Centella asiatica Protects d-Galactose/AlCl3 Mediated Alzheimer’s Disease-Like Rats via PP2A/GSK-3β Signaling Pathway in Their Hippocampus
Abstract
1. Introduction
2. Results
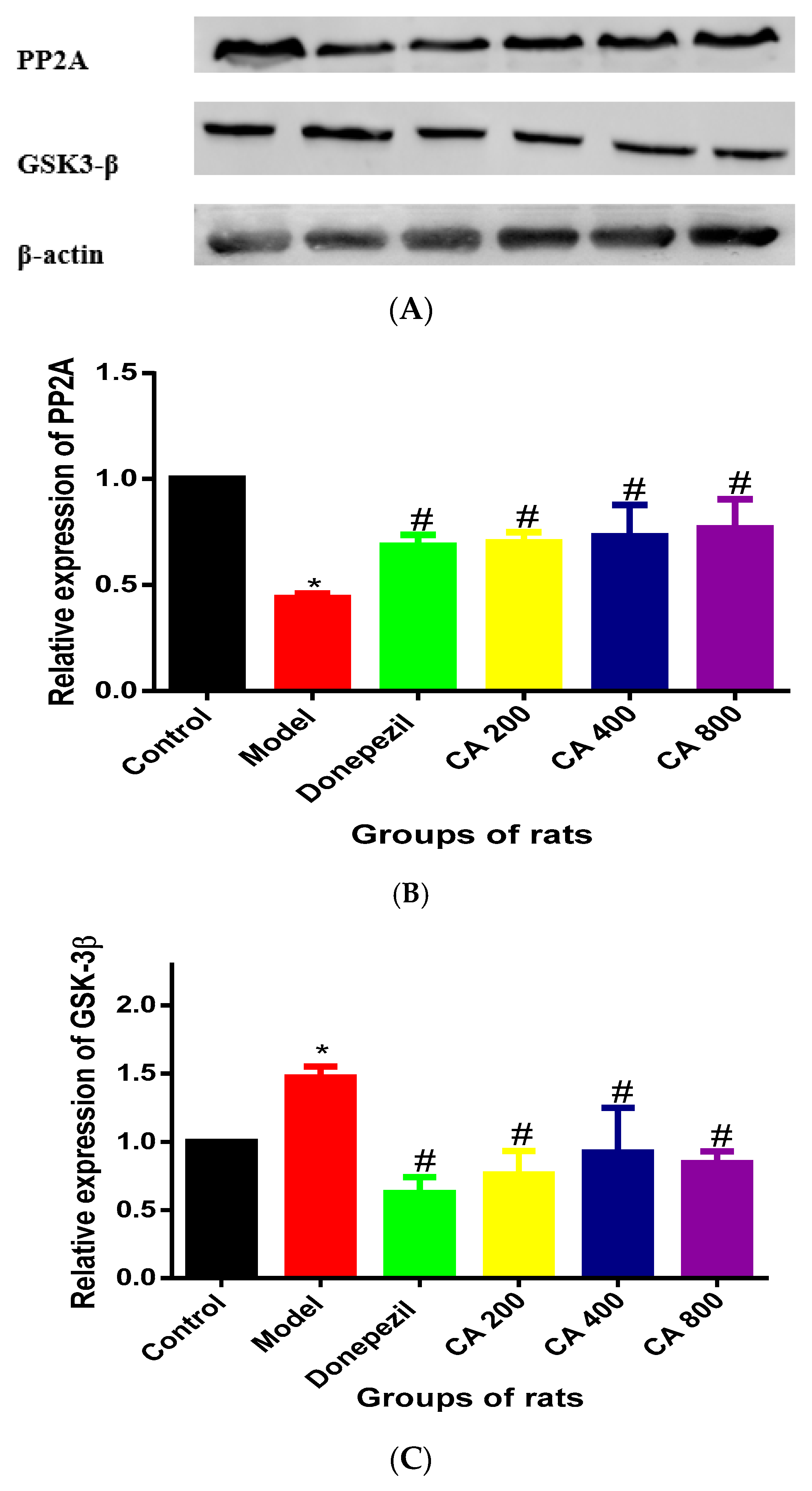
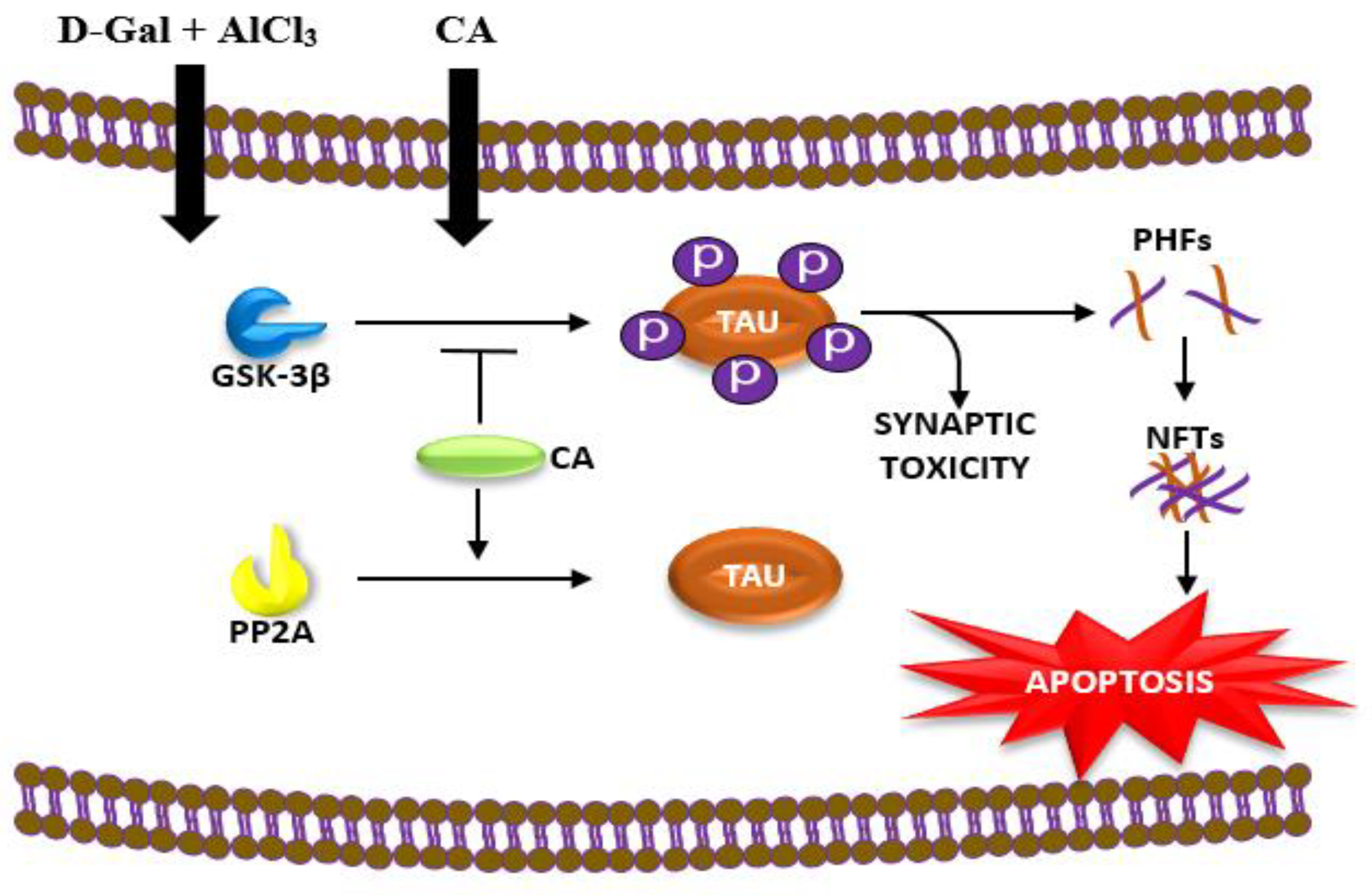
2.1. CA Increased the Activity of PP2A and Decreased the Activity of GSK-3β in Hippocampus of Rats Exposed to d-Gal and AlCl3
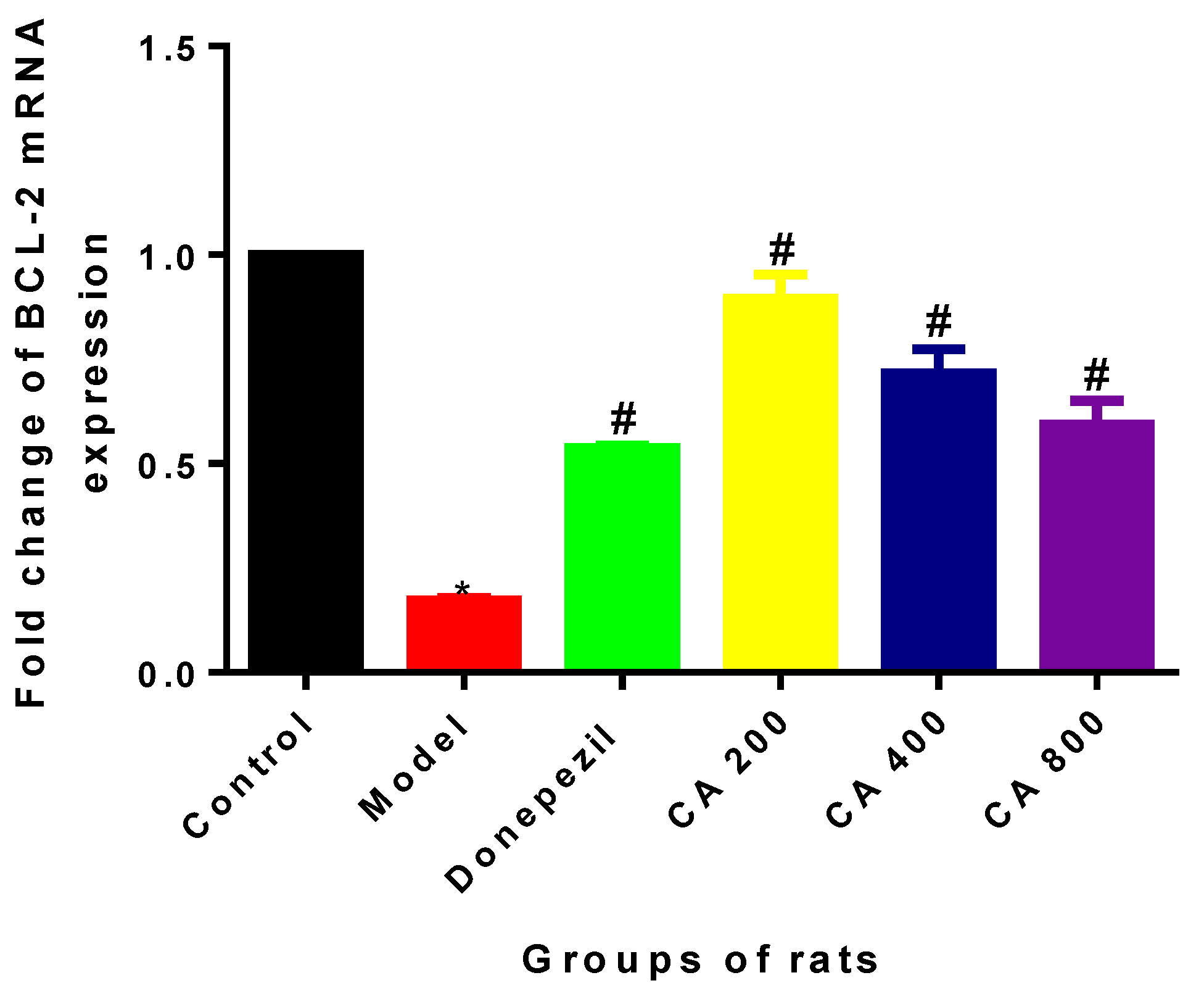
2.2. Effects of CA on Intrinsic Mitochondria Mediated Apoptosis Related Genes of Rat Hippocampus Exposed to d-Gal and AlCl3
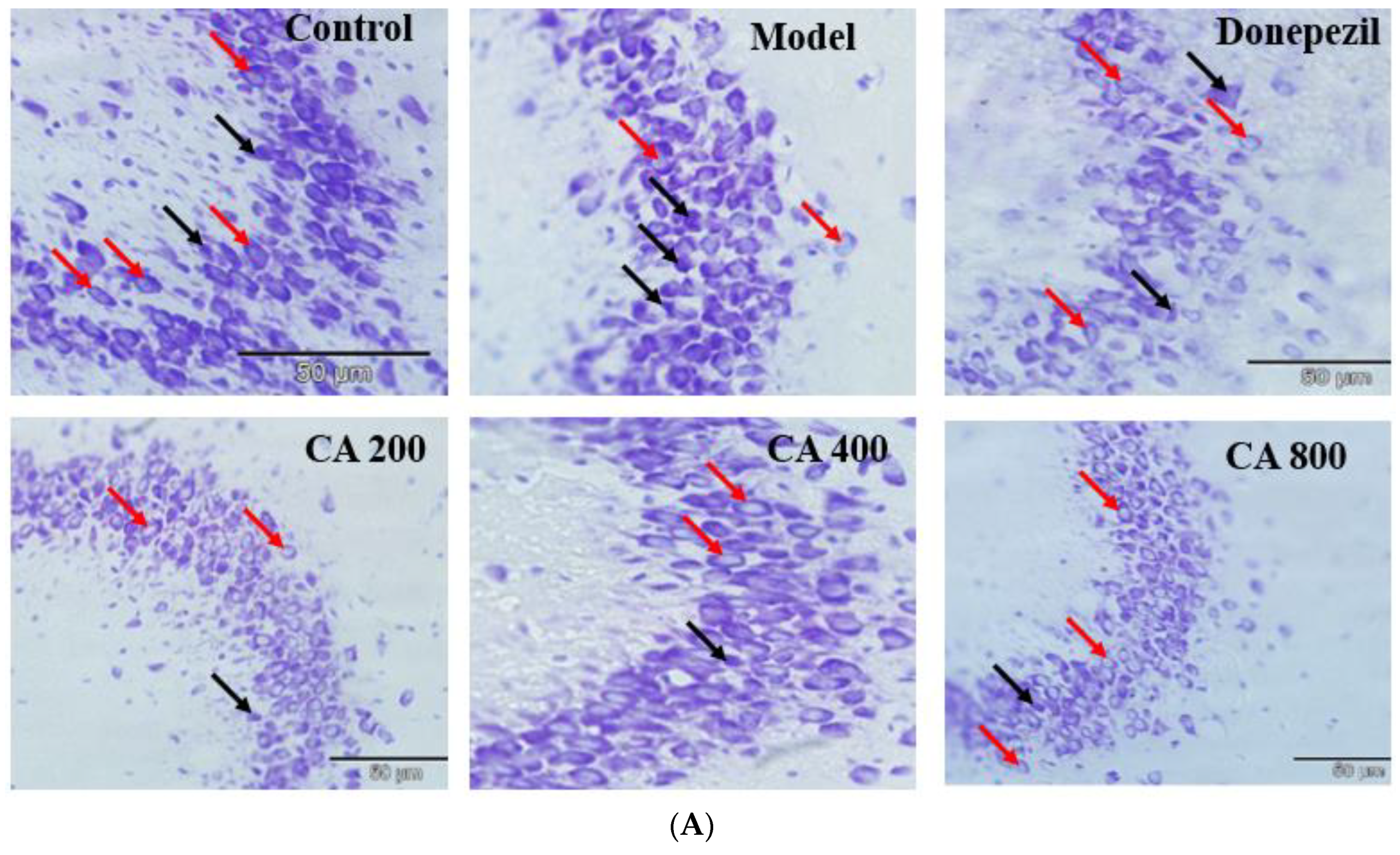
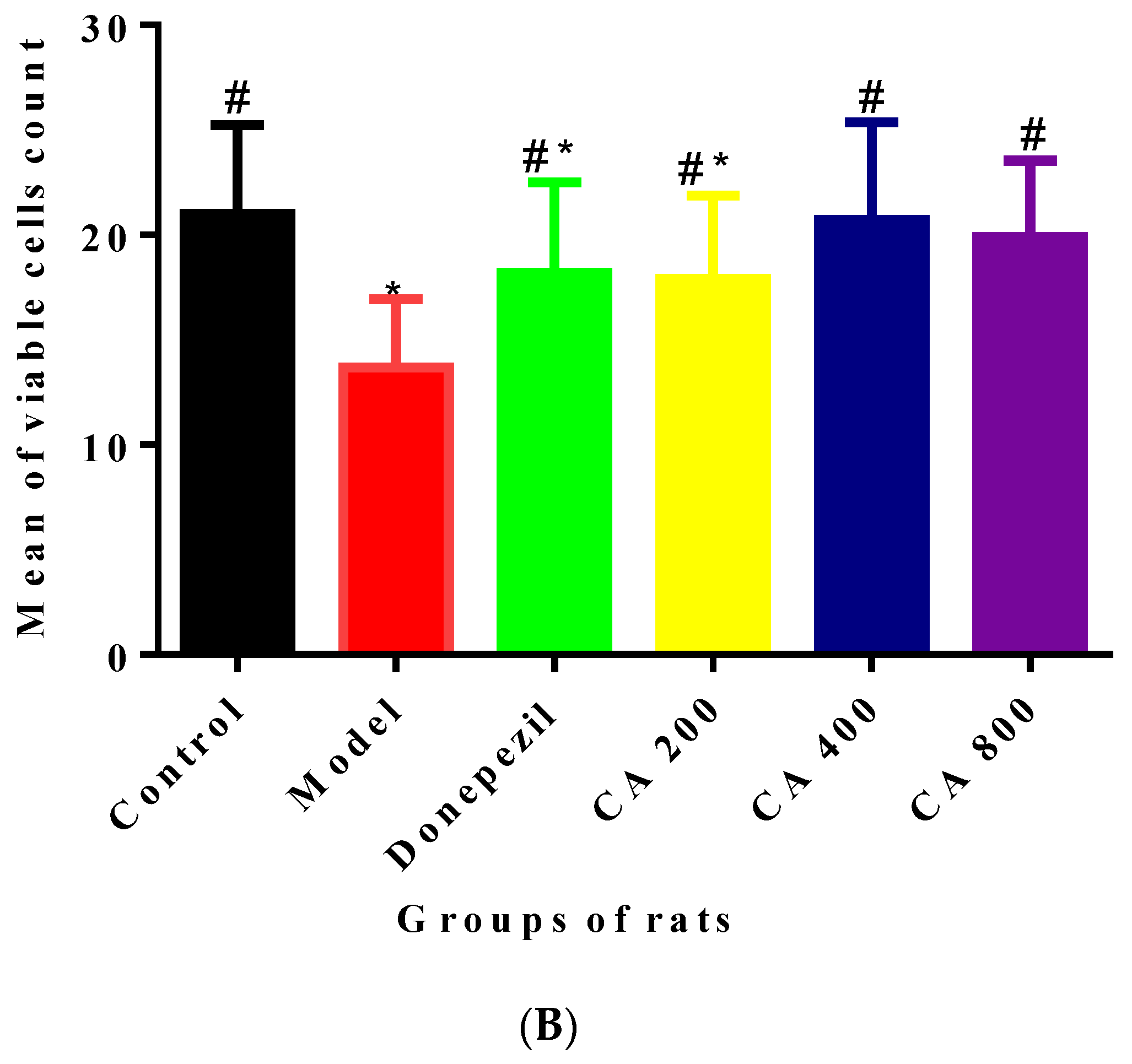
2.3. CA Protects against d-Gal and AlCl3 Induced Pyramidal Cells Loss in CA 3 Subregions of Hippocampus of Rats
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Chemicals and Reagents
4.3. Experimental Design and Treatment Protocol
4.4. Protein Estimation
4.5. Western Blotting Analysis
4.6. RNA Extraction and cDNA Synthesis
4.7. Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
4.8. Cresyl Violet Staining and Scoring
4.9. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| AlCl3 | Aluminium chloride |
| AMPAR | α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor |
| ANOVA | Analysis of variance |
| ATP | Adenosine triphosphate |
| BAK | Bcl-2 antagonist/killer 1 |
| Bax | BCL2-Associated X Protein |
| Bcl-2 | B-cell lymphoma 2 |
| CA | Centella asiatica |
| CA3 | Connus ammonis 3 |
| Caspase 3 | Cysteine-aspartic acid protease 3 |
| Cdk5 | Cyclin-Dependent Kinase 5 |
| Cyt c | Cytochrome c |
| ddH2O | Double distilled water |
| d-Gal | d-galactose |
| GSK-3β | Glycogen synthase kinase-3 beta |
| HRP | Horseradish peroxidase |
| mRNA | Messenger Ribonucleic Acid |
| MOM | Mitochondrial outer membrane |
| MOMP | Mitochondrial outer membrane permealisation |
| n | Number of rats per group |
| NFTs | Neutofibrillary tangles |
| NO | Nitric oxide |
| PP2A | Protein phosphatase 2 |
| PVDF | Polyvinylidene difluoride |
| ROS | Reactive oxygen species |
| RT PCR | Real time polymerase chain reaction |
| SD | Standard deviation |
| SDS | Sodium dodecyl sulfate |
| TBST | Tri-buffered saline, 0.1% tween 20 |
| SDS-PAGE | Sodium dodecyl sulfate polyacrilmide gel electrophoresis |
| Tyr | Tyrosine |
References
