Resveratrol as a Multifunctional Topical Hypopigmenting Agent
Abstract
1. Introduction
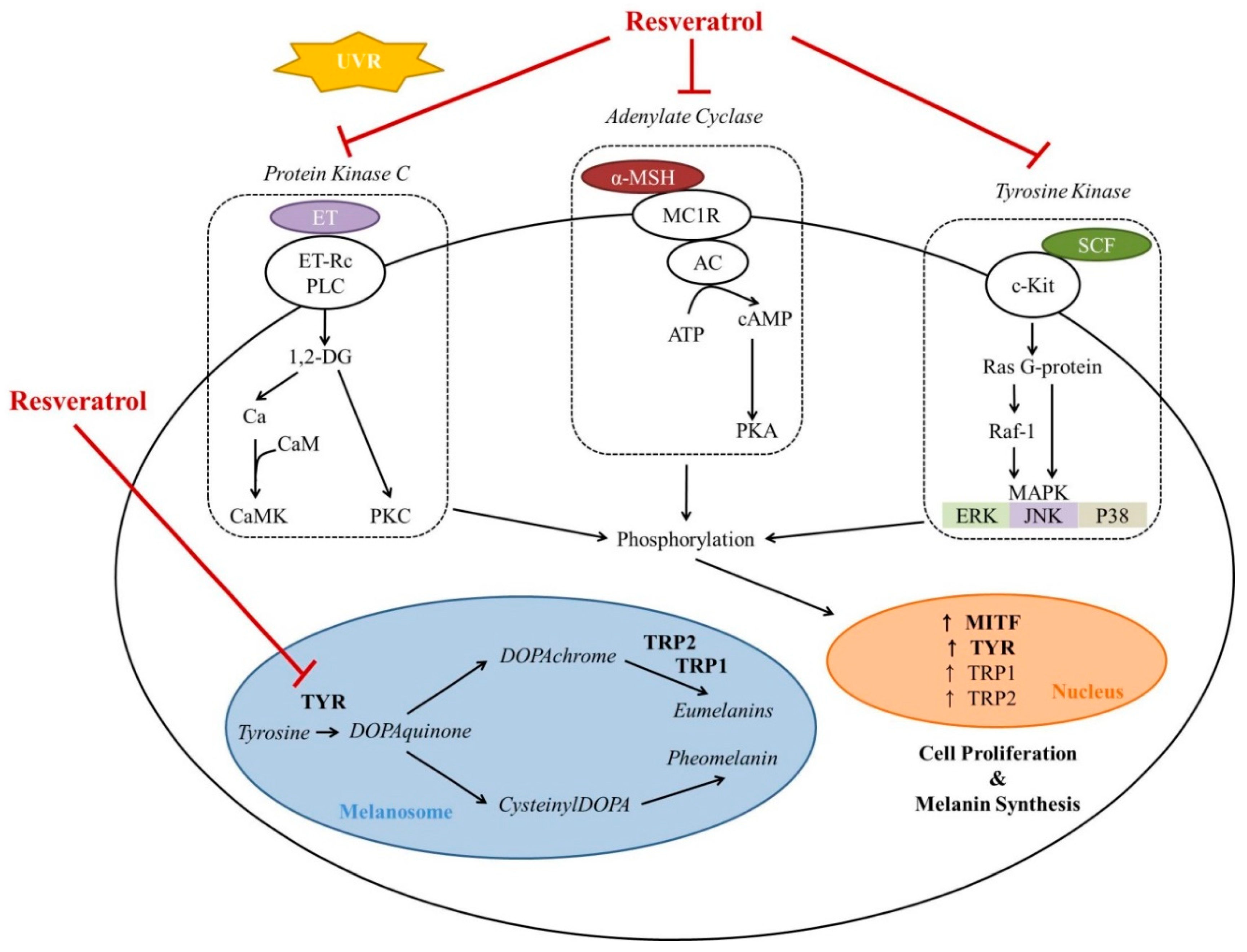
2. Effects on Melanocytes
2.1. Resveratrol as a Direct Tyrosinase Inhibitor
2.2. Resveratrol as an Indirect Tyrosinase Inhibitor
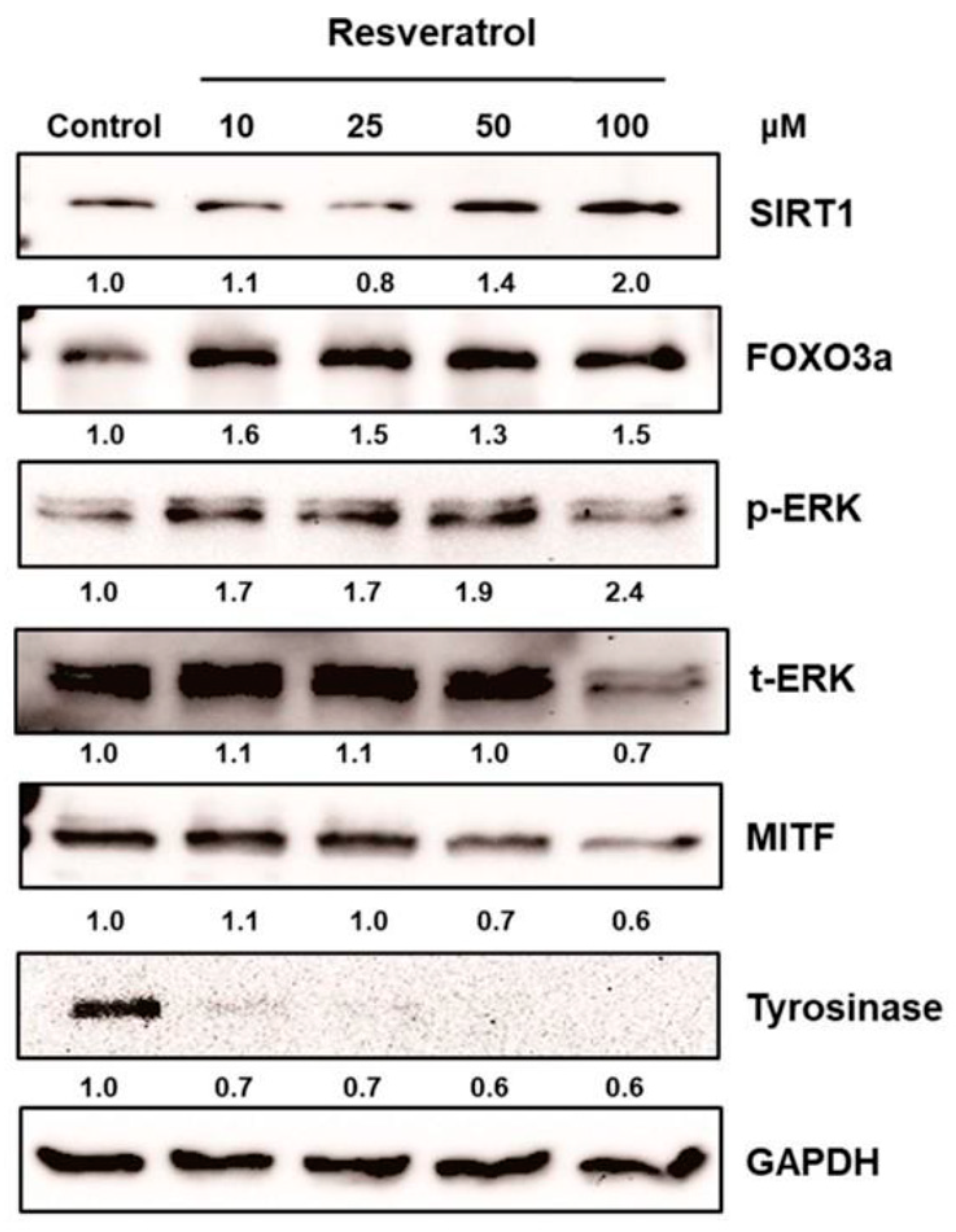
2.2.1. Resveratrol as an Inhibitor of Tyrosinase Transcription
2.2.2. Resveratrol as a Post-Transcriptional Regulator of Tyrosinase
2.3. Summary
3. Effects on Keratinocytes
3.1. Resveratrol as an Anti-Inflammatory Regulator
3.2. Resveratrol as a UV Protectant
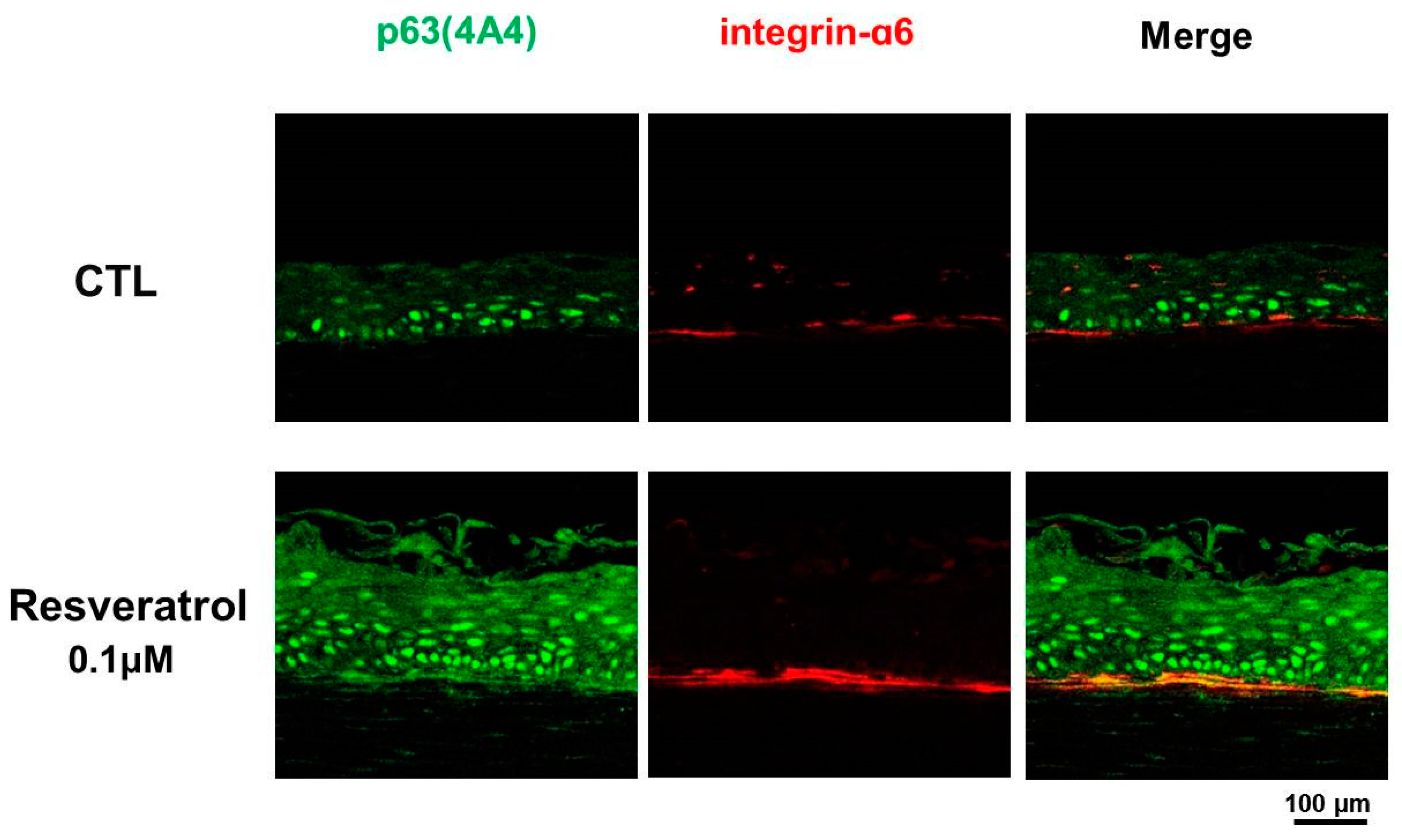
3.3. Resveratrol as a Niche Modulator
3.4. Summary
4. The Antimelanoma Activity of Resveratrol
4.1. The Role of Melanin in Melanoma Biology
4.2. Resveratrol as a Potential Antimelanoma Agent
5. Potential to be Used as a Promising Topical Hypopigmenting Agent
5.1. Bioavailability of Resveratrol Following Topical Application
5.2. Toxicity of Resveratrol Following Topical Application
6. Conclusions
Funding
Conflicts of Interest
References
- Gupta, A.K.; Gover, M.D.; Nouri, K.; Taylor, S. The treatment of melasma: A review of clinical trials. J. Am. Acad. Dermatol. 2006, 55, 1048–1065. [Google Scholar] [CrossRef] [PubMed]
- Amer, M.; Metwalli, M. Topical hydroquinone in the treatment of some hyperpigmentary disorders. Int. J. Dermatol. 1998, 37, 449–450. [Google Scholar] [CrossRef]
- Romaguera, C.; Grimalt, F. Leukoderma from hydroquinone. Contact Dermatitis 1985, 12, 183. [Google Scholar] [CrossRef] [PubMed]
- Engasser, P.G. Ochronosis caused by bleaching creams. J. Am. Acad. Dermatol. 1984, 10, 1072–1073. [Google Scholar] [CrossRef]
- Kim, H.; Choi, H.R.; Kim, D.S.; Park, K.C. Topical hypopigmenting agents for pigmentary disorders and their mechanisms of action. Ann. Dermatol. 2012, 24, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Suleria, H.A.R.; Ahmad, B.; Peters, D.G.; Mubarak, M.S. A comprehensive review of the health perspectives of resveratrol. Food Funct. 2017, 8, 4284–4305. [Google Scholar] [CrossRef] [PubMed]
- Ratz-Lyko, A.; Arct, J. Resveratrol as an active ingredient for cosmetic and dermatological applications: A review. J. Cosmet. Laser. Ther. 2018, 21, 84–90. [Google Scholar] [CrossRef]
- Fenoll, L.G.; Rodriguez-Lopez, J.N.; Varon, R.; Garcia-Ruiz, P.A.; Garcia-Canovas, F.; Tudela, J. Action mechanism of tyrosinase on meta- and para-hydroxylated monophenols. Biol. Chem. 2000, 381, 313–320. [Google Scholar] [CrossRef]
- Lin, C.B.; Babiarz, L.; Liebel, F.; Roydon Price, E.; Kizoulis, M.; Gendimenico, G.J.; Fisher, D.E.; Seiberg, M. Modulation of microphthalmia-associated transcription factor gene expression alters skin pigmentation. J. Investig. Dermatol. 2002, 119, 1330–1340. [Google Scholar] [CrossRef]
- Newton, R.A.; Cook, A.L.; Roberts, D.W.; Leonard, J.H.; Sturm, R.A. Post-transcriptional regulation of melanin biosynthetic enzymes by cAMP and resveratrol in human melanocytes. J. Investig. Dermatol. 2007, 127, 2216–2227. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y. Resveratrol alleviates LPS-induced injury in human keratinocyte cell line HaCaT by up-regulation of miR-17. Biochem. Biophys. Res. Commun. 2018, 501, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Kang, J.H.; Seo, J.O.; Baek, S.H.; Moh, S.H.; Chae, J.K.; Park, Y.U.; Ko, Y.T.; Kim, S.Y. Anti-melanogenic potentials of nanoparticles from calli of resveratrol-enriched rice against UVB-induced hyperpigmentation in guinea pig skin. Biomol. Ther. 2016, 24, 85–93. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef] [PubMed]
