Pinitol Improves Lipopolysaccharide-Induced Cellular Damage in Human Dermal Microvascular Endothelial Cells
Abstract
1. Introduction
2. Results
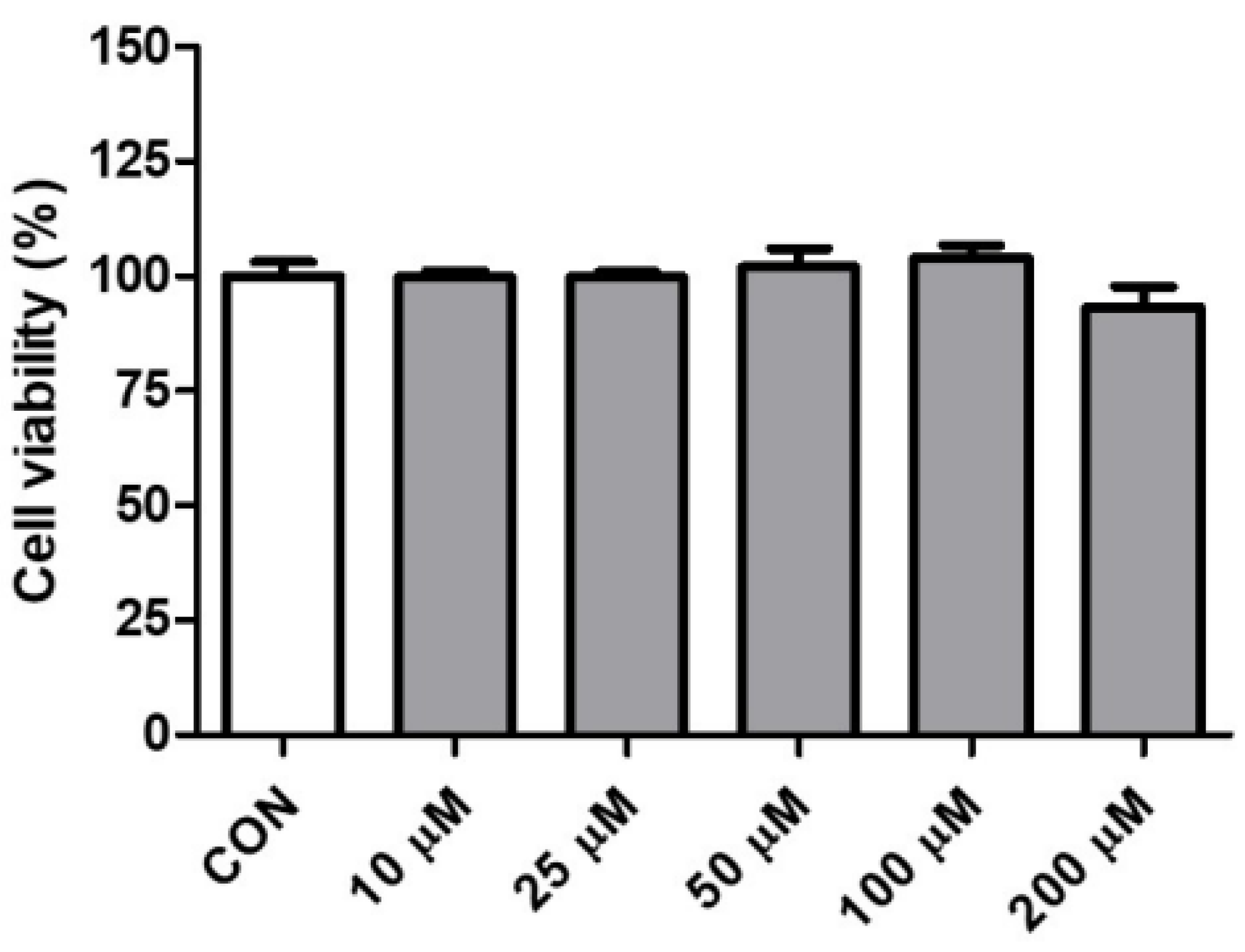
2.1. Effect of Pinitol on the Cell Viability in HDMECs
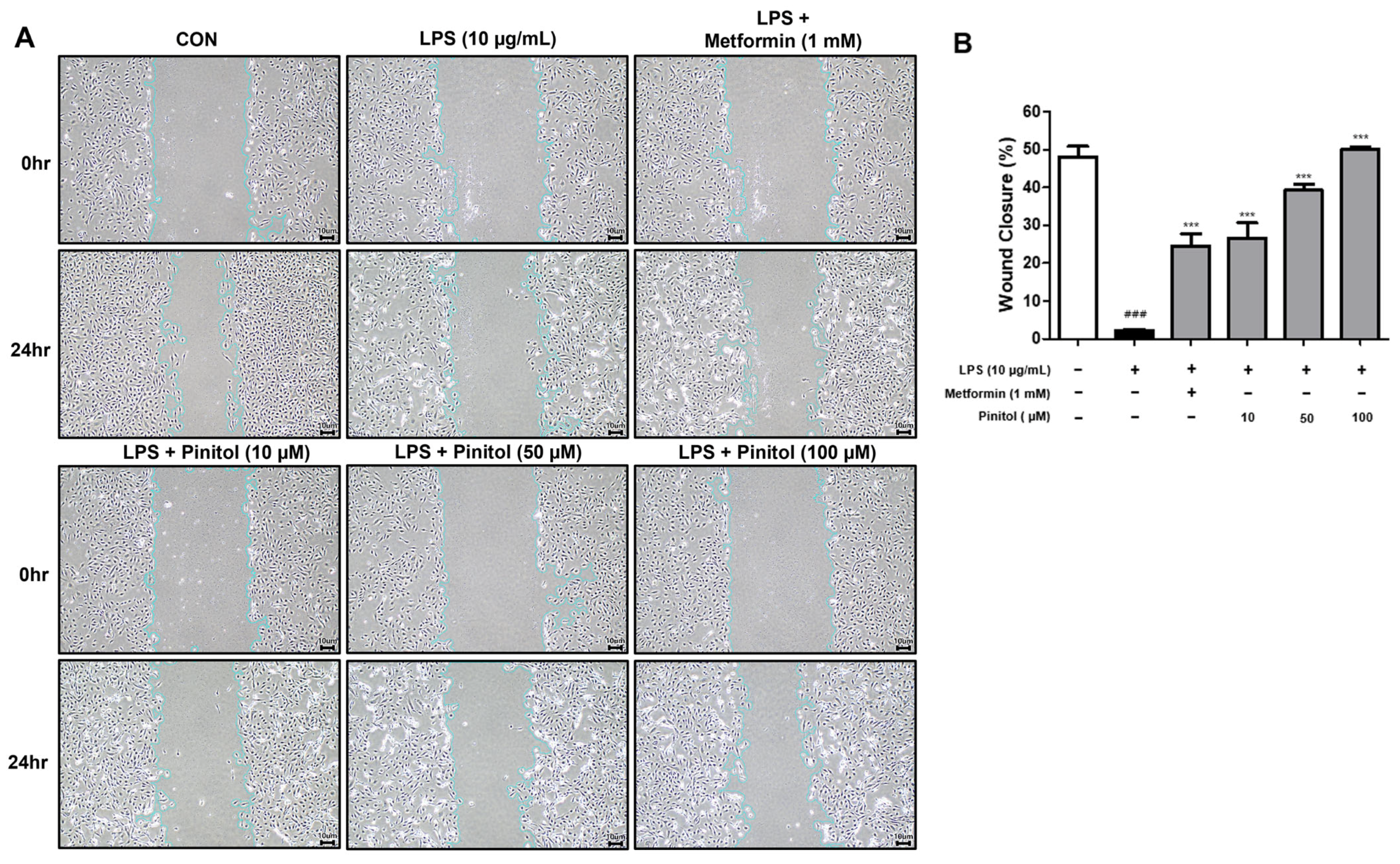
2.2. Pinitol Improved Wound Healing in LPS-Damaged HDMECs
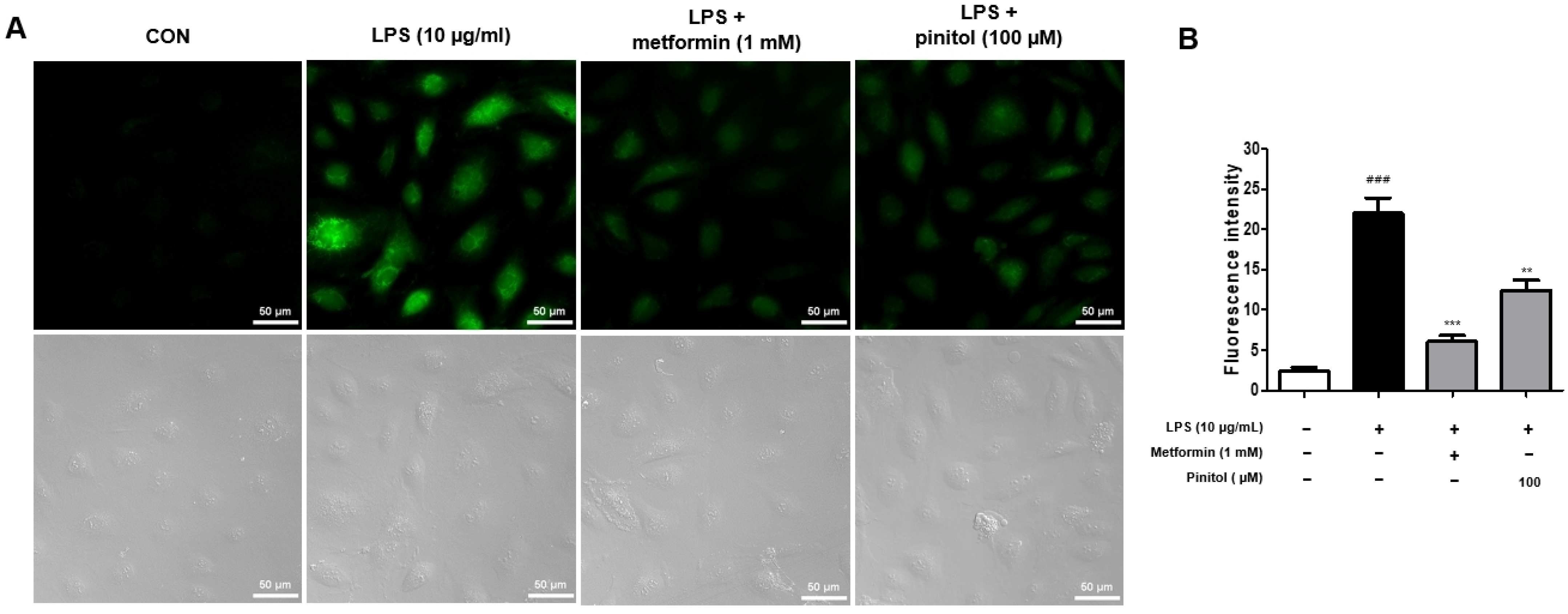
2.3. Pinitol Recovered ROS Levels in LPS-Damaged HDMECs
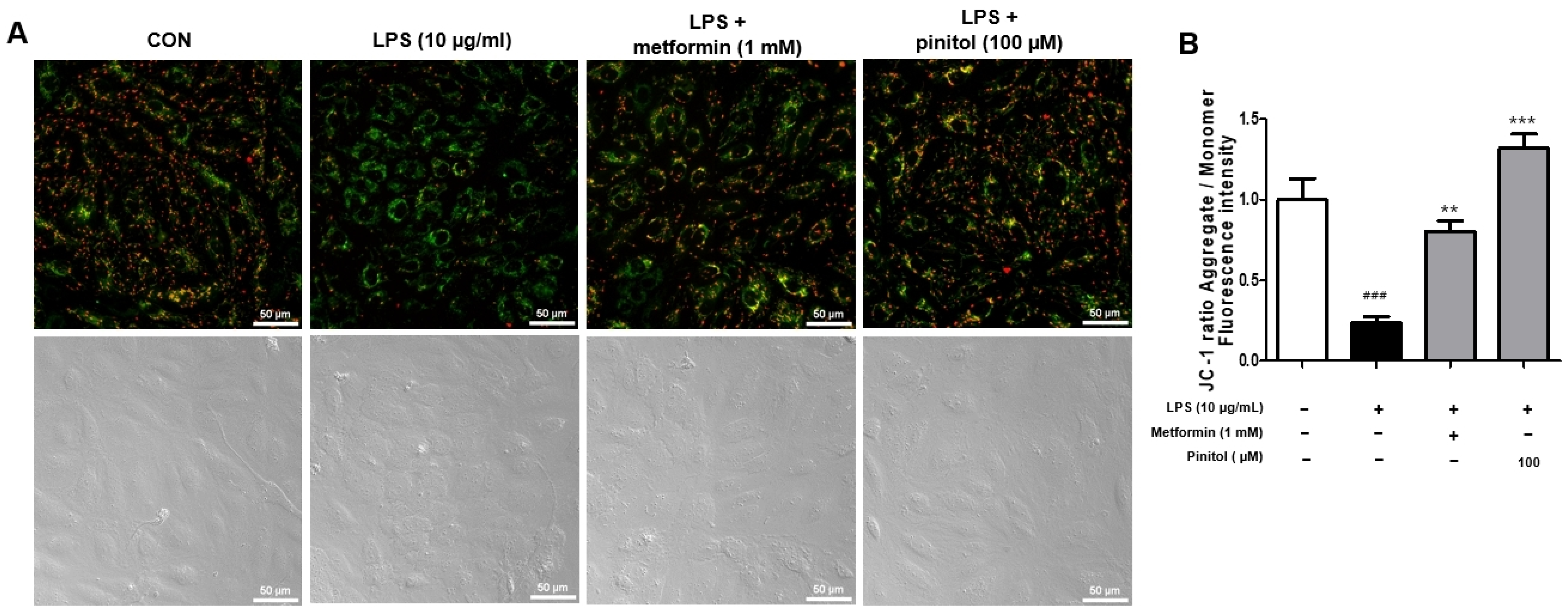
2.4. Pinitol Ameliorated the Membrane Potential of Mitochondria in LPS-Damaged HDMECs
2.5. Pinitol Inhibited NF-κB Pathway in LPS-Damaged HDMECs
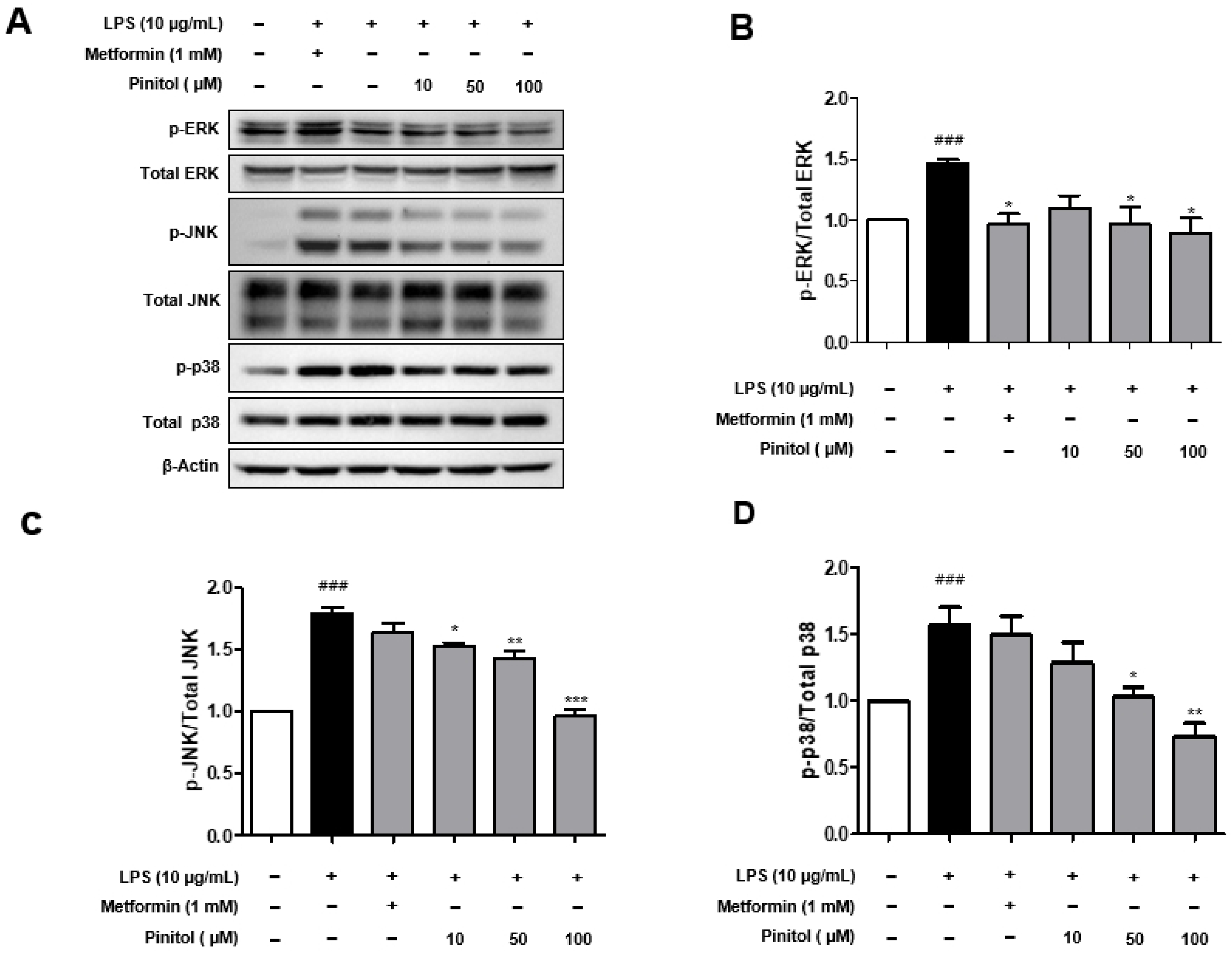
2.6. Pinitol Significantly Decreased the Phosphorylation Levels of MAPKs in LPS-Damaged HDMECs
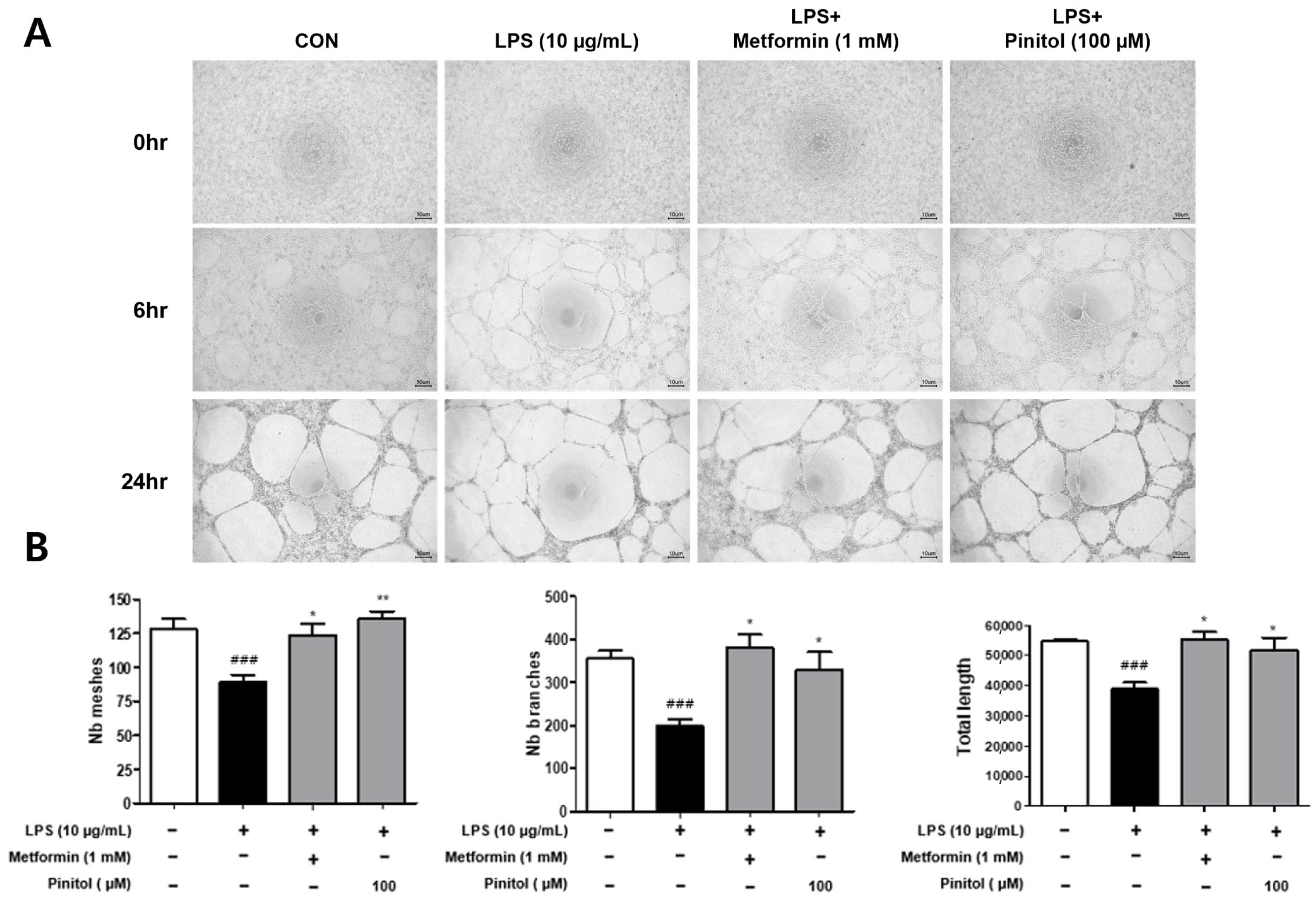
2.7. Pinitol Improved Tube Formation in LPS-Damaged HDMECs
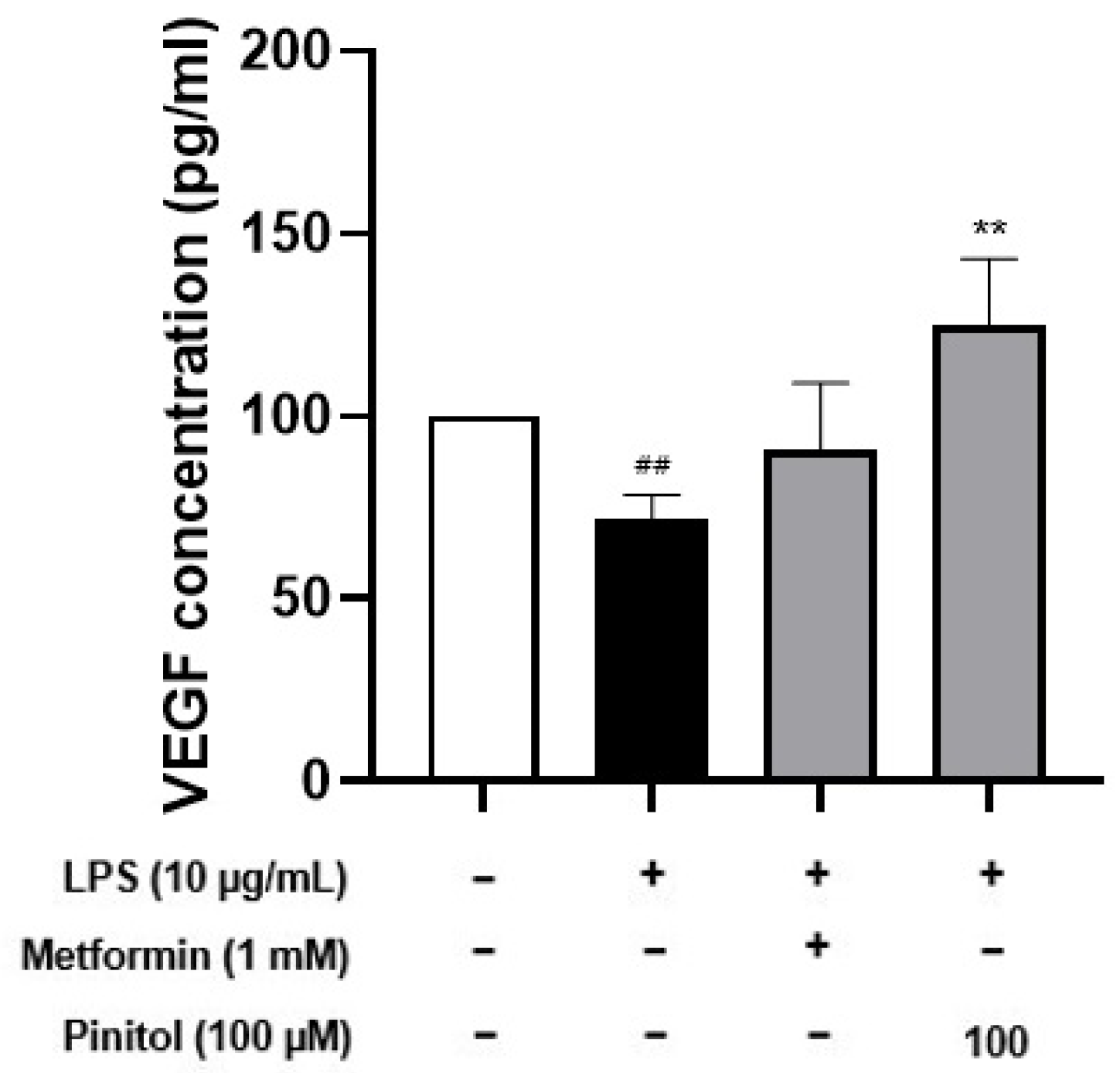
2.8. Pinitol Improved Specific Cytokine Levels in LPS-Damaged HDMECs
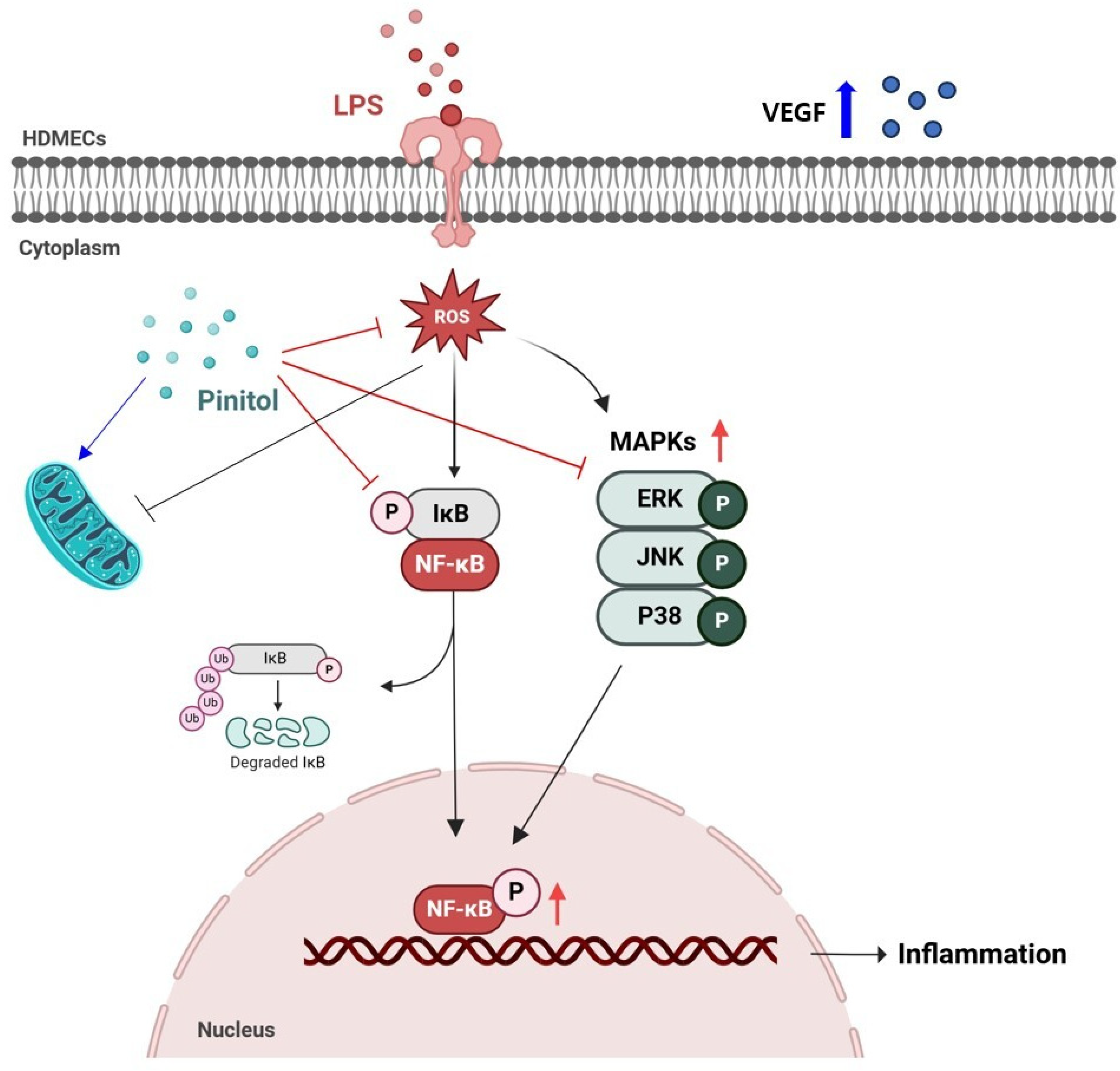
