Functional Analysis of Peptidyl-prolyl cis-trans Isomerase from Aspergillus flavus
Abstract
1. Introduction
2. Results
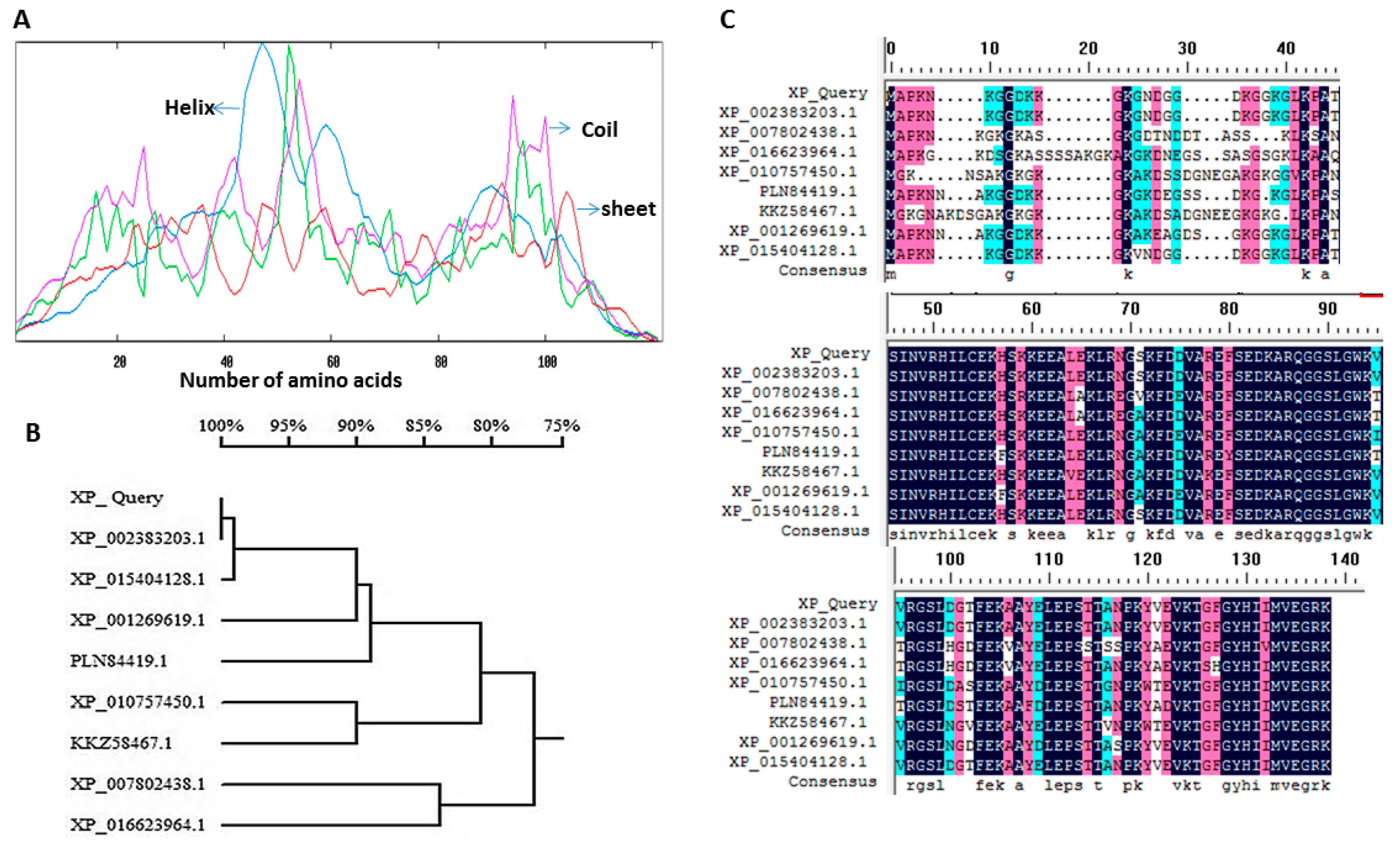
2.1. Bioinformatics Analysis of the Sequences
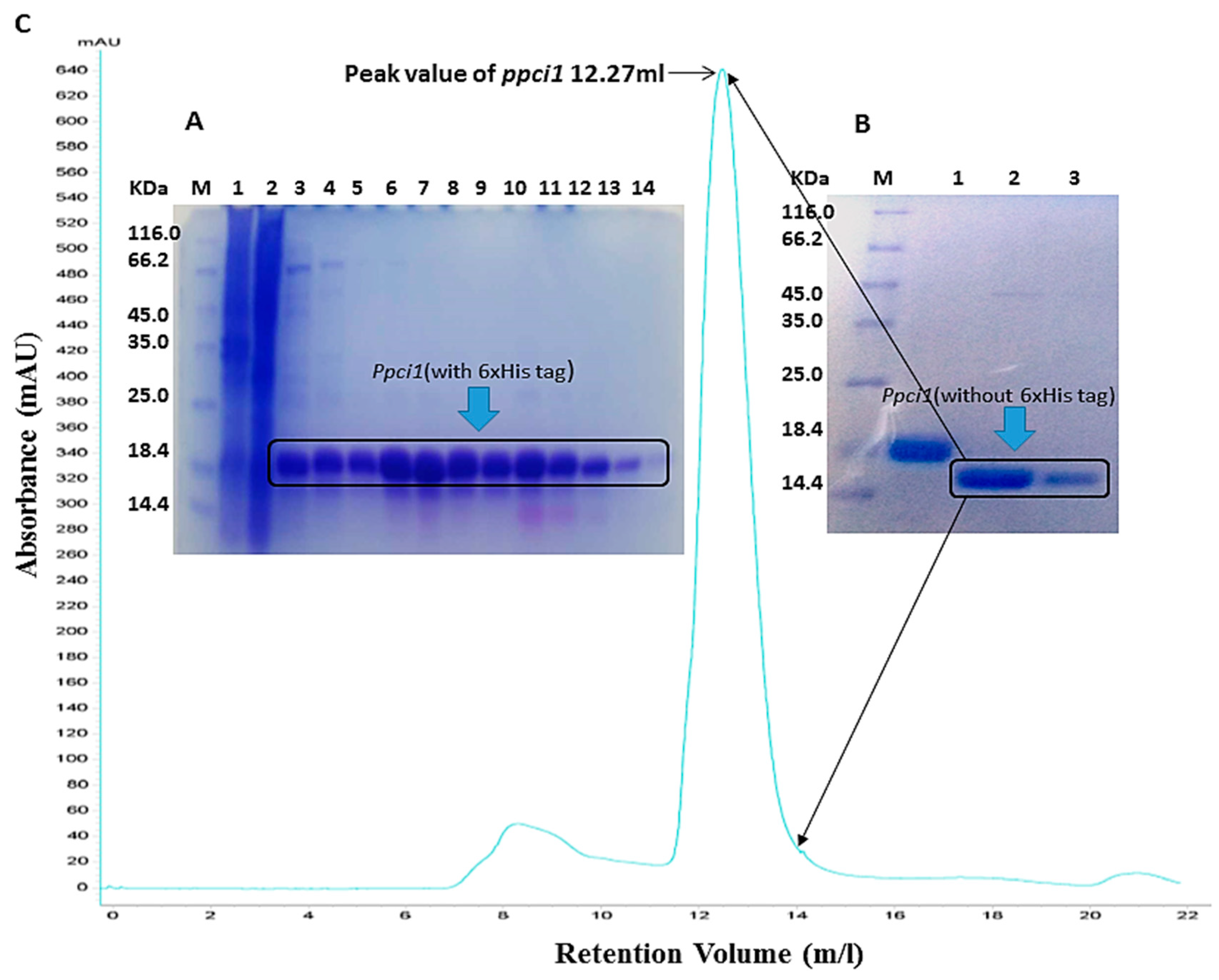
2.2. Protein Expression and Purification
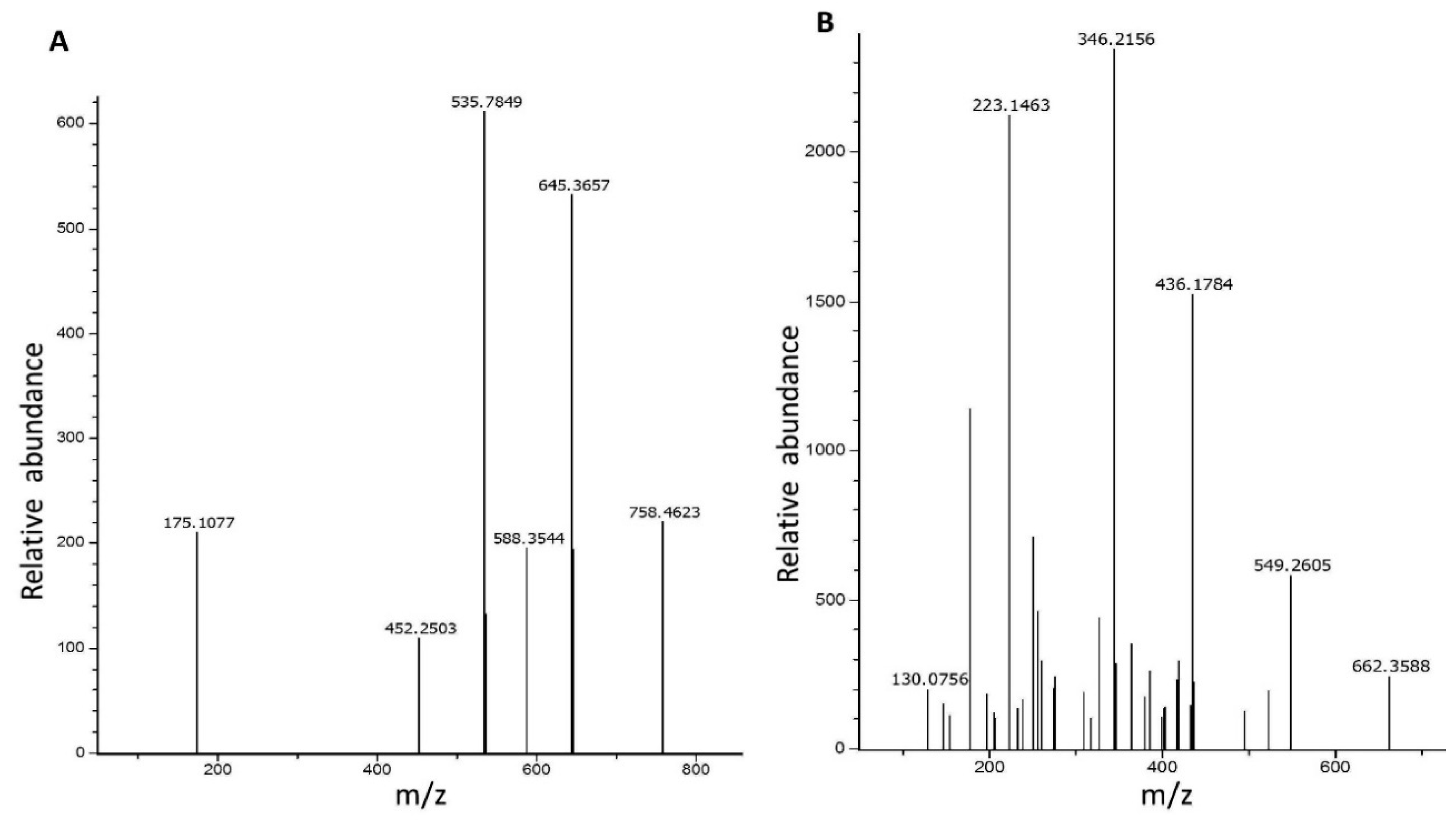
2.3. Peptides Identification by Mass Spectrometry
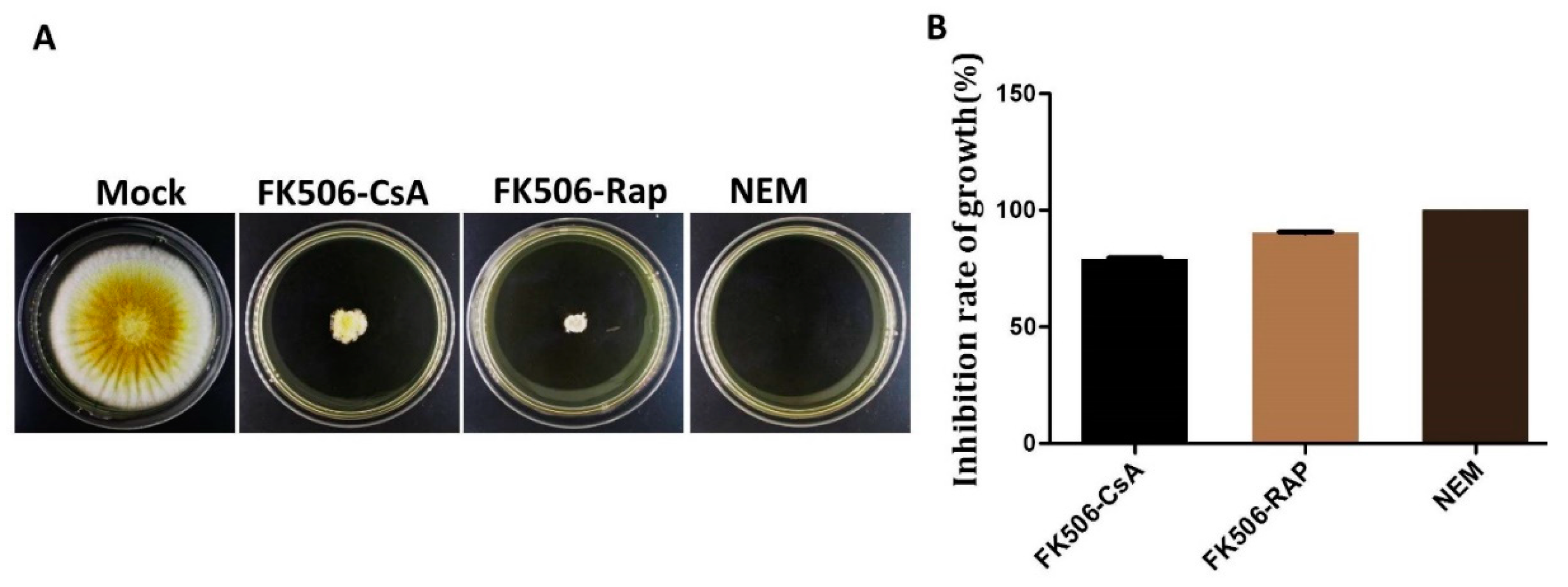
2.4. Substrate Specificity and Effect of Inhibitor On ppci1
2.5. 3D Model of Ppci1
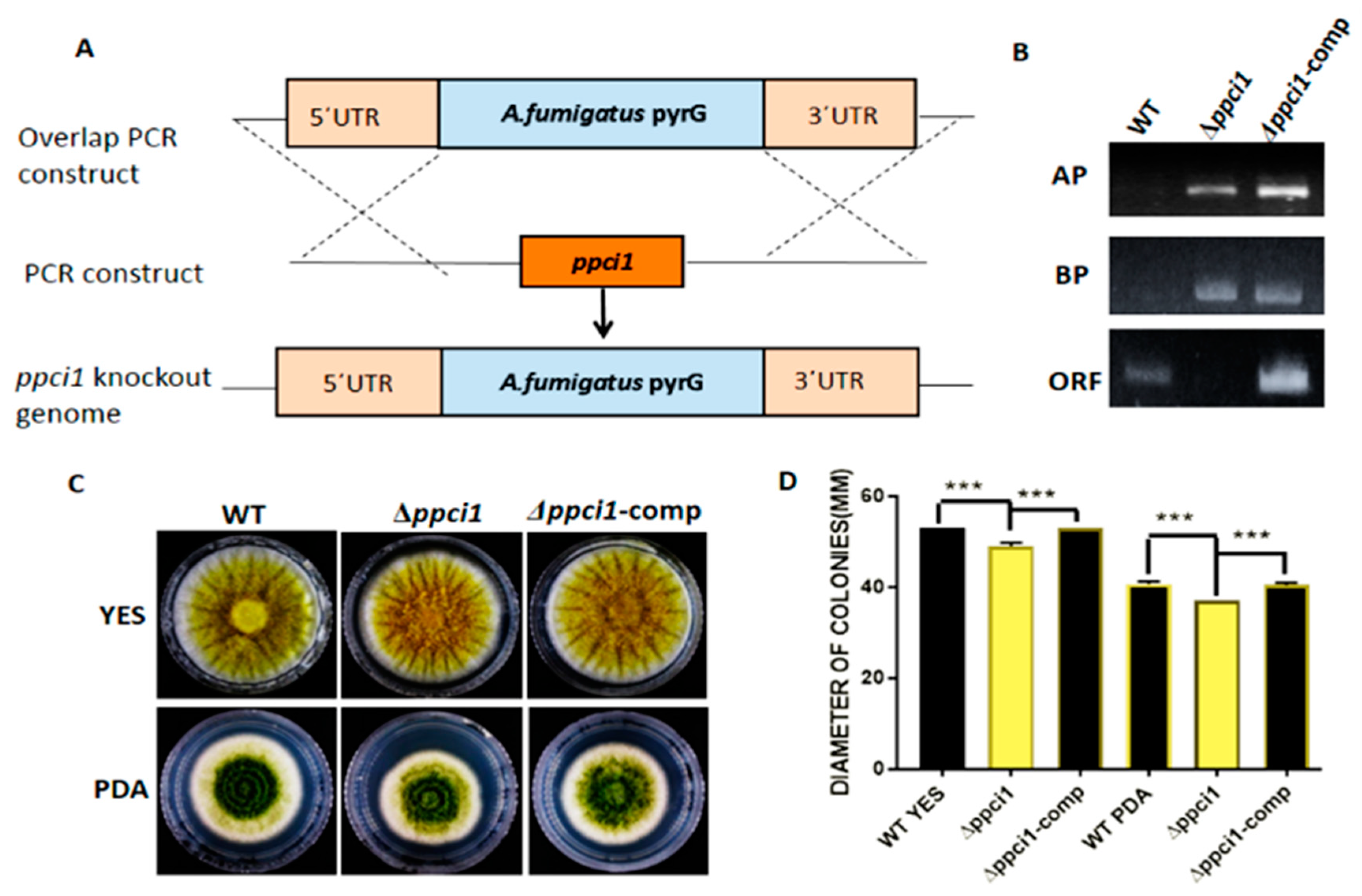
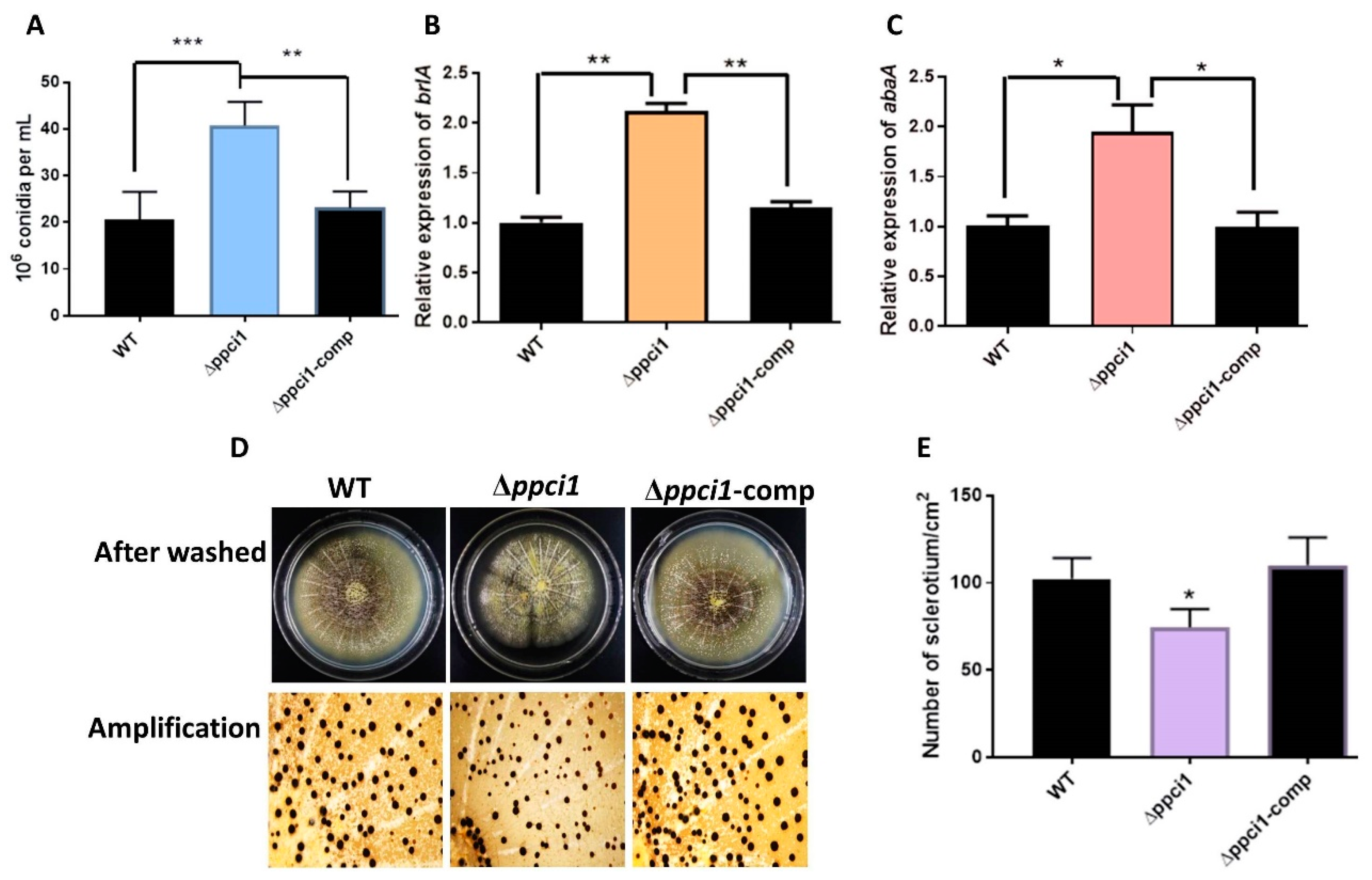
2.6. Construction of Δppci1 Deletion Mutant
2.7. Effect of Inhibitors on the Growth of Fungal Strain
2.8. Ppci1 is Important for Conidiation and Sclerotia Formation in A. flavus
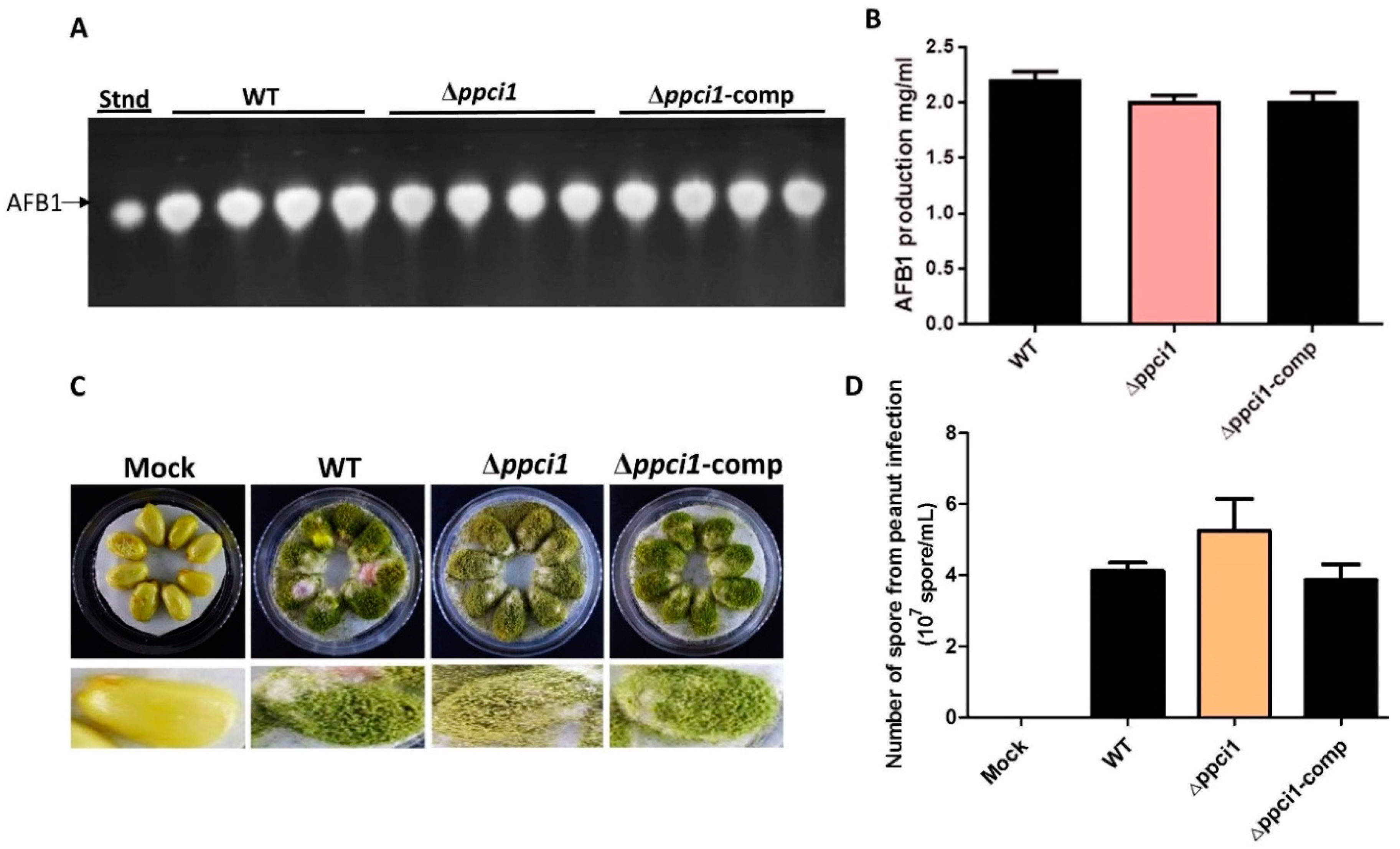
2.9. Effects of ppci1 on Aflatoxin Biosy$nthesis and Pathogenicity
3. Discussion
4. Materials and Methods
4.1. DNA Cloning, Protein Expression and Purification
4.2. Mass Spectrometry
4.3. PPIase Activity Assay
4.4. Reconstruction of Phylogeny Based on Sequence
4.5. Structure Determination and Functional Analysis
4.6. Gene Deletion and Complementation of Ppci1
4.7. Physiological Growth, Sclerotia and Conidiation, Analysis
4.8. Effect of Inhibitors
4.9. Aflatoxin and Seeds Infection Assays
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Payne, G.A.; Yu, J. Ecology, development and gene regulation in A. flavus. Aspergillus: Mol. Mol. Biol. Genom. 2010, 5, 157–171. [Google Scholar]
- Ramírez-Camejo, L.A.; Zuluaga-Montero, A.; Lázaro-Escudero, M.; Hernández-Kendall, V.; Bayman, P. Phylogeography of the cosmopolitan fungus A. flavus: Is everything everywhere? Fungal Biol. 2012, 116, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Hedayati, M.; Pasqualotto, A.; Warn, P.; Bowyer, P.; Denning, D.A. flavus: Human pathogen, allergen and mycotoxin producer. Microbiology 2007, 153, 1677–1692. [Google Scholar] [CrossRef]
