Development, Characterization, and Exploitation in Food Systems of Functional Ingredients Obtained from Artichoke By-Products Phenolic Extracts
Abstract
1. Introduction
2. Results and Discussion
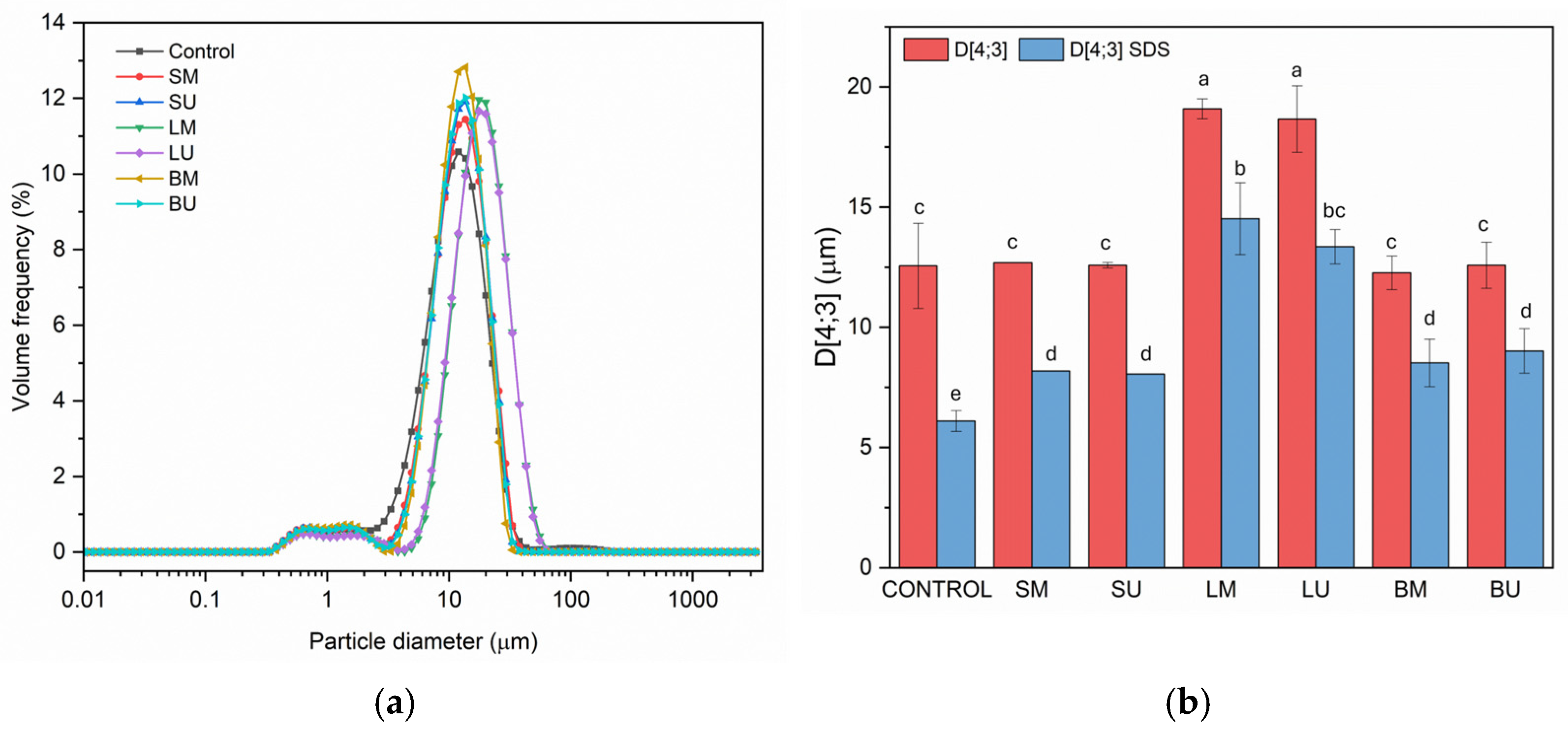
2.1. Technological Functionality in Oil-in-Water Emulsified Systems
2.1.1. Surface Properties and Emulsifying Capacity
2.1.2. Emulsions Flow Curves
2.1.3. Oxidative Stability of Emulsions
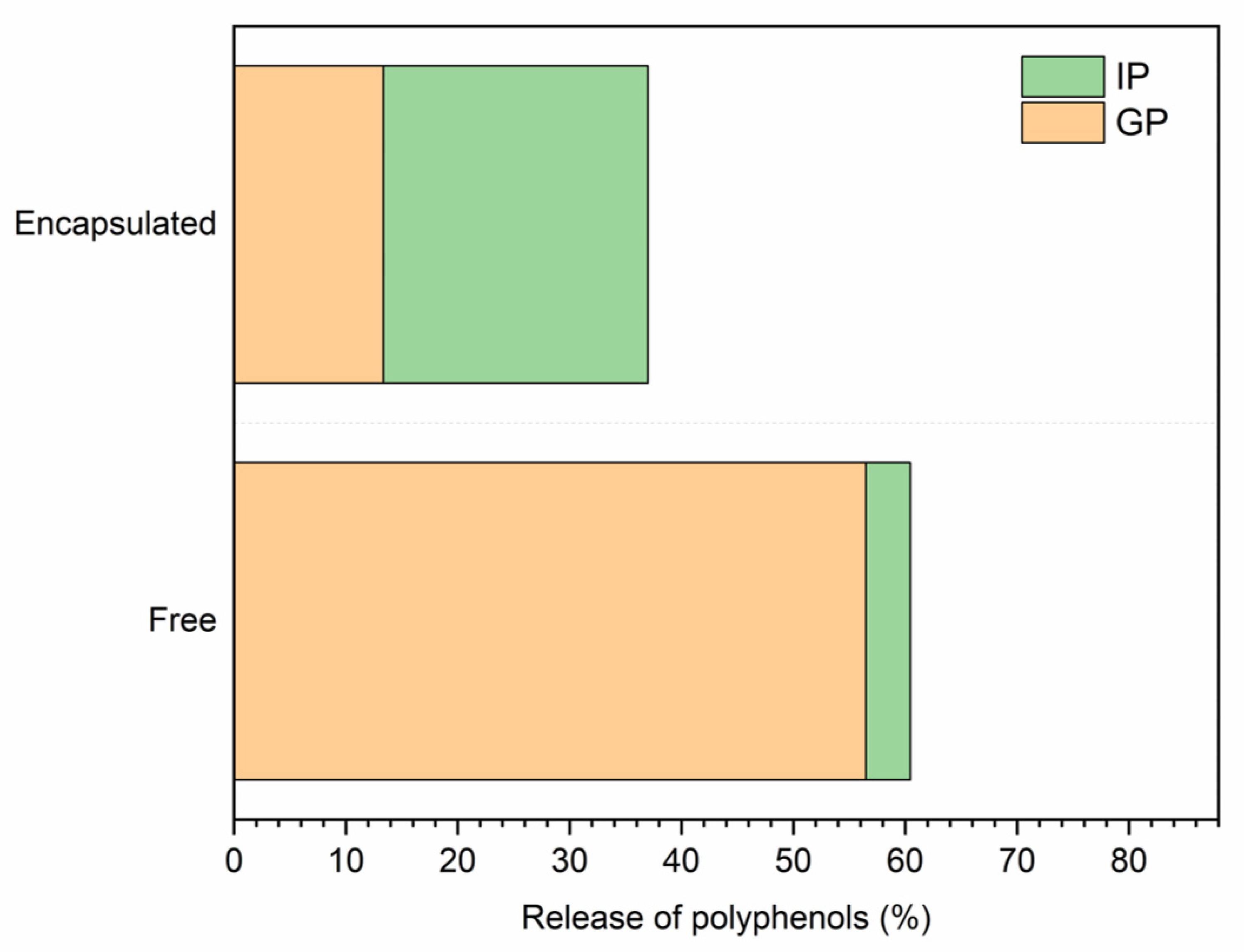
2.2. Extract Encapsulation by Spray Drying, Exploitation in a Real Matrix, and In Vitro Digestion
3. Materials and Methods
3.1. Materials
3.2. Surface Tension of the Phenolic-Rich Extracts
3.3. Emulsion Preparation
3.4. Emulsion Particle Size Distribution
3.5. Emulsions Flow Behavior
3.6. Oxidative Stability Evaluation
3.7. Extract Encapsulation by Spray-Drying
3.8. Encapsulation Efficiency and Load Yield of Total Phenolic Content
3.9. Determination of Dry Matter of the Encapsulated Powdered Extract
3.10. Enrichment of Commercial Vegan Mayonnaise
3.11. In Vitro Digestion
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. The State of Food and Agriculture. 2019, Moving Forward on Food Loss and Waste Reduction; FAO: Rome, Italy, 2019. [Google Scholar]
- García, S.L.R.; Raghavan, V. Green extraction techniques from fruit and vegetable waste to obtain bioactive compounds—A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 6446–6466. [Google Scholar] [CrossRef]
- Marcillo-Parra, V.; Tupuna-Yerovi, D.S.; González, Z.; Ruales, J. Encapsulation of bioactive compounds from fruit and vegetable by-products for food application—A review. Trends Food Sci. Technol. 2021, 116, 11–23. [Google Scholar] [CrossRef]
- “FAOSTAT Statistical Database. 2022. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 20 April 2023).
- Salem, M.B.; Affes, H.; Ksouda, K.; Dhouibi, R.; Sahnoun, Z.; Hammami, S.; Zeghal, K.M. Pharmacological Studies of Artichoke Leaf Extract and Their Health Benefits. Plant Foods Hum. Nutr. 2015, 70, 441–453. [Google Scholar] [CrossRef]
