Impact of High Salt Diet on Cerebral Vascular Function and Stroke in Tff3−/−/C57BL/6N Knockout and WT (C57BL/6N) Control Mice
Abstract
1. Introduction
2. Results
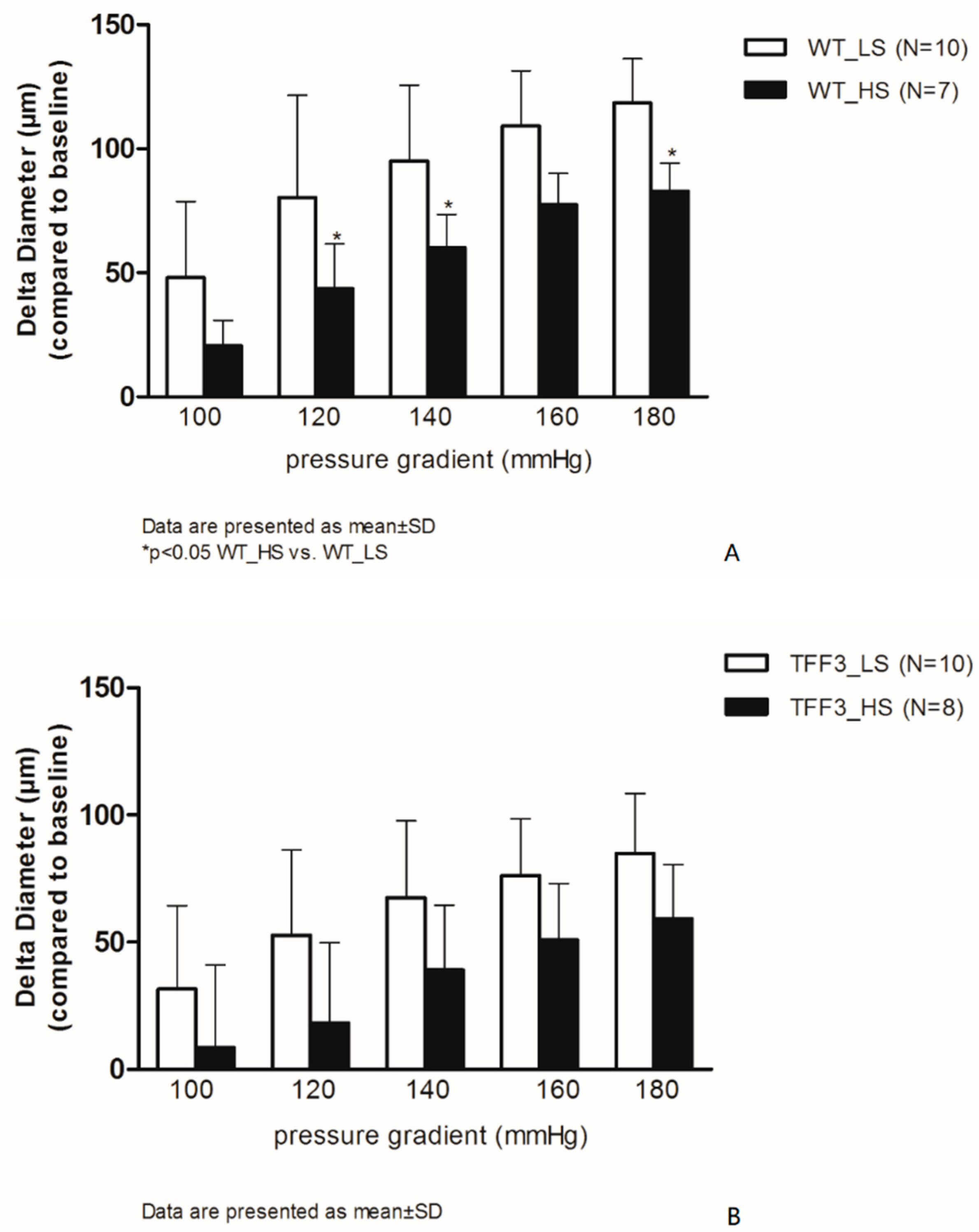
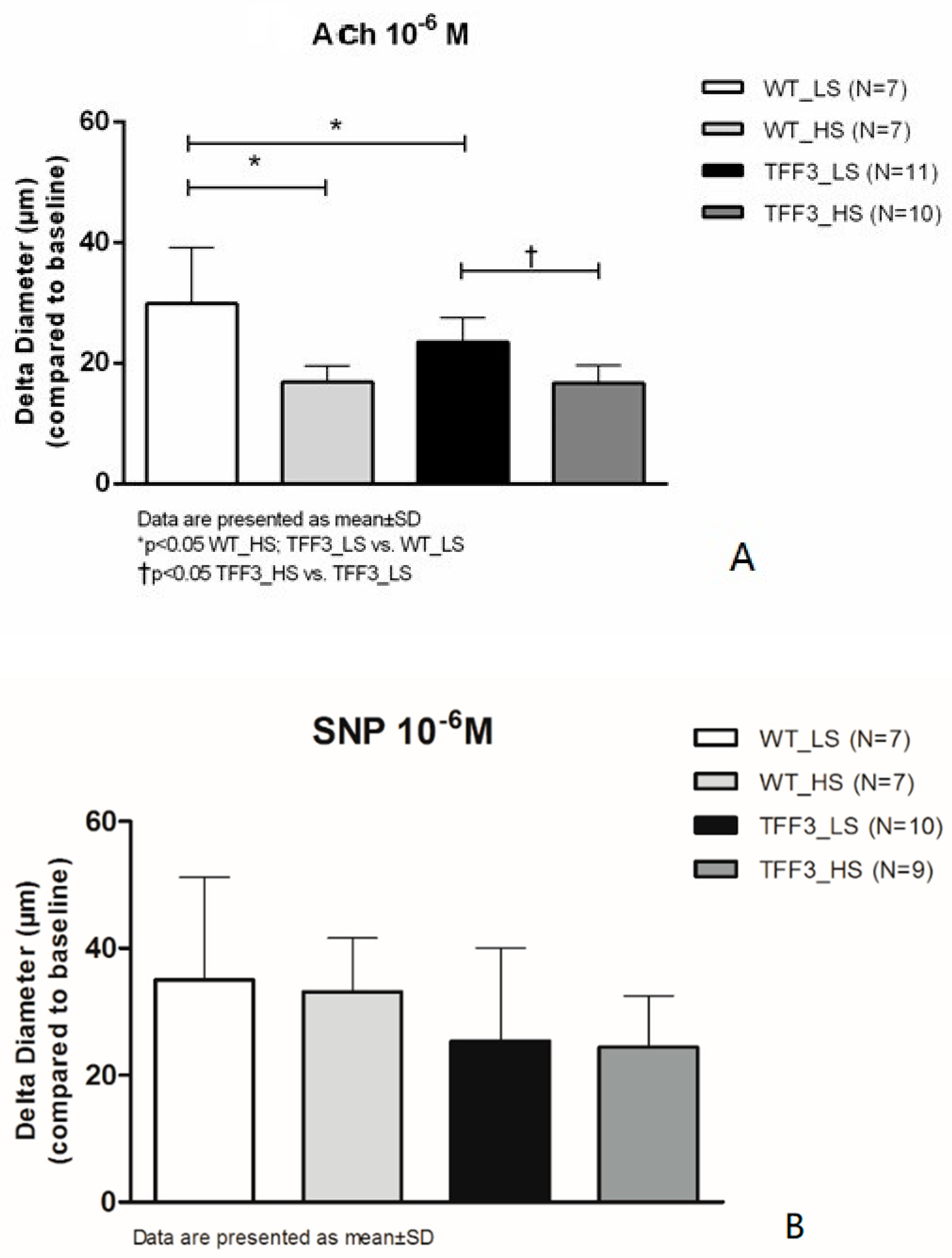
2.1. Flow-Induced Relaxation of Carotid Arteries
2.2. In Situ Evaluation of Nitric Oxide (NO) Levels in Carotid Arteries by Fluorescence Microscopy
2.3. Biochemical Analysis of the Sera
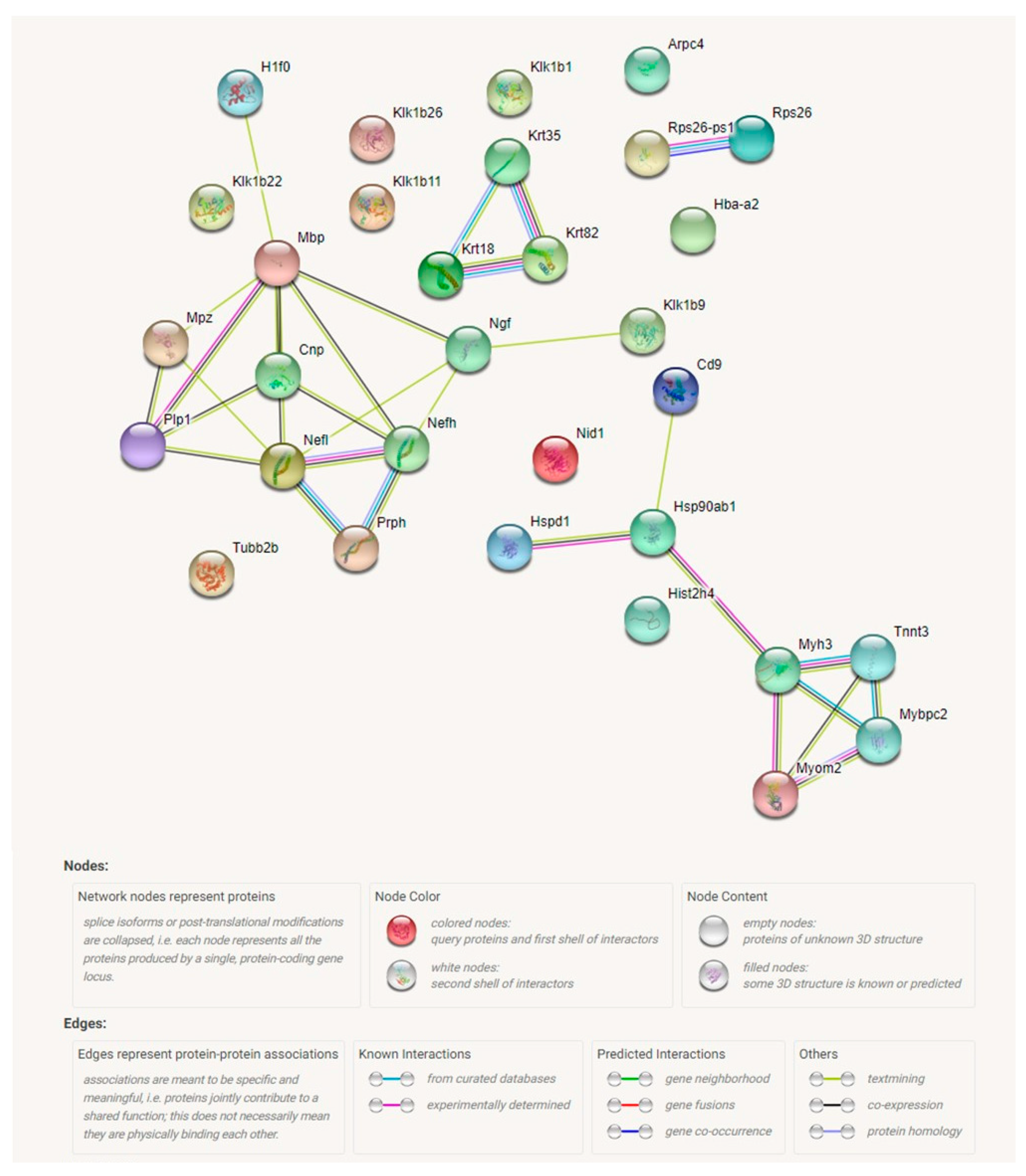
2.4. Proteomic Analysis Revealed a Significant Effect of Tff3 Gene Deficiency on the HS Diet Induced Changes in Protein Expression in Carotid Arteries
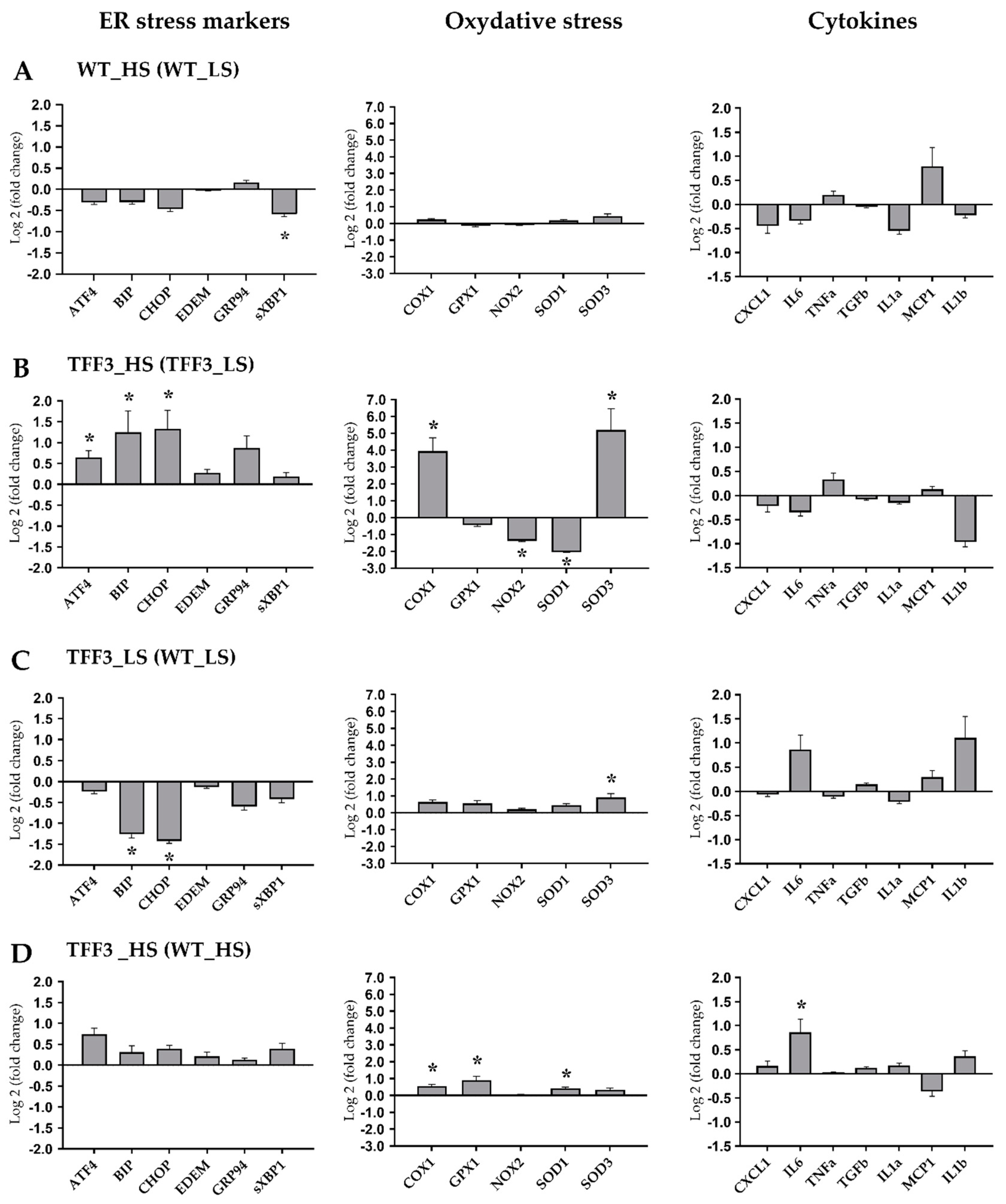
2.5. Expression Levels of Genes Encoding for ER Stress Markers, Oxidative Stress, and Cytokines
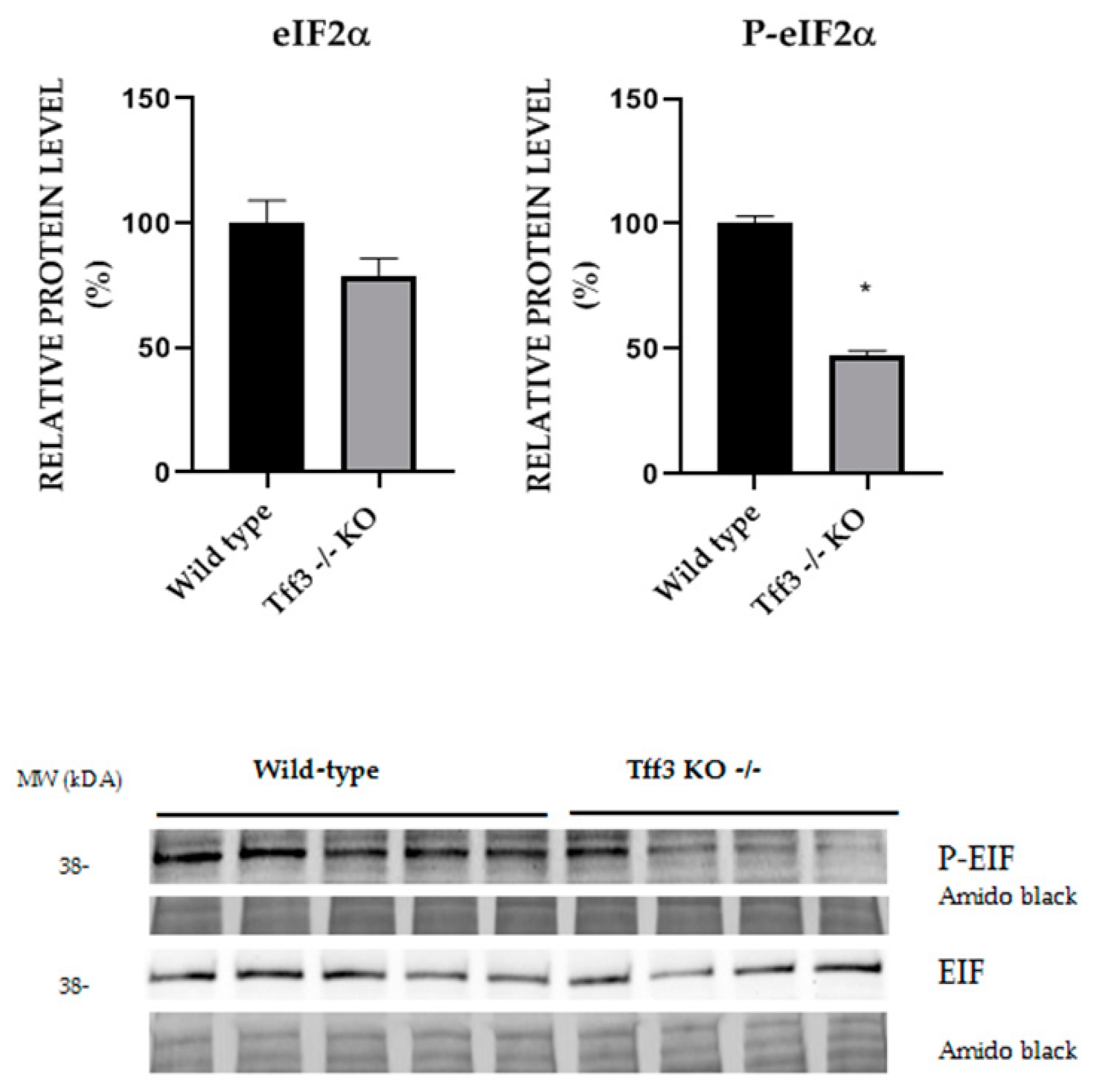
2.6. Activation of ER Stress Relevant eIF2α Protein
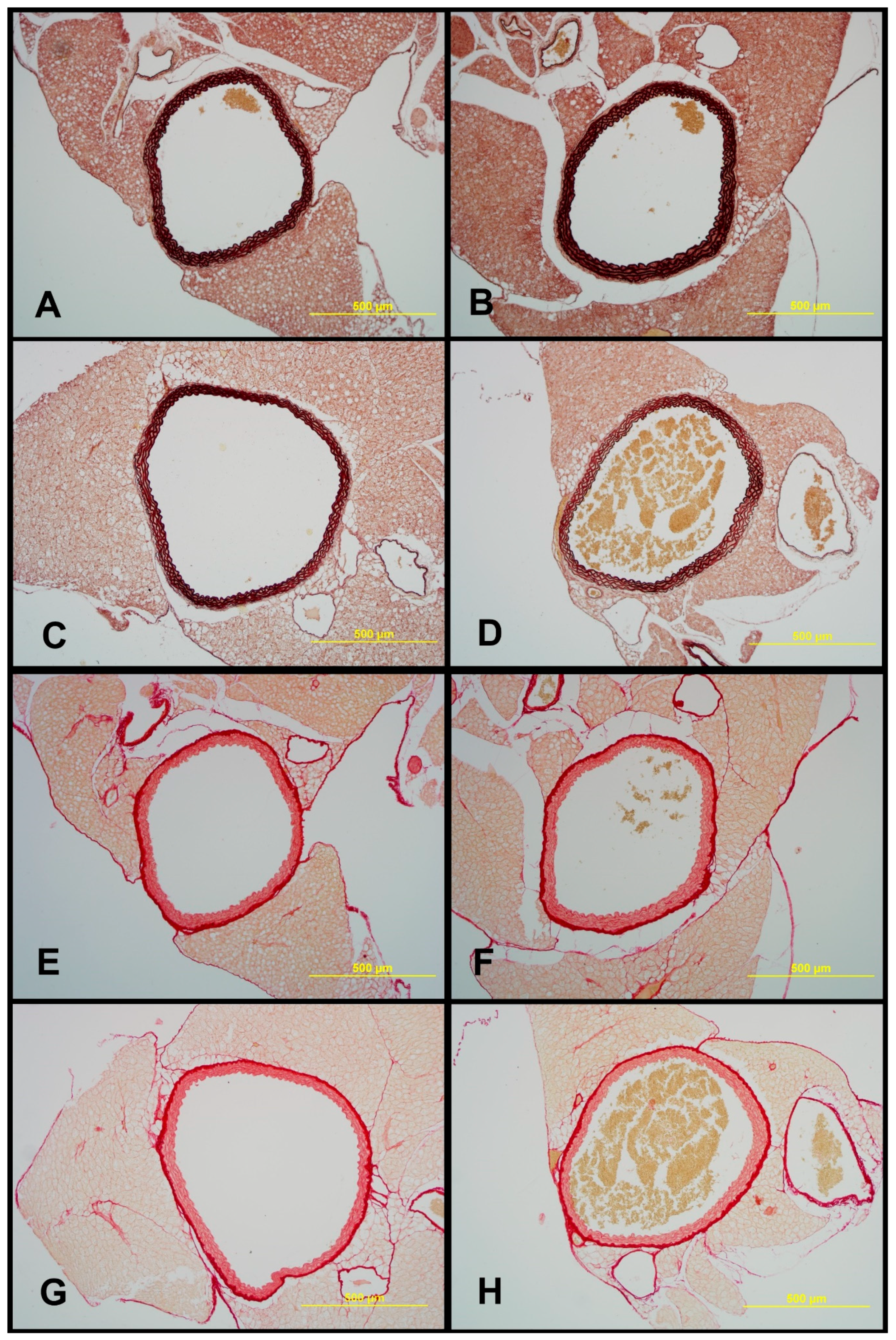
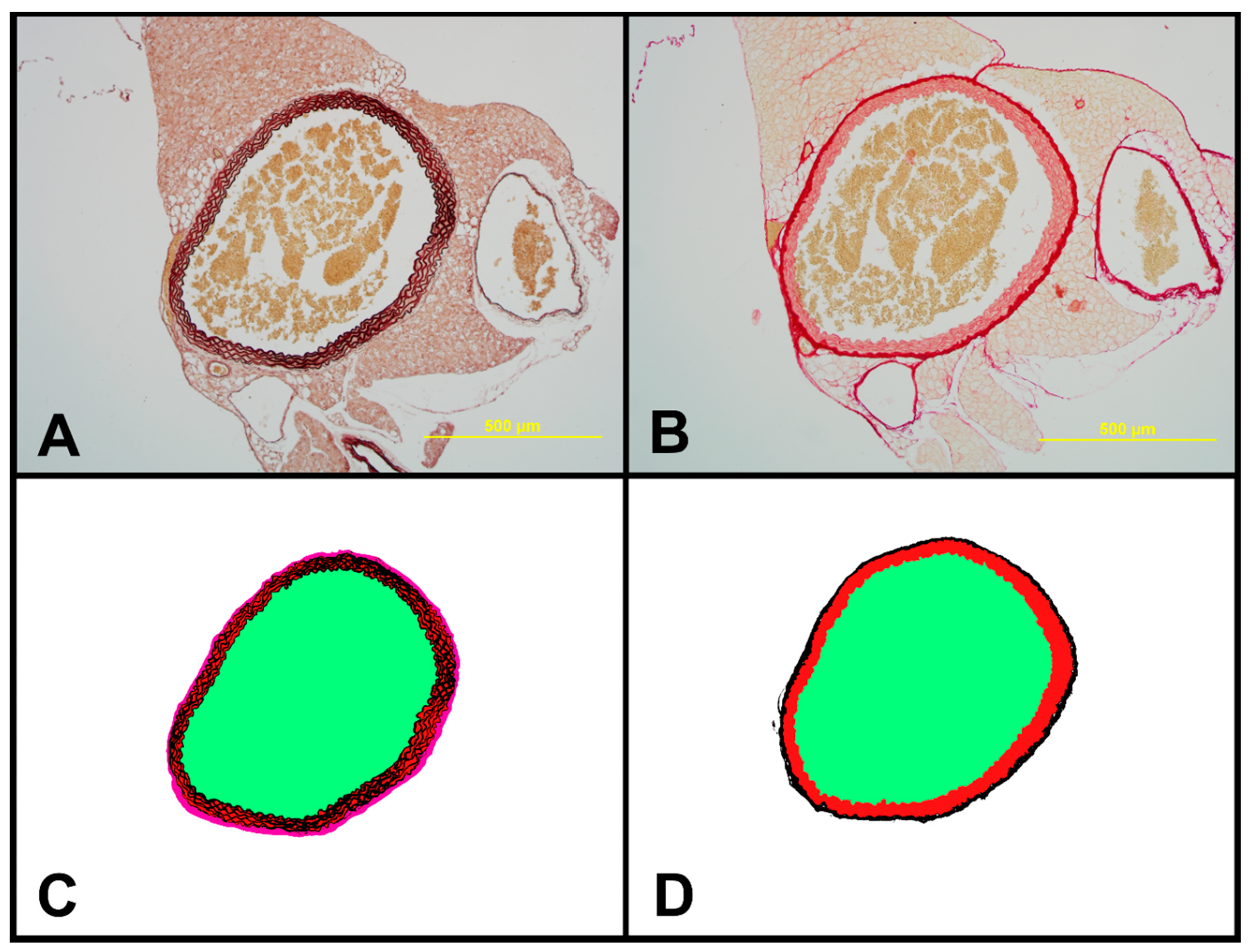
2.7. Structural Characteristics of Aortas of Tff3−/− and WT Mice on LS and HS Diet
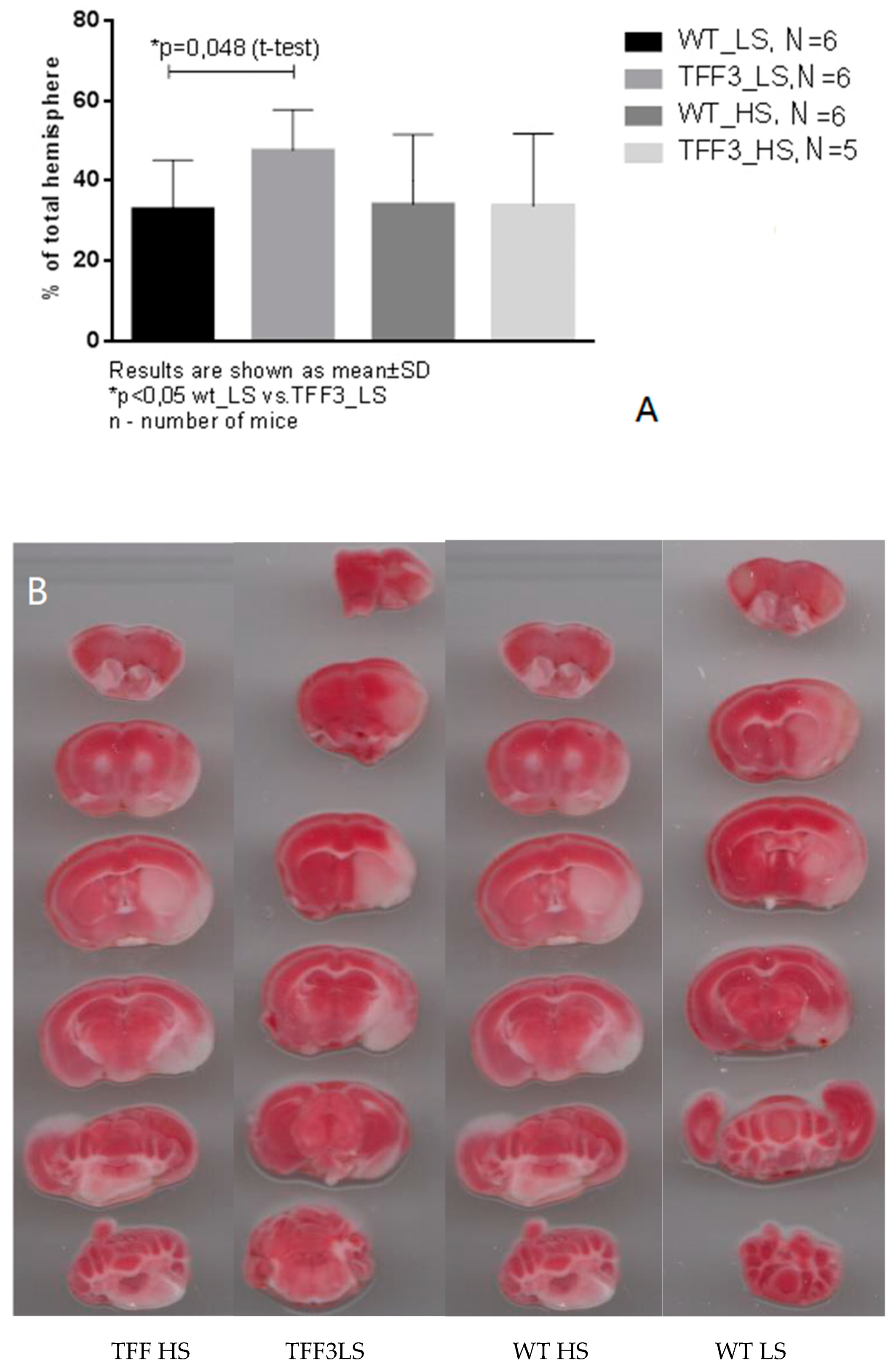
2.8. Effects of Dietary Protocol and Strain Difference on the Brain Infarct Volume in Tff3−/− and WT Mice
3. Discussion
3.1. High Dietary Salt Intake and Vascular Function
3.2. Vascular Relaxation Responses and the Role of NO in Flow-Induced Relaxation of Carotid Arteries
3.3. Biochemical Analysis of the Sera
3.4. Salt Intake and Tff3 Gene Depletion Affect the Structural Components of the Vessel
3.5. The Effects of Diet and Gene Depletion on Systemic Inflammatory Response and Oxidative Stress
3.6. Tff3 Gene Depletion, but not Short-Term High Salt Diet Increases the Stroke Volume; the Role of Proteome Modification
4. Materials and Methods
4.1. Dietary Protocols
4.2. Endothelium-Dependent and Endothelium-Independent Relaxation Responses of Carotid Arteries of TFF3 and WT Mice on LS and HS Diet
Isolated Carotid Artery Protocols
4.3. In Situ Evaluation of Nitric Oxide (NO) Levels in Carotid Arteries by Fluorescence Microscopy
4.4. Biochemical Serum Analysis
4.5. Proteomic Analysis of Carotid Artery Tissue
Protein Extraction and Sample Preparation
4.6. LC-MS/MS Analysis
4.7. Proteomic Data Analysis
4.8. Liver mRNA Expression Studies with Real Time PCR
4.9. Liver Protein Expression Analysis of eIF2α Activation with Western Blot
4.10. Histomorphological Analysis of the Aorta
4.11. Experimental Stroke Model—Transient Occlusion of Middle Cerebral Artery (toMCAO) Experiments
4.12. Neurological Scoring
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Drenjancevic-Peric, I.; Frisbee, J.C.; Lombard, J.H. Skeletal muscle arteriolar reactivity in SS BN13 consomic rats and Dahl salt-sensitive rats. Hypertension 2003, 41, 1012–1015. [Google Scholar] [CrossRef] [PubMed]