- Briganti, S.; Camera, E.; Picardo, M. Chemical and instrumental approaches to treat hyperpigmentation. Pigment Cell Res. 2003, 16, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.H.; Solano, F.; Penafiel, R.; Lozano, J.A. Half-lives of tyrosinase isozymes from Harding-Passey mouse melanoma. Cancer Lett 1988, 38, 339–346. [Google Scholar] [CrossRef]
- Rendon, M.I. Utilizing combination therapy to optimize melasma outcomes. J. Drugs Dermatol. 2004, 3, S27–S34. [Google Scholar] [PubMed]
- Bolognia, J.L.; Sodi, S.A.; Osber, M.P.; Pawelek, J.M. Enhancement of the depigmenting effect of hydroquinone by cystamine and buthionine sulfoximine. Br. J. Dermatol. 1995, 133, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Rivas, S.; Pandya, A.G. Treatment of melasma with topical agents, peels and lasers: An evidence-based review. Am. J. Clin. Dermatol. 2013, 14, 359–376. [Google Scholar] [CrossRef] [PubMed]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.L.; et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef]
- Ebanks, J.P.; Wickett, R.R.; Boissy, R.E. Mechanisms regulating skin pigmentation: The rise and fall of complexion coloration. Int. J. Mol. Sci. 2009, 10, 4066–4087. [Google Scholar] [CrossRef] [PubMed]
- Satooka, H.; Kubo, I. Resveratrol as a kcat type inhibitor for tyrosinase: Potentiated melanogenesis inhibitor. Bioorg. Med. Chem. 2012, 20, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, K.; Okura, M.; Sumikawa, Y.; Hida, T.; Kuno, A.; Horio, Y.; Yamashita, T. Biochemical effects of the flavanol-rich lychee fruit extract on the melanin biosynthesis and reactive oxygen species. J. Dermatol. 2016, 43, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Kolbe, L.; Mann, T.; Gerwat, W.; Batzer, J.; Ahlheit, S.; Scherner, C.; Wenck, H.; Stab, F. 4-n-butylresorcinol, a highly effective tyrosinase inhibitor for the topical treatment of hyperpigmentation. J. Eur. Acad. Dermatol. Venereol 2013, 27, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Park, J.H.; Suh, H.J.; Lee, I.C.; Koh, J.; Boo, Y.C. Effects of resveratrol, oxyresveratrol, and their acetylated derivatives on cellular melanogenesis. Arch. Dermatol. Res. 2014, 306, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.H.; Ryu, S.Y.; Choi, E.J.; Kang, S.H.; Chang, I.M.; Min, K.R.; Kim, Y. Oxyresveratrol as the potent inhibitor on dopa oxidase activity of mushroom tyrosinase. Biochem. Biophys. Res. Commun. 1998, 243, 801–803. [Google Scholar] [CrossRef] [PubMed]
- Yasumoto, K.; Yokoyama, K.; Takahashi, K.; Tomita, Y.; Shibahara, S. Functional analysis of microphthalmia-associated transcription factor in pigment cell-specific transcription of the human tyrosinase family genes. J. Biol. Chem. 1997, 272, 503–509. [Google Scholar] [CrossRef]
- Vachtenheim, J.; Borovansky, J. “Transcription physiology” of pigment formation in melanocytes: Central role of MITF. Exp. Dermatol. 2010, 19, 617–627. [Google Scholar] [CrossRef]
- Bharti, K.; Gasper, M.; Ou, J.; Brucato, M.; Clore-Gronenborn, K.; Pickel, J.; Arnheiter, H. A regulatory loop involving PAX6, MITF, and WNT signaling controls retinal pigment epithelium development. PLoS Genet. 2012, 8, e1002757. [Google Scholar] [CrossRef]
