New Opportunity to Formulate Intranasal Vaccines and Drug Delivery Systems Based on Chitosan
Abstract
1. Introduction
2. Properties of Chitosan and Its Derivatives
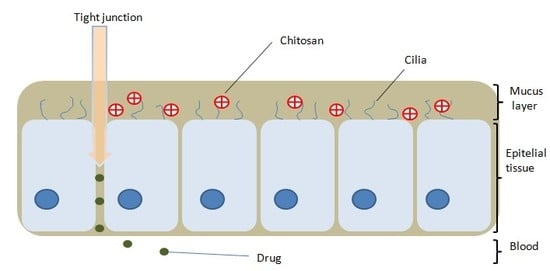
2.1. The Mucoadhesive Action and Permeability Enhancer Effect of Chitosan
2.2. Superficial Tension
2.3. Zeta Potential
2.4. Interactions of Chitosan with Other Molecules
2.4.1. Proteins
2.4.2. Lipids
2.4.3. Other
2.5. Antibacterial and Antifungal Action
2.6. Immunostimulatory Effect
2.7. Antitumor Action
2.8. Hemostatic Effect
2.9. Antioxidant Action
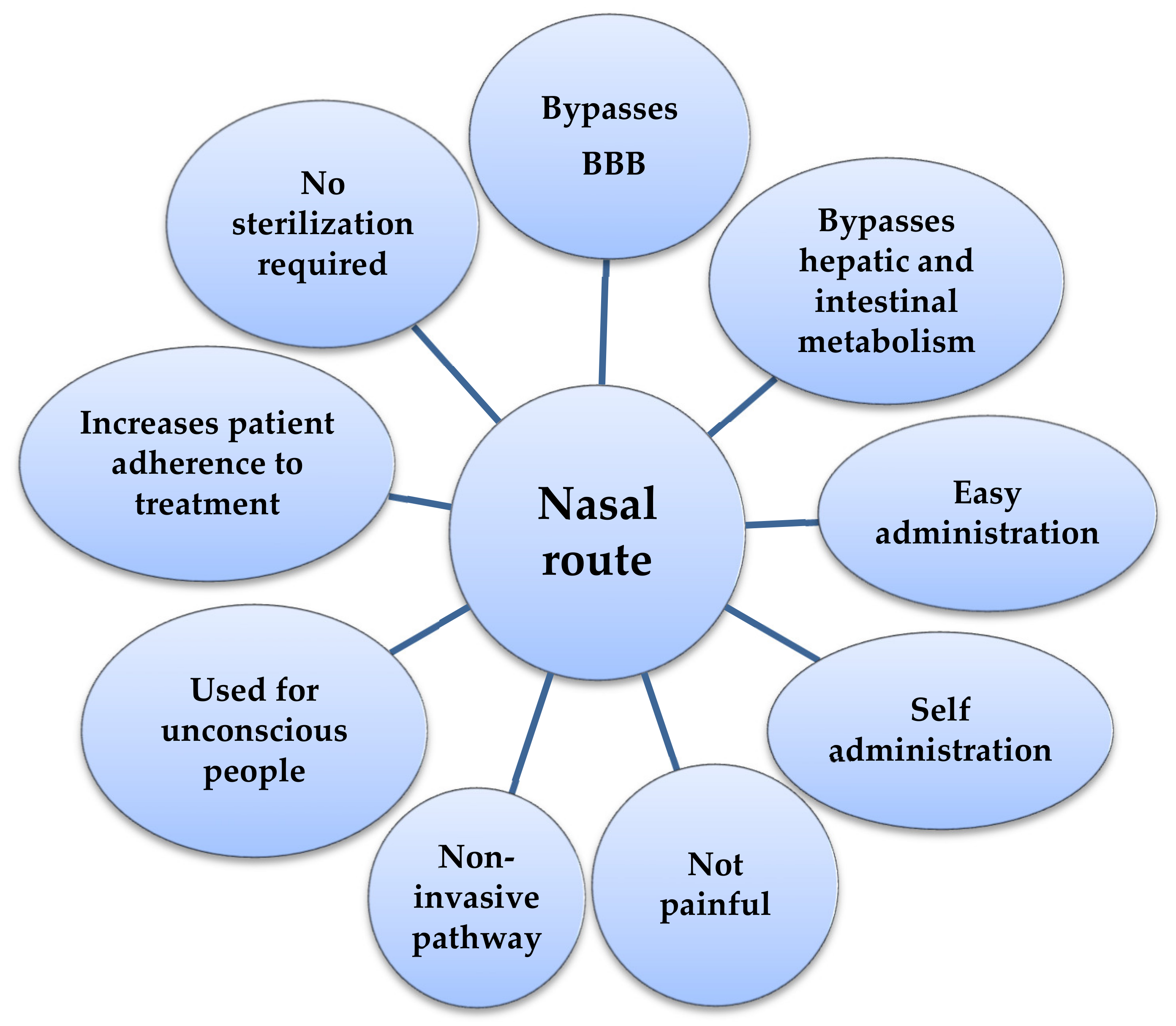
3. The Nasal Route
4. Chitosan in the Development of Intranasal Vaccines
5. Chitosan-Based Formulations for Intranasal Administration
5.1. Hydrogels
Hybrid Hydrogels
5.2. Nanoparticles
5.3. Microparticles
5.4. Powders and Microspheres
5.5. Emulsions
5.6. Nano-Lipid Carriers
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pella, M.C.G.; Lima-Tenorio, M.K.; Tenorio-Neto, E.T.; Guilherme, M.R.; Muniz, E.C.; Rubira, A.F. Chitosan-based hydrogels: From preparation to biomedical applications. Carbohydr. Polym. 2018, 196, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Mati-Baouche, N.; Elchinger, P.H.; de Baynast, H.; Pierre, G.; Delattre, C.; Michaud, P. Chitosan as an adhesive. Eur. Polym. J. 2014, 60, 198–212. [Google Scholar] [CrossRef]
- Ramirez Barragan, C.A.; Balleza, E.R.M.; Garcia-Uriostegui, L.; Ortega, J.A.A.; Toriz, G.; Delgado, E. Rheological characterization of new thermosensitive hydrogels formed by chitosan, glycerophosphate, and phosphorylated beta-cyclodextrin. Carbohydr. Polym. 2018, 201, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Gao, Q.; Lu, X.; Zhou, H. In situ forming hydrogels based on chitosan for drug delivery and tissue regeneration. Asian J. Pharm. Sci. 2016, 11, 673–683. [Google Scholar] [CrossRef]
- Elsabee, M.Z.; Morsi, R.E.; Al-Sabagh, A.M. Surface active properties of chitosan and its derivatives. Colloids Surf B Biointerfaces 2009, 74, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Pacho, M.N.; Manzano, V.E.; D’Accorso, N.B. Synthesis of micro- and nanoparticles of alginate and chitosan for controlled release of drugs. In Natural Polysaccharides in Drug Delivery and Biomedical Applications; Elsevier Science Publishing Co. Inc.: San Diego, CA, USA, 2019; pp. 363–398. [Google Scholar] [CrossRef]
- Irimia, T.; Ghica, M.V.; Popa, L.; Anuta, V.; Arsene, A.L.; Dinu-Pirvu, C.E. Strategies for Improving Ocular Drug Bioavailability and Corneal Wound Healing with Chitosan-Based Delivery Systems. Polymers 2018, 10, 1221. [Google Scholar] [CrossRef]
- Nilsen-Nygaard, J.; Strand, S.; Vårum, K.; Draget, K.; Nordgård, C. Chitosan: Gels and Interfacial Properties. Polymers 2015, 7, 552–579. [Google Scholar] [CrossRef]
- Pacheco, C.; Sousa, F.; Sarmento, B. Chitosan-based nanomedicine for brain delivery: Where are we heading? React. Funct. Polym. 2020, 146, 104430. [Google Scholar] [CrossRef]
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef]
- Pandey, S.P.; Shukla, T.; Dhote, V.K.; Mishra, D.K.; Maheshwari, R.; Tekade, R.K. Use of Polymers in Controlled Release of Active Agents. In Basic Fundamentals of Drug Delivery; Elsevier Science Publishing Co. Inc.: San Diego, CA, USA, 2019; pp. 113–172. [Google Scholar] [CrossRef]
- Pascu, B.; Ardean, C.; Davidescu, C.M.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Rusu, G. Modified Chitosan for Silver Recovery-Kinetics, Thermodynamic, and Equilibrium Studies. Materials 2020, 13, 657. [Google Scholar] [CrossRef]
- Su, S.; Kang, P.M. Systemic Review of Biodegradable Nanomaterials in Nanomedicine. Nanomaterials 2020, 10, 656. [Google Scholar] [CrossRef] [PubMed]
- Rosli, N.A.H.; Loh, K.S.; Wong, W.Y.; Yunus, R.M.; Lee, T.K.; Ahmad, A.; Chong, S.T. Review of Chitosan-Based Polymers as Proton Exchange Membranes and Roles of Chitosan-Supported Ionic Liquids. Int. J. Mol. Sci. 2020, 21, 632. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef]
- Matica, M.A.; Aachmann, F.L.; Tondervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef]
- Li, J.; Wu, X.; Shi, Q.; Li, C.; Chen, X. Effects of hydroxybutyl chitosan on improving immunocompetence and antibacterial activities. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110086. [Google Scholar] [CrossRef]
