A review. Food Eng. Rev. 2011, 3, 1–16. [Google Scholar] [CrossRef]
- Llana-Ruiz-Cabello, M.; Pichardo, S.; Bermúdez, J.M.; Baños, A.; Núñez, C.; Aucejo, S.; Cameán, A.M. Development of PLA films containing oregano essential oil (Origanum vulgare L. virens) intended for use in food packaging. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2016, 33, 1374–1386. [Google Scholar] [CrossRef]
- Seydim, A.C.; Sarikus, G. Antimicrobial activity of whey protein based edible films incorporated with oregano, rosemary and garlic essential oils. Food Res. Int. 2006, 39, 639–644. [Google Scholar] [CrossRef]
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan based nanocomposite films and coatings: Emerging antimicrobial food packaging alternatives. Trends Food Sci. Technol. 2020, 97, 196–209. [Google Scholar] [CrossRef]
- Munteanu, S.B.; Vasile, C. Vegetable additives in food packaging polymeric materials. Polymers 2020, 12, 28. [Google Scholar] [CrossRef]
- Krkić, N.; Lazić, V.; Savatić, S.; Šojić, B.; Petrović, L.; Šuput, D. Application of chitosan coating with oregano essential oil on dry fermented sausage. J. Food Nutr. Res. 2012, 51, 60–68. [Google Scholar]
- Yegan Mohammadi, M.; Khanjari, A.; Akhondzadeh Basti, A.; Bokaie, S.; Cheragi, N.; Fayazfar, S.; Shoja gharebagh, S.; Ghadami, F. Evaluation of the antimicrobial effect of chitosan and whey proteins isolate films containing free and nanoliposomal garlic essential oils against Listeria monocytogenes, E. coli O157:H7 and Staphylococcus aures. Iran. J. Med. Microbiol. 2016, 10, 45–51. [Google Scholar]
- Shoja Gharehbagh, S.; Khanjar, A.; Yeganmohammadi Davaji, M.; Akhonzadeh Basti, A. The use of chitosan and whey protein isolate edible films incorporated with Zataria multiflora Boiss. Essential oil as an active packaging ingredient against some common foodborne bacteria. J. Food Biosci. Technol. 2017, 7, 41–48. [Google Scholar]
- Oymacı, P. Development of Whey Protein Isolate Based Nanocomposite Food Packaging Film Incorporated with Chitosan and Zein Nanoparticles. Master’s Thesis, İzmir Institute of Technology, İzmir, Turkey, 2014. [Google Scholar]
- Vahedikia, N.; Garavand, F.; Tajeddin, B.; Cacciotti, I.; Jafari, S.M.; Omidi, T.; Zahedi, Z. Biodegradable zein film composites reinforced with chitosan nanoparticles and cinnamon essential oil: Physical, mechanical, structural and antimicrobial attributes. Colloids Surf. B Biointerfaces 2019, 177, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Cinelli, P.; Schmid, M.; Bugnicourt, E.; Wildner, J.; Bazzichi, A.; Anguillesi, I.; Lazzeri, A. Whey protein layer applied on biodegradable packaging film to improve barrier properties while maintaining biodegradability. Polym. Degrad. Stab. 2014, 108, 151–157. [Google Scholar] [CrossRef]
- Hong, S.I.; Krochta, J.M. Whey protein isolate coating on LDPE film as a novel oxygen barrier in the composite structure. Packag. Technol. Sci. 2004, 17, 13–21. [Google Scholar] [CrossRef]
- Ajdnik, U.; Fras Zemljič, L.; Bračič, M.; Maver, U.; Plohl, O.; Rebol, J. Functionalisation of silicone by drug-embedded chitosan nanoparticles for potential applications in othorinolaryngology. Materials 2019, 12, 847. [Google Scholar] [CrossRef]
