Effects of Extract of Arrabidaea chica Verlot on an Experimental Model of Osteoarthritis
Abstract
1. Introduction
2. Results
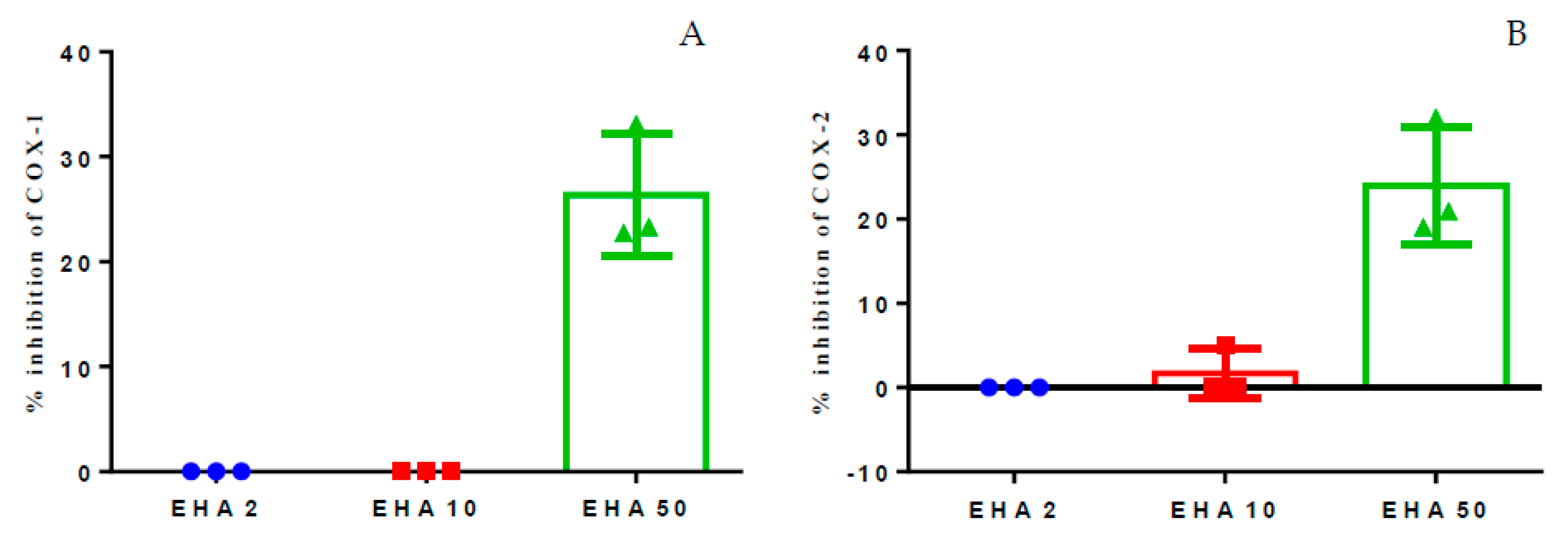
2.1. Inhibition of Cyclooxygenase 1 and 2
2.2. Clinical Evaluations
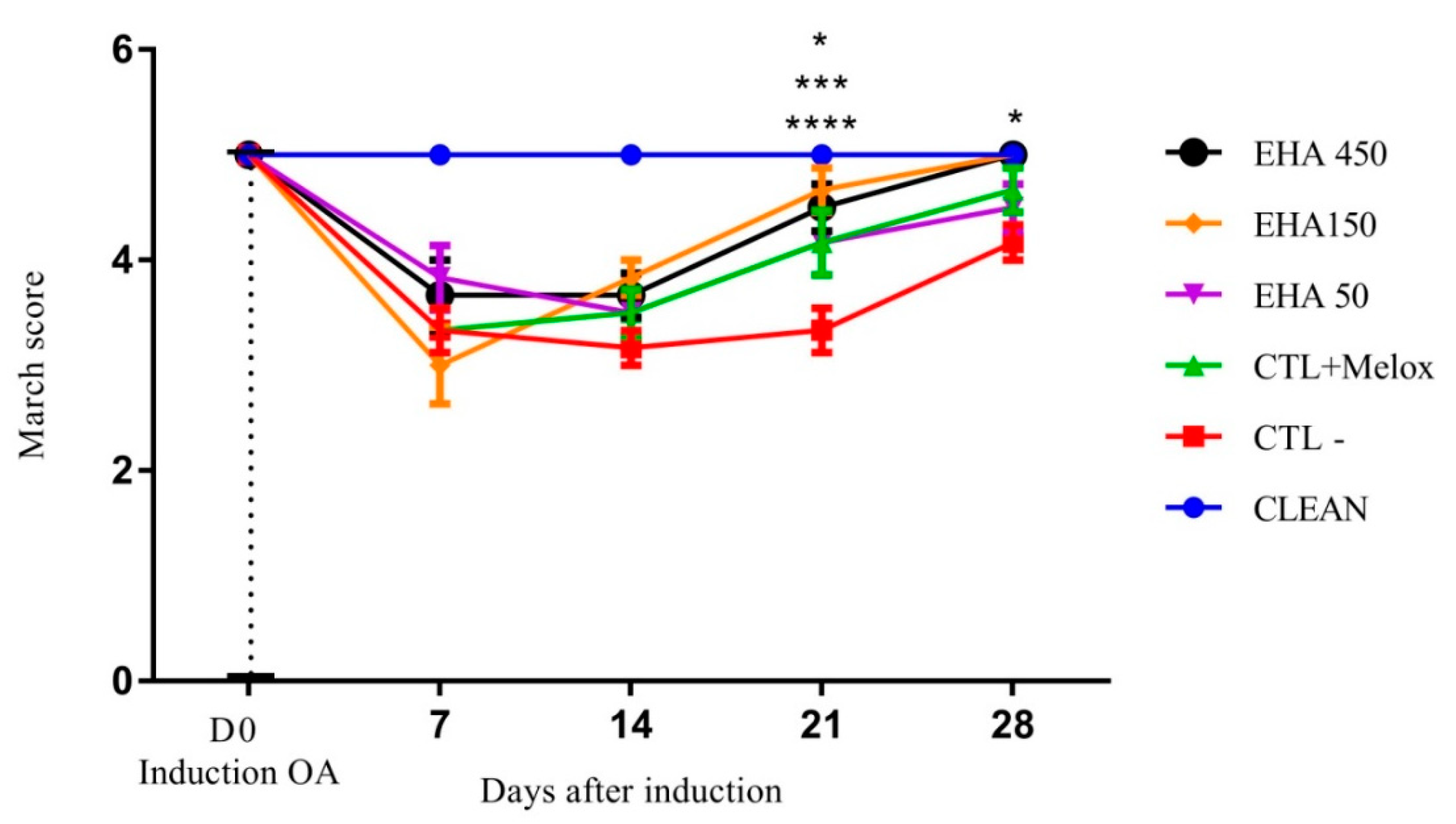
2.2.1. Evaluation of Motor Activity/Forced Deambulation (Rotarod Test)
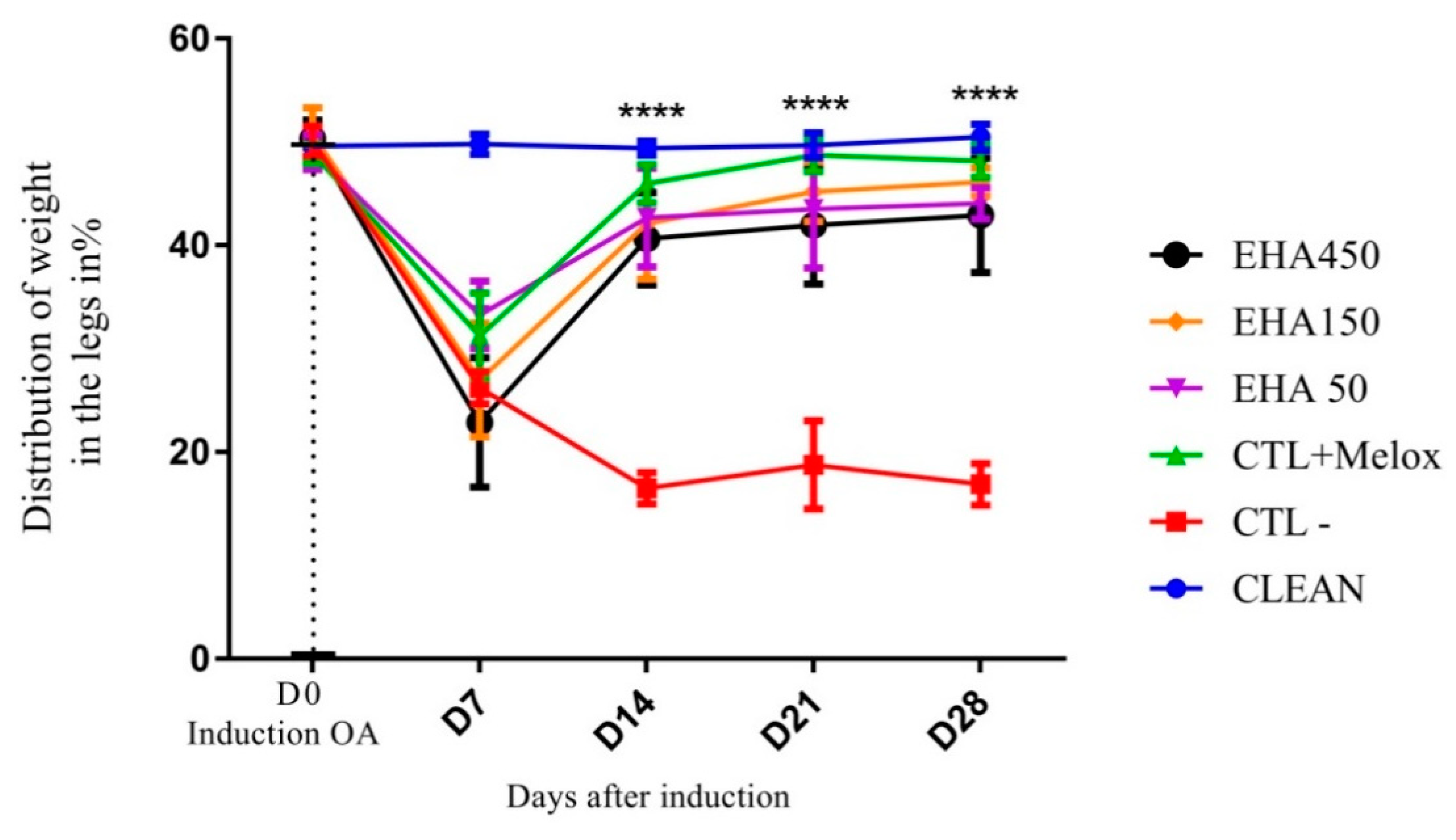
2.2.2. Incapacitation/Weight Distribution Test on Hind Legs (Weight Bearing)
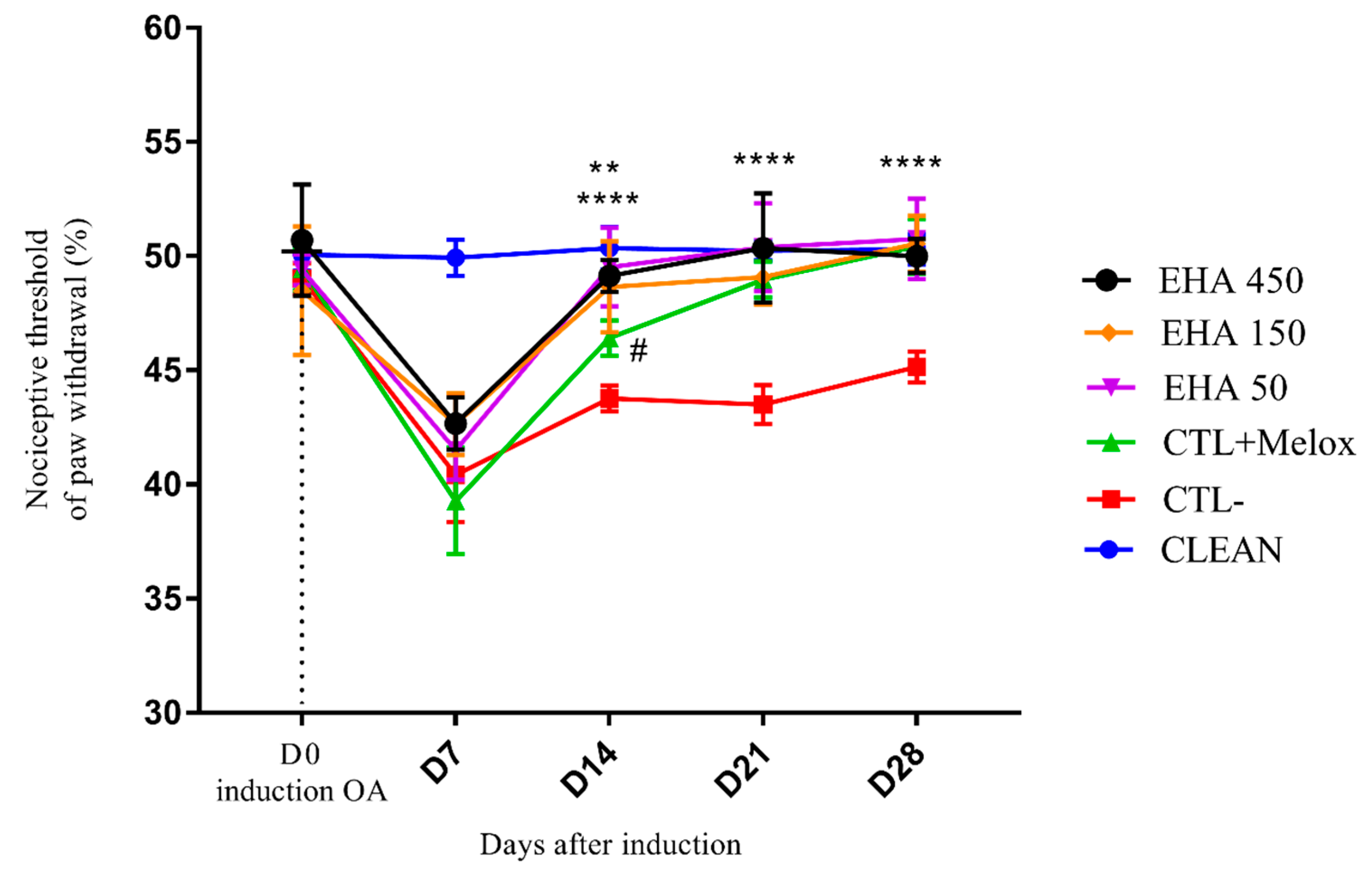
2.2.3. Mechanical Hyperalgesia (Randall Selitto Test)
2.2.4. Mechanical Allodynia (von Frey Test)
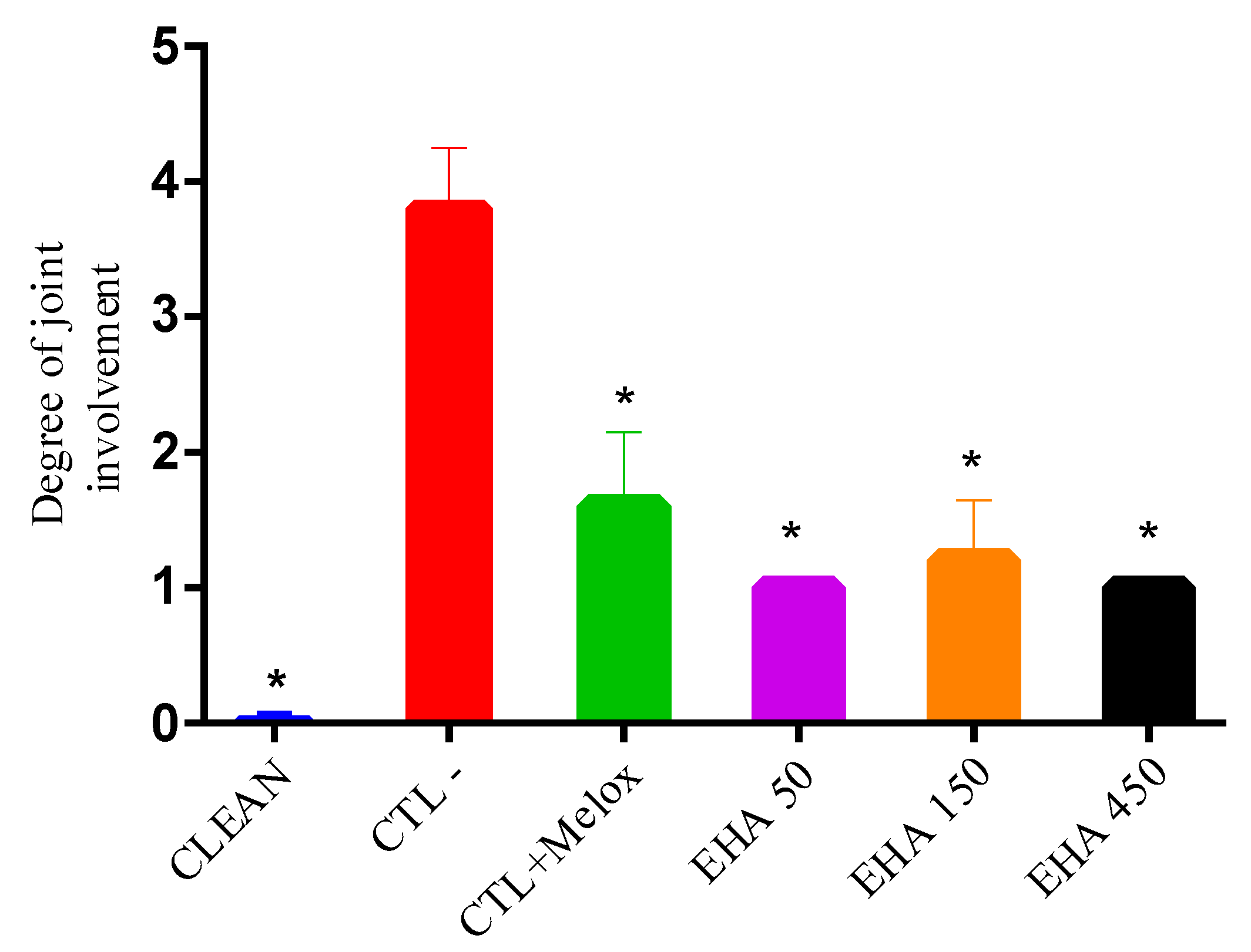
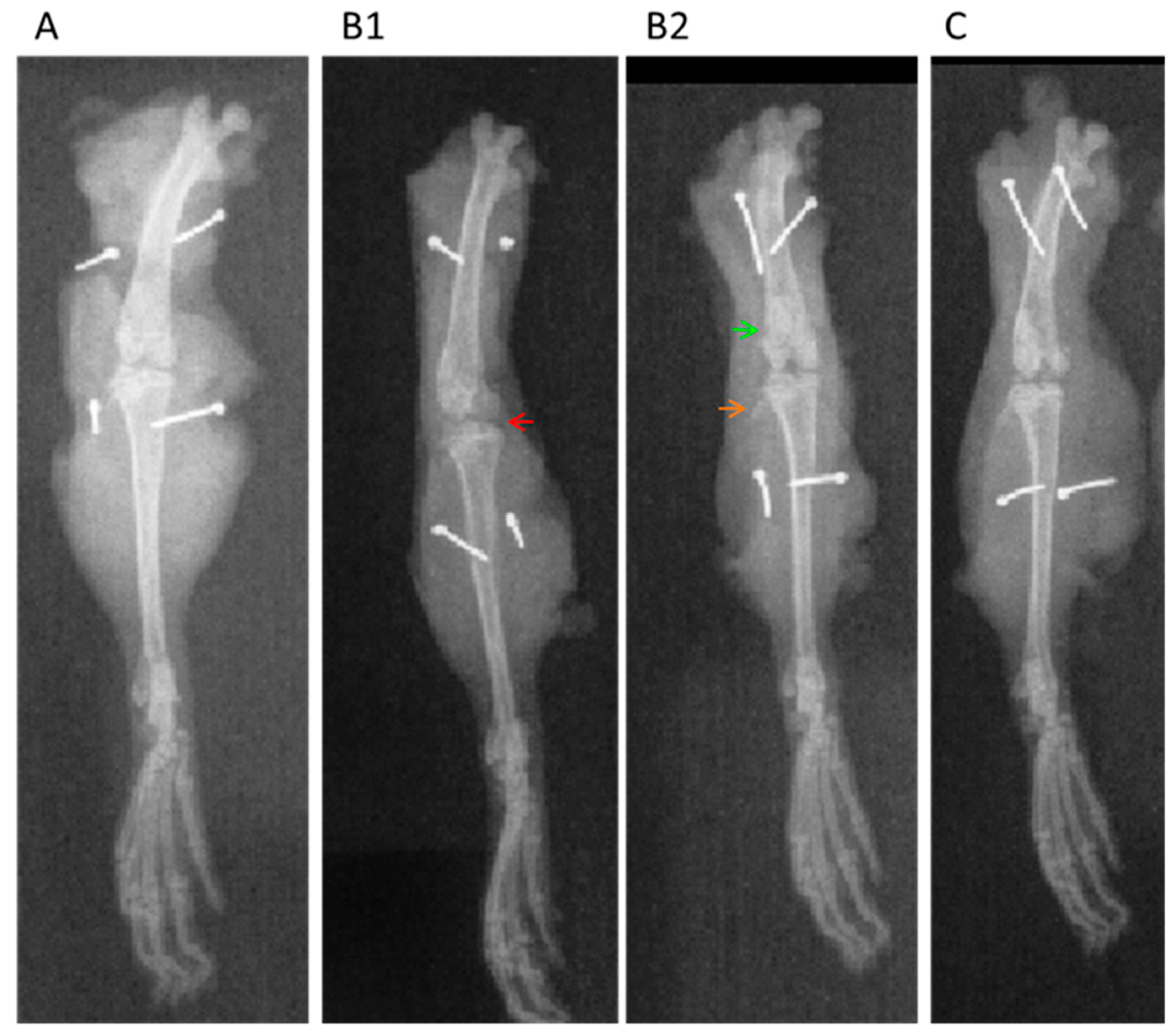
2.3. Radiographic Analysis
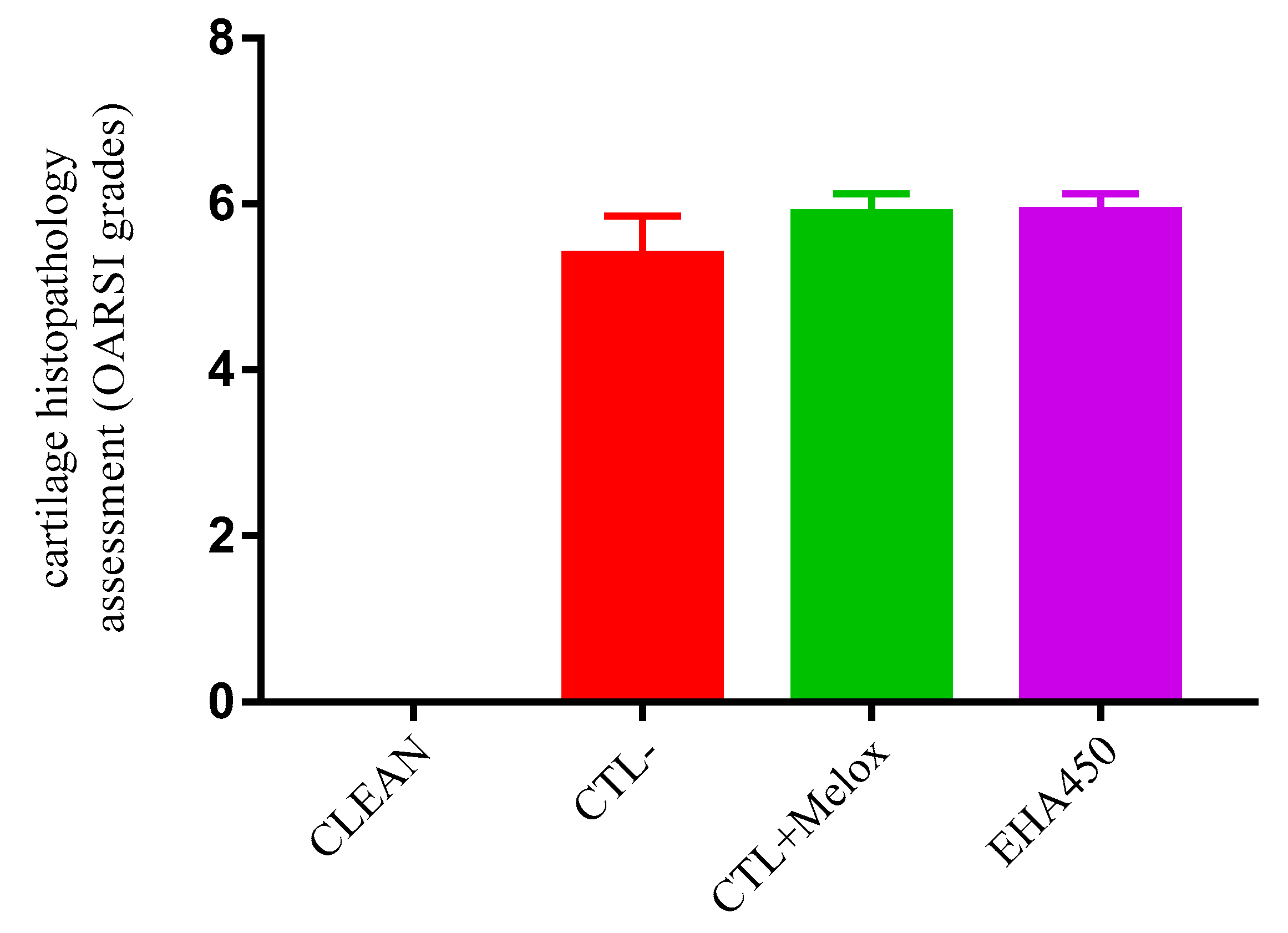
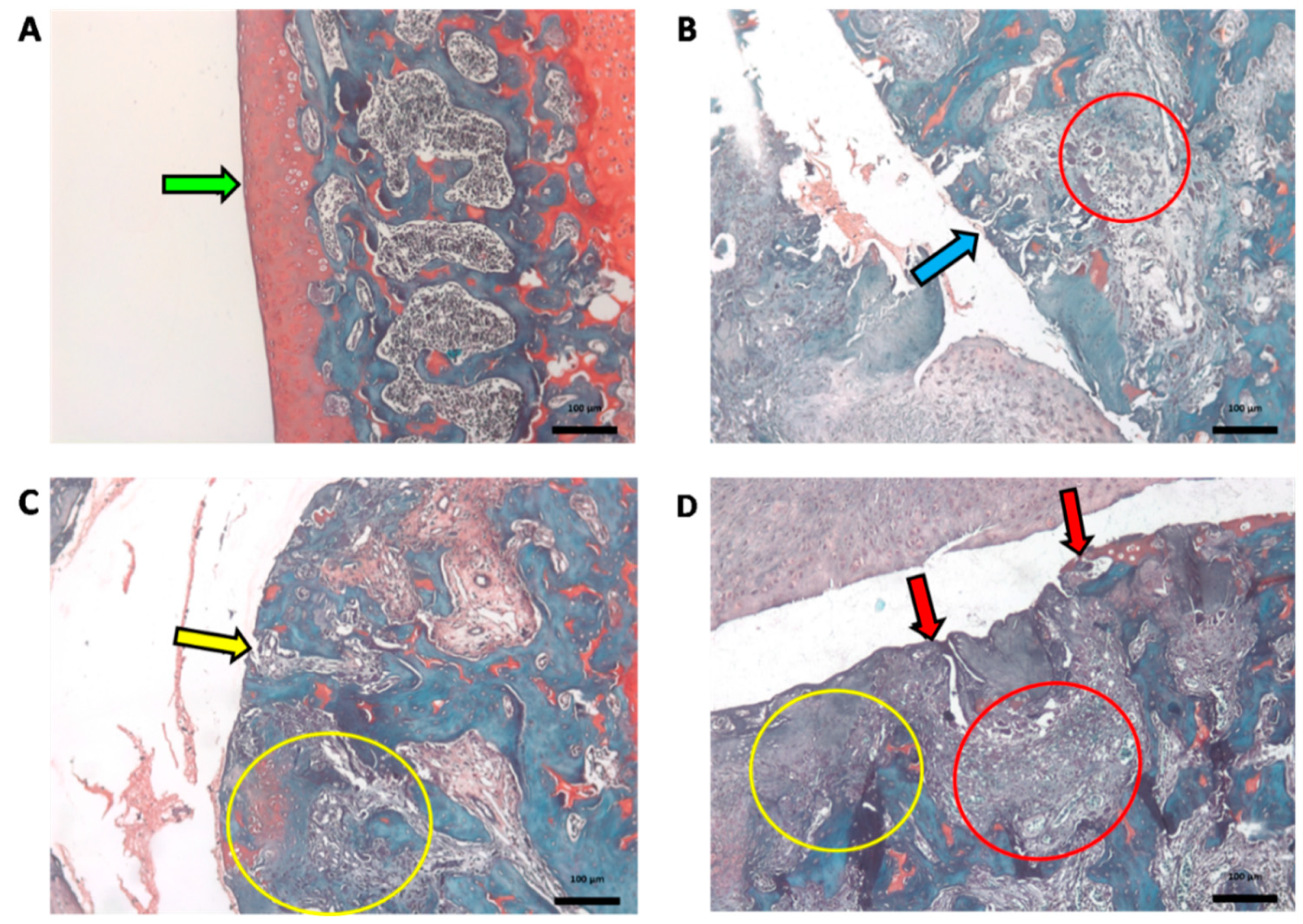
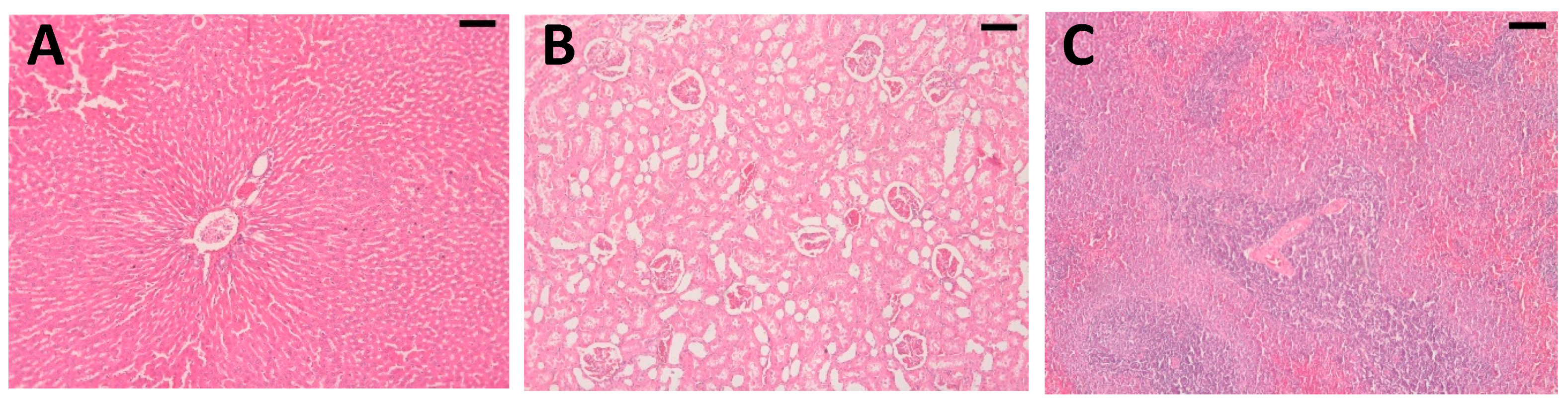
2.4. Histopathological Analysis
2.5. Chemical Analysis
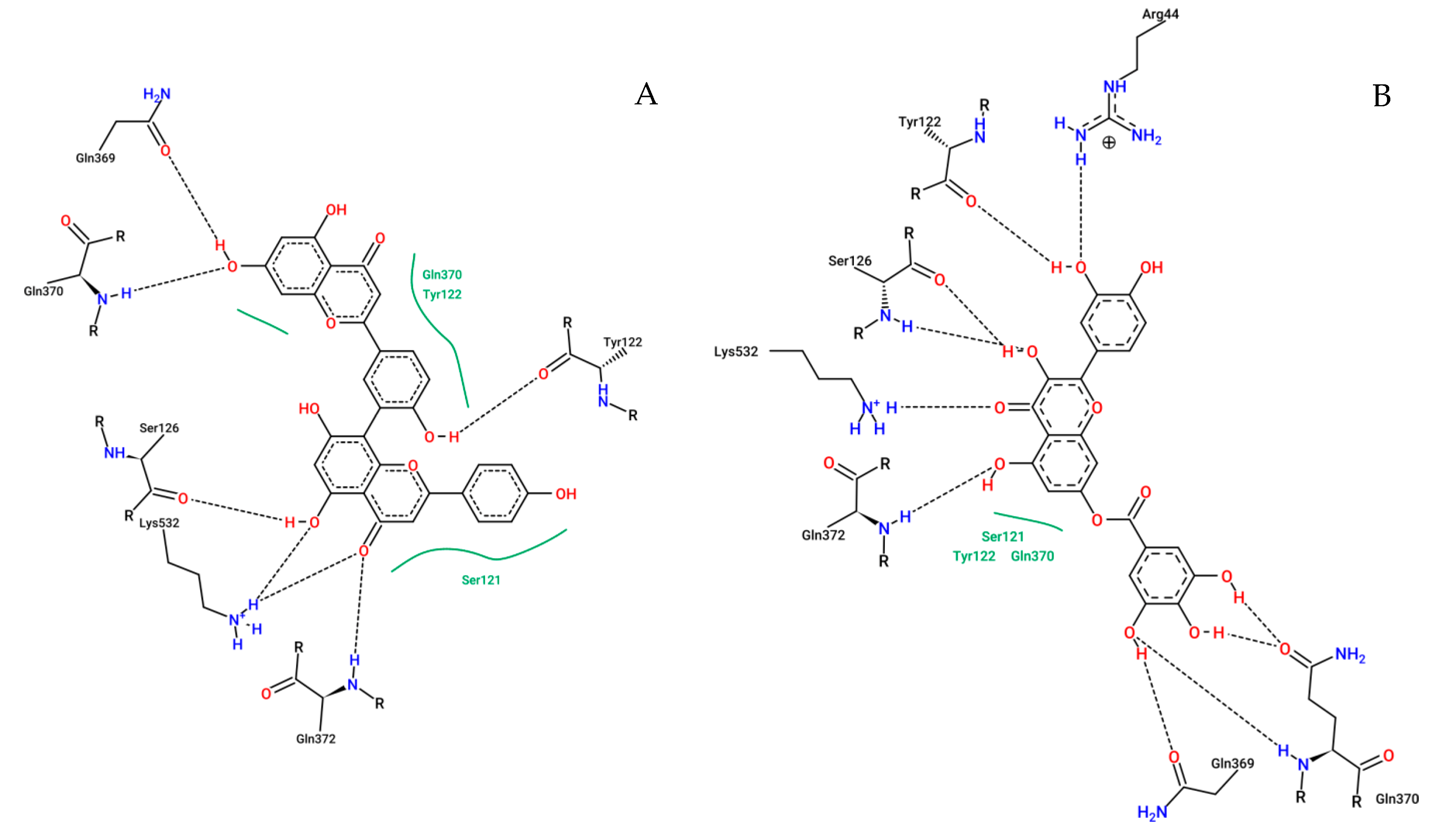
2.6. In silico Analysis
3. Discussion
4. Materials and Methods
4.1. Collection and Processing of Plant Species
4.2. In vitro Activity on Cyclooxygenase
4.3. In vivo Experimental Studies
4.3.1. Animals
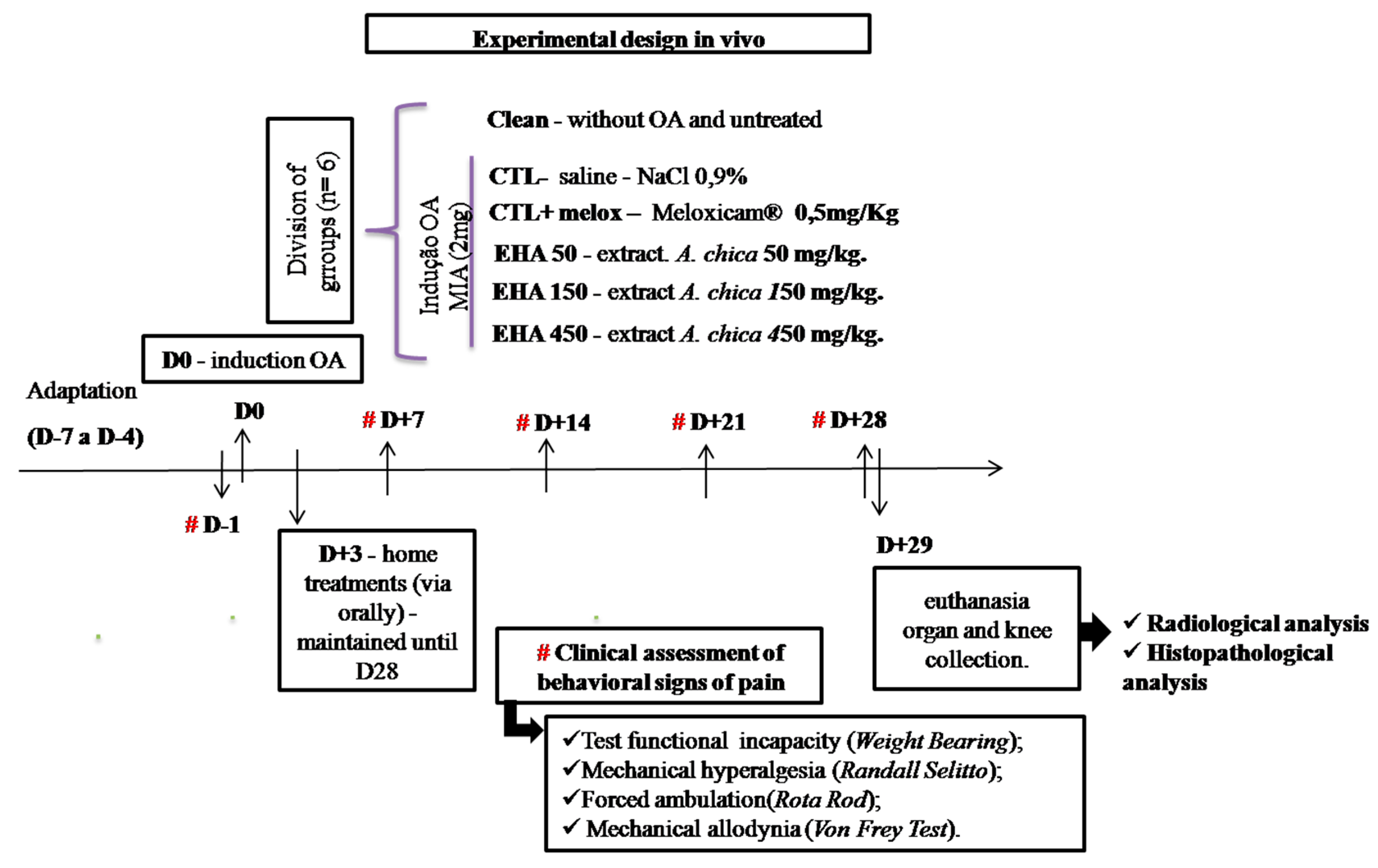
4.3.2. Experimental Design
4.3.3. MIA-Induced OA Model
4.3.4. Clinical Evaluations
Evaluation of Motor Activity/Forced Deambulation (Rotarod Test)
Incapacitation/Weight Distribution Test on Hind Legs (Weight Bearing)
Mechanical Hyperalgesia (Randall–SelittoTest)
Mechanical Allodynia (von Frey Test)
4.4. Radiological Analysis
4.5. Histopathological Analysis of Articular Cartilage
4.6. FIA-ESI-IT-MS/MSn and HPLC-ESI-IT-MS Analysis Instrumentation
4.7. In silico Studies
4.7.1. Predictive Models and Theoretical Calculations
4.7.2. Molecular Docking
4.8. Statistics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Calixto, J.B. Twenty-five years of research on medicinal plants in Latin America: A personal view. J. Ethnopharmacol. 2005, 100, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Joly, C.; Haddad, C.; Verdade, L.; Oliveira, M.; Bolzani, V.; Berlinck, R. Diagnóstico da pesquisa em biodiversidade no Brasil. Rev. USP 2011, 89, 114–133. [Google Scholar] [CrossRef]
- Filardi, F.L.R.; de Barros, F.; Baumgratz, J.F.A.; Bicudo, C.E.M.; Cavalcanti, T.B.; Coelho, M.A.N.; Costa, A.F.; Costa, D.P.; Goldenberg, R.; Labiak, P.H.; et al. Brazilian Flora 2020: Innovation and collaboration to meet Target 1 of the Global Strategy for Plant Conservation (GSPC). Rodriguésia 2018, 69, 1513–1527. [Google Scholar]
- Rocha, C.Q.; Vilela, F.C.; Cavalcante, G.P.; Santa-Cecilia, F.V.; Santos-e-Silva, L.; dos Santos, M.H.; Giusti-Paiva, A. Anti-inflammatory and antinociceptive effects of Arrabidaea brachypoda (DC.) Bureau roots. J. Ethnopharmacol. 2011, 133, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi, H.; Matos, F.J.A. Plantas medicinais no Brasil: Nativas e exóticas, 2nd ed.; Instituto Plantarum: Nova Odessa, Brazil, 2008. [Google Scholar]