- Payne, G.; Nierman, W.; Wortman, J.; Pritchard, B.; Brown, D.; Dean, R.; Bhatnagar, D.; Cleveland, T.; Machida, M.; Yu, J. Whole genome comparison of A. flavus and A. oryzae. Med Mycol. 2006, 44, S9–S11. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Chang, P.-K.; Ehrlich, K.C.; Cary, J.W.; Bhatnagar, D.; Cleveland, T.E.; Payne, G.A.; Linz, J.E.; Woloshuk, C.P.; Bennett, J.W. Clustered pathway genes in aflatoxin biosynthesis. Appl. Environ. Microbiol. 2004, 70, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Egal, S.; Hounsa, A.; Gong, Y.; Turner, P.; Wild, C.; Hall, A.; Hell, K.; Cardwell, K. Dietary exposure to aflatoxin from maize and groundnut in young children from Benin and Togo, West Africa. Int. J. Food Microbiol. 2005, 104, 215–224. [Google Scholar] [CrossRef]
- Amare, M.G.; Keller, N.P. Molecular mechanisms of A. flavus secondary metabolism and development. Fungal Genet. Biol. 2014, 66, 11–18. [Google Scholar] [CrossRef]
- Bhatnagar-Mathur, P.; Sunkara, S.; Bhatnagar-Panwar, M.; Waliyar, F.; Sharma, K.K. Biotechnological advances for combating A. flavus and aflatoxin contamination in crops. Plant Sci. 2015, 234, 119–132. [Google Scholar] [CrossRef]
- Fischer, G.; Berger, E.; Bang, H. Kinetic β-deuterium isotope effects suggest a covalent mechanism for the protein folding enzyme peptidylprolyl cis/trans-isomerase. FEBS Lett. 1989, 250, 267–270. [Google Scholar] [CrossRef]
- Pemberton, T.J. Identification and comparative analysis of sixteen fungal peptidyl-prolyl cis/trans isomerase repertoires. BMC Genom. 2006, 7, 244. [Google Scholar] [CrossRef]
- Xia, J.; Levy, R.M. Molecular dynamics of the proline switch and its role in Crk signaling. J. Phys. Chem. B 2014, 118, 4535–4545. [Google Scholar] [CrossRef] [PubMed]
- Walter, S.; Buchner, J. Molecular chaperones cellular machines for protein folding. Angew. Chem. Int. Ed. 2002, 41, 1098–1113. [Google Scholar] [CrossRef]