- Yang, W.; Shi, L.; Chen, L.; Zhang, B.; Ma, K.; Liu, Y.; Qian, Y. Protective effects of perindopril on d-galactose and aluminum trichloride induced neurotoxicity via the apoptosis of mitochondria-mediated intrinsic pathway in the hippocampus of mice. Brain Res. Bull. 2014, 109, 46–53. [Google Scholar] [CrossRef]
- Xing, Z.; He, Z.; Wang, S.; Yan, Y.; Zhu, H.; Gao, Y.; Zhao, Y.; Zhang, L. Ameliorative effects and possible molecular mechanisms of action of fibrauretine from Fibraurea recisa Pierre on d-galactose/AlCl3-mediated Alzheimer’s disease. RSC Adv. 2018, 8, 31646–31657. [Google Scholar] [CrossRef]
- Yang, W.N.; Han, H.; Hu, X.D.; Feng, G.F.; Qian, Y.H. The effects of perindopril on cognitive impairment induced by d-galactose and aluminum trichloride via inhibition of acetylcholinesterase activity and oxidative stress. Pharmacol. Biochem. Behav. 2013, 114–115, 31–36. [Google Scholar] [CrossRef]
- Kumar, A.; Dogra, S.; Prakash, A. Protective effect of curcumin (Curcuma longa), against aluminium toxicity: Possible behavioral and biochemical alterations in rats. Behav. Brain Res. 2009, 205, 384–390. [Google Scholar] [CrossRef]
- Zhang, Y.; Pi, Z.; Song, F.; Liu, Z. Ginsenosides attenuate d-galactose- and AlCl3-inducedspatial memory impairment by restoring the dysfunction of the neurotransmitter systems in the rat model of Alzheimer’s disease. J. Ethnopharmacol. 2016, 194, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, X.; Jin, G.; Yang, X.; Zhang, Y. Polysaccharides from Pleurotus ostreatus alleviate cognitive impairment in a rat model of Alzheimer’s disease. Int. J. Biol. Macromol. 2016, 92, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Bilgic, Y.; Demir, E.A.; Bilgic, N.; Dogan, H.; Tutuk, O.; Tumer, C. Detrimental effects of chia (Salvia hispanica L.) seeds on learning and memory in aluminum chloride-induced experimental Alzheimer’s disease. Acta Neurobiol. Exp. Wars 2018, 78, 322–331. [Google Scholar] [CrossRef]
- Chiroma, S.M.; Mohd Moklas, M.A.; Mat Taib, C.N.; Baharuldin, M.T.H.; Amon, Z. d-galactose and aluminium chloride induced rat model with cognitive impairments. Biomed. Pharmacother. 2018, 103, 1602–1608. [Google Scholar] [CrossRef]