- De Falco, E.; Senatore, A.; Roscigno, G.; Pergola, M. The Artichoke ‘Bianco di Pertosa’: The Enhancement of Crop Residues through Environmentally Friendly Uses. Horticulturae 2022, 8, 900. [Google Scholar] [CrossRef]
- Borsini, A.A.; Llavata, B.; Umaña, M.; Cárcel, J.A. Artichoke by products as a source of antioxidant and fiber: How it can be affected by drying temperature. Foods 2021, 10, 459. [Google Scholar] [CrossRef]
- Lutz, M.; Henríquez, C.; Escobar, M. Chemical composition and antioxidant properties of mature and baby artichokes (Cynara scolymus L.), raw and cooked. J. Food Compos. Anal. 2011, 24, 49–54. [Google Scholar] [CrossRef]
- López-Salas, L.; Borrás-Linares, I.; Quintin, D.; García-Gomez, P.; Giménez-Martínez, R.; Segura-Carretero, A.; Lozano-Sánchez, J. Artichoke by-products as natural source of phenolic food ingredient. Appl. Sci. 2021, 11, 3788. [Google Scholar] [CrossRef]
- Lattanzio, V.; Kroon, P.A.; Linsalata, V.; Cardinali, A. Globe artichoke: A functional food and source of nutraceutical ingredients. J. Funct. Foods 2009, 1, 131–144. [Google Scholar] [CrossRef]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Cannas, M.; Conte, P.; Piga, A.; Del Caro, A. Green recovery optimization of phenolic compounds from ‘Spinoso sardo’ globe artichoke by-products using response surface methodology. Front. Sustain. Food Syst. 2023, 7, 1215809. [Google Scholar] [CrossRef]
- Boubaker, M.; Omri, A.E.L.; Blecker, C.; Bouzouita, N. Fibre concentrate from artichoke (Cynara scolymus L.) stem by-products: Characterization and application as a bakery product ingredient. Food Sci. Technol. Int. 2016, 22, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Rejeb, I.B.; Dhen, N.; Gargouri, M.; Boulila, A. Chemical Composition, Antioxidant Potential and Enzymes Inhibitory Properties of Globe Artichoke By-Products. Chem. Biodivers. 2020, 17, e2000073. [Google Scholar] [CrossRef] [PubMed]
