Anti-Inflammatory and Antinociceptive Activity of Pollen Extract Collected by Stingless Bee Melipona fasciculata
Abstract
1. Introduction
2. Results
2.1. Total Phenolic Content, Total Flavonoids Content and Antioxidant Activity
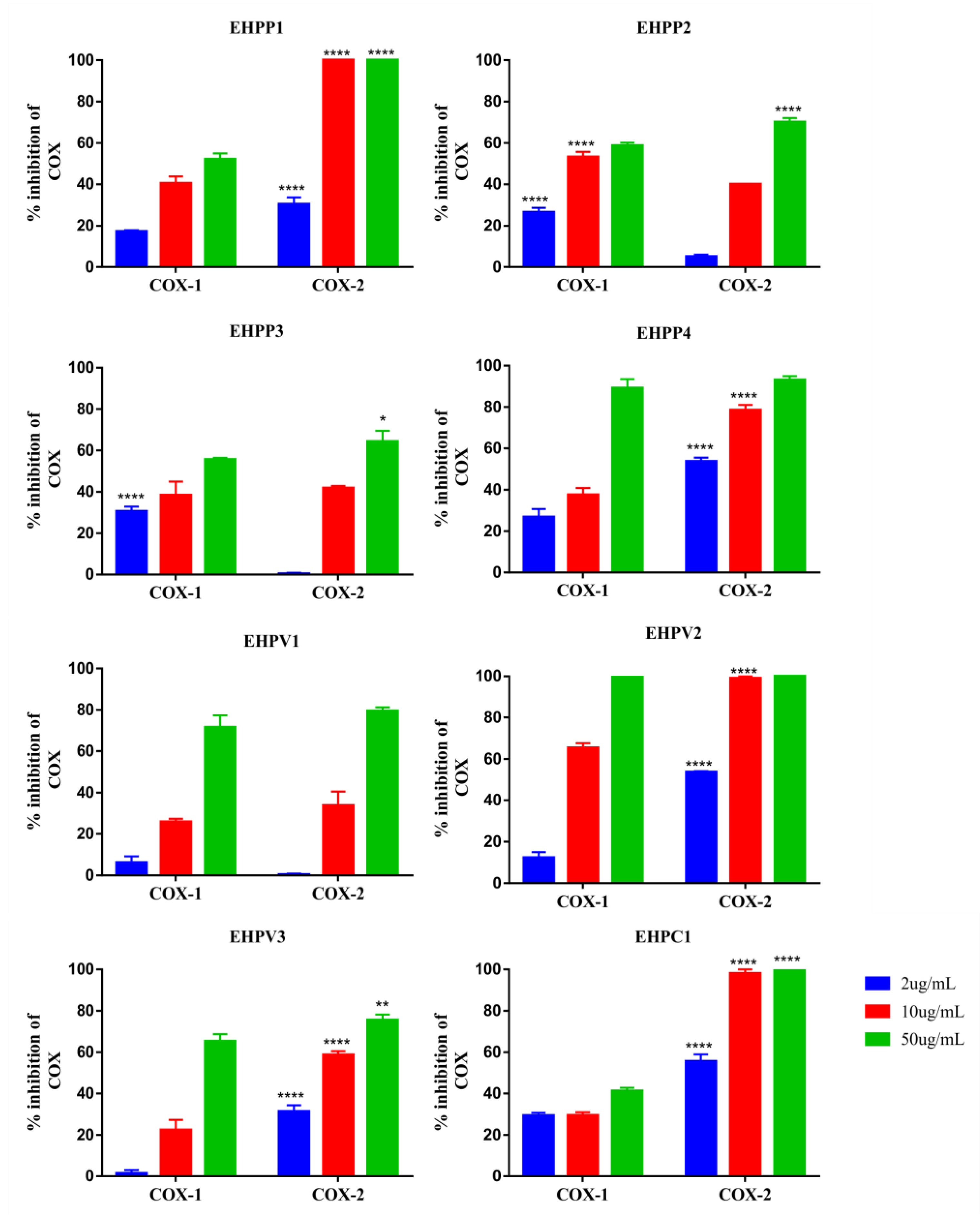
2.2. COX–1 and 2 Inhibition Assay
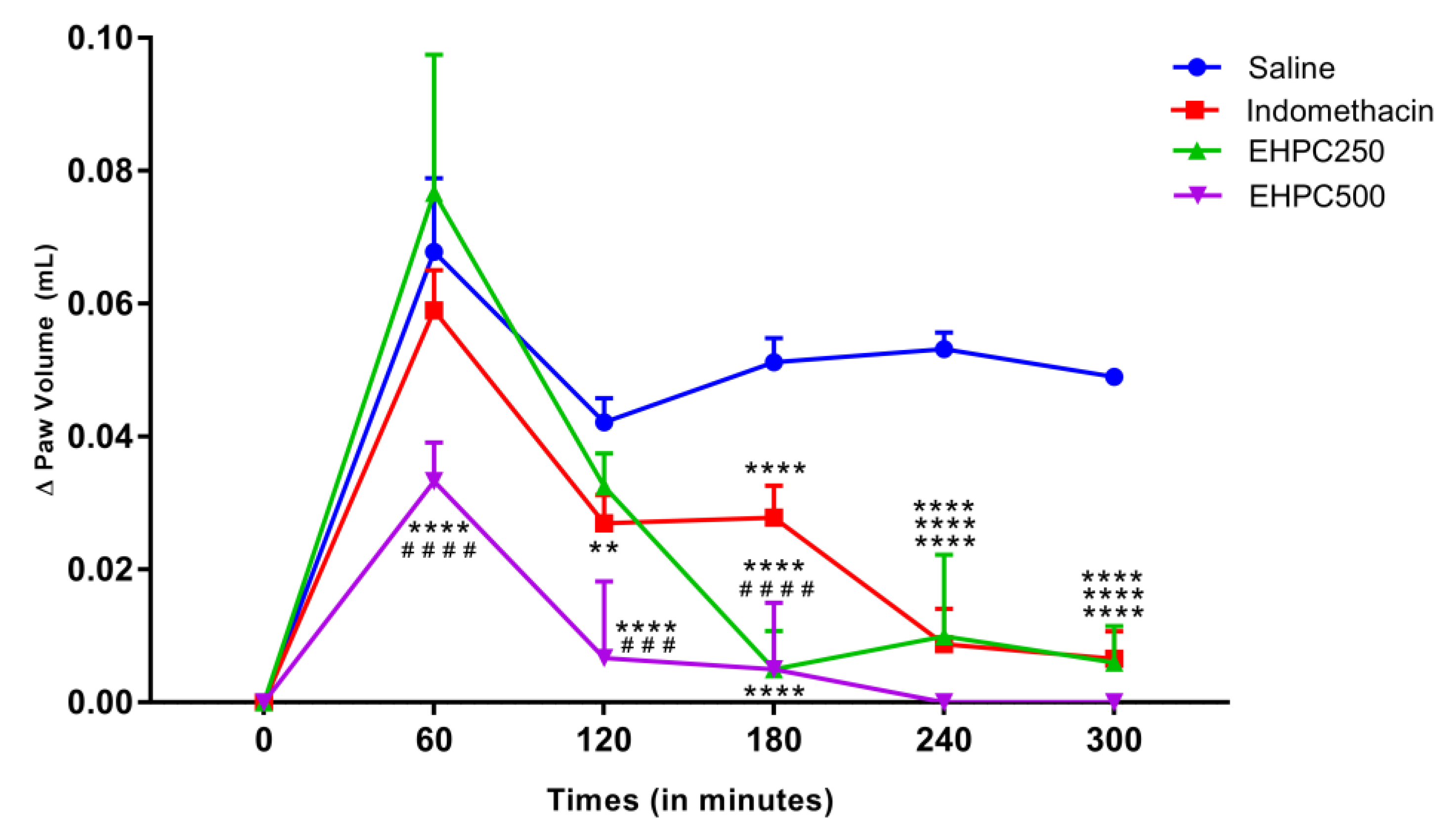
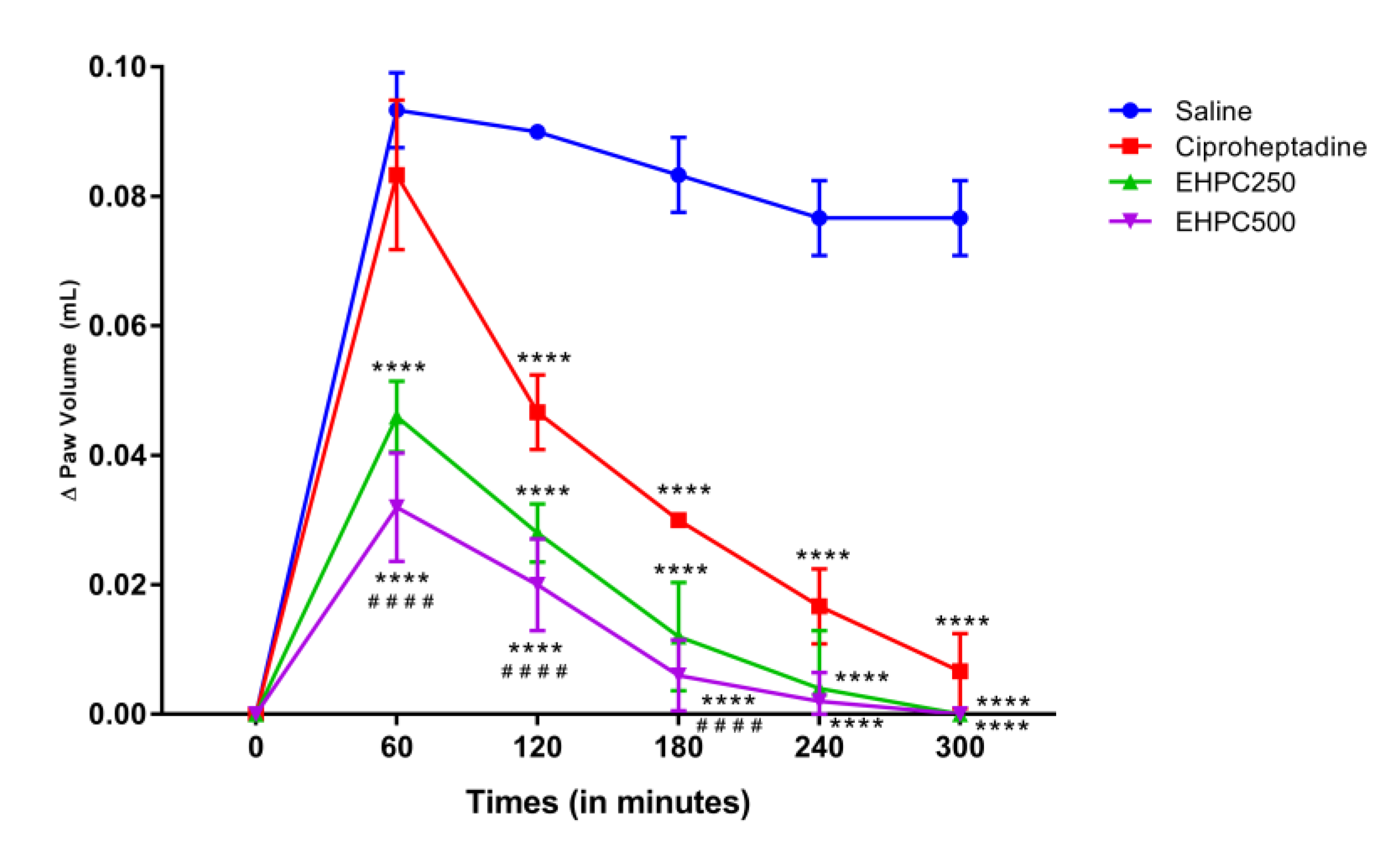
2.3. In Vivo Anti–Inflammatory Activity
2.3.1. Carrageenan–Induced Paw Edema Test
2.3.2. Dextran–Induced Paw Edema Test
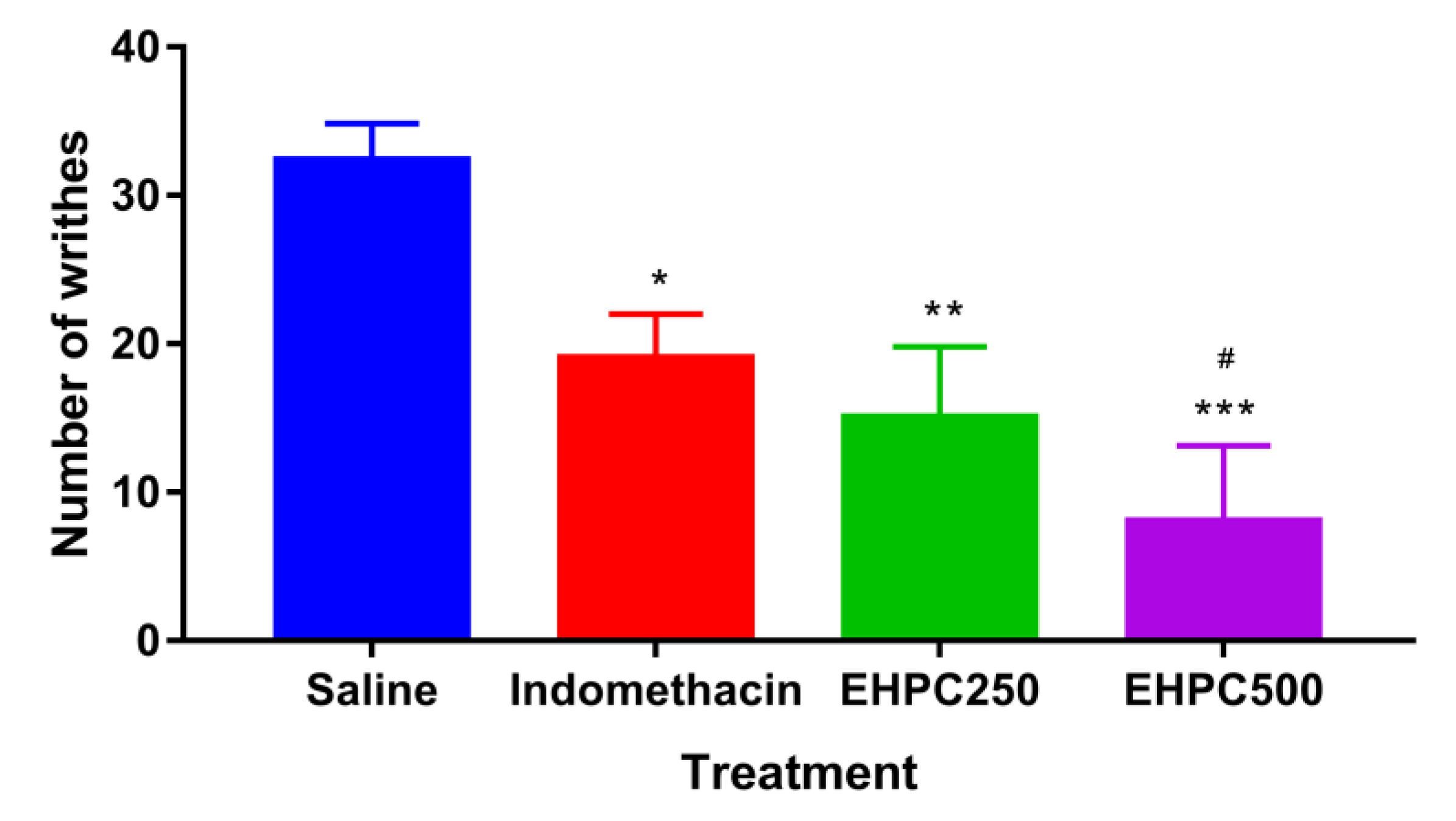
2.4. In Vivo Anti–Nociceptive Activity
2.4.1. Acetic Acid Writhing Test
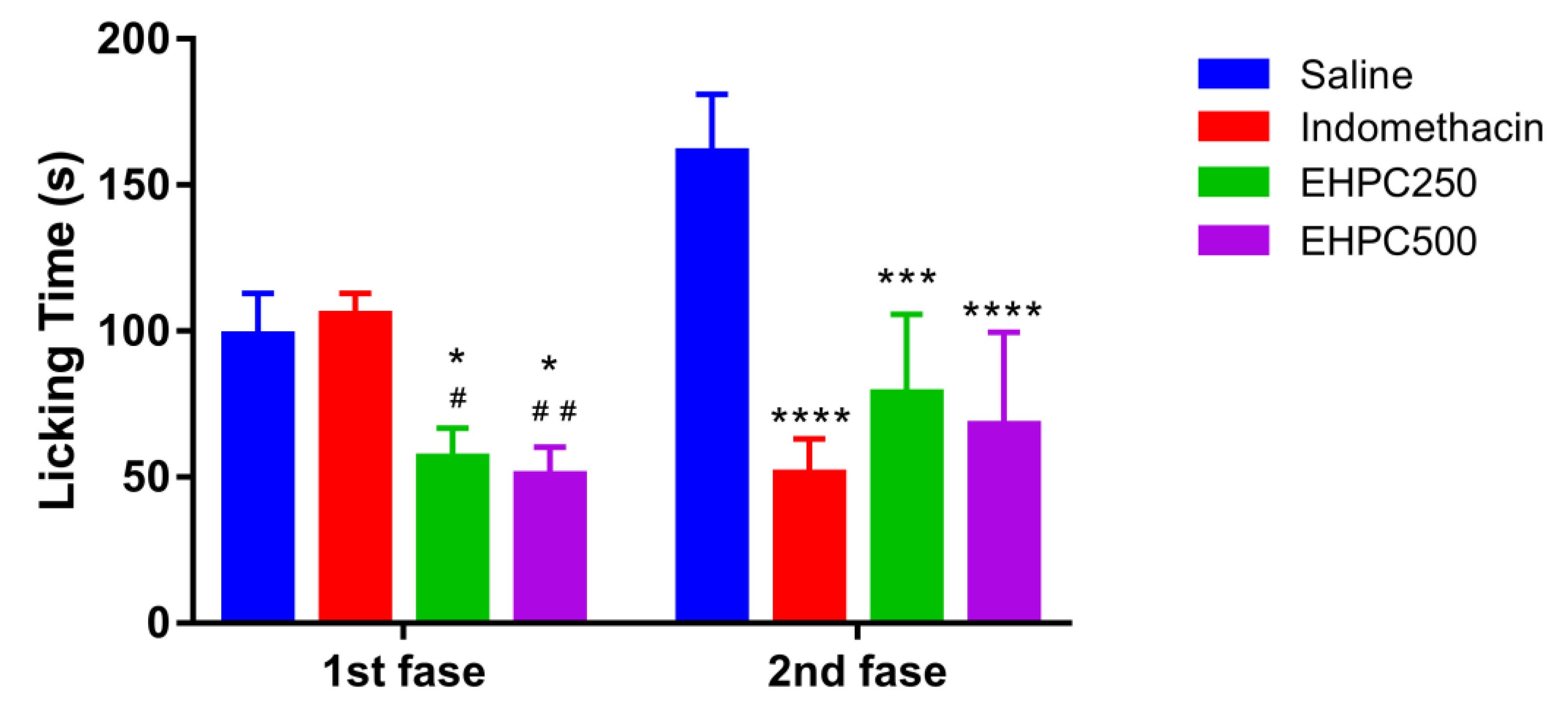
2.4.2. Formalin Test
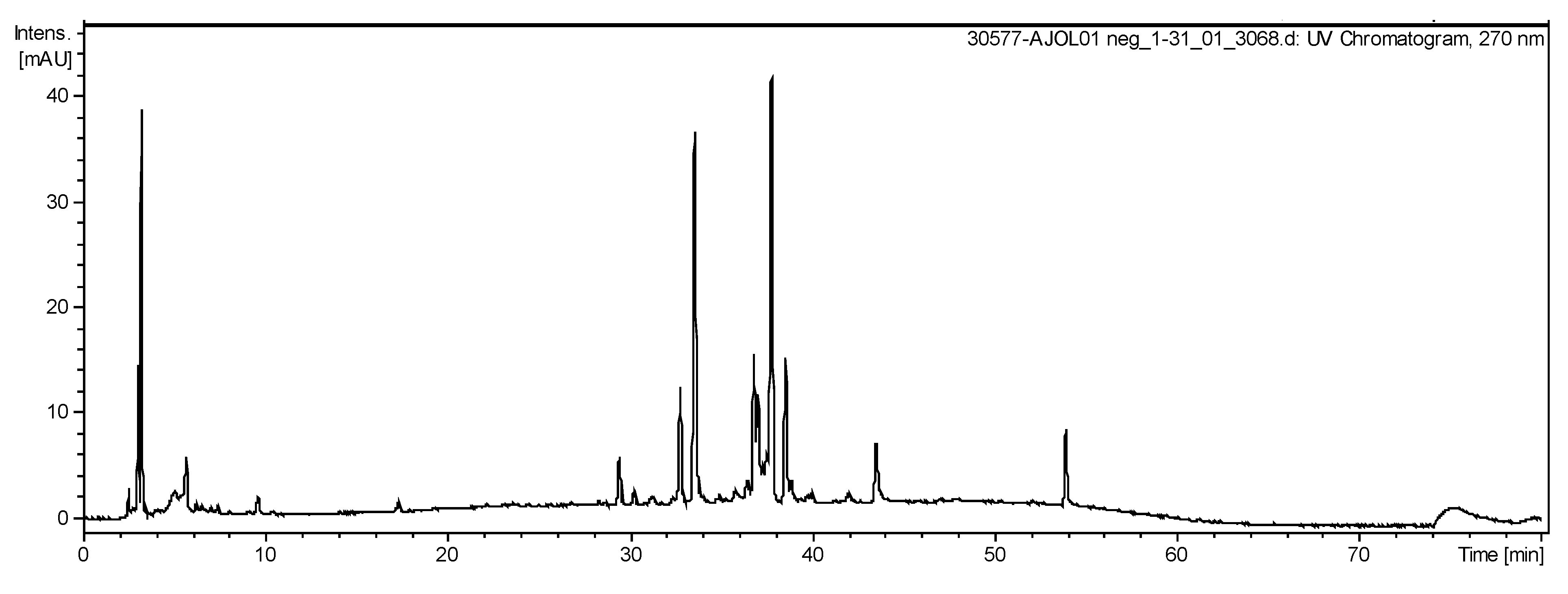
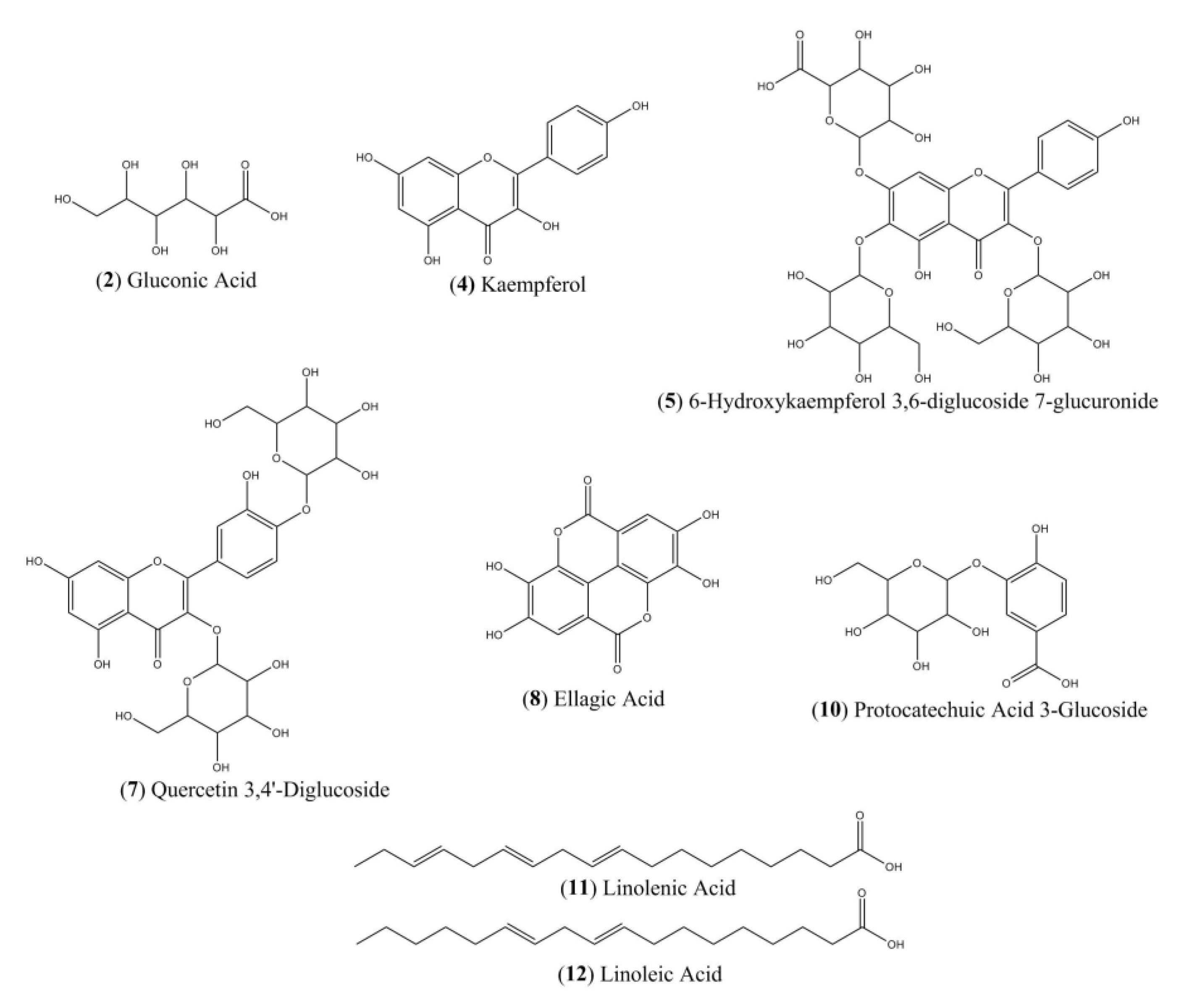
2.5. LC-ESI-IT-MS/MS Analysis
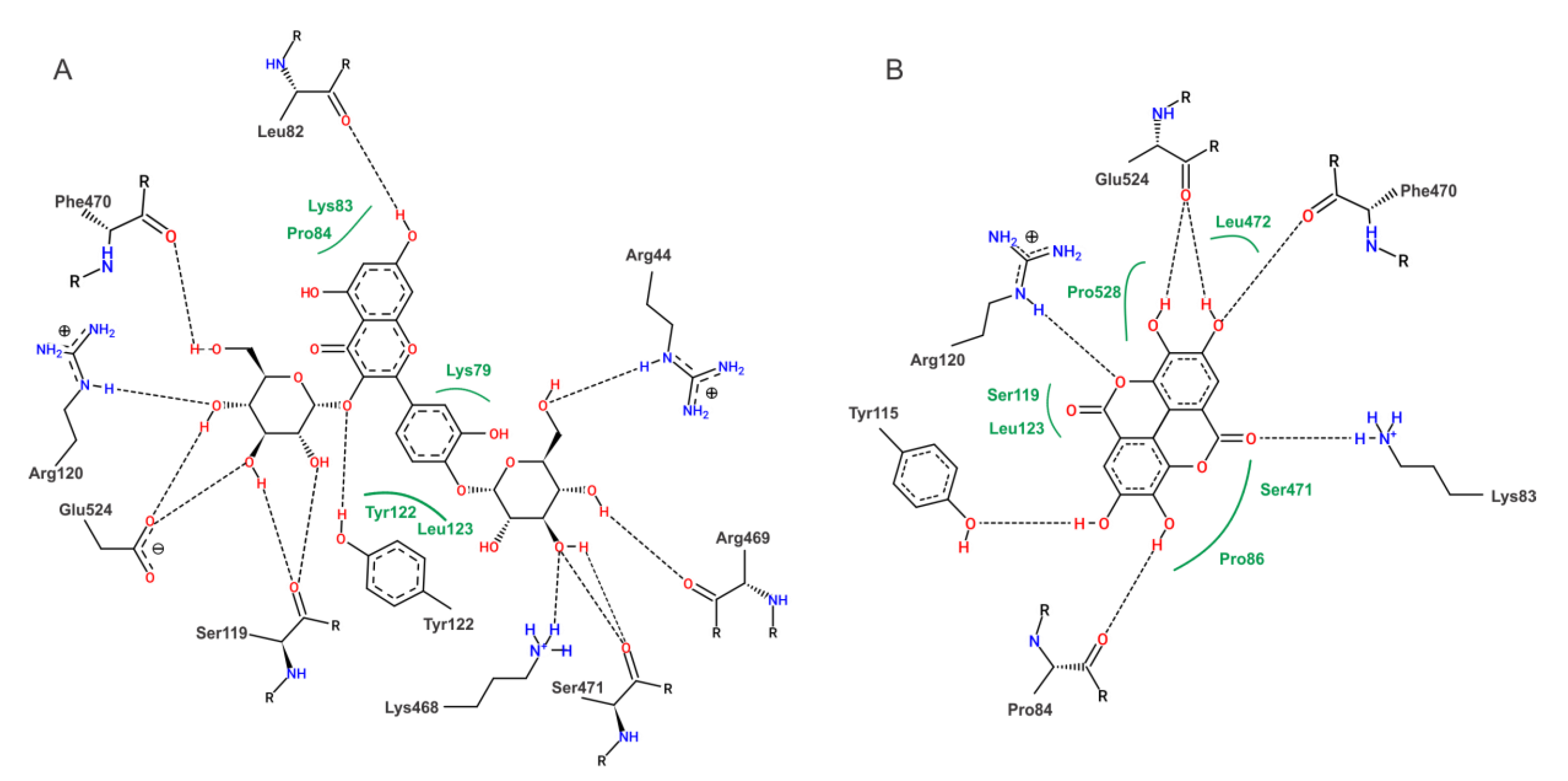
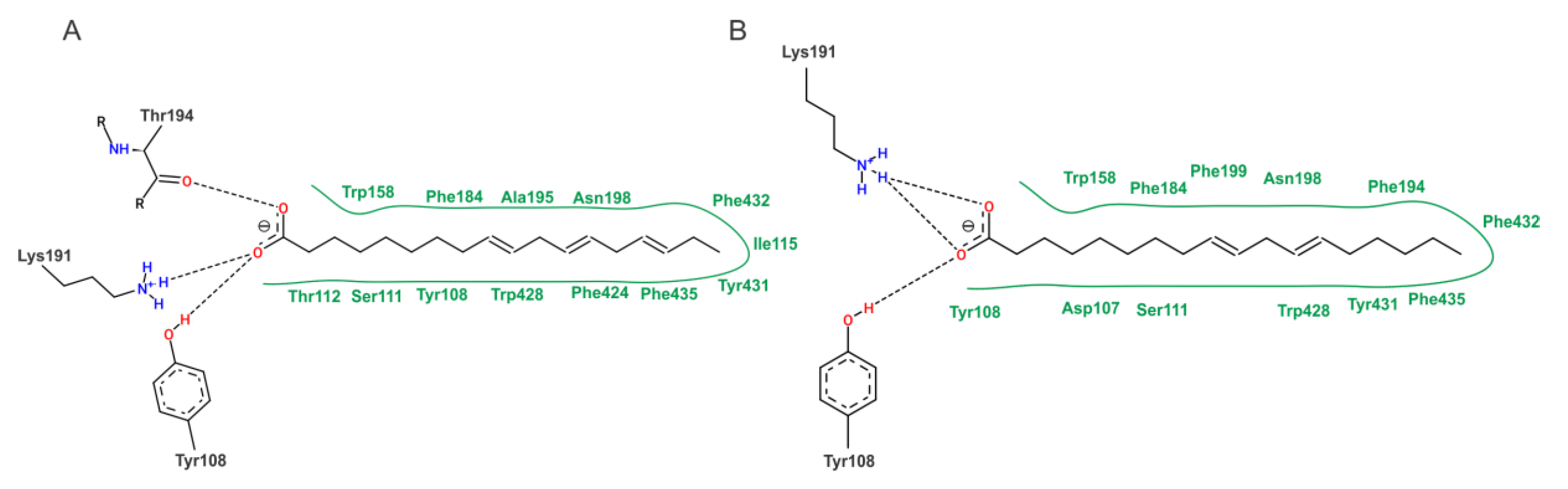
2.6. Molecular Docking
3. Discussion
4. Materials and Methods
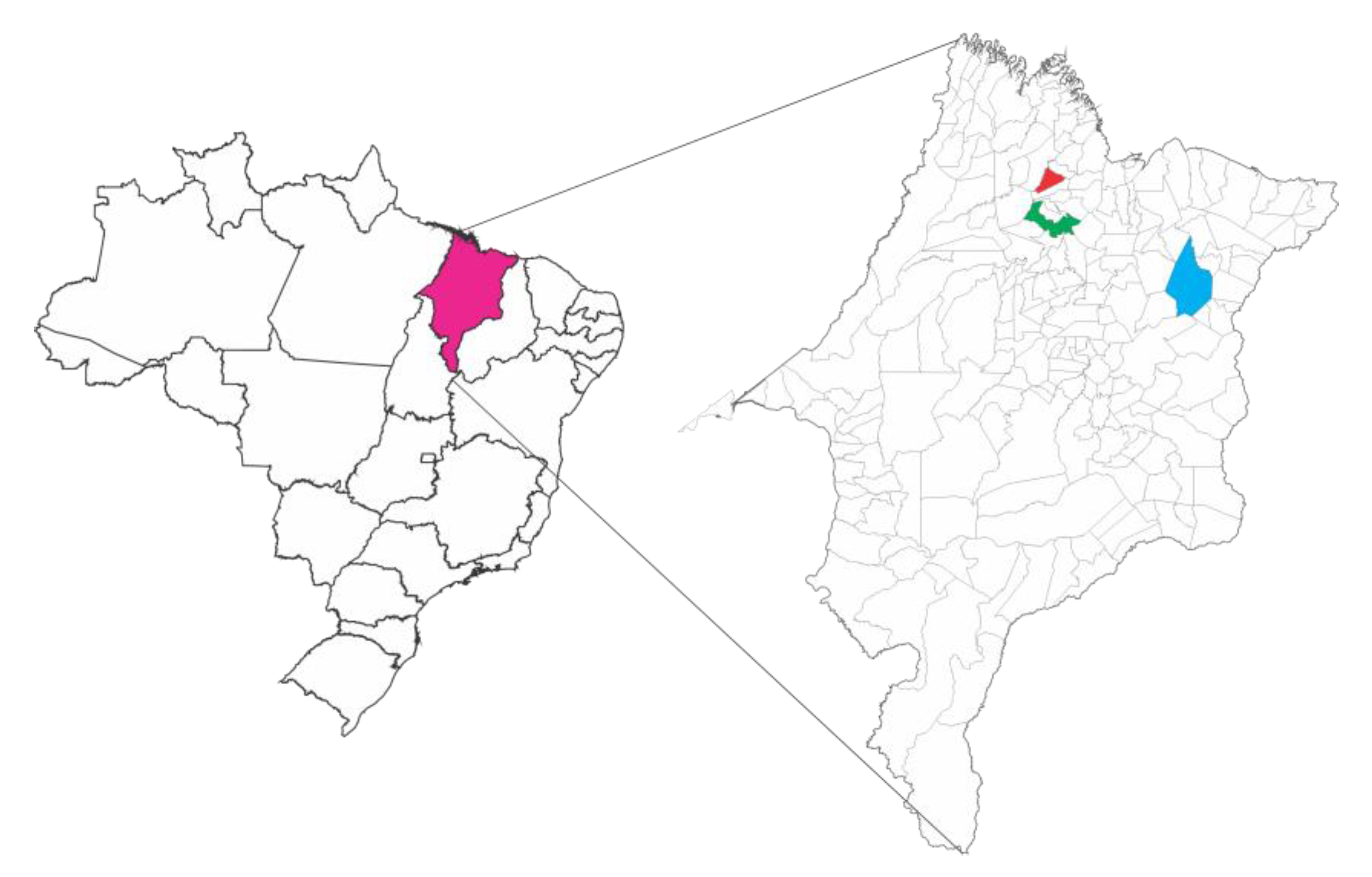
4.1. Obtaining Pollen and Preparing Extracts
4.2. Determination of Total Phenolic Content (TPC) in Hydroethanolic Pollen Extracts
4.3. Determinations of Total Flavonoid Concentration (TFC) in Hydroethanolic Pollen Extracts
4.4. Determination of Antioxidant Activity
4.4.1. DPPH• Radical Scavenging Activity
4.4.2. Ferric Reducing Antioxidant Power Assay (FRAP)
4.4.3. ABTS•+ Assay
4.5. COX Inhibition Assay
4.6. Animals
4.7. Anti–Inflammatory Activity
4.7.1. Carrageenan–Induced Paw Edema Test
4.7.2. Dextran–Induced Paw Edema Test
4.8. Anti–Nociceptive Activity
4.8.1. Acetic Acid Writhing Test
4.8.2. Formalin Test
4.9. HPLC– ESI–MS/MS Analysis
4.10. Computational Study
4.10.1. Predictive Models and Theoretical Calculations
4.10.2. Molecular Docking
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| LPS | lipopolysaccharide |
| NO | Nitric oxide |
| TNF-α | tumor necrosis fator α |
| IL | Interleukin |
| DPPH• | 2,2-difenil-1-picrilhidrazil |
| FRAP | Ferric reducing antioxidant power |
| ABTS | 2,2′-azinobis-3-ethylbenzotiazoline-6-sulfonic acid |
| EHPP | Hydroethanolic pollen extract from Palmeirândia–MA, Brazil |
| EHPV | Hydroethanolic pollen extract from Viana–MA, Brazil |
| EHPC | Hydroethanolic pollen extract from Chapadinha–MA, Brazil |
| IC50 | Concentration of sample necessary to cause 50% inhibition |
| COX | Cyclooxygenase |
| TPC | Total Phenolic Content |
| TFC | Total Flavonoid Concentration |
| NI | Not identified |
| HPLC | High performance liquid chromatography |
| LC-ESI-IT-MS/MS | liquid chromatography coupled to mass spectrometer with electrospray ionization and ion trap analyzer |
| RMSD | root mean square deviation |
| LGA | Lamarckian genetic algorithm |
| DFT | Density Functional Theory |
| UFMA | Federal University of Maranhão |
| CONCEA | National Council for Animal Experimentation Control |
| CEUA | Ethics in Animal Use Committee |
| AlCl3 | Aluminum chloride |
