Effect of Zinc Supplementation on Renal Anemia in 5/6-Nephrectomized Rats and a Comparison with Treatment with Recombinant Human Erythropoietin
Abstract
:1. Introduction
2. Results
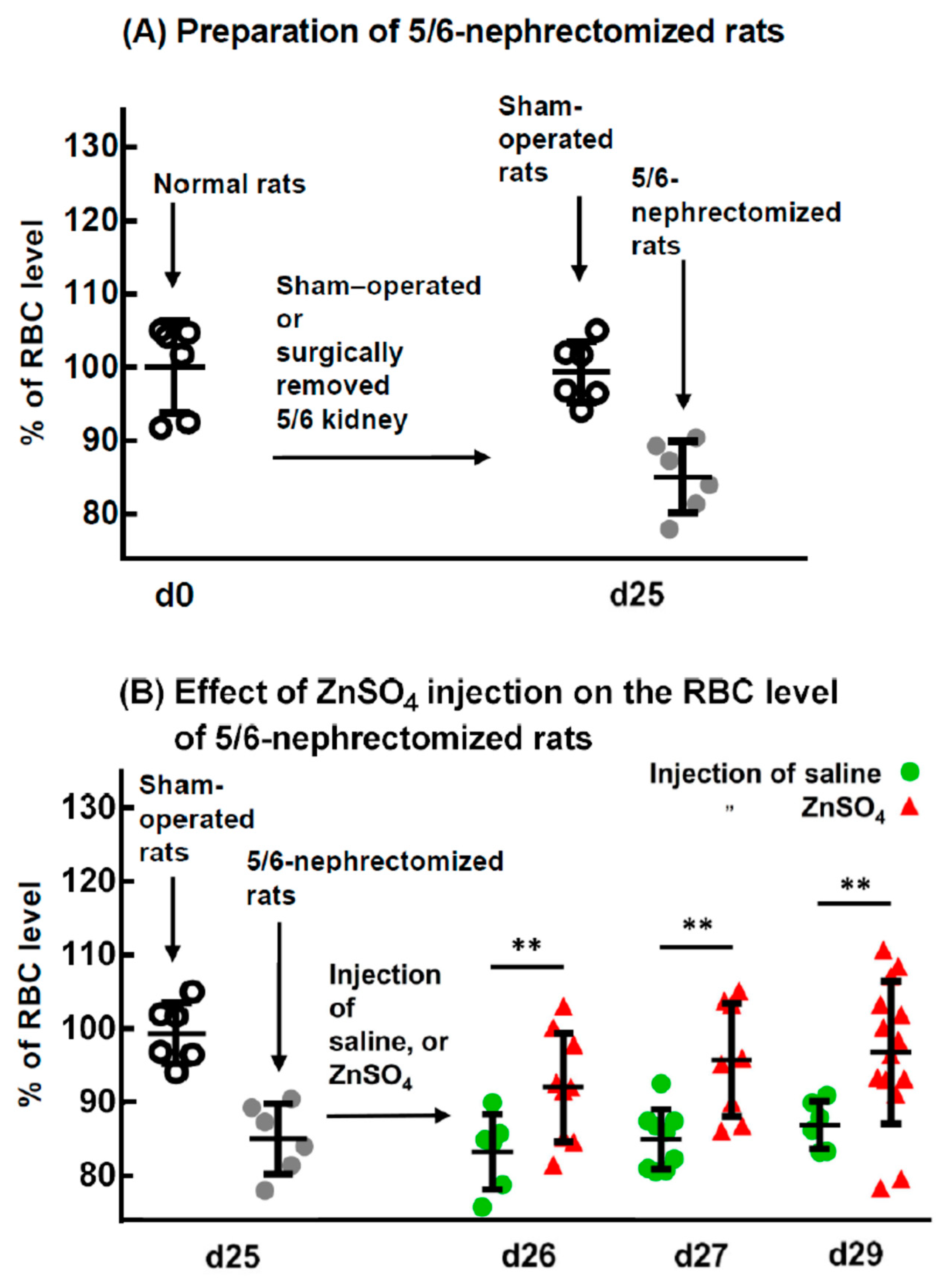
2.1. Effect of ZnSO4 Injection on the RBC Level of 5/6-Nephrectomized Rats
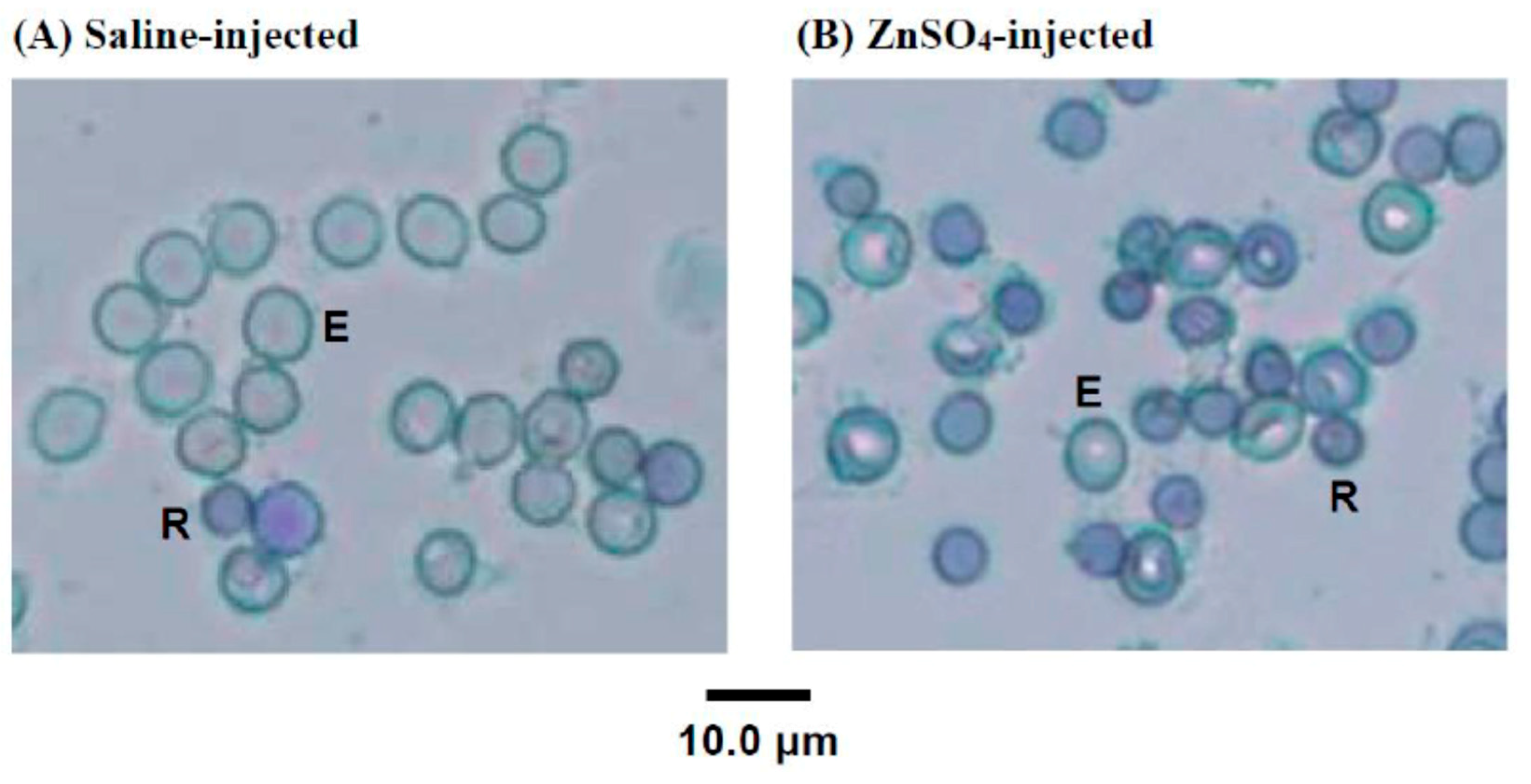
2.2. New Methylene Blue Staining of the Blood Cells of 5/6-Nephrectomized Rats Injected with Either Saline or ZnSO4
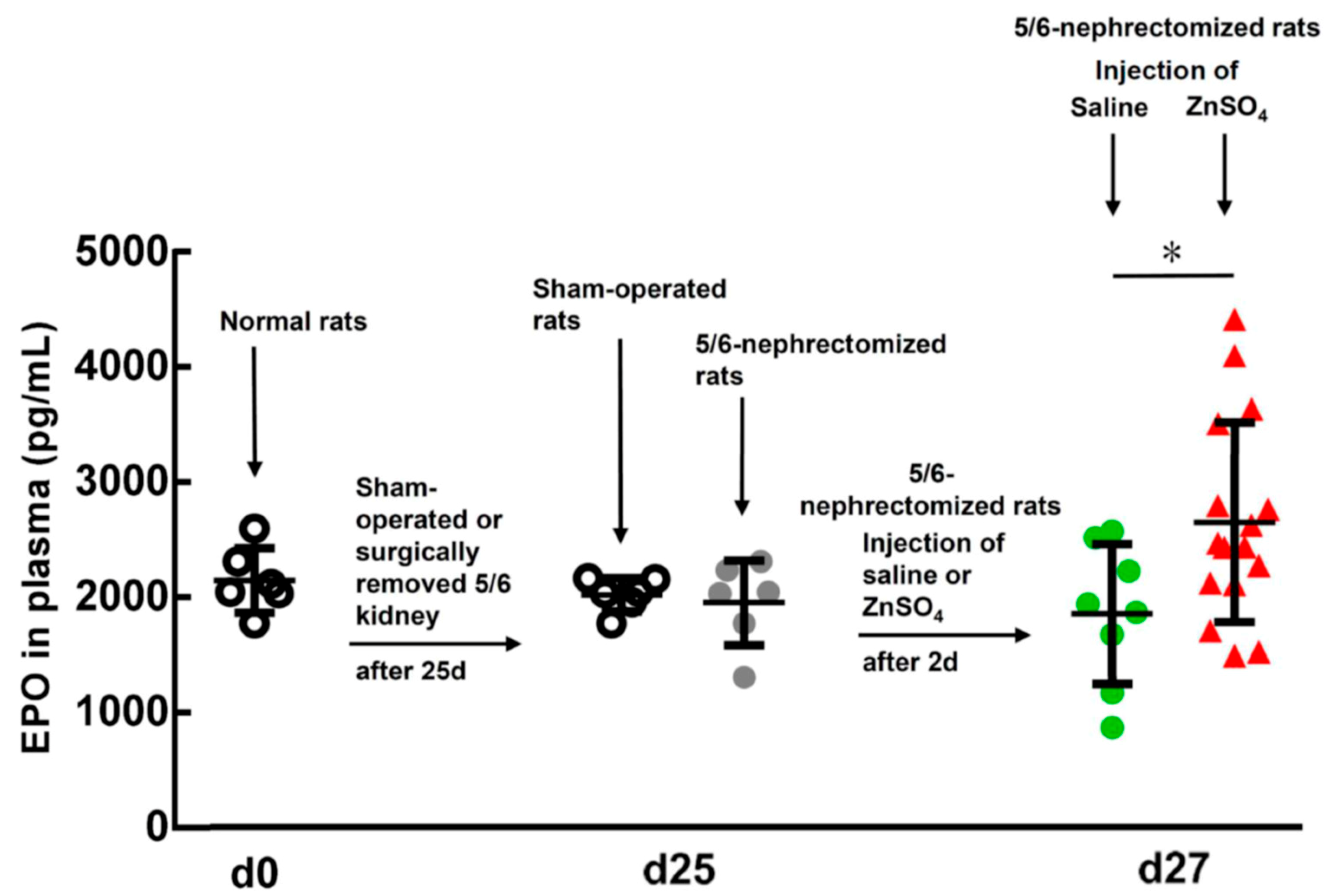
2.3. Effect of ZnSO4 Injection on the EPO Level in the 5/6-Nephrectomized Rats
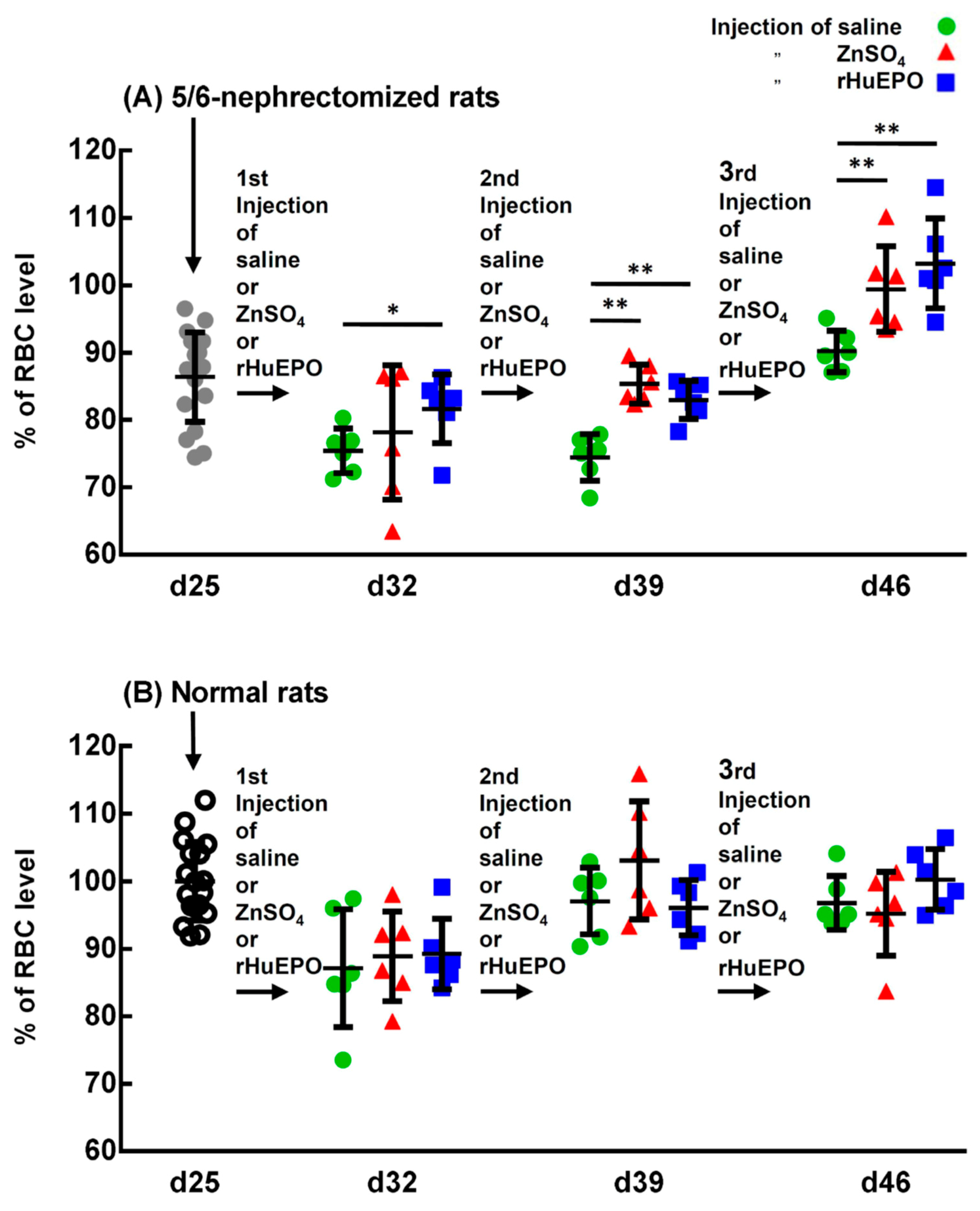
2.4. In Vivo Comparison of the Injection of Saline, ZnSO4, or rHuEPO on the RBC Levels of 5/6-Nephrectomized and Normal Rats
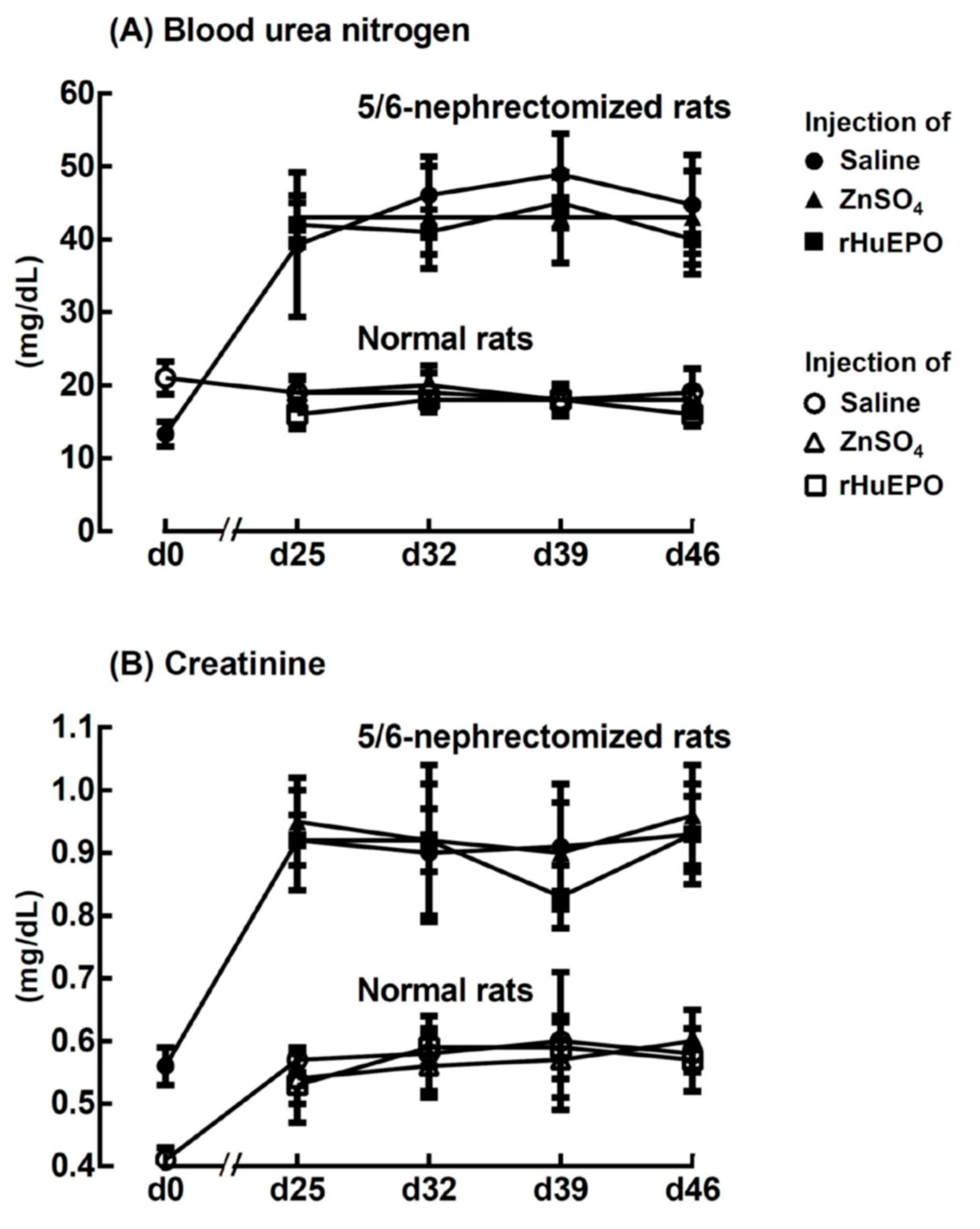
2.5. Effect of ZnSO4 or rHuEPO Injection on Blood Urea Nitrogen and Creatinine of Rats
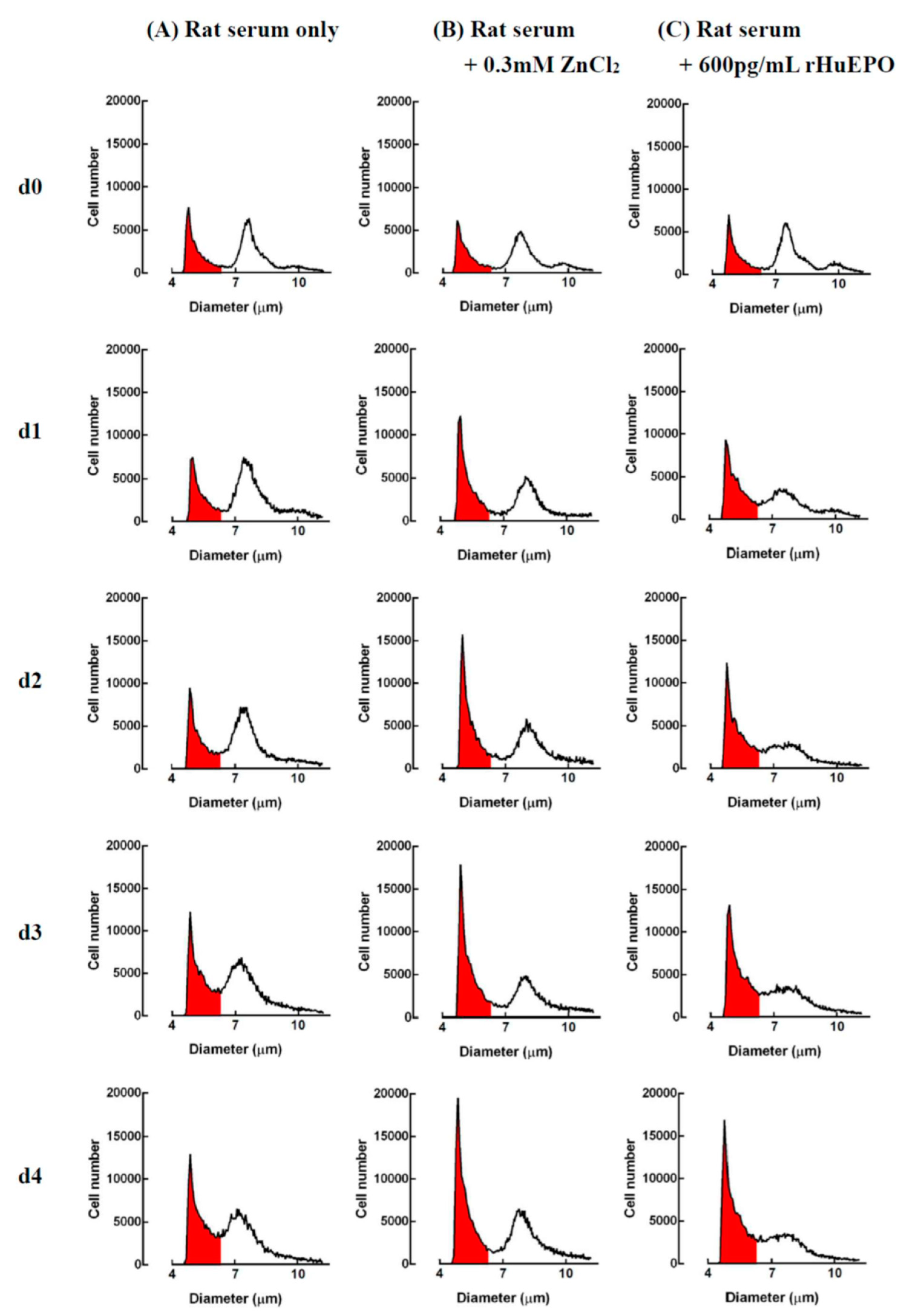
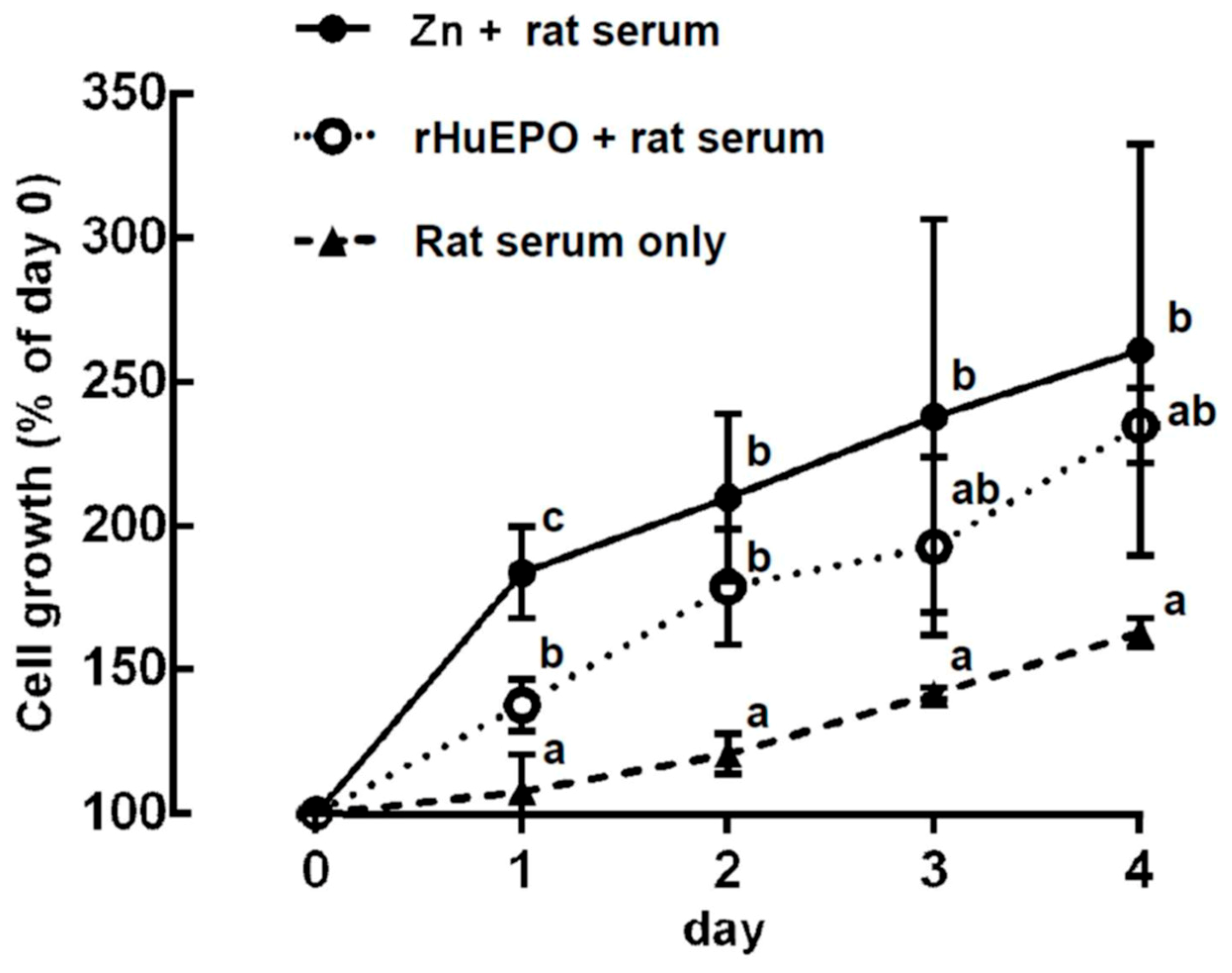
2.6. In Vitro Comparison of Zinc- and rHuEPO-Induced Erythropoiesis in Rat Bone Marrow Cells
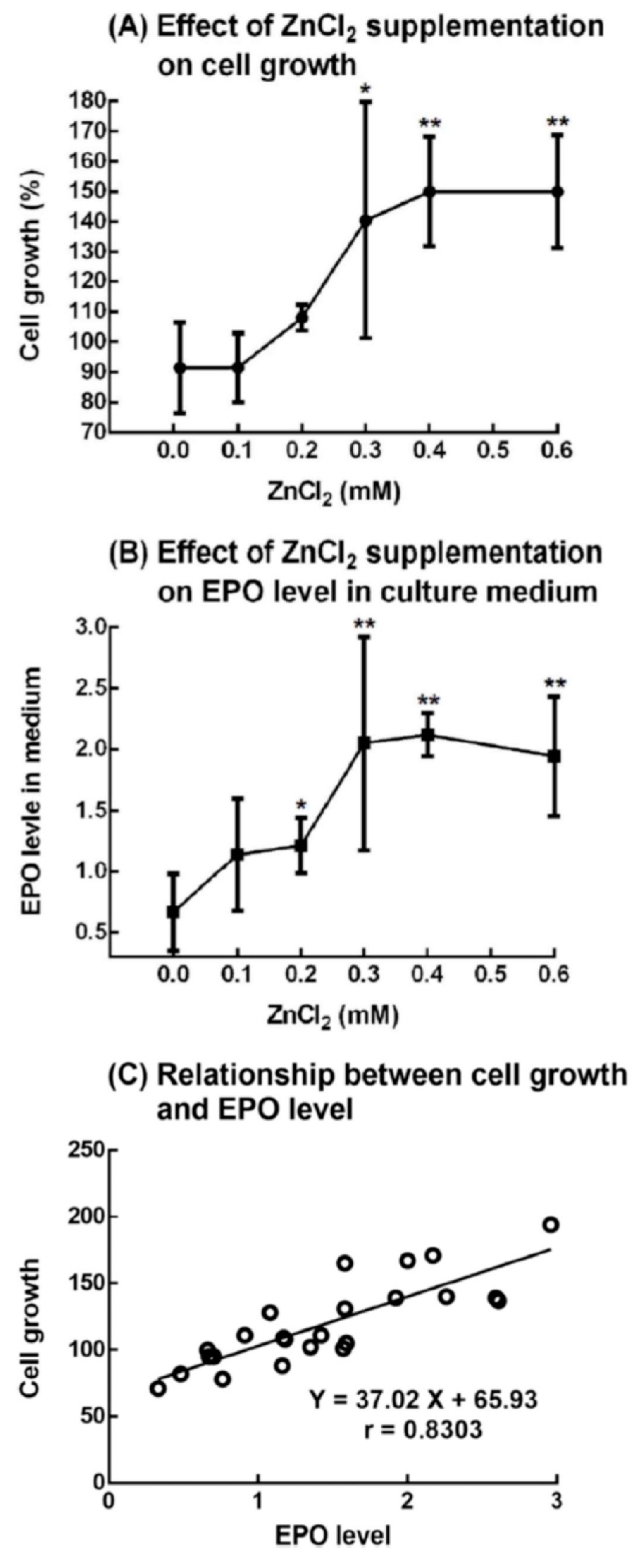
