Exploration of miRNA and mRNA Profiles in Fresh and Frozen-Thawed Boar Sperm by Transcriptome and Small RNA Sequencing
Abstract
1. Introduction
2. Results
2.1. Analysis of Small RNA Sequences
2.2. Analysis of Transcriptome Sequences
2.3. Combined Analysis of Transcriptome and Small RNA Sequencing
2.4. QRT-PCR Validation
3. Discussion
4. Materials and Methods
4.1. Semen Collection
4.2. Semen Cryopreservation
4.3. RNA Preparation and Small RNA Sequencing
4.4. Identification of Known/Novel miRNAs and Target Gene Prediction
4.5. Transcriptome Library Construction, Sequencing and Analysis
4.6. Quantitative Real-Time Reverse Transcription PCR (qRT-PCR) Validation of Differentially Expressed mRNAs and miRNAs
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bailey, J.L.; Lessard, C.; Jacques, J.; Brèque, C.; Dobrinski, I.; Zeng, W.; Galantino-Homer, H.L. Cryopreservation of boar semen and its future importance to the industry. Theriogenology 2008, 70, 1251–1259. [Google Scholar] [CrossRef]
- Yeste, M. Recent advances in boar sperm cryopreservation: State of the art and current perspectives. Reprod. Domest. Anim. 2015, 50, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, Y.-J.; Kim, Y.-J. Changes in sperm membrane and ROS following cryopreservation of liquid boar semen stored at 15 C. Anim. Reprod. Sci. 2011, 124, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Yeste, M. Sperm cryopreservation update: Cryodamage, markers, and factors affecting the sperm freezability in pigs. Theriogenology 2016, 85, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Mazur, P.; Leibo, S.; Seidel, G.E., Jr. Cryopreservation of the germplasm of animals used in biological and medical research: Importance, impact, status, and future directions. Biol. Reprod. 2008, 78, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Kopeika, J.; Thornhill, A.; Khalaf, Y. The effect of cryopreservation on the genome of gametes and embryos: Principles of cryobiology and critical appraisal of the evidence. Hum. Reprod. Update 2015, 21, 209–227. [Google Scholar] [CrossRef] [PubMed]
- O’connell, M.; McClure, N.; Lewis, S. The effects of cryopreservation on sperm morphology, motility and mitochondrial function. Hum. Reprod. 2002, 17, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Tongdee, P.; Sukprasert, M.; Satirapod, C.; Wongkularb, A.; Choktanasiri, W. Comparison of cryopreserved human sperm between ultra rapid freezing and slow programmable freezing: Effect on motility, morphology and DNA integrity. J. Med. Assoc. Thail. 2015, 98, S33–S42. [Google Scholar]
- Bailey, J.L.; BLODEAU, J.F.; CORMIER, N. Semen cryopreservation in domestic animals: A damaging and capacitating phenomenon minireview. J. Androl. 2000, 21, 1–7. [Google Scholar] [PubMed]
