Abstract
Hypoxia, or lack of oxygen, can occur in both physiological (high altitude) and pathological conditions (respiratory diseases). In this narrative review, we introduce high altitude pulmonary edema (HAPE), acute respiratory distress syndrome (ARDS), Chronic Obstructive Pulmonary Disease (COPD), and Cystic Fibrosis (CF) as examples of maladaptation to hypoxia, and highlight some of the potential mechanisms influencing the prognosis of the affected patients. Among the specific pathways modulated in response to hypoxia, iron metabolism has been widely explored in recent years. Recent evidence emphasizes hepcidin as highly involved in the compensatory response to hypoxia in healthy subjects. A less investigated field in the adaptation to hypoxia is the sphingolipid (SPL) metabolism, especially through Ceramide and sphingosine 1 phosphate. Both individually and in concert, iron and SPL are active players of the (mal)adaptation to physiological hypoxia, which can result in the pathological HAPE. Our aim is to identify some pathways and/or markers involved in the physiological adaptation to low atmospheric pressures (high altitudes) that could be involved in pathological adaptation to hypoxia as it occurs in pulmonary inflammatory diseases. Hepcidin, Cer, S1P, and their interplay in hypoxia are raising growing interest both as prognostic factors and therapeutical targets.
1. Introduction
Hypoxia is characterized by a decrease in the partial pressure of oxygen in the blood and a consequent decreased supply of oxygen to the tissues. This condition can occur both in para-physiological conditions (subjects exposed to low atmospheric pressures, at high altitudes) and as a consequence of respiratory pathologies such as Acute Respiratory Distress Syndrome (ARDS), Chronic Obstructive Pulmonary Disease (COPD), and Cystic Fibrosis (CF). In such pathologies, as long as in the maladaptation to high altitude which results in High Altitude Pulmonary Edema (HAPE), both the hypoxia-induced and inflammatory pathways play a key role in determining the disease progression. Inflammation is one of the main mechanisms influencing the maladaptation to hypoxia, especially when causing a chronic decrease in hemoglobin (Hb) production, a phenomenon known as anemia of inflammation.
Alveolar hypoxia itself is a strong inducer of pulmonary inflammation. It is well recognized that it generates reactive oxygen species (ROS) and that it promotes nuclear factor kappa B (NFĸB) activation, which in turn upregulates numerous pro-inflammatory cytokines such as IL-1, IL-6, and TNFα [1]. Excess ROS production, enhanced by the presence of unbound iron through the Fenton reaction [2], results in lipid peroxidation [3] and in the decrease in vasodilator NO pool [2]. Chen and colleagues observed that alveolar hypoxia triggers pulmonary vasoconstriction and systemic inflammation by activating alveolar macrophages that in turn promote the ET-1, iNOS, and NO/cGMP pathways [4]. In a model of inflammation following aspiration-induced lung injury, HIF-1α robust increase upregulates the release of pro-inflammatory cytokines depending on NF-κB signaling in type 2 alveolar epithelial cells [5].
In this narrative review, we mention respiratory pathologies as examples of either acute (HAPE and ARDS) or chronic (CF and COPD) hypoxia, and highlight some of the potential mechanisms influencing the prognosis of patients. We will focus on the roles of iron and sphingolipid (SPL) metabolism in determining the adaptation or maladaptation to hypoxia, either physiological or pathological. Upon an overview of the role of iron and SPL in hypoxia, we started by reviewing studies investigating the effects of hypoxia on healthy subjects at high altitude. Then, we looked for studies assessing the most relevant markers that could correlate with prognosis and therapy in hypoxic patients. Our aim is to identify pathways and/or markers involved in the para-physiological adaptation to low atmospheric pressures (high altitudes) that could play a key role in pathological adaptation to hypoxia conditions such as HAPE, or respiratory pathologies such as ARDS, COPD, and CF.
2. Maladaptation to Hypoxia: The Case of HAPE and Respiratory Pathologies
2.1. High-Altitude Pulmonary Edema
HAPE is a potentially fatal condition, occurring at altitudes greater than 3,000 m. It affects rapidly ascending, non-acclimatized healthy individuals and it is the leading cause of death due to high-altitude illnesses [6]. At 3000 m, the reduction in mitochondrial PO2 due to the hypobaric hypoxia induces dysfunctions into the electron transport chain that fails in providing cellular energy. As recently reviewed by Montgomery and colleagues [7], the hypoxic pulmonary vasoconstriction (HPV) that redistributes the pulmonary perfusion and the activation of HIF-1α-mediated pathways that ameliorate the oxygen availability can override the regional hypoxia in healthy subjects. In the case of HAPE, hypoxia is not regional, and this leads to exaggerated HPV which may end up in overperfusion of the non-constricted pulmonary vessels [8] and eventually in pulmonary hypertension and edema. Currently, misdiagnosis is quite frequent due to the broad heterogeneity of clinical symptoms and the characterization of metabolites involved in the pathophysiology of HAPE would be of great value.
2.2. Acute Respiratory Distress Syndrome
ARDS is an acute condition characterized by sudden onset of severe hypoxemia without evidence of heart failure or volume overload [9]. As it is a severe condition with multiple and complex etiology, it requires hospitalization in the Intensive Care Unit (ICU), and has a high mortality (35–46%) [10]. The main feature of ARDS is an increase in pulmonary capillary permeability. Protein-rich fluids accumulate inside the alveoli, as a result of the damage to the capillary endothelium and alveolar epithelium [11]. In several patients, ARDS is associated with low Hb despite persisting hypoxia, down to severe anemia (<8 g/dl Hb), which correlates with worse prognosis [12,13]. Frequent among ICU patients [14], this anemia may be due to inflammation.
2.3. Chronic Obstructive Pulmonary Disease
COPD is a prevalent airway disease functionally characterized by non or partly reversible proximal bronchial obstruction that is a major cause of respiratory disability [15]. Exposure to inhaled pollutants and most often to cigarette smoke [16] leads to chronic airway and lung inflammation that is regarded to promote structural changes, obstructions, and respiratory symptoms [17]. Small airways (inner diameter less than 2 mm) give the major contribution to airflow limitation in COPD since intraluminal obstruction could occur for mucus or substances deposition or for increased wall thickness [18]. Moreover, loss of alveolar attachment could alter the luminal diameter by means of small airway distortion [19]. Pulmonary vascular dysfunction, vascular remodeling, and pulmonary hypertension precede development of alveolar destruction, as seen in the lungs of a mouse model of emphysema and in lung tissue from humans with end-stage COPD [20].
Beyond small airway obstruction, pulmonary vascular abnormalities triggered by the inflammatory process may participate in COPD disability. Hypoxia-induced vascular changes may characterize the early onset as well as the disease evolution of COPD, contributing to pulmonary hypertension and heart dysfunction [21]. Due to hypoxia-mediated lung vessel remodeling, COPD patients exhibit structural alterations of the intimal layer in small pulmonary arteries [22], vessel wall thickening, proliferation of vascular smooth muscle, and infiltration of inflammatory cells [23].
Low levels of oxygen activate HIF-1α that controls the transcription of genes regulating angiogenesis, vascular remodeling, and glucose metabolism [24]. In response to HIF-1α, platelet derived growth factor β (PDGF β) is released in the endothelium, thus favoring the vasodilation of pulmonary arterial smooth muscle [25] together with vascular endothelial growth factor (VEGF), involved in tissue remodeling and angiogenesis in COPD [26].
2.4. Cystic Fibrosis
CF is a fatal genetic disorder caused by dysfunction of the anion transporter cystic fibrosis transmembrane conductance regulator (CFTR), which is expressed on the apical membrane of epithelial secretory cells. It involves several organs, but mortality is mainly due to lung disease. The reduced epithelial chloride transport and the excessive sodium reabsorption lead to dehydrated surface airway liquid, increased viscosity of the mucus layer, and plugging of the airway lumen [27,28]. Lumen obstruction caused by mucus plaques together with increased epithelial oxygen consumption probably due to enhanced ENaC-mediated sodium transport [27,29], create regional hypoxic niches within airway epithelial cells [30]. CF patients are characterized by perfusion deficit and the dysfunctional CFTR impairs the HIF-1α stabilization and activity thus affecting the adaptive response to hypoxia. The unresolved regional hypoxia exacerbates neutrophilic sterile inflammation that is triggered by the release of IL-1α. In fact, the hypoxic stimulus promotes the necrosis of airway cells and the consequent passive release of IL-1α together with the activation of NLRP3 inflammasome that in turn results in the active release of IL-1α [7].
3. The Variegate Pathways Involved in Hypoxia Adaptation
Hypoxia activates rho kinase, inducing vasoconstriction [31]. Hypoxic pulmonary vasoconstriction (HPV) is known as an adaptive mechanism that optimizes pulmonary ventilation-perfusion matching in regional hypoxia. Such phenomenon can be both protective and harmful, depending on the hypoxia condition to which the subject is exposed. In case of global hypoxia, as it happens at high altitude, or if the respiratory impairment involves most of the lung area, HPV can be harmful, as a potential cause of pulmonary hypertension [8,32]. On the other hand, when hypoxia is restricted to a confined area of the lung, HPV allows the blood flow to be deviated to the better ventilated compartments, in order to compensate the local lack of O2. The mechanism triggering HPV involves an impairment in the redox equilibrium that triggers the smooth cells’ contraction [8].
