Cytotoxic Acetogenins from the Roots of Annona purpurea
Abstract
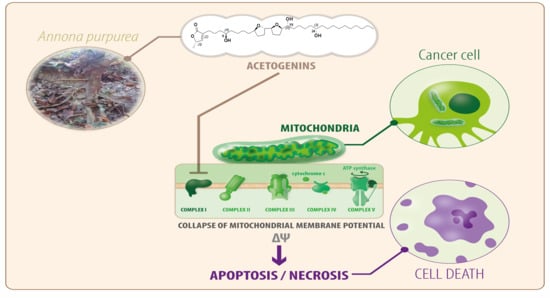
1. Introduction
2. Results and Discussion
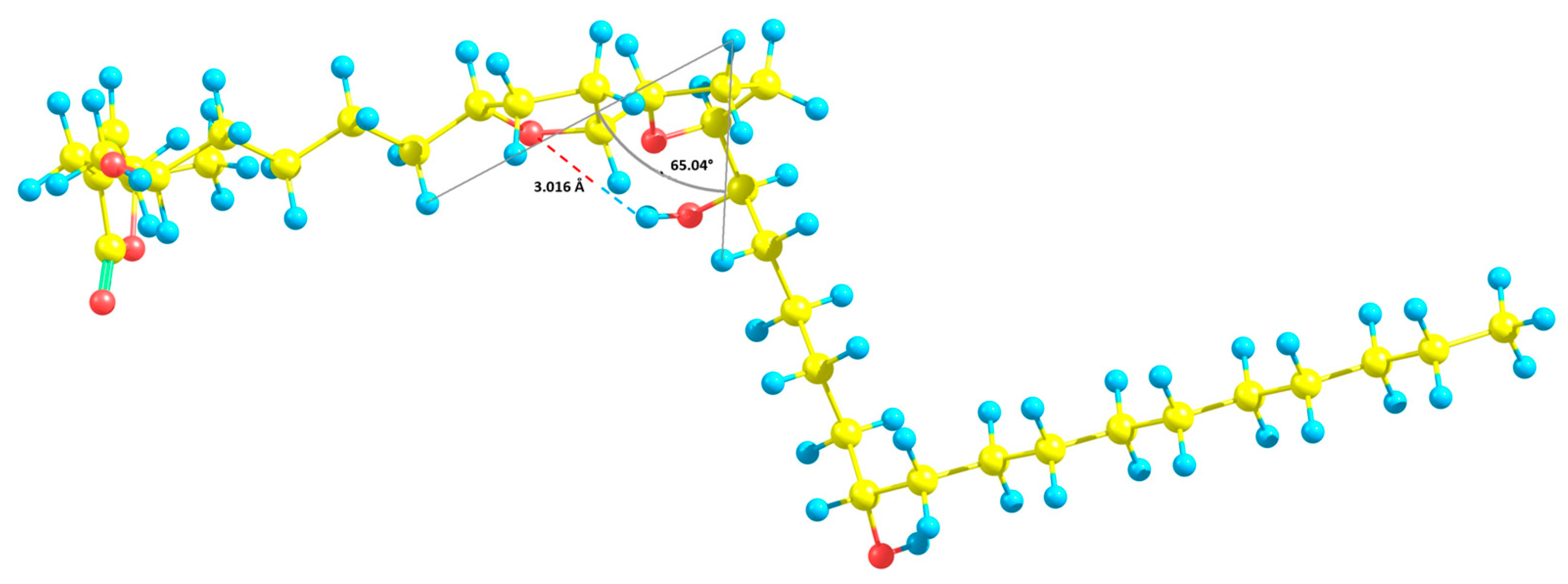
2.1. Isolation and Structure Elucidation
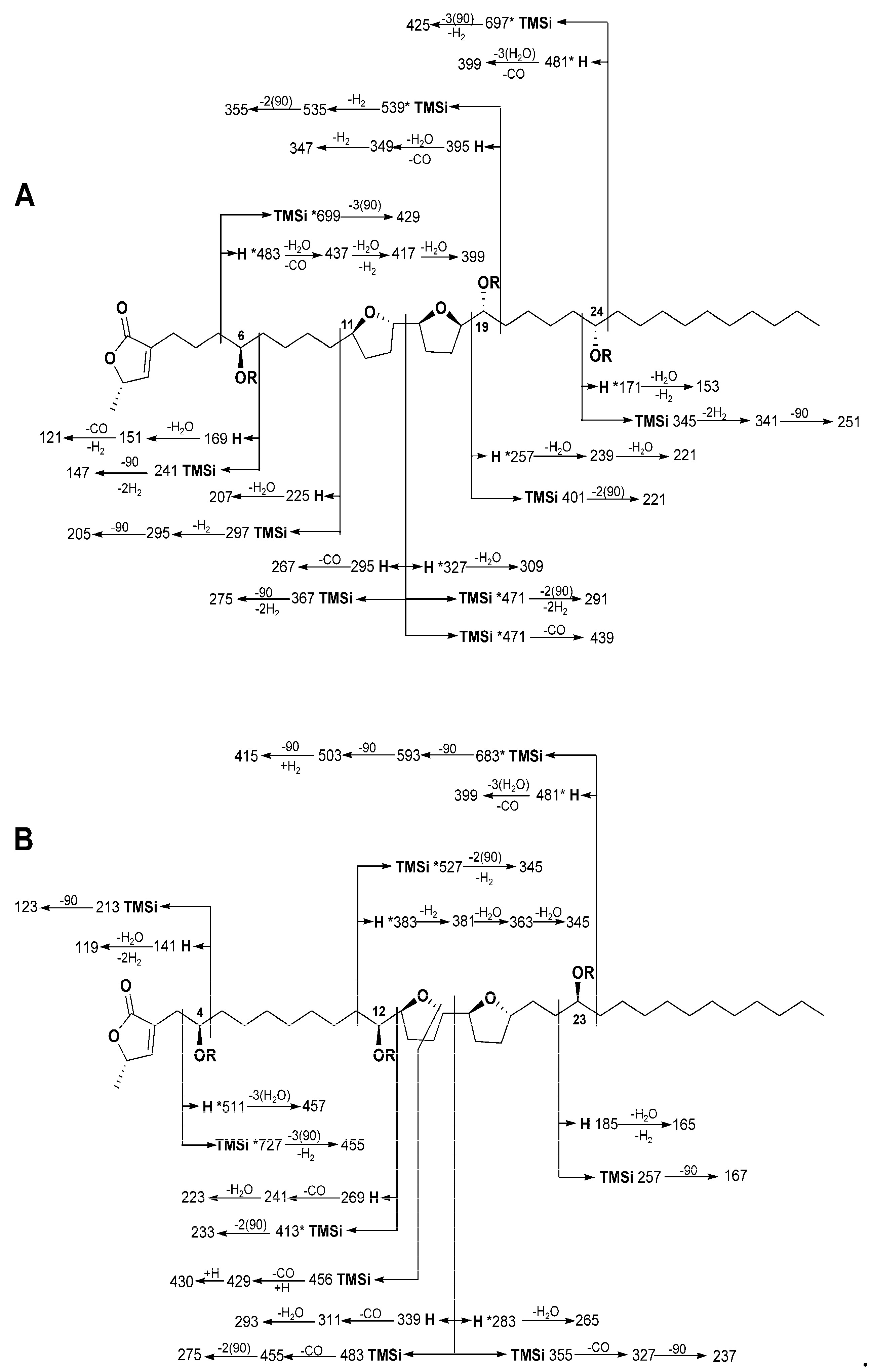
2.1.1. Annopurpuricin A (1)
2.1.2. Annopurpuricin B (2)
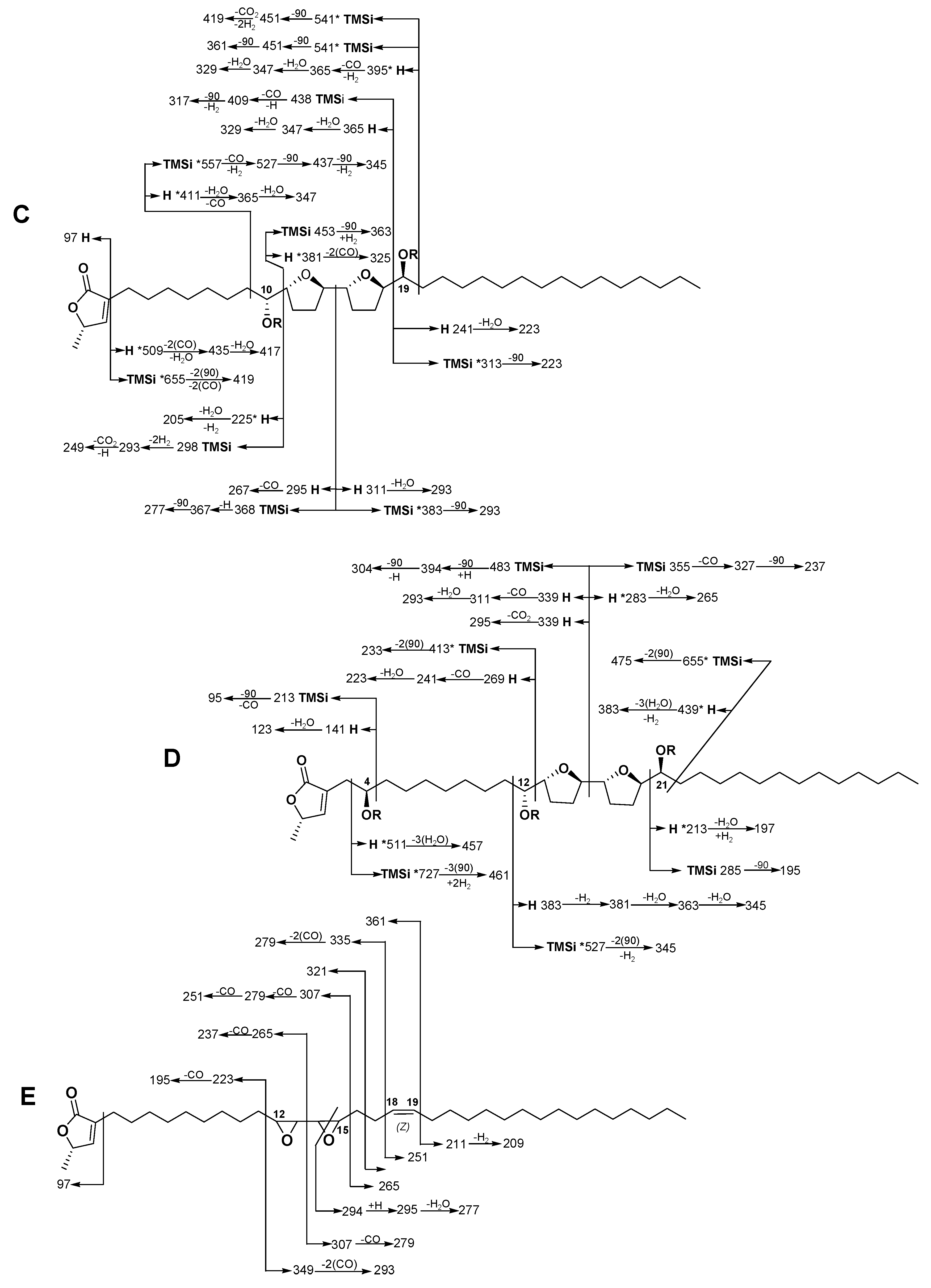
2.1.3. Annopurpuricin C (3)
2.1.4. Annopurpuricin D (4)
2.1.5. Annopurpuricin E (5)
2.2. Antiproliferative Activity
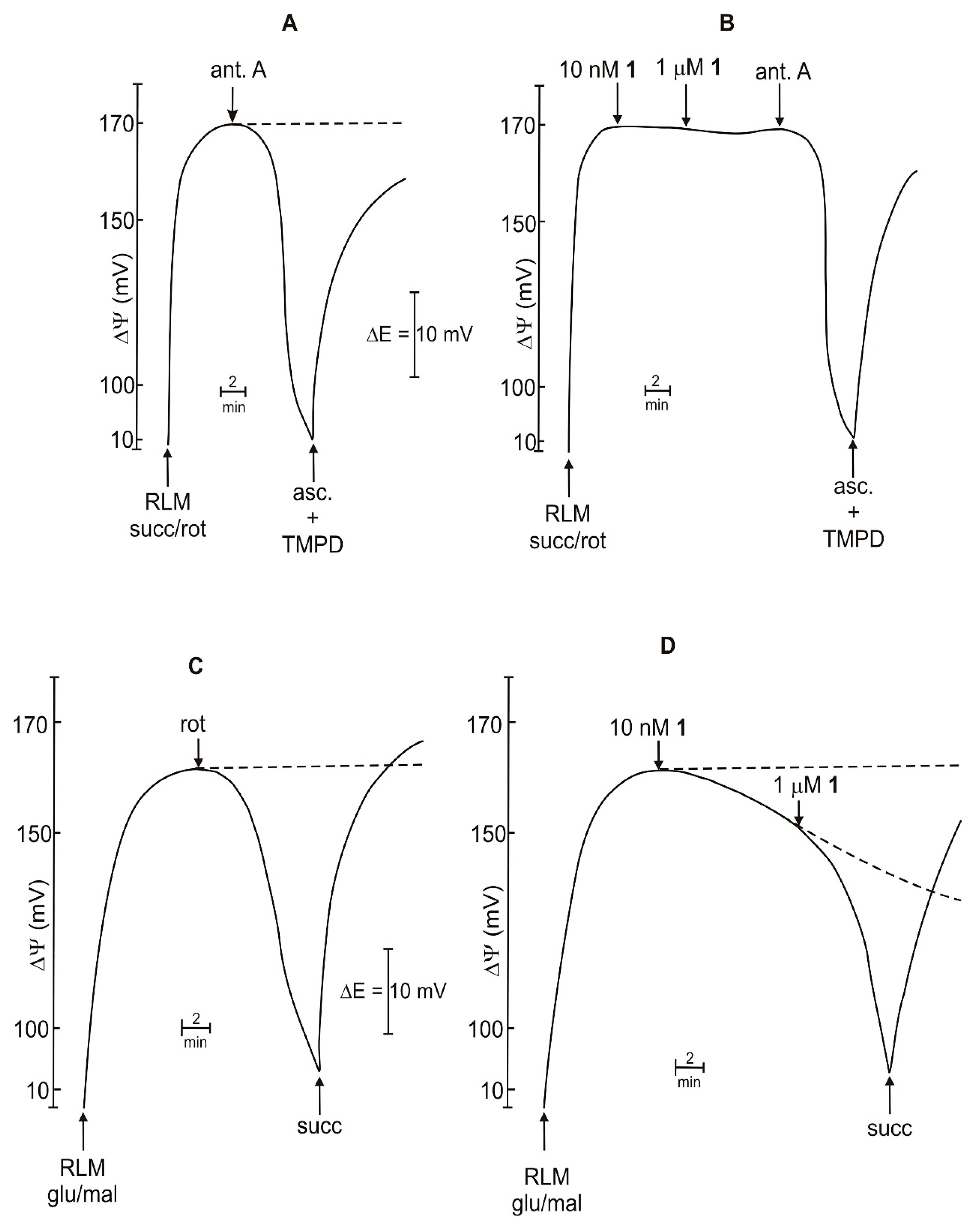
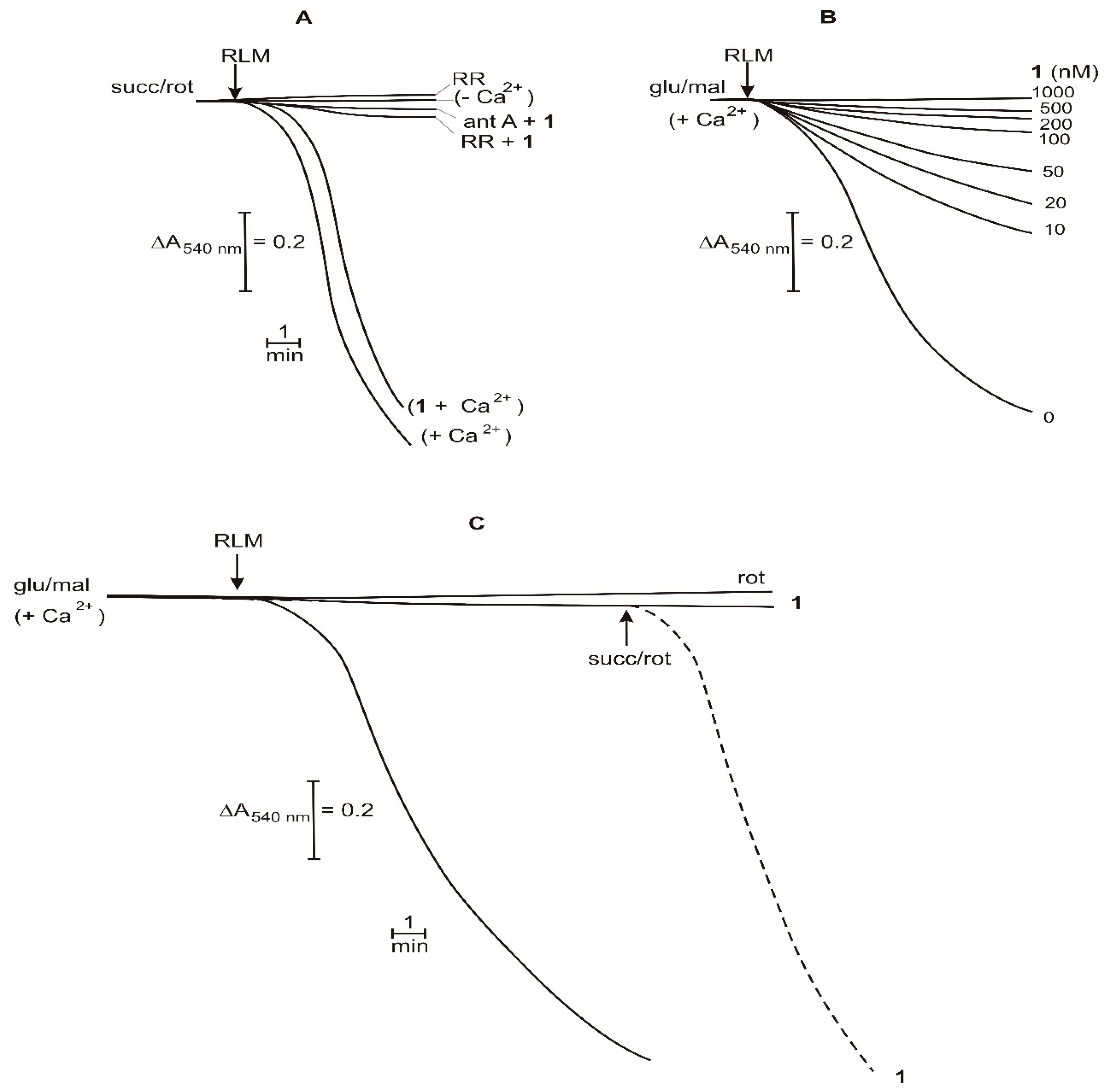
2.3. Effect on Mitochondrial Membrane Potential
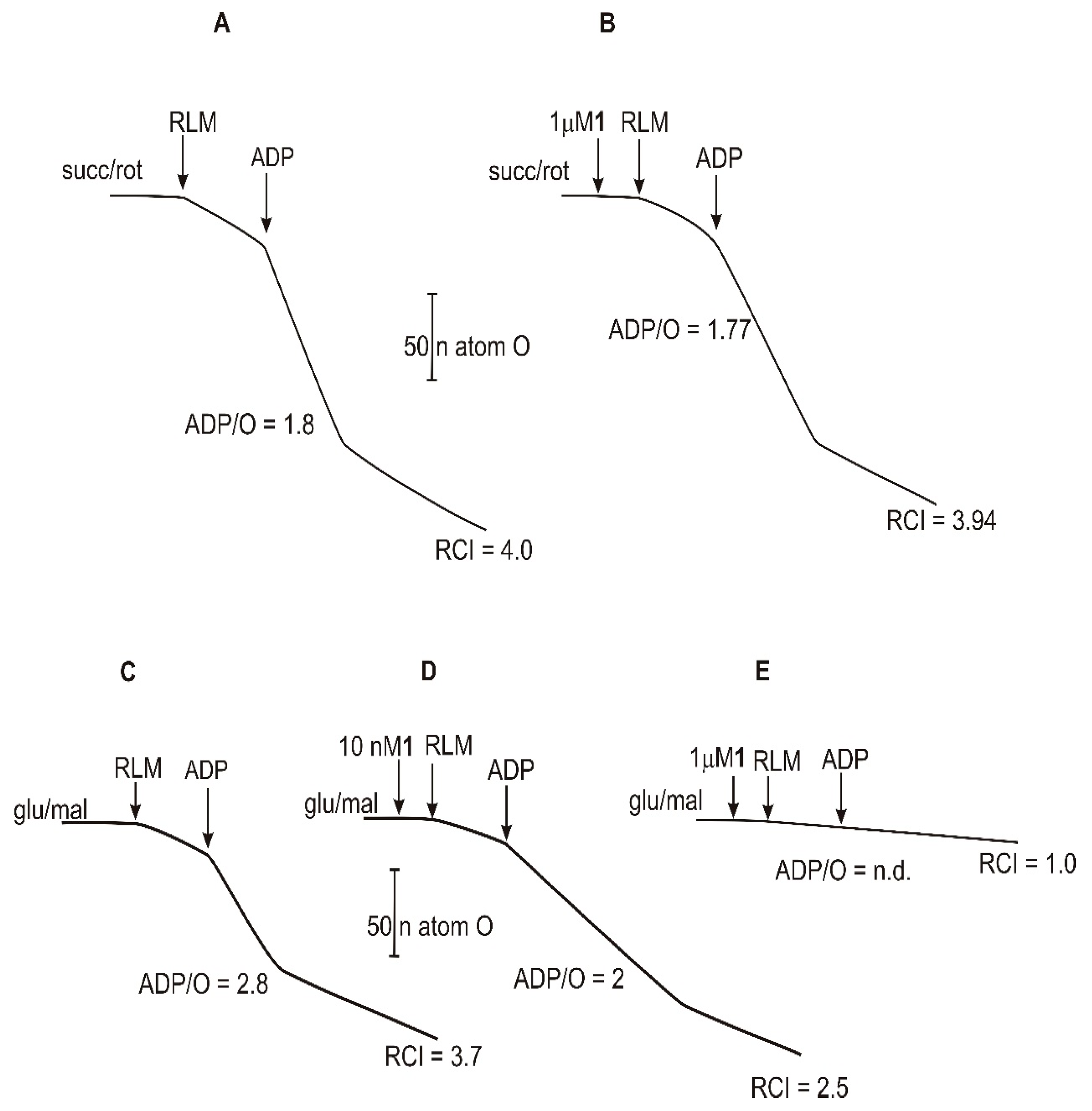
2.4. Mitochondrial Respiration Parameters
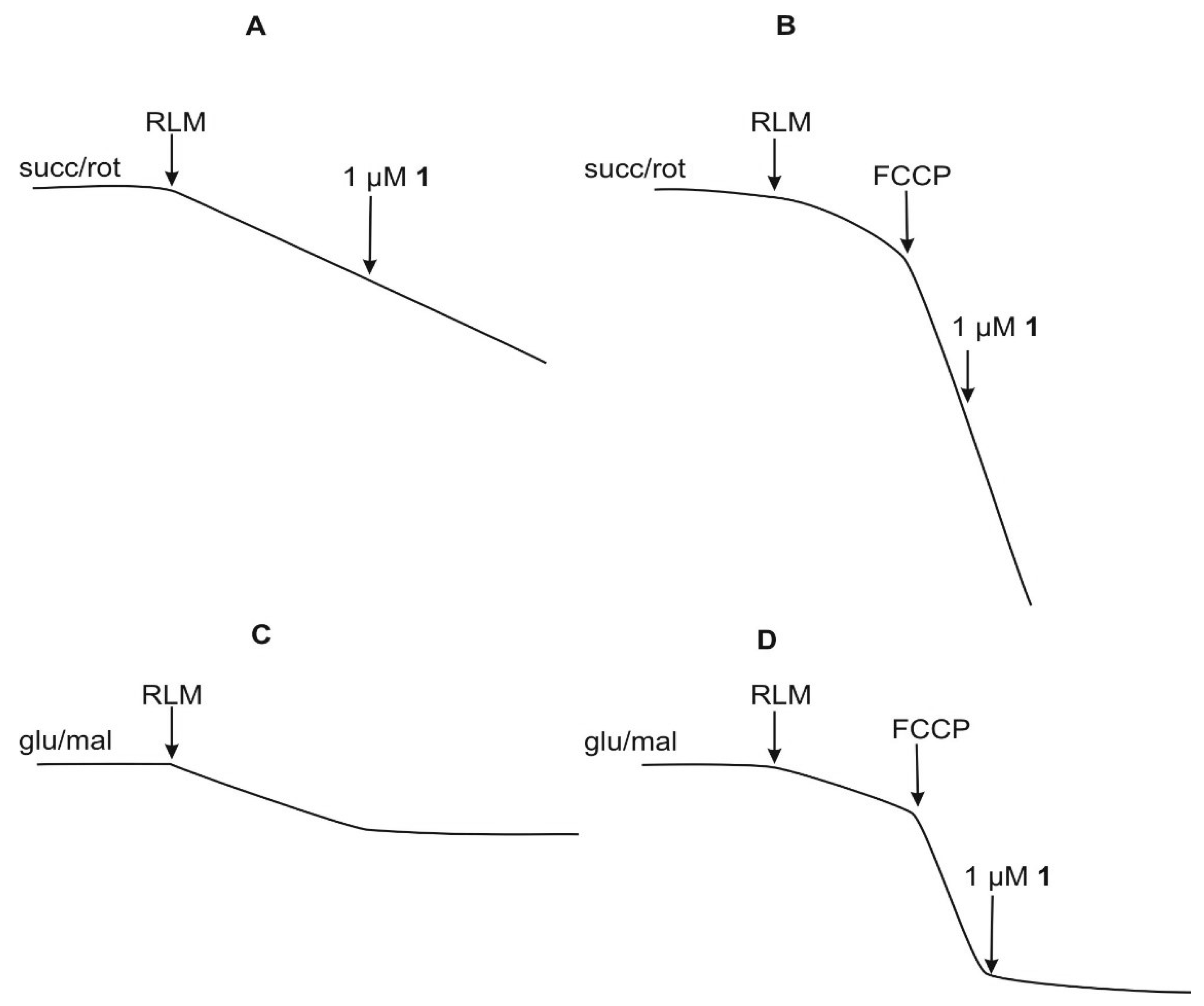
2.5. Mitochondrial Permeability Transition
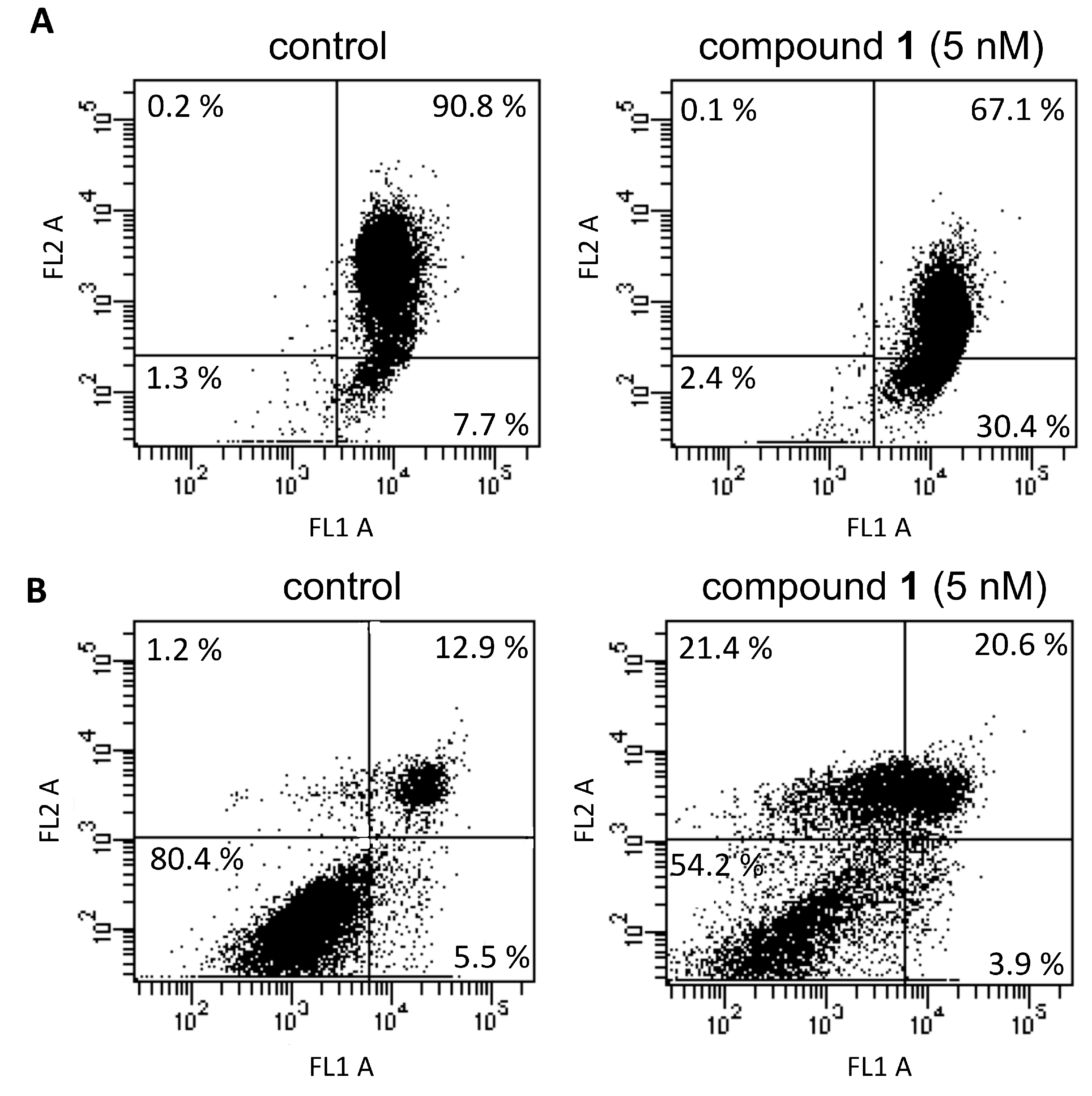
2.6. Cytofluorimetric Analysis
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.3.1. Annopurpuricin A (1)
3.3.2. Annopurpuricin B (2)
3.3.3. Annopurpuricin C (3)
3.3.4. Annopurpuricin D (4)
3.3.5. Annopurpuricin E (5)
3.4. Preparation of Derivatives
3.4.1. Acetyl derivatives (1a–4a)
3.4.2. Trimethylsilyl Derivative
3.4.3. (R) - and (S)-MTPA Ester Derivatives
3.5. Computational Details
3.6. Inhibition Growth Assay
3.7. Mitochondrial Isolation and Standard Incubation Procedures
3.8. Determination of Mitochondrial Functions
3.9. Determination of Mitochondrial Membrane Potential on Whole Cells
3.10. Evaluation of Apoptotic Cell Death
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADP | Adenosine diphosphate |
| ant A | Antimycin A |
| asc | Ascorbate |
| Bis-THF | Bis tetrahydrofuran |
| BSTFA | N,O-Bis(trimethylsilyl)trifluoroacetamide |
| B3LYP | Becke, three-parameter, Lee-Yang-Parr |
| OTMSi | O-trimethylsilyl ether |
| CD | Circular dichroism |
| C6D6 | Benzene-d6 |
| COSY | Correlated spectroscopy |
| DCM | Dichloromethane |
| ESI-TOF | Electrospray ionization time-of-flight mass spectrometry |
| FADH2 | flavin adenine dinucleotide (reduced form) |
| FCCP | Carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone |
| FITC | Fluorescein isotiocyanate |
| FTIR | Fourier transform infrared spectroscopy |
| glu | Glutamate |
| HEPES | 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid |
| HMBC | Heteronuclear multiple bond correlation |
| HRMS | High-resolution mass spectra |
| HSQC | Heteronuclear single-quantum correlation |
| Hz | Hertz |
| JC-1 | 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolocarbo-cyanine iodide |
| mal | Malate |
| mp | Melting point |
| MPT | Mitochondrial permeability transition |
| NADH | Nicotinamide adenine dinucleotide reduced form |
| 1H NMR | Proton nuclear magnetic resonance |
| 13C NMR | Carbon-13 nuclear magnetic resonance |
| S-MTPA | (S)-(−)-α-methoxy-α-(trifluoromethyl)phenylacetic acid |
| ppm | Parts per million |
| RCI | Respiration control index |
| rot | Rotenone |
| R-MTPA | (R)-(−)-α-methoxy-α-(trifluoromethyl)phenylacetic acid |
| RLM | Rat liver mitochondria |
| RR | Ruthenium red |
| succ | Succinate |