- Yan, X.; Zhou, M.; Yu, S.; Jin, Z.; Zhao, K. An overview of biodegradable nanomaterials and applications in vaccines. Vaccine 2020, 38, 1096–1104. [Google Scholar] [CrossRef]
- Szymanska, E.; Winnicka, K. Stability of chitosan-a challenge for pharmaceutical and biomedical applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef]
- Cheung, R.C.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef] [PubMed]
- Martau, G.A.; Mihai, M.; Vodnar, D.C. The Use of Chitosan, Alginate, and Pectin in the Biomedical and Food Sector-Biocompatibility, Bioadhesiveness, and Biodegradability. Polymers 2019, 11, 1837. [Google Scholar] [CrossRef]
- George, A.; Shah, P.A.; Shrivastav, P.S. Natural biodegradable polymers based nano-formulations for drug delivery: A review. Int. J. Pharm. 2019, 561, 244–264. [Google Scholar] [CrossRef] [PubMed]
- Bruinsmann, F.A.; Pigana, S.; Aguirre, T.; Souto, G.D.; Pereira, G.G.; Bianchera, A.; Fasiolo, L.T.; Colombo, G.; Marques, M.; Pohlmann, A.R.; et al. Chitosan-Coated Nanoparticles: Effect of Chitosan Molecular Weight on Nasal Transmucosal Delivery. Pharmaceutics 2019, 11, 86. [Google Scholar] [CrossRef] [PubMed]
- Di Colo, G.; Zambito, Y.; Zaino, C. Polymeric enhancers of mucosal epithelia permeability: Synthesis, transepithelial penetration-enhancing properties, mechanism of action, safety issues. J. Pharm. Sci. 2008, 97, 1652–1680. [Google Scholar] [CrossRef] [PubMed]
- TM, M.W.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers 2018, 10, 267. [Google Scholar]
- Kolawole, O.M.; Lau, W.M.; Khutoryanskiy, V.V. Methacrylated chitosan as a polymer with enhanced mucoadhesive properties for transmucosal drug delivery. Int. J. Pharm. 2018, 550, 123–129. [Google Scholar] [CrossRef]
- Krishna, R.; Yu, L.X. Biopharmaceutics Applications in Drug Development; Springer: New York, NY, USA, 2008; pp. 1–416. [Google Scholar]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef]
- Sahariah, P.; Masson, M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure-Activity Relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef]
- Zheng, M.; Qu, D.; Wang, H.; Sun, Z.; Liu, X.; Chen, J.; Li, C.; Li, X.; Chen, Z. Intranasal Administration of Chitosan Against Influenza A (H7N9) Virus Infection in a Mouse Model. Sci. Rep. 2016, 6, 28729. [Google Scholar] [CrossRef]
- Frank, L.A.; Onzi, G.R.; Morawski, A.S.; Pohlmann, A.R.; Guterres, S.S.; Contri, R.V. Chitosan as a coating material for nanoparticles intended for biomedical applications. React. Funct. Polym. 2020, 147, 104459. [Google Scholar] [CrossRef]
- Hamedi, H.; Moradi, S.; Hudson, S.M.; Tonelli, A.E. Chitosan based hydrogels and their applications for drug delivery in wound dressings: A review. Carbohydr. Polym. 2018, 199, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Irimia, T.; Dinu-Pirvu, C.E.; Ghica, M.V.; Lupuleasa, D.; Muntean, D.L.; Udeanu, D.I.; Popa, L. Chitosan-Based In Situ Gels for Ocular Delivery of Therapeutics: A State-of-the-Art Review. Mar. Drugs 2018, 16, 373. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, J.; Jiang, Z.; Tong, R.; Duan, X.; Bai, L.; Shi, J.Y. Chitosan N,N,N-trimethyl chitosan (TMC) and 2-hydroxypropyltrimethyl ammonium chloride chitosan (HTCC): The potential immune adjuvants and nano carriers. Int. J. Biol. Macromol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Tekade, M.; Maheshwari, N.; Youngren-Ortiz, S.R.; Pandey, V.; Chourasiya, Y.; Soni, V.; Deb, P.K.; Sharma, M.C. Thiolated-Chitosan: A Novel Mucoadhesive Polymer for Better-Targeted Drug Delivery. In Biomaterials and Bionanotechnology; Elsevier Science Publishing Co. Inc.: San Diego, CA, USA, 2019; pp. 459–493. [Google Scholar] [CrossRef]
- Shariatinia, Z. Pharmaceutical applications of chitosan. Adv. Colloid Interface Sci. 2019, 263, 131–194. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Li, L.M.; Gao, J.Q. Biomaterials for local drug delivery in central nervous system. Int. J. Pharm. 2019, 560, 92–100. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Ding, F. Recent advances in engineered chitosan-based nanogels for biomedical applications. J. Mater. Chem. B 2017, 5, 6986–7007. [Google Scholar] [CrossRef] [PubMed]
