Dietary Fatty Acids Affect Red Blood Cell Membrane Composition and Red Blood Cell ATP Release in Dairy Cows
Abstract
1. Introduction
2. Results
2.1. Animal Performance Data
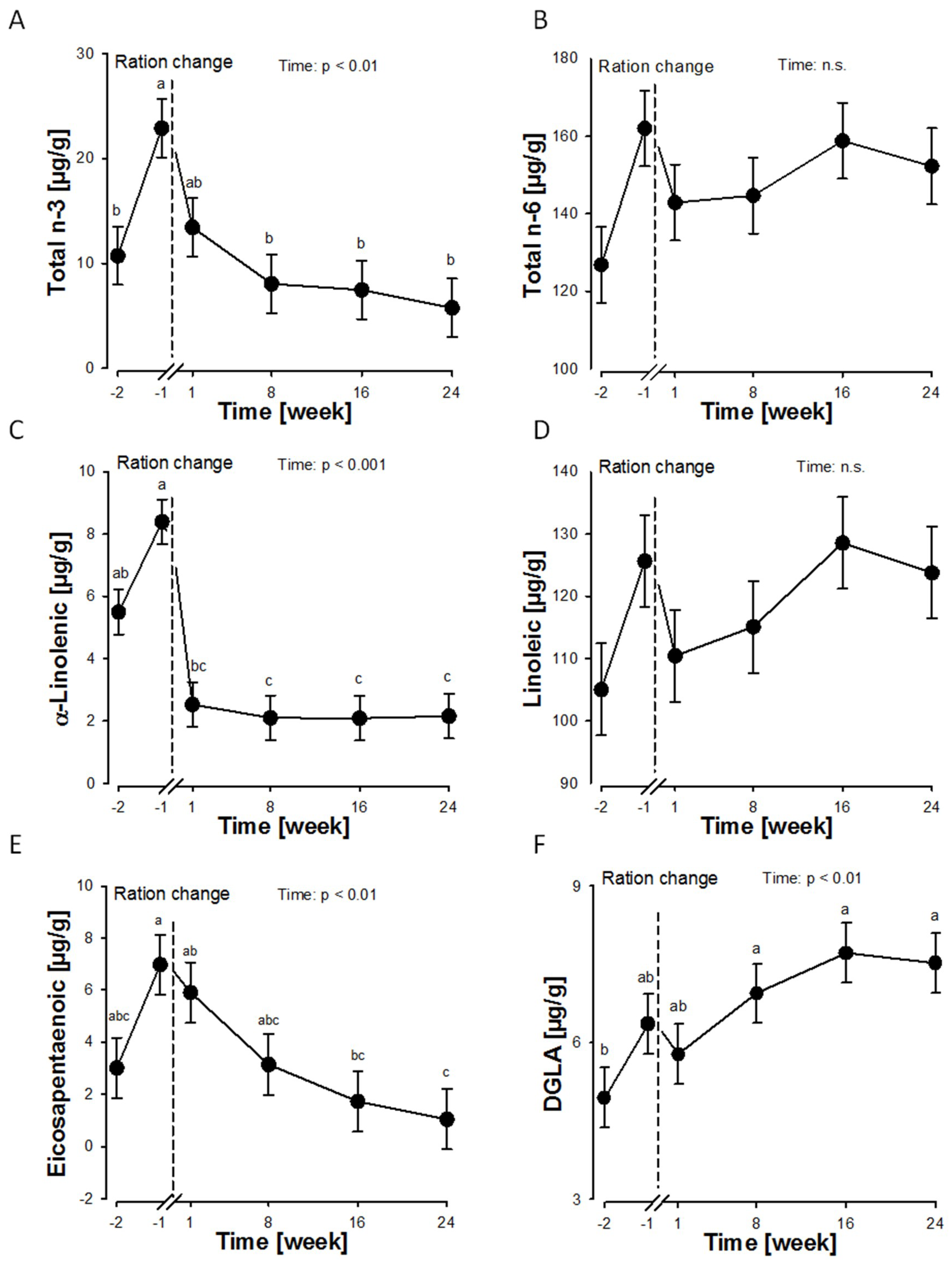
2.2. Effects of an n-3 Fatty Acid Reduced Diet and an Additional Fatty Acid Supplementation on the Fatty Acid Profile of the Plasma Membrane of RBCs
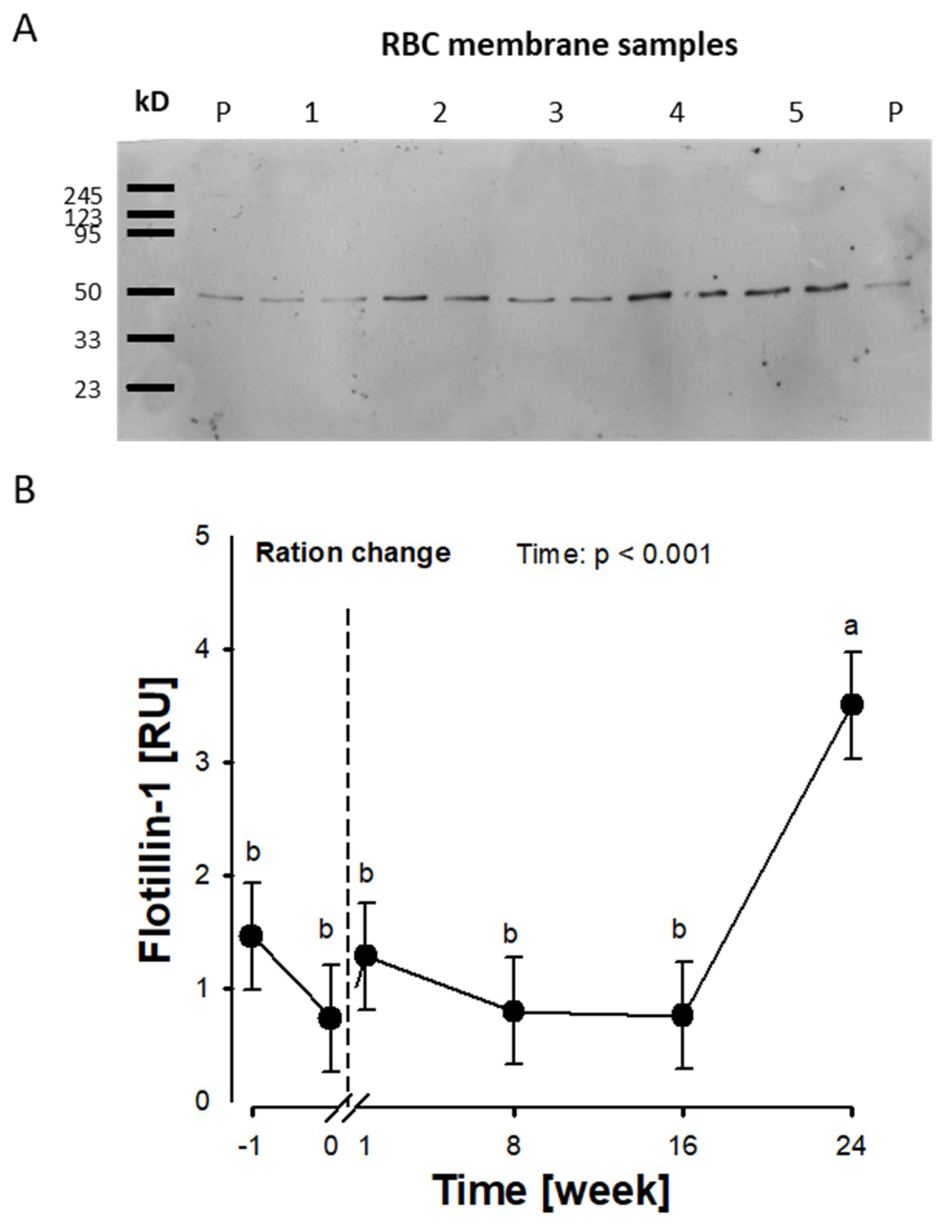
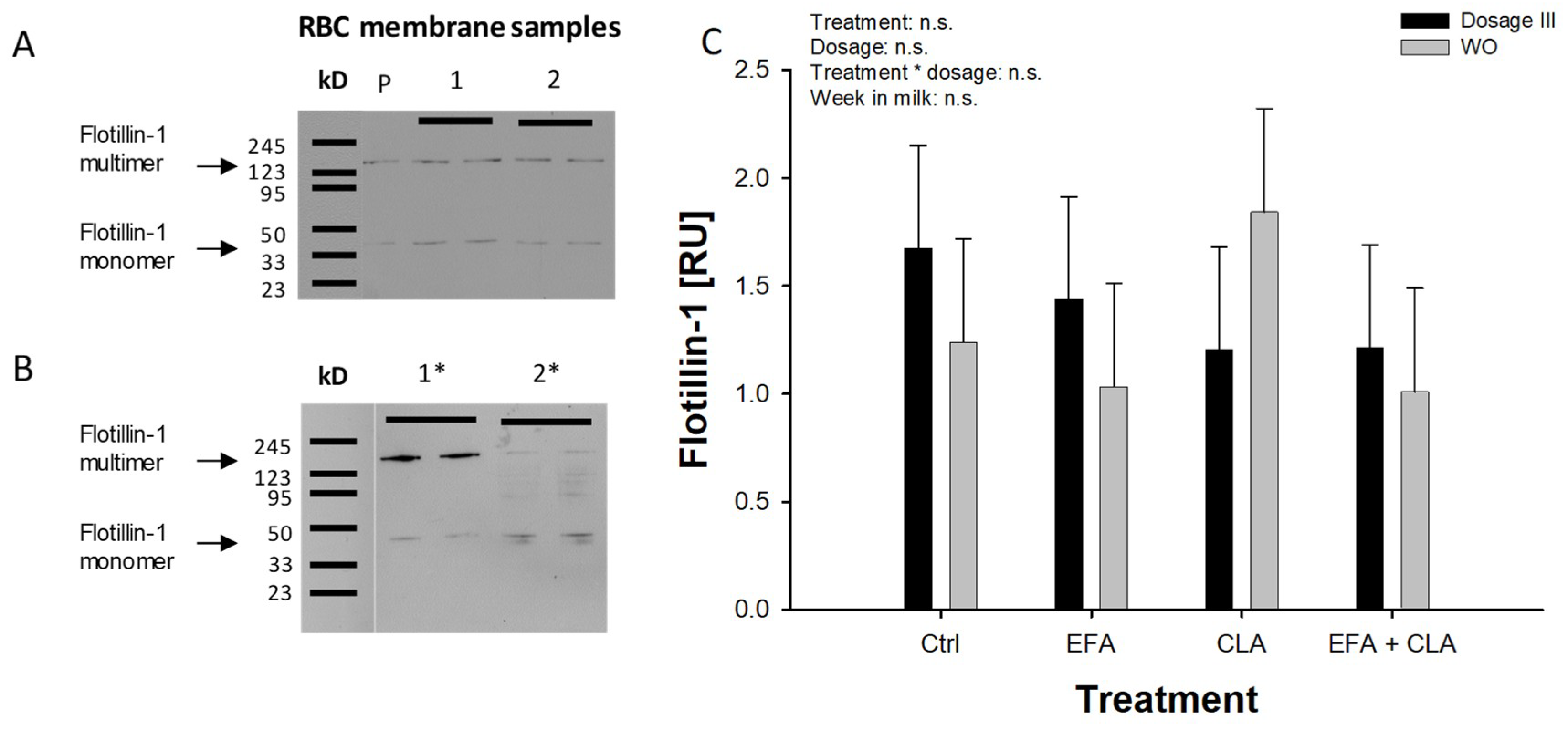
2.3. Flotillin-1 and Pannexin-1 Abundance in RBC Membranes
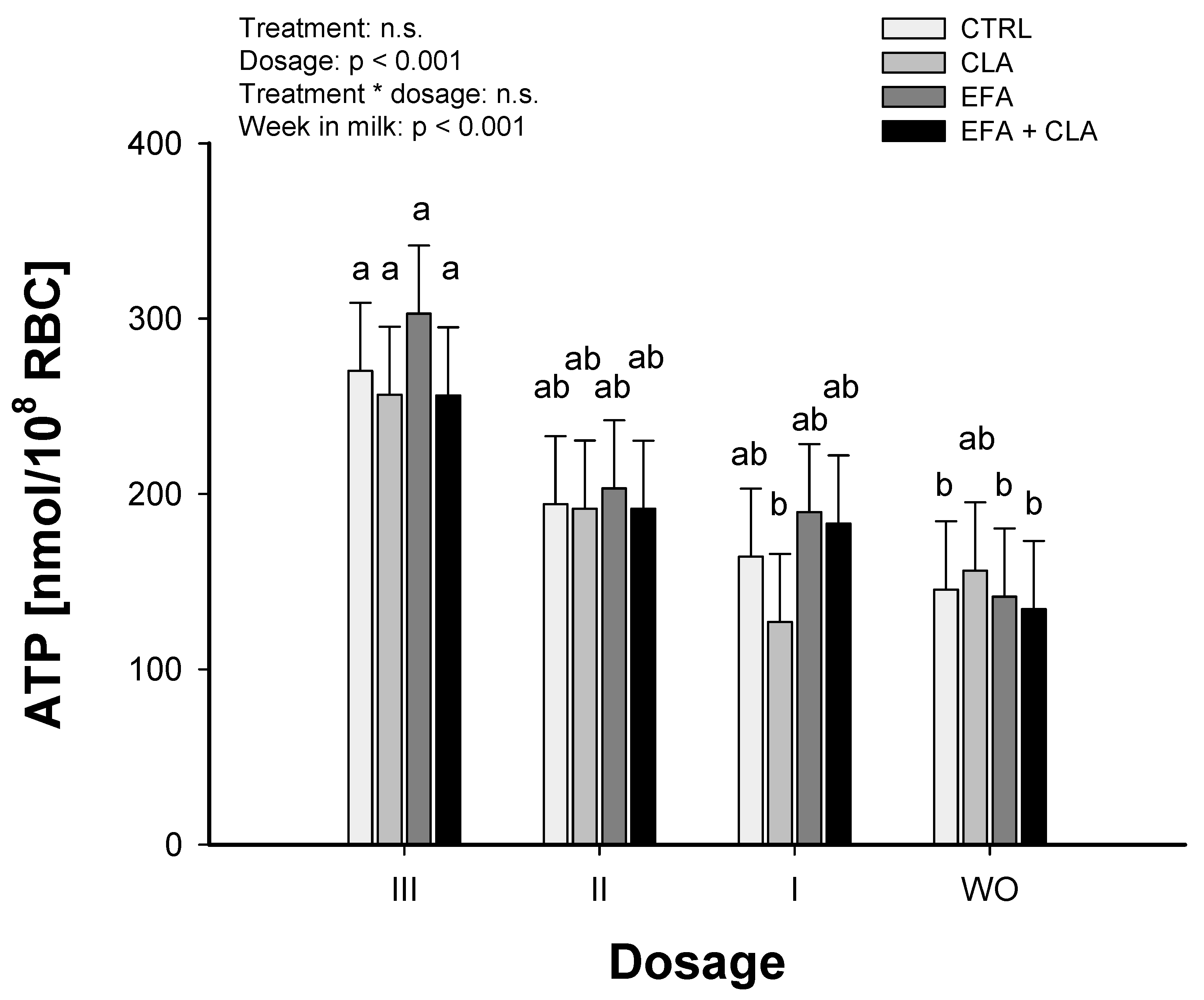
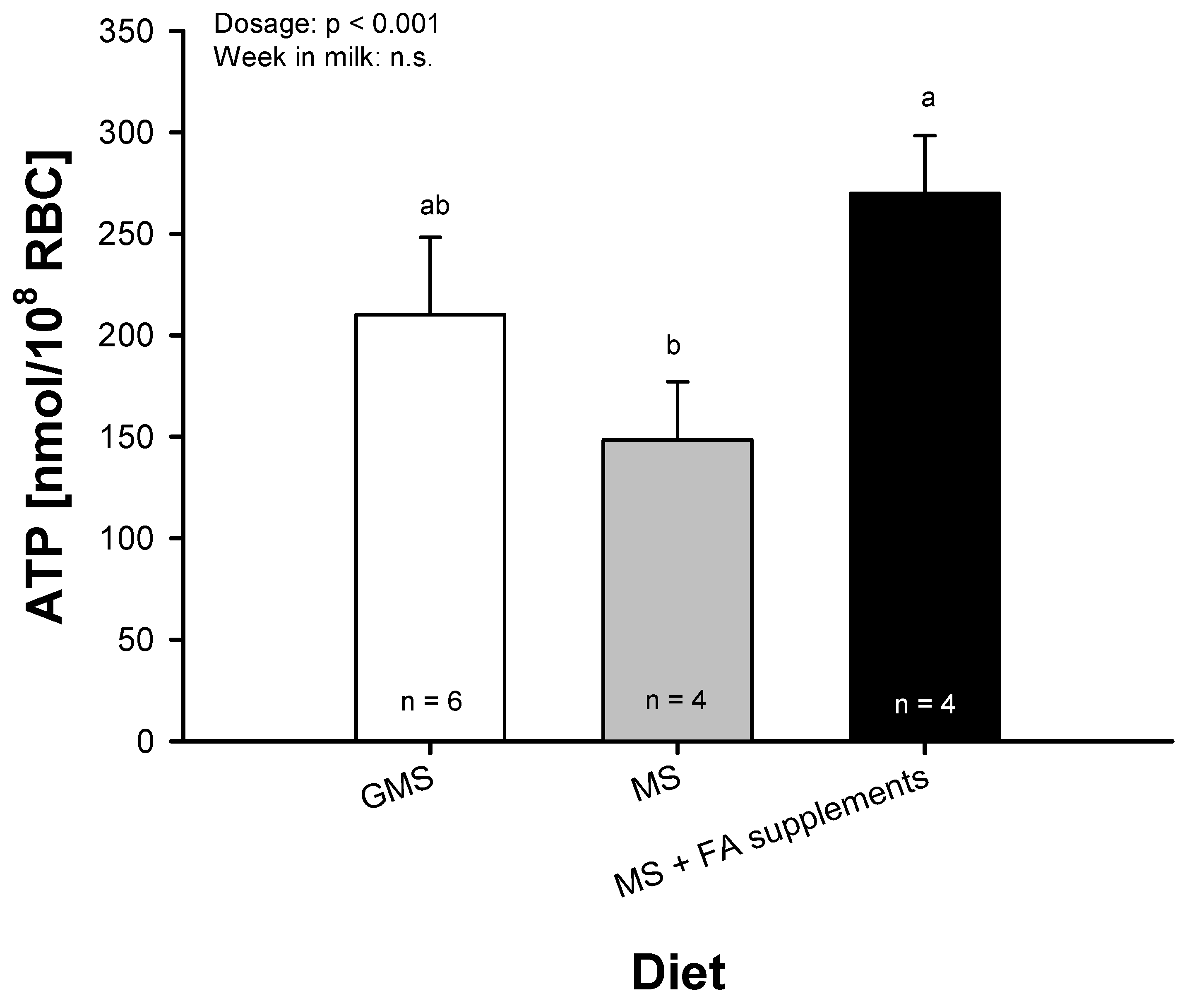
2.4. ATP Release from Bovine RBC
3. Discussion
4. Materials and Methods
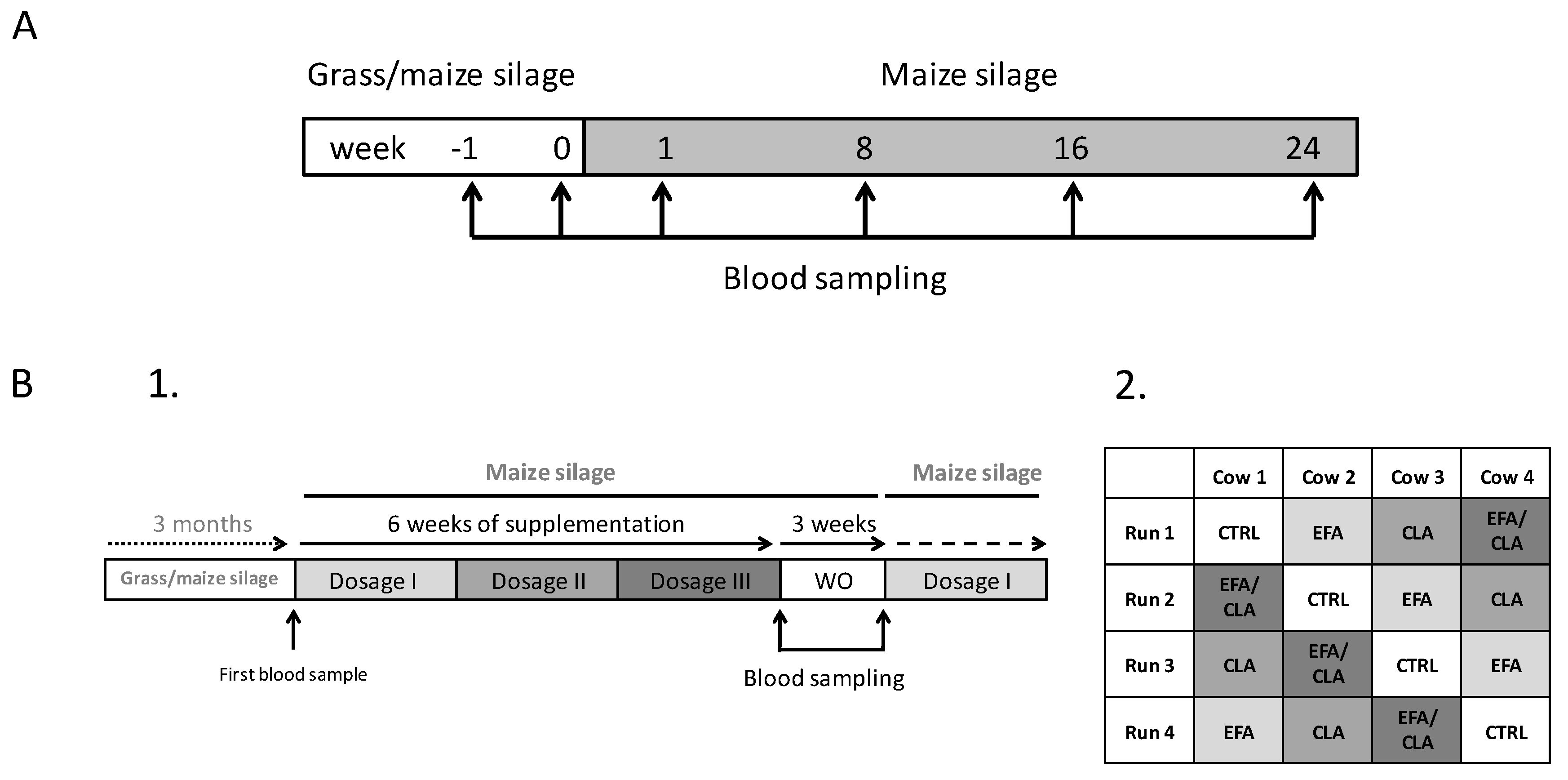
4.1. Animals and Study Design
4.2. Blood Sampling and Plasma Membrane Extraction of RBC
4.3. Fatty Acid Analysis of Total Membrane Lipids
4.4. RBC Isolation and Measurement of ATP Release
4.5. Stimulated ATP Release from RBC in vitro, Incubated with LA and ALA
4.6. SDS-PAGE and Western Blot Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stubbs, C.D.; Smith, A.D. The modification of mammalian membrane polyunsaturated fatty acid composition in relation to membrane fluidity and function. Biochim. Biophys. Acta 1984, 779, 89–137. [Google Scholar] [CrossRef]
- Lee, A.G. How lipids affect the activities of integral membrane proteins. Biochim. Biophys. Acta 2004, 1666, 62–87. [Google Scholar] [CrossRef] [PubMed]
- Poon, R.; Richards, J.M.; Clark, W.R. The relationship between plasma membrane lipid composition and physical-chemical properties. II. Effect of phospholipid fatty acid modulation on plasma membrane physical properties and enzymatic activities. Biochim. Biophys. Acta 1981, 649, 58–66. [Google Scholar] [CrossRef]
- Lenaz, G. Lipid fluidity and membrane protein dynamics. Biosci. Rep. 1987, 7, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Zarate, R.; El Jaber-Vazdekis, N.; Tejera, N.; Perez, J.A.; Rodriguez, C. Significance of long chain polyunsaturated fatty acids in human health. Clin. Transl. Med. 2017, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S. The Omega-6:Omega-3 ratio: A critical appraisal and possible successor. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 34–40. [Google Scholar] [CrossRef]
- Sailaja, Y.R.; Baskar, R.; Srinivas Rao, C.S.; Saralakumari, D. Membrane lipids and protein-bound carbohydrates status during the maturation of reticulocytes to erythrocytes in type 2 diabetics. Clin. Chim. Acta 2004, 341, 185–192. [Google Scholar] [CrossRef]