- Drenjancevic-Peric, I.; Lombard, J.H. Reduced angiotensin II and oxidative stress contribute to impaired vasodilation in dahl salt-sensitive rats on low-salt diet. Hypertension 2005, 45, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Drenjancevic-Peric, I.; Jelakovic, B.; Lombard, J.H.; Kunert, M.P.; Kibel, A.; Gros, M. High-salt diet and hypertension: Focus on the renin-angiotensin system. Kid Blood Press Res. 2011, 34, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Durand, M.J.; Lombard, J.H. Low-dose angiotensin II infusion restores vascular function in cerebral arteries of high salt-fed rats by increasing copper/zinc superoxide dimutase expression. Am. J. Hypertens. 2013, 26, 739–747. [Google Scholar] [CrossRef]
- Matic, A.; Jukic, I.; Stupin, A.; Baric, L.; Mihaljevic, Z.; Unfirer, S.; Tartaro Bujak, I.; Mihaljevic, B.; Lombard, J.H.; Drenjancevic, I. High salt intake shifts the mechanisms of flow-induced dilation in the middle cerebral arteries of Sprague-Dawley rats. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H718–H730. [Google Scholar] [CrossRef] [PubMed]
- Cavka, A.; Jukic, I.; Ali, M.; Goslawski, M.; Bian, J.T.; Wang, E.; Drenjancevic, I.; Phillips, S.A. Short-term high salt intake reduces brachial artery and microvascular function in the absence of changes in blood pressure. J. Hypertens. 2016, 34, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Cosic, A.; Jukic, I.; Stupin, A.; Mihalj, M.; Mihaljevic, Z.; Novak, S.; Vukovic, R.; Drenjancevic, I. Attenuated flow-induced dilatation of middle cerebral arteries is related to increased vascular oxidative stress in rats on a short-term high salt diet. J. Physiol. 2016, 594, 4917–4931. [Google Scholar] [CrossRef]
- Matoba, T.; Shimokawa, H.; Kubota, H.; Morikawa, K.; Fujiki, T.; Kunihiro, I.; Mukai, Y.; Hirakawa, Y.; Takeshita, A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in human mesenteric arteries. Biochem. Biophys. Res. Commun. 2002, 290, 909–913. [Google Scholar] [CrossRef]
- Larsen, B.T.; Campbell, W.B.; Gutterman, D.D. Beyond vasodilatation: Non-vasomotor roles of epoxyeicosatrienoic acids in the cardiovascular system. Trends Pharmacol. Sci. 2007, 28, 32–38. [Google Scholar] [CrossRef]
- Liu, Y.; Bubolz, A.H.; Mendoza, S.; Zhang, D.X.; Gutterman, D.D. H2O2 is the transferrable factor mediating flow-induced dilation in human coronary arterioles. Circ. Res. 2011, 108, 566–573. [Google Scholar] [CrossRef]
- Allen, C.L.; Bayraktutan, U. Oxidative Stress and Its Role in the Pathogenesis of Ischaemic Stroke. Int. J. Stroke. 2009, 4, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, M.A.; Lo, E.H.; Iadecola, C. The science of stroke: Mechanisms in search of treatments. Neuron 2010, 67, 181–198. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, P.M. The Interrelation between carotid, femoral and coronary artery disease. Eur. Heart J. 2001, 22, 11–14. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vamos, Z.; Cseplo, P.; Ivic, I.; Matics, R.; Hamar, J.; Koller, A. Age Determines the Magnitudes of Angiotensin II-Induced Contractions, mRNA, and Protein Expression of Angiotensin Type 1 Receptors in Rat Carotid Arteries. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Kraft, P.; Göb, E.; Schuhmann, M.K.; Göbel, K.; Deppermann, C.; Thielmann, I. FTY720 ameliorates acute ischemic stroke in mice by reducing thrombo-inflammation but not by direct neuroprotection. Stroke 2013, 44, 3202–3210. [Google Scholar] [CrossRef] [PubMed]