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Wound Healing Assay
4.5. Tube Formation Assay
4.6. DCF-DA ROS Assay
4.7. Mitochondrial Membrane Potential Assay
4.8. Immunofluorescence
4.9. Western Blot Analysis
4.10. ELISA Assay
4.11. Quantitative Real-Time Polymerase Chain Reaction
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prisby, R.D. Mechanical, Hormonal, and Metabolic Influences on Blood Vessels, Blood Flow and Bone. J. Endocrinol. 2017, 235, R77–R100. [Google Scholar] [CrossRef] [PubMed]
- Eelen, G.; Treps, L.; Li, X.; Carmeliet, P. Basic and Therapeutic Aspects of Angiogenesis Updated. Circ. Res. 2020, 127, 310–329. [Google Scholar] [CrossRef] [PubMed]
- Rousselle, P.; Montmasson, M.; Garnier, C. Extracellular Matrix Contribution to Skin Wound Re-Epithelialization. Matrix Biol. 2019, 75–76, 12–26. [Google Scholar] [CrossRef]
- Lindley, L.E.; Stojadinovic, O.; Pastar, I.; Tomic-Canic, M. Biology and Biomarkers for Wound Healing. Plast. Reconstr. Surg. 2016, 138, 18S–28S. [Google Scholar] [CrossRef]