- Vokurkova, M.; Rauchova, H.; Dobesova, Z.; Loukotova, J.; Novakova, O.; Kunes, J.; Zicha, J. The influence of erythrocyte maturity on ion transport and membrane lipid composition in the rat. Physiol. Res. 2016, 65, 91–99. [Google Scholar]
- Sun, Q.; Ma, J.; Campos, H.; Hankinson, S.E.; Hu, F.B. Comparison between plasma and erythrocyte fatty acid content as biomarkers of fatty acid intake in US women. Am. J. Clin. Nutr. 2007, 86, 74–81. [Google Scholar] [CrossRef]
- Adderley, S.P.; Sridharan, M.; Bowles, E.A.; Stephenson, A.H.; Sprague, R.S.; Ellsworth, M.L. Inhibition of ATP release from erythrocytes: A role for EPACs and PKC. Microcirculation 2011, 18, 128–135. [Google Scholar] [CrossRef]
- Ellsworth, M.L.; Ellis, C.G.; Sprague, R.S. Role of erythrocyte-released ATP in the regulation of microvascular oxygen supply in skeletal muscle. Acta Physiol. 2016, 216, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Sprague, R.S.; Ellsworth, M.L. Erythrocyte-derived ATP and perfusion distribution: Role of intracellular and intercellular communication. Microcirculation 2012, 19, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Ellsworth, M.L.; Sprague, R.S. Regulation of blood flow distribution in skeletal muscle: Role of erythrocyte-released ATP. J. Physiol. 2012, 590, 4985–4991. [Google Scholar] [CrossRef] [PubMed]
- Sprague, R.S.; Stephenson, A.H.; Bowles, E.A.; Stumpf, M.S.; Lonigro, A.J. Reduced expression of G(i) in erythrocytes of humans with type 2 diabetes is associated with impairment of both cAMP generation and ATP release. Diabetes 2006, 55, 3588–3593. [Google Scholar] [CrossRef] [PubMed]
- Bodin, S.; Planchon, D.; Rios Morris, E.; Comunale, F.; Gauthier-Rouviere, C. Flotillins in intercellular adhesion—From cellular physiology to human diseases. J. Cell Sci. 2014, 127, 5139–5147. [Google Scholar] [CrossRef] [PubMed]
- Bach, J.N.; Bramkamp, M. Flotillins functionally organize the bacterial membrane. Mol. Microbiol. 2013, 88, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Ibarguren, M.; Lopez, D.J.; Escriba, P.V. The effect of natural and synthetic fatty acids on membrane structure, microdomain organization, cellular functions and human health. Biochim. Biophys. Acta 2014, 1838, 1518–1528. [Google Scholar] [CrossRef]