The main mechanism of adaptation involves the inducible transcription factors HIF-1α and HIF-2α, which enhance the transcription of several proteins with the aim of increasing oxygen availability [33]. One of these proteins, upregulated through HIF-2 is erythropoietin [34], which causes an increase in hematopoiesis and circulating Hb, as an attempt to optimize the transport of the available oxygen [35], Hb levels increase with altitude in acclimatized newcomers, but vary among high-altitude populations [36]. Higher Hb values are common among the Oromo population of East Africa, which lives at 4000 m above sea level, as compared to the values of a population living at lower altitude [37]. An excessive increase in hemoglobin can promote cardiovascular disease and pulmonary hypertension in those populations [38].
Recently, iron and SPL metabolisms have been considered as potentially involved in hypoxia adaptation response.
The relationship between hypoxia and iron metabolism has been widely explored as therapeutic targets for anemic patients [39]. Indeed, the PubMed hits for the terms “hypoxia” AND “iron” total more than 2500. A less explored field is the role of SPL in hypoxia (224 PubMed hits). This heterogeneous class of lipids plays, on the other hand, a key role in the inflammation enhancing hypoxia in respiratory diseases, and in hypoxia itself [40,41].
3.1. Hypoxia and Iron Metabolism: Role of Hepcidin
Since iron is an essential component of Hb, the response to hypoxia also increases the need for iron. In fact, HPV and the potential consequent hypoxic pulmonary hypertension may be reduced by iron supplementation and exacerbated in case of iron deficiency [42,43]. In order to optimize iron use for hematopoiesis, the degradation of ferroportin (Fpn) must be reduced. Fpn is the transmembrane protein that allows the release of iron from the cells, mediating its intestinal absorption, and subsequent release into the circulation, bound to its transporter, the transferrin. Fpn degradation is mediated by the regulating peptide hepcidin, whose decrease, in healthy subjects, contributes to a good compensatory response to hypoxia and to iron deficiency [44]. A decrease in hepcidin has indeed been observed in healthy subjects exposed to high altitude hypoxia, both after acute (hours) and chronic (weeks) exposure [45,46]. However, in the clinical setting, hepcidin is not yet considered among the routine parameters for the assessment of iron metabolism, although some studies are considering its decrease as a factor suggestive of the need for iron-based intravenous therapy [47]. The values described in literature are very variable and denote a marked difference between healthy subjects [48] or anemic subjects hospitalized in Intensive Care Unit, in which high values are observed [49].
As circulating hepcidin is mainly produced by the liver, most of the in vitro studies about hepcidin expression are performed on hepatic cell lines, such as HepG2. Hepcidin expression is enhanced by a treatment with the iron chelant deferoxamine [50], despite the main effect of such compound being a reduced iron availability. Interestingly, deferoxamine is also used in several in vitro studies as a hypoxia mimetic as it activates the HIF-1 α pathway [51]. This suggests that, other than an increased iron request, there are several mechanisms that play active roles in regulating hepcidin production and action on iron storage and use. HIF-2α expression in intestinal cells is influenced by a decrease in hepcidin expression other than by hypoxia. Inducible deletion of hepatic hepcidin in a mouse model has indeed been found to activate intestinal HIF-2α and rapid iron accumulation [52]. HIF2 inhibitors have been proposed to treat iron accumulation diseases characterized by dysfunction of the hepcidin/FPN axis [53].
3.1.1. Iron Metabolism and HAPE
Hepcidin can be proposed as one of the potential markers of HAPE, as higher hepcidin values have been found in subjects developing HAPE, when compared to a group of subjects reaching the same altitude with the same timings, but without any sign of misadaptation [54].
3.1.2. Iron Metabolism and ARDS
Anemic ICU patients, among which patients with ARDS, have higher values of hepcidin than healthy subjects [49]. In addition, ferritin, the protein involved in intracellular iron storage and now known as an acute phase protein, is increased in the plasma of ARDS patients [55]. Inflammation, particularly through the pro-inflammatory cytokine IL-6, is a cause of increased hepcidin production [50], which may therefore interfere with the previously described hematopoietic compensation mechanism.
3.1.3. Iron Metabolism and COPD
In contrast to the physiological adaptation to hypoxia that should increase iron absorption and utilization with consequent compensatory Hb elevation [44], this pattern is observed only in a limited fraction of COPD patients: 40–50% of COPD patients instead develop iron deficiency, with anemia representing a predictive risk factor of worse outcome in 5–30% of cases [56,57]. Several studies demonstrated that COPD patients with chronic hypoxia have a reduced production of erythropoietin at either the level of the kidney or bone marrow. This response may correlate with an increase in systemic inflammatory markers though the interplay between iron, hypoxia, inflammation, and erythropoietin is complex and needs to be elucidated [58,59,60].
3.1.4. Iron Metabolism and CF
Hypoxia maladaptation in CF has been correlated to altered iron homeostasis that comprises systemic iron deficiency as well as increased iron accumulation and defective iron sequestration in the airway cells. Furthermore, there is preliminary evidence that iron deficiency in CF patients prevents secondary erythrocytosis [61]. In in vitro CF airway epithelial cells, the deficiency of heme-oxygenase-1 (HO-1), which plays a pivotal role in regulating cellular iron, correlates with increased iron concentration [62]. This in turn impairs HIF-1α stability by promoting its prolyl hydroxylase-mediated polyubiquitination and proteasomal degradation leading to an altered cellular response to hypoxia [62].
In a study performed on a small cohort of CF patients with mild to moderate pulmonary dysfunction and borderline hypoxia [63], hemoglobin and hematocrit values did not significantly differ from normal control subjects while serum iron and iron-binding capacity were lower, leading to a tendency to anemia. A prospective observational study by Fischer et al. showed that a hypoxia driven increase in red cells mass is absent in CF patients who often exhibit normal or even decreased hemoglobin levels. The occurrence of subclinical anemia may involve the absolute iron deficiency due to gastrointestinal malabsorption, loss via sputum, a lack of vitamin E, and inflammation [61].
3.2. Hypoxia and Sphingolipid Metabolism
SPLs are a minor class of lipids of all mammal cells composed by a hydrophilic head group protruding into the extracellular environment and a hydrophobic moiety, the ceramide (Cer), located into the membrane bilayer [64]. As the main components of plasma and intercellular organelle membranes, they have structural roles, but they also act as signaling molecules regulating cellular processes such as cell growth and death, senescence, inflammatory response [65,66]. Cer, the central hub of the SPL metabolism, is synthesized in the de novo pathway, from palmitoyl-CoA and serine, in the sphingomyelin (SM) hydrolysis pathway, which generates Cer from SMs and in the salvage pathway, from the catabolism of complex glycosphingolipids [67,68]. Cer can be cleaved by a ceramidase (CDase) to produce sphingosine (Sph) that, in turn, can be phosphorylated by two enzymes, sphingosine kinases 1 (SK1) and 2 (SK2) for the synthesis of sphingosine 1 phosphate (S1P) that is a crucial bioactive SPL.
Similar to iron, Cer, and S1P must be finely tuned to preserve cellular homeostasis. Several lines of evidence outlined the opposite cellular effects of Cer and S1P in response and adaptation to hypoxia.
3.2.1. Role of Ceramide
Cer levels increase in hypoxia and other forms of cell stress. Cer accumulated via activation of both de novo synthesis or SM hydrolysis pathway mediates hypoxia-derived cytotoxicity in different cellular models [69,70,71,72]. In neonatal mice exposed to 10% of oxygen (close to oxygen level at 5,260 m), cardiac Cer content was dependent on the acute (1 day) or chronic (1; 4; 8 weeks) hypoxia condition, suggesting a role for Cer decrease [73] together with its upstream precursor dihydroceramide increase [74] in the right ventricle adaptive response to chronic hypoxia. Such studies not only support a key role for Cer de novo synthetic pathway, but also propose the enzyme dihydroceramide desaturase which converts dihydroceramide to Cer as an important regulator in the adaptation to hypoxia [73,74]. Interestingly, this enzyme is oxygen-dependent [75]. Other evidence indicates that the augmented sphingomyelinase activity critically contributes to the hypoxia-induced increases in Cer content [76]. Klevstig and colleagues found that cardiac Cer accumulation was reduced in sphingomyelinase deficient mice under ischemic conditions [76]. In addition, Cer has been described as a promoter of hypoxic pulmonary vasoconstriction, although its vascular effects deserve further investigations [77]. These results would suggest that enhanced Cer synthesis and accumulation contribute in mediating the downstream toxic signals in response to hypoxia.
Although S1P has intracellular targets, it acts commonly as an autocrine or paracrine mediator by binding to five cell surface S1P receptors [78]. In lung disease, S1P receptors 1 (S1PR1) are of particular interest since they enhance vascular barrier functions and they counteract apoptosis [79]. In a mouse model of emphysema induced by VEGF receptor blockade, Diab and colleagues reported that activation of S1P-S1PR1 signaling axis reduced the deleterious effects of ceramide on airspace enlargement [4]. Intracellular S1P accumulation, derived from exogenous S1P-activated SK1, prevents lung cell apoptosis by promoting the expression of HIF-1α and VEGF. This effect contributes to prevent the airspace enlargement together with the S1P-S1PR1 axis that maintains the alveolar septal integrity and mediate S1P effects on endothelial cell differentiation and integrity (together with S1PR3) via the Akt pathway [80]. In a model of fenretinide-induced emphysema, the protective effect of S1P injection was mediated by preservation of the transcription factor HIF-1α expression and its target gene VEGF and by the increase of Nrf2 expression [81].