- Göb, E.; Reymann, S.; Langhauser, F. Blocking of plasma kallikrein ameliorates stroke by reducing thromboinflammation. Ann. Neurol. 2015, 77, 784–803. [Google Scholar] [CrossRef] [PubMed]
- Thammisetty, S.S.; Pedragosa, J.; Weng, Y.C.; Calon, F.; Planas, A.; Kriz, J. Age-related deregulation of TDP-43 after stroke enhances NF-κB-mediated inflammation and neuronal damage. J. Neuroinflammation 2018, 15, 312. [Google Scholar] [CrossRef]
- Noll, J.M.; Li, Y.; Distel, T.J.; Ford, G.D.; Ford, B.D. Neuroprotection by Exogenous and Endogenous Neuregulin-1 in Mouse Models of Focal Ischemic Stroke. J. Mol. Neurosci. 2019, 69, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Bujak, M.; Tartaro Bujak, I.; Sobočanec, S.; Mihalj, M.; Novak, S.; Ćosić, A.; Tolušić Levak, M.; Kopačin, V.; Mihaljević, B.; Balog, T.; et al. Trefoil Factor 3 Deficiency Affects Liver Lipid Metabolism. Cell. Physiol. Biochem. 2018, 47, 827–841. [Google Scholar] [CrossRef] [PubMed]
- Kozina, N.; Jukić, I. Trefoil Factor Family (TFF): Peptides with Numerous Functions. SEEMEDJ 2019, 3, 69–77. [Google Scholar] [CrossRef]
- Drenjančević, I.; Jukić, I.; Mihaljević, Z.; Ćosić, A.; Kibel, A. The Metabolites of Arachidonic Acid in Microvascular Function, Microcirculation Revisited, 1st ed.; IntechOpen: Rijeka, Croatia, 2016; pp. 101–133. [Google Scholar] [CrossRef]
- Wang, S.; Kaufman, R.J. The impact of the unfolded protein response on human disease. J. Cell Biol. 2012, 197, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Q.; Tefft, B.J.; Zhang, D.; Roberts, D.; Schuster, D.J.; Wu, A. Cardioprotective mechanisms activated in response to myocardial ischemia. Mol. Cell Biomech. 2011, 8, 319–338. [Google Scholar] [PubMed]
- Zhang, B.H.; Yu, H.G.; Sheng, Z.X.; Luo, H.S.; Yu, J.P. The therapeutic effect of recombinant human trefoil factor 3 on hypoxia-induced necrotizing enterocolitis in immature rat. Regul. Pept. 2003, 116, 53–60. [Google Scholar] [CrossRef]
- Intersalt Cooperative Research Group. Intersalt: An international study of electro-lyte excretion and blood pressure. Results for 24 h urinary sodium and potassium excre-tion. Intersalt Cooperative Research Group. BMJ 1988, 297, 319–328. [Google Scholar] [CrossRef]
- Elliott, P.; Brown, I.J.; Tzoulaki, I.; Candeias, V. Salt intakes around the world: implications for public health. Int. J. Epidemiol. 2009, 38, 791–813. [Google Scholar] [CrossRef]
- Strazzullo, P.; D’Elia, L.; Kandala, N.B.; Cappuccio, F.P. Salt intake, stroke, and cardiovascular disease: Meta-analysis of prospective studies. BMJ 2009, 339, b4567. [Google Scholar] [CrossRef]
- Priestley, J.R.; Kautenburg, K.E.; Casati, M.C.; Endres, B.T.; Geurts, A.M.; Lombard, J.H. The NRF2 knockout rat: A new animal model to study endothelial dysfunction, oxidant stress, and microvascular rarefaction. Am. J. Physiol. Heart Circ. Physiol. 2016, 15, H478–H487. [Google Scholar] [CrossRef]