2.7. Effect of ZnCl2 Supplementation on the Growth of 5.1-μm Cells (Immature Reticulocytes) and Production of EPO from Rat Bone Marrow Cells
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Effect of ZnSO4 Injection on the RBC Level of 5/6-Nephretomized Rats
4.3. Microscopic Observation of Rat Blood Cells Using New Methylene Blue Staining
4.4. Comparison between the Injection of Saline, ZnSO4, or rHuEPO on the RBC Level of 5/6-Nephrectomized Rats
4.5. Determination of Blood Urea Nitrogen and Creatinine Contents
4.6. Comparison of Cell Growth Induced Using ZnCl2 or rHuEPO Supplementation in Rat Bone Marrow Cells
4.7. Effect of ZnCl2 Supplementation on the Growth of 5.1-μm Cells (Immature Reticulocytes) and Production of EPO From Rat Bone Marrow Cells
4.8. Determination of the EPO Concentration
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CKD | chronic kidney disease |
| EPO | erythropoietin |
| rHuEPO | recombinant human erythropoietin |
| RBC | red blood cell |
| PHZ | phenylhydrazine |
References
- Stevens, L.A.; Levin, A. Anaemia, cardiovascular disease and kidney disease: Integrating new knowledge in 2002. Curr. Opin. Nephrol. Hypertens. 2003, 12, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Toto, R.D. Anemia of chronic disease: Past, present, and future: Management of comorbidities in kidney disease in the 21st century: Anemia and bone disease. Kidney Int. 2003, 64, S20–S23. [Google Scholar] [CrossRef]
- Kimmel, P.L. Zinc and chronic renal disease. Semin. Dialy 1989, 2, 253–259. [Google Scholar] [CrossRef]
- Mahajan, S.K. Zinc in kidney disease. J. Am. Coll. Nutr. 1989, 8, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; Wiebe, N.; Hemmelgarn, B.; Klarenbach, S.; Field, C.; Manns, B.; Thadhani, R.; Gill, J. Trace elements in hemodialysis patients: A systematic review and meta-analysis. Bmc. Med. 2009, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.-H.; Wang, C.-L.; Chen, P.-C. Chapter 10. Micronutrient metabolism in hemodialysis patients. In Hemodialysis-Different Aspects; InTech: Rijeka, Croatia, 2011; pp. 173–204. [Google Scholar]
- Neto, L.; Bacci, M.; Sverzutt, L.; Costa, M.; Alves, B.; Fonseca, F. The role of zinc in chronic kidney disease patients on hemodialysis: A systematic review. Health 2016, 8, 344. [Google Scholar] [CrossRef]