- Jiang, M.; Jia, L.; Jiang, W.; Hu, X.; Zhou, H.; Gao, X.; Lu, Z.; Zhang, Z. Protein disregulation in red blood cell membranes of type 2 diabetic patients. Biochem. Biophys. Res. Commun. 2003, 309, 196–200. [Google Scholar] [CrossRef]
- Khan, N.A.; Yu, P.; Ali, M.; Cone, J.W.; Hendriks, W.H. Nutritive value of maize silage in relation to dairy cow performance and milk quality. J. Sci. Food Agric. 2015, 95, 238–252. [Google Scholar] [CrossRef]
- Phipps, R.H.; Sutton, J.D.; Jones, B.A. Forage mixtures for dairy cows: the effect on dry-matter intake and milk production of incorporating either fermented or urea-treated whole-crop wheat, brewers’ grains, fodder beet or maize silage into diets based on grass silage. Anim. Sci. 1995, 61, 491–496. [Google Scholar] [CrossRef]
- Hart, K.J.; Huntington, J.A.; Wilkinson, R.G.; Bartram, C.G.; Sinclair, L.A. The influence of grass silage-to-maize silage ratio and concentrate composition on methane emissions, performance and milk composition of dairy cows. Animal 2015, 9, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Rego, O.A.; Portugal, P.V.; Sousa, M.B.; Rosa, H.J.D.; Vouzela, C.M.; Borba, A.E.S.; Bessa, R.J.B. Effect of diet on the fatty acid pattern of milk from dairy cows. Anim. Res. 2004, 53, 213–220. [Google Scholar] [CrossRef]
- Weber, C.; Tröscher, A.; Kienberger, H.; Rychlik, M.; Hammon, H.M. Performance and fatty acid status in dairy cows fed a diet with reduced essential fatty acid content. In Energy and Protein Metabolism and Nutrition; Skomial, J., Lapierre, H., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2016; Volume 137, pp. 245–246. [Google Scholar]
- Plewes, M.R.; Burns, P.D.; Graham, P.E.; Bruemmer, J.E.; Engle, T.E.; Barisas, B.G. Effect of fish meal supplementation on spatial distribution of lipid microdomains and on the lateral mobility of membrane-bound prostaglandin F2alpha receptors in bovine corpora lutea. Domest. Anim. Endocrinol. 2017, 60, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Palmquist, D.L. Essential fatty acids in ruminant diets. In Proceedings of the 21st Annual Florida Ruminant Nutrition Symposium, Gainesville, FL, USA, 2–3 February 2010; pp. 127–141. [Google Scholar]
- Sigl, T.; Schlamberger, G.; Kienberger, H.; Wiedemann, S.; Meyer, H.H.; Kaske, M. Rumen-protected conjugated linoleic acid supplementation to dairy cows in late pregnancy and early lactation: Effects on milk composition, milk yield, blood metabolites and gene expression in liver. Acta Vet. Scand. 2010, 52, 16. [Google Scholar] [CrossRef] [PubMed]