- Kim, D.S.; Park, S.H.; Park, K.C. Transforming growth factor-beta1 decreases melanin synthesis via delayed extracellular signal-regulated kinase activation. Int. J. Biochem. Cell Biol. 2004, 36, 1482–1491. [Google Scholar]
- Kim, D.S.; Park, S.H.; Kwon, S.B.; Youn, S.W.; Park, K.C. Effects of lysophosphatidic acid on melanogenesis. Chem. Phys. Lipids. 2004, 127, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Han, W.S.; Yoo, J.Y.; Youn, S.W.; Kim, D.S.; Park, K.C.; Kim, S.Y.; Kim, K.H. Effects of C2-ceramide on the Malme-3M melanoma cell line. J. Dermatol. Sci. 2002, 30, 10–19. [Google Scholar] [CrossRef]
- Kim, D.S.; Kim, S.Y.; Chung, J.H.; Kim, K.H.; Eun, H.C.; Park, K.C. Delayed ERK activation by ceramide reduces melanin synthesis in human melanocytes. Cell Signal. 2002, 14, 779–785. [Google Scholar] [CrossRef]
- Chen, Y.J.; Chen, Y.Y.; Lin, Y.F.; Hu, H.Y.; Liao, H.F. Resveratrol inhibits alpha-melanocyte-stimulating hormone signaling, viability, and invasiveness in melanoma cells. Evid.-Based Complement. Altern. Med. 2013, 2013, 632121. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Seo, J.O.; Baek, S.H.; Kim, S.Y. Inhibitory effects of resveratrol on melanin synthesis in ultraviolet B-induced pigmentation in Guinea pig skin. Biomol. Ther. 2014, 22, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Chang, H.; Choi, H.; Shin, J.H.; Park, S.J.; Jo, Y.K.; Choi, E.S.; Baek, S.Y.; Kim, B.G.; Chang, J.W.; et al. Autophagy induced by resveratrol suppresses alpha-MSH-induced melanogenesis. Exp. Dermatol. 2014, 23, 204–206. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.T.; Tseng, Y.T.; Wong, W.J.; Liu, C.M.; Lo, Y.C. Resveratrol prevents nanoparticles-induced inflammation and oxidative stress via downregulation of PKC-alpha and NADPH oxidase in lung epithelial A549 cells. BMC Complement. Altern. Med. 2018, 18, 211. [Google Scholar] [CrossRef]
- Pavel, S. Dynamics of melanogenesis intermediates. J. Investig. Dermatol. 1993, 100, 162S–165S. [Google Scholar] [CrossRef]
- Park, H.Y.; Wu, H.; Killoran, C.E.; Gilchrest, B.A. The receptor for activated C-kinase-I (RACK-I) anchors activated PKC-beta on melanosomes. J. Cell Sci. 2004, 117, 3659–3668. [Google Scholar] [CrossRef]
- Kim, D.S.; Hwang, E.S.; Lee, J.E.; Kim, S.Y.; Kwon, S.B.; Park, K.C. Sphingosine-1-phosphate decreases melanin synthesis via sustained ERK activation and subsequent MITF degradation. J. Cell Sci. 2003, 116, 1699–1706. [Google Scholar] [CrossRef]
- Park, K.; Elias, P.M.; Hupe, M.; Borkowski, A.W.; Gallo, R.L.; Shin, K.O.; Lee, Y.M.; Holleran, W.M.; Uchida, Y. Resveratrol stimulates sphingosine-1-phosphate signaling of cathelicidin production. J. Investig. Dermatol. 2013, 133, 1942–1949. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.H.; Choi, H.R.; Kang, Y.A.; Park, K.C. Depigmenting effect of resveratrol is dependent on FOXO3a activation without SIRT1 activation. Int. J. Mol. Sci. 2017, 18, 1213. [Google Scholar] [CrossRef] [PubMed]
- Ando, H.; Ryu, A.; Hashimoto, A.; Oka, M.; Ichihashi, M. Linoleic acid and alpha-linolenic acid lightens ultraviolet-induced hyperpigmentation of the skin. Arch. Dermatol. Res. 1998, 290, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Ando, H.; Funasaka, Y.; Oka, M.; Ohashi, A.; Furumura, M.; Matsunaga, J.; Matsunaga, N.; Hearing, V.J.; Ichihashi, M. Possible involvement of proteolytic degradation of tyrosinase in the regulatory effect of fatty acids on melanogenesis. J. Lipid Res. 1999, 40, 1312–1316. [Google Scholar] [PubMed]