- Lummis, S.C.; Beene, D.L.; Lee, L.W.; Lester, H.A. Cis-trans isomerization at a proline opens the pore of a neurotransmitter-gated ion channel. Nature 2005, 438, 248. [Google Scholar] [CrossRef]
- Auerbach, A. The energy and work of a ligand-gated ion channel. J. Mol. Biol. 2013, 425, 1461–1475. [Google Scholar] [CrossRef] [PubMed]
- Rabenstein, D.L.; Shi, T.; Spain, S. Intramolecular Catalysis of the cis-trans Isomerization of Proline Peptide Bonds in Cyclic Disulfide-Containing Peptides. J. Am. Chem. Soc. 2000, 122, 2401–2402. [Google Scholar] [CrossRef]
- Su, X.; Aprahamian, I. Zinc (II)-Regulation of Hydrazone Switch Isomerization Kinetics. Org. Lett. 2013, 15, 5952–5955. [Google Scholar] [CrossRef]
- Edlich, F.; Weiwad, M.; Erdmann, F.; Fanghänel, J.; Jarczowski, F.; Rahfeld, J.U.; Fischer, G. Bcl-2 regulator FKBP38 is activated by Ca2+/calmodulin. EMBO J. 2005, 24, 2688–2699. [Google Scholar] [CrossRef]
- Kromina, K.; Ignatov, A.; Abdeeva, I. Role of peptidyl-prolyl-cis/trans-isomerases in pathologic processes. Biochem. (Moscow) Suppl. Ser. A Membr. Cell Biol. 2008, 2, 195–202. [Google Scholar] [CrossRef]
- Arevalo-Rodriguez, M.; Heitman, J. Cyclophilin A is localized to the nucleus and controls meiosis in Saccharomyces cerevisiae. Eukaryot. Cell 2005, 4, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Viaud, M.C.; Balhadere, P.V.; Talbot, N.J. A Magnaporthe grisea cyclophilin acts as a virulence determinant during plant infection. Plant Cell 2002, 14, 917–930. [Google Scholar] [CrossRef]
- Chen, M.M.; Jiang, M.G.; Shang, J.J.; Lan, X.W.; Yang, F.; Huang, J.K.; Nuss, D.L.; Chen, B.S. CYP1, a hypovirus-regulated cyclophilin, is required for virulence in the chestnut blight fungus. Mol. Plant Pathol. 2011, 12, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Wedemeyer, W.J.; Welker, E.; Scheraga, H.A. Proline cis-trans isomerization and protein folding. Biochemistry 2002, 41, 14637–14644. [Google Scholar] [CrossRef]
- Maruyama, T.; Furutani, M. Archaeal peptidyl prolyl cis-trans isomerases (PPIases). Front Biosci. 2000, 5, 821–836. [Google Scholar]