- Riu, J.; Giussani, B. Electrochemical biosensors for the detection of pathogenic bacteria in food. TrAC Trends Anal. Chem. 2020, 126, 116126. [Google Scholar] [CrossRef]
- Cesewski, E.; Johnson, B.N. Electrochemical biosensors for pathogen detection. Biosens. Bioelectron. 2020, 159, 112214. [Google Scholar] [CrossRef]
- Schuerer, N.; Stein, E.; Inic-Kanada, A.; Ghasemian, E.; Stojanovic, M.; Montanaro, J.; Bintner, N.; Hohenadl, C.; Sachsenhofer, R.; Barisani-Asenbauer, T. Effects of chitosan and chitosan N-acetylcysteine solutions on conjunctival epithelial cells. J. EuCornea 2018, 1, 12–18. [Google Scholar] [CrossRef]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2019. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.A.; Rodrigues, C.; Coelhoso, I.M.; Fernando, A.L. Chitosan Composites in Packaging Industry-Current Trends and Future Challenges. Polymers 2020, 12, 417. [Google Scholar] [CrossRef]
- Ngo, T.M.P.; Nguyen, T.H.; Dang, T.M.Q.; Tran, T.X.; Rachtanapun, P. Characteristics and Antimicrobial Properties of Active Edible Films Based on Pectin and Nanochitosan. Int. J. Mol. Sci. 2020, 21, 2224. [Google Scholar] [CrossRef]
- Al Harthi, S.; Alavi, S.E.; Radwan, M.A.; el Khatib, M.M.; AlSarra, I.A. Nasal delivery of donepezil HCl-loaded hydrogels for the treatment of Alzheimer’s disease. Sci. Rep. 2019, 9, 9563. [Google Scholar] [CrossRef]
- Fu, J.; Yang, F.; Guo, Z. The chitosan hydrogels: From structure to function. New J. Chem. 2018, 42, 17162–17180. [Google Scholar] [CrossRef]
- Shariatinia, Z.; Jalali, A.M. Chitosan-based hydrogels: Preparation, properties and applications. Int. J. Biol. Macromol. 2018, 115, 194–220. [Google Scholar] [CrossRef]
- Popa, L. Periodontal Chitosan-Gels Designed for Improved Local Intra-Pocket Drug Delivery. Farmacia 2013, 61, 240–250. [Google Scholar]
- Bshara, H.; Osman, R.; Mansour, S.; El-Shamy Ael, H. Chitosan and cyclodextrin in intranasal microemulsion for improved brain buspirone hydrochloride pharmacokinetics in rats. Carbohydr. Polym. 2014, 99, 297–305. [Google Scholar] [CrossRef]
- Vilchez, A.; Acevedo, F.; Cea, M.; Seeger, M.; Navia, R. Applications of Electrospun Nanofibers with Antioxidant Properties: A Review. Nanomaterials 2020, 10, 175. [Google Scholar] [CrossRef]
- Kucukoglu, V.; Uzuner, H.; Kenar, H.; Karadenizli, A. In vitro antibacterial activity of ciprofloxacin loaded chitosan microparticles and their effects on human lung epithelial cells. Int. J. Pharm. 2019, 569, 118578. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef]
- Asgari, S.; Saberi, A.H.; McClements, D.J.; Lin, M. Microemulsions as nanoreactors for synthesis of biopolymer nanoparticles. Trends Food Sci. Technol. 2019, 86, 118–130. [Google Scholar] [CrossRef]
- Khutoryanskiy, V.V. Advances in mucoadhesion and mucoadhesive polymers. Macromol. Biosci. 2011, 11, 748–764. [Google Scholar] [CrossRef]
- Huang, T.W.; Li, S.T.; Young, T.H. Chitosan-hyaluronan: Promotion of mucociliary differentiation of respiratory epithelial cells and development of olfactory receptor neurons. Artif. Cells Nanomed. Biotechnol. 2019, 47, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.W.; Wei, C.K.; Su, H.W.; Fang, K.M. Chitosan promotes aquaporin formation and inhibits mucociliary differentiation of nasal epithelial cells through increased TGF-beta1 production. J. Tissue Eng. Regen. Med. 2017, 11, 3567–3575. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, J.; Lyu, X.; Yuan, Y.; Wang, G.; Zhao, B. Chitosan-based thermosensitive hydrogel for nasal delivery of exenatide: Effect of magnesium chloride. Int. J. Pharm. 2018, 553, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Irimia, T. Contributions on Formulation and Preliminary Evaluation of Ocular Colloidal Systems of Chitosan and Poloxamer 407 with Bupivacaine Hydrochloride. Farmacia 2019, 67, 702–708. [Google Scholar] [CrossRef]
- Martins, P.P.; Smyth, H.D.C.; Cui, Z. Strategies to facilitate or block nose-to-brain drug delivery. Int. J. Pharm. 2019, 570, 118635. [Google Scholar] [CrossRef] [PubMed]
- Mura, P.; Mennini, N.; Nativi, C.; Richichi, B. In situ mucoadhesive-thermosensitive liposomal gel as a novel vehicle for nasal extended delivery of opiorphin. Eur. J. Pharm. Biopharm. 2018, 122, 54–61. [Google Scholar] [CrossRef]