| NaCO3 | Sodium carbonate |
| 5-HT | 5-hydroxytryptamine |
References
- Kibble, M.; Saarinen, N.; Tang, J.; Wennerberg, K.; Makela, S.; Aittokallio, T. Network pharmacology applications to map the unexplored target space and therapeutic potential of natural products. Nat. Prod. Rep. 2015, 32, 1249–1266. [Google Scholar] [CrossRef] [PubMed]
- Mastinu, A.; Bonini, S.A.; Rungratanawanich, W.; Aria, F.; Marziano, M.; Maccarinelli, G.; Abate, G.; Premoli, M.; Memo, M.; Uberti, D. Gamma-oryzanol Prevents LPS-induced Brain Inflammation and Cognitive Impairment in Adult Mice. Nutrients 2019, 11, 728. [Google Scholar] [CrossRef] [PubMed]
- Bonini, S.A.; Premoli, M.; Tambaro, S.; Kumar, A.; Maccarinelli, G.; Memo, M.; Mastinu, A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018, 227, 300–315. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Premoli, M.; Aria, F.; Bonini, S.A.; Maccarinelli, G.; Gianoncelli, A.; Memo, M.; Mastinu, A. Cannabimimetic plants: Are they new cannabinoidergic modulators? Planta 2019, 249, 1681–1694. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.V.; Krishnan, K.T.; Salleh, N.; Gan, S.H. Biological and therapeutic effects of honey produced by honey bees and stingless bees: A comparative review. Rev. Bras. Farmacogn. 2016, 26, 657–664. [Google Scholar] [CrossRef]
- Gabriele, M.; Parri, E.; Felicioli, A.; Sagona, S.; Pozzo, L.; Biondi, C.; Domenici, V.; Pucci, L. Phytochemical composition and antioxidant activity of Tuscan bee pollen of different botanic origins. Ital. J. Food Sci. 2015, 27, 248–259. [Google Scholar]
- Komosinska-Vassev, K.; Olczyk, P.; Kazmierczak, J.; Mencner, L.; Olczyk, K. Bee pollen: Chemical composition and therapeutic application. Evid. Based Complement. Altern. Med. 2015, 2015, 297425. [Google Scholar] [CrossRef]
- Yang, K.; Wu, D.; Ye, X.; Liu, D.; Chen, J.; Sun, P. Characterization of chemical composition of bee pollen in China. J. Agric. Food Chem. 2013, 61, 708–718. [Google Scholar] [CrossRef]
- Fatrcova-Sramkova, K.; Nozkova, J.; Kacaniova, M.; Mariassyova, M.; Rovna, K.; Stricik, M. Antioxidant and antimicrobial properties of monofloral bee pollen. J. Environ. Sci. Health Part B 2013, 48, 133–138. [Google Scholar] [CrossRef]
- Denisow, B.; Denisow-Pietrzyk, M. Biological and therapeutic properties of bee pollen: A review. J. Sci. Food Agric. 2016, 96, 4303–4309. [Google Scholar] [CrossRef]
- Sattler, J.A.G.; de Melo, I.L.P.; Granato, D.; Araújo, E.; da Silva de Freitas, A.; Barth, O.M.; Sattler, A.; de Almeida-Muradian, L.B. Impact of origin on bioactive compounds and nutritional composition of bee pollen from southern Brazil: A screening study. Food Res. Int. 2015, 77, 82–91. [Google Scholar] [CrossRef]
- Villas-Bôas, J. Manual Tecnológico de Aproveitamento Integral dos Produtos das Nativas Sem Ferrão, 2nd ed.; Instituto Sociedade, População e Natureza (ISPN): Brasília, Brazil, 2018; ISBN 9788563288080. [Google Scholar]