- Ando, H.; Wen, Z.M.; Kim, H.Y.; Valencia, J.C.; Costin, G.E.; Watabe, H.; Yasumoto, K.; Niki, Y.; Kondoh, H.; Ichihashi, M.; et al. Intracellular composition of fatty acid affects the processing and function of tyrosinase through the ubiquitin-proteasome pathway. Biochem. J. 2006, 394, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Ando, H.; Watabe, H.; Valencia, J.C.; Yasumoto, K.; Furumura, M.; Funasaka, Y.; Oka, M.; Ichihashi, M.; Hearing, V.J. Fatty acids regulate pigmentation via proteasomal degradation of tyrosinase: A new aspect of ubiquitin-proteasome function. J. Biol. Chem. 2004, 279, 15427–15433. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.H.; Jin, Z.H. Paracrine regulation of melanogenesis. Br. J. Dermatol. 2018, 178, 632–639. [Google Scholar] [CrossRef]
- Serre, C.; Busuttil, V.; Botto, J.M. Intrinsic and extrinsic regulation of human skin melanogenesis and pigmentation. Int. J. Cosmet. Sci. 2018, 40, 328–347. [Google Scholar] [CrossRef]
- Yokota, T.; Nishio, H.; Kubota, Y.; Mizoguchi, M. The inhibitory effect of glabridin from licorice extracts on melanogenesis and inflammation. Pigment Cell Res. 1998, 11, 355–361. [Google Scholar] [CrossRef]
- Nam, J.H.; Lee, D.U. Foeniculum vulgare extract and its constituent, trans-anethole, inhibit UV-induced melanogenesis via ORAI1 channel inhibition. J. Dermatol. Sci. 2016, 84, 305–313. [Google Scholar] [CrossRef]
- Davis, E.C.; Callender, V.D. Postinflammatory hyperpigmentation: A review of the epidemiology, clinical features, and treatment options in skin of color. J. Clin. Aesthet. Dermatol. 2010, 3, 20–31. [Google Scholar] [PubMed]
- Kligman, A.M.; Willis, I. A new formula for depigmenting human skin. Arch. Dermatol. 1975, 111, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Thiel, G.; Ulrich, M.; Mukaida, N.; Rossler, O.G. Resveratrol stimulation induces interleukin-8 gene transcription via NF-kappaB. Pharmacol. Res. 2018, 134, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.; Savouret, J.F.; Widerak, M.; Corvol, M.T.; Rannou, F. Resveratrol, potential therapeutic interest in joint disorders: A critical narrative review. Nutrients 2017, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Soeur, J.; Eilstein, J.; Lereaux, G.; Jones, C.; Marrot, L. Skin resistance to oxidative stress induced by resveratrol: From Nrf2 activation to GSH biosynthesis. Free Radic. Biol. Med. 2015, 78, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Vitale, N.; Kisslinger, A.; Paladino, S.; Procaccini, C.; Matarese, G.; Pierantoni, G.M.; Mancini, F.P.; Tramontano, D. Resveratrol couples apoptosis with autophagy in UVB-irradiated HaCaT cells. PLoS ONE 2013, 8, e80728. [Google Scholar] [CrossRef] [PubMed]
- Sticozzi, C.; Cervellati, F.; Muresan, X.M.; Cervellati, C.; Valacchi, G. Resveratrol prevents cigarette smoke-induced keratinocytes damage. Food Funct. 2014, 5, 2348–2356. [Google Scholar] [CrossRef]
- Kwon, S.H.; Hwang, Y.J.; Lee, S.K.; Park, K.C. Heterogeneous pathology of melasma and Its clinical implications. Int. J. Mol. Sci. 2016, 17, 824. [Google Scholar] [CrossRef]
- Pellegrini, G.; Dellambra, E.; Golisano, O.; Martinelli, E.; Fantozzi, I.; Bondanza, S.; Ponzin, D.; McKeon, F.; De Luca, M. p63 identifies keratinocyte stem cells. Proc. Natl. Acad. Sci. USA 2001, 98, 3156–3161. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, Y.C.; Lee, E.S.; Park, K.C.; Kang, H.Y. Acquired bilateral melanosis of the neck in perimenopausal women. Br. J. Dermatol. 2012, 166, 662–665. [Google Scholar] [CrossRef]