- Lochhead, J.J.; Kellohen, K.L.; Ronaldson, P.T.; Davis, T.P. Distribution of insulin in trigeminal nerve and brain after intranasal administration. Sci. Rep. 2019, 9, 2621. [Google Scholar] [CrossRef]
- Akel, H.; Ismail, R.; Csoka, I. Progress and perspectives of brain-targeting lipid-based nanosystems via the nasal route in Alzheimer’s disease. Eur. J. Pharm. Biopharm. 2020. [Google Scholar] [CrossRef] [PubMed]
- Mndlovu, H.; Toit, L.C.d.; Kumar, P.; Marimuthu, T.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. Development of a fluid-absorptive alginate-chitosan bioplatform for potential application as a wound dressing. Carbohydr. Polym. 2019, 222, 114988. [Google Scholar] [CrossRef]
- Chang, S.H.; Lin, H.T.; Wu, G.J.; Tsai, G.J. pH Effects on solubility, zeta potential, and correlation between antibacterial activity and molecular weight of chitosan. Carbohydr. Polym. 2015, 134, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Gartziandia, O.; Herran, E.; Pedraz, J.L.; Carro, E.; Igartua, M.; Hernandez, R.M. Chitosan coated nanostructured lipid carriers for brain delivery of proteins by intranasal administration. Colloids Surf. B Biointerfaces 2015, 134, 304–313. [Google Scholar] [CrossRef]
- Safdar, R.; Omar, A.A.; Arunagiri, A.; Regupathi, I.; Thanabalan, M. Potential of Chitosan and its derivatives for controlled drug release applications—A review. J. Drug Deliv. Sci. Technol. 2019, 49, 642–659. [Google Scholar] [CrossRef]
- Azevedo, J.R.; RSizilio, H.; Brito, M.B.; Costa, A.M.B.; Serafini, M.R.; Araújo, A.A.S.; Santos, M.R.V.; Lira, A.A.M.; Nunes, R.S. Physical and chemical characterization insulin-loaded chitosan-TPP nanoparticles. J. Therm. Anal. Calorim. 2011, 106, 685–689. [Google Scholar] [CrossRef]
- Boonsongrit, Y.; Mitrevej, A.; Mueller, B.W. Chitosan drug binding by ionic interaction. Eur. J. Pharm. Biopharm. 2006, 62, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Vemireddy, S.; Pallavi, M.C.P.; Halmuthur, M.S. Chitosan stabilized nasal emulsion delivery system for effective humoral and cellular response against recombinant tetravalent dengue antigen. Carbohydr. Polym. 2018, 190, 129–138. [Google Scholar] [CrossRef]
- Anush, S.M.; Vishalakshi, B.; Kalluraya, B.; Manju, N. Synthesis of pyrazole-based Schiff bases of Chitosan: Evaluation of antimicrobial activity. Int. J. Biol. Macromol. 2018, 119, 446–452. [Google Scholar] [CrossRef]
- Lim, J.Y.C.; Lin, Q.; Xue, K.; Loh, X.J. Recent advances in supramolecular hydrogels for biomedical applications. Mater. Today Adv. 2019, 3, 100021. [Google Scholar] [CrossRef]
- Fini, A.; Orienti, I. The Role of Chitosan in Drug Delivery. Am. J. Drug Deliv. 2003, 1, 43–59. [Google Scholar] [CrossRef]
- Benltoufa, S.; Miled, W.; Trad, M.; Slama, R.B.; Fayala, F. Chitosan hydrogel-coated cellulosic fabric for medical end-use: Antibacterial properties, basic mechanical and comfort properties. Carbohydr. Polym. 2020, 227, 115352. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Qian, J.; Fan, J.; Guo, H.; Gou, L.; Yang, H.; Liang, C. Preparation nanoparticle by ionic cross-linked emulsified chitosan and its antibacterial activity. Colloids Surf. A Physicochem. Eng. Asp. 2019, 568, 362–370. [Google Scholar] [CrossRef]
- Ardila, N.; Daigle, F.; Heuzey, M.C.; Ajji, A. Effect of Chitosan Physical Form on Its Antibacterial Activity Against Pathogenic Bacteria. J. Food Sci. 2017, 82, 679–686. [Google Scholar] [CrossRef]
- Alavi, M.; Nokhodchi, A. An overview on antimicrobial and wound healing properties of ZnO nanobiofilms, hydrogels, and bionanocomposites based on cellulose, chitosan, and alginate polymers. Carbohydr. Polym. 2020, 227, 115349. [Google Scholar] [CrossRef]
- Yu, Z.; Rao, G.; Wei, Y.; Yu, J.; Wu, S.; Fang, Y. Preparation, characterization, and antibacterial properties of biofilms comprising chitosan and epsilon-polylysine. Int. J. Biol. Macromol. 2019, 141, 545–552. [Google Scholar] [CrossRef]
- Cao, W.; Yue, L.; Wang, Z. High antibacterial activity of chitosan - molybdenum disulfide nanocomposite. Carbohydr. Polym. 2019, 215, 226–234. [Google Scholar] [CrossRef]