- Lee, K.-H.; Kim, A.-J.; Choi, E.-M. Antioxidant and antiinflammatory activity of pine pollen extract in vitro. Phytother. Res. 2009, 23, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Kanashiro, A.; Souza, J.G.; Kabeya, L.M.; Azzolini, A.E.C.S.; Lucisano-Valim, Y.M. Elastase release by stimulated neutrophils inhibited by flavonoids: Importance of the catechol group. Z. Naturforsch. C 2007, 62, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Sahin, H.; Aliyazicioglu, R.; Yildiz, O.; Kolayli, S.; Innocenti, A.; Supuran, C.T. Honey, pollen, and propolis extracts show potent inhibitory activity against the zinc metalloenzyme carbonic anhydrase. J. Enzyme Inhib. Med. Chem. 2011, 26, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Dutra, R.P.; Nascimento, F.R.F.; Guerra, R.N.M.; De Barros Abreu, B.V.; Cunha, M.S.; Batista, M.C.A.; Ribeiro, M.N.S.; Torres, L.M.B. Phenolic acids, hydrolyzable tannins, and antioxidant activity of geopropolis from the stingless bee melipona fasciculata smith. J. Agric. Food Chem. 2014, 62, 2549–2557. [Google Scholar] [CrossRef] [PubMed]
- Batista, M.C.A.; Abreu, B.V.; De, B.; Dutra, R.P.; Cunha, M.S.; Amaral, F.M.M.D.; Torres, L.M.B.; Ribeiro, M.N.; De, S. Chemical composition and antioxidant activity of geopropolis produced by Melipona fasciculata (Meliponinae) in flooded fields and cerrado areas of MaranhÃ\poundso State, northeastern Brazil. Acta Amaz. 2016, 46, 315–322. [Google Scholar] [CrossRef]
- Dutra, R.P.; Bezerra, J.L.; Silva, M.C.P.; Batista, M.C.A.; Patrício, F.J.B.; Nascimento, F.R.F.; Ribeiro, M.N.S.; Guerra, R.N.M. Antileishmanial activity and chemical composition from Brazilian geopropolis produced by stingless bee Melipona fasciculata. Rev. Bras. Farmacogn. 2019, 29, 287–293. [Google Scholar] [CrossRef]
- Liberio, S.A.; Pereira, A.J.O.L.A.; Dutra, R.P.; Reis, A.S.; Araujo, M.J.A.M.; Mattar, N.S.; Silva, L.A.; Ribeiro, M.N.S.; Nascimento, F.R.F.; Guerra, R.N.M.; et al. Antimicrobial activity against oral pathogens and immunomodulatory effects and toxicity of geopropolis produced by the stingless bee Melipona fasciculata Smith. BMC Complement. Altern. Med. 2011, 11, 108. [Google Scholar] [CrossRef]
- Cunha, M.S. Composição Química e Atividade Antitumoral da Geoprópolis de Melipona fasciculata Smith. Ph.D. Thesis, Universidade Federal do Maranhão, São Luís, MA, Brazil, 2017. [Google Scholar]
- Batista, M.C.A. Bioprospecção Anti-Helmíntica de Geoprópolis de Melipona fasciculata Smith em Testes In Vitro com Ovos e Larvas de Haemochus Contortus de Pequenos Ruminantes. Ph.D. Thesis, Universidade Federal do Maranhão, São Luís, MA, Brazil, 2016. [Google Scholar]
- Carpes, S.T.; Mourão, G.B.; De Alencar, S.M.; Masson, M.L. Chemical composition and free radical scavenging activity of Apis mellifera bee pollen from Southern Brazil. Braz. J. Food Technol. 2009, 12, 220–229. [Google Scholar] [CrossRef]