- Jang, Y.H.; Park, J.Y.; Park, Y.J.; Kang, H.Y. Changes in melanin and melanocytes in mottled hypopigmentation after Low-fluence 1,064-nm Q-switched Nd:YAG laser treatment for melasma. Ann. Dermatol. 2015, 27, 340–342. [Google Scholar] [CrossRef] [PubMed]
- Kretzschmar, K.; Watt, F.M. Markers of epidermal stem cell subpopulations in adult mammalian skin. Cold Spring Harb. Perspect. Med. 2014, 4, a013631. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Choi, H.R.; Nam, K.M.; Lee, H.S.; Kim, S.A.; Joe, H.J.; Kazumi, T.; Park, K.C. The co-expression pattern of p63 and HDAC1: A potential way to disclose stem cells in interfollicular epidermis. Int. J. Mol. Sci. 2017, 18, 1360. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.; Fisher, D.E. Current status of diagnostic and prognostic markers in melanoma. Methods Mol. Biol. 2014, 1102, 177–197. [Google Scholar]
- Kwong, L.N.; Davies, M.A. Targeted therapy for melanoma: Rational combinatorial approaches. Oncogene 2014, 33, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Zmijewski, M.A.; Pawelek, J. L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 2012, 25, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Zbytek, B.; Slominski, R. Inhibitors of melanogenesis increase toxicity of cyclophosphamide and lymphocytes against melanoma cells. Inter. J. Cancer 2009, 124, 1470–1477. [Google Scholar] [CrossRef]
- Scislowski, P.W.; Slominski, A.; Bomirski, A. Biochemical characterization of three hamster melanoma variants--II. Glycolysis and oxygen consumption. Inter. J. Biochem. 1984, 16, 327–331. [Google Scholar] [CrossRef]
- Scislowski, P.W.; Slominski, A.; Bomirski, A.; Zydowo, M. Metabolic characterization of three hamster melanoma variants. Neoplasma 1985, 32, 593–598. [Google Scholar]
- Slominski, R.M.; Zmijewski, M.A.; Slominski, A.T. The role of melanin pigment in melanoma. Exp. Dermatol. 2015, 24, 258–259. [Google Scholar] [CrossRef]
- Slominski, A.T.; Carlson, J.A. Melanoma resistance: A bright future for academicians and a challenge for patient advocates. Mayo Clin. Proc. 2014, 89, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Brozyna, A.A.; Jozwicki, W.; Carlson, J.A.; Slominski, A.T. Melanogenesis affects overall and disease-free survival in patients with stage III and IV melanoma. Hum. Pathol. 2013, 44, 2071–2074. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.H.; Sethi, G.; Um, J.Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The role of resveratrol in cancer therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, H.; Moriyama, M.; Ninomiya, K.; Morikawa, T.; Hayakawa, T. Inhibitory effects of oligostilbenoids from the bark of shorea roxburghii on malignant melanoma cell growth: Implications for novel topical anticancer candidates. Biol. Pharm. Bull. 2016, 39, 1675–1682. [Google Scholar] [CrossRef]
- Gatouillat, G.; Balasse, E.; Joseph-Pietras, D.; Morjani, H.; Madoulet, C. Resveratrol induces cell-cycle disruption and apoptosis in chemoresistant B16 melanoma. J. Cell. Biochem. 2010, 110, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F.; Adhami, V.M.; Ahmad, N. Prevention of short-term ultraviolet B radiation-mediated damages by resveratrol in SKH-1 hairless mice. Toxicol. Appl. Pharmacl. 2003, 186, 28–37. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Afaq, F.; Aziz, M.H.; Ahmad, N. Modulations of critical cell cycle regulatory events during chemoprevention of ultraviolet B-mediated responses by resveratrol in SKH-1 hairless mouse skin. Oncogene 2004, 23, 5151–5160. [Google Scholar] [CrossRef]