- Wang, H.; Wang, Q.; Yang, M.; Yang, L.; Wang, W.; Ding, H.; Zhang, D.; Xu, J.; Tang, X.; Ding, H.; et al. Histomorphology and innate immunity during the progression of osteoarthritis: Does synovitis affect cartilage degradation? J. Cell. Physiol. 2018, 233, 1342–1358. [Google Scholar] [CrossRef] [PubMed]
- Ximenes, A.C.; Melo, A.M.P.; Custodio, D.M.E. Dor: Princípios e Prática, 1st ed.; Artmed: Porto Alegre, Brazil, 2009. [Google Scholar]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Siebuhr, A.S.; Bay-Jensen, A.C.; Jordan, J.M.; Kjelgaard-Petersen, C.F.; Christiansen, C.; Abramson, S.B.; Attur, M.; Berenbaum, F.; Kraus, V.; Karsdal, M.A. Inflammation (or synovitis)-driven osteoarthritis: An opportunity for personalizing prognosis and treatment? Scand. J. Rheumatol. 2016, 45, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Bjordal, J.M.; Klovning, A.; Ljunggren, A.E.; Slordal, L. Short-term efficacy of pharmacotherapeutic interventions in osteoarthritic knee pain: A meta-analysis of randomised placebo-controlled trials. Eur. J. Pain 2007, 11, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.H. Research of Pathogenesis and Novel Therapeutics in Arthritis. Int. J. Mol. Sci. 2019, 20, 1646. [Google Scholar] [CrossRef]
- Chou, R.; Helfand, M.; Peterson, K.; Dana, T.; Roberts, C.; Health, O.; Helfand, M. Drug Class Review on Cyclo-Oxygenase (COX)-2 Inhibitors and Non-Steroidal Anti-Inflammatory Drugs (NSAIDs); Oregon Health & Science University: Portland, OR, USA, 2006. [Google Scholar]
- Muri, E.; Sposito, M.; Metsavaht, L. Antiinflamatórios não-esteroidais e sua farmacologia local. Acta Fisiátr. 2009, 16, 186–190. [Google Scholar]
- Sharma, L.; Kapoor, D.; Issa, S. Epidemiology of osteoarthritis: An update. Curr. Opin. Rheumatol. 2006, 18, 147–156. [Google Scholar] [CrossRef] [PubMed]
- De Rosis, R.G.; Kairalla, M. Osteoartrite: Avaliação clínica e epidemiológica de pacientes idosos em instituição de longa permanência. Rev. Bras. Clin. Med. 2010, 8, 101–108. [Google Scholar]
- Kawano, M.M.; Araújo, I.L.A.; Castro, M.C.; Matos, M.A. Assessment of quality of life in patients with knee osteoarthritis. Acta Ortop. Bras. 2015, 23, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Chapman, E.; Perkin, A.; Robinson, R. The coloring matters of carajuruna. J Chem Soc 1927, 3015–3040. [Google Scholar] [CrossRef]
- Zorn, B.; Garcia-Pineres, A.J.; Castro, V.; Murillo, R.; Mora, G.; Merfort, I. 3-Desoxyanthocyanidins from Arrabidaea chica. Phytochemistry 2001, 56, 831–835. [Google Scholar] [CrossRef]
- Devia, B.; Llabres, G.; Wouters, J.; Dupont, L.; Escribano-Bailon, M.T.; de Pascual-Teresa, S.; Angenot, L.; Tits, M. New 3-deoxyanthocyanidins from leaves of Arrabidaea chica. Phytochem. Anal. 2002, 13, 114–119. [Google Scholar] [CrossRef]
- Horai, H.; Arita, M.; Kanaya, S.; Nihei, Y.; Ikeda, T.; Suwa, K.; Ojima, Y.; Tanaka, K.; Tanaka, S.; Aoshima, K.; et al. MassBank: A public repository for sharing mass spectral data for life sciences. J. Mass Spectrom. 2010, 45, 703–714. [Google Scholar] [CrossRef]