- Velnar, T.; Gradisnik, L. Tissue Augmentation in Wound Healing: The Role of Endothelial and Epithelial Cells. Med. Arch. 2018, 72, 444–448. [Google Scholar] [CrossRef]
- Todorova, D.; Simoncini, S.; Lacroix, R.; Sabatier, F.; Dignat-George, F. Extracellular Vesicles in Angiogenesis. Circ. Res. 2017, 120, 1658–1673. [Google Scholar] [CrossRef]
- Yuge, S.; Ishii, T.; Noishiki, C.; Fukuhara, S. Novel Regulatory Mechanisms Underlying Angiogenesis during Wound Healing Revealed by Fluorescence-Based Live-Imaging in Zebrafish. J. Biochem. 2023, 174, 5–12. [Google Scholar] [CrossRef]
- Nosrati, H.; Aramideh Khouy, R.; Nosrati, A.; Khodaei, M.; Banitalebi-Dehkordi, M.; Ashrafi-Dehkordi, K.; Sanami, S.; Alizadeh, Z. Nanocomposite Scaffolds for Accelerating Chronic Wound Healing by Enhancing Angiogenesis. J. Nanobiotechnol. 2021, 19, 1. [Google Scholar] [CrossRef]
- Vilas Boas, P.; Cerroni, L.; Requena, L. Intravascular Cutaneous Disorders. A Clinicopathologic Review. Am. J. Dermatopathol. 2021, 43, 119–136. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Q.; Zhu, J.; Xiao, Q.; Zhang, L. Reactive Oxygen Species: Key Regulators in Vascular Health and Diseases. Br. J. Pharmacol. 2018, 175, 1279–1292. [Google Scholar] [CrossRef]
- Cracowski, J.; Roustit, M. Human Skin Microcirculation. Compr. Physiol. 2020, 10, 1105–1154. [Google Scholar] [PubMed]
- Papi, M.; Papi, C. Vasculitic Ulcers. Int. J. Low. Extrem. Wounds 2016, 15, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Raharja, A.; Mahil, S.K.; Barker, J.N. Psoriasis: A Brief Overview. Clin. Med. 2021, 21, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Herrick, A.L.; Wigley, F.M. Raynaud’s Phenomenon. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101474. [Google Scholar] [CrossRef]
- Calderon, L.M.; Pope, J.E. Scleroderma Epidemiology Update. Curr. Opin. Rheumatol. 2021, 33, 122–127. [Google Scholar] [CrossRef]
- Burgess, J.L.; Wyant, W.A.; Abdo Abujamra, B.; Kirsner, R.S.; Jozic, I. Diabetic Wound-Healing Science. Medicina 2021, 57, 1072. [Google Scholar] [CrossRef]
- Larouche, J.; Sheoran, S.; Maruyama, K.; Martino, M.M. Immune Regulation of Skin Wound Healing: Mechanisms and Novel Therapeutic Targets. Adv. Wound Care 2018, 7, 209–231. [Google Scholar] [CrossRef]
- Orecchia, A.; Scarponi, C.; Di Felice, F.; Cesarini, E.; Avitabile, S.; Mai, A.; Mauro, M.L.; Sirri, V.; Zambruno, G.; Albanesi, C.; et al. Sirtinol Treatment Reduces Inflammation in Human Dermal Microvascular Endothelial Cells. PLoS ONE 2011, 6, e24307. [Google Scholar] [CrossRef]
- Baselet, B.; Sonveaux, P.; Baatout, S.; Aerts, A. Pathological Effects of Ionizing Radiation: Endothelial Activation and Dysfunction. Cell Mol. Life Sci. 2019, 76, 699–728. [Google Scholar] [CrossRef]
- Ribatti, D.; Tamma, R.; Annese, T. The Role of Vascular Niche and Endothelial Cells in Organogenesis and Regeneration. Exp. Cell Res. 2021, 398, 112398. [Google Scholar] [CrossRef]
- Immanuel, J.; Yun, S. Vascular Inflammatory Diseases and Endothelial Phenotypes. Cells 2023, 12, 1640. [Google Scholar] [CrossRef] [PubMed]