- Carriquiry, M.; Weber, W.J.; Fahrenkrug, S.C.; Crooker, B.A. Hepatic gene expression in multiparous Holstein cows treated with bovine somatotropin and fed n-3 fatty acids in early lactation. J. Dairy Sci. 2009, 92, 4889–4900. [Google Scholar] [CrossRef] [PubMed]
- do Prado, R.M.; Palin, M.F.; do Prado, I.N.; Dos Santos, G.T.; Benchaar, C.; Petit, H.V. Milk yield, milk composition, and hepatic lipid metabolism in transition dairy cows fed flaxseed or linola. J. Dairy Sci. 2016, 99, 8831–8846. [Google Scholar] [CrossRef]
- Dänicke, S.; Kowalczyk, J.; Renner, L.; Pappritz, J.; Meyer, U.; Kramer, R.; Weber, E.M.; Doll, S.; Rehage, J.; Jahreis, G. Effects of conjugated linoleic acids fed to dairy cows during early gestation on hematological, immunological, and metabolic characteristics of cows and their calves. J. Dairy Sci. 2012, 95, 3938–3953. [Google Scholar] [CrossRef]
- Duan, Y.; Li, F.; Li, L.; Fan, J.; Sun, X.; Yin, Y. n-6:n-3 PUFA ratio is involved in regulating lipid metabolism and inflammation in pigs. Br. J. Nutr. 2014, 111, 445–451. [Google Scholar] [CrossRef]
- Simopoulos, A.P. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef]
- Rodriguez, A.; Sarda, P.; Nessmann, C.; Boulot, P.; Leger, C.L.; Descomps, B. Delta6- and delta5-desaturase activities in the human fetal liver: Kinetic aspects. J. Lipid Res. 1998, 39, 1825–1832. [Google Scholar]
- Cao, J.; Schwichtenberg, K.A.; Hanson, N.Q.; Tsai, M.Y. Incorporation and clearance of omega-3 fatty acids in erythrocyte membranes and plasma phospholipids. Clin. Chem. 2006, 52, 2265–2272. [Google Scholar] [CrossRef]
- Ferlay, A.; Bernard, L.; Meynadier, A.; Malpuech-Brugere, C. Production of trans and conjugated fatty acids in dairy ruminants and their putative effects on human health: A review. Biochimie 2017, 141, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Hötger, K.; Hammon, H.M.; Weber, C.; Gors, S.; Tröscher, A.; Bruckmaier, R.M.; Metges, C.C. Supplementation of conjugated linoleic acid in dairy cows reduces endogenous glucose production during early lactation. J. Dairy Sci. 2013, 96, 2258–2270. [Google Scholar] [CrossRef] [PubMed]
