Different Forms of TFF3 in the Human Saliva: Heterodimerization with IgG Fc Binding Protein (FCGBP)
Abstract
:1. Introduction
2. Results
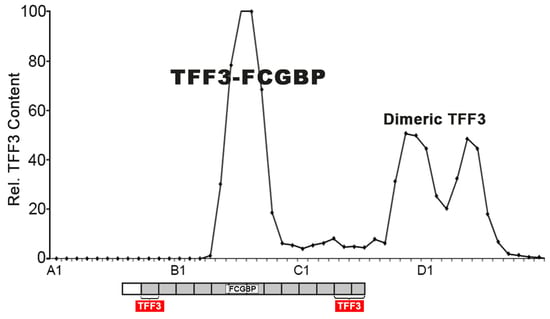
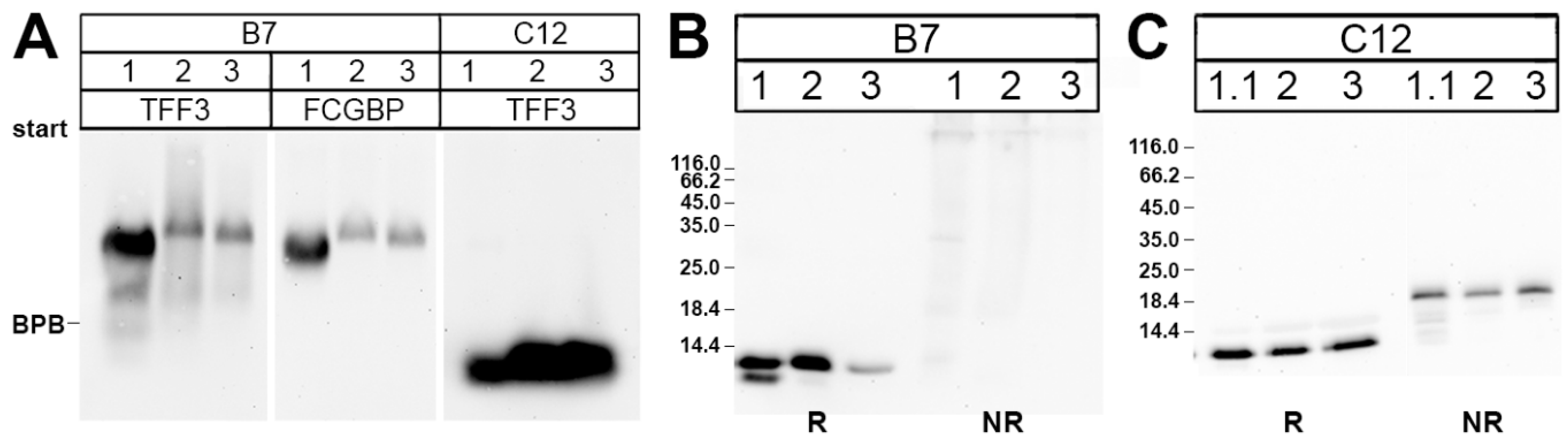
2.1. Characterization of TFF3 in Human Saliva by SEC and Western Blot Analysis
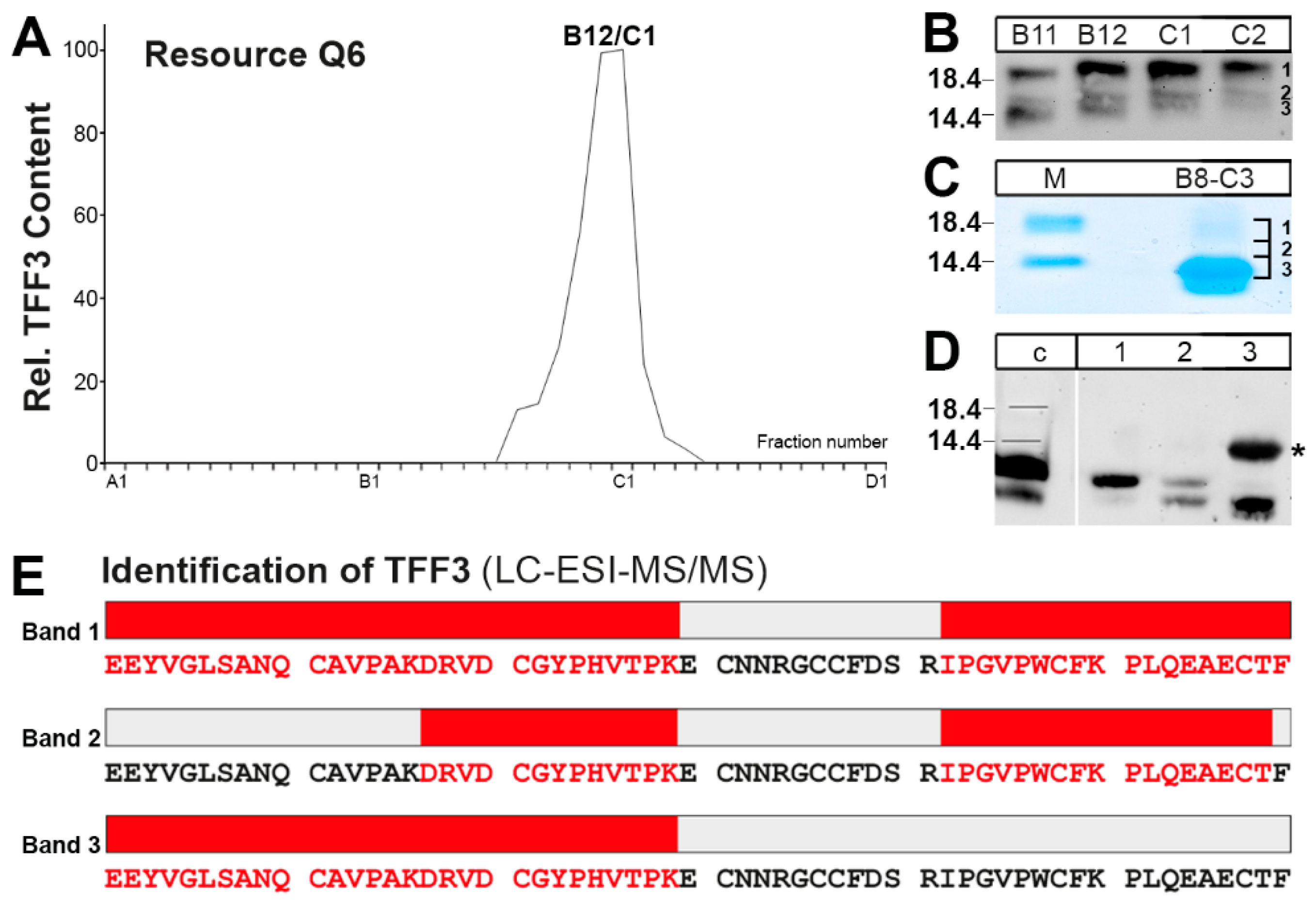
2.2. Purification of the Low-Molecular-Mass Form of TFF3 and Characterization by Mass Spectrometric Proteomics
3. Discussion
3.1. Salivary TFF3 Forms High-Molecular-Mass Heterodimers with FCGBP
3.2. TFF3 Homodimers and Degradation in Human Saliva
4. Materials and Methods
4.1. Human Saliva
4.2. Protein Purification by FPLC
4.3. SDS-PAGE, Agarose Gel Electrophoresis, and Western Blot Analysis
4.4. Mass Spectrometric Proteomics of in-Gel Digested Proteins, Database Searching
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AgGE | Agarose gel electrophoresis |
| DMBT | Deleted in Malignant Brain Tumor |
| FCGBP | IgG Fc binding protein |
| FPLC | Fast protein liquid chromatography |
| LC-ESI-MS/MS | Liquid chromatography-electrospray ionization-tandem mass spectrometry |
| PAS | Periodic acid-Schiff |
| SDS-PAGE | Sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| SEC | Size exclusion chromatography |
| TFF | Trefoil factor family |
References
- Wang, S.S.; Tang, Y.L.; Pang, X.; Zheng, M.; Tang, Y.J.; Liang, X.H. The maintenance of an oral epithelial barrier. Life Sci. 2019, 227, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, S.P.; Williamson, R.T. A review of saliva: Normal composition, flow, and function. J. Prosthet. Dent. 2001, 85, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Nieuw Amerongen, A.V.; Veerman, E.C.I. Saliva—The defender of the oral cavity. Oral Dis. 2002, 8, 12–22. [Google Scholar] [CrossRef]
- de Almeida, P.D.V.; Gregio, A.M.T.; Machado, M.A.N.; de Lima, A.A.S.; Azevedo, L.R. Saliva composition and functions: A comprehensive review. J. Contemp. Dent. Pract. 2008, 9, 72–80. [Google Scholar]
- Lynge Pedersen, A.M.; Belstrøm, D. The role of natural salivary defences in maintaining a healthy oral microbiota. J. Dent. 2019, 80, S3–S12. [Google Scholar] [CrossRef] [PubMed]
- Nieuw Amerongen, A.V.; Bolscher, J.G.; Veerman, E.C.I. Salivary proteins: Protective and diagnostic value in cariology? Caries Res. 2004, 38, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Bräuer, L.; Möschter, S.; Beileke, S.; Jäger, K.; Garreis, F.; Paulsen, F.P. Human parotid and submandibular glands express and secrete surfactant proteins A., B., C and D. Histochem. Cell Biol. 2009, 132, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Amado, F.M.L.; Ferreira, R.P.; Vitorino, R. One decade of salivary proteomics: Current approaches and outstanding challenges. Clin. Biochem. 2013, 46, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, W.; Jagla, W. Cell type specific expression of secretory TFF peptides: Colocalization with mucins and synthesis in the brain. Int. Rev. Cytol. 2002, 213, 147–188. [Google Scholar] [PubMed]