3.2.2. Role of Sphingosine 1 Phosphate
S1P is mainly considered a pro-survival and pro-proliferative factor. Since mature blood cells contain the biosynthetic machinery but not the degrading enzyme, they represent a large source of S1P for the plasma [82]. Intra erythrocyte S1P enhances the glycolytic metabolic fluxes leading to the generation of more 2,3-biphosphoglicerate that in turn promotes O2 release to protect against tissue hypoxia [82]. Human healthy volunteers brought to high altitude at 5260 m for up to 16 days, showed a time-dependent increase in plasma levels of S1P concurrently with elevated hemoglobin capacity to release oxygen [83]. Exogenous S1P has been proved effective as a potential preconditioning agent favoring adaptation to hypobaric hypoxia in in vivo models of different pathologies, such as respiratory, cardiovascular, and cerebral [84]. A pioneering study by Chawla et al. shows that S1P pre-treatment facilitates hypoxia adaptation in rats remained at 7620 m of altitude for 6 h. They propose that the beneficial effects of S1P rely on the enhanced blood oxygen carrying potential, mediated by HIF-1α stabilization and consequent dependent transcription of adaptive gene expression [85]. A recent work by Barbacini and colleagues investigated the potential role of SPL in metabolic adaptation of Andean children, born and living at 3775 m of altitude. They exhibit a prevalence of dyslipidemia and hypertension as compared to children at lower altitudes, suggesting that they undergo different metabolic adaptations. They observed that high density lipoprotein-cholesterol (HDL-C), Cer and S1P are involved in hypoxia adaptation though they propose S1P as the main actor in this process [86]. Further studies are needed to deepen in the complex mechanisms of SPL involvement in hypoxia adaptation though, up to now, several pieces of evidence would indicate S1P, instead of Cer, as a good candidate to counteract hypoxia-induced tissue damage.
3.2.3. SPL Metabolism and HAPE
To the best of our knowledge, only one study reports the correlation between Cer and S1P alterations and HAPE. The metabolomics analysis of plasma metabolites from HAPE subjects and healthy controls by Guo and colleagues revealed that, among others, C-8 Cer and sphingosine were significantly higher in HAPE subjects [87]. The accumulation of these SPL metabolites could play a role in hypoxia maladaptation occurring in HAPE since it has been proven that Cer may contribute to pulmonary endothelial decreased barrier function, lung inflammation and edema [88]. Therefore, reestablishing the SPL homeostasis could represent a novel therapeutic target to improve acclimatization.
3.2.4. SPL Metabolism and ARDS
Sphingolipids contribute to the modulation of endothelial barrier integrity [89,90]. In ARDS, endothelial barrier is disrupted and functionally altered. In a neonatal piglet ARDS model [91], aSMase hyper-activity and the consequent ceramide-C16/C18 accumulation in lung tissues correlate with inflammasome NLRP3 oligomerization, NF-κB and pro-fibrotic pathway activation. By inhibiting aSMase function with inositol 1,2,6-trisphosphate (IP3), Cer generation and NLRP3 oligomerization was completely blocked, resulting in improved oxygenation. Pandolfi and colleagues described a new aSMase-IL-6 pathway as pathogenic mediator of pulmonary vascular dysfunction in rat or human pulmonary artery smooth muscle cells (PASMCs) model of ARDS [92]. In a mouse model of ARDS, S1P analogue FTY720 reduces inflammatory lung injury induced by lipopolysaccharide (LPS) treatment [93]. Camp and coworkers synthesized novel FTY720 analogs and demonstrated that their effect in reversing the pulmonary vascular leak that characterizes ARDS in human pulmonary artery endothelial cells (HPAEC) is mediated by S1PR1-dependent receptor ligation [94]. Combination therapy of human umbilical cord (hUC-MSCs) and FTY720 in ALI/ARDS mice model induced by LPS resulted in higher survival rates and attenuated lung injuries [95].
3.2.5. SPL Metabolism and COPD
Omics data on sphingolipid, arachidonic acid, hypoxia and energy signaling network in sputum of COPD patients, showed that upregulation of Cer, together with a dysfunctional response to hypoxia, strongly influence cellular energy metabolism. This is probably achieved by inhibiting mediators in energy metabolism and lipid trafficking such as fatty acid binding protein 4 and uncoupling protein 2 [96]. In COPD patients, oxygen limitation and airway inflammation correlate with sphingolipid imbalance whose main feature is the alveolar Cer accumulation [41,97]. Several pieces of evidence demonstrate that increased Cer further aggravates the compromised airway homeostasis [88]. Very recently, Bodas et al. showed that Cer/Sph ratio was elevated in COPD subjects as well as in mice exposed to cigarette smoke, worsening COPD-emphysema severity [98]. Excessive Cer content triggers damages of structural airway epithelial cells [99,100,101], which is involved in pulmonary tissue destruction [102] and contributes to maintain pulmonary inflammation [103].
A critical role for S1PR mediated signaling pathway has been demonstrated in the control of airway function in COPD though the precise mechanisms underlying the effects of S1PR modulation in COPD have to be further clarified. Cigarette smoke induced pulmonary S1P pathway upregulation in a mouse model of mild COPD, with an evident increase in S1PR2 and S1PR3 expressions [104]. Defective ability to phagocytose apoptotic cells in alveolar COPD macrophages has been related to the increased expression of the S1PR5 gene that correlates with its reduced methylation status [105]. On the contrary, the analysis of lung tissue samples from 25 patients with COPD showed that the relative mRNA expression of S1PR5 was reduced in COPD. Moreover, a positive correlation was found between the mRNA expression of S1PR5, S1PR1, and S1PR3, and between S1PR3 and S1PR2. In conclusion, the authors suggest S1PR5 as a possible novel target for pharmacotherapy [106]. S1PR1 has been found to be downregulated in human pulmonary diseases such as COPD [79] and induction of the S1PR1 receptor activity increases survival and vascular permeability [107].
3.2.6. SPL Metabolism and CF
Though several in vitro and in vivo CF studies describe the involvement of Cer and S1P metabolism dysregulation in worsening the pulmonary conditions (for a comprehensive review, please refer to Aureli et al. [108]), the role of SPLs in mediating the adaptation to hypoxia is still a poorly investigated field. There is only one paper, by Tabelling et al. [40] that focused on the interplay between SPLs, hypoxia adaptation, and CF. The authors proposed a dual role for SPL signaling in CFTR-mediated HPV, suggesting an nSMase-derived Cer and SphK1-dependent conversion to S1P as the mediators of this hypoxia adaptation response. They demonstrated that, in CF, CFTR dysfunction impairs HPV by inhibiting the Cer-mediated translocation and the S1P-mediated activation of TRPC6 (transient receptor potential canonical 6) that plays a crucial role in the contraction of pulmonary artery smooth muscle cells [40].
4. Perspectives: Sphingolipid and Iron Metabolism in the Prognosis and Treatment of Chronic Respiratory Diseases
4.1. Sphingolipids and Iron Interplay
Recently, SPL alterations in response to dysregulated iron pathways have been observed in different animal models and patients. As reviewed by Rockfield and colleagues [109], iron toxicity correlates with increased synthesis of SPLs in different eukaryotic organisms as well as in mammalian cell lines.
In D. melanogaster and M. musculus, iron-induced toxicity that recapitulates neurodegenerative damages seems to be mediated by SPLs and downstream signaling pathways [110]. In Neurodegeneration with Brain Iron Accumulation (NBIA) disease, a gene network analysis found an SPL enrichment as well as high iron levels in basal ganglia [111]. Iron accumulation triggers Cer production (via activation of SM hydrolysis) that in turn promotes hepcidin expression by transcriptional upregulation of HAMP gene (Hepcidin Antimicrobial Peptide) in HepG2 cells [112]. Thus, hepcidin entangles iron and Cer in a vicious loop that sustains the high levels of both mediators. Iron deficiency was found to reduce SPLs synthesis in S. cerevisiae strains, probably due to the inactivation of SPL biosynthetic enzymes that require iron as an essential cofactor [113]. Such interplay between iron and SPL, under hypoxia and inflammation conditions, is shown in Figure 1.
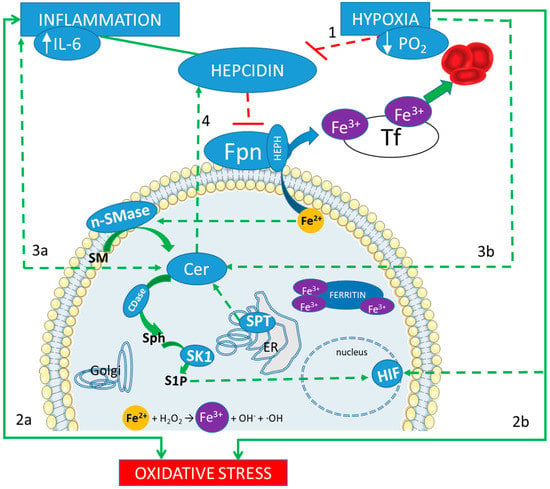
Figure 1.
Iron and sphingolipids interplay in response to inflammation and hypoxia. A correct adaptation to hypoxia results in the inhibition of the regulator peptide hepcidin (line 1). Hepcidin main action is the reduction of the outflow of the intracellular ferrous iron (Fe2+), which is mediated by ferroportin (Fpn). Therefore, if Fpn is less inhibited, iron can be released in the blood stream, bound to the trasporter fransferrin (Tf) in its ferric form (Fe3+), and then reach the bone marrow, to contribute to the hematopoietic response. On the other hand, inflammation induces an increase in hepcidin, which blocks such adaptation. Both inflammation and hypoxia are sources of oxidative stress (lines 2a and 2b). An excess of intracellular iron can be a further source of oxidative stress, through the Fenton reaction (showed at the bottom). Both inflammation and hypoxia increase the production of Ceramide (Cer, lines 3a and 3b) derived by a de novo biosynthetic pathway, mediated by serin palmitoyl transferase (SPT) in the endoplasmic reticulum (ER), and by the hydrolysis of sphingomyelin (SM), mediated by neutral sphingomyelinase (nSMase). Cer accumulation promotes hepcidin expression (line 4) with a consequent increase in intracellular iron content, which, in turn, triggers Cer production (via activation of SM hydrolysis) in a vicious loop. Furthermore, ceramidase (CDase) converts Cer in sphingosine (Sph), which is phosphorylated by sphingosine kinase 1 (SK1) to produce sphingosine 1 phosphate (S1P). S1P acts as an oxygen-independent regulator of HIFs.