- Drenjancevic-Peric, I.; Brian, D.; Weinberg, A.; Greene, S.; Lombard, J.H. Restoration of Cerebral Vascular Relaxation in Renin Congenic Rats by Introgression of the Dahl R Renin Gene. Am. J. Hypertens. 2010, 23, 243–248. [Google Scholar] [CrossRef]
- Dhar, D.K.; Wang, T.C.; Tabara, H.; Tonomoto, Y.; Maruyama, R.; Tachibana, M.; Kubota, H.; Nagasue, N. Expression of trefoil factor family members correlates with patient prognosis and neoangiogenesis. Clin. Cancer Res. 2005, 11, 6472–6478. [Google Scholar] [CrossRef]
- Hung, L.Y.; Oniskey, T.K.; Sen, D.; Krummel, M.F.; Vaughan, A.E.; Cohen, N.A.; Herbert, D.R. Trefoil Factor 2 Promotes Type 2 Immunity and Lung Repair through Intrinsic Roles in Hematopoietic and Nonhematopoietic Cells. Am. J. Pathol. 2018, 188, 1161–1170. [Google Scholar] [CrossRef]
- Bijelić, N.; Belovari, T.; Tolušić Levak, M.; Baus Lončar, M.; Bosn, J.B. Localization of trefoil factor family peptide 3 (TFF3) in epithelial tissues originating from the three germ layers of developing mouse embryo. Med. Sci. 2017, 17, 241–247. [Google Scholar] [CrossRef]
- Vallance, P.; Collier, J.; Moncada, S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet 1989, 2, 997–1000. [Google Scholar] [CrossRef]
- Moncada, S.; Higgs, E.A. The discovery of nitric oxide and its role in vascular biology. Br. J. Pharmacol. 2006, 147, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Ignarro, L.J. Nitric oxide as a unique signaling molecule in the vascular system: A historical overview. J. Physiol. Pharmacol. 2002, 53, 503–514. [Google Scholar] [PubMed]
- Tran, Q.K.; Ohashi, K.; Watanabe, H. Calcium signalling in endothelial cells. Cardiovasc. Res. 2000, 48, 13–22. [Google Scholar] [CrossRef]
- Rubanyi, G.M.; Romero, J.C.; Vanhoutte, P.M. Flow-induced release of endothelium-derived relaxing factor. Am. J. Physiol. Heart Circ. Physiol. 1986, 250, H1145–H1149. [Google Scholar] [CrossRef] [PubMed]
- Flammer, A.J.; Lüscher, T.F. Three decades of endothelium research. From the detection of nitric oxide to the everyday implementation of endothelial function measurements in cardiovascular diseases. Swiss Med. Wkly. 2010, 140, w13122. [Google Scholar]
- Crimi, E.; Ignarro, L.J.; Napoli, C. Microcirculation and oxidative stress. Free Radic. Res. 2007, 41, 1364–1375. [Google Scholar] [CrossRef]
- Sandoo, A.; van Zanten, J.J.; Metsios, G.S.; Carroll, D.; Kitas, G.D. The endothelium and its role in regulating vascular tone. Open Cardiovasc. Med. J. 2010, 4, 302–312. [Google Scholar] [CrossRef]
- Rösler, S.; Haase, T.; Claassen, H.; Schulze, U.; Schicht, M.; Riemann, D. Trefoil factor 3 is induced during degenerative and inflammatory joint disease, activates matrix metalloproteinases, and enhances apoptosis of articular cartilage chondrocytes. Arthritis Rheum. 2010, 62, 815–825. [Google Scholar] [CrossRef]
- Bijelić, N.; Perić Kačarević, Ž.; Belovari, T.; Radić, R. Trefoil factor family protein 3 affects cancellous bone formation in the secondary centers of ossification of mouse tibiae. Period. Biol. 2015, 117, 59–64. [Google Scholar]