- Aziz, M.H.; Reagan-Shaw, S.; Wu, J.; Longley, B.J.; Ahmad, N. Chemoprevention of skin cancer by grape constituent resveratrol: Relevance to human disease? FASEB J. 2005, 19, 1193–1195. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.H.; Afaq, F.; Ahmad, N. Prevention of ultraviolet-B radiation damage by resveratrol in mouse skin is mediated via modulation in surviving. Photochem. Photobiol. 2005, 81, 25–31. [Google Scholar] [CrossRef]
- Park, S.; Seok, J.K.; Kwak, J.Y.; Choi, Y.H.; Hong, S.S.; Suh, H.J.; Park, W.; Boo, Y.C. Anti-melanogenic effects of resveratryl triglycolate, a novel hybrid compound derived by esterification of resveratrol with glycolic acid. Arch. Dermatol. Res. 2016, 308, 325–334. [Google Scholar] [CrossRef]
- Jo, D.J.; Seok, J.K.; Kim, S.Y.; Park, W.; Baek, J.H.; Kim, Y.M.; Boo, Y.C. Human skin-depigmenting effects of resveratryl triglycolate, a hybrid compound of resveratrol and glycolic acid. Int. J. Cosmet. Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Seok, J.K.; An, S.M.; Baek, J.H.; Koh, J.S.; Boo, Y.C. A study of the human skin-whitening effects of resveratryl triacetate. Arch. Dermatol. Res. 2015, 307, 239–247. [Google Scholar] [CrossRef]
- Kim, S.Y.; Park, K.C.; Kwon, S.B.; Kim, D.S. Hypopigmentary effects of 4-n-butylresorcinol and resveratrol in combination. Pharmazie 2012, 67, 542–546. [Google Scholar] [PubMed]
- Alonso, C.; Marti, M.; Barba, C.; Carrer, V.; Rubio, L.; Coderch, L. Skin permeation and antioxidant efficacy of topically applied resveratrol. Arch. Dermatol. Res. 2017, 309, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Murakami, I.; Chaleckis, R.; Pluskal, T.; Ito, K.; Hori, K.; Ebe, M.; Yanagida, M.; Kondoh, H. Metabolism of skin-absorbed resveratrol into its glucuronized form in mouse skin. PLoS ONE 2014, 9, e115359. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.F.; Lin, Y.K.; Huang, Z.R.; Fang, J.Y. Delivery of resveratrol, a red wine polyphenol, from solutions and hydrogels via the skin. Biol. Pharm. Bull. 2008, 31, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Moyano-Mendez, J.R.; Fabbrocini, G.; De Stefano, D.; Mazzella, C.; Mayol, L.; Scognamiglio, I.; Carnuccio, R.; Ayala, F.; La Rotonda, M.I.; De Rosa, G. Enhanced antioxidant effect of trans-resveratrol: Potential of binary systems with polyethylene glycol and cyclodextrin. Drug Dev. Ind. Pharm. 2014, 40, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Pando, D.; Caddeo, C.; Manconi, M.; Fadda, A.M.; Pazos, C. Nanodesign of olein vesicles for the topical delivery of the antioxidant resveratrol. J. Pharm. Pharmacol. 2013, 65, 1158–1167. [Google Scholar] [CrossRef] [PubMed]
- Scognamiglio, I.; De Stefano, D.; Campani, V.; Mayol, L.; Carnuccio, R.; Fabbrocini, G.; Ayala, F.; La Rotonda, M.I.; De Rosa, G. Nanocarriers for topical administration of resveratrol: A comparative study. Int. J. Pharm. 2013, 440, 179–187. [Google Scholar] [CrossRef]
- Tsai, M.J.; Lu, I.J.; Fu, Y.S.; Fang, Y.P.; Huang, Y.B.; Wu, P.C. Nanocarriers enhance the transdermal bioavailability of resveratrol: In-vitro and in-vivo study. Colloids Surf. B Biointerfaces 2016, 148, 650–656. [Google Scholar] [CrossRef]