- Norville, I.H. The identification and characterisation of PPIases from Burkholderia pseudomallei and Burkholderia thailandensis. EXETER 2011, 4, 64–67. [Google Scholar]
- Lundemose, A.G.; Kay, J.E.; Pearce, J.H. Chlamydia trachomatis Mip-like protein has peptidylprolyl cis/trans isomerase activity that is inhibited by FK506 and rapamycin and is implicated in initiation of chlamydial infection. Mol. Microbiol. 1993, 7, 777–783. [Google Scholar] [CrossRef]
- Justice, S.S.; Hunstad, D.A.; Harper, J.R.; Duguay, A.R.; Pinkner, J.S.; Bann, J.; Frieden, C.; Silhavy, T.J.; Hultgren, S.J. Periplasmic peptidyl prolyl cis-trans isomerases are not essential for viability, but SurA is required for pilus biogenesis in E. coli. J. Bacteriol. 2005, 187, 7680–7686. [Google Scholar] [CrossRef] [PubMed]
- Guy, N.C.; Garcia, Y.A.; Cox, M.B. Therapeutic targeting of the FKBP52 co-chaperone in steroid hormone receptor-regulated physiology and disease. Curr. Mol. Pharmacol. 2016, 9, 109–125. [Google Scholar] [CrossRef]
- Behrens, S.; Maier, R.; de Cock, H.; Schmid, F.X.; Gross, C.A. The SurA periplasmic PPIase lacking its parvulin domains functions in vivo and has chaperone activity. EMBO J. 2001, 20, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.; Sali, A. Protein structure prediction and structural genomics. Science 2001, 294, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-K.; Kwon, N.-J.; Lee, I.-S.; Jung, S.; Kim, S.-C.; Yu, J.-H. Negative regulation and developmental competence in Aspergillus. Sci. Rep. 2016, 6, 28874. [Google Scholar] [CrossRef]
- Fischer, G.; Wittmann-Liebold, B.; Lang, K.; Kiefhaber, T.; Schmid, F.X. Cyclophilin and peptidyl-prolyl cis-trans isomerase are probably identical proteins. Nature 1989, 337, 476. [Google Scholar] [CrossRef]