- Morais, M.; Moreira, L.; Feas, X.; Estevinho, L.M. Honeybee-collected pollen from five Portuguese Natural Parks: Palynological origin, phenolic content, antioxidant properties and antimicrobial activity. Food Chem. Toxicol. 2011, 49, 1096–1101. [Google Scholar] [CrossRef]
- Akin, M.; Nalbantoglu, S.; Saki, N. Total phenols, antioxidant potential and tyrosinase inhibitory activity of honeybee collected pollen from Turkey. Res. J. Biotechnol. 2013, 8, 15–18. [Google Scholar]
- LeBlanc, B.W.; Davis, O.K.; Boue, S.; DeLucca, A.; Deeby, T. Antioxidant activity of Sonoran Desert bee pollen. Food Chem. 2009, 115, 1299–1305. [Google Scholar] [CrossRef]
- Mărghitaş, L.A.; Stanciu, O.G.; Dezmirean, D.S.; Bobiş, O.; Popescu, O.; Bogdanov, S.; Campos, M.G. In vitro antioxidant capacity of honeybee-collected pollen of selected floral origin harvested from Romania. Food Chem. 2009, 115, 878–883. [Google Scholar] [CrossRef]
- de Pascual-Teresa, S.; Johnston, K.L.; DuPont, M.S.; O’Leary, K.A.; Needs, P.W.; Morgan, L.M.; Clifford, M.N.; Bao, Y.; Williamson, G. Quercetin Metabolites Downregulate Cyclooxygenase-2 Transcription in Human Lymphocytes Ex Vivo but Not In Vivo. J. Nutr. 2004, 134, 552–557. [Google Scholar] [CrossRef]
- Chandrasekharan, N.V.; Dai, H.; Roos, K.L.T.; Evanson, N.K.; Tomsik, J.; Elton, T.S.; Simmons, D.L. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: Cloning, structure, and expression. Proc. Natl. Acad. Sci. USA 2002, 99, 13926–13931. [Google Scholar] [CrossRef]
- Carvalho, W.A.; Carvalho, R.D.S.; Rios-Santos, F. Analgésicos inibidores específicos da ciclooxigenase-2: Avanços terapêuticos. Rev. Bras. Anestesiol. 2004, 54, 448–464. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, J.; Zhong, L. Hydroxytyrosol inhibits pro-inflammatory cytokines, iNOS, and COX-2 expression in human monocytic cells. Naunyn. Schmiedeberg’s Arch. Pharmacol. 2009, 379, 581–586. [Google Scholar] [CrossRef]
- Maruyama, H.; Sakamoto, T.; Araki, Y.; Hara, H. Anti-inflammatory effect of bee pollen ethanol extract from Cistus sp. of Spanish on carrageenan-induced rat hind paw edema. BMC Complement. Altern. Med. 2010, 10, 30. [Google Scholar] [CrossRef]
- Lee, J.-H. Intracellular Antioxidant Activity and Inhibition of Bee Pollens on the Production of Inflammatory Mediators (P06-081-19). Curr. Dev. Nutr. 2019, 3, 596. [Google Scholar] [CrossRef]
- Vinegar, R.; Schreiber, W.; Hugo, R. Biphasic development of carrageenin edema in rats. J. Pharmacol. Exp. Ther. 1969, 166, 96–103. [Google Scholar]
- Vinegar, R.; Truax, J.F.; Selph, J.L.; Johnston, P.R.; Venable, A.J.O.L.; McKenzie, K.K. Pathway to carrageenan-induced inflammation in the hind limb of the rat. Fed. Proc. 1987, 46, 118–126. [Google Scholar]
- Lo, T.N.; Almeida, A.P.; Beaven, M.A. Dextran and carrageenan evoke different inflammatory responses in rat with respect to composition of infiltrates and effect of indomethacin. J. Pharmacol. Exp. Ther. 1982, 221, 261–267. [Google Scholar]