- Braga Emidio, N.; Hoffmann, W.; Brierley, S.M.; Muttenthaler, M. Trefoil factor family: Unresolved questions and clinical perspectives. Trends Biochem. Sci. 2019, 44, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Jagla, W.; Wiede, A.; Hinz, M.; Dietzmann, K.; Gülicher, D.; Gerlach, K.L.; Hoffmann, W. Secretion of TFF-Peptides by human salivary glands. Cell Tissue Res. 1999, 298, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Devine, D.A.; High, A.S.; Owen, P.J.; Poulsom, R.; Bonass, W.A. Trefoil factor expression in normal and diseased human salivary glands. Hum. Pathol. 2000, 31, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Storesund, T.; Schreurs, O.; Messelt, E.B.; Kolltveit, K.M.; Schenck, K. Trefoil factor family 3 expression in the oral cavity. Eur. J. Oral Sci. 2009, 117, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Kouznetsova, I.; Gerlach, K.L.; Zahl, C.; Hoffmann, W. Expression analysis of human salivary glands by laser microdissection: Differences between submandibular and labial glands. Cell. Physiol. Biochem. 2010, 26, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Kutta, H.; May, J.; Jaehne, M.; Münscher, A.; Paulsen, F.P. Antimicrobial defence mechanisms of the human parotid duct. J. Anat. 2006, 208, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Chaiyarit, P.; Utrawichian, A.; Leelayuwat, C.; Vatanasapt, P.; Chanchareonsook, N.; Samson, M.H.; Giraud, A.S. Investigation of trefoil factor expression in saliva and oral mucosal tissues of patients with oral squamous cell carcinoma. Clin. Oral Investig. 2012, 16, 1549–1556. [Google Scholar] [CrossRef]
- Chaiyarit, P.; Chayasadom, A.; Wara-Aswapati, N.; Hormdee, D.; Sittisomwong, S.; Nakaresisoon, S.; Samson, M.H.; Pitiphat, W.; Giraud, A.S. Trefoil factors in saliva and gingival tissues of patients with chronic periodontitis. J. Periodontol. 2012, 83, 1129–1138. [Google Scholar] [CrossRef]
- Chaiyarit, P.; Klanrit, P.; Phothipakdee, P.; Subarnbhesaj, A.; Thongprasom, K.; Giraud, A.S. Trefoil factor expression by immunohistochemistry in patients with oral lichen planus. Asian Biomed. 2014, 8, 743–749. [Google Scholar] [CrossRef]