- Gallo, R.; Viglizzo, G.; Vecchio, F.; Parodi, A. Allergic contact dermatitis from pentylene glycol in an emollient cream, with possible co-sensitization to resveratrol. Contact Dermatitis 2003, 48, 176–177. [Google Scholar] [CrossRef] [PubMed]
- Shrotriya, S.N.; Ranpise, N.S.; Vidhate, B.V. Skin targeting of resveratrol utilizing solid lipid nanoparticle-engrossed gel for chemically induced irritant contact dermatitis. Drug Deliv. Transl. Res. 2017, 7, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.L.; Lulli, D.; Passarelli, F.; Pastore, S. Topical plant polyphenols prevent type I interferon signaling in the skin and suppress contact hypersensitivity. Int. J. Mol. Sci. 2018, 19, 2652. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Baek, K.; Lee, J.E.; Kim, B.G. Using tyrosinase as a monophenol monooxygenase: A combined strategy for effective inhibition of melanin formation. Biotechnol. Bioeng. 2016, 113, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Nishigori, C.; Aoyama, Y.; Ito, A.; Suzuki, K.; Suzuki, T.; Tanemura, A.; Ito, M.; Katayama, I.; Oiso, N.; Kagohashi, Y.; et al. Guide for medical professionals (i.e., dermatologists) for the management of Rhododenol-induced leukoderma. J. Dermatol. 2015, 42, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Wakamatsu, K. A convenient screening method to differentiate phenolic skin whitening tyrosinase inhibitors from leukoderma-inducing phenols. J. Dermatol. Sci. 2015, 80, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Wakamatsu, K. Biochemical mechanism of rhododendrol-induced leukoderma. Int. J. Mol. Sci. 2018, 19, 552. [Google Scholar]
- Goto, N.; Tsujimoto, M.; Nagai, H.; Masaki, T.; Ito, S.; Wakamatsu, K.; Nishigori, C. 4-(4-Hydroxyphenyl)-2-butanol (rhododendrol)-induced melanocyte cytotoxicity is enhanced by UVB exposure through generation of oxidative stress. Exp. Dermatol. 2018, 27, 754–762. [Google Scholar] [CrossRef]
- Fujimura, A.T.; Martinez, R.M.; Pinho-Ribeiro, F.A.; Lopes Dias da Silva, A.M.; Baracat, M.M.; Georgetti, S.R.; Verri, W.A.; Jr Chorilli, M.; Casagrande, R. Resveratrol-loaded liquid-crystalline system inhibits UVB-induced skin inflammation and oxidative stress in mice. J. Nat. Prod. 2016, 79, 1329–1338. [Google Scholar] [CrossRef]
- Lee, J.H.; Chen, H.; Kolev, V.; Aull, K.H.; Jung, I.; Wang, J.; Miyamoto, S.; Hosoi, J.; Mandinova, A.; Fisher, D.E. High-throughput, high-content screening for novel pigmentation regulators using a keratinocyte/melanocyte co-culture system. Exp. Dermatol. 2014, 23, 125–129. [Google Scholar] [CrossRef]
- Okura, M.; Yamashita, T.; Ishii-Osai, Y.; Yoshikawa, M.; Sumikawa, Y.; Wakamatsu, K.; Ito, S. Effects of rhododendrol and its metabolic products on melanocytic cell growth. J. Dermatol. Sci. 2015, 80, 142–149. [Google Scholar] [CrossRef] [PubMed]
| Mechanisms of Action | Hypopigmenting Agent |
|---|---|
| Before melanin synthesis | |
| Regulation of tyrosinase transcription | TGF-β1, TNFα, IL-1α, IL-1β, IL-6, Lysophosphatidic acid, C2-Ceramides, Sphingosine-1-phosphate, Sphingosylphosphorylcholine, Tretinoin |
| Inhibition of tyrosinase maturation | Glucosamine, Tunicamycin, Glycosphingolipid, Calcium D-pantetheine-S-sulfonate |
| During melanin synthesis | |
| Inhibition of tyrosinase activity | Hydroquinone, Arbutin, Kojic acid, 4-n-Butylresorcinol, Phenolic compounds, 4-Hydroxyanisole, Methyl-gentisate, 4-S-CAP & derivatives, Ellagic acid, Oxyresveratrol, Resveratrol, Aloesin, Azelaic acid, Zinc |