- Lallo, V.; Bracaglia, L.G. Influencing Endothelial Cells’ Roles in Inflammation and Wound Healing Through Nucleic Acid Delivery. Tissue Eng. Part A 2024, 30, 272–286. [Google Scholar] [CrossRef] [PubMed]
- Cayrol, C.; Girard, J. Interleukin-33 (IL-33): A Critical Review of its Biology and the Mechanisms Involved in its Release as a Potent Extracellular Cytokine. Cytokine 2022, 156, 155891. [Google Scholar] [PubMed]
- Du, H.; Li, S.; Lu, J.; Tang, L.; Jiang, X.; He, X.; Liang, J.; Liao, X.; Cui, T.; Huang, Y.; et al. Single-Cell RNA-Seq and Bulk-Seq Identify RAB17 as a Potential Regulator of Angiogenesis by Human Dermal Microvascular Endothelial Cells in Diabetic Foot Ulcers. Burn. Trauma 2023, 11, tkad020. [Google Scholar] [CrossRef]
- Gonzalez-Mauraza, N.H.; Leon-Gonzalez, A.J.; Espartero, J.L.; Gallego-Fernandez, J.B.; Sanchez-Hidalgo, M.; Martin-Cordero, C. Isolation and Quantification of Pinitol, a Bioactive Cyclitol, in Retama spp. Nat. Prod. Commun. 2016, 11, 405–406. [Google Scholar] [CrossRef]
- Azab, A. D-Pinitol-Active Natural Product from Carob with Notable Insulin Regulation. Nutrients 2022, 14, 1453. [Google Scholar] [CrossRef]