- Ristić, T.; Zabret, A.; Fras Zemljič, L.; Peršin, Z. Chitosan nanoparticles as a potential drug delivery system attached to viscose cellulose fibers. Cellulose 2017, 24, 739–753. [Google Scholar] [CrossRef]
- Fras Zemljič, L.; Potrč, S.; Plohl, O.; Vesel, A.; Luxbacher, T.; Potrč, S. Physicochemical characterization of packaging foils coated by chitosan and polyphenols colloidal formulation. Int. J. Mol. Sci. 2020, 21, 495. [Google Scholar] [CrossRef]
- Cinelli, P.; Schmid, M.; Bugnicourt, E.; Coltelli, M.B.; Lazzeri, A. Recyclability of PET/WPI/PE multilayer films by removal of whey protein isolate-based coatings with enzymatic detergents. Materials 2016, 9, 473. [Google Scholar] [CrossRef]
- Kraševac Glaser, T.; Plohl, O.; Vesel, A.; Ajdnik, U.; Poklar Ulrih, N.; Knez Hrnčič, M.; Bren, U.; Fras Zemljič, L. Functionalization of polyethylene (PE) and polypropylene (PP) material using chitosan nanoparticles with incorporated resveratrol as potential active packaging. Materials 2019, 12, 2118. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Wu, H. Analysis and evaluation of essential oil components of cinnamon barks using GC-MS and FTIR spectroscopy. Ind. Crops Prod. 2013, 41, 269–278. [Google Scholar] [CrossRef]
- Wen, P.; Zhu, D.H.; Wu, H.; Zong, M.H.; Jing, Y.R.; Han, S.Y. Encapsulation of cinnamon essential oil in electrospun nanofibrous film for active food packaging. Food Control 2016, 59, 366–376. [Google Scholar] [CrossRef]
- Topala, C.M.; Tataru, L.D. ATR-FTIR Study of thyme and rosemary oils extracted by supercritical carbon dioxide. Rev. Chim. (Bucharest) 2016, 67, 842–846. [Google Scholar]
- Schmid, M.; Dallman, K.; Bugnicourt, E.; Cordoni, D.; Wild, F.; Lazzeri, A.; Noller, K. Properties of whey-protein-coated films and laminated as novel recyclable food packaging materials with excellent barrier properties. Int. J. Polym. Sci. 2012, 2012, 562381. [Google Scholar] [CrossRef]
- Ristić, T. Antimicrobial Medical Textiles Based on Chitosan Nanoparticles for Gynaecological Treatment. Ph.D. Thesis, University of Maribor, Maribor, Slovenia, 2014. [Google Scholar]
- Liu, M.; Zhang, Y.; Wu, C.; Xiong, S.; Zhou, C. Chitosan/halloysite nanotubes bionanocomposites: Structure, mechanical properties and biocompatibility. Int. J. Biol. Macromol. 2012, 51, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Helander, I.M.; Nurmiaho-Lassila, E.L.; Ahvenainen, R.; Rhoades, J.; Roller, S. Chitosan disrupts the barrier properties of the outer membrane of gram-negative bacteria. Int. J. Food Microbiol. 2001, 70, 235–244. [Google Scholar] [CrossRef]
- Riaz, A.; Lei, S.; Akhtar, H.M.S.; Wan, P.; Chen, D.; Jabbar, S.; Abid, M.; Hashim, M.M.; Zeng, X. Preparation and characterization of chitosan-based antimicrobial active food packaging film incorporated with apple peel polyphenols. Int. J. Biol. Macromol. 2018, 114, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food. Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Rabea, E.I.; Badawy, M.E.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Xu, Z.; Jiang, X.; Hu, C.; Zou, X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr. Res. 2004, 339, 2693–2700. [Google Scholar] [CrossRef]