| After melanin synthesis | |
| Post-transcriptional control of tyrosinase | Linoleic acid, α-Linolenic acid, Phospholipase D2 |
| Inhibition of melanosome transfer | Niacinamide (Vitamin B3), serine protease inhibitors, lecthins and neoglycoproteins, RW-50353, soybean.milk extracts |
| Regulation of melanocyte environment | Corticosteroids, Glabridin |
| Antioxidants | α-Tocopherol, Ascorbic acid, 6-Hydroxy-3,4-dihydrocoumarins, α-Lipoic acid, Methimazole, Phenol/catechol, Ascorbic acid palmitate, Decursin, L-α TF, VC-PMG, Thioctic acid |
| Study Models | Biological Activities of Resveratrol | References |
|---|---|---|
| Enzyme inhibition | ||
| inhibits mushroom tyrosinase | [22,26] | |
| inhibits human tyrosinase | [25,10] | |
| Cell culture | ||
| reduces melanin production in B16 murine melanoma cells | [22,25,34] | |
| reduces melanin production in human epidermal melanocytes | [25] | |
| reduces MITF and tyrosinase transcription in B16 murine melanoma cells | [34,35] | |
| reduces MITF and tyrosinase in human epidermal melanocytes | [9,42] | |
| induces autophagy and reduces α-MSH-induced melanogenesis in melan-A cells | [24] | |
| downregulates PKC-α in lung epithelial A549 cells | [37] | |
| increases phosphorylation of ERK in human epidermal melanocytes | [42] | |
| reduces the post-transcriptional process of tyrosinase in human epidermal melanocytes | [10] | |
| reduces inflammatory injury in HaCaT cells | [11] | |
| reduces UVB-induced injury in HaCaT cells | [56] | |
| prevents oxidative stress-induced injury in human keratinocytes | [57] | |
| Reconstructed skin model | ||
| reduces melanin production in a reconstituted human skin model | [25] | |
| Animal model | ||
| reduces skin pigmentation in Yucatan swine, 1% topical resveratrol for 8 weeks | [9] | |
| reduces UVB-induced skin pigmentation in guinea pig, 1% topical resveratrol for 2 weeks | [35] | |
| reduces UVB-induced skin pigmentation in guinea pig, topical application of callus of resveratrol-enriched rice for 15 days | [12] | |
| Human clinical trials | ||
| enhances depigmentation after UV-induced tanning (p < 0.05), topical 0.4% resveratrol triglycolate vs. control cream, 22 subjects for 8 weeks | [81] | |
| decreases hyperpigmented spots on the face (p < 0.05), topical 0.4% resveratrol triacetate vs. control cream, 21 subjects for 8 weeks | [82] | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Na, J.-I.; Shin, J.-W.; Choi, H.-R.; Kwon, S.-H.; Park, K.-C. Resveratrol as a Multifunctional Topical Hypopigmenting Agent. Int. J. Mol. Sci. 2019, 20, 956. https://doi.org/10.3390/ijms20040956
Na J-I, Shin J-W, Choi H-R, Kwon S-H, Park K-C. Resveratrol as a Multifunctional Topical Hypopigmenting Agent. International Journal of Molecular Sciences. 2019; 20(4):956. https://doi.org/10.3390/ijms20040956
Chicago/Turabian StyleNa, Jung-Im, Jung-Won Shin, Hye-Ryung Choi, Soon-Hyo Kwon, and Kyung-Chan Park. 2019. "Resveratrol as a Multifunctional Topical Hypopigmenting Agent" International Journal of Molecular Sciences 20, no. 4: 956. https://doi.org/10.3390/ijms20040956
APA StyleNa, J.-I., Shin, J.-W., Choi, H.-R., Kwon, S.-H., & Park, K.-C. (2019). Resveratrol as a Multifunctional Topical Hypopigmenting Agent. International Journal of Molecular Sciences, 20(4), 956. https://doi.org/10.3390/ijms20040956