- Bilodeau, J.F.; Chatterjee, S.; Sirard, M.A.; Gagnon, C. Levels of antioxidant defenses are decreased in bovine spermatozoa after a cycle of freezing and thawing. Mol. Reprod. Dev. 2000, 55, 282–288. [Google Scholar] [CrossRef]
- Kim, S.-H.; Yu, D.-H.; Kim, Y.-J. Effects of cryopreservation on phosphatidylserine translocation, intracellular hydrogen peroxide, and DNA integrity in canine sperm. Theriogenology 2010, 73, 282–292. [Google Scholar] [CrossRef]
- Gürler, H.; Malama, E.; Heppelmann, M.; Calisici, O.; Leiding, C.; Kastelic, J.; Bollwein, H. Effects of cryopreservation on sperm viability, synthesis of reactive oxygen species, and DNA damage of bovine sperm. Theriogenology 2016, 86, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Bucak, M.N.; Tuncer, P.B.; Sariozkan, S.; Baspinar, N.; Taspinar, M.; Coyan, K.; Bilgili, A.; Akalin, P.P.; Buyukleblebici, S.; Aydos, S.; et al. Effects of antioxidants on post-thawed bovine sperm and oxidative stress parameters: Antioxidants protect DNA integrity against cryodamage. Cryobiology 2010, 61, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, H.; Welch, G. Determination of intracellular reactive oxygen species and high mitochondrial membrane potential in Percoll-treated viable boar sperm using fluorescence-activated flow cytometry. J. Anim. Sci. 2006, 84, 2089–2100. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, H.; Welch, G. Effects of reactive oxygen species on sperm function. Theriogenology 2012, 78, 1700–1708. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Alborcia, M.J.; Valverde, A.; Parrilla, I.; Vazquez, J.M.; Martinez, E.A.; Roca, J. Detrimental effects of non-functional spermatozoa on the freezability of functional spermatozoa from boar ejaculate. PLoS ONE 2012, 7, e36550. [Google Scholar] [CrossRef]
- Cross, N.L. Role of cholesterol in sperm capacitation. Biol. Reprod. 1998, 59, 7–11. [Google Scholar] [CrossRef]
- Vadnais, M.L.; Althouse, G.C. Characterization of capacitation, cryoinjury, and the role of seminal plasma in porcine sperm. Theriogenology 2011, 76, 1508–1516. [Google Scholar] [CrossRef]
- Buhr, M.; Curtis, E.; Kakuda, N.S. Composition and behavior of head membrane lipids of fresh and cryopreserved boar sperm. Cryobiology 1994, 31, 224–238. [Google Scholar] [CrossRef]
- Cerolini, S.; Maldjian, A.; Surai, P.; Noble, R. Viability, susceptibility to peroxidation and fatty acid composition of boar semen during liquid storage1. Anim. Reprod. Sci. 2000, 58, 99–111. [Google Scholar] [CrossRef]
- Brouwers, J.F.; Silva, P.F.; Gadella, B.M. New assays for detection and localization of endogenous lipid peroxidation products in living boar sperm after BTS dilution or after freeze-thawing. Theriogenology 2005, 63, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Di Santo, M.; Tarozzi, N.; Nadalini, M.; Borini, A. Human sperm cryopreservation: Update on techniques, effect on DNA integrity, and implications for ART. Adv. Urol. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Flores, E.; Fernández-Novell, J.; Peña, A.; Rigau, T.; Rodríguez-Gil, J. Cryopreservation-induced alterations in boar spermatozoa mitochondrial function are related to changes in the expression and location of midpiece mitofusin-2 and actin network. Theriogenology 2010, 74, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Ferrusola, C.O.; Fernández, L.G.; Morrell, J.; Sandoval, C.S.; García, B.M.; Rodríguez-Martinez, H.; Tapia, J.; Peña, F. Lipid peroxidation, assessed with BODIPY-C11, increases after cryopreservation of stallion spermatozoa, is stallion-dependent and is related to apoptotic-like changes. Reproduction 2009, 138, 55–63. [Google Scholar] [CrossRef]
- Pena, F.; Plaza Davila, M.; Ball, B.; Squires, E.; Martin Munoz, P.; Ortega Ferrusola, C.; Balao da Silva, C. The impact of reproductive technologies on stallion mitochondrial function. Reprod. Domest. Anim. 2015, 50, 529–537. [Google Scholar] [CrossRef]
- Yeste, M.; Estrada, E.; Rocha, L.; Marín, H.; Rodríguez-Gil, J.; Miró, J. Cryotolerance of stallion spermatozoa is related to ROS production and mitochondrial membrane potential rather than to the integrity of sperm nucleus. Andrology 2015, 3, 395–407. [Google Scholar] [CrossRef]
- Said, T.M.; Gaglani, A.; Agarwal, A. Implication of apoptosis in sperm cryoinjury. Reprod. Biomed. Online 2010, 21, 456–462. [Google Scholar] [CrossRef]
- Castro, L.; Hamilton, T.; Mendes, C.; Nichi, M.; Barnabe, V.; Visintin, J.; Assumpção, M. Sperm cryodamage occurs after rapid freezing phase: Flow cytometry approach and antioxidant enzymes activity at different stages of cryopreservation. J. Anim. Sci. Biotechnol. 2016, 7, 17. [Google Scholar] [CrossRef]
- Sieme, H.; Oldenhof, H.; Wolkers, W.F. Mode of action of cryoprotectants for sperm preservation. Anim. Reprod. Sci. 2016, 169, 2–5. [Google Scholar] [CrossRef]
- Yang, C.-H.; Wu, T.-W.; Cheng, F.-P.; Wang, J.-H.; Wu, J.-T. Effects of different cryoprotectants and freezing methods on post-thaw boar semen quality. Reprod. Biol. 2016, 16, 41–46. [Google Scholar] [CrossRef]
- Zhang, W.; Yi, K.; Chen, C.; Hou, X.; Zhou, X. Application of antioxidants and centrifugation for cryopreservation of boar spermatozoa. Anim. Reprod. Sci. 2012, 132, 123–128. [Google Scholar] [CrossRef] [PubMed]
- De Vantéry Arrighi, C.; Lucas, H.; Chardonnens, D.; De Agostini, A. Removal of spermatozoa with externalized phosphatidylserine from sperm preparation in human assisted medical procreation: Effects on viability, motility and mitochondrial membrane potential. Reprod. Biol. Endocrinol. 2009, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Valcarce, D.; Cartón-García, F.; Herráez, M.; Robles, V. Effect of cryopreservation on human sperm messenger RNAs crucial for fertilization and early embryo development. Cryobiology 2013, 67, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Card, C.J.; Anderson, E.J.; Zamberlan, S.; Krieger, K.E.; Kaproth, M.; Sartini, B.L. Cryopreserved bovine spermatozoal transcript profile as revealed by high-throughput ribonucleic acid sequencing. Biol. Reprod. 