- Ergezer, H.; Serdaroğlu, M. Antioxidant potential of artichoke (Cynara scolymus L.) byproducts extracts in raw beef patties during refrigerated storage. J. Food Meas. Charact. 2018, 12, 982–991. [Google Scholar] [CrossRef]
- McClements, D.J.; Jafari, S.M. Improving emulsion formation, stability and performance using mixed emulsifiers: A review. Adv. Colloid. Interface Sci. 2018, 251, 55–79. [Google Scholar] [CrossRef]
- Iervese, F.; Flamminii, F.; D’Alessio, G.; Neri, L.; De Bruno, A.; Imeneo, V.; Valbonetti, L.; Di Mattia, C.D. Flavonoid- and limonoid-rich extracts from lemon pomace by-products: Technological properties for the formulation of o/w emulsions. Food Biosci. 2024, 59, 104030. [Google Scholar] [CrossRef]
- Velderrain-Rodríguez, G.R.; Salvia-Trujillo, L.; González-Aguilar, G.A.; Martín-Belloso, O. Interfacial activity of phenolic-rich extracts from avocado fruit waste: Influence on the colloidal and oxidative stability of emulsions and nanoemulsions. Innov. Food Sci. Emerg. Technol. 2021, 69, 102665. [Google Scholar] [CrossRef]
- Giacintucci, V.; Di Mattia, C.; Sacchetti, G.; Neri, L.; Pittia, P. Role of olive oil phenolics in physical properties and stability of mayonnaise-like emulsions. Food Chem. 2016, 213, 369–377. [Google Scholar] [CrossRef]
- Flamminii, F.; Di Mattia, C.D.; Difonzo, G.; Neri, L.; Faieta, M.; Caponio, F.; Pittia, P. From by-product to food ingredient: Evaluation of compositional and technological properties of olive-leaf phenolic extracts. J. Sci. Food Agric. 2019, 99, 6620–6627. [Google Scholar] [CrossRef]
- Di Mattia, C.D.; Sacchetti, G.; Mastrocola, D.; Sarker, D.K.; Pittia, P. Surface properties of phenolic compounds and their influence on the dispersion degree and oxidative stability of olive oil O/W emulsions. Food Hydrocoll. 2010, 24, 652–658. [Google Scholar] [CrossRef]
- Caballero, S.; Li, Y.O.; McClements, D.J.; Davidov-Pardo, G. Encapsulation and delivery of bioactive citrus pomace polyphenols: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 8028–8044. [Google Scholar] [CrossRef]