- Siber-Hoogeboom, R.; Schicht, M.; Hoogeboom, S.; Paulsen, F.P.; Traxdorf, M. Obstructive sleep apnea and rhonchopathy are associated with downregulation of trefoil factor family peptide 3 (TFF3)–Implications of changes in oral mucus composition. PLoS ONE 2017, 12, e0185200. [Google Scholar] [CrossRef]
- Hauser, F.; Poulsom, R.; Chinery, R.; Rogers, L.A.; Hanby, A.M.; Wright, N.A.; Hoffmann, W. hP1.B, a human P-Domain peptide homologous with rat intestinal trefoil factor, is expressed also in the Ulcer-Associated cell lineage and the uterus. Proc. Natl. Acad. Sci. USA 1993, 90, 6961–6965. [Google Scholar] [CrossRef]
- Taupin, D.; Podolsky, D.K. Trefoil factors: Initiators of mucosal healing. Nat. Rev. Mol. Cell. Biol. 2003, 4, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, W. TFF Peptides. In Handbook of Biologically Active Peptides, 2nd ed.; Kastin, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1338–1345. [Google Scholar]
- Albert, T.K.; Laubinger, W.; Müller, S.; Hanisch, F.G.; Kalinski, T.; Meyer, F.; Hoffmann, W. Human intestinal TFF3 forms disulfide-linked heteromers with the Mucus-Associated FCGBP protein and is released by hydrogen sulfide. J. Proteome Res. 2010, 9, 3108–3117. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.; Smitha, C.N.; Suresh, D.K. Trefoils: An unexplored natural protective shield of oral cavity. J. Oral Biol. Craniofacial Res. 2015, 5, 226–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chwieralski, C.E.; Schnurra, I.; Thim, L.; Hoffmann, W. Epidermal growth factor and trefoil factor family 2 synergistically trigger chemotaxis on BEAS-2B cells via different signaling cascades. Am. J. Respir. Cell. Mol. Biol. 2004, 31, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, W. Trefoil factors TFF (trefoil factor family) peptide-triggered signals promoting mucosal restitution. Cell. Mol. Life Sci. 2005, 62, 2932–2938. [Google Scholar] [CrossRef] [PubMed]
- Dürer, U.; Hartig, R.; Bang, S.; Thim, L.; Hoffmann, W. TFF3 and EGF induce different migration patterns of intestinal epithelial cells in vitro and trigger increased internalization of E-Cadherin. Cell. Physiol. Biochem. 2007, 20, 329–346. [Google Scholar] [CrossRef] [PubMed]
- Storesund, T.; Hayashi, K.; Kolltveit, K.M.; Bryne, M.; Schenck, K. Salivary trefoil factor 3 enhances migration of oral keratinocytes. Eur. J. Oral Sci. 2008, 116, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Storesund, T.; Schenck, K.; Osmundsen, H.; Røed, A.; Helgeland, K.; Kolltveit, K.M. Signal transduction and gene transcription induced by TFF3 in oral keratinocytes. Eur. J. Oral Sci. 2009, 117, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, M.; Sato, M.; Ishikawa, M.; Maeno, M.; Otsuka, K. Physiologic levels of epidermal growth factor in saliva stimulate cell migration of an oral epithelial cell line, HO-1-N-1. Eur. J. Oral Sci. 2002, 110, 130–136. [Google Scholar] [CrossRef]