- Galamb, E.; Faigl, V.; Keresztes, M.; Csillik, Z.; Tröscher, A.; Elek, P.; Kulcsar, M.; Huszenicza, G.; Febel, H.; Husveth, F. Effect of pre- and post-partum supplementation with lipid-encapsulated conjugated linoleic acid on milk yield and metabolic status in multiparous high-producing dairy cows. J. Anim. Physiol. Anim. Nutr. 2017, 101, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Kramer, R.; Wolf, S.; Petri, T.; von Soosten, D.; Dänicke, S.; Weber, E.M.; Zimmer, R.; Rehage, J.; Jahreis, G. A commonly used rumen-protected conjugated linoleic acid supplement marginally affects fatty acid distribution of body tissues and gene expression of mammary gland in heifers during early lactation. Lipids Health Dis. 2013, 12, 96. [Google Scholar] [CrossRef] [PubMed]
- Baumann, C.A.; Ribon, V.; Kanzaki, M.; Thurmond, D.C.; Mora, S.; Shigematsu, S.; Bickel, P.E.; Pessin, J.E.; Saltiel, A.R. CAP defines a second signalling pathway required for insulin-stimulated glucose transport. Nature 2000, 407, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Babuke, T.; Tikkanen, R. Dissecting the molecular function of reggie/flotillin proteins. Eur. J. Cell Biol. 2007, 86, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Roland, L.; Drillich, M.; Iwersen, M. Hematology as a diagnostic tool in bovine medicine. J. Vet. Diagn. Investig. 2014, 26, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Solis, G.P.; Hoegg, M.; Munderloh, C.; Schrock, Y.; Malaga-Trillo, E.; Rivera-Milla, E.; Stuermer, C.A. Reggie/flotillin proteins are organized into stable tetramers in membrane microdomains. Biochem. J. 2007, 403, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Leal Denis, M.F.; Alvarez, H.A.; Lauri, N.; Alvarez, C.L.; Chara, O.; Schwarzbaum, P.J. Dynamic Regulation of Cell Volume and Extracellular ATP of Human Erythrocytes. PLoS ONE 2016, 11, e0158305. [Google Scholar] [CrossRef] [PubMed]
- Berlin, E.; Bhathena, S.J.; McClure, D.; Peters, R.C. Dietary menhaden and corn oils and the red blood cell membrane lipid composition and fluidity in hyper- and normocholesterolemic miniature swine. J. Nutr. 1998, 128, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Berger, H.; Lietzau, M.; Tichy, A.; Herzog, K. Investigations of mammary and uterine blood flow in relation to milk yield, postpartum disease, and pregnancy result in dairy cows. Theriogenology 2016, 86, 1906–1912. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, A.M.; Wan, J.; Owrutsky, P.D.; Abkarian, M.; Stone, H.A. Multiscale approach to link red blood cell dynamics, shear viscosity, and ATP release. Proc. Natl. Acad. Sci. USA 2011, 108, 10986–10991. [Google Scholar] [CrossRef] [PubMed]