- Kegere, J.; Ouf, A.; Siam, R.; Mamdouh, W. Fabrication of Poly(vinyl alcohol)/Chitosan/Bidens pilosa Composite Electrospun Nanofibers with Enhanced Antibacterial Activities. ACS Omega 2019, 4, 8778–8785. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Sun, Y.; Cui, H.; Zhu, S.J.; Qiu, H.J. Mucosal vaccines: Strategies and challenges. Immunol. Lett. 2020, 217, 116–125. [Google Scholar] [CrossRef]
- Yang, Y.; Xing, R.; Liu, S.; Qin, Y.; Li, K.; Yu, H.; Li, P. Chitosan hydroxypropyltrimethyl ammonium chloride chitosan and sulfated chitosan nanoparticles as adjuvants for inactivated Newcastle disease vaccine. Carbohydr. Polym. 2020, 229, 115423. [Google Scholar] [CrossRef]
- Miranda-Calderón, J.E.; Macías-Rosales, L.; Gracia-Mora, I.; Ruiz-Azuara, L.; Faustino-Vega, A.; Gracia-Mora, J.; Bernad-Bernad, M.J. Effect of casiopein III-ia loaded into chitosan nanoparticles on tumor growth inhibition. J. Drug Deliv. Sci. Technol. 2018, 48, 1–8. [Google Scholar] [CrossRef]
- Radwan-Pragłowska, J.; Pi, M.; Deineka, V.; Janus, Ł.; Korniienko, V.; Husak, E.; Holubnycha, V.; Liubchak, I.; Zhurba, V.; Sierakowska, A.; et al. Chitosan-Based Bioactive Hemostatic Agents with Antibacterial Properties—Synthesis and Characterization. Molecules 2019, 24, 2629. [Google Scholar] [CrossRef]
- Guo, X.; Wang, C.; Sun, T.; Zhong, R.; Ma, L.; You, C.; Li, H.; Tian, M. Effects of Chitosan Oligosaccharides on Human Blood Components. Front. Pharmacol. 2018, 9, 1412. [Google Scholar] [CrossRef]
- Hu, Z.; Lu, S.; Cheng, Y.; Kong, S.; Li, S.; Li, C.; Yang, L. Investigation of the Effects of Molecular Parameters on the Hemostatic Properties of Chitosan. Molecules 2018, 23, 3147. [Google Scholar] [CrossRef]
- Tamer, T.M.; Valachová, K.; Mohyeldin, M.S.; Soltes, L. Free radical scavenger activity of chitosan and its aminated derivative. J. Appl. Pharm. Sci. 2016, 6, 195–201. [Google Scholar] [CrossRef]
- Luan, F.; Wei, L.; Zhang, J.; Mi, Y.; Dong, F.; Li, Q.; Guo, Z. Antioxidant Activity and Antifungal Activity of Chitosan Derivatives with Propane Sulfonate Groups. Polymers 2018, 10, 395. [Google Scholar] [CrossRef]
- Hua, Y.; Ma, C.; Wei, T.; Zhang, L.; Shen, J. Collagen/Chitosan Complexes: Preparation, Antioxidant Activity, Tyrosinase Inhibition Activity, and Melanin Synthesis. Int. J. Mol. Sci. 2020, 21, 313. [Google Scholar] [CrossRef]
- Trotta, V.; Pavan, B.; Ferraro, L.; Beggiato, S.; Traini, D.; Reis, L.G.D.; Scalia, S.; Dalpiaz, A. Brain targeting of resveratrol by nasal administration of chitosan-coated lipid microparticles. Eur. J. Pharm. Biopharm. 2018, 127, 250–259. [Google Scholar] [CrossRef]
- Rohrer, J.; Lupo, N.; Bernkop-Schnurch, A. Advanced formulations for intranasal delivery of biologics. Int. J. Pharm. 2018, 553, 8–20. [Google Scholar] [CrossRef]
- Feng, Y.; He, H.; Li, F.; Lu, Y.; Qi, J.; Wu, W. An update on the role of nanovehicles in nose-to-brain drug delivery. Drug Discov. Today 2018, 23, 1079–1088. [Google Scholar] [CrossRef]
- Chaud, M.V.; Rios, A.C.; Santos, C.A.d.; de Barros, C.T.; de Souza, J.F.; Alves, T.F.R. Nanostructure self-assembly for direct nose-to-brain drug delivery. In Nanomycotoxicology; Elsevier Science Publishing Co. Inc.: San Diego, CA, USA, 2020; pp. 449–480. [Google Scholar] [CrossRef]
- Karavasili, C.; Fatouros, D.G. Smart materials: In situ gel-forming systems for nasal delivery. Drug Discov. Today 2016, 21, 157–166. [Google Scholar] [CrossRef]
- Kurti, L.; Gaspar, R.; Marki, A.; Kapolna, E.; Bocsik, A.; Veszelka, S.; Bartos, C.; Ambrus, R.; Vastag, M.; Deli, M.A.; et al. In vitro and in vivo characterization of meloxicam nanoparticles designed for nasal administration. Eur. J. Pharm. Sci. 2013, 50, 86–92. [Google Scholar] [CrossRef]
- Dalpiaz, A.; Fogagnolo, M.; Ferraro, L.; Capuzzo, A.; Pavan, B.; Rassu, G.; Salis, A.; Giunchedi, P.; Gavini, E. Nasal chitosan microparticles target a zidovudine prodrug to brain HIV sanctuaries. Antivir. Res. 2015, 123, 146–157. [Google Scholar] [CrossRef]
- Rassu, G.; Soddu, E.; Cossu, M.; Gavini, E.; Giunchedi, P.; Dalpiaz, A. Particulate formulations based on chitosan for nose-to-brain delivery of drugs. A review. J. Drug Deliv. Sci. Technol. 2016, 32, 77–87. [Google Scholar] [CrossRef]
- Abdel Mouez, M.; Zaki, N.M.; Mansour, S.; Geneidi, A.S. Bioavailability enhancement of verapamil HCl via intranasal chitosan microspheres. Eur. J. Pharm. Sci. 2014, 51, 59–66. [Google Scholar] [CrossRef]