- Vasconcelos, S.M.M.; Sales, G.T.M.; Lima, N.; Lobato, R.D.F.G.; Macêdo, S.D.; Barbosa-Filho, J.M.; Leal, L.K.A.M.; Fonteles, M.M.F.; Sousa, F.C.F.; Oliveira, J.L.; et al. Anti-inflammatory activities of the hydroalcoholic extracts from Erythrina velutina and E. mulungu in mice. Rev. Bras. Farmacogn. 2011, 21, 1155–1158. [Google Scholar] [CrossRef]
- Arslan, R.; Bektas, N.; Ozturk, Y. Antinociceptive activity of methanol extract of fruits of Capparis ovata in mice. J. Ethnopharmacol. 2010, 131, 28–32. [Google Scholar] [CrossRef]
- Bahamonde, S.M.A.; Flores, M.L.; CÃ\textthreesuperiorrdoba, O.L.; Taira, C.A.; Gorzalczany, S. Antinociceptive and anti-inflammatory activities of an aqueous extract of Chiliotrichum diffusum. Rev. Bras. Farmacogn. 2013, 23, 699–705. [Google Scholar] [CrossRef]
- Abdulmalik, I.A.; Sule, M.I.; Musa, A.M.; Yaro, A.H.; Abdullahi, M.I.; Abdulkadir, M.F.; Yusuf, H. Evaluation of analgesic and anti-inflammatory effects of ethanol extract of Ficus iteophylla leaves in rodents. Afr. J. Tradit. Complement. Altern. Med. AJTCAM 2011, 8, 462–466. [Google Scholar] [CrossRef][Green Version]
- Parandin, R.; Daroogari, S. Anti-Inflammatory and Antinociceptive Activities of the Ethanolic Extract of Propolis in Male Mice and Rats. Zahedan J. Res. Med. Sci. 2019, 21. [Google Scholar] [CrossRef]
- Sulaiman, M.R.; Hussain, M.K.; Zakaria, Z.A.; Somchit, M.N.; Moin, S.; Mohamad, A.S.; Israf, D.A. Evaluation of the antinociceptive activity of Ficus deltoidea aqueous extract. Fitoterapia 2008, 79, 557–561. [Google Scholar] [CrossRef]
- Milano, J.; Oliveira, S.M.; Rossato, M.F.; Sauzem, P.D.; Machado, P.; Beck, P.; Zanatta, N.; Martins, M.A.P.; Mello, C.F.; Rubin, M.A.; et al. Antinociceptive effect of novel trihalomethyl-substituted pyrazoline methyl esters in formalin and hot-plate tests in mice. Eur. J. Pharmacol. 2008, 581, 86–96. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Khatun, A.; Imam, M.Z. Evaluation of Antinociceptive Activity of Ethanol Extract of Leaves of Adenanthera pavonina. Evid. Based. Complement. Altern. Med. 2015, 2015, 412497. [Google Scholar] [CrossRef]
- Choi, E.-M. Antinociceptive and antiinflammatory activities of pine (Pinus densiflora) pollen extract. Phytother. Res. 2007, 21, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Ambriz-Pérez, D.L.; Leyva-López, N.; Gutierrez-Grijalva, E.P.; Heredia, J.B. Phenolic compounds: Natural alternative in inflammation treatment. A Review. Cogent Food Agric. 2016, 2, 1131412. [Google Scholar]
- Złotek, U.; Gawlik-Dziki, U.; Dziki, D.; Świeca, M.; Nowak, R.; Martinez, E. Influence of Drying Temperature on Phenolic Acids Composition and Antioxidant Activity of Sprouts and Leaves of White and Red Quinoa. J. Chem. 2019. [Google Scholar] [CrossRef]