The inflammatory cascade, particularly through the pro-inflammatory cytokine IL-6, can increase hepcidin production [50], which may therefore interfere with the previously described hematopoietic compensation mechanism. Failure to regulate the mechanism of hepcidin decrease in response to hypoxia may limit the effectiveness of iron-based therapies or transfusions [49]. In fact, even a red blood cell transfusion has an inducing effect on hepcidin blood concentrations, in addition to increasing the concentration of free iron (non-transferrin-bound iron, NTBI), without however having effects on transferrin (Tf) saturation [114]. Tf, by binding iron, allows a reduction of toxicity and a more effective use by cells. Furthermore, its receptor (TfR) that allows the transport from the extracellular to the intracellular compartment increases in physiological response to iron deficiency. Tf saturation is often used for a more precise evaluation of the presence of iron in the blood, together with the total serum iron, which measures both the iron bound to transferrin, and therefore recruited for the hematopoiesis, and the NTBI. The increase in NTBI is one of the harmful effects of irregular iron metabolism as it can cause oxidative stress, catalyzing the formation of reactive oxygen species [2].
The link between iron/hepcidin content and SPL metabolism in inflammation is further strengthened since inflammatory hypoxia has been proved to modulate the synthesis of Cer and S1P and, in turn, to be modulated by these lipid molecules. Cer and S1P are both described as important signaling mediators in inflammation [115]. Cer accumulation induces inflammation [116] and hepcidin expression [112], while S1P acts as an oxygen-independent regulator of HIFs [117,118].
These data suggest that there is a unique relationship between SPL levels and iron-mediated cellular toxicity, since downregulating SPL metabolism is sufficient to allow survival in high iron conditions. Whether alterations in other elements of iron signaling pathway are induced in response to Cer and other SPLs is actually an open field.
4.2. The Potential Prognostic Factors
Table 1 summarizes the main iron and sphingolipids metabolism markers and their role in influencing the adaptation to hypoxia. Further investigation on their role in determining the respiratory diseases prognosis are still required, especially for ceramide and intra-erythrocyte S1P. Here, we propose to compare the adaptation to high altitude in healthy subjects to the one to respiratory disease, in order to propose new biomarkers. Ceramide, measurable in plasma samples through mass spectrometry, has already been proposed as a biomarker of pulmonary and hepatic metastasis response to specific radiochemotherapy [119]. A metabolomic study of plasma shows that ceramides, sphingomyelin, and gangliosides levels are altered in specific COPD phenotypes such as emphysema and in COPD exacerbations [120]. Cystic Fibrosis is the first disease in which excessive airway epithelial cell ceramide was found to play a role in airway pathophysiology [116,121,122]. In the lung parenchyma, the accumulation of ceramide but also the ceramide to S1P balance influences cell fate and lung remodeling responses. High ceramide-to-S1P ratio due to either ceramide increase [4] or S1P decrease [123] correlates with emphysema-like pathology and cell death.

Table 1.
Iron and SPL metabolism markers and their trend in comparison to the normal values, in response to different hypoxic conditions.
4.3. Enhancing Hypoxia Adaptive Mechanism for the Treatment of Inflammatory Anemia
HIF and hepcidin are both already considered as therapeutic targets for inflammatory anemia, also in subjects without any respiratory disease [39]. The clinical utility of enhancing the HIF pathway to increase and optimize hemopoiesis is already under assessment through phase II clinical studies. Roxadustat, a small molecule which inhibit HIF prolyl-4-hydroxylases, therefore increasing HIF action, has been showing its effectiveness in patients with chronic kidney disease, and, as a secondary effect, was found to decrease circulating cholesterol [131]. Lexaptepid, an L-oligoribonucleotide with a strong affinity to hepcidin mRNA, has been assessed in healthy subjects for its ability of decreasing hepcidin levels [132], and might be useful in treating anemic COPD patients too. On the other hand, a decrease in hepcidin has been suggested as a potential criteria to discriminate the patients who may need iron-based intravenous therapy [47]. Iron infusion has an acute increasing effect on hepcidin concentration, but has been shown to reduce the hypoxia-induced increase in Pulmonary Artery Systolic Pressure [43].
Although the ubiquitous SPL expression could be a limitation, there are ongoing preclinical and clinical studies targeting both Cer and S1P in CF [133,134]. S1PR1 agonists may represent a new therapeutic strategy in the treatment COPD [4]. As reviewed by Becker and colleagues [133,134], systemic treatment with amitriptyline, an inhibitor of acid sphingomyelinase, is used in clinical trials to improve lung function in CF patients. Moreover, the application of recombinant acid ceramidase, a Cer degrading enzyme, is currently under development for clinical use in children with Farber disease, and it could be evaluated in CF patients since it has been proven to prevent infection in CF mice.
5. Conclusions
Among the various mechanisms involved in the patho-physiological adaptation to hypoxia, iron and SPL pathways play a key role, as described in pathological conditions such as HAPE, ARDS, COPD, and CF. Hepcidin, Cer, S1P, and their interplay in hypoxia are raising growing interest both as prognostic factors and therapeutical targets.
Funding
This research received no external funding.
Acknowledgments
S.O. was supported by the PhD program in “Translational Medicine” of the University of Milan, Italy. A.Z.’s post doc position was funded by Fondazione Umberto Veronesi.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| CDase | ceramidase |
| Cer | ceramide |
| CF | Cystic Fibrosis |
| CFTR | Cystic Fibrosis Transmembrane conductance Regulator |
| COPD | Chronic Obstructive Pulmonary Disease |
| Fpn | ferroportin |
| HAPE | High altitude pulmonary edema |
| Hb | hemoglobin |
| HIF | Hypoxia-inducible factor |
| HPV | Hypoxic Pulmonary Vasocostriction |
| nSMase | neutral sphingomyelinase |
| O2 | oxygen |
| S1P | sphingosine 1 phosphate |
| Sph | sphingosine |
| SPL | sphingolipids |
References
- Nanduri, J.; Yuan, G.; Kumar, G.K.; Semenza, G.L.; Prabhakar, N.R. Transcriptional responses to intermittent hypoxia. Respir Physiol Neurobiol. Transcriptional responses to intermittent hypoxia. Respir. Physiol. Neurobiol. 2008, 164, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Koskenkorva-Frank, T.S.; Weiss, G.; Koppenol, W.H.; Burckhardt, S. The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: Insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free Radic. Biol. Med. 2013, 65, 1174–1194. [Google Scholar] [CrossRef] [PubMed]
- Ademowo, O.S.; Dias, H.K.I.; Burton, D.G.A.; Griffiths, H.R. Lipid (per) oxidation in mitochondria: An emerging target in the ageing process? Biogerontology 2017, 18, 859–879. [Google Scholar] [CrossRef] [PubMed]
- Diab, K.J.; Adamowicz, J.J.; Kamocki, K.; Rush, N.I.; Garrison, J.; Gu, Y.; Schweitzer, K.S.; Skobeleva, A.; Rajashekhar, G.; Hubbard, W.C.; et al. Stimulation of sphingosine 1-phosphate signaling as an alveolar cell survival strategy in emphysema. Am. J. Respir. Crit. Care Med. 2010, 181, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Suresh, M.V.; Balijepalli, S.; Zhang, B.; Singh, V.V.; Swamy, S.; Panicker, S.; Dolgachev, V.A.; Subramanian, C.; Ramakrishnan, S.K.; Thomas, B.; et al. Hypoxia-Inducible Factor (HIF)-1alpha Promotes Inflammation and Injury Following Aspiration-Induced Lung Injury in Mice. Shock 2019, 52, 612–621. [Google Scholar] [CrossRef]