- Liang, H.N.; Tang, C.H. PH-dependent emulsifying properties of pea [Pisum sativum (L.)] proteins. Food Hydrocoll. 2013, 33, 309–319. [Google Scholar] [CrossRef]
- Ladjal-Ettoumi, Y.; Boudries, H.; Chibane, M.; Romero, A. Pea, Chickpea and Lentil Protein Isolates: Physicochemical Characterization and Emulsifying Properties. Food Biophys. 2016, 11, 43–51. [Google Scholar] [CrossRef]
- McClements, D.J. Food Emulsions: Principles, Practices, and Techniques; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef]
- Meng, Y.; Li, C. Conformational changes and functional properties of whey protein isolate-polyphenol complexes formed by non-covalent interaction. Food Chem. 2021, 364, 129622. [Google Scholar] [CrossRef]
- Di Mattia, C.; Paradiso, V.M.; Andrich, L.; Giarnetti, M.; Caponio, F.; Pittia, P. Effect of Olive Oil Phenolic Compounds and Maltodextrins on the Physical Properties and Oxidative Stability of Olive Oil O/W Emulsions. Food Biophys. 2014, 9, 396–405. [Google Scholar] [CrossRef]
- Katsouli, M.; Polychniatou, V.; Tzia, C. Influence of surface-active phenolic acids and aqueous phase ratio on w/o nano-emulsions properties; model fitting and prediction of nano-emulsions oxidation stability. J. Food Eng. 2017, 214, 40–46. [Google Scholar] [CrossRef]
- Jiménez-Moreno, N.; Cimminelli, M.J.; Volpe, F.; Ansó, R.; Esparza, I.; Mármol, I.; Rodríguez-Yoldi, M.J.; Ancín-Azpilicueta, C. Phenolic composition of artichoke waste and its antioxidant capacity on differentiated Caco-2 cells. Nutrients 2019, 11, 1723. [Google Scholar] [CrossRef]
- Marques, P.; Marto, J.; Gonçalves, L.M.; Pacheco, R.; Fitas, M.; Pinto, P.; Serralheiro, M.L.; Ribeiro, H. Cynara scolymus L.: A promising Mediterranean extract for topical anti-aging prevention. Ind. Crops Prod. 2017, 109, 699–706. [Google Scholar] [CrossRef]