- Dieckow, J.; Brandt, W.; Hattermann, K.; Schob, S.; Schulze, U.; Mentlein, R.; Ackermann, P.; Sel, S.; Paulsen, F.P. CXCR4 and CXCR7 mediate TFF3-Induced cell migration independently from the ERK1/2 signaling pathway. Investig. Ophthalmol. Sci. 2016, 57, 56–65. [Google Scholar] [CrossRef]
- Reeves, E.P.; Ali, T.; Leonard, P.; Hearty, S.; O’Kennedy, R.; May, F.E.B.; Westley, B.R.; Josenhans, C.; Rust, M.; Suerbaum, S.; et al. Helicobacter pylori lipopolysaccharide interacts with TFF1 in a pH-dependent manner. Gastroenterology 2008, 135, 2043–2054. [Google Scholar] [CrossRef] [PubMed]
- Dolan, B.; Naughton, J.; Tegtmeyer, N.; May, F.E.B.; Clyne, M. The interaction of Helicobacter pylori with the adherent mucus gel layer secreted by polarized HT29-MTX-E12 cells. PLoS ONE 2012, 7, e47300. [Google Scholar] [CrossRef] [PubMed]
- Beck, P.L.; Wong, J.F.; Li, Y.; Swaminathan, S.; Xavier, R.J.; Devaney, K.L.; Podolsky, D.K. Chemotherapy- and radiotherapy-induced intestinal damage is regulated by intestinal trefoil factor. Gastroenterology 2004, 126, 796–808. [Google Scholar] [CrossRef]
- Caluwaerts, S.; Vandenbroucke, K.; Steidler, L.; Neirynck, S.; Vanhoenacker, P.; Corveleyn, S.; Watkins, B.; Sonis, S.; Coulie, B.; Rottiers, P. AG013, a mouth rinse formulation of Lactococcus lactis secreting human Trefoil Factor 1, provides a safe and efficacious therapeutic tool for treating oral mucositis. Oral Oncol. 2010, 46, 564–570. [Google Scholar] [CrossRef]
- Peterson, D.E.; Barker, N.P.; Akhmadullina, L.I.; Rodionova, I.; Sherman, N.Z.; Davidenko, I.S.; Rakovskaya, G.N.; Gotovkin, E.A.; Shinkarev, S.A.; Kopp, M.V.; et al. Phase II, randomized, Double-Blind, Placebo-Controlled study of recombinant human intestinal trefoil factor oral spray for prevention of oral mucositis in patients with colorectal cancer who are receiving fluorouracil-based chemotherapy. J. Clin. Oncol. 2009, 27, 4333–4338. [Google Scholar] [CrossRef] [PubMed]
- Stürmer, R.; Harder, S.; Schlüter, H.; Hoffmann, W. Commercial porcine gastric mucin preparations, also used as artificial saliva, are a rich source for the lectin TFF2: In vitro binding studies. ChemBioChem 2018, 19, 2598–2608. [Google Scholar] [CrossRef]
- Mashimo, H.; Wu, D.C.; Podolsky, D.K.; Fishman, M.C. Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science 1996, 274, 262–265. [Google Scholar] [CrossRef]
- Johansson, M.E.; Hansson, G.C. Immunological aspects of intestinal mucus and mucins. Nat. Rev. Immunol. 2016, 16, 639–649. [Google Scholar] [CrossRef]