| THF | Tetrahydrofuran |
| TMPD | N,N,N′,N′-tetramethyl-p-phenylenediamine |
| TPP+ | tetraphenylphosphonium cation |
| TMSi | Trimethylsilyl |
| UV | Ultraviolet–visible spectrophotometry |
| ΔΨ | Membrane potential |
References
- Bories, C.; Loiseau, P.; Cortes, D.; Myint, S.; Hocquemiller, R.; Gayral, P.; Cavé, A.; Laurens, A. Antiparasitic activity of Annona muricata and Annona cherimolia seeds. Planta Med. 1991, 57, 434–436. [Google Scholar] [CrossRef] [PubMed]
- Bourne, R.K.; Egbe, P.C. A preliminary study of the sedative effects of Annona muricata (sour sop). West Indian Med. J. 1979, 28, 106–110. [Google Scholar] [PubMed]
- Cronquist, A. An Integrated System of Classification of Flowering Plants; Columbia University Press: New York, NY, USA, 1981; ISBN 0-231-03880-1. [Google Scholar]
- Hasrat, J.A.; Pieters, L.; De Backer, J.-P.; Vauquelin, G.; Vlietinck, A.J. Screening of medicinal plants from Suriname for 5-HT1A ligands: Bioactive isoquinoline alkaloids from the fruit of Annona muricata. Phytomedicine 1997, 4, 133–140. [Google Scholar] [CrossRef]
- Martínez, M. Las Plantas Medicinales de México, 6th ed.; Ediciones Botas: México, D.F., Mexico, 1989; ISBN 968-6334-07-6. [Google Scholar]
- Emanuel, R.V. Árboles de Minatitlán, Colima: Guía de usos tradicionales, 1st ed.; PACMYC: Colima, México, 2016; Volume 1. [Google Scholar]
- Zafra-Polo, M.C.; González, M.C.; Estornell, E.; Sahpaz, S.; Cortes, D. Acetogenins from annonaceae, inhibitors of mitochondrial complex I. Phytochemistry 1996, 42, 253–271. [Google Scholar] [CrossRef]
- Zafra-Polo, M.C.; Figadère, B.; Gallardo, T.; Tormo, J.; Cortes, D. Natural acetogenins from annonaceae, synthesis and mechanisms of action. Phytochemistry 1998, 48, 1087–1117. [Google Scholar] [CrossRef]
- Melot, A.; Fall, D.; Gleye, C.; Champy, P. Apolar annonaceous acetogenins from the fruit pulp of Annona muricata. Molecules 2009, 14, 4387–4395. [Google Scholar] [CrossRef] [PubMed]
- Mangal, M.; Khan, M.I.; Agarwal, S.M. Acetogenins as potential anticancer agents. Anticancer Agents Med. Chem. 2015, 16, 138–159. [Google Scholar] [CrossRef]
- Oberlies, N.H.; Chang, C.; McLaughlin, J.L. Structure−Activity relationships of diverse annonaceous acetogenins against multidrug resistant human mammary adenocarcinoma (MCF-7/Adr) Cells. J. Med. Chem. 1997, 40, 2102–2106. [Google Scholar] [CrossRef]
- Wu, T.-Y.; Yang, I.-H.; Tsai, Y.-T.; Wang, J.-Y.; Shiurba, R.; Hsieh, T.-J.; Chang, F.-R.; Chang, W.-C. Isodesacetyluvaricin, an annonaceous acetogenin, specifically inhibits gene expression of cyclooxygenase-2. J. Nat. Prod. 2012, 75, 572–576. [Google Scholar] [CrossRef]
- Kojima, N.; Fushimi, T.; Tatsukawa, T.; Tanaka, T.; Okamura, M.; Akatsuka, A.; Yamori, T.; Dan, S.; Iwasaki, H.; Yamashita, M. Thiophene-3-carboxamide analogue of annonaceous acetogenins as antitumor drug lead. Eur. J. Med. Chem. 2014, 86, 684–689. [Google Scholar] [CrossRef]