- Francavilla, M.; Marone, M.; Marasco, P.; Contillo, F.; Monteleone, M. Artichoke biorefinery: From food to advanced technological applications. Foods 2021, 10, 112. [Google Scholar] [CrossRef]
- Bertelsen, A.S.; Laursen, A.; Knudsen, T.A.; Møller, S.; Kidmose, U. Bitter taste masking of enzyme-treated soy protein in water and bread. J. Sci. Food Agric. 2018, 98, 3860–3869. [Google Scholar] [CrossRef]
- Eljounaidi, K.; Comino, C.; Moglia, A.; Cankar, K.; Genre, A.; Hehn, A.; Bourgaud, F.; Beekwilder, J.; Lanteri, S. Accumulation of cynaropicrin in globe artichoke and localization of enzymes involved in its biosynthesis. Plant Sci. 2015, 239, 128–136. [Google Scholar] [CrossRef]
- Cravotto, G.; Nano, G.M.; Binello, A.; Spagliardi, P.; Seu, G. Chemical and biological modification of cynaropicrin and grosheimin: A structure-bitterness relationship study. J. Sci. Food Agric. 2005, 85, 1757–1764. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols—A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Ćujić-Nikolić, N.; Stanisavljević, N.; Šavikin, K.; Kalušević, A.; Nedović, V.; Samardžić, J.; Janković, T. Chokeberry polyphenols preservation using spray drying: Effect of encapsulation using maltodextrin and skimmed milk on their recovery following in vitro digestion. J. Microencapsul. 2019, 36, 693–703. [Google Scholar] [CrossRef]
- Stoica, F.; Condurache, N.N.; Aprodu, I.; Andronoiu, D.G.; Enachi, E.; Stănciuc, N.; Bahrim, G.E.; Croitoru, C.; Râpeanu, G. Value-added salad dressing enriched with red onion skin anthocyanins entrapped in different biopolymers. Food Chem. X 2022, 15, 100374. [Google Scholar] [CrossRef]
- González, E.; Gómez-Caravaca, A.M.; Giménez, B.; Cebrián, R.; Maqueda, M.; Parada, J.; Martínez-Férez, A.; Segura-Carretero, A.; Robert, P. Role of maltodextrin and inulin as encapsulating agents on the protection of oleuropein during in vitro gastrointestinal digestion. Food Chem. 2020, 310, 125976. [Google Scholar] [CrossRef]