- Rucker, D.; Thadhani, R.; Tonelli, M. Trace element status in hemodialysis patients. Semin. Dial. 2010, 23, 389–395. [Google Scholar] [CrossRef]
- Tonelli, M.; Wiebe, N.; Thompson, S.; Kinniburgh, D.; Klarenbach, S.W.; Walsh, M.; Bello, A.K.; Faruque, L.; Field, C.; Manns, B.J. Trace element supplementation in hemodialysis patients: A randomized controlled trial. BMC Nephrol. 2015, 16, 52. [Google Scholar] [CrossRef]
- Wang, L.-J.; Wang, M.-Q.; Hu, R.; Yang, Y.; Huang, Y.-S.; Xian, S.-X.; Lu, L. Effect of zinc supplementation on maintenance hemodialysis patients: A systematic review and meta-analysis of 15 randomized controlled trials. Biomed Res. Int. 2017. [Google Scholar] [CrossRef]
- Argani, H.; Mahdavi, R.; Ghorbani-haghjo, A.; Razzaghi, R.; Nikniaz, L.; Gaemmaghami, S.J. Effects of zinc supplementation on serum zinc and leptin levels, BMI, and body composition in hemodialysis patients. J. Trace Elem. Med. Biol. 2014, 28, 35–38. [Google Scholar] [CrossRef]