- Fischer, G.; Bang, H.; Ludwig, B.; Mann, K.; Hacker, J. Mip protein of Legionella pneumophila exhibits peptidyl-prolyl-cis/trans isomerase (PPIase) activity. Mol. Microbiol. 1992, 6, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.; Monaghan, P.; Page, A.P. Peptidyl-prolyl cis-trans isomerases (immunophilins) and their roles in parasite biochemistry, host–parasite interaction and antiparasitic drug action. Int. J. Parasitol. 2006, 36, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Gavini, N.; Tungtur, S.; Pulakat, L. Peptidyl-prolyl cis/trans isomerase-independent functional NifH mutant of Azotobacter vinelandii. J. Bacteriol. 2006, 188, 6020–6025. [Google Scholar] [CrossRef]
- Terpe, K. Overview of bacterial expression systems for heterologous protein production: From molecular and biochemical fundamentals to commercial systems. Appl. Microbiol. Biotechnol. 2006, 72, 211. [Google Scholar] [CrossRef]
- Wang, S.; Li, B.W.; Jiang, L.G.; Wang, S.H.; Zhang, Y.F.; Wang, Y. Expression, Purification, Crystallization and Preliminary Crystallographic Analysis of Nucleoside Diphosphate Kinase (NDK) from Aspergillus Flavus. Chin. J. Struc. Chem. 2016, 35, 1708–1713. [Google Scholar] [CrossRef]
- Rahfeld, J.-U.; Schierhorn, A.; Mann, K.; Fischer, G. A novel peptidyl-prolyl cis/trans isomerase from Escherichia coli. FEBS Lett. 1994, 343, 65–69. [Google Scholar] [CrossRef]
- Kaur, G.; Singh, S.; Singh, H.; Chawla, M.; Dutta, T.; Kaur, H.; Bender, K.; Snedden, W.; Kapoor, S.; Pareek, A. Characterization of peptidyl-prolyl cis-trans isomerase-and calmodulin-binding activity of a cytosolic Arabidopsis thaliana cyclophilin AtCyp19-3. PLoS ONE 2015, 10, e0136692. [Google Scholar] [CrossRef] [PubMed]
- Blecher, O.; Erel, N.; Callebaut, I.; Aviezer, K.; Breiman, A. A novel plant peptidyl-prolyl-cis-trans-isomerase (PPIase): cDNA cloning, structural analysis, enzymatic activity and expression. Plant Mol. Biol. 1996, 32, 493–504. [Google Scholar] [CrossRef]
- Harding, M.W.; Galat, A.; Uehling, D.E.; Schreiber, S.L. A receptor for the immuno-suppressant FK 506 is a cis-trans peptidyl-prolyl isomerase. Nature 1989, 341, 758–760. [Google Scholar] [CrossRef]