- Miyoshi, H.; Ohshima, M.; Shimada, H.; Akagi, T.; Iwamura, H.; McLaughlin, J.L. Essential structural factors of annonaceous acetogenins as potent inhibitors of mitochondrial complex I. Biochim. Biophys. Acta BBA—Bioenerg. 1998, 1365, 443–452. [Google Scholar] [CrossRef]
- Nakanishi, S.; Abe, M.; Yamamoto, S.; Murai, M.; Miyoshi, H. Bis-THF motif of acetogenin binds to the third matrix-side loop of ND1 subunit in mitochondrial NADH-ubiquinone oxidoreductase. Biochim. Biophys. Acta BBA—Bioenerg. 2011, 1807, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- Cepleanu, F.; Ohtani, K.; Hamburger, M.; Hostettmann, K.; Gupta, M.P.; Solis, P. Novel acetogenins from the leaves of Annona purpurea. Helv. Chim. Acta 1993, 76, 1379–1388. [Google Scholar] [CrossRef]
- Chang, F.R.; Chen, C.Y.; Wu, P.H.; Kuo, R.Y.; Chang, Y.C.; Wu, Y.C. New alkaloids from Annona purpurea. J. Nat. Prod. 2000, 63, 746–748. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.-R.; Wei, J.-L.; Teng, C.-M.; Wu, Y.-C. Antiplatelet aggregation constituents from Annona purpurea. J. Nat. Prod. 1998, 61, 1457–1461. [Google Scholar] [CrossRef] [PubMed]
- Chávez, D.; Mata, R. Purpuracenin: A new cytotoxic adjacent bis-tetrahydrofuran annonaceous acetogenin from the seeds of Annona purpurea. Phytochemistry 1999, 50, 823–828. [Google Scholar] [CrossRef]
- Chávez, D.; Mata, R. Purpurediolin and purpurenin, two new cytotoxic adjacent bis-tetrahydrofuran annonaceous acetogenins from the seeds of Annona purpurea. J. Nat. Prod. 1998, 61, 580–584. [Google Scholar] [CrossRef]
- Rejón-Orantes, J.D.C.; González-Esquinca, A.R.; de la Mora, M.P.; Roldan Roldan, G.; Cortes, D. Annomontine, an alkaloid isolated from Annona purpurea, has anxiolytic-like effects in the elevated plus-maze. Planta Med. 2011, 77, 322–327. [Google Scholar] [CrossRef]
- Alali, F.Q.; Zhang, Y.; Rogers, L.; McLaughlin, J.L. Mono-tetrahydrofuran acetogenins from Goniothalamus giganteus. Phytochemistry 1998, 49, 761–768. [Google Scholar] [CrossRef]
- Gawroński, J.; Wu, Y.-C. A note on the determination of absolute configuration of acetogenins by circular dichroism. Pol. J. Chem. 1999, 73, 241–243. [Google Scholar]
- Cortes, D.; Figadere, B.; Cavé, A. Bis-tetrahydrofuran acetogenins from annonaceae. Phytochemistry 1993, 32, 1467–1473. [Google Scholar] [CrossRef]
- Duret, P.; Waechter, A.-I.; Figadère, B.; Hocquemiller, R.; Cavé, A. Determination of absolute configurations of carbinols of annonaceous acetogenins with 2-naphthylmethoxyacetic acid esters. J. Org. Chem. 1998, 63, 4717–4720. [Google Scholar] [CrossRef]
- Rieser, M.J.; Hui, Y.H.; Rupprecht, J.K.; Kozlowski, J.F.; Wood, K.V.; McLaughlin, J.L.; Hanson, P.R.; Zhuang, Z.; Hoye, T.R. Determination of absolute configuration of stereogenic carbinol centers in annonaceous acetogenins by proton and fluorine 19-NMR analysis of Mosher ester derivatives. J. Am. Chem. Soc. 1992, 114, 10203–10213. [Google Scholar] [CrossRef]
- Gallardo, T.; Saez, J.; Granados, H.; Tormo, J.R.; Velez, I.D.; Brun, N.; Torres, B.; Cortes, D. 10-Oximeguanacone, the first nitrogenated acetogenin derivative found to be a potent inhibitor of mitochondrial complex I. J. Nat. Prod. 1998, 61, 1001–1005. [Google Scholar] [CrossRef]