- Bijelić, N.; Belovari, T.; Stolnik, D.; Lovrić, I.; Baus Lončar, M. Histomorphometric Parameters of the Growth Plate and Trabecular Bone in Wild-Type and Trefoil Factor Family 3 (Tff3)-Deficient Mice Analyzed by Free and Open-Source Image Processing Software. Microsc. Microanal. 2017, 23, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.R.H.; Griffiths, A.B.; Tilby, M.T.; Westley, B.R. TFF3 is a normal breast epithelial protein and is associated with differentiated phenotype in early breast cancer but predisposes to invasion and metastasis in advanced disease. Am. J. Pathol. 2012, 180, 904–916. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.H.; Pandey, V.; Kong, X.; Wang, X.N.; Wu, Z.; Zhu, T. Trefoil Factor-3 (TFF3) Stimulates De Novo Angiogenesis in Mammary Carcinoma both Directly and Indirectly via IL-8/CXCR2. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Balcer-Kubiczek, E.K.; Harrison, G.H.; Xu, J.F.; Gutierrez, P.L. Coordinate late expression of trefoil peptide genes (pS2/TFF1 and ITF/TFF3) in human breast, colon, and gastric tumor cells exposed to X-rays. Mol. Cancer Ther. 2002, 1, 405–415. [Google Scholar] [PubMed]
- Tadžić, R.; Mihalj, M.; Včev, A.; Ennen, J.; Tadžić, A.; Drenjančević, I. The Effects of Arterial Blood Pressure Reduction on Endocan and Soluble Endothelial Cell Adhesion Molecules (CAMs) and CAMs Ligands Expression in Hypertensive Patients on Ca-Channel Blocker Therapy. Kidney Blood Press. Res. 2013, 37, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Gruys, E.; Toussaint, M.J.M.; Niewold, T.A.; Koopmans, S.J.; Zhejiang, J. Acute phase reaction and acute phase proteins. J. Zhejiang Univ. Sci. B 2005, 6, 1045–1056. [Google Scholar] [CrossRef]
- Jakic, B.; Buszko, M.; Cappellano, G.; Wick, G. Elevated sodium leads to the increased expression of HSP60 and induces apoptosis in HUVECs. PLoS ONE 2017, 12, e0179383. [Google Scholar] [CrossRef]
- Allen, L.A.; Schmidt, J.R.; Thompson, C.T.; Carlson, B.E.; Beard, D.A.; Lombard, J.H. High salt diet impairs cerebral blood flow regulation via salt-induced angiotensin II suppression. Microcirculation 2019, 26, e12518. [Google Scholar] [CrossRef]
- James, N.L.; Milijasevic, Z.; Ujhazy, A.; Edwards, G.; Jermyn, K.; Mynard, J.P.; Celermajer, D.S. The common carotid artery provides significant pressure wave dampening in the young adult sheep. Int. J. Cardiol. Heart Vasc. 2019, 23, 100343. [Google Scholar] [CrossRef]
- Fu, X.; Li, X.; Xiong, L.; Li, X.; Huang, R.; Gao, Q. Cerebral Arterial Stiffness as A New Marker of Early Stage Atherosclerosis of The Cerebral Large Artery in Acute Stroke. J. Atheroscler. Thromb. 2019. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Q.; Tefft, B.J.; Roberts, D.T.; Zhang, L.Q.; Ren, Y.; Li, Y.C. Cardioprotective proteins upregulated in the liver in response to experimental myocardial ischemia. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H1446–H1458. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Q.; Roberts, D.; Zhang, B.; Ren, Y.; Zhang, L.Q.; Wu, Y.H. Trefoil factor 3 as an endocrine neuroprotective factor from the liver in experimental cerebral ischemia/reperfusion injury. PLoS ONE 2013, 8, e77732. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Zhang, H.L.; Zhang, X.P.; Jiang, H.L. Arachidonic acid attenuates brain damage in a rat model of ischemia/reperfusion by inhibiting inflammatory response and oxidative stress. Hum. Exp. Toxicol. 2018, 37, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Oslowski, C.M.; Urano, F. Measuring ER Stress and the Unfolded Protein Response Using Mammalian Tissue Culture System. Meth. Enzymol. 2011, 490, 71–92. [Google Scholar]
- Mihaljević, Z.; Matić, A.; Stupin, A.; Barić, L.; Jukić, I.; Drenjančević, I. Acute hyperbaric oxygenation, contrary to intermittent hyperbaric oxygenation, adversely affects vasorelaxation in healthy Sprague- Dawley rats due to increased oxidative stress. Oxid. Med. Cell. Longev. 2018, 2018, 7406027. [Google Scholar] [CrossRef]
- Collins, T.J. ImageJ for microscopy. Biotechniques. 2007, 43, 25–30. [Google Scholar] [CrossRef]
- Girish, V.; Vijayalakshmi, A. Affordable image analysis using NIH Image/ImageJ. Indian J. Cancer. 2004, 41, 47. [Google Scholar]
- Horvatić, A.; Guillemin, N.; Kaab, H.; McKeegan, D.; O’Reilly, E.; Bain, M.; Kuleš, J.; Eckersall, P.D. Quantitative proteomics using tandem mass tags in relation to the acute phase protein response in chicken challenged with Escherichia coli lipopolysaccharide endotoxin. J. Proteomics 2019, 192, 64–77. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Rueden, C.T.; Hiner, M.C.; Eliceiri, K.W. The ImageJ ecosystem: An open platform for biomedical image analysis. Mol. Reprod. Dev. 2015, 82, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Adamczak, J.M.; Schneider, G.; Nelles, M.; Que, I.; Suidgeest, E.; van der Weerd, L.; Löwik, C.; Hoehn, M. In vivo bioluminescence imaging of vascular remodeling after stroke. Front. Cell. Neurosci. 2014, 8, 274. [Google Scholar] [CrossRef] [PubMed]
- Bahmani, P.; Schellenberger, E.; Klohs, J.; Steinbrink, J.; Cordell, R.; Zille, M.; Müller, J.; Harhausen, D.; Hofstra, L.; Reutelingsperger, C.; et al. Visualization of cell death in mice with focal cerebral ischemia using fluorescent annexin A5, propidium iodide, and TUNEL staining. J. Cereb. Blood Flow Metab. 2011, 31, 1311–1320. [Google Scholar] [CrossRef]
- Longa, E.Z.; Weinstein, P.R.; Carlson, S.; Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989, 20, 84–91. [Google Scholar] [CrossRef]


| Group | ANOVA | |||||
|---|---|---|---|---|---|---|
| Parameter | Unit | WT LS | Tff3−/− LS | WT HS | Tff3−/− HS | p = |
| Uric acid | µmol/L | 189.8 ± 74.2 | 217.6 ± 54.2 | 164.1 ± 19.4 | 201.6 ± 25.9 | 0.157 |
| Blood glucose level | mmol/L | 6.23 ± 3.64 | 5.96 ± 2.97 | 6.99 ± 1.85 | 6.43 ± 3.04 | 0.954 |
| AST | U/L | 353.6 ± 216.4 | 487.3 ± 90.74 | 389.5 ± 214.6 | 413.3 ± 230.3 | 0.630 |
| ALT | U/L | 42.6 ± 21.1 | 48.7 ± 4.93 | 39.8 ± 11.1 | 39.8 ± 14.9 | 0.835 |
| CRP | mg/L | 0.14 ± 0.02 | 0.15 ± 0.03 | 0.12 ± 0.01 | 0.12 ± 0.01 | 0.054 |
| Urea | mmol/L | 5.18 ± 0.73 | 3.63 ± 0.87 | 4.62 ± 0.73 | 5.10 ± 1.57 | 0.233 |