- Fukushima, T.; Horike, H.; Fujiki, S.; Kitada, S.; Sasaki, T.; Kashihara, N. Zinc deficiency anemia and effects of zinc therapy in maintenance hemodialysis patients. Apher. Dial. 2009, 13, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Abe, M.; Okada, K.; Tei, R.; Maruyama, N.; Kikuchi, F.; Higuchi, T.; Soma, M. Oral zinc supplementation reduces the erythropoietin responsiveness index in patients on hemodialysis. Nutrients 2015, 7, 3783–3795. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.C.; Nogueira-Pedro, A.; Santos, E.W.; Hastreiter, A.; Silva, G.B.; Borelli, P.; Fock, R.A. A review of select minerals influencing the haematopoietic process. Nutr. Res. Rev. 2018, 31, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Feng, H.-L.; Jeng, S.-S. Zinc supplementation stimulates red blood cell formation in rats. Int. J. Mol. Sci. 2018, 19, 2824. [Google Scholar] [CrossRef] [PubMed]
- Anagnostou, A.; Vercellotti, G.; Barone, J.; Fried, W. Factors which affect erythropoiesis in partially nephrectomized and sham-operated rats. Blood 1976, 48, 425–433. [Google Scholar] [PubMed]
- Mason, C.; Thomas, T. A model for erythropoiesis in experimental chronic renal failure. Br. J. Haematol. 1984, 58, 729–740. [Google Scholar] [CrossRef]