- Shen, D.; Wu, Q.; Wang, M.; Yang, Y.; Lavoie, E.J.; Simon, J.E. Determination of the predominant catechins in Acacia catechu by liquid chromatography/electrospray ionization-mass spectrometry. J. Agric. Food Chem. 2006, 54, 3219–3224. [Google Scholar] [CrossRef]
- Li, Z.H.; Guo, H.; Xu, W.-B.; Ge, J.; Li, X.; Alimu, M.; He, D.J. Rapid Identification of Flavonoid Constituents Directly from PTP1B Inhibitive Extract of Raspberry (Rubus idaeus L.) Leaves by HPLC-ESI-QTOF-MS-MS. J. Chromatogr. Sci. 2016, 54, 805–810. [Google Scholar] [CrossRef]
- Bahia, M.V.; David, J.P.; David, J.M. Occurrence of biflavones in leaves of Caesalpinia pyramidalis specimens. Química Nov. 2010, 33, 1297–1300. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, H.; Wu, H.; Pan, Y.; Wang, K.; Jin, Y.; Zhang, C. Characterization and Quantification by LC-MS/MS of the Chemical Components of the Heating Products of the Flavonoids Extract in Pollen Typhae for Transformation Rule Exploration. Molecules 2015, 20, 18352–18366. [Google Scholar] [CrossRef] [PubMed]
- Plazonic, A.; Bucar, F.; Males, Z.; Mornar, A.; Nigovic, B.; Kujundzic, N. Identification and quantification of flavonoids and phenolic acids in burr parsley (Caucalis platycarpos L.), using high-performance liquid chromatography with diode array detection and electrospray ionization mass spectrometry. Molecules 2009, 14, 2466–2490. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, W.L.R.; do Nascimento Pinto, L.; Quignard, E.; dos Santos Vieira, J.M.; Silva, J.O.C., Jr.; Albuquerque, S. Arrabidaea chica (HBK) Verlot: Phytochemical approach, antifungal and trypanocidal activities. Rev. Bras. Farmacogn. 2008, 18, 544–548. [Google Scholar]
- Iwashina, T.; Smirnov, S.V.; Damdinsuren, O.; Kondo, K. Saussurea Species from the Altai Mountains and Adjacent Area, and Their Flavonoid Diversity. Bull. Natl. Mus. Nat. Sci. Ser. B 2010, 36, 141–154. [Google Scholar]
- Takemura, O.S.; Iinuma, M.; Tosa, H.; Miguel, O.G.; Moreira, E.A.; Nozawa, Y. A flavone from leaves of Arrabidaea chica f. cuprea. Phytochemistry 1995, 38, 1299–1300. [Google Scholar] [CrossRef]
- Siraichi, J.T.G.; Felipe, D.F.; Brambilla, L.Z.S.; Gatto, M.J.; Terra, V.A.; Cecchini, A.L.; Cortez, L.E.R.; Rodrigues-Filho, E.; Cortez, D.A.G. Antioxidant capacity of the leaf extract obtained from Arrabidaea chica cultivated in Southern Brazil. PLoS ONE 2013, 8, e72733. [Google Scholar] [CrossRef] [PubMed]
- Hasrat, J.A.; Pieters, L.; Claeys, M.; Vlietinck, A.; De Backer, J.P.; Vauquelin, G. Adenosine-1 active ligands: Cirsimarin, a flavone glycoside from Microtea debilis. J. Nat. Prod. 1997, 60, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Rodriguez, J.P.; Lee, K.H.; Park, J.Y.; Kang, K.S.; Hahm, D.-H.; Huh, C.K.; Lee, S.C.; Lee, S. Determination of flavonoids from Cirsium japonicum var. maackii and their inhibitory activities against aldose reductase. Appl. Biol. Chem. 2017, 60, 487–496. [Google Scholar] [CrossRef]