2013, 88, 49. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.Y.; Mulligan, B.P.; Kim, H.-M.; Yang, B.-C.; Lee, C.-K. Quantitative analysis of sperm mRNA in the pig: Relationship with early embryo development and capacitation. Reprod. Fertil. Dev. 2013, 25, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Peng, W.; Ding, L.; He, L.; Zhang, Y.; Fang, D.; Tang, K. A preliminary study on epigenetic changes during boar spermatozoa cryopreservation. Cryobiology 2014, 69, 119–127. [Google Scholar] [CrossRef]
- Kim, V.N.; Han, J.; Siomi, M.C. Biogenesis of small RNAs in animals. Nat. Rev. Mol. Cell Biol. 2009, 10, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.I.; Miyazono, K. Emerging complexity of microRNA generation cascades. J. Biochem. 2010, 149, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Curry, E.; Safranski, T.J.; Pratt, S.L. Differential expression of porcine sperm microRNAs and their association with sperm morphology and motility. Theriogenology 2011, 76, 1532–1539. [Google Scholar] [CrossRef]
- Capra, E.; Turri, F.; Lazzari, B.; Cremonesi, P.; Gliozzi, T.; Fojadelli, I.; Stella, A.; Pizzi, F. Small RNA sequencing of cryopreserved semen from single bull revealed altered miRNAs and piRNAs expression between High-and Low-motile sperm populations. BMC Genom. 2017, 18, 14. [Google Scholar] [CrossRef] [PubMed]
- Ran, M.-X.; Li, Y.; Zhang, Y.; Liang, K.; Ren, Y.-N.; Zhang, M.; Zhou, G.-B.; Zhou, Y.-M.; Wu, K.; Wang, C.-D. Transcriptome Sequencing Reveals the Differentially Expressed lncRNAs and mRNAs Involved in Cryoinjuries in Frozen-Thawed Giant Panda (Ailuropoda melanoleuca) Sperm. Int. J. Mol. Sci. 2018, 19, 3066. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, R.H.; Ran, M.X.; Zhang, Y.; Liang, K.; Ren, Y.N.; He, W.C.; Zhang, M.; Zhou, G.B.; Qazi, I.H.; et al. High throughput small RNA and transcriptome sequencing reveal capacitation-related microRNAs and mRNA in boar sperm. BMC Genom. 2018, 19, 736. [Google Scholar] [CrossRef]
- Aitken, R.J.; Gordon, E.; Harkiss, D.; Twigg, J.P.; Milne, P.; Jennings, Z.; Irvine, D.S. Relative impact of oxidative stress on the functional competence and genomic integrity of human spermatozoa. Biol. Reprod. 1998, 59, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Cordova, A.; Perez, J.; Lleo, B.; Artiga, C.G.; Rillo, S.M. In vitro fertilizing capacity of deep frozen boar semen packaged in 0.5 and 5 ml straws. Reprod. Domest. Anim. 2001, 36, 199–202. [Google Scholar] [CrossRef]
- Fraser, L. Markers for Sperm Freezability and Relevance of Transcriptome Studies in Semen Cryopreservation: A Review. In Theriogenology; Carreira, R.P., Ed.; IN TECH: Rijeka, Croatia, 2017; Chapter 3; pp. 47–62. [Google Scholar]