- Fras Zemljič, L.; Kokol, V.; Čakara, D. Antimicrobial and antioxidant properties od chitosan-based viscose fibres enzymatically functionalized with flavonoids. Text. Res. J. 2011, 81, 1532–1540. [Google Scholar] [CrossRef]
- Ristić, T.; Hribernik, S.; Fras Zemljič, L. Electrokinetic properties of fibres functionalised by chitosan and chitosan nanoparticles. Cellulose 2015, 22, 3811–3823. [Google Scholar] [CrossRef]
- ISO22196. Plastics–Measurement of Antibacterial Activity on Plastics Surfaces; Organization for Standardization: Geneva, Switzerland, 2007. [Google Scholar]
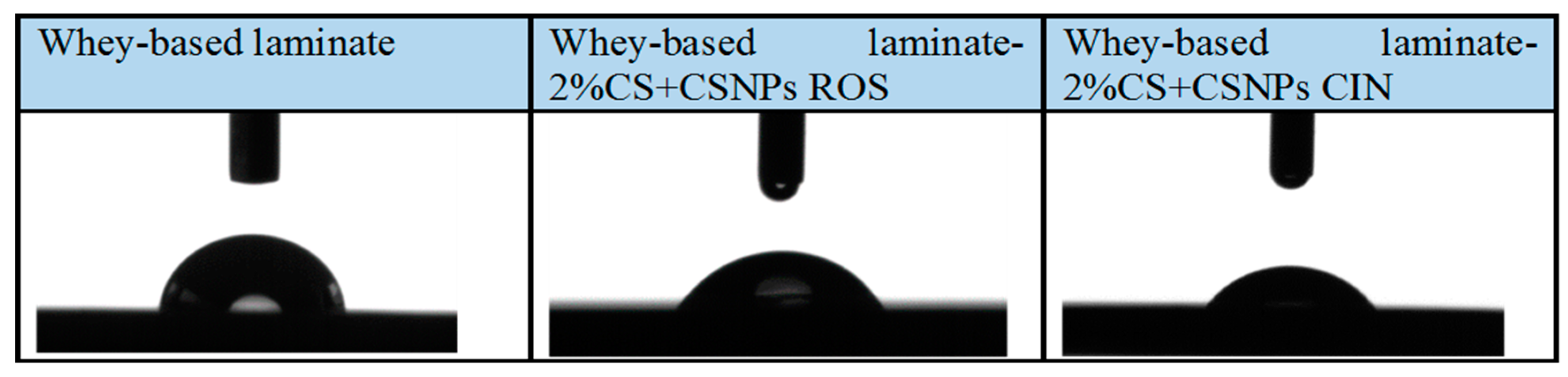
| Sample | Average Angle (α/°) | Difference (°) |
|---|---|---|
| Whey-based laminate | 75.63° ± 1.60° | / |
| Whey-based laminate-2%CS-CSNPs ROS | 65.40° ± 1.30° | −10.23° |
| Whey-based laminate-2%CS-CSNPs CIN | 58.90° ± 1.20° | −16.73° |
| Sample | O2GTR (cm3/(m224h)) | STDV O2GTR | Thickness (mm) |
|---|---|---|---|
| Whey-based laminate | 6.585 | 0.523 | 0.4 |
| Whey-based laminate-2%CS-CSNPs ROS | 84.667 | 4.272 | 0.4 |
| Whey-based laminate-2%CS-CSNPs CIN | 12.679 | 1.047 | 0.4 |
| Sample | Description |
|---|---|
| Whey-based laminate | Laminate consisting of polyethylene (PE), polyethylene terephthalate (PET) and whey protein-based layer (inner part) |
| Whey-based laminate-2%CS-CSNPs ROS | Whey-based laminate functionalised with 2% chitosan solution (2% CS) for “1. Layer” and chitosan nanoparticles with embedded rosemary extract (CSNPs ROS) for “2. Layer” |
| Whey-based laminate-2%CS-CSNPs ROS + ROS | Whey-based laminate-2% CS + CSNPs ROS additionally coated with rosemary ethanolic solution |
| Whey-based laminate-2%CS-CSNPs CIN | Whey-based laminate functionalised with 2% chitosan solution (2%CS) and chitosan nanoparticles with embedded cinnamon extract (CSNPs CIN) |
| Whey-based laminate-2%CS-CSNPs CIN + CIN | Whey-based laminate-2%CS + CSNPs CIN additionally coated with cinnamon ethanolic solution |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potrč, S.; Fras Zemljič, L.; Sterniša, M.; Smole Možina, S.; Plohl, O. Development of Biodegradable Whey-Based Laminate Functionalised by Chitosan–Natural Extract Formulations. Int. J. Mol. Sci. 2020, 21, 3668. https://doi.org/10.3390/ijms21103668
Potrč S, Fras Zemljič L, Sterniša M, Smole Možina S, Plohl O. Development of Biodegradable Whey-Based Laminate Functionalised by Chitosan–Natural Extract Formulations. International Journal of Molecular Sciences. 2020; 21(10):3668. https://doi.org/10.3390/ijms21103668
Chicago/Turabian StylePotrč, Sanja, Lidija Fras Zemljič, Meta Sterniša, Sonja Smole Možina, and Olivija Plohl. 2020. "Development of Biodegradable Whey-Based Laminate Functionalised by Chitosan–Natural Extract Formulations" International Journal of Molecular Sciences 21, no. 10: 3668. https://doi.org/10.3390/ijms21103668
APA StylePotrč, S., Fras Zemljič, L., Sterniša, M., Smole Možina, S., & Plohl, O. (2020). Development of Biodegradable Whey-Based Laminate Functionalised by Chitosan–Natural Extract Formulations. International Journal of Molecular Sciences, 21(10), 3668. https://doi.org/10.3390/ijms21103668