- Hackett, P.H.; Roach, R.C. High-altitude illness. N. Engl. J. Med. 2001, 345, 107–114. [Google Scholar] [CrossRef]
- Montgomery, S.T.; Mall, M.A.; Kicic, A.; Stick, S.M.; AREST, C.F. Hypoxia and sterile inflammation in cystic fibrosis airways: Mechanisms and potential therapies. Eur. Respir. J. 2017, 49. [Google Scholar] [CrossRef]
- Dunham-Snary, K.J.; Wu, D.; Sykes, E.A.; Thakrar, A.; Parlow, L.R.G.; Mewburn, J.D.; Parlow, J.L.; Archer, S.L. Hypoxic Pulmonary Vasoconstriction: From Molecular Mechanisms to Medicine. Chest 2017, 151, 181–192. [Google Scholar] [CrossRef]
- Force, A.D.T.; Ranieri, V.M.; Rubenfeld, G.D. Acute Respiratory Distress Syndrome. JAMA 2012, 307, 2526–2533. [Google Scholar]
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; van Haren, F.; Larsson, A.; McAuley, D.F.; et al. Epidemiology, Patterns of Care, and Mortality for Patients with Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016, 315, 788–800. [Google Scholar] [CrossRef]
- Umbrello, M.; Formenti, P.; Bolgiaghi, L.; Chiumello, D. Current Concepts of ARDS: A Narrative Review. Int. J. Mol. Sci. 2016, 18, 64. [Google Scholar] [CrossRef] [PubMed]
- Jenq, C.C.; Tsai, F.C.; Tsai, T.Y.; Hsieh, S.Y.; Lai, Y.W.; Tian, Y.C.; Chang, M.Y.; Lin, C.Y.; Fang, J.T.; Yang, C.W.; et al. Effect of Anemia on Prognosis in Patients on Extracorporeal Membrane Oxygenation. Artif. Organs 2018, 42, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Silver, M.R. Anemia in the long-term ventilator-dependent patient with respiratory failure. Chest 2005, 128, 568S–575S. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.V.; Bota, D.P.; Mélot, C.; Vincent, J.L. Time course of hemoglobin concentrations in nonbleeding intensive care unit patients. Crit. Care Med. 2003, 31, 406–410. [Google Scholar] [PubMed]
- Barnes, J.P.; Celli, B.R. Systemic manifestations and comorbidities of COPD. Eur. Respir. J. 2009, 33, 1165–1185. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, C.; Peto, R. The natural history of chronic airflow obstruction. Br. Med. J. 1977, 1, 1645–1648. [Google Scholar] [CrossRef] [PubMed]
- Rovina, N.; Koutsoukou, A.; Koulouris, N.G. Inflammation and immune response in COPD: Where do we stand? Mediat. Inflamm. 2013, 2013, 413735. [Google Scholar] [CrossRef]
- Burgel, P.R. The role of small airways in obstructive airway diseases. Eur. Respir. Rev. 2011, 20, 23–33. [Google Scholar] [CrossRef]
- Burgel, P.R.; Bourdin, A.; Chanez, P.; Chabot, F.; Chaouat, A.; Chinet, T.; de Blic, J.; Devillier, P.; Deschildre, A.; Didier, A.; et al. Update on the roles of distal airways in COPD. Eur. Respir. Rev. 2011, 20, 7–22. [Google Scholar] [CrossRef]
- Seimetz, M.; Parajuli, N.; Pichl, A.; Veit, F.; Kwapiszewska, G.; Weisel, F.C.; Milger, K.; Egemnazarov, B.; Turowska, A.; Fuchs, B.; et al. Inducible NOS Inhibition Reverses Tobacco-Smoke-Induced Emphysema and Pulmonary Hypertension in Mice. Cell 2011, 147, 293–305. [Google Scholar] [CrossRef]
- Barr, R.G. The epidemiology of vascular dysfunction relating to chronic obstructive pulmonary disease and emphysema. Proc. Am. Thorac. Soc. 2011, 8, 522–527. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Peinado, V.I.; Barbera, J.A.; Ramirez, J.; Gomez, F.P.; Roca, J.; Jover, L.; Gimferrer, J.M.; Rodriguez-Roisin, R. Endothelial dysfunction in pulmonary arteries of patients with mild COPD. Am. J. Physiol. 1998, 274, L908–L913. [Google Scholar] [CrossRef] [PubMed]
- Peinado, V.I.; Pizarro, S.; Barbera, J.A. Pulmonary vascular involvement in COPD. Chest 2008, 134, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Hypoxia-inducible factor 1 and cardiovascular disease. Annu. Rev. Physiol. 2014, 76, 39–56. [Google Scholar] [CrossRef]
- Sheikh, A.Q.; Saddouk, F.Z.; Ntokou, A.; Mazurek, R.; Greif, D.M. Cell Autonomous and Non-cell Autonomous Regulation of SMC Progenitors in Pulmonary Hypertension. Cell Rep. 2018, 23, 1152–1165. [Google Scholar] [CrossRef]
- Youn, B.S.; Mantel, C.; Broxmeyer, H.E. Chemokines, chemokine receptors and hematopoiesis. Immunol. Rev. 2000, 177, 150–174. [Google Scholar] [CrossRef]
- Matsui, H.; Grubb, B.R.; Tarran, R.; Randell, S.H.; Gatzy, J.T.; Davis, C.W.; Boucher, R.C. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell 1998, 95, 1005–1015. [Google Scholar] [CrossRef]
- Worlitzsch, D.; Tarran, R.; Ulrich, M.; Schwab, U.; Cekici, A.; Meyer, K.C.; Birrer, P.; Bellon, G.; Berger, J.; Weiss, T.; et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J. Clin. Investig. 2002, 109, 317–325. [Google Scholar] [CrossRef]
- Sly, P.D.; Gangell, C.L.; Chen, L.; Ware, R.S.; Ranganathan, S.; Mott, L.S.; Murray, C.P.; Stick, S.M.; AREST CF Investigators. Risk factors for bronchiectasis in children with cystic fibrosis. N. Engl. J. Med. 2013, 368, 1963–1970. [Google Scholar] [CrossRef]
- Fritzsching, B.; Zhou-Suckow, Z.; Trojanek, J.B.; Schubert, S.C.; Schatterny, J.; Hirtz, S.; Agrawal, R.; Muley, T.; Kahn, N.; Sticht, C.; et al. Hypoxic epithelial necrosis triggers neutrophilic inflammation via IL-1 receptor signaling in cystic fibrosis lung disease. Am. J. Respir. Crit. Care Med. 2015, 191, 902–913. [Google Scholar] [CrossRef]
- Wang, Z.; Jin, N.; Ganguli, S.; Swartz, D.R.; Li, L.; Rhoades, R.A. Rho-kinase activation is involved in hypoxia-induced pulmonary vasoconstriction. Am. J. Respir. Cell Mol. Biol. 2001, 25, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Young, J.M.; Williams, D.R.; Thompson, A.A.R. Thin Air, Thick Vessels: Historical and Current Perspectives on Hypoxic Pulmonary Hypertension. Front. Med. 2019, 6, 93. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. HIF-1: Mediator of physiological and pathophysiological responses to hypoxia. J. Appl. Physiol. 2000, 88, 1474–1480. [Google Scholar] [CrossRef]
- Rankin, E.B.; Biju, M.P.; Liu, Q.; Unger, T.L.; Rha, J.; Johnson, R.S.; Simon, M.C.; Keith, B.; Haase, V.H. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J. Clin. Investig. 2007, 117, 1068–1077. [Google Scholar] [CrossRef]
- Wang, L.G.; Semenza, G.L. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc. Natl. Acad. Sci. USA 1993, 90, 4304–4308. [Google Scholar] [CrossRef]
- Moore, L.G. Measuring high-altitude adaptation. J. Appl. Physiol. 2017, 123, 1371–1385. [Google Scholar] [CrossRef] [PubMed]
- Cheong, H.I.; Janocha, A.J.; Monocello, L.T.; Garchar, A.C.; Gebremedhin, A.; Erzurum, S.C.; Beall, C.M. Alternative hematological and vascular adaptive responses to high-altitude hypoxia in East African highlanders. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 312, L172–L177. [Google Scholar] [CrossRef] [PubMed]
- Corante, N.; Anza-Ramírez, C.; Figueroa-Mujíca, R.; Macarlupú, J.L.; Vizcardo-Galindo, G.; Bilo, G.; Parati, G.; Gamboa, J.L.; León-Velarde, F.; Villafuerte, F.C. Excessive Erythrocytosis and Cardiovascular Risk in Andean Highlanders. High Alt. Med. Biol. 2018, 19, 221–231. [Google Scholar] [CrossRef]
- Ganz, T. Anemia of Inflammation. N. Engl. J. Med. 2019, 381, 1148–1157. [Google Scholar] [CrossRef]
- Tabeling, C.; Yu, H.; Wang, L.; Ranke, H.; Goldenberg, N.M.; Zabini, D.; Noe, E.; Krauszman, A.; Gutbier, B.; Yin, J.; et al. CFTR and sphingolipids mediate hypoxic pulmonary vasoconstriction. Proc. Natl. Acad. Sci. USA 2015, 112, E1614–E1623. [Google Scholar] [CrossRef]
- Ghidoni, R.; Caretti, A.; Signorelli, P. Role of Sphingolipids in the Pathobiology of Lung Inflammation. Mediat. Inflamm. 2015, 2015, 487508. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.G.; Talbot, N.P.; Privat, C.; Rivera-Ch, M.; Nickol, A.H.; Ratcliffe, P.J.; Dorrington, K.L.; León-Velarde, F.; Robbins, P.A. Effects of iron supplementation and depletion on hypoxic pulmonary hypertension: Two randomized controlled trials. JAMA 2009, 302, 1444–1450. [Google Scholar] [CrossRef] [PubMed]
- Bart, N.K.; Curtis, M.K.; Cheng, H.Y.; Hungerford, S.L.; McLaren, R.; Petousi, N.; Dorrington, K.L.; Robbins, P.A. Elevation of iron storage in humans attenuates the pulmonary vascular response to hypoxia. J. Appl. Physiol. 2016, 121, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Gassmann, M.; Muckenthaler, M.U. Adaptation of iron requirement to hypoxic conditions at high altitude. J. Appl. Physiol. 2015, 119, 1432–1440. [Google Scholar] [CrossRef]