- Mitsou, E.; Pletsa, V.; Sotiroudis, G.T.; Panine, P.; Zoumpanioti, M.; Xenakis, A. Development of a microemulsion for encapsulation and delivery of gallic acid. The role of chitosan. Colloids Surf. B Biointerfaces 2020, 190, 110974. [Google Scholar] [CrossRef]
- Salade, L.; Wauthoz, N.; Vermeersch, M.; Amighi, K.; Goole, J. Chitosan-coated liposome dry-powder formulations loaded with ghrelin for nose-to-brain delivery. Eur. J. Pharm. Biopharm. 2018, 129, 257–266. [Google Scholar] [CrossRef]
- Dehghan, S.; Tafaghodi, M.; Bolourieh, T.; Mazaheri, V.; Torabi, A.; Abnous, K.; Kheiri, M.T. Rabbit nasal immunization against influenza by dry-powder form of chitosan nanospheres encapsulated with influenza whole virus and adjuvants. Int. J. Pharm. 2014, 475, 1–8. [Google Scholar] [CrossRef]
- Rajput, A.P.; Butani, S.B. Resveratrol anchored nanostructured lipid carrier loaded in situ gel via nasal route: Formulation, optimization and in vivo characterization. J. Drug Deliv. Sci. Technol. 2019, 51, 214–223. [Google Scholar] [CrossRef]
- Sonje, A.G.; Mahajan, H.S. Nasal inserts containing ondansetron hydrochloride based on Chitosan-gellan gum polyelectrolyte complex: In vitro-in vivo studies. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 64, 329–335. [Google Scholar] [CrossRef]
- Wang, Q.; Zuo, Z.; Cheung, C.K.C.; Leung, S.S.Y. Updates on thermosensitive hydrogel for nasal, ocular and cutaneous delivery. Int. J. Pharm. 2019, 559, 86–101. [Google Scholar] [CrossRef]
- Khosa, A.; Saha, R.N.; Singhvi, G. Drug delivery to the brain. In Nanomaterials for Drug Delivery and Therapy; Elsevier Science Publishing Co. Inc.: San Diego, CA, USA, 2019; pp. 461–514. [Google Scholar] [CrossRef]
- Fan, L.W.; Carter, K.; Bhatt, A.; Pang, Y. Rapid transport of insulin to the brain following intranasal administration in rats. Neural Regen. Res. 2019, 14, 1046–1051. [Google Scholar]
- Soni, V.; Pandey, V.; Asati, S.; Jain, P.; Tekade, R.K. Design and Fabrication of Brain-Targeted Drug Delivery. In Basic Fundamentals of Drug Delivery; Elsevier Science Publishing Co. Inc.: San Diego, CA, USA, 2019; pp. 539–593. [Google Scholar] [CrossRef]
- Khan, A.R.; Liu, M.; Khan, M.W.; Zhai, G. Progress in brain targeting drug delivery system by nasal route. J. Control. Release 2017, 268, 364–389. [Google Scholar] [CrossRef]
- Santiago, J.C.P.; Hallschmid, M. Outcomes and clinical implications of intranasal insulin administration to the central nervous system. Exp. Neurol. 2019, 317, 180–190. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Tripathi, P.; Gupta, R.; Pandey, S. Niosomes: A review on niosomal research in the last decade. J. Drug Deliv. Sci. Technol. 2020, 56, 101581. [Google Scholar] [CrossRef]
- Zheng, H.; Pan, L.; Lv, J.; Zhang, Z.; Wang, Y.; Hu, W.; Liu, X.; Zhou, P.; Wang, Y.; Zhang, Y. Comparison of immune responses in guinea pigs by intranasal delivery with different nanoparticles-loaded FMDV DNA vaccine. Microb. Pathog. 2020, 142, 104061. [Google Scholar] [CrossRef]
- Wu, D.; Zhu, L.; Li, Y.; Zhang, X.; Xu, S.; Yang, G.; Delair, T. Chitosan-based Colloidal Polyelectrolyte Complexes for Drug Delivery: A Review. Carbohydr. Polym. 2020. [Google Scholar] [CrossRef]
- Gilavand, F.; Marzban, A.; Ebrahimipour, G.; Soleimani, N.; Goudarzi, M. Designation of chitosan nano-vaccine based on MxiH antigen of Shigella flexneri with increased immunization capacity. Carbohydr. Polym. 2020, 232, 115813. [Google Scholar] [CrossRef]
- Kulkarni, A.D.; Patel, H.M.; Surana, S.J.; Vanjari, Y.H.; Belgamwar, V.S.; Pardeshi, C.V. N,N,N-Trimethyl chitosan: An advanced polymer with myriad of opportunities in nanomedicine. Carbohydr. Polym. 2017, 157, 875–902. [Google Scholar] [CrossRef]
- Mosafer, J.; Sabbaghi, A.H.; Badiee, A.; Dehghan, S.; Tafaghodi, M. Preparation, characterization and in vivo evaluation of alginate-coated chitosan and trimethylchitosan nanoparticles loaded with PR8 influenza virus for nasal immunization. Asian J. Pharm. Sci. 2019, 14, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wei, L.; Wang, H. Progress and perspectives on nanoplatforms for drug delivery to the brain. J. Drug Deliv. Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Huang, H.; Qi, X.; Chen, Y.; Wu, Z. Thermo-sensitive hydrogels for delivering biotherapeutic molecules: A review. Saudi Pharm. J. 2019, 27, 990–999. [Google Scholar] [CrossRef]