- Petersson, J.; Schreiber, O.; Hansson, G.C.; Gendler, S.J.; Velcich, A.; Lundberg, J.O.; Roos, S.; Holm, L.; Phillipson, M. Importance and regulation of the colonic mucus barrier in a mouse model of colitis. Am. J. Physiol. 2011, 300, G327–G333. [Google Scholar] [CrossRef] [Green Version]
- Johansson, M.E.V.; Gustafsson, J.K.; Sjöberg, K.E.; Petersson, J.; Holm, L.; Sjövall, H.; Hansson, G.C. Bacteria penetrate the inner mucus layer before inflammation in the dextran sulfate colitis model. PLoS ONE 2010, 5, e12238. [Google Scholar] [CrossRef]
- Feng, Z.M.; Fang, D.C.; Chen, W.S.; Wang, R.Q. Rodent IRR-219 (IgGFcγBP) and rTFF3, expressed mainly in the intestinal mucosa, depleted during dextran sulfate sodium-induced colitis. Dig. Dis. Sci. 2007, 52, 2104–2112. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Y.; Stappenbeck, T.S. Mucus, it is not just a static barrier. Sci. Signal. 2014, 7, pe11. [Google Scholar] [CrossRef] [PubMed]
- Denny, P.; Hagen, F.K.; Hardt, M.; Liao, L.; Yan, W.; Arellanno, M.; Bassilian, S.; Bedi, G.S.; Boontheung, P.; Cociorva, D.; et al. The proteomes of human parotid and submandibular/sublingual gland salivas collected as the ductal secretions. J. Proteome Res. 2008, 7, 1994–2006. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Ogata, H.; Morikawa, M.; Iijima, S.; Harada, N.; Yoshida, T.; Brown, W.R.; Inoue, N.; Hamada, Y.; Ishii, H.; et al. Distribution and partial characterisation of IgG Fc binding protein in various mucin producing cells and body fluids. Gut 2002, 51, 169–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansson, M.E.V.; Thomsson, K.A.; Hansson, G.C. Proteomic analyses of the two mucus layers of the colon barrier reveal that their main component, the Muc2 mucin, is strongly bound to the Fcgbp protein. J. Proteome Res. 2009, 8, 3549–3557. [Google Scholar] [CrossRef]
- Schwartz, J.L. Fcgbp—A potential viral trap in RV144. Open AIDS J. 2014, 8, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, R.; Su, B.; Luo, Y.; Terhune, J.; Beck, B.; Peatman, E. Evasion of mucosal defenses during Aeromonas hydrophila infection of channel catfish (Ictalurus punctatus) skin. Dev. Comp. Immunol. 2013, 39, 447–455. [Google Scholar] [CrossRef]
- Wesener, D.A.; Dugan, A.; Kiessling, L.L. Recognition of microbial glycans by soluble human lectins. Curr. Opin. Struct. Biol. 2017, 44, 168–178. [Google Scholar] [CrossRef]
- Madsen, J.; Sorensen, G.L.; Nielsen, O.; Tornøe, I.; Thim, L.; Fenger, C.; Mollenhauer, J.; Holmskov, U. A variant form of the human deleted in malignant brain tumor 1 (DMBT1) gene shows increased expression in inflammatory bowel diseases and interacts with dimeric trefoil factor 3 (TFF3). PLoS ONE 2013, 8, e64441. [Google Scholar] [CrossRef]