- Goetze, O.; Schmitt, J.; Spliethoff, K.; Theurl, I.; Weiss, G.; Swinkelsu, D.W.; Tjalsma, H.; Maggiorini, M.; Krayenbühl, P.; Rau, M.; et al. Adaptation of iron transport and metabolism to acute high-altitude hypoxia in mountaineers. Hepatology 2013, 58, 2153–2162. [Google Scholar] [CrossRef]
- Piperno, A.; Galimberti, S.; Mariani, R.; Pelucchi, S.; Ravasi, G.; Lombardi, C.; Bilo, G.; Revera, M.; Giuliano, A.; Faini, A.; et al. Modulation of hepcidin production during hypoxia-induced erythropoiesis in humans in vivo: Data from the HIGHCARE project. Blood 2011, 117, 2953–2959. [Google Scholar] [CrossRef]
- Lasocki, S.; Puy, H.; Mercier, G.; Lehmann, S.; Hepcidane study group. Impact of iron deficiency diagnosis using hepcidin mass spectrometry dosage methods on hospital stay and costs after a prolonged ICU stay: Study protocol for a multicentre, randomised, single-blinded medico-economic trial. Anaesth. Crit. Care Pain Med. 2017, 36, 391–396. [Google Scholar] [CrossRef]
- Galesloot, T.E.; Vermeulen, S.H.; Geurts-Moespot, A.J.; Klaver, S.M.; Kroot, J.J.; van Tienoven, D.; Wetzels, J.F.; Kiemeney, L.A.; Sweep, F.C.; den Heijer, M.; et al. Serum hepcidin: Reference ranges and biochemical correlates in the general population. Blood 2011, 117, e218–e225. [Google Scholar] [CrossRef]
- Lasocki, S.; Baron, G.; Driss, F.; Westerman, M.; Puy, H.; Boutron, I.; Beaumont, C.; Montravers, P. Diagnostic accuracy of serum hepcidin for iron deficiency in critically ill patients with anemia. Intensive Care Med. 2010, 36, 1044–1048. [Google Scholar] [CrossRef]
- Fein, E.; Merle, U.; Ehehalt, R.; Herrmann, T.; Kulaksiz, H. Regulation of hepcidin in HepG2 and RINm5F cells. Peptides 2007, 28, 951–957. [Google Scholar] [CrossRef]
- Vrtacnik, P.; Marc, J.; Ostanek, B. Hypoxia mimetic deferoxamine influences the expression of histone acetylation- and DNA methylation-associated genes in osteoblasts. Connect. Tissue Res. 2015, 56, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.J.; Das, N.K.; Ramakrishnan, S.K.; Jain, C.; Jurkovic, M.T.; Wu, J.; Nemeth, E.; Lakhal-Littleton, S.; Colacino, J.A.; Shah, Y.M. Hepatic hepcidin/intestinal HIF-2α axis maintains iron absorption during iron deficiency and overload. J. Clin. Investig. 2018, 129, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Renassia, C.; Peyssonnaux, C. New insights into the links between hypoxia and iron homeostasis. Curr. Opin. Hematol. 2019, 26, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Altamura, S.; Bärtsch, P.; Dehnert, C.; Maggiorini, M.; Weiss, G.; Theurl, I.; Muckenthaler, M.U.; Mairbäurl, H. Increased hepcidin levels in high-altitude pulmonary edema. J. Appl. Physiol. 2015, 118, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Connelly, K.G.; Moss, M.; Parsons, P.E.; Moore, E.E.; Moore, F.A.; Giclas, P.C.; Seligman, P.A.; Repine, J.E. Serum ferritin as a predictor of the acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 1997, 155, 21–25. [Google Scholar] [CrossRef]
- Nickol, A.H.; Frise, M.C.; Cheng, H.Y.; McGahey, A.; McFadyen, B.M.; Harris-Wright, T.; Bart, N.K.; Curtis, M.K.; Khandwala, S.; O’Neill, D.P.; et al. A cross-sectional study of the prevalence and associations of iron deficiency in a cohort of patients with chronic obstructive pulmonary disease. BMJ Open 2015, 5, e007911. [Google Scholar] [CrossRef]
- Cloonan, S.M.; Mumby, S.; Adcock, I.M.; Choi, A.M.K.; Chung, K.F.; Quinlan, G.J. The “Iron”-y of Iron Overload and Iron Deficiency in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2017, 196, 1103–1112. [Google Scholar] [CrossRef]
- Tassiopoulos, S.; Kontos, A.; Konstantopoulos, K.; Hadzistavrou, C.; Vaiopoulos, G.; Aessopos, A.; Tassiopoulos, T. Erythropoietic response to hypoxaemia in diffuse idiopathic pulmonary fibrosis, as opposed to chronic obstructive pulmonary disease. Respir. Med. 2001, 95, 471–475. [Google Scholar] [CrossRef][Green Version]
- Pavlisa, G.; Vrbanic, V.; Kusec, V.; Jaksic, B. Erythropoietin response after correction of severe hypoxaemia due to acute respiratory failure in chronic obstructive pulmonary disease patients. Clin. Sci. 2004, 106, 43–51. [Google Scholar] [CrossRef]
- John, M.; Hoernig, S.; Doehner, W.; Okonko, D.D.; Witt, C.; Anker, S.D. Anemia and inflammation in COPD. Chest 2005, 127, 825–829. [Google Scholar] [CrossRef]
- Fischer, R.; Simmerlein, R.; Huber, R.M.; Schiffl, H.; Lang, S.M. Lung disease severity, chronic inflammation, iron deficiency, and erythropoietin response in adults with cystic fibrosis. Pediatr. Pulmonol. 2007, 42, 1193–1197. [Google Scholar] [CrossRef] [PubMed]
- Chillappagari, S.; Venkatesan, S.; Garapati, V.; Mahavadi, P.; Munder, A.; Seubert, A.; Sarode, G.; Guenther, A.; Schmeck, B.T.; Tümmler, B.; et al. Impaired TLR4 and HIF expression in cystic fibrosis bronchial epithelial cells downregulates hemeoxygenase-1 and alters iron homeostasis in vitro. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 307, L791–L799. [Google Scholar] [CrossRef] [PubMed]
- O’connor, T.M.; McGrath, D.S.; Short, C.; O’donnell, M.J.; Sheehy, M.; Bredin, C.P. Subclinical anaemia of chronic disease in adult patients with cystic fibrosis. J. Cyst. Fibros. 2002, 1, 31–34. [Google Scholar] [CrossRef]
- Feizi, T. Demonstration by monoclonal antibodies that carbohydrate structures of glycoproteins and glycolipids are onco-developmental antigens. Nature 1985, 314, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Hannun, A.Y.; Obeid, L.M. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat. Rev. Mol. Cell. Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef]
- Chiricozzi, E.; Loberto, N.; Schiumarini, D.; Samarani, M.; Mancini, G.; Tamanini, A.; Lippi, G.; Dechecchi, M.C.; Bassi, R.; Giussani, P.; et al. Sphingolipids role in the regulation of inflammatory response: From leukocyte biology to bacterial infection. J. Leukoc. Biol. 2018, 103, 445–456. [Google Scholar] [CrossRef]
- Gault, R.C.; Obeid, L.M.; Hannun, Y.A. An overview of sphingolipid metabolism: From synthesis to breakdown. Adv. Exp. Med. Biol. 2010, 688, 1–23. [Google Scholar]
- Tettamanti, G.; Bassi, R.; Viani, P.; Riboni, L. Salvage pathways in glycosphingolipid metabolism. Biochimie 2003, 85, 423–437. [Google Scholar] [CrossRef]
- Ueda, N.; Kaushal, G.P.; Hong, X.; Shah, S.V. Role of enhanced ceramide generation in DNA damage and cell death in chemical hypoxic injury to LLC-PK1 cells. Kidney Int. 1998, 54, 399–406. [Google Scholar] [CrossRef]
- Yoshimura, S.; Banno, Y.; Nakashima, S.; Hayashi, K.; Yamakawa, H.; Sawada, M.; Sakai, N.; Nozawa, Y. Inhibition of neutral sphingomyelinase activation and ceramide formation by glutathione in hypoxic PC12 cell death. J. Neurochem. 1999, 73, 675–683. [Google Scholar] [CrossRef]
- Basnakian, A.G.; Ueda, N.; Hong, X.; Galitovsky, V.E.; Yin, X.; Shah, S.V. Ceramide synthase is essential for endonuclease-mediated death of renal tubular epithelial cells induced by hypoxia-reoxygenation. Am. J. Physiol. Ren. Physiol. 2005, 288, F308–F314. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Ahn, K.H.; Kim, S.K.; Jeon, H.J.; Ji, J.E.; Choi, J.M.; Jung, K.M.; Jung, S.Y.; Kim, D.K. Hypoxia-induced neuronal apoptosis is mediated by de novo synthesis of ceramide through activation of serine palmitoyltransferase. Cell. Signal. 2010, 22, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Noureddine, L.; Azzam, R.; Nemer, G.; Bielawski, J.; Nasser, M.; Bitar, F.; Dbaibo, G.S. Modulation of total ceramide and constituent ceramide species in the acutely and chronically hypoxic mouse heart at different ages. Prostaglandins Other Lipid Mediat. 2008, 86, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Azzam, R.; Hariri, F.; El-Hachem, N.; Kamar, A.; Dbaibo, G.; Nemer, G.; Bitar, F. Regulation of de novo ceramide synthesis: The role of dihydroceramide desaturase and transcriptional factors NFATC and Hand2 in the hypoxic mouse heart. DNA Cell Biol. 2013, 32, 310–319. [Google Scholar] [CrossRef]