- Li, H.; Kang, T.; Qi, B.; Kong, L.; Jiao, Y.; Cao, Y.; Zhang, J.; Yang, J. Neuroprotective effects of ginseng protein on PI3K/Akt signaling pathway in the hippocampus of d-galactose/AlCl3 inducing rats model of Alzheimer’ s disease. J. Ethnopharmacol. 2016, 179, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Pascoal, T.A.; Mathotaarachchi, S.; Shin, M.; Benedet, A.L.; Mohades, S.; Wang, S.; Beaudry, T.; Kang, M.S.; Soucy, J.; Labbe, A.; et al. Synergistic interaction between amyloid and tau predicts the progression to dementia. Alzheimer’s Dement. 2017, 13, 644–653. [Google Scholar] [CrossRef]
- Reddy, P.H. Abnormal tau, mitochondrial dysfunction, impaired axonal transport of mitochondria, and synaptic deprivation in Alzheimer’s disease. Brain Res. 2011, 1415, 136–148. [Google Scholar] [CrossRef]
- Liu, F.; Grundke-Iqbal, I.; Iqbal, K.; Gong, C. Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau phosphorylation. Eur. J. Neurosci. 2005, 22, 1942–1950. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, F.; Lucas, J.J.; Avila, J. GSK3 and tau: Two convergence points in Alzheimer’s disease. J. Alzheimer’s Dis. 2013, 33, S141–S144. [Google Scholar] [CrossRef] [PubMed]
- Sontag, E.; Luangpirom, A.; Hladik, C.; Mudrak, I.; Ogris, E.; Speciale, S.; White, C.L., III. Altered Expression Levels of the Protein Phosphatase 2A ABαC Enzyme Are Associated with Alzheimer Disease Pathology. J. Neuropathol. Exp. Neurol. 2004, 63, 287–301. [Google Scholar] [CrossRef]
- Subaraja, M.; Vanisree, A.J. The novel phytocomponent Asiaticoside-D isolated from Centella asiatica exhibits monoamine oxidase–B inhibiting potential in the rotenone degenerated cerebral ganglions of Lumbricus terretris. Phytomedicine 2019, 152833, in press. [Google Scholar] [CrossRef] [PubMed]
- Rasid, N.A.M.; Nazmi, N.N.M.; Isa, M.I.N.; Sarbon, N.M. Rheological, functional and antioxidant properties of films forming solution and active gelatin films incorporated with Centella asiatica (L.) urban extract. Food Packag. Shelf Life 2018, 18, 115–124. [Google Scholar] [CrossRef]
- Dev, R.D.O.; Mohamed, S.; Hambali, Z.; Samah, B.A. Comparison on cognitive effects of Centella asiatica in healthy middle age female and male volunteers. Eur. J. Sci. Res. 2009, 31, 553–565. [Google Scholar]