| Total cholesterol | mmol/L | 4.03 ± 0.52 | 3.97 ± 0.47 | 3.46 ± 0.69 | 3.65 ± 0.45 | 0.253 |
| LDL | mmol/L | 1.66 ± 0.40 | 1.57 ± 0.55 | 1.32 ± 0.44 | 1.33 ± 0.21 | 0.423 |
| HDL | mmol/L | 1.92 ± 0.23 | 2.05 ± 0.10 | 1.64 ± 0.35 | 1.83 ± 0.38 | 0.295 |
| Triglycerides | mmol/L | 0.98 ± 0.21 | 0.77 ± 0.07 | 1.11 ± 0.58 | 1.07 ± 0.22 | 0.261 |
| Alkaline phosphatase | U/L | 26.8 ± 2.68 | 25.3 ± 5.69 | 34.8 ± 12.9 | 27.7 ± 4.18 | 0.263 |
| Total proteins | g/L | 55.0 ± 2.83 | 54.0 ± 3.46 | 52.3 ± 3.44 | 50.2 ± 6.43 | 0.352 |
| Corresponding Gene Symbol | Abudance Ratio | p Value |
|---|---|---|
| Hspd1 | 9.625 | 1.08 × 10−15 |
| Nefl | 4.248 | 1.08 × 10−15 |
| Ngf | 3.339 | 1.08 × 10−15 |
| Krt18 | 3.299 | 1.08 × 10−15 |
| Tubb2b | 3.293 | 1.08 × 10−15 |
| Nid1 | 3.207 | 1.08 × 10−15 |
| Mpz | 2.962 | 1.08 × 10−15 |
| Krt18 | 2.727 | 3.59 × 10−13 |
| Mpz | 2.688 | 8.81 × 10−13 |
| Klk1b26 | 2.683 | 9.04 × 10−13 |
| Mbp | 2.432 | 2.27 × 10−10 |
| Plp1 | 2.389 | 5.80 × 10−10 |
| Nefh | 2.270 | 7.15 × 10−09 |
| Mbp | 2.193 | 3.89 × 10−08 |
| Klk1b1 | 2.187 | 4.35 × 10−08 |
| Cnp | 2.138 | 1.23 × 10−07 |
| Nefh | 1.982 | 3.64 × 10−06 |
| Hsp90ab1 | 1.892 | 2.31 × 10−05 |
| Prph | 1.86 | 4.48 × 10−05 |
| Mbp | 1.849 | 5.42 × 10−05 |
| Arpc4 | 1.848 | 5.42 × 10−05 |
| Arpc4 | 1.848 | 5.42 × 10−05 |
| Tnnt3 | 1.824 | 6.44 × 10−05 |
| Myom2 | 1.756 | 2.58 × 10−04 |
| Klk1b11 | 1.742 | 3.41 × 10−04 |
| Klk1b22 | 1.741 | 3.48 × 10−04 |
| Prph | 1.734 | 4.01 × 10−04 |
| Cd9 | 1.682 | 0.0011 |
| Klk1b9 | 1.677 | 0.0012 |
| Hist2h4 | 1.661 | 0.0016 |
| H1f0 | 1.655 | 0.0017 |
| Prph | 1.606 | 0.044 |
| Mybpc2 | 1.533 | 0.017 |
| Myh3 | 1.501 | 0.029 |
| Rps26 | 1.479 | 0.042 |
| Rps26; Rps26-ps1 | 1.479 | 0.042 |
| Gm6654 | 1.479 | 0.042 |
| Nid1 | 1.478 | 0.042 |
| Hba-a2 | 0.623 | 0.0035 |
| Krt82 | 0.336 | 1.08 × 10−15 |
| Krt35 | 0.221 | 1.08 × 10−15 |
| Lumen Area [μm2] | Elastin [%] | Media [%] | ADV [%] | Wall Surface [μm2] | Collagen [%] | Wall to Lumen Ratio [%] | |
|---|---|---|---|---|---|---|---|
| WT LS | 714,834.57 ± 128,116.62 | 52.908 ± 4.44 | 70.669 ± 3,71 | 29.331 ± 3.71 | 379,241.52 ± 51,995.64 | 32.23 ± 4.32 | 54.00 ± 9.19 |
| WT HS | 772,048.33 ± 100,916.49 | 59.53 ± 4.69 * | 76.83 ± 4.77 * | 23.165 ± 4.77 | 374,726.27 ± 54,361.98 | 28.51 ± 4.59 | 48.8 ± 6.58 |
| Tff3−/−LS | 912,555.53 ± 256,729.08 | 60.74 ± 6.65 * | 76.49 ± 3.90* | 23.50 ± 3.90 * | 345,104.12 ± 39,500.88 | 30.01 ± 4.66 | 39.9 ± 10.03 * |
| Tff3−/−HS | 739,867.67 ± 182,518.08 | 57.06 ± 4.56 | 77.48 ± 1.79 * | 22.51 ± 1.79 * | 362,517.18 ± 39,568.26 | 27.24 ± 2.45 * | 53.5 ± 22.37 |
| Gene Symbol | Accession No. | Primer sequence Forward (5′-3′) Reverse (5′-3′) | Optimized PCR Condition (Annealing Temp/MgCl2) |
|---|---|---|---|
| ER stress markers | |||
| ATF4 | NM_009716.3 | CCACTCCAGAGCATTCCTTTAG CTCCTTTACACATGGAGGGATTAG | 59 °C; 3,5 mM |
| BIP | NM_001163434.1 | GAGACTGCTGAGGCGTATTT CAGCATCTTTGGTTGCTTGTC | 58 °C; 3,5 mM |
| CHOP | NM_007837.4 | TTGAGCCTAACACGTCGATTAT CACTTCCTTCTGGAACACTCTC | 58 °C; 3 mM |
| EDEM | NM_138677.2 | TGAAAGCATGTGAGGGTAGTG GAGAGAAGGGAAGACAGGATAGA | 61 °C; 3,5 mM |