- Pandi, A.; Sattu, K.; Kalappan, V.M.; Lal, V.; Varikasuvu, S.R.; Ganguly, A.; Prasad, J. Pharmacological Effects of D-Pinitol—A Comprehensive Review. J. Food Biochem. 2022, 46, e14282. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, M.; Wu, T.; Xu, M.; Cai, H.; Zhang, Z. Effects of D-Pinitol on Insulin Resistance through the PI3K/Akt Signaling Pathway in Type 2 Diabetes Mellitus Rats. J. Agric. Food Chem. 2015, 63, 6019–6026. [Google Scholar]
- Sivakumar, S.; Palsamy, P.; Subramanian, S.P. Impact of D-Pinitol on the Attenuation of Proinflammatory Cytokines, Hyperglycemia-Mediated Oxidative Stress and Protection of Kidney Tissue Ultrastructure in Streptozotocin-Induced Diabetic Rats. Chem.-Biol. Interact. 2010, 188, 237–245. [Google Scholar] [CrossRef]
- Haque, M.F.; El-Nashar, H.A.S.; Akbor, M.S.; Alfaifi, M.; Bappi, M.H.; Chowdhury, A.K.; Hossain, M.K.; El-Shazly, M.; Albayouk, T.; Saleh, N.; et al. Anti-Inflammatory Activity of D-Pinitol Possibly through Inhibiting COX-2 Enzyme: In Vivo and in Silico Studies. Front. Chem. 2024, 12, 1366844. [Google Scholar]