- Flores, E.; Cifuentes, D.; Fernandez-Novell, J.M.; Medrano, A.; Bonet, S.; Briz, M.D.; Pinart, E.; Pena, A.; Rigau, T.; Rodriguez-Gil, J.E. Freeze-thawing induces alterations in the protamine-1/DNA overall structure in boar sperm. Theriogenology 2008, 69, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Flores, E.; Ramió-Lluch, L.; Bucci, D.; Fernández-Novell, J.; Peña, A.; Rodríguez-Gil, J. Freezing-thawing induces alterations in histone H1-DNA binding and the breaking of protein-DNA disulfide bonds in boar sperm. Theriogenology 2011, 76, 1450–1464. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Wang, H.Y.; Greenawalt, D.M.; Azaro, M.A.; Luo, M.; Tereshchenko, I.V.; Cui, X.; Yang, Q.; Gao, R.; Shen, L. AccuTyping: New algorithms for automated analysis of data from high-throughput genotyping with oligonucleotide microarrays. Nucleic Acids Res. 2006, 34, e116. [Google Scholar] [CrossRef][Green Version]
- Curry, E.; Ellis, S.; Pratt, S. Detection of porcine sperm microRNAs using a heterologous microRNA microarray and reverse transcriptase polymerase chain reaction. Mol. Reprod. Dev. 2009, 76, 218–219. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, C.-J.; He, L.; Ding, L.; Tang, K.-Y.; Peng, W.-P. Selection of endogenous reference microRNA genes for quantitative reverse transcription polymerase chain reaction studies of boar spermatozoa cryopreservation. Theriogenology 2015, 83, 634–641. [Google Scholar] [CrossRef]
- Govindaraju, A.; Uzun, A.; Robertson, L.; Atli, M.O.; Kaya, A.; Topper, E.; Crate, E.A.; Padbury, J.; Perkins, A.; Memili, E. Dynamics of microRNAs in bull spermatozoa. Reprod. Biol. Endocrinol. 2012, 10, 82. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, D.; Chang, Y.; Li, Y.; Zhang, M.; Zhou, G.; Peng, Z.; Zeng, C. Cryopreservation of boar sperm induces differential microRNAs expression. Cryobiology 2017, 76, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Huse, J.T.; Brennan, C.; Hambardzumyan, D.; Wee, B.; Pena, J.; Rouhanifard, S.H.; Sohn-Lee, C.; Le Sage, C.; Agami, R.; Tuschl, T. The PTEN-regulating microRNA miR-26a is amplified in high-grade glioma and facilitates gliomagenesis in vivo. Genes Dev. 2009, 23, 1327–1337. [Google Scholar] [CrossRef] [PubMed]
- Shoshan, E.; Mobley, A.K.; Braeuer, R.R.; Kamiya, T.; Huang, L.; Vasquez, M.E.; Salameh, A.; Lee, H.J.; Kim, S.J.; Ivan, C. Reduced adenosine-to-inosine miR-455-5p editing promotes melanoma growth and metastasis. Nat. Cell Biol. 2015, 17, 311. [Google Scholar] [CrossRef] [PubMed]
- Aquila, S.; Middea, E.; Catalano, S.; Marsico, S.; Lanzino, M.; Casaburi, I.; Barone, I.; Bruno, R.; Zupo, S.; Ando, S. Human sperm express a functional androgen receptor: Effects on PI3K/AKT pathway. Hum. Reprod. 2007, 22, 2594–2605. [Google Scholar] [CrossRef] [PubMed]