- Gu, Z.; Zhou, D.; Lewis, N.J.; Wu, J.; Shi, G.; McLaughlin, J.L. Isolation of new bioactive annonaceous acetogenins from Rollinia mucosa guided by liquid chromatography/mass spectrometry. Bioorg. Med. Chem. 1997, 5, 1911–1916. [Google Scholar] [CrossRef]
- Liu, X.-X.; Alali, F.Q.; Hopp, D.C.; Rogers, L.L.; Pilarinou, E.; McLaughlin, J.L. Glabracins A and B, two new acetogenins from Annona glabra. Bioorg. Med. Chem. 1998, 6, 959–965. [Google Scholar] [CrossRef]
- Navrátilová, H.; de Gelder, R.; Kříž, Z. Enantiodiscrimination in NMR Spectra and X-Ray Structures of Diastereomeric Salts of Trans-4-(4-Fluorophenyl)-3-Hydroxymethyl-1-Methylpiperidine with (S)-Mosher Acid. J. Chem. Soc. Perkin Trans. 2002, 2, 2093–2099. [Google Scholar] [CrossRef]
- Zhao, G.-X.; Gu, Z.-M.; Zeng, L.; Chao, J.-F.; Kozlowski, J.F.; Wood, K.V.; McLaughlin, J.L. The absolute configuration of trilobacin and trilobin, a novel highly potent acetogenin from the stem bark of Asimina triloba (Annonaceae). Tetrahedron 1995, 51, 7149–7160. [Google Scholar] [CrossRef]
- Raynaud, S.; Fourneau, C.; Hocquemiller, R.; Sévenet, T.; Hadi, H.A.; Cavé, A. Acetogenins from the bark of Uvaria pauci-ovulata. Phytochemistry 1997, 46, 321–326. [Google Scholar] [CrossRef]
- Alali, F.Q.; Liu, X.-X.; McLaughlin, J.L. Annonaceous acetogenins: Recent progress. J. Nat. Prod. 1999, 62, 504–540. [Google Scholar] [CrossRef]
- Chih, H.-W.; Chiu, H.-F.; Tang, K.-S.; Chang, F.-R.; Wu, Y.-C. Bullatacin, a potent antitumor annonaceous acetogenin, inhibits proliferation of human hepatocarcinoma cell line 2.2.15 by apoptosis induction. Life Sci. 2001, 69, 1321–1331. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Chang, F.-R.; Yen, H.-F.; Wu, Y.-C. Epomusenins A and B, two acetogenins from fruits of Rollinia mucosa. Phytochemistry 1996, 42, 1081–1083. [Google Scholar] [CrossRef]
- Gleye, C.; Laurens, A.; Hocquemiller, R.; Laprévote, O.; Serani, L.; Cavé, A. Cohibins A and B, acetogenins from roots of Annona muricata. Phytochemistry 1997, 44, 1541–1545. [Google Scholar] [CrossRef]
- Meneses da Silva, E.L.; Roblot, F.; Mahuteau, J.; Cavé, A. Coriadienin, the First Annonaceous Acetogenin with two double bonds isolated from Annona coriaceae. J. Nat. Prod. 1996, 59, 528–530. [Google Scholar] [CrossRef]
- Tormo, J.R.; Zafra-Polo, M.C.; Serrano, A.; Estornell, E.; Cortes, D. Epoxy-acetogenins and other polyketide epoxy derivatives as inhibitors of the mitochondrial respiratory chain complex I. Planta Med. 2000, 66, 318–323. [Google Scholar] [CrossRef]
- Vázquez-Vuelvas, O.F.; Hernández-Madrigal, J.V.; Gaviño, R.; Tlenkopatchev, M.A.; Morales-Morales, D.; Germán-Acacio, J.M.; Gomez-Sandoval, Z.; Garcias-Morales, C.; Ariza-Castolo, A.; Pineda-Contreras, A. X-ray, DFT, FTIR and NMR structural study of 2, 3-dihydro-2-(R-phenylacylidene)-1, 3, 3-trimethyl-1H-indole. J. Mol. Struc. 2011, 987, 106–118. [Google Scholar] [CrossRef]