- Pandey, V.; Gadeval, A.; Asati, S.; Jain, P.; Jain, N.; Roy, R.K.; Tekade, M.; Soni, V.; Tekade, R.K. Formulation strategies for nose-to-brain delivery of therapeutic molecules. In Drug Delivery Systems; Elsevier Science Publishing Co. Inc.: San Diego, CA, USA, 2020; pp. 291–332. [Google Scholar] [CrossRef]
- Ghasemi Tahrir, F.; Ganji, F.; Mani, A.R.; Khodaverdi, E. In vitro and in vivo evaluation of thermosensitive chitosan hydrogel for sustained release of insulin. Drug Deliv. 2016, 23, 1038–1046. [Google Scholar] [CrossRef]
- Naik, A.; Nair, H. Formulation and Evaluation of Thermosensitive Biogels for Nose to Brain Delivery of Doxepin. BioMed Res. Int. 2014, 2014, 847547. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.K.; Gupta, P.N.; Khanna, A.; Sharma, R.K.; Chandrabanshi, H.K.; Gupta, N.; Patil, U.K.; Yadav, S.K. Development and characterization of in situ gel system for nasal insulin delivery. Pharmazie 2010, 65, 188–193. [Google Scholar] [PubMed]
- Palmese, L.L.; Thapa, R.K.; Sullivan, M.O.; Kiick, K.L. Hybrid hydrogels for biomedical applications. Curr. Opin. Chem. Eng. 2019, 24, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Aderibigbe, B.A. In Situ-Based Gels for Nose to Brain Delivery for the Treatment of Neurological Diseases. Pharmaceutics 2018, 10, 40. [Google Scholar] [CrossRef]
- Bhattamisra, S.K.; Shak, A.T.; Xi, L.W.; Safian, N.H.; Choudhury, H.; Lim, W.M.; Shahzad, N.; Alhakamy, N.A.; Anwer, M.K.; Radhakrishnan, A.K.; et al. Nose to brain delivery of rotigotine loaded chitosan nanoparticles in human SH-SY5Y neuroblastoma cells and animal model of Parkinson’s disease. Int. J. Pharm. 2020, 579, 119148. [Google Scholar] [CrossRef]
- Kulkarni, A.D.; Bari, D.B.; Surana, S.J.; Pardeshi, C.V. In vitro, ex vivo and in vivo performance of chitosan-based spray-dried nasal mucoadhesive microspheres of diltiazem hydrochloride. J. Drug Deliv. Sci. Technol. 2016, 31, 108–117. [Google Scholar] [CrossRef]
- Fachel, F.N.S.; Medeiros-Neves, B.; Pra, M.D.; Schuh, R.S.; Veras, K.S.; Bassani, V.L.; Koester, L.S.; Henriques, A.T.; Braganhol, E.; Teixeira, H.F. Box-Behnken design optimization of mucoadhesive chitosan-coated nanoemulsions for rosmarinic acid nasal delivery-In vitro studies. Carbohydr. Polym. 2018, 199, 572–582. [Google Scholar] [CrossRef]
- Choudhury, H.; Zakaria, N.F.B.; Tilang, P.A.B.; Tzeyung, A.S.; Pandey, M.; Chatterjee, B.; Alhakamy, N.A.; Bhattamishra, S.K.; Kesharwani, P.; Gorain, B.; et al. Formulation development and evaluation of rotigotine mucoadhesive nanoemulsion for intranasal delivery. J. Drug Deliv. Sci. Technol. 2019, 54, 101301. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Y.; Pang, Z. Chitosan in drug delivery applications. In Natural Polysaccharides in Drug Delivery and Biomedical Applications; Elsevier Science Publishing Co. Inc.: San Diego, CA, USA, 2019; pp. 101–119. [Google Scholar] [CrossRef]
- Yu, S.; Xu, X.; Feng, J.; Liu, M.; Hu, K. Chitosan and chitosan coating nanoparticles for the treatment of brain disease. Int. J. Pharm. 2019, 560, 282–293. [Google Scholar] [CrossRef]
- Ansari, R.; Sadati, S.M.; Mozafari, N.; Ashrafi, H.; Azadi, A. Carbohydrate polymer-based nanoparticle application in drug delivery for CNS-related disorders. Eur. Polym. J. 2020, 128, 109607. [Google Scholar] [CrossRef]
- Tiozzo Fasiolo, L.; Manniello, M.D.; Tratta, E.; Buttini, F.; Rossi, A.; Sonvico, F.; Bortolotti, F.; Russo, P.; Colombo, G. Opportunity and challenges of nasal powders: Drug formulation and delivery. Eur. J. Pharm. Sci. 2018, 113, 2–17. [Google Scholar] [CrossRef]
- Deb, P.K.; Al-Attraqchi, O.; Chandrasekaran, B.; Paradkar, A.; Tekade, R.K. Protein/Peptide Drug Delivery Systems. In Basic Fundamentals of Drug Delivery; Elsevier Science Publishing Co. Inc.: San Diego, CA, USA, 2019; pp. 651–684. [Google Scholar] [CrossRef]
- Rassu, G.; Ferraro, L.; Pavan, B.; Giunchedi, P.; Gavini, E.; Dalpiaz, A. The Role of Combined Penetration Enhancers in Nasal Microspheres on In Vivo Drug Bioavailability. Pharmaceutics 2018, 10, 206. [Google Scholar] [CrossRef] [PubMed]
- Nirale, P.; Paul, A.; Yadav, K.S. Nanoemulsions for targeting the neurodegenerative diseases: Alzheimer’s, Parkinson’s and Prion’s. Life Sci. 2020, 245, 117394. [Google Scholar] [CrossRef] [PubMed]