- Kurmasheva, R.T.; Harwood, F.C.; Houghton, P.J. Differential regulation of vascular endothelial growth factor by Akt and mammalian target of rapamycin inhibitors in cell lines derived from childhood solid tumors. Mol. Cancer Therap. 2007, 6, 1620–1628. [Google Scholar] [CrossRef]
- Tang, Y.; Nakada, M.T.; Rafferty, P.; Laraio, J.; McCabe, F.L.; Millar, H.; Cunningham, M.; Snyder, L.A.; Bugelski, P.; Yan, L. Regulation of vascular endothelial growth factor expression by EMMPRIN via the PI3K-Akt signaling pathway. Mol. Cancer Res. 2006, 4, 371–377. [Google Scholar] [CrossRef]
- Iyibozkurt, A.C.; Balcik, P.; Bulgurcuoglu, S.; Arslan, B.K.; Attar, R.; Attar, E. Effect of vascular endothelial growth factor on sperm motility and survival. Reprod. Biomed. Online 2009, 19, 784–788. [Google Scholar] [CrossRef]
- Lee, T.-C.; Ho, H.-C. Effects of prostaglandin E2 and vascular endothelial growth factor on sperm might lead to endometriosis-associated infertility. Fertil. Steril. 2011, 95, 360–362. [Google Scholar] [CrossRef]
- Sargent, K.M.; Clopton, D.T.; Lu, N.; Pohlmeier, W.E.; Cupp, A.S. VEGFA splicing: Divergent isoforms regulate spermatogonial stem cell maintenance. Cell Tissue Res. 2016, 363, 31–45. [Google Scholar] [CrossRef]
- Min, Y.H.; Cheong, J.-W.; Kim, J.Y.; Eom, J.I.; Lee, S.T.; Hahn, J.S.; Ko, Y.W.; Lee, M.H. Cytoplasmic mislocalization of p27Kip1 protein is associated with constitutive phosphorylation of Akt or protein kinase B and poor prognosis in acute myelogenous leukemia. Cancer Res. 2004, 64, 5225–5231. [Google Scholar] [CrossRef]
- Prasad, S.B.; Yadav, S.S.; Das, M.; Modi, A.; Kumari, S.; Pandey, L.K.; Singh, S.; Pradhan, S.; Narayan, G. PI3K/AKT pathway-mediated regulation of p27 Kip1 is associated with cell cycle arrest and apoptosis in cervical cancer. Cell. Oncol. 2015, 38, 215–225. [Google Scholar] [CrossRef]
- Krakowiak, P.A.; Wassif, C.A.; Kratz, L.; Cozma, D.; Kovářová, M.; Harris, G.; Grinberg, A.; Yang, Y.; Hunter, A.G.; Tsokos, M. Lathosterolosis: An inborn error of human and murine cholesterol synthesis due to lathosterol 5-desaturase deficiency. Hum. Mol. Genet. 2003, 12, 1631–1641. [Google Scholar] [CrossRef] [PubMed]
- Lang-Ouellette, D.; Richard, T.; Morin, P. Mammalian hibernation and regulation of lipid metabolism: A focus on non-coding RNAs. Biochemistry 2014, 79, 1161–1171. [Google Scholar] [CrossRef]
- Schmidt, S.; Corydon, T.J.; Pedersen, C.B.; Bross, P.; Gregersen, N. Misfolding of short-chain acyl-CoA dehydrogenase leads to mitochondrial fission and oxidative stress. Mol. Genet. Metabol. 2010, 100, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, L.; Schmidt, R.E.; Su, C.; Huang, X.; Gould, K.; Cao, G. Characterization of HSCD5, a novel human stearoyl-CoA desaturase unique to primates. Biochem. Biophys. Res. Commun. 