- Mazza, A.; Beccalli, E.M.; Contini, A.; Garcia-Argaez, A.N.; Dalla Via, L.; Gelmi, M.L. A new scaffold of topoisomerase I inhibitors: Design, synthesis and biological evaluation. Eur. J. Med. Chem. 2016, 124, 326–339. [Google Scholar] [CrossRef]
- Afonso, S.; Silva, F.B.; Silva, A.F.; Scarminio, I.S.; Bruns, R.E. Infrared spectral evidence and DFT calculations of hydrogen-bonding and molecular structures of acetogenins. J. Mol. Struct. 2017, 1130, 174–180. [Google Scholar] [CrossRef]
- Zoratti, M.; Szabò, I. The mitochondrial permeability transition. Biochim. Biophys. Acta 1995, 1241, 139–176. [Google Scholar] [CrossRef]
- Susin, S.A.; Lorenzo, H.K.; Zamzami, N.; Marzo, I.; Snow, B.E.; Brothers, G.M.; Mangion, J.; Jacotot, E.; Costantini, P.; Loeffler, M.; et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 1999, 397, 441–446. [Google Scholar] [CrossRef]
- Schummer, C.; Delhomme, O.; Appenzeller, B.M.R.; Wennig, R.; Millet, M. Comparison of MTBSTFA and BSTFA in derivatization reactions of polar compounds prior to GC/MS analysis. Talanta 2009, 77, 1473–1482. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Chai, J.D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef]
- Scott, A.P.; Radom, L. Harmonic vibrational frequencies: An evaluation of Hartree−Fock, Møller−Plesset, quadratic configuration interaction, density functional theory, and semiempirical scale factors. J. Phys. Chem. 1996, 100, 16502–16513. [Google Scholar] [CrossRef]
- NIST, N. Computational chemistry comparison and benchmark database. In NIST Standard Reference Database Number 101; NIST: Gaithersburg, MD, USA, 2010. [Google Scholar]
- Schneider, W.C.; Hogeboom, G.H. Cytochemical studies of mammalian tissues; the isolation of cell components by differential centrifugation: A review. Cancer Res. 1951, 11, 1–22. [Google Scholar]
- Gornall, A.G.; Bardawill, C.J.; David, M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar]
- Kamo, N.; Muratsugu, M.; Hongoh, R.; Kobatake, Y. Membrane potential of mitochondria measured with an electrode sensitive to tetraphenyl phosphonium and relationship between proton electrochemical potential and phosphorylation potential in steady state. J. Membr. Biol. 1979, 49, 105–121. [Google Scholar] [CrossRef]
- Palmieri, F.; Klingenberg, M. Direct methods for measuring metabolite transport and distribution in mitochondria. In Biomembranes Part G: Bioenergetics: Biogenesis of Mitochondria, Organization, and Transport; Methods, E., Fleisher, S., Packer, L., Eds.; Academic Press: Cambridge, MA, USA, 1979; Volume 56, pp. 279–301. [Google Scholar]
- Gunter, T.E.; Jensen, B.D. The efficiencies of the component steps of oxidative phosphorylation. I. A simple steady state theory. Arch. Biochem. Biophys. 1986, 248, 289–304. [Google Scholar] [CrossRef]
- Estabrook, R.W. Mitochondrial respiratory control and the polarographic measurement of ADP: O ratios. In Oxidation and Phosphorylation; Methods, E., Estabrook, R.W., Pullman, M.E., Eds.; Academic Press: Cambridge, MA, USA, 1967; Volume 10, pp. 41–47. [Google Scholar]