- Lima, J.C.S.; de Oliveira, R.G.; Silva, V.C.; de Sousa, P.T.J.; Violante, I.M.P.; Macho, A.; de Oliveira Martins, D.T. Anti-inflammatory activity of 4′,6,7-trihydroxy-5-methoxyflavone from Fridericia chica (Bonpl.) L.G.Lohmann. Nat. Prod. Res. [CrossRef]
- Miranda, N.; Gerola, A.P.; Novello, C.R.; Ueda-Nakamura, T.; de Oliveira Silva, S.; Dias-Filho, B.P.; Hioka, N.; de Mello, J.C.P.; Nakamura, C.V. Pheophorbide a, a compound isolated from the leaves of Arrabidaea chica, induces photodynamic inactivation of Trypanosoma cruzi. Photodiagnosis Photodyn. Ther. 2017, 19, 256–265. [Google Scholar] [CrossRef]
- Liu, X.; Machado, G.C.; Eyles, J.P.; Ravi, V.; Hunter, D.J. Dietary supplements for treating osteoarthritis: A systematic review and meta-analysis. Br. J. Sports Med. 2018, 52, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Maksimović, Z.; Samardžić, S. Herbal Medicinal Products in the Treatment of Osteoarthritis. In Osteoarthritis Biomarkers and Treatments; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Ribeiro, A.F.C. Avaliação das atividades anti-inflamatoria, antiangiogenica e anti-tumoral de extratos de Arrabidaea chica (Humb. & Bonpl.) B. Verlot. Ph.D. Thesis, Universidade Federal de Minas Gerais—UFMG, Belo Horizonte, Brazil, 2012. [Google Scholar]
- Michel, A.F.R.M.; Melo, M.M.; Campos, P.P.; Oliveira, M.S.; Oliveira, F.A.S.; Cassali, G.D.; Ferraz, V.P.; Cota, B.B.; Andrade, S.P.; Souza-Fagundes, E.M. Evaluation of anti-inflammatory, antiangiogenic and antiproliferative activities of Arrabidaea chica crude extracts. J. Ethnopharmacol. 2015, 165, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Lima, F.C.V.M. Efeito de Arrabidaea chica Verlot na dor neuropática póstraumática em ratos. Ph.D. Thesis, Universidade Federal do Maranhão, São Luís, Brazil, 2018. [Google Scholar]
- Hohjoh, H.; Inazumi, T.; Tsuchiya, S.; Sugimoto, Y. Prostanoid receptors and acute inflammation in skin. Biochimie. 2014, 107, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, D.; Kothari, P.; Tripathi, A.K.; Singh, S.; Adhikary, S.; Ahmad, N.; Kumar, S.; Dev, K.; Mishra, V.K.; Shukla, S.; et al. Spinacia oleracea extract attenuates disease progression and sub-chondral bone changes in monosodium iodoacetate-induced osteoarthritis in rats. BMC Complement. Altern. Med. 2018, 18, 69. [Google Scholar] [CrossRef] [PubMed]
- Chien, T.-Y.; Huang, S.K.-H.; Lee, C.-J.; Tsai, P.-W.; Wang, C.-C. Antinociceptive and Anti-Inflammatory Effects of Zerumbone against Mono-Iodoacetate-Induced Arthritis. Int. J. Mol. Sci. 2016, 17, 249. [Google Scholar] [CrossRef] [PubMed]
- Verri, W.A.J.; Cunha, T.M.; Parada, C.A.; Poole, S.; Cunha, F.Q.; Ferreira, S.H. Hypernociceptive role of cytokines and chemokines: Targets for analgesic drug development? Pharmacol. Ther. 2006, 112, 116–138. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.R.; More, A.S.; Gupta, G.; Lingaraju, M.C.; Balaganur, V.; Kumar, P.; Kumar, D.; Sharma, A.K.; Mishra, S.K.; Tandan, S.K. Effect of alcoholic extract of Entada pursaetha DC on monosodium iodoacetate-induced osteoarthritis pain in rats. Indian J. Med. Res. 2015, 141, 454–462. [Google Scholar] [PubMed]