- Devlin, C.M.; Lahm, T.; Hubbard, W.C.; Van Demark, M.; Wang, K.C.; Wu, X.; Bielawska, A.; Obeid, L.M.; Ivan, M.; Petrache, I. Dihydroceramide-based response to hypoxia. J. Biol. Chem. 2011, 286, 38069–38078. [Google Scholar] [CrossRef]
- Klevstig, M.; Ståhlman, M.; Lundqvist, A.; Scharin Täng, M.; Fogelstrand, P.; Adiels, M.; Andersson, L.; Kolesnick, R.; Jeppsson, A.; Borén, J.; et al. Targeting acid sphingomyelinase reduces cardiac ceramide accumulation in the post-ischemic heart. J. Mol. Cell Cardiol. 2016, 93, 69–72. [Google Scholar] [CrossRef]
- Cogolludo, A.; Villamor, E.; Perez-Vizcaino, F.; Moreno, L. Ceramide and Regulation of Vascular Tone. Int. J. Mol. Sci. 2019, 20, 411. [Google Scholar] [CrossRef]
- Ebenezer, L.D.; Fu, P.; Natarajan, V. Targeting sphingosine-1-phosphate signaling in lung diseases. Pharmacol. Ther. 2016, 168, 143–157. [Google Scholar] [CrossRef]
- Yang, Y.; Uhlig, S. The role of sphingolipids in respiratory disease. Ther. Adv. Respir. Dis. 2011, 5, 325–344. [Google Scholar] [CrossRef]
- Takuwa, Y.; Takuwa, N.; Sugimoto, N. The Edg family G protein-coupled receptors for lysophospholipids: Their signaling properties and biological activities. J. Biochem. 2002, 131, 767–771. [Google Scholar] [CrossRef]
- Yasuo, M.; Mizuno, S.; Allegood, J.; Kraskauskas, D.; Bogaard, H.J.; Spiegel, S.; Voelkel, N.F. Fenretinide causes emphysema, which is prevented by sphingosine 1-phoshate. PLoS ONE 2013, 8, e53927. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Berka, V.; Song, A.; Sun, K.; Wang, W.; Zhang, W.; Ning, C.; Li, C.; Zhang, Q.; Bogdanov, M.; et al. Elevated sphingosine-1-phosphate promotes sickling and sickle cell disease progression. J. Clin. Investig. 2014, 124, 2750–2761. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Zhang, Y.; D’Alessandro, A.; Nemkov, T.; Song, A.; Wu, H.; Liu, H.; Adebiyi, M.; Huang, A.; Wen, Y.E.; et al. Sphingosine-1-phosphate promotes erythrocyte glycolysis and oxygen release for adaptation to high-altitude hypoxia. Nat. Commun. 2016, 7, 12086. [Google Scholar] [CrossRef] [PubMed]
- Takuwa, Y.; Okamoto, Y.; Yoshioka, K.; Takuwa, N. Sphingosine-1-phosphate signaling in physiology and diseases. Biofactors 2012, 38, 329–337. [Google Scholar] [CrossRef]
- Chawla, S.; Rahar, B.; Singh, M.; Bansal, A.; Saraswat, D.; Saxena, S. Exogenous sphingosine-1-phosphate boosts acclimatization in rats exposed to acute hypobaric hypoxia: Assessment of haematological and metabolic effects. PLoS ONE 2014, 9, e98025. [Google Scholar] [CrossRef]
- Barbacini, P.; Casas, J.; Torretta, E.; Capitanio, D.; Maccallini, G.; Hirschler, V.; Gelfi, C. Regulation of Serum Sphingolipids in Andean Children Born and Living at High Altitude (3775 m). Int. J. Mol. Sci. 2019, 20, 2835. [Google Scholar] [CrossRef]
- Guo, L.; Tan, G.; Liu, P.; Li, H.; Tang, L.; Huang, L.; Ren, Q. Three plasma metabolite signatures for diagnosing high altitude pulmonary edema. Sci. Rep. 2015, 5, 15126. [Google Scholar] [CrossRef] [PubMed]
- Petrache, I.; Natarajan, V.; Zhen, L.; Medler, T.R.; Richter, A.T.; Cho, C.; Hubbard, W.C.; Berdyshev, E.V.; Tuder, R.M. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat. Med. 2005, 11, 491–498. [Google Scholar] [CrossRef]
- Singleton, P.A.; Dudek, S.M.; Chiang, E.T.; Garcia, J.G. Regulation of sphingosine 1-phosphate-induced endothelial cytoskeletal rearrangement and barrier enhancement by S1P1 receptor, PI3 kinase, Tiam1/Rac1, and alpha-actinin. FASEB J. 2005, 19, 1646–1656. [Google Scholar] [CrossRef]
- Kolliputi, N.; Galam, L.; Parthasarathy, P.T.; Tipparaju, S.M.; Lockey, R.F. NALP-3 inflammasome silencing attenuates ceramide-induced transepithelial permeability. J. Cell. Physiol. 2012, 227, 3310–3316. [Google Scholar] [CrossRef]
- Spengler, D.; Winoto-Morbach, S.; Kupsch, S.; Vock, C.; Blöchle, K.; Frank, S.; Rintz, N.; Diekötter, M.; Janga, H.; Weckmann, M.; et al. Novel therapeutic roles for surfactant-inositols and -phosphatidylglycerols in a neonatal piglet ARDS model: A translational study. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 314, L32–L53. [Google Scholar] [CrossRef] [PubMed]
- Pandolfi, R.; Barreira, B.; Moreno, E.; Lara-Acedo, V.; Morales-Cano, D.; Martínez-Ramas, A.; de Olaiz Navarro, B.; Herrero, R.; Lorente, J.Á.; Cogolludo, Á.; et al. Role of acid sphingomyelinase and IL-6 as mediators of endotoxin-induced pulmonary vascular dysfunction. Thorax 2017, 72, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Liu, H.; Zhang, X.; Wen, G.; Zhu, C.; Zhao, Y.; Niu, W.; Qin, Y.; Chen, H.; Bai, C.; et al. Transcriptomic analysis of lung tissues after hUC-MSCs and FTY720 treatment of lipopolysaccharide-induced acute lung injury in mouse models. Int. Immunopharmacol. 2018, 63, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Camp, S.M.; Chiang, E.T.; Sun, C.; Usatyuk, P.V.; Bittman, R.; Natarajan, V.; Garcia, J.G.; Dudek, S.M. Pulmonary Endothelial Cell Barrier Enhancement by Novel FTY720 Analogs: Methoxy-FTY720, Fluoro-FTY720, and beta-Glucuronide-FTY720. Chem. Phys. Lipids 2016, 194, 85–93. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, W.; Heng, Z.; Zheng, J.; Li, P.; Yuan, X.; Niu, W.; Bai, C.; Liu, H. Combination therapy of human umbilical cord mesenchymal stem cells and FTY720 attenuates acute lung injury induced by lipopolysaccharide in a murine model. Oncotarget 2017, 8, 77407–77414. [Google Scholar] [CrossRef][Green Version]
- Jamalkandi, S.A.; Mirzaie, M.; Jafari, M.; Mehrani, H.; Shariati, P.; Khodabandeh, M. Signaling network of lipids as a comprehensive scaffold for omics data integration in sputum of COPD patients. Biochim. Biophys. Acta 2015, 1851, 1383–1393. [Google Scholar]
- Scarpa, M.C.; Baraldo, S.; Marian, E.; Turato, G.; Calabrese, F.; Saetta, M.; Maestrelli, P. Ceramide expression and cell homeostasis in chronic obstructive pulmonary disease. Respiration 2013, 85, 342–349. [Google Scholar] [CrossRef]
- Bodas, M.; Pehote, G.; Silverberg, D.; Gulbins, E.; Vij, N. Autophagy augmentation alleviates cigarette smoke-induced CFTR-dysfunction, ceramide-accumulation and COPD-emphysema pathogenesis. Free Radic. Biol. Med. 2019, 131, 81–97. [Google Scholar] [CrossRef]
- Arana, L.; Gangoiti, P.; Ouro, A.; Trueba, M.; Gómez-Muñoz, A. Ceramide and ceramide 1-phosphate in health and disease. Lipids Health Dis. 2010, 9, 15. [Google Scholar] [CrossRef]
- Filosto, S.; Castillo, S.; Danielson, A.; Franzi, L.; Khan, E.; Kenyon, N.; Last, J.; Pinkerton, K.; Tuder, R.; Goldkorn, T. Neutral sphingomyelinase 2: A novel target in cigarette smoke-induced apoptosis and lung injury. Am. J. Respir. Cell. Mol. Biol. 2011, 44, 350–360. [Google Scholar] [CrossRef]
- Zulueta, A.; Caretti, A.; Campisi, G.M.; Brizzolari, A.; Abad, J.L.; Paroni, R.; Signorelli, P.; Ghidoni, R. Inhibitors of ceramide de novo biosynthesis rescue damages induced by cigarette smoke in airways epithelia. Naunyn Schmiedebergs Arch. Pharmacol. 2017, 390, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Demedts, I.K.; Demoor, T.; Bracke, K.R.; Joos, G.F.; Brusselle, G.G. Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respir. Res. 2006, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Karandashova, S.; Kummarapurugu, A.B.; Zheng, S.; Chalfant, C.E.; Voynow, J.A. Neutrophil elastase increases airway ceramide levels via upregulation of serine palmitoyltransferase. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 314, L206–L214. [Google Scholar] [CrossRef] [PubMed]
- De Cunto, G.; Brancaleone, V.; Riemma, M.A.; Cerqua, I.; Vellecco, V.; Spaziano, G.; Cavarra, E.; Bartalesi, B.; D’Agostino, B.; Lungarella, G.; et al. Functional contribution of sphingosine-1-phosphate to airway pathology in cigarette smoke-exposed mice. Br. J. Pharmacol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Barnawi, J.; Jersmann, H.; Haberberger, R.; Hodge, S.; Meech, R. Reduced DNA methylation of sphingosine-1 phosphate receptor 5 in alveolar macrophages in COPD: A potential link to failed efferocytosis. Respirology 2017, 22, 315–321. [Google Scholar] [CrossRef]