2005, 332, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yanzhu, Y.; Yuguang, S. Characterization of human SCD2, an oligomeric desaturase with improved stability and enzyme activity by cross-linking in intact cells. Biochem. J. 2005, 388, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.Q.; Ruden, D.M.; Lu, X.Y. PKD2 cation channel is required for directional sperm movement and male fertility. Curr. Biol. 2003, 13, 2175–2178. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.C.; Phadnis, N. Parallel evolution of sperm hyper-activation ca2+ channels. Genome Bio. Evol. 2017, 9, 1938–1949. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lu, X.Y. Drosophila sperm motility in the reproductive tract. Bio. Reprod. 2011, 84, 1005–1015. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Zhu, H.; Hao, H.; Zhao, X.; Qin, T.; Wang, D. Comparative transcript profiling of gene expression of fresh and frozen-thawed bull sperm. Theriogenology. 2015, 4, 504–511. [Google Scholar] [CrossRef]
- Card, C.J.; Kreiger, K.E.; Kaproth, M.; Sartini, B.L. Oligo-dT selected spermatozoal transcript profiles differ among higher and lower fertility dairy sires. Anim. Reprod. Sci. 2017, 177, 105–123. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Chen, X.; Wang, Z.; Wang, D. Is transcription in sperm stationary or dynamic? J. Reprod. Dev. 2017, 63, 439–443. [Google Scholar] [CrossRef]
- Dzeja, C.; Hagen, V.; Kaupp, U.B.; Frings, S. Ca2+ permeation in cyclic nucleotide-gated channels. EMBO J. 1999, 18, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhao, H.; Xu, B.; Jiang, Y. Odorant receptor might be related to sperm DNA integrity in Apis cerana cerana. Anim. Reprod. Sci. 2018, 193, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Moreno, J.; Esteso, M.C.; Pradiee, J.; Castano, C.; Toledano-Diaz, A.; O’Brien, E.; Lopez-Sebastian, A.; Martinez-Nevado, E.; Delclaux, M.; Fernandez-Moran, J.; et al. Giant panda (Ailuropoda melanoleuca) sperm morphometry and function after repeated freezing and thawing. Andrologia 2016, 48, 470–474. [Google Scholar] [CrossRef] [PubMed]
- King, G.J.; Macpherson, J.W. A comparison of two methods for boar semen collection. J. Anim. Sci. 1973, 36, 563–565. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; He, L.; Peng, W.; Ding, L.; Tang, K.; Fang, D.; Zhang, Y. Selection of optimal reference genes for quantitative RT-PCR studies of boar spermatozoa cryopreservation. Cryobiology 2014, 68, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]


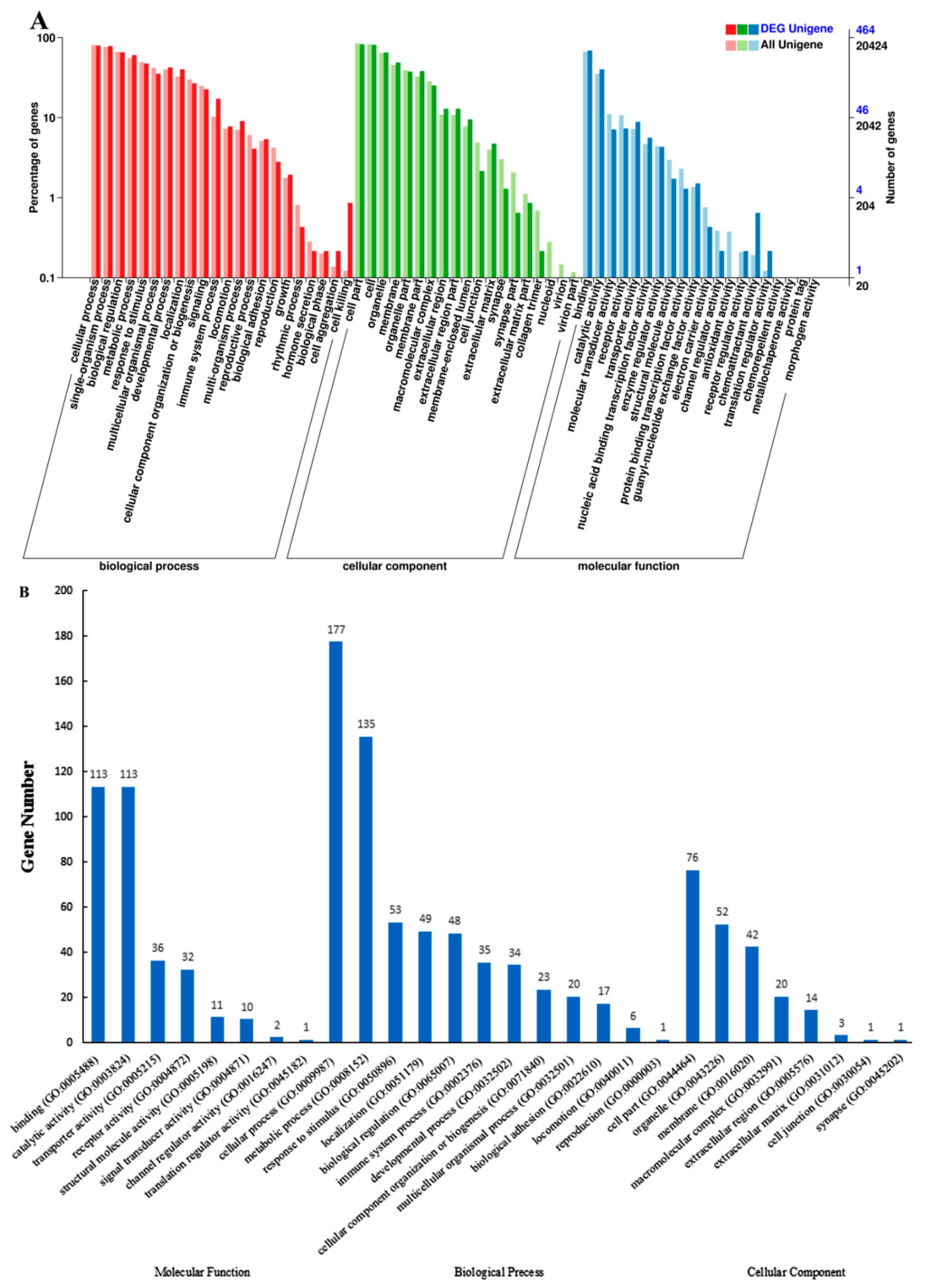

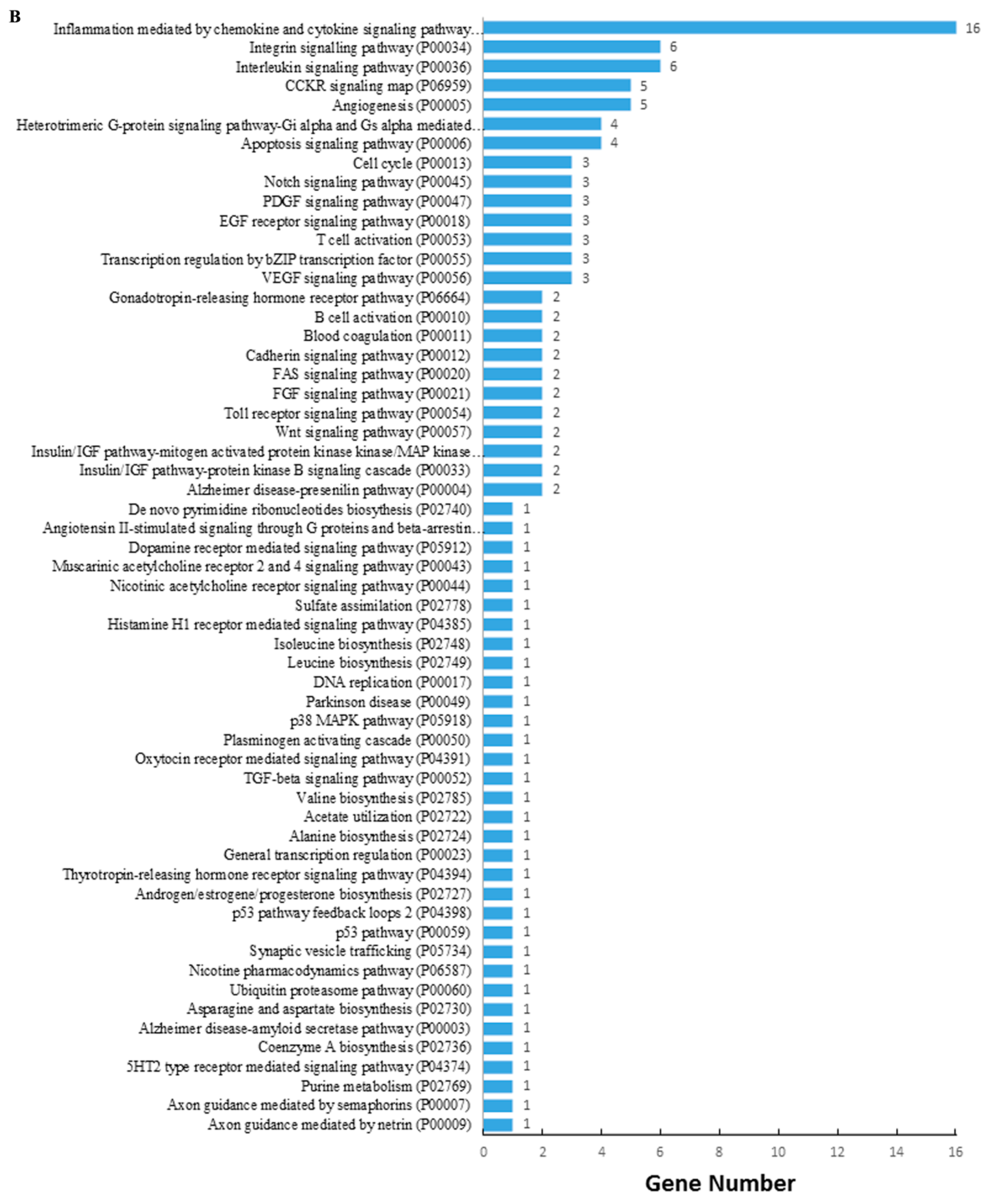
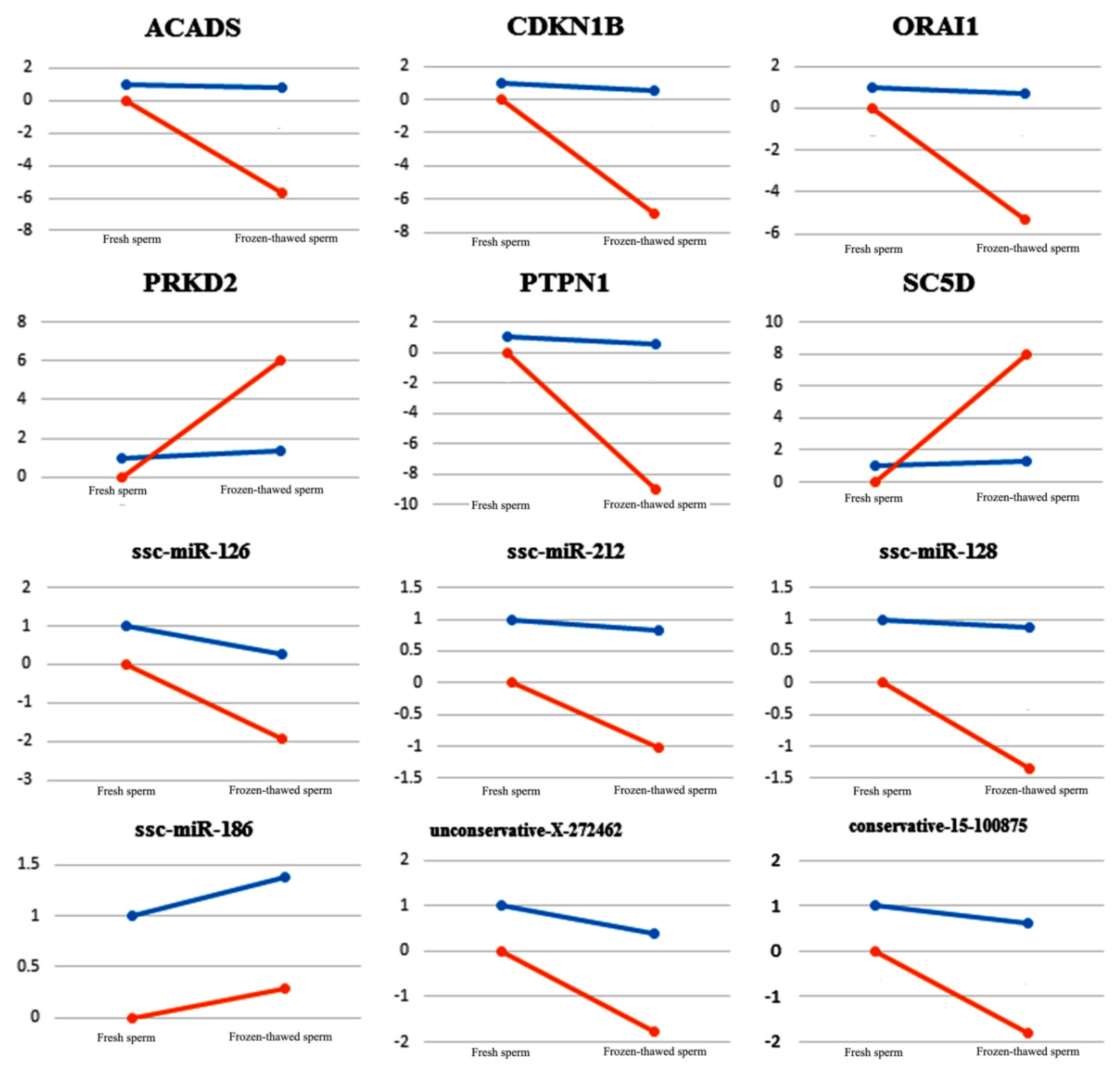
| Group | Raw Reads | Clean Reads | Q30 (%) | Mapped Reads | Total miRNAs | Known miRNAs | Novel miRNAs |
|---|---|---|---|---|---|---|---|
| Fresh sperm [42] | 18,956,444 | 12,561,033 | 86 | 3,027,230 | 1028 | 259 | 769 |
| Frozen-thawed sperm | 16,507,275 | 11,100,601 | 85 | 2,377,337 | 984 | 246 | 738 |