- Tiwari, S.; Singh, S.; Patwardhan, K.; Gehlot, S.; Gambhir, I.S. Effect of Centella asiatica on mild cognitive impairment (MCI) and other common age-related clinical problems. Dig. J. Nanomater. Biostruct. 2008, 3, 215–220. [Google Scholar]
- Soumyanath, A.; Zhong, Y.-P.; Henson, E.; Wadsworth, T.; Bishop, J.; Gold, B.G.; Quinn, J.F. Centella asiatica Extract Improves Behavioral Deficits in a Mouse Model of Alzheimer’s Disease: Investigation of a Possible Mechanism of Action. Int. J. Alzheimer’s Dis. 2012, 2012, 381974. [Google Scholar]
- Gupta, R.; Flora, S.J.S. Effect of Centella asiatica on arsenic induced oxidative stress and metal distribution in rats. J. Appl. Toxicol. 2006, 26, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Gray, N.E.; Morré, J.; Kelley, J.; Maier, C.S.; Stevens, J.F.; Quinn, J.F.; Soumyanath, A. Caffeoylquinic acids in centella asiatica protect against amyloid-β toxicity. J. Alzheimer’s Dis. 2014, 40, 359–373. [Google Scholar] [CrossRef]
- Lokanathan, Y.; Omar, N.; Ahmad Puz, N.N.; Saim, A.; Hj Idrus, R. Recent updates in neuroprotective and neuroregenerative potential of Centella asiatica. Malays. J. Med. Sci. 2016, 23, 4–14. [Google Scholar]
- Gray, N.E.; Zweig, J.A.; Caruso, M.; Zhu, J.Y.; Wright, K.M.; Quinn, J.F.; Soumyanath, A. Centella asiatica attenuates hippocampal mitochondrial dysfunction and improves memory and executive function in β-amyloid overexpressing mice. Mol. Cell Neurosci. 2018, 93, 1–9. [Google Scholar] [CrossRef]
- Wong, J.H.; Muthuraju, S.; Reza, F.; Senik, M.H.; Zhang, J.; Mohd Yusuf Yeo, N.A.B.; Chuang, H.G.; Jaafar, H.; Yusof, S.R.; Mohamad, H.; et al. Differential expression of entorhinal cortex and hippocampal subfields α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-d-aspartate (NMDA) receptors enhanced learning and memory of rats following administration of Centella asiatica. Biomed. Pharmacother. 2019, 110, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Chiroma, S.M.; Baharuldin, M.T.H.; Taib, C.N.M.; Amom, Z.; Jagadeesan, S.; Adenan, M.I.; Moklas, M.A.M. Protective effect of Centella asiatica against d-galactose and aluminium chloride induced rats: Behavioral and ultrastructural approaches. Biomed. Pharmacother. 2019, 109, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Hanger, D.P.; Anderton, B.H.; Noble, W. Tau phosphorylation: The therapeutic challenge for neurodegenerative disease. Trends Mol. Med. 2009, 15, 112–119. [Google Scholar] [CrossRef]