- Calado, G.P.; Lopes, A.J.O.; Costa Junior, L.M.; Lima, F.D.C.A.; Silva, L.A.; Pereira, W.S.; do Amaral, F.M.M.; Garcia, J.B.S.; Cartágenes, M.D.S.D.S.; Nascimento, F.R.F. Chenopodium ambrosioides L. Reduces Synovial Inflammation and Pain in Experimental Osteoarthritis. PLoS ONE 2015, 10, e0141886. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Momin, R.A.; Nair, M.G.; Bourquin, L.D. Cyclooxygenase inhibitory and antioxidant cyanidin glycosides in cherries and berries. Phytomedicine 2001, 8, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Dougados, M.; Nguyen, M.; Berdah, L.; Mazieres, B.; Vignon, E.; Lequesne, M. Evaluation of the structure-modifying effects of diacerein in hip osteoarthritis: ECHODIAH, a three-year, placebo-controlled trial. Evaluation of the Chondromodulating Effect of Diacerein in OA of the Hip. Arthritis Rheum. 2001, 44, 2539–2547. [Google Scholar] [CrossRef]
- Dieppe, P.A.; Lohmander, L.S. Pathogenesis and management of pain in osteoarthritis. Lancet 2005, 365, 965–973. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Marques, J.I.; Alves, J.S.F.; Torres-Rêgo, M.; Furtado, A.A.; da Silva Siqueira, E.M.; Galinari, E.; de Souza Araújo, D.F.; Guerra, G.C.B.; de Azevedo, E.P.; Fernandes-Pedrosa, M.D.F.; et al. Phytochemical Analysis by HPLC–HRESI-MS and Anti-Inflammatory Activity of Tabernaemontana catharinensis. Int. J. Mol. Sci. 2018, 19, 636. [Google Scholar] [CrossRef]
- Lopes, A.J.O.; Vasconcelos, C.C.; Pereira, F.A.N.; Silva, R.H.M.; Queiroz, P.F.S.; Fernandes, C.V.; Garcia, J.B.S.; Ramos, R.M.; Rocha, C.Q.; Lima, S.T.J.R.M.; et al. Anti-Inflammatory and Antinociceptive Activity of Pollen Extract Collected by Stingless Bee Melipona fasciculata. Int. J. Mol. Sci. 2019, 20, 4512. [Google Scholar] [CrossRef]
- Rowlinson, S.W.; Kiefer, J.R.; Prusakiewicz, J.J.; Pawlitz, J.L.; Kozak, K.R.; Kalgutkar, A.S.; Stallings, W.C.; Kurumbail, R.G.; Marnett, L.J. A novel mechanism of cyclooxygenase-2 inhibition involving interactions with Ser-530 and Tyr-385. J. Biol. Chem. 2003, 278, 45763–45769. [Google Scholar] [CrossRef]
- Xu, S.; Hermanson, D.J.; Banerjee, S.; Ghebreselasie, K.; Clayton, G.M.; Garavito, R.M.; Marnett, L.J. Oxicams bind in a novel mode to the cyclooxygenase active site via a two-water-mediated H-bonding Network. J. Biol. Chem. 2014, 289, 6799–6808. [Google Scholar] [CrossRef]
- Silva, R.H.M.; Lima, N.D.F.M.; Lopes, A.J.O.; Vasconcelos, C.C.; de Mesquita, J.W.C.; de Mesquita, L.S.S.; Lima, F.C.V.M.; Ribeiro, M.N.D.S.; Ramos, R.M.; Cartágenes, M.D.S.D.S.; et al. Antinociceptive activity of Borreria verticillata: In vivo and in silico studies. Front. Pharmacol. 2017, 8, 1–14. [Google Scholar]
- Oh, J.; Rho, H.S.; Yang, Y.; Yoon, J.Y.; Lee, J.; Hong, Y.D.; Kim, H.C.; Choi, S.S.; Kim, T.W.; Shin, S.S.; et al. Extracellular signal-regulated kinase is a direct target of the anti-inflammatory compound amentoflavone derived from Torreya nucifera. Mediators Inflamm. 2013, 2013, 761506. [Google Scholar] [CrossRef]