- Cossarizza, A.; Baccaranicontri, M.; Kalashnikova, G.; Franceschi, C. A new method for the cytofluorometric analysis of mitochondrial membrane potential using the J-aggregate forming lipophilic cation 5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide (JC-1). Biochem. Biophys. Res. Commun. 1993, 197, 40–45. [Google Scholar] [CrossRef]
- Van Engeland, M.; Nieland, L.J.; Ramaekers, F.C.; Schutte, B.; Reutelingsperger, C.P. Annexin V-affinity assay: A review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry 1998, 31, 1–9. [Google Scholar] [CrossRef]
| 1 | 2 | |||
|---|---|---|---|---|
| Position | δ H (J Hz) | δ C | δ H (J Hz) | δ C |
| 1 | - | 174.0 C | - | 174.7 C |
| 2 | - | 134.4 C | - | 131.3 C |
| 3a | 2.24 br t | 25.1 CH2 | 2.38 m | 33.4 CH2 |
| 3b | 2.24 br t | 25.1 CH2 | 2.51 m | 33.4 CH2 |
| 4 | 1.51 m | 25.3 CH2 | 3.81 m | 70.0 CH |
| 5 | 1.29 m | 29.4 CH2 | 1.62 m | 29.6 CH2 |
| 6 | 3.82 m | 71.4 CH | 1.22 br s | 29.6–29.8 CH2 |
| 7 | 1.33 m | 32.0 CH2 | 1.22 br s | 29.6–29.8 CH2 |
| 8 | 1.23 br s | 29.5 CH2 | 1.22 br s | 29.6–29.8 CH2 |
| 9 | 1.23 br s | 29.5 CH2 | 1.22 br s | 29.6–29.8 CH2 |
| 10 | 1.53 m | 27.3 CH2 | 1.22 br s | 29.6–29.8 CH2 |
| 11 | 3.88 m | 82.5 CH | 1.35 m | 33.4 CH2 |
| 12 | 1.93 m | 29.0 CH2 | 3.37 m | 74.2 CH |
| 13 | 1.77 m | 24.6 CH2 | 3.79 m | 83.2 CH |
| 14 | 3.91 m | 82.7 CH | 1.93 m | 29.1 CH2 |
| 15 | 3.78 m | 82.1 CH | 1.56 m | 28.5 CH2 |
| 16 | 1.56 m | 29.0 CH2 | 3.81 m | 82.2 CH |
| 17 | 1.94 m | 28.5 CH2 | 3.91 m | 82.8 CH |
| 18 | 3.79 m | 83.2 CH | 1.81 m | 24.6 CH2 |
| 19 | 3.38 m | 74.1 CH | 1.97 m | 29.0 CH2 |
| 20 | 1.35 m | 33.2 CH2 | 3.90 m | 82.4 CH |
| 21 | 1.23 br s | 29.5 CH2 | 1.59 m | 29.4 CH2 |
| 22 | 1.23 br s | 29.5 CH2 | 1.45 m | 37.5 CH2 |
| 23 | 1.40 m | 37.3 CH2 | 3.84 m | 71.5 CH |
| 24 | 3.55 m | 71.7 CH | 1.32 m | 32.0 CH2 |
| 25 | 1.39 m | 37.4 CH2 | 1.22 br s | 29.6–29.8 CH2 |
| 26 | 1.23 br s | 25.1–29.2 CH2 | 1.22 br s | 29.6–29.8 CH2 |
| 27 | 1.23 br s | 25.1–29.2 CH2 | 1.22 br s | 29.6–29.8 CH2 |
| 28 | 1.23 br s | 25.1–29.2 CH2 | 1.22 br s | 29.6–29.8 CH2 |
| 29 | 1.23 br s | 25.1–29.2 CH2 | 1.22 br s | 29.6–29.8 CH2 |
| 30 | 1.23 br s | 25.1–29.2 CH2 | 1.22 br s | 29.6–29.8 CH2 |
| 31 | 1.23 br s | 25.1–29.2 CH2 | 1.22 br s | 29.6–29.8 CH2 |
| 32 | 1.26 m | 22.7 CH2 | 1.22 br s | 29.6–29.8 CH2 |
| 33 | 1.27 m | 32.0 CH2 | 1.28 m | 32.5 CH2 |
| 34 | 0.86 t (6.76) | 14.1 CH3 | 0.86 t (6.9) | 14.2 CH3 |
| 35 | 6.97 br q (1.48) | 149.0 CH | 7.18 br d (1.3) | 151.9 CH |
| 36 | 4.98 dq (6.53, 5.12, 6.76) | 77.4 CH | 5.05 qq (5.48, 6.8, 6.84) | 78.0 CH |
| 37 | 1.38 d (6.8) | 19.2 CH3 | 1.42 d (6.8) | 19.2 CH3 |
| 3 | 4 | 5 | ||||
|---|---|---|---|---|---|---|
| Position | δ H (J Hz) | δ C | δ H (J Hz) | δ C | δ H (J Hz) | δ C |
| 1 | - | 174.0 C | - | 174.6 C | - | 174.0 C |
| 2 | - | 134.5 C | - | 131.3 C | - | 134.5 C |
| 3a | 2.25 t | 25.3 CH2 | 2.37 m | 33.4 CH2 | 2.25 m | 25.4 CH2 |
| 3b | 2.25 t | 25.3 CH2 | 2.50 m | 33.4 CH2 | 2.25 m | 25.4 CH2 |
| 4 | 1.51 m | 27.5 CH2 | 3.82 m | 70.0 CH | 1.52 m | 26.7 CH2 |