- Xiao, F.; Li, X.-G.; Zhang, X.-Y.; Hou, J.-D.; Lin, L.-F.; Gao, Q.; Luo, H.M. Combined administration of d-galactose and aluminium induces Alzheimerlike lesions in brain. Neurosci. Bull. 2011, 27, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Eldar-Finkelman, H. Glycogen synthase kinase 3: An emerging therapeutic target. Trends Mol. Med. 2002, 8, 126–132. [Google Scholar] [CrossRef]
- Lovell, M.A.; Xiong, S.; Xie, C.; Davies, P.; Markesbery, W.R. Induction of hyperphosphorylated tau in primary rat cortical neuron cultures mediated by oxidative stress and glycogen synthase kinase-3. J. Alzheimer’s Dis. 2004, 6, 659–671. [Google Scholar] [CrossRef]
- Hanger, D.P.; Noble, W. Functional implications of glycogen synthase kinase-3-mediated tau phosphorylation. Int. J. Alzheimers Dis. 2011, 2011, 352805. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Araiza, C.; Amorim, M.A.R.; Camacho-Arroyo, I.; Garcia-Segura, L.M. Effects of progesterone and its reduced metabolites, dihydroprogesterone and tetrahydroprogesterone, on the expression and phosphorylation of glycogen synthase kinase-3 and the microtubule-associated protein tau in the rat cerebellum. Dev. Neurobiol. 2007, 67, 510–520. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, X.; Cui, X.; Zuo, P. d-galactose injured neurogenesis in the hippocampus of adult mice. Neurol. Res. 2005, 27, 552–556. [Google Scholar] [CrossRef]
- Tanzi, R.E.; Moir, R.D.; Wagner, S.L. Clearance of Alzheimer’s Aβ peptide: The many roads to perdition. Neuron 2004, 43, 605–608. [Google Scholar] [PubMed]
- Pompl, P.N.; Yemul, S.; Xiang, Z.; Ho, L.; Haroutunian, V.; Purohit, D.; Mohs, R.; Pasinetti, G.M. Caspase gene expression in the brain as a function of the clinical progression of Alzheimer disease. Arch. Neurol. 2003, 60, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Blomgren, K. Mitochondrial cell death control in familial Parkinson disease. PLoS Biol. 2007, 5, e206. [Google Scholar] [CrossRef]
- Adams, J.M.; Cory, S. The Bcl-2 protein family: Arbiters of cell survival. Science 1998, 281, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Yang, W.; Gao, S.; Lin, J.; Wang, T.; Zhou, K.; Hu, H. Ginsenoside Rb1 inhibit apoptosis in rat model of alzheimer’s disease induced by Aβ1-40. Am. J. Transl. Res. 2018, 10, 796. [Google Scholar]