- Kawasaki, M.; Toyoda, M.; Teshima, R.; Sawada, J.; Saito, Y. Effect of alpha-linolenic acid on the metabolism of omega-3 and omega-6 polyunsaturated fatty acids and histamine release in RBL-2H3 cells. Biol. Pharm. Bull. 1994, 17, 1321–1325. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tasaka, K.; Akagi, M.; Miyoshi, K.; Mio, M.; Makino, T. Anti-allergic constituents in the culture medium of Ganoderma lucidum. (I). Inhibitory effect of oleic acid on histamine release. Agents Actions 1988, 23, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Fagali, N.; Catala, A. Antioxidant activity of conjugated linoleic acid isomers, linoleic acid and its methyl ester determined by photoemission and DPPH techniques. Biophys. Chem. 2008, 137, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.Y.; Nile, S.H.; Jung, Y.-S.; Keum, Y.S. Antioxidant and antiplatelet potential of different methanol fractions and flavonols extracted from onion (Allium cepa L.). 3 Biotech 2018, 8, 155. [Google Scholar] [CrossRef] [PubMed]
- Nile, S.H.; Nile, A.S.; Keum, Y.S.; Sharma, K. Utilization of quercetin and quercetin glycosides from onion (Allium cepa L.) solid waste as an antioxidant, urease and xanthine oxidase inhibitors. Food Chem. 2017, 235, 119–126. [Google Scholar] [CrossRef]
- Nile, A.; Nile, S.H.; Kim, D.H.; Keum, Y.S.; Seok, P.G.; Sharma, K. Valorization of onion solid waste and their flavonols for assessment of cytotoxicity, enzyme inhibitory and antioxidant activities. Food Chem. Toxicol. 2018, 119, 281–289. [Google Scholar] [CrossRef]
- García-Niño, W.R.; Zazueta, C. Ellagic acid: Pharmacological activities and molecular mechanisms involved in liver protection. Pharmacol. Res. 2015, 97, 84–103. [Google Scholar] [CrossRef]
- El-Shitany, N.A.; El-Bastawissy, E.A.; El-desoky, K. Ellagic acid protects against carrageenan-induced acute inflammation through inhibition of nuclear factor kappa B, inducible cyclooxygenase and proinflammatory cytokines and enhancement of interleukin-10 via an antioxidant mechanism. Int. Immunopharmacol. 2014, 19, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Dutra, R.P.; Nogueira, A.M.C.; Marques, R.R.D.O.; Costa, M.C.P.; Ribeiro, M.N.S. Avaliação farmacognóstica de geoprópolis de Melipona fasciculata Smith da Baixada maranhense, Brasil. Braz. J. Pharmacogn. 2008, 18, 557–562. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxid. Power”: The Frap Assay. Anal. Biochem. 1996, 76, 70–76. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved Abts Radical Cation Decolorization Assay Roberta. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Chagas, V.T.; de Sousa Coelho, R.M.R.; Gaspar, R.S.; da Silva, S.A.; Mastrogiovanni, M.; de Jesus Mendonça, C.; de Sousa Ribeiro, M.N.; de Andrade Paes, A.M.; Trostchansky, A. Corrigendum to “Protective Effects of a Polyphenol-Rich Extract from Syzygium cumini (L.) Skeels Leaf on Oxidative Stress-Induced Diabetic Rats”. Oxid. Med. Cell. Longev. 2019, 2019, 5785798. [Google Scholar] [PubMed]