- Yang, K.; Qin, Q.; Liu, Y.; Zhang, L.; Liang, L.; Lan, H.; Chen, C.; You, Y.; Zhang, F.; Wang, S. Adenylate cyclase AcyA regulates development, aflatoxin biosynthesis and fungal virulence in Aspergillus Flavus. Front. Cell. Infect. Microbiol. 2016, 6, 190. [Google Scholar] [CrossRef]
- Han, X.; Qiu, M.; Wang, B.; Yin, W.-B.; Nie, X.; Qin, Q.; Ren, S.; Yang, K.; Zhang, F.; Zhuang, Z. Functional analysis of the nitrogen metabolite repression regulator gene nmrA in Aspergillus flavus. Front. Microbiol. 2016, 7, 1794. [Google Scholar] [CrossRef]
- Pildain, M.B.; Frisvad, J.C.; Vaamonde, G.; Cabral, D.; Varga, J.; Samson, R.A. Two novel aflatoxin-producing Aspergillus species from Argentinean peanuts. Int. J. Syst. Evol. Microbiol. 2008, 58, 725–735. [Google Scholar] [CrossRef]
- Yuan, J.; Chen, Z.; Guo, Z.; Li, D.; Zhang, F.; Shen, J.; Zhang, Y.; Wang, S.; Zhuang, Z. PbsB regulates morphogenesis, Aflatoxin B1 biosynthesis and pathogenicity of A. flavus. Front. Cell. Infect. Microbiol. 2018, 8, 162. [Google Scholar] [CrossRef]
- Tumukunde, E.; Li, D.; Qin, L.; Li, Y.; Shen, J.; Wang, S.; Yuan, J. Osmotic-Adaptation Response of sakA/hogA Gene to Aflatoxin Biosynthesis, Morphology Development and Pathogenicity in A. Flavus. Toxins 2019, 11, 41. [Google Scholar] [CrossRef]
- Shilov, I.V.; Seymour, S.L.; Patel, A.A.; Loboda, A.; Tang, W.H.; Keating, S.P.; Hunter, C.L.; Nuwaysir, L.M.; Schaeffer, D.A. The Paragon Algorithm, a next generation search engine that uses sequence temperature values and feature probabilities to identify peptides from tandem mass spectra. Mol. Cell. Proteom. 2007, 6, 1638–1655. [Google Scholar] [CrossRef]
- Gourlay, L.J.; Angelucci, F.; Baiocco, P.; Boumis, G.; Brunori, M.; Bellelli, A.; Miele, A.E. The Three-dimensional Structure of Two Redox States of Cyclophilin A from Schistosoma mansoni evidence for redox regulation of peptidyl-prolyl cis-trans isomerase activity. J. Biol. Chem. 2007, 282, 24851–24857. [Google Scholar] [CrossRef]