- Bourganis, V.; Kammona, O.; Alexopoulos, A.; Kiparissides, C. Recent advances in carrier mediated nose-to-brain delivery of pharmaceutics. Eur. J. Pharm. Biopharm. 2018, 128, 337–362. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Saraf, S.; Saraf, S.; Dubey, S.K.; Puri, A.; Patel, R.J.; Ajazuddin; Ravichandiran, V.; Murty, U.S.; Alexander, A. Recent strategies and advances in the fabrication of nano lipid carriers and their application towards brain targeting. J. Control. Release 2020, 321, 372–415. [Google Scholar] [CrossRef]
- Salvi, V.R.; Pawar, P. Nanostructured lipid carriers (NLC) system: A novel drug targeting carrier. J. Drug Deliv. Sci. Technol. 2019, 51, 255–267. [Google Scholar] [CrossRef]


| No. | Polymer | Molecule | Binding Type | Outcomes | Index |
|---|---|---|---|---|---|
| 1 | Chitosan | Insulin | Hydrophobic interactions Electrostatic interactions Hydrogen bonds | Conformational alteration | [67] |
| 2 | Insulin | Ionic bonds | Increased absorption | [68] | |
| 3 | Trypsin and trypsin inhibitors | Van der Waals bonds Hydrogen bonds | Improves stability | [36] | |
| 4 | HSA | Electrostatic interactions | Conformational changes | [36] | |
| 5 | BSA | Hydrophobic interactions | Conformational modification | [36] | |
| 6 | Lipids | Electrostatic interactions Hydrophobic interactions Hydrogen bonds | Improves system stability | [5] | |
| 7 | Indomethacin | Ionic bonds Electrostatic interactions | Increase solubility | [68] | |
| 8 | Pyrazole-4-carbaldehyde | Substitution reaction | Shiff base with superior antibacterial and antifungal effects | [70] | |
| 9 | Pluronic-CHO-benzaldehyde | Substitution reaction | Schiff Base; hydrogel with joint-like properties | [71] | |
| 10 | Carboxymethyl chitosan | PEG | Substitution reaction | Schiff Base; hemostatic material | [71] |
| 11 | N,O-carboxymethyl chitosan | Oxidized hyaluronic acid | Substitution reaction | Schiff Base: hydrogel that prevents postoperative adhesion | [71] |
| Drug Delivery Systems | Content of Chitosan (% w/v) | Drug | Results | References |
|---|---|---|---|---|
| Hydrogel | 2 | Doxepin | The residence time in the nasal cavity Is increased and the drug bypass the blood brain barrier. | [119] |
| Hydrogel | 1; 2; 3; 4; 5 | Insulin | Decrease the mucociliary clearance leading to a slow release; insulin didn’t suffer any degradation during transport to the brain. | [120] |
| Nanoparticle | 0.05 | Rotigotine | Increase bioavailability and a higher concentration in the brain is achieved. | [123] |
| Microparticle | 1.75 | Resveratrol | The absorption of the drug is increased by opening the tight epithelial junction and enhanced the contact time. | [89] |
| Microsphere | 2 | Diltiazem | The effective dose required is less than the oral dose. | [124] |
| Emulsion | 0.1; 0.3; 0.5 | Rosmarinic acid | The penetration capacity is higher, and the release is slow. | [125] |
| Emulsion | 1 | Rotigotine | Prolonged release and increased absorption. | [126] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popescu, R.; Ghica, M.V.; Dinu-Pîrvu, C.-E.; Anuța, V.; Lupuliasa, D.; Popa, L. New Opportunity to Formulate Intranasal Vaccines and Drug Delivery Systems Based on Chitosan. Int. J. Mol. Sci. 2020, 21, 5016. https://doi.org/10.3390/ijms21145016
Popescu R, Ghica MV, Dinu-Pîrvu C-E, Anuța V, Lupuliasa D, Popa L. New Opportunity to Formulate Intranasal Vaccines and Drug Delivery Systems Based on Chitosan. International Journal of Molecular Sciences. 2020; 21(14):5016. https://doi.org/10.3390/ijms21145016
Chicago/Turabian StylePopescu, Roxana, Mihaela Violeta Ghica, Cristina-Elena Dinu-Pîrvu, Valentina Anuța, Dumitru Lupuliasa, and Lăcrămioara Popa. 2020. "New Opportunity to Formulate Intranasal Vaccines and Drug Delivery Systems Based on Chitosan" International Journal of Molecular Sciences 21, no. 14: 5016. https://doi.org/10.3390/ijms21145016
APA StylePopescu, R., Ghica, M. V., Dinu-Pîrvu, C.-E., Anuța, V., Lupuliasa, D., & Popa, L. (2020). New Opportunity to Formulate Intranasal Vaccines and Drug Delivery Systems Based on Chitosan. International Journal of Molecular Sciences, 21(14), 5016. https://doi.org/10.3390/ijms21145016