- Cordts, F.; Pitson, S.; Tabeling, C.; Gibbins, I.; Moffat, D.F.; Jersmann, H.; Hodge, S.; Haberberger, R.V. Expression profile of the sphingosine kinase signalling system in the lung of patients with chronic obstructive pulmonary disease. Life Sci. 2011, 89, 806–811. [Google Scholar] [CrossRef]
- Josipovic, I.; Pflüger, B.; Fork, C.; Vasconez, A.E.; Oo, J.A.; Hitzel, J.; Seredinski, S.; Gamen, E.; Heringdorf, D.M.Z.; Chen, W.; et al. Long noncoding RNA LISPR1 is required for S1P signaling and endothelial cell function. J. Mol. Cell. Cardiol. 2018, 116, 57–68. [Google Scholar] [CrossRef]
- Aureli, M.; Schiumarini, D.; Loberto, N.; Bassi, R.; Tamanini, A.; Mancini, G.; Tironi, M.; Munari, S.; Cabrini, G.; Dechecchi, M.C.; et al. Unravelling the role of sphingolipids in cystic fibrosis lung disease. Chem. Phys. Lipids 2016, 200, 94–103. [Google Scholar] [CrossRef]
- Rockfield, S.; Chhabra, R.; Robertson, M.; Rehman, N.; Bisht, R.; Nanjundan, M. Links Between Iron and Lipids: Implications in Some Major Human Diseases. Pharm. (Basel) 2018, 11, 113. [Google Scholar] [CrossRef]
- Chen, K.; Ho, T.S.; Lin, G.; Tan, K.L.; Rasband, M.N.; Bellen, H.J. Loss of Frataxin activates the iron/sphingolipid/PDK1/Mef2 pathway in mammals. Elife 2016, 5, e20732. [Google Scholar] [CrossRef]
- Bettencourt, C.; Forabosco, P.; Wiethoff, S.; Heidari, M.; Johnstone, D.M.; Botía, J.A.; Collingwood, J.F.; Hardy, J.; UK Brain Expression Consortium (UKBEC); Milward, E.A.; et al. Gene co-expression networks shed light into diseases of brain iron accumulation. Neurobiol. Dis. 2016, 87, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Natarajan, S.K.; Mott, J.L.; Kharbanda, K.K.; Harrison-Findik, D.D. Ceramide Induces Human Hepcidin Gene Transcription through JAK/STAT3 Pathway. PLoS ONE 2016, 11, e0147474. [Google Scholar] [CrossRef] [PubMed]
- Shakoury-Elizeh, M.; Protchenko, O.; Berger, A.; Cox, J.; Gable, K.; Dunn, T.M.; Prinz, W.A.; Bard, M.; Philpott, C.C. Metabolic response to iron deficiency in Saccharomyces cerevisiae. J. Biol. Chem. 2010, 285, 14823–14833. [Google Scholar] [CrossRef] [PubMed]
- Boshuizen, M.; van Hezel, M.E.; van Manen, L.; Straat, M.; Somsen, Y.B.O.; Spoelstra-de Man, A.M.E.; Blumberg, N.; van Bruggen, R.; Juffermans, N.P. The effect of red blood cell transfusion on iron metabolism in critically ill patients. Transfusion 2019, 59, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Maceyka, M.; Spiegel, S. Sphingolipid metabolites in inflammatory disease. Nature 2014, 510, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Teichgräber, V.; Ulrich, M.; Endlich, N.; Riethmüller, J.; Wilker, B.; De Oliveira-Munding, C.C.; van Heeckeren, A.M.; Barr, M.L.; von Kürthy, G.; Schmid, K.W.; et al. Ceramide accumulation mediates inflammation, cell death and infection susceptibility in cystic fibrosis. Nat. Med. 2008, 14, 382–391. [Google Scholar] [CrossRef]
- Michaud, M.D.; Robitaille, G.A.; Gratton, J.P.; Richard, D.E. Sphingosine-1-phosphate: A novel nonhypoxic activator of hypoxia-inducible factor-1 in vascular cells. Arter. Thromb. Vasc. Biol. 2009, 29, 902–908. [Google Scholar] [CrossRef]
- Glaser, U.G.; Fandrey, J. Sphingolipids in inflammatory hypoxia. Biol. Chem. 2018, 399, 1169–1174. [Google Scholar] [CrossRef]
- Dubois, N.; Rio, E.; Ripoche, N.; Ferchaud-Roucher, V.; Gaugler, M.H.; Campion, L.; Krempf, M.; Carrie, C.; Mahé, M.; Mirabel, X.; et al. Plasma ceramide, a real-time predictive marker of pulmonary and hepatic metastases response to stereotactic body radiation therapy combined with irinotecan. Radiother. Oncol. 2016, 119, 229–235. [Google Scholar] [CrossRef]
- Bowler, R.P.; Jacobson, S.; Cruickshank, C.; Hughes, G.J.; Siska, C.; Ory, D.S.; Petrache, I.; Schaffer, J.E.; Reisdorph, N.; Kechris, K. Plasma sphingolipids associated with chronic obstructive pulmonary disease phenotypes. Am. J. Respir. Crit. Care Med. 2015, 191, 275–284. [Google Scholar] [CrossRef]
- Brodlie, M.; McKean, M.C.; Johnson, G.E.; Gray, J.; Fisher, A.J.; Corris, P.A.; Lordan, J.L.; Ward, C. Ceramide is increased in the lower airway epithelium of people with advanced cystic fibrosis lung disease. Am. J. Respir. Crit. Care Med. 2010, 182, 369–375. [Google Scholar] [CrossRef]
- Grassme, H.; Riethmuller, J.; Gulbins, E. Ceramide in cystic fibrosis. Handb. Exp. Pharmacol. 2013, 216, 265–274. [Google Scholar]
- Christofidou-Solomidou, M.; Pietrofesa, R.A.; Arguiri, E.; Schweitzer, K.S.; Berdyshev, E.V.; McCarthy, M.; Corbitt, A.; Alwood, J.S.; Yu, Y.; Globus, R.K.; et al. Space radiation-associated lung injury in a murine model. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 308, L416–L428. [Google Scholar] [CrossRef] [PubMed]
- Duru, S.; Bilgin, E.; Ardic, S. Hepcidin: A useful marker in chronic obstructive pulmonary disease. Ann. Thorac. Med. 2012, 7, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Tandara, L.; Grubisic, T.Z.; Ivan, G.; Jurisic, Z.; Tandara, M.; Gugo, K.; Mladinov, S.; Salamunic, I. Systemic inflammation up-regulates serum hepcidin in exacerbations and stabile chronic obstructive pulmonary disease. Clin. Biochem. 2015, 48, 1252–1257. [Google Scholar] [CrossRef] [PubMed]
- Uijterschout, L.; Swinkels, D.W.; Akkermans, M.D.; Zandstra, T.; Nuijsink, M.; Hendriks, D.; Hudig, C.; Tjalsma, H.; Vos, R.; van Goudoever, J.B.; et al. The value of soluble transferrin receptor and hepcidin in the assessment of iron status in children with cystic fibrosis. J. Cyst. Fibros. 2014, 13, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Basu, M.; Malhotra, A.S.; Pal, K.; Prasad, R.; Kumar, R.; Prasad, B.A.; Sawhney, R.C. Erythropoietin levels in lowlanders and high-altitude natives at 3450 m. Aviat. Space Environ. Med. 2007, 78, 963–967. [Google Scholar] [CrossRef]
- Markoulaki, D.; Kostikas, K.; Papatheodorou, G.; Koutsokera, A.; Alchanatis, M.; Bakakos, P.; Gourgoulianis, K.I.; Roussos, C.; Koulouris, N.G.; Loukides, S. Hemoglobin, erythropoietin and systemic inflammation in exacerbations of chronic obstructive pulmonary disease. Eur. J. Intern. Med. 2011, 22, 103–107. [Google Scholar] [CrossRef]
- Sun, X.; Ma, S.F.; Wade, M.S.; Acosta-Herrera, M.; Villar, J.; Pino-Yanes, M.; Zhou, T.; Liu, B.; Belvitch, P.; Moitra, J.; et al. Functional promoter variants in sphingosine 1-phosphate receptor 3 associate with susceptibility to sepsis-associated acute respiratory distress syndrome. Am. J. Physiol. Lung Cell Mol. Physiol. 2013, 305, L467–L477. [Google Scholar] [CrossRef]
- Lea, S.R.; Metcalfe, H.J.; Plumb, J.; Beerli, C.; Poll, C.; Singh, D.; Abbott-Banner, K.H. Neutral sphingomyelinase-2, acid sphingomyelinase, and ceramide levels in COPD patients compared to controls. Int. J. Chron. Obs. Pulmon. Dis. 2016, 11, 2139–2147. [Google Scholar] [CrossRef]
- Chen, N.; Hao, C.; Peng, X.; Lin, H.; Yin, A.; Hao, L.; Tao, Y.; Liang, X.; Liu, Z.; Xing, C.; et al. Roxadustat for Anemia in Patients with Kidney Disease Not Receiving Dialysis. N. Eng. J. Med. 2019, 381, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Boyce, M.; Warrington, S.; Cortezi, B.; Zöllner, S.; Vauléon, S.; Swinkels, D.W.; Summo, L.; Schwoebel, F.; Riecke, K. Safety, Pharmacokinetics and pharmacodynamics of the anti-hepcidin Spiegelmer lexaptepid pegol in healthy subjects. Br. J. Pharmacol. 2016, 173, 1580–1588. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.; Harikumar, K.B. Sphingosine 1-Phosphate: A Novel Target for Lung Disorders. Front. Immunol. 2017, 8, 296. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.A.; Riethmüller, J.; Seitz, A.P.; Gardner, A.; Boudreau, R.; Kamler, M.; Kleuser, B.; Schuchman, E.; Caldwell, C.C.; Edwards, M.J.; et al. Sphingolipids as targets for inhalation treatment of cystic fibrosis. Adv. Drug Deliv. Rev. 2018, 133, 66–75. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).