- Chen, A.; He, S.; Li, F.; Li, Z.; Ding, M.; Liu, Q.; Rong, J. Analyses of the sucrose synthase gene family in cotton: Structure, phylogeny and expression patterns. BMC Plant Biol. 2012, 12, 85. [Google Scholar] [CrossRef]
- Westbrook, J.; Feng, Z.; Jain, S.; Bhat, T.; Thanki, N.; Ravichandran, V.; Gilliland, G.L.; Bluhm, W.; Weissig, H.; Greer, D.S. The protein data bank: Unifying the archive. Nucleic Acids Res. 2002, 30, 245–248. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Rullmann, J.A.C.; MacArthur, M.W.; Kaptein, R.; Thornton, J.M. AQUA and PROCHECK-NMR: Programs for checking the quality of protein structures solved by NMR. J. Biomol. NMR 1996, 8, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Liang, L.; Ran, F.; Liu, Y.; Li, Z.; Lan, H.; Gao, P.; Zhuang, Z.; Zhang, F.; Nie, X. The DmtA methyltransferase contributes to A. flavus conidiation, sclerotial production, aflatoxin biosynthesis and virulence. Sci. Rep. 2016, 6, 23259. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.-K.; Scharfenstein, L.L.; Wei, Q.; Bhatnagar, D. Development and refinement of a high-efficiency gene-targeting system for Aspergillus flavus. J. Microbiol. Methods 2010, 81, 240–246. [Google Scholar] [CrossRef]
- Lan, H.; Sun, R.; Fan, K.; Yang, K.; Zhang, F.; Nie, X.Y.; Wang, X.; Zhuang, Z.; Wang, S. The A. flavus histone acetyltransferase AflGcnE regulates morphogenesis, aflatoxin biosynthesis, and pathogenicity. Front. Microbiol. 2016, 7, 1324. [Google Scholar] [CrossRef]


| Source Organism | A. flavus NRRL3357 |
|---|---|
| Primer F | 5′-ATGGCGCCCAAAAACA3′ |
| Primer R | 5′-TTACTTCCGCCCCTCGA3′ |
| Cloning and Expression Vector | pRSFDuet-1 expression vector (Novagen) |
| Expression Host | E. coli BL21(DE3) |
| Complete amino acids sequence of the constructed product | MAPKNKGGDKKGKGNDGGDKGGKGLKPATSINVRHILCEKHSKKEEALEKLRNGSKFDDVAREFSEDKARQGGSLGWKVRGSLDGTFEKAAYELEPSTTANPKYVEVKTGFGYHIIMVEGRK |