- Winter, C.A.; Risley, E.A.; Nuss, G.W. Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc. Soc. Exp. Biol. Med. 1962, 111, 544–547. [Google Scholar] [CrossRef]
- Silva, R.H.M.; Lima, N.D.; Lopes, A.J.O.; Vasconcelos, C.C.; de Mesquita, J.W.C.; de Mesquita, L.S.S.; Lima, F.C.V.M.; Ribeiro, M.N.S.; Ramos, R.M.; Cartagenes, M.D.S.S.; et al. Antinociceptive Activity of Borreria verticillata: In vivo and in silico Studies. Front. Pharmacol. 2017, 8, 283. [Google Scholar] [CrossRef]
- Koster, R.; Anderson, M.; De Beer, E.J. Acetic Acid-Induced Analgesic Screening. Fed. Proc. 1959, 18, 418–420. [Google Scholar]
- Hunskaar, S.; Hole, K. The formalin test in mice: Dissociation between inflammatory and non-inflammatory pain. Pain 1987, 30, 103–114. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView5; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Goodsell, D.S.; Morris, G.M.; Olson, A.J. Automated docking of flexible ligands: Applications of AutoDock. J. Mol. Recognit. 1996, 9, 1–5. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Olson, A.J. Using AutoDock for ligand-receptor docking. Curr. Protoc. Bioinform. 2008, 8, 8–14. [Google Scholar]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Calado, G.P.; Lopes, A.J.O.; Costa Junior, L.M.; Lima, F.; Silva, L.A.; Pereira, W.S.; Amaral, F.M.; Garcia, J.B.; Cartagenes, M.S.; Nascimento, F.R. Chenopodium ambrosioides L. Reduces Synovial Inflammation and Pain in Experimental Osteoarthritis. PLoS ONE 2015, 10, e0141886. [Google Scholar] [CrossRef]
| Extrato | CPT (%) a,b | CFT (%) a,c | DPPH• IC50 (μg/mL) | FRAP (mmol Fe2+/g) | ABTS•+ IC50 (μg/mL) |
|---|---|---|---|---|---|
| EHPP1 | 11.06 ± 0.08 | 1.47 ± 0.06 | 205.17 ± 0.08 c | 0.99 ± 0.05 b | 34.30 ± 0.22 b |
| EHPP2 | 8.36 ± 0.82 | 0.94 ± 0.01 | 373.56 ± 1.32 d | 0.83 ± 0.08 b | - |
| EHPP3 | 10.22 ± 0.54 | 1.16 ± 0.03 | 178.91 ± 1.09 e | 0.84 ± 0.09 b | 103.93 ± 0.14 d |
| EHPP4 | 8.87 ± 0.22 | 0.65 ± 0.01 | 269.73 ± 0.05 f | 0.62 ± 0.05 c | - |
| EHPV1 | 6.10 ± 0.31 | 0.40 ± 0.00 | 557.53 ± 0.61 g | 0.15 ± 0.08 e | 235.19 ± 0.19 f |
| EHPV2 | 9.01 ± 1.05 | 0.35 ± 001 | 597.93 ± 0.96 h | 0.34 ± 0.03 d | - |
| EHPV3 | 9.01 ± 0.02 | 0.30 ± 002 | 560.82 ± 0.20 i | 0.30 ± 0.03 d | 202.60 ± 0.15 e |
| EHPC | 11.4 ± 0.31 | 2.09 ± 0.02 | 117 ± 0.03 b | 0.84 ± 0.03 b | 70.77 ± 0.15 c |
| Trolox | - | - | 3.05 ± 0.61 a | 8.74 ± 0.13 a | 3.42 ± 0.41 a |
| Nº | Time Retention (min) | [M-H]− | MSn Ion m/z (−) | Tentative Identification |
|---|---|---|---|---|
| 1 | 2.9 | 539 | 195 | gluconic acid derivate |
| 2 | 3.0 | 195 | 177; 129 | gluconic acid |
| 3 | 29.4 | 571 | 285 | kaempeferol derivative |
| 4 | 29.5 | 285 | 255 | kaempferol |
| 5 | 30,1 | 801 | 539; 285 | 6-hydroxykaempferol 3,6-diglucoside 7-glucuronide |
| 6 | 33.6 | 603 | 301 | ellagic acid dimer |