- Marginedas-Freixa, I.; Alvarez, C.L.; Moras, M.; Leal Denis, M.F.; Hattab, C.; Halle, F.; Bihel, F.; Mouro-Chanteloup, I.; Lefevre, S.D.; Le Van Kim, C.; et al. Human erythrocytes release ATP by a novel pathway involving VDAC oligomerization independent of pannexin-1. Sci. Rep. 2018, 8, 11384. [Google Scholar] [CrossRef] [PubMed]
- Donabedian, R.K.; Karmen, A. Fatty acid transport and incorporation into human erythrocytes in vitro. J. Clin. Investig. 1967, 46, 1017–1027. [Google Scholar] [CrossRef]
- Sikora, J.; Orlov, S.N.; Furuya, K.; Grygorczyk, R. Response: Hemolysis is a primary and physiologically relevant ATP release mechanism in human erythrocytes. Blood 2015, 125, 1845–1846. [Google Scholar] [CrossRef]
- Keller, A.S.; Diederich, L.; Panknin, C.; DeLalio, L.J.; Drake, J.C.; Sherman, R.; Jackson, E.K.; Yan, Z.; Kelm, M.; Cortese-Krott, M.M.; et al. Possible roles for ATP release from RBCs exclude the cAMP-mediated Panx1 pathway. Am. J. Physiol. Cell Physiol. 2017, 313, C593–C603. [Google Scholar] [CrossRef]
- Leal Denis, M.F.; Incicco, J.J.; Espelt, M.V.; Verstraeten, S.V.; Pignataro, O.P.; Lazarowski, E.R.; Schwarzbaum, P.J. Kinetics of extracellular ATP in mastoparan 7-activated human erythrocytes. Biochim. Biophys. Acta 2013, 1830, 4692–4707. [Google Scholar] [CrossRef]
- Olearczyk, J.J.; Stephenson, A.H.; Lonigro, A.J.; Sprague, R.S. Heterotrimeric G protein Gi is involved in a signal transduction pathway for ATP release from erythrocytes. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H940–H945. [Google Scholar] [CrossRef]
- Haubold, S.; Kröger-Koch, C.; Tuchscherer, A.; Tröscher, A.; Starke, A.; Kienberger, H.; Rychlik, M.; Hoeflich, A.; Hammon, H.M. Effects of abomasal infusion of essential fatty acids and conjugated linoleic acid on performance, fatty acid status and metabolism in dairy cows fed a ration with reduced essential fatty acid content. In Proceedings of the Society of Nutrition Physiology, Göttingen, Germany, 14–16 March 2017; p. 155. [Google Scholar]
- German Society of Nutrition Physiology (GfE). New equations for predicting metabolisable energy of grass and maize products for ruminants. Mitteilung des Ausschusses für Bedarfsnormen der Gesellschaft für Ernährungsphysiologie. Proc. Soc. Nutr. Physiol. 2008, 17, 191–198. [Google Scholar]