| Binding Buffer A | 50 mM Tris-HCl, 500 mM NaCl, 20 mM Imidazole pH 8.0 |
| Eluted Buffer A | 50 mM Tris-HCl, 500 mM NaCl, 20 mM Imidazole pH 8.0 |
| Eluted Buffer B | 50 mM Tris-HCl, 500 mM NaCl, 50 mM Imidazole pH 8.0 |
| Eluted Buffer C | 50 mM Tris-HCl, 500 mM NaCl, 300 mM Imidazole pH 8.0 |
| Dialyzed Buffer D | 50 mM Tris-HCl, 500 mM NaCl |
| Primers | Sequence (5′–3′) |
|---|---|
| ppci1 AF | CCTAGCGACTCAAAGCG |
| PPci1 AR | GGGTGAAGAGCATTGTTTGAGGCTTGGGTAACGGTAAGTGC |
| ppci1 ORF/F: | AACAAAGGCGGAGACAA |
| ppci1 ORF/R: | AAGGAAAGGAGACGAAAG |
| ppci1 BF | GCATCAGTGCCTCCTCTCAGACGCATTACTTTACTGGCTCTT |
| ppci1 BR | GTCTACATTTGCCGCTAT |
| pyrg F: | GCCTCAAACAATGCTCTTCACCC |
| pyrg R | GTCTGAGAGGAGGCACTGATGC |
| Comp F: | ACAAGCGTTCCAAGCCA |
| Comp R: | TTCCGCCCCTCGACCAT |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, S.; Wang, S.; Wu, W.; Yang, K.; Zhang, Y.; Tumukunde, E.; Wang, S.; Wang, Y. Functional Analysis of Peptidyl-prolyl cis-trans Isomerase from Aspergillus flavus. Int. J. Mol. Sci. 2019, 20, 2206. https://doi.org/10.3390/ijms20092206
Ahmad S, Wang S, Wu W, Yang K, Zhang Y, Tumukunde E, Wang S, Wang Y. Functional Analysis of Peptidyl-prolyl cis-trans Isomerase from Aspergillus flavus. International Journal of Molecular Sciences. 2019; 20(9):2206. https://doi.org/10.3390/ijms20092206
Chicago/Turabian StyleAhmad, Saleem, Sen Wang, Weizhong Wu, Kunlong Yang, YanFeng Zhang, Elisabeth Tumukunde, Shihua Wang, and Yu Wang. 2019. "Functional Analysis of Peptidyl-prolyl cis-trans Isomerase from Aspergillus flavus" International Journal of Molecular Sciences 20, no. 9: 2206. https://doi.org/10.3390/ijms20092206
APA StyleAhmad, S., Wang, S., Wu, W., Yang, K., Zhang, Y., Tumukunde, E., Wang, S., & Wang, Y. (2019). Functional Analysis of Peptidyl-prolyl cis-trans Isomerase from Aspergillus flavus. International Journal of Molecular Sciences, 20(9), 2206. https://doi.org/10.3390/ijms20092206