- Banerjee, T.; Van der Vliet, A.; Ziboh, V.A. Downregulation of COX-2 and iNOS by amentoflavone and quercetin in A549 human lung adenocarcinoma cell line. Prostaglandins. Leukot. Essent. Fatty Acids 2002, 66, 485–492. [Google Scholar] [CrossRef]
- Sakthivel, K.M.; Guruvayoorappan, C. Amentoflavone inhibits iNOS, COX-2 expression and modulates cytokine profile, NF-kappaB signal transduction pathways in rats with ulcerative colitis. Int. Immunopharmacol. 2013, 17, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Nagy, E.; Vajda, E.; Vari, C.; Sipka, S.; Farr, A.-M.; Horvath, E. Meloxicam ameliorates the cartilage and subchondral bone deterioration in monoiodoacetate-induced rat osteoarthritis. PeerJ 2017, 5, e3185. [Google Scholar] [CrossRef] [PubMed]
- Fernihough, J.; Gentry, C.; Malcangio, M.; Fox, A.; Rediske, J.; Pellas, T.; Kidd, B.; Bevan, S.; Winter, J. Pain related behaviour in two models of osteoarthritis in the rat knee. Pain 2004, 112, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Andersen, M.L.; Tufik, S. Sleep pattern in an experimental model of osteoarthritis. Pain 2008, 140, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Monville, C.; Torres, E.M.; Dunnett, S.B. Comparison of incremental and accelerating protocols of the rotarod test for the assessment of motor deficits in the 6-OHDA model. J. Neurosci. Methods 2006, 158, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Schott, E.; Berge, O.G.; Angeby-Moller, K.; Hammarstrom, G.; Dalsgaard, C.J.; Brodin, E. Weight bearing as an objective measure of arthritic pain in the rat. J. Pharmacol. Toxicol. Methods 1994, 31, 79–83. [Google Scholar] [CrossRef]
- Santos-Nogueira, E.; Redondo Castro, E.; Mancuso, R.; Navarro, X. Randall-Selitto test: A new approach for the detection of neuropathic pain after spinal cord injury. J. Neurotrauma 2012, 29, 898–904. [Google Scholar] [CrossRef]
- Randall, L.O.; Selitto, J.J. A method for measurement of analgesic activity on inflamed tissue. Arch. Int. Pharmacodyn. Ther. 1957, 111, 409–419. [Google Scholar]
- Moller, K.A.; Johansson, B.; Berge, O.G. Assessing mechanical allodynia in the rat paw with a new electronic algometer. J. Neurosci. Methods 1998, 84, 41–47. [Google Scholar] [CrossRef]
- Kellgren, J.H.; Lawrence, J.S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef]
- Pritzker, K.P.H.; Gay, S.; Jimenez, S.A.; Ostergaard, K.; Pelletier, J.-P.; Revell, P.A.; Salter, D.; van den Berg, W.B. Osteoarthritis cartilage histopathology: Grading and staging. Osteoarthr. Cartil. 2006, 14, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, UK, 2009. [Google Scholar]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView5; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Olson, A.J. Using AutoDock for ligand-receptor docking. Curr. Protoc. Bioinform. 2008, 24, 8.14.1–8.14.40. [Google Scholar]