- Love, S. Apoptosis and brain ischaemia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2003, 27, 267–282. [Google Scholar] [CrossRef]
- Woo, R.-S.; Lee, J.-H.; Yu, H.-N.; Song, D.-Y.; Baik, T.-K. Expression of ErbB4 in the apoptotic neurons of Alzheimer’s disease brain. Anat. Cell Biol. 2010, 43, 332–339. [Google Scholar] [CrossRef][Green Version]
- Kilbride, S.M.; Prehn, J.H.M. Central roles of apoptotic proteins in mitochondrial function. Oncogene 2013, 32, 2703. [Google Scholar] [CrossRef]
- Anilkumar, U.; Prehn, J.H.M. Anti-apoptotic BCL-2 family proteins in acute neural injury. Front. Cell Neurosci. 2014, 8, 281. [Google Scholar] [CrossRef]
- Surgucheva, I.; Shestopalov, V.I.; Surguchov, A. Effect of γ-synuclein silencing on apoptotic pathways in retinal ganglion cells. J. Biol. Chem. 2008, 283, 36377–36385. [Google Scholar] [CrossRef]
- D’Amelio, M.; Sheng, M.; Cecconi, F. Caspase-3 in the central nervous system: Beyond apoptosis. Trends Neurosci. 2012, 35, 700–709. [Google Scholar] [CrossRef]
- Sharma, D.R.; Wani, W.Y.; Sunkaria, A.; Kandimalla, R.J.; Sharma, R.K.; Verma, D.; Bal, A.; Gill, K.D. Quercetin attenuates neuronal death against aluminum-induced neurodegeneration in the rat hippocampus. Neuroscience 2016, 324, 163–176. [Google Scholar] [CrossRef]
- Ravi, S.K.; Ramesh, B.N.; Mundugaru, R.; Vincent, B. Multiple pharmacological activities of Caesalpinia crista against aluminium-induced neurodegeneration in rats: Relevance for Alzheimer’s disease. Environ. Toxicol. Pharmacol. 2018, 58, 202–211. [Google Scholar] [CrossRef]
- Donahue, J.E.; Flaherty, S.L.; Johanson, C.E.; Duncan, J.A.; Silverberg, G.D.; Miller, M.C.; Tavares, R.; Yang, W.; Wu, Q.; Sabo, E.; et al. RAGE, LRP-1, and amyloid-beta protein in Alzheimer’s disease. Acta Neuropathol. 2006, 112, 405–415. [Google Scholar] [CrossRef]
- Smith, J.V.; Luo, Y. Elevation of oxidative free radicals in Alzheimer’s disease models can be attenuated by Ginkgo biloba extract EGb 761. J. Alzheimer’s Dis. 2003, 5, 287–300. [Google Scholar] [CrossRef]