- Gutiérrez-Grijalva, E.P.; Antunes-Ricardo, M.; Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Heredia, J.B. Cellular antioxidant activity and in vitro inhibition of α-glucosidase, α-amylase and pancreatic lipase of oregano polyphenols under simulated gastrointestinal digestion. Food Res. Int. 2019, 116, 676–686. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- D’Alessio, G.; Flamminii, F.; Faieta, M.; Pittia, P.; Di Mattia, C.D. Pea protein isolates: Emulsification properties as affected by preliminary pretreatments. Ital. J. Food Sci. 2022, 34, 25–32. [Google Scholar] [CrossRef]
- Tatasciore, S.; Santarelli, V.; Neri, L.; González Ortega, R.; Faieta, M.; Di Mattia, C.D.; Di Michele, A.; Pittia, P. Freeze-Drying Microencapsulation of Hop Extract: Effect of Carrier Composition on Physical, Techno-Functional, and Stability Properties. Antioxidants 2023, 12, 442. [Google Scholar] [CrossRef]
- Ravichai, K.; Muangrat, R. Effect of different coating materials on freeze-drying encapsulation of bioactive compounds from fermented tea leaf wastewater. J. Food Process Preserv. 2019, 43, e14145. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food-an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Xie, M.; Wan, P.; Chen, D.; Ye, H.; Chen, L.; Zeng, X.; Liu, Z. Digestion under saliva, simulated gastric and small intestinal conditions and fermentation in vitro by human intestinal microbiota of polysaccharides from Fuzhuan brick tea. Food Chem. 2018, 244, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Bu, G.; Ren, M.; Zuo, Y.; Zhao, C. Functional characteristics and structural properties of soybean protein isolate–maltose conjugates. Cereal Chem. 2022, 99, 100–110. [Google Scholar] [CrossRef]

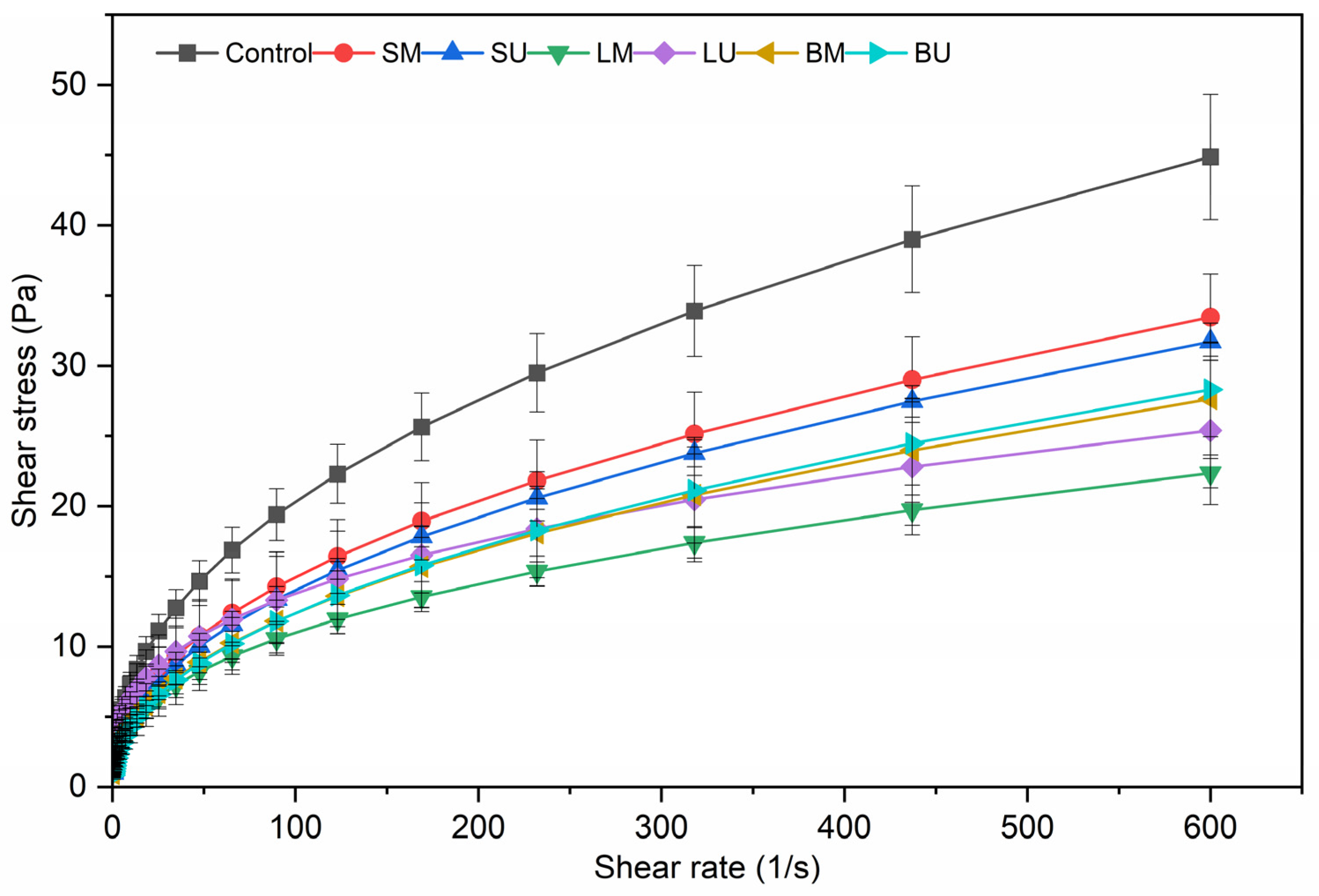

| Sample | k | n | R2 |
|---|---|---|---|
| Control | 2.692 ± 0.432 a | 0.441 ± 0.028 a | 0.999 ± 2.89 × 10−4 |
| SM | 1.943 ± 0.716 ab | 0.454 ± 0.052 a | 0.999 ± 8.29 × 10−5 |
| SU | 1.732 ± 0.121 ab | 0.455 ± 0.01 a | 0.999 ± 1.36 × 10−4 |