- German Society of Nutrition Physiology (GfE). Recommended Energy and Nutrient Supply for Dairy Cows and Growing Cattle; DLG-Verlag: Frankfurt am Main, Germany, 2001. [Google Scholar]
- Reist, M.; Erdin, D.; von Euw, D.; Tschuemperlin, K.; Leuenberger, H.; Delavaud, C.; Chilliard, Y.; Hammon, H.M.; Kuenzi, N.; Blum, J.W. Concentrate feeding strategy in lactating dairy cows: Metabolic and endocrine changes with emphasis on leptin. J. Dairy Sci. 2003, 86, 1690–1706. [Google Scholar] [CrossRef]
- Kamata, K.; Manno, S.; Ozaki, M.; Takakuwa, Y. Functional evidence for presence of lipid rafts in erythrocyte membranes: Gsalpha in rafts is essential for signal transduction. Am. J. Hematol. 2008, 83, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Firl, N.; Kienberger, H.; Hauser, T.; Rychlik, M. Determination of the fatty acid profile of neutral lipids, free fatty acids and phospholipids in human plasma. Clin. Chem. Lab. Med. 2013, 51, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Ni, D.; Xu, P.; Gallagher, S. Immunoblotting and Immunodetection. Curr. Protoc. Mol. Biol. 2016, 114, 10.8.1–10.8.37. [Google Scholar] [CrossRef]
- Locher, L.F.; Rehage, J.; Khraim, N.; Meyer, U.; Danicke, S.; Hansen, K.; Huber, K. Lipolysis in early lactation is associated with an increase in phosphorylation of adenosine monophosphate-activated protein kinase (AMPK)alpha1 in adipose tissue of dairy cows. J. Dairy Sci. 2012, 95, 2497–2504. [Google Scholar] [CrossRef] [PubMed]
| Treatment | P-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fatty Acid in RBC Membranes (µg/g) | ω | CTRL 1 | CLA 2 | EFA 3 | CLA + EFA 4 | SE 5 | Treatment | Dosage | Treatment × Dosage | Time 6 |
| 18:2, 9-cis,trans-11 | 0.042 | 0.289 | 0.248 | 0.000 | ||||||
| Dosage III | 4.90 | 7.01 | 6.51 | 5.63 | 0.85 | |||||
| Washout | 5.81 | 6.04 | 6.23 | 3.83 | 0.86 | |||||
| 18:2, trans-10,cis-12 | 0.250 | 0.157 | 0.290 | 0.000 | ||||||
| Dosage III | 0.85 | 1.59 | 0.98 | 1.47 * | 0.27 | |||||
| Washout | 0.92 | 1.24 | 0.99 | 0.57 | 0.27 | |||||
| 18:3 cis-9, cis-12, cis-15 | n3 | 0.161 | 0.008 | 0.000 | 0.000 | |||||
| Dosage III | 8.32 b | 9.69 b | 17.25 *a | 16.56 *a | 1.34 | |||||
| Washout | 12.51 * | 11.47 | 8.63 | 8.05 | 1.35 | |||||
| 20:3 cis-8,cis-11,cis-14 | n6 | 0.401 | 0.292 | 0.082 | 0.192 | |||||
| Dosage III | 13.04 | 11.26 | 9.41 | 8.59 | 1.61 | |||||
| Washout | 10.98 | 10.46 | 14.03 * | 11.13 | 1.63 | |||||