- Wilcock, G.; Howe, I.; Coles, H.; Lilienfeld, S.; Truyen, L.; Zhu, Y.; Bullock, R.; Kershaw, P. A long-term comparison of galantamine and donepezil in the treatment of Alzheimer’s disease. Drugs Aging 2003, 20, 777–789. [Google Scholar] [CrossRef]
- Mathiyazahan, D.B.; Justin Thenmozhi, A.; Manivasagam, T. Protective effect of black tea extract against aluminium chloride-induced Alzheimer’s disease in rats: A behavioural, biochemical and molecular approach. J. Funct. Foods 2015, 16, 423–435. [Google Scholar] [CrossRef]
- Chen, C.L.; Tsai, W.H.; Chen, C.J.; Pan, T.M. Centella asiatica extract protects against amyloidβ1–40-induced neurotoxicity in neuronal cells by activating the antioxidative defence system. J. Tradit. Complement. Med. 2016, 6, 362–369. [Google Scholar] [CrossRef]
- Deloncle, R.; Huguet, F.; Babin, P.; Fernandez, B.; Quellard, N.; Guillard, O. Chronic administration of aluminium L-glutamate in young mature rats: Effects on iron levels and lipid peroxidation in selected brain areas. Toxicol. Lett. 1999, 104, 65–73. [Google Scholar] [CrossRef]
- Binti Mohd Yusuf Yeo, N.A.; Muthuraju, S.; Wong, J.H.; Mohammed, F.R.; Senik, M.H.; Zhang, J.; Yusof, S.R.; Jaafar, H.; Adenan, M.L.; Mohamad, H.; et al. Hippocampal amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid GluA1 (AMPA GluA1) receptor subunit involves in learning and memory improvement following treatment with Centella asiatica extract in adolescent rats. Brain Behav. 2018, 8, 1–14. [Google Scholar] [CrossRef]
- van Rijn, C.M.; Krijnen, H.; Menting-Hermeling, S.; Coenen, A.M.L. Decapitation in Rats: Latency to Unconsciousness and the ‘Wave of Death’. PLoS ONE 2011, 6, e16514. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Adeli, S.; Zahmatkesh, M.; Tavoosidana, G.; Karimian, M.; Hassanzadeh, G. Simvastatin enhances the hippocampal klotho in a rat model of streptozotocin-induced cognitive decline. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 72, 87–94. [Google Scholar] [CrossRef]
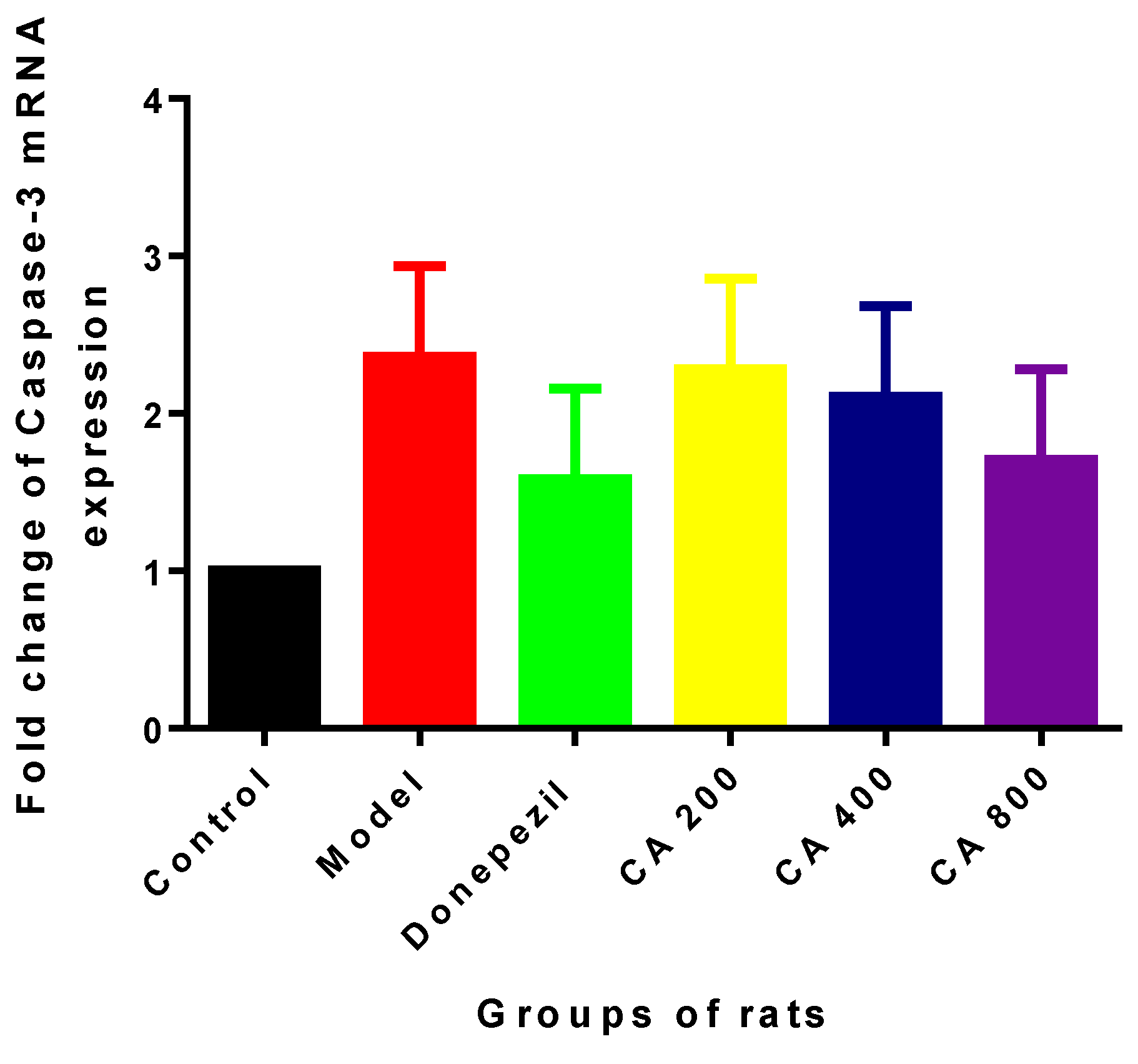
| Groups | Description | Treatment i.p | Treatment p.o |
|---|---|---|---|
| I | Control | Saline | Distilled water |
| II | Model | d-gal 60 mg/kg∙bwt | AlCl3 200 mg/kg∙bwt |
| III | Donepezil | d-gal 60 mg/kg∙bwt | AlCl3 200 mg/kg∙bw + Done 1 mg/kg∙bwt |
| IV | CA 200 | d-gal 60 mg/kg∙bwt | AlCl3 200 mg/kg∙bw + CA 200 mg/kg∙bwt |