- Yang, H.-C.; Zuo, Y.; Fogo, A.B. Models of chronic kidney disease. Drug Discov. Today Dis. Models 2010, 7, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Anagnostou, A.; Barone, J.; Kedo, A.; Fried, W. Effect of erythropoietin therapy on the red cell volume of uraemic and non-uraemic rats. Br. J. Haematol. 1977, 37, 85–91. [Google Scholar] [CrossRef]
- Kawamura, A.; Higuchi, M.; Imai, N.; Kawaguchi, T.; Ogura, Y. Effect of purified recombinant human erythropoietin on anemia in rats with experimental renal failure induced by five-sixth nephrectomy. Biotherapy 1990, 2, 77–85. [Google Scholar] [CrossRef]
- Kono, M.; Kondo, T.; Takagi, Y.; Wada, A.; Fujimoto, K. Morphological definition of CD71 positive reticulocytes by various staining techniques and electron microscopy compared to reticulocytes detected by an automated hematology analyzer. Clin. Chim. Acta 2009, 404, 105–110. [Google Scholar] [CrossRef]
- Jelkmann, W. Erythropoietin: Structure, control of production, and function. Physiol. Rev. 1992, 72, 449–489. [Google Scholar] [CrossRef] [PubMed]
- Weidemann, A.; Johnson, R.S. Nonrenal regulation of EPO synthesis. Kidney Int. 2009, 75, 682–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fandrey, J. Oxygen-dependent and tissue-specific regulation of erythropoietin gene expression. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R977–R988. [Google Scholar] [CrossRef] [PubMed]
- Hermine, O.; Beru, N.; Pech, N.; Goldwasser, E. An autocrine role for erythropoietin in mouse hematopoietic cell differentiation. Blood 1991, 78, 2253–2260. [Google Scholar] [PubMed]
- Stopka, T.; Zivny, J.; Stopkova, P.; Prchal, J.; Prchal, J. Human hematopoietic progenitors express erythropoietin. Blood 1998, 91, 3766–3772. [Google Scholar] [PubMed]
- Vogt, C.; Pentz, S.; Rich, I.N. A role for the macrophage in normal hemopoiesis: III. In vitro and in vivo erythropoietin gene expression in macrophages detected by in situ hybridization. Exp. Hematol. 1989, 17, 391–397. [Google Scholar] [PubMed]
- Bunn, H.F. Erythropoietin. Cold Spring Harb Perspect. Med. 2013, 3, a011619. [Google Scholar] [CrossRef]