- Neubert, H.; Gale, J.; Muirhead, D. Online High-Flow peptide immunoaffinity enrichment and nanoflow LC-MS/MS: Assay development for total salivary pepsin/pepsinogen. Clin. Chem. 2010, 56, 1413–1423. [Google Scholar] [CrossRef]
- Kinoshita, M.; Kume, E.; Igarashi, S.; Saito, N.; Narita, H. Role of salivary mucin in the protection of rat esophageal mucosa from acid and pepsin-induced injury. Am. J. Physiol. 1999, 277, G796–G800. [Google Scholar] [CrossRef] [PubMed]
- Chinery, R.; Playford, R.J. Combined intestinal trefoil factor and epidermal growth factor is prophylactic against Indomethacin-Induced gastric damage in the rat. Clin. Sci. 1995, 88, 401–403. [Google Scholar] [CrossRef] [PubMed]
- Łysik, D.; Niemirowicz-Laskowska, K.; Bucki, R.; Tokajuk, G.; Mystkowska, J. Artificial saliva: Challenges and future perspectives for the treatment of xerostomia. Int. J. Mol. Sci. 2019, 20, 3199. [Google Scholar] [CrossRef] [PubMed]
- Stürmer, R.; Müller, S.; Hanisch, F.-G.; Hoffmann, W. Porcine gastric TFF2 is a mucus constituent and differs from pancreatic TFF2. Cell. Physiol. Biochem. 2014, 33, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Wiede, A.; Jagla, W.; Welte, T.; Kohnlein, T.; Busk, H.; Hoffmann, W. Localization of TFF3, a new Mucus-Associated peptide of the human respiratory tract. Am. J. Respir. Crit. Care Med. 1999, 159, 1330–1335. [Google Scholar] [CrossRef]
- Kouznetsova, I.; Laubinger, W.; Kalbacher, H.; Kalinski, T.; Meyer, F.; Roessner, A.; Hoffmann, W. Biosynthesis of Gastrokine-2 in the human gastric mucosa: Restricted spatial expression along the antral gland axis and differential interaction with TFF1, TFF1 and mucins. Cell. Physiol. Biochem. 2007, 20, 899–908. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Houben, T.; Harder, S.; Schlüter, H.; Kalbacher, H.; Hoffmann, W. Different Forms of TFF3 in the Human Saliva: Heterodimerization with IgG Fc Binding Protein (FCGBP). Int. J. Mol. Sci. 2019, 20, 5000. https://doi.org/10.3390/ijms20205000
Houben T, Harder S, Schlüter H, Kalbacher H, Hoffmann W. Different Forms of TFF3 in the Human Saliva: Heterodimerization with IgG Fc Binding Protein (FCGBP). International Journal of Molecular Sciences. 2019; 20(20):5000. https://doi.org/10.3390/ijms20205000
Chicago/Turabian StyleHouben, Till, Sönke Harder, Harmut Schlüter, Hubert Kalbacher, and Werner Hoffmann. 2019. "Different Forms of TFF3 in the Human Saliva: Heterodimerization with IgG Fc Binding Protein (FCGBP)" International Journal of Molecular Sciences 20, no. 20: 5000. https://doi.org/10.3390/ijms20205000