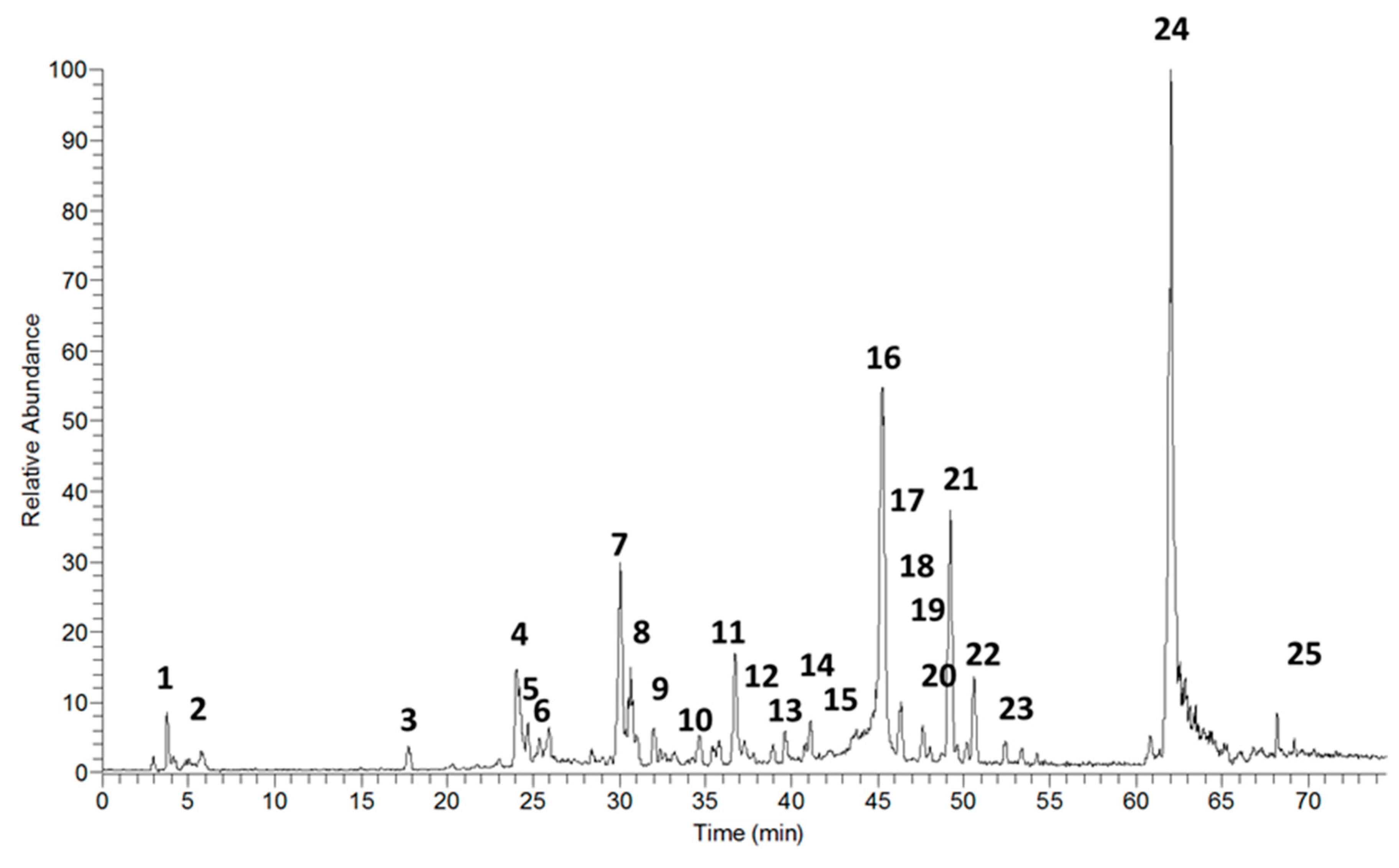
| N | RT | [M+H] | MSn Fragments | Name Suggestion for Structure | Reference |
|---|---|---|---|---|---|
| 1 | 4.48 | 299 | 289, 287, 160 | carajurin | [17,18,19] |
| 2 | 5.38 | 315 | 313, 289 | 6,7,3′-trihydroxy-5,4′-dimethoxy-flavylium | [18] |
| 3 | 17.61 | 289 | 205, 188 | 2′-hydroxy-a-naphthoflavone | [20] ID BML00331 |
| 4 | 24.13 | 289 | catechin | [21] | |
| 5 | 24.69 | 301 | 289 | 5,7-dimethoxy-4′-hydroxyflavone | |
| 6 | 25.42 | 289 | epicatechin | [21] | |
| 7 | 29.98 | 477 | 301, 289 | quercetin-o-gallate | |
| 8 | 31.12 | 465 | 301 | Quercetin-o-glucoside | [22] |
| 9 | 32.04 | 540 | 460, 301, 289 | amentoflavone | [23] |
| 10 | 34.72 | 479 | 301, 279 | isorhamnetin-3-o-glucoside | [24] |
| 11 | 36.62 | 301 | 279 | chrysoeriol | [25] |
| 12 | 37.64 | 287 | 279 | kaempferol | [26] |
| 13 | 39.65 | 463 | 330, 301 | chrysoeriol-o-glucoside | [27] |
| 14 | 41.24 | 317 | 301, 279 | isorhamnetin | [24] |
| 15 | 42.99 | 287 | 279 | luteolin | [28,29] |
| 16 | 45.25 | 303 | 6-hydroxyluteolin | [29] | |
| 17 | 46.18 | 477 | 328, 301, 279 | cirsimarin | [30,31] |
| 18 | 47.42 | 617 | 601, 301, 279 | hyperin 6″ gallate | |
| 19 | 47.64 | 301 | 279 | 6,7,3′,4′-tetrahydroxy-5-metoxy-flavilium | [32] |
| 20 | 47.94 | 302 | 301, 288, 285 | hispidulin | [29] |
| 21 | 49.23 | n.i. | |||
| 22 | 50.31–50.96 | 601 | n.i. | ||
| 23 | 52.72–54.29 | 577 | 301, 289 | catechin dimer | [29] |
| 24 | 62.14 | 819 | n.i. | ||
| 25 | 72.66 | 593 | 421, 399 | feoforbide A | [33] |
| Ligand | ΔGbind (kcal·mol−1) * | Ki (μM) ** |
|---|---|---|
| amentoflavone | −9.21 | 0.11 |
| quercetin-o-gallate | −8.86 | 0.32 |
| chrysoeriol-o-glucoside | −8.45 | 0.63 |
| catechin dimer | −8.33 | 0.78 |
| 2′-hydroxy-a-naphthoflavone | −7.98 | 1.43 |
| 6-hydroxyluteolin | −7.95 | 1.48 |
| hispidulin | −7.81 | 1.88 |
| cirsimarin | −7.61 | 2.64 |
| isorhamnetin-3-o-glucoside | −7.56 | 2.87 |
| epicatechin | −7.49 | 3.25 |
| catechin | −7.46 | 3.40 |
| hyperin 6″ gallate | −7.39 | 3.81 |
| 6,7,3′,4′-tetrahydroxy-5-metoxy-flavylium | −7.21 | 5.19 |
| 6,7,3′-trihydroxy-5,4′-dimethoxy-flavylium | −7.11 | 6.11 |
| carajurin | −7.06 | 6.71 |
| kaempferol | −7.06 | 6.73 |
| luteolin | −7.05 | 6.76 |
| chrysoeriol | −6.90 | 8.72 |
| quercetin-o-glucoside | −6.85 | 9.46 |
| isorhamnetin | −6.72 | 11.86 |
| meloxicam | −8.82 | 0.34 |
| Grade 0 | No arthrosis—Normal radiology |
| Grade I | Doubtful arthrosis—Doubtful articular space narrowing and possible |
| Grade II | Minimal osteoarthritis—Possible joint narrowing, defined osteophytes |
| Grade III | Moderate arthrosis—Defined joint narrowing, multiple moderate osteophytes, some subchondral sclerosis and possible deformity in the bone contour |
| Grade IV | Severe arthrosis—Significant articular space narrowing, severe subchondral sclerosis, defined deformity in the bone contour and large osteophytes |
| Grade (Key Feature) | Subgrade (Optional) | Associated Criteria (Tissue Reaction) |