| 5 | 1.30 m | 26.1 CH2 | 1.45 m | 37.5 CH2 | 1.32 br s | 27.4 CH2 |
| 6 | 1.24 br s | 29.3-29.8 CH2 | 1.24 br s | 29.6-29.8 CH2 | 1.32 br s | 29.7-28.8 CH2 |
| 7 | 1.24 br s | 29.3-29.8 CH2 | 1.24 br s | 29.6-29.8 CH2 | 1.32 br s | 29.7-28.8 CH2 |
| 8 | 1.24 br s | 29.3-29.8 CH2 | 1.24 br s | 29.6-29.8 CH2 | 1.32 br s | 29.7-28.8 CH2 |
| 9 | 1.34 m | 32.6 CH2 | 1.24 br s | 29.6-29.8 CH2 | 1.32 br s | 29.7-28.8 CH2 |
| 10 | 3.88 m | 71.4 CH | 1.45 m | 25.76 CH2 | 1.32 br s | 29.7-28.8 CH2 |
| 11 | 3.82 m | 82.2 CH | 1.37 m | 33.3 CH2 | 1.51 m | 27.9 CH2 |
| 12 | 1.94 m | 29.1 CH2 | 3.38 m | 74.2 CH | 2.97 m | 57.0 CH |
| 13 | 1.78, 1.87 m | 24.6 CH2 | 3.81 m | 83.3 CH | 2.95 m | 56.9 CH |
| 14 | 3.92 m | 82.8 CH | 1.95 m | 29.0 CH2 | 2.95 m | 56.8 CH |
| 15 | 3.90 m | 82.5 CH | 1.59 m | 28.5 CH2 | 2.95 m | 57.4 CH |
| 16 | 1.58, 1.95 m | 29.0 CH2 | 3.80 m | 82.3 CH | 1.59 m | 28.1 CH2 |
| 17 | 1.61 m | 28.5 CH2 | 3.91 m | 82.6 CH | 2.21 m | 24.4 CH2 |
| 18 | 3.80 m | 83.3 CH | 1.56, 1.96 m | 29.0 CH2 | 5.37 m | 128.2 CH |
| 19 | 3.39 m | 74.2 CH | 1.88 m | 24.6 CH2 | 5.40 m | 131.3 CH |
| 20 | 1.37 m | 33.5 CH2 | 3.93 m | 82.9 CH | 2.02 m | 27.5 CH2 |
| 21 | 1.24 br s | 29.3–29.8 CH2 | 3.84 m | 71.5 CH | 1.32 m | 29.4 CH2 |
| 22 | 1.24 br s | 29.3–29.8 CH2 | 1.33 m | 32.5 CH2 | 1.27 br s | 29.7–28.8 CH2 |
| 23 | 1.24 br s | 29.3–29.8 CH2 | 1.24 br s | 29.6-29.8 CH2 | 1.27 br s | 29.7–28.8 CH2 |
| 24 | 1.24 br s | 29.3–29.8 CH2 | 1.24 br s | 29.6-29.8 CH2 | 1.27 br s | 29.7–28.8 CH2 |
| 25 | 1.24 br s | 29.3–29.8 CH2 | 1.24 br s | 29.6-29.8 CH2 | 1.27 br s | 29.7–28.8 CH2 |
| 26 | 1.24 br s | 29.3–29.8 CH2 | 1.24 br s | 29.6-29.8 CH2 | 1.27 br s | 29.7–28.8 CH2 |
| 27 | 1.24 br s | 29.3–29.8 CH2 | 1.24 br s | 29.6-29.8 CH2 | 1.27 br s | 29.7–28.8 CH2 |
| 28 | 1.24 br s | 29.3–29.8 CH2 | 1.24 br s | 29.6-29.8 CH2 | 1.27 br s | 29.7–28.8 CH2 |
| 29 | 1.24 br s | 29.3–29.8 CH2 | 1.24 br s | 29.6-29.8 CH2 | 1.27 br s | 29.7–28.8 CH2 |
| 30 | 1.24 br s | 29.3–29.8 CH2 | 1.24 br s | 29.6-29.8 CH2 | 1.27 br s | 29.7–28.8 CH2 |
| 31 | 1.24 br s | 29.3–29.8 CH2 | 1.24 br s | 29.6-29.8 CH2 | 1.27 br s | 29.7–28.8 CH2 |
| 32 | 1.27 m | 22.8 CH2 | 1.24 br s | 32.0 CH2 | 1.24 br s | 32.0 CH2 |
| 33 | 1.26 m | 32.0 CH2 | 1.27 m | 22.8 CH2 | 1.26 m | 22.8 CH2 |
| 34 | 0.86 t (6.9) | 14.2 CH3 | 0.85 t (6.9) | 14.2 CH3 | 0.87 t (6.9) | 14.2 CH3 |
| 35 | 6.98 q (1.48, 1.56, 1.52) | 148.9 CH | 7.18 br d (1.3) | 151.8 CH | 6.98 q (1.48, 1.56, 1.52) | 148.9 CH |
| 36 | 4.99 qq (5.08, 1.72, 5.08) | 77.4 CH | 5.04 qq (5.48, 6.8, 6.84) | 78.0 CH | 4.99 qq (5.08, 1.72, 5.08) | 77.4 CH |
| 37 | 1.39 d (6.8) | 19.3 CH3 | 1.41 d (6.8) | 19.2 CH3 | 1.40 d (6.8) | 19.3 CH3 |
| MTPA Config. | Proton Chemical Shifts | Carbinol Configuration | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H (4) | H (5) | H (7) | H (10) | H (17) | H (18) | H (20) | H (23) | H (25) | 6R | 19R | 24S | |
| 1 S-MTPA | 1.52 | 1.33 | 1.61 | 1.15 | 1.38 | 3.38 | 1.63 | 1.99 | 1.80 | |||
| 1 R-MTPA | 1.56 | 1.35 | 1.57 | 1.13 | 1.39 | 3.39 | 1.59 | 1.97 | 1.85 | |||