| V | CA 400 | d-gal 60 mg/kg∙bwt | AlCl3 200 mg/kg∙bw + CA 400 mg/kg∙bwt |
| VI | CA 800 | d-gal 60 mg/kg∙bwt | AlCl3 200 mg/kg∙bw + CA 800 mg/kg∙bwt |
| Accession No. | Gene Symbol | Primer | Sequence | Length | Tm | Amplicon Size |
|---|---|---|---|---|---|---|
| L14680.1 | Bcl-2 | Forward | 5′-GGTGGACAACATCGCTCT-3′ | 18 | 57.01 | 143 bp |
| Reverse | 5′-GAGACAGCCAGGAGAAATCA-3′ | 20 | 57.94 | |||
| NM_012922.2 | Caspase-3 | Forward | 5′-GAGCGTAAGGAAAGGAGAGG-3′ | 20 | 58.15 | 140 bp |
| Reverse | 5′-GACATCATCCACACAGACCAG-3′ | 21 | 58.96 | |||
| AY618569.1 | B-Actin | Forward | 5′-TGGCTCTGTGGCTTCTACTG-3′ | 20 | 58.16 | 192 bp |
| Reverse | 5′-TACCTTCCCAACTCCTCACC-3′ | 20 | 58.97 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiroma, S.M.; Baharuldin, M.T.H.; Mat Taib, C.N.; Amom, Z.; Jagadeesan, S.; Ilham Adenan, M.; Mahdi, O.; Moklas, M.A.M. Centella asiatica Protects d-Galactose/AlCl3 Mediated Alzheimer’s Disease-Like Rats via PP2A/GSK-3β Signaling Pathway in Their Hippocampus. Int. J. Mol. Sci. 2019, 20, 1871. https://doi.org/10.3390/ijms20081871
Chiroma SM, Baharuldin MTH, Mat Taib CN, Amom Z, Jagadeesan S, Ilham Adenan M, Mahdi O, Moklas MAM. Centella asiatica Protects d-Galactose/AlCl3 Mediated Alzheimer’s Disease-Like Rats via PP2A/GSK-3β Signaling Pathway in Their Hippocampus. International Journal of Molecular Sciences. 2019; 20(8):1871. https://doi.org/10.3390/ijms20081871
Chicago/Turabian StyleChiroma, Samaila Musa, Mohamad Taufik Hidayat Baharuldin, Che Norma Mat Taib, Zulkhairi Amom, Saravanan Jagadeesan, Mohd Ilham Adenan, Onesimus Mahdi, and Mohamad Aris Mohd Moklas. 2019. "Centella asiatica Protects d-Galactose/AlCl3 Mediated Alzheimer’s Disease-Like Rats via PP2A/GSK-3β Signaling Pathway in Their Hippocampus" International Journal of Molecular Sciences 20, no. 8: 1871. https://doi.org/10.3390/ijms20081871
APA StyleChiroma, S. M., Baharuldin, M. T. H., Mat Taib, C. N., Amom, Z., Jagadeesan, S., Ilham Adenan, M., Mahdi, O., & Moklas, M. A. M. (2019). Centella asiatica Protects d-Galactose/AlCl3 Mediated Alzheimer’s Disease-Like Rats via PP2A/GSK-3β Signaling Pathway in Their Hippocampus. International Journal of Molecular Sciences, 20(8), 1871. https://doi.org/10.3390/ijms20081871