| LM | 1.808 ± 0.392 ab | 0.396 ± 0.032 ab | 0.999 ± 2.63 × 10−4 |
| LU | 2.899 ± 0.853 a | 0.342 ± 0.017 b | 0.999 ± 2.68 × 10−4 |
| BM | 1.601 ± 0.586 b | 0.451 ± 0.032 a | 0.999 ± 4.39 × 10−5 |
| BU | 1.489 ± 0.255 b | 0.461 ± 0.006 a | 0.999 ± 5.25 × 10−5 |
| Method | By-Product | Legend | Parameters | TPC (mg/gdm) | |||
|---|---|---|---|---|---|---|---|
| Frequency (Hz) | Power (W) | Ethanol (%) | Time (min) | ||||
| M | Stems | SM | 53 | 60 | 2604 ± 10 a | ||
| Leaves | LM | 45 | 60 | 1863 ± 6 d | |||
| Bracts | BM | 50 | 60 | 1866 ± 5 d | |||
| UAE | Stems | SU | 40 | 144 | 42 | 10 | 2516 ± 4 b |
| Leaves | LU | 20 | 10 | 1723 ± 20 e | |||
| Bracts | BU | 64 | 41 | 2014 ± 31 c | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iervese, F.; Paluzzi, A.; Cannas, M.; D’Alessio, G.; Piga, A.; Di Mattia, C. Development, Characterization, and Exploitation in Food Systems of Functional Ingredients Obtained from Artichoke By-Products Phenolic Extracts. Molecules 2025, 30, 1514. https://doi.org/10.3390/molecules30071514
Iervese F, Paluzzi A, Cannas M, D’Alessio G, Piga A, Di Mattia C. Development, Characterization, and Exploitation in Food Systems of Functional Ingredients Obtained from Artichoke By-Products Phenolic Extracts. Molecules. 2025; 30(7):1514. https://doi.org/10.3390/molecules30071514
Chicago/Turabian StyleIervese, Francesco, Arianna Paluzzi, Michela Cannas, Giulia D’Alessio, Antonio Piga, and Carla Di Mattia. 2025. "Development, Characterization, and Exploitation in Food Systems of Functional Ingredients Obtained from Artichoke By-Products Phenolic Extracts" Molecules 30, no. 7: 1514. https://doi.org/10.3390/molecules30071514
APA StyleIervese, F., Paluzzi, A., Cannas, M., D’Alessio, G., Piga, A., & Di Mattia, C. (2025). Development, Characterization, and Exploitation in Food Systems of Functional Ingredients Obtained from Artichoke By-Products Phenolic Extracts. Molecules, 30(7), 1514. https://doi.org/10.3390/molecules30071514