- Sugihara, T.; Sawada, S.; Hakura, A.; Hori, Y.; Uchida, K.; Sagami, F. A staining procedure for micronucleus test using new methylene blue and acridine orange: Specimens that are supravitally stained with possible long-term storage. Mutat. Res. Genet. Toxicol. Env. Mutagen. 2000, 470, 103–108. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, H.-L.; Chen, Y.-H.; Jeng, S.-S. Effect of Zinc Supplementation on Renal Anemia in 5/6-Nephrectomized Rats and a Comparison with Treatment with Recombinant Human Erythropoietin. Int. J. Mol. Sci. 2019, 20, 4985. https://doi.org/10.3390/ijms20204985
Feng H-L, Chen Y-H, Jeng S-S. Effect of Zinc Supplementation on Renal Anemia in 5/6-Nephrectomized Rats and a Comparison with Treatment with Recombinant Human Erythropoietin. International Journal of Molecular Sciences. 2019; 20(20):4985. https://doi.org/10.3390/ijms20204985
Chicago/Turabian StyleFeng, Hui-Lin, Yen-Hua Chen, and Sen-Shyong Jeng. 2019. "Effect of Zinc Supplementation on Renal Anemia in 5/6-Nephrectomized Rats and a Comparison with Treatment with Recombinant Human Erythropoietin" International Journal of Molecular Sciences 20, no. 20: 4985. https://doi.org/10.3390/ijms20204985