| ∆δH | −0.04 | −0.02 | +0.04 | +0.02 | −0.01 | −0.01 | +0.04 | +0.02 | −0.05 | |||
| H (3) b | H (5) b | H (11) | H (13) | H (16) | H (17) | H (21) | H (24) | H (35) | 4R | 12S | 23S | |
| 2 S-MTPA | 2.58 | 1.61 | 1.41 | 3.91 | 3.82 | 3.39 | 1.92 | 1.62 | 6.73 | |||
| 2 R-MTPA | 2.64 | 1.59 | 1.61 | 4.05 | 3.76 | 3.37 | 1.58 | 1.63 | 6.98 | |||
| ∆δH | −0.06 | +0.02 | −0.20 | −0.14 | +0.06 | +0.02 | +0.34 | −0.01 | −0.25 | |||
| H (4) | H (9) | H (11) | H (17) | H (18) | H (20) | H (33) | H (34) | 10R | 19S | |||
| 3 S-MTPA | 1.52 | 1.64 | 3.34 | 1.38 | 3.34 | 1.64 | 1.41 | 0.92 | ||||
| 3 R-MTPA | 1.54 | 1.58 | 3.41 | 1.94 | 3.41 | 1.58 | 1.33 | 0.89 | ||||
| ∆δH | −0.02 | +0.06 | −0.07 | −0.56 | −0.07 | +0.06 | +0.08 | +0.02 | ||||
| H (3) | H (5) | H (11) | H (13) | H (16) | H (17) | H (20) | H (22) | H (35) | 4R | 12R | 21S | |
| 4 S-MTPA | 2.59 | 1.65 | 1.63 | 3.40 | 3.72 | 3.67 | 3.85 | 1.36 | 6.72 | |||
| 4 R-MTPA | 2.64 | 1.61 | 1.48 | 3.99 | 3.82 | 3.64 | 3.93 | 1.59 | 6.97 | |||
| ∆δH | −0.05 | +0.04 | +0.23 | −0.59 | −0.1 | +0.03 | −0.08 | −0.2 | −0.25 | |||
| Compound | Cell Lines GI50 a (nM) | ||
|---|---|---|---|
| MSTO-211H | HeLa | HepG2 | |
| 1 | 25.9 ± 4.5 | 0.06 ± 0.01 | 0.45 ± 0.16 |
| 2 | 6.9 ± 1.9 | 0.09 ± 0.02 | 0.66 ± 0.09 |
| 3 | 7.2 ± 1.6 | 0.41 ± 0.16 | 3.0 ± 1.3 |
| 4 | 7.3 ± 2.1 | 0.35 ± 0.06 | 2.5 ± 0.2 |
| 5 | >50 | >50 | >50 |
| Camptothecin | 2.1 ± 0.1 * | 5.4 ± 0.2 * | 3.00 ± 0.20 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Fuentes, G.A.; García-Argáez, A.N.; Peraza Campos, A.L.; Delgado-Enciso, I.; Muñiz-Valencia, R.; Martínez-Martínez, F.J.; Toninello, A.; Gómez-Sandoval, Z.; Mojica-Sánchez, J.P.; Dalla Via, L.; et al. Cytotoxic Acetogenins from the Roots of Annona purpurea. Int. J. Mol. Sci. 2019, 20, 1870. https://doi.org/10.3390/ijms20081870
Hernández-Fuentes GA, García-Argáez AN, Peraza Campos AL, Delgado-Enciso I, Muñiz-Valencia R, Martínez-Martínez FJ, Toninello A, Gómez-Sandoval Z, Mojica-Sánchez JP, Dalla Via L, et al. Cytotoxic Acetogenins from the Roots of Annona purpurea. International Journal of Molecular Sciences. 2019; 20(8):1870. https://doi.org/10.3390/ijms20081870
Chicago/Turabian StyleHernández-Fuentes, Gustavo Alejandro, Aída Nelly García-Argáez, Ana Lilia Peraza Campos, Iván Delgado-Enciso, Roberto Muñiz-Valencia, Francisco Javier Martínez-Martínez, Antonio Toninello, Zeferino Gómez-Sandoval, Juan Pablo Mojica-Sánchez, Lisa Dalla Via, and et al. 2019. "Cytotoxic Acetogenins from the Roots of Annona purpurea" International Journal of Molecular Sciences 20, no. 8: 1870. https://doi.org/10.3390/ijms20081870
APA StyleHernández-Fuentes, G. A., García-Argáez, A. N., Peraza Campos, A. L., Delgado-Enciso, I., Muñiz-Valencia, R., Martínez-Martínez, F. J., Toninello, A., Gómez-Sandoval, Z., Mojica-Sánchez, J. P., Dalla Via, L., & Parra-Delgado, H. (2019). Cytotoxic Acetogenins from the Roots of Annona purpurea. International Journal of Molecular Sciences, 20(8), 1870. https://doi.org/10.3390/ijms20081870