| GRP94 | NM_011631.1 | AAGAATGAAGGAAAAACAGGACAAAA CAAATGGAGAAGATTCCGCC | 58 °C; 3 mM |
| sXBP1 | NM_008934.4 | GAGTCCGCAGCAGGTG GTGTCAGAGTCCATGGGA | 56 °C; 3 mM |
| Cytokines | |||
| CXCL1 | NM_008176.3 | GTGTCAACCACTGTGCTAGT CACACATGTCCTCACCCTAATAC | 61 °C; 3,5 mM |
| IL1α | NM_010554.4 | CCTTACACCTACCAGAGTGATTT CCTTACACCTACCAGAGTGATTT | 65 °C; 3 mM |
| IL1β | NM_008361.4 | ATGGGCAACCACTTACCTATTT GTTCTAGAGAGTGCTGCCTAATG | 64 °C; 3 mM |
| IL6 | NM_031168.2 | GATAAGCTGGAGTCACAGAAGG TTGCCGAGTAGATCTCAAAGTG | 59 °C; 3,5 mM |
| MCP1 | NM_011333.3 | CCTGGATCGGAACCAAATGA CGGGTCAACTTCACATTCAAAG | 62 °C; 3 mM |
| TGFα | NM_031199.4 | CTTTAGGAAGGACCTGGGTTG GTGTGTCCAGGCTCCAAATA | 66 °C; 3 mM |
| TNFα | NM_013693.3 | GTCTCAGAATGAGGCTGGATAAG CATTGCACCTCAGGGAAGAA | 63 °C; 2,5 mM |
| Oxidative Stress Markers | |||
| COX1 | NM_008969.4 | GTGCCAGAACCAGGGTGTCT GTAGCCCGTGCGAGTACAATC | 58 °C 3 mM |
| GPX1 | NM_008160.6 | GGTTCGAGCCCAATTTTACA CATTCCGCAGGAAGGTAAAG | 58 °C 2,5 mM |
| NOX2 | NM_007807.5 | ACTCCTTGGGTCAGCACTGG GTTCCTGTCCAGTTGTCTTCG | 62 °C 3 mM |
| SOD1 | NM_011434.2 | GCCTTCTGCTCGAAGTGGAT GGAAGCATGGCGATGAAAGC | 59 °C 3,5 mM |
| SOD3 | NM_011435.3 | TGGCTGATGGTTGTACCCTG TGAGAAGATAGGCGACACGC | 60 °C 2,5 mM |
| Housekeeping Genes | |||
| ActB | NM_007393.5 | GCAAGCAGGAGTACGATGAG CCATGCCAATGTTGTCTCTT | 61 °C; 3,5mM |
| B2M | NM_009735.3 | CCTGCAGAGTTAAGCATGACAGT TCATGATGCTTGATCACATGTCT | 60 °C; 3mM |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozina, N.; Mihaljević, Z.; Baus Lončar, M.; Mihalj, M.; Mišir, M.; Radmilović, M.D.; Justić, H.; Gajović, S.; Šešelja, K.; Bazina, I.; et al. Impact of High Salt Diet on Cerebral Vascular Function and Stroke in Tff3−/−/C57BL/6N Knockout and WT (C57BL/6N) Control Mice. Int. J. Mol. Sci. 2019, 20, 5188. https://doi.org/10.3390/ijms20205188
Kozina N, Mihaljević Z, Baus Lončar M, Mihalj M, Mišir M, Radmilović MD, Justić H, Gajović S, Šešelja K, Bazina I, et al. Impact of High Salt Diet on Cerebral Vascular Function and Stroke in Tff3−/−/C57BL/6N Knockout and WT (C57BL/6N) Control Mice. International Journal of Molecular Sciences. 2019; 20(20):5188. https://doi.org/10.3390/ijms20205188
Chicago/Turabian StyleKozina, Nataša, Zrinka Mihaljević, Mirela Baus Lončar, Martina Mihalj, Mihael Mišir, Marina Dobrivojević Radmilović, Helena Justić, Srećko Gajović, Kate Šešelja, Iva Bazina, and et al. 2019. "Impact of High Salt Diet on Cerebral Vascular Function and Stroke in Tff3−/−/C57BL/6N Knockout and WT (C57BL/6N) Control Mice" International Journal of Molecular Sciences 20, no. 20: 5188. https://doi.org/10.3390/ijms20205188
APA StyleKozina, N., Mihaljević, Z., Baus Lončar, M., Mihalj, M., Mišir, M., Radmilović, M. D., Justić, H., Gajović, S., Šešelja, K., Bazina, I., Horvatić, A., Matić, A., Bijelić, N., Rođak, E., Jukić, I., & Drenjančević, I. (2019). Impact of High Salt Diet on Cerebral Vascular Function and Stroke in Tff3−/−/C57BL/6N Knockout and WT (C57BL/6N) Control Mice. International Journal of Molecular Sciences, 20(20), 5188. https://doi.org/10.3390/ijms20205188