|---|---|---|
| Grade 0: Surface intact, Cartilage intact | No subgrade | Intact, uninvolved cartilage |
| Grade 1: Surface intact | 1.0 Cells intact 1.5 Cell death | Matrix: superficial zone intact, edema and/or fibrillation; Cells:proliferation (clusters),hypertrophy; Reaction must be more than superficial fibrillation only. |
| Grade 2: Surfacediscontinuity | 2.0 Fibrillation through superficial zone 2.5 Surface abrasion with matrix loss within superficial zone | As above +Discontinuity at superficial zone; ±Cationic stain matrix depletion (Safranin O or Toluidine Blue) upper 1/3 of cartilage (mid zone); ±Disorientation of chondron columns |
| Grade 3: Verticalfissures | 3.0 Simple fissures 3.5 Branched/complex fissures | As above; ±Cationic stain depletion (Safranin O or Toluidine Blue) into lower 2/3 of cartilage (deep zone); ±New collagen formation (polarized light microscopy, Picro Sirius Red stain) |
| Grade 4: Erosion | 4.0 Superficial zone delamination 4.5 Mid zone excavation | Cartilage matrix loss, cyst formation within cartilage matrix |
| Grade 5: Denudation | 5.0 Bone surface intact 5.5 Reparative tissue surface present | Surface is sclerotic bone or reparative tissue including fibrocartilage. |
| Grade 6: Deformation | 6.0 Joint margin osteophytes 6.5 Joint margin and central osteophytes | Bone remodeling; Deformation of articular surface contour (more than osteophyte formation only); Includes: microfracture and repair |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasconcelos, C.C.; Lopes, A.J.O.; Sousa, E.L.F.; Camelo, D.S.; Lima, F.C.V.M.; Rocha, C.Q.d.; Silva, G.E.B.; Garcia, J.B.S.; Cartágenes, M.d.S.d.S. Effects of Extract of Arrabidaea chica Verlot on an Experimental Model of Osteoarthritis. Int. J. Mol. Sci. 2019, 20, 4717. https://doi.org/10.3390/ijms20194717
Vasconcelos CC, Lopes AJO, Sousa ELF, Camelo DS, Lima FCVM, Rocha CQd, Silva GEB, Garcia JBS, Cartágenes MdSdS. Effects of Extract of Arrabidaea chica Verlot on an Experimental Model of Osteoarthritis. International Journal of Molecular Sciences. 2019; 20(19):4717. https://doi.org/10.3390/ijms20194717
Chicago/Turabian StyleVasconcelos, Cleydlenne Costa, Alberto Jorge Oliveira Lopes, Emerson Lucas Frazão Sousa, Darleno Sousa Camelo, Fernando César Vilhena Moreira Lima, Cláudia Quintino da Rocha, Gyl Eanes Barros Silva, João Batista Santos Garcia, and Maria do Socorro de Sousa Cartágenes. 2019. "Effects of Extract of Arrabidaea chica Verlot on an Experimental Model of Osteoarthritis" International Journal of Molecular Sciences 20, no. 19: 4717. https://doi.org/10.3390/ijms20194717
APA StyleVasconcelos, C. C., Lopes, A. J. O., Sousa, E. L. F., Camelo, D. S., Lima, F. C. V. M., Rocha, C. Q. d., Silva, G. E. B., Garcia, J. B. S., & Cartágenes, M. d. S. d. S. (2019). Effects of Extract of Arrabidaea chica Verlot on an Experimental Model of Osteoarthritis. International Journal of Molecular Sciences, 20(19), 4717. https://doi.org/10.3390/ijms20194717