| Group | Clean Reads (Pair-End) | Clean Bases | GC Content | Q30 (%) | Mapped Reads (Single-End) | Unique Mapped Reads |
|---|---|---|---|---|---|---|
| Fresh sperm [42] | 26,843,452 | 6,642,110,360 | 48.62% | 86.90 | 30,016,749 (55.91%) | 28,565,403 (53.21%) |
| Frozen-thawed sperm | 24,611,191 | 6,084,424,468 | 45.49% | 86.05 | 24,785,693 (50.35%) | 23,864,342 (48.48%) |
| Gene Name | Primer Sequence | Amplicon (bp) | GenBank/miRBase Accession Number |
|---|---|---|---|
| PPIA | F: CACAAACGGTTCCCAGTTTT | 171 | NM_214353 |
| R: TGTCCACAGTCAGCAATGGT | |||
| ACADS | F: CCAGGGCATCCAGTTCAAGT | 102 | NM_213898 |
| R: TTGCCGGCTCCTTGATGAAT | |||
| CDKN1B | F: TGGAGGGCAAATACGAGTGG | 150 | NM_214316 |
| R: CAATTAAAGGCACCGCCTGG | |||
| ORAI1 | F: TGCATCTGTTTGCGCTGATG | 168 | NM_001173519 |
| R: CCAGGAAGAGCAGTGTACCG | |||
| PRKD2 | F: GGAAAACGTGTTGTTGGCGT | 157 | XM_021094608 |
| R: GTTGTAGCCCTGGTTGAGCA | |||
| PTPN1 | F: TACACCGTCCGACAGCTAGA | 149 | DQ239903 |
| R: CCCGACTCACGGACTTTGAA | |||
| SC5D | F: CGGCTGGTTTCGACTCCTT | 175 | AY609684.1 |
| R: AGCCATCCAGAGGGTGAAAAG | |||
| U6 | F: TTATGGGTCCTAGCCTGAC | - | EU520423 |
| R: CACTATTGCGGGTCTGC | |||
| ssc-miR-212 | ACCTTGGCTCTAGACTGCTTACT | - | MI0022140 |
| ssc-miR-186 | CAAAGAATTCTCCTTTTGGGCTT | - | MI0002456 |
| ssc-miR-128 | TCACAGTGAACCGGTCTCTTT | - | MIMAT0002157 |
| ssc-miR-126 | TCGTACCGTGAGTAATAATGCG | - | MI0016619 |
| unconservative-X-272462 | TGAACGGTGCCTGTGTGGCTAGA | - | / |
| conservative-15-100875 | TCTCTGCTGCGCTCTTTCCTGA | - | / |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, D.-H.; Qazi, I.H.; Ran, M.-X.; Liang, K.; Zhang, Y.; Zhang, M.; Zhou, G.-B.; Angel, C.; Zeng, C.-J. Exploration of miRNA and mRNA Profiles in Fresh and Frozen-Thawed Boar Sperm by Transcriptome and Small RNA Sequencing. Int. J. Mol. Sci. 2019, 20, 802. https://doi.org/10.3390/ijms20040802
Dai D-H, Qazi IH, Ran M-X, Liang K, Zhang Y, Zhang M, Zhou G-B, Angel C, Zeng C-J. Exploration of miRNA and mRNA Profiles in Fresh and Frozen-Thawed Boar Sperm by Transcriptome and Small RNA Sequencing. International Journal of Molecular Sciences. 2019; 20(4):802. https://doi.org/10.3390/ijms20040802
Chicago/Turabian StyleDai, Ding-Hui, Izhar Hyder Qazi, Ming-Xia Ran, Kai Liang, Yan Zhang, Ming Zhang, Guang-Bin Zhou, Christiana Angel, and Chang-Jun Zeng. 2019. "Exploration of miRNA and mRNA Profiles in Fresh and Frozen-Thawed Boar Sperm by Transcriptome and Small RNA Sequencing" International Journal of Molecular Sciences 20, no. 4: 802. https://doi.org/10.3390/ijms20040802
APA StyleDai, D.-H., Qazi, I. H., Ran, M.-X., Liang, K., Zhang, Y., Zhang, M., Zhou, G.-B., Angel, C., & Zeng, C.-J. (2019). Exploration of miRNA and mRNA Profiles in Fresh and Frozen-Thawed Boar Sperm by Transcriptome and Small RNA Sequencing. International Journal of Molecular Sciences, 20(4), 802. https://doi.org/10.3390/ijms20040802