| 22:5 cis-7,cis-10,cis-13,cis-16,cis-19 | n3 | 0.503 | 0.227 | 0.108 | 0.575 | |||||
| Dosage III | 6.55 | 9.81 | 12.47 | 12.40 * | 3.61 | |||||
| Washout | 8.41 | 12.61 | 6.76 | 3.85 | 3.63 | |||||
| 24:1, 15c | 0.453 | 0.420 | 0.224 | 0.000 | ||||||
| Dosage III | 0.25 | 0.49 | 0.21 | 0.55 * | 0.18 | |||||
| Washout | 0.23 | 0.50 | 0.36 | −0.01 | 0.18 | |||||
| Sum CLA | 0.082 | 0.220 | 0.228 | 0.000 | ||||||
| Dosage III | 5.75 | 8.60 | 7.49 | 7.10 * | 1.05 | |||||
| Washout | 6.73 | 7.28 | 7.21 | 4.40 | 1.06 | |||||
| Sum n-3 | 0.583 | 0.058 | 0.004 | 0.108 | ||||||
| Dosage III | 21.31 b | 27.18 ab | 37.42 *a | 35.90 *a | 4.66 | |||||
| Washout | 28.76 | 31.04 | 21.96 | 18.19 | 4.69 | |||||
| Sum n-6 | 0.696 | 0.937 | 0.777 | 0.751 | ||||||
| Dosage III | 280.37 | 289.61 | 264.85 | 247.21 | 33.95 | |||||
| Washout | 268.46 | 258.45 | 304.72 | 242.09 | 34.30 | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Revskij, D.; Haubold, S.; Viergutz, T.; Kröger-Koch, C.; Tuchscherer, A.; Kienberger, H.; Rychlik, M.; Tröscher, A.; Hammon, H.M.; Schuberth, H.-J.; et al. Dietary Fatty Acids Affect Red Blood Cell Membrane Composition and Red Blood Cell ATP Release in Dairy Cows. Int. J. Mol. Sci. 2019, 20, 2769. https://doi.org/10.3390/ijms20112769
Revskij D, Haubold S, Viergutz T, Kröger-Koch C, Tuchscherer A, Kienberger H, Rychlik M, Tröscher A, Hammon HM, Schuberth H-J, et al. Dietary Fatty Acids Affect Red Blood Cell Membrane Composition and Red Blood Cell ATP Release in Dairy Cows. International Journal of Molecular Sciences. 2019; 20(11):2769. https://doi.org/10.3390/ijms20112769
Chicago/Turabian StyleRevskij, Denis, Susanne Haubold, Torsten Viergutz, Claudia Kröger-Koch, Armin Tuchscherer, Hermine Kienberger, Michael Rychlik, Arnulf Tröscher, Harald M. Hammon, Hans-Joachim Schuberth, and et al. 2019. "Dietary Fatty Acids Affect Red Blood Cell Membrane Composition and Red Blood Cell ATP Release in Dairy Cows" International Journal of Molecular Sciences 20, no. 11: 2769. https://doi.org/10.3390/ijms20112769
APA StyleRevskij, D., Haubold, S., Viergutz, T., Kröger-Koch, C., Tuchscherer, A., Kienberger, H., Rychlik, M., Tröscher, A., Hammon, H. M., Schuberth, H.-J., & Mielenz, M. (2019). Dietary Fatty Acids Affect Red Blood Cell Membrane Composition and Red Blood Cell ATP Release in Dairy Cows. International Journal of Molecular Sciences, 20(11), 2769. https://doi.org/10.3390/ijms20112769