| 7 | 33.6 | 625 | 301 | quercetin 3,4’-diglucoside |
| 8 | 33.6 | 301 | - | ellagic acid |
| 9 | 36.8 | 1345 | 672; 522; 372 | NI |
| 10 | 36.9 | 315 | 299; 153 | protocatechuic acid 3-glucoside |
| 11 | 39.2 | 277 | 233; 179 | linolenic acid |
| 12 | 42.3 | 279 | 261 | linoleic acid |
| COX-2 | Histamine H1 Receptor | ||||
|---|---|---|---|---|---|
| Ligand | ΔGbind (kcal/mol) | Ki (μM) | Ligand | ΔGbind (kcal/mol) | Ki (μM) |
| Quercetin 3,4’-diglucoside | −8.13 | 1.11 | Linolenic acid | −9.15 | 0.18 |
| Ellagic acid | −7.60 | 2.68 | Linoleic acid | −8.72 | 0.40 |
| Kaempferol | −7.44 | 3.54 | Kaempferol | −8.32 | 0.80 |
| 6-Hydroxykaempferol 3,6-diglucoside 7-glucuronide | −7.07 | 6.57 | Protocatechuic acid 3-glucoside | −7.49 | 3.25 |
| Protocatechuic acid 3-glucoside | −6.91 | 8.59 | Ellagic acid | −6.51 | 23.74 |
| Linolenic acid | −6.62 | 13.99 | Quercetin 3,4’-diglucoside | −2.31 | 201 |
| Linoleic acid | −5.96 | 42.49 | 6-Hydroxykaempferol 3,6-diglucoside 7-glucuronide | −1.18 | 1370 |
| Gluconic acid | −4.51 | 491.05 | Gluconic acid | 0.77 | 2719 |
| Meloxicam | −8.63 | 0.49 | Doxepin | −10.36 | 0.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, A.J.O.; Vasconcelos, C.C.; Pereira, F.A.N.; Silva, R.H.M.; Queiroz, P.F.d.S.; Fernandes, C.V.; Garcia, J.B.S.; Ramos, R.M.; Rocha, C.Q.d.; Lima, S.T.d.J.R.M.; et al. Anti-Inflammatory and Antinociceptive Activity of Pollen Extract Collected by Stingless Bee Melipona fasciculata. Int. J. Mol. Sci. 2019, 20, 4512. https://doi.org/10.3390/ijms20184512
Lopes AJO, Vasconcelos CC, Pereira FAN, Silva RHM, Queiroz PFdS, Fernandes CV, Garcia JBS, Ramos RM, Rocha CQd, Lima STdJRM, et al. Anti-Inflammatory and Antinociceptive Activity of Pollen Extract Collected by Stingless Bee Melipona fasciculata. International Journal of Molecular Sciences. 2019; 20(18):4512. https://doi.org/10.3390/ijms20184512
Chicago/Turabian StyleLopes, Alberto Jorge Oliveira, Cleydlenne Costa Vasconcelos, Francisco Assis Nascimento Pereira, Rosa Helena Moraes Silva, Pedro Felipe dos Santos Queiroz, Caio Viana Fernandes, João Batista Santos Garcia, Ricardo Martins Ramos, Cláudia Quintino da Rocha, Silvia Tereza de Jesus Rodrigues Moreira Lima, and et al. 2019. "Anti-Inflammatory and Antinociceptive Activity of Pollen Extract Collected by Stingless Bee Melipona fasciculata" International Journal of Molecular Sciences 20, no. 18: 4512. https://doi.org/10.3390/ijms20184512
APA StyleLopes, A. J. O., Vasconcelos, C. C., Pereira, F. A. N., Silva, R. H. M., Queiroz, P. F. d. S., Fernandes, C. V., Garcia, J. B. S., Ramos, R. M., Rocha, C. Q. d., Lima, S. T. d. J. R. M., Cartágenes, M. d. S. d. S., & Ribeiro, M. N. d. S. (2019). Anti-Inflammatory and Antinociceptive Activity of Pollen Extract Collected by Stingless Bee Melipona fasciculata. International Journal of Molecular Sciences, 20(18), 4512. https://doi.org/10.3390/ijms20184512