- Jung, J.; Shim, J.H.; Cho, S.H.; Bae, I.; Yang, S.H.; Kim, J.; Lim, H.W.; Shin, D.W. The Anti-Diabetic Pinitol Improves Damaged Fibroblasts. Biomol. Ther. 2024, 32, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Go, M.Y.; Jeon, C.Y.; Shin, J.U.; Kim, M.; Lim, H.W.; Shin, D.W. Pinitol Improves Diabetic Foot Ulcers in Streptozotocin-Induced Diabetes Rats Through Upregulation of Nrf2/HO-1 Signaling. Antioxidants 2024, 14, 15. [Google Scholar] [CrossRef] [PubMed]
- Hawthorne, B.; Simmons, J.K.; Stuart, B.; Tung, R.; Zamierowski, D.S.; Mellott, A.J. Enhancing Wound Healing Dressing Development through Interdisciplinary Collaboration. J. Biomed. Mater. Res. B. Appl. Biomater. 2021, 109, 1967–1985. [Google Scholar] [PubMed]
- DiPietro, L.A. Angiogenesis and Wound Repair: When enough is Enough. J. Leukoc. Biol. 2016, 100, 979–984. [Google Scholar]
- Wang, P.; Huang, B.; Horng, H.; Yeh, C.; Chen, Y. Wound Healing. J. Chin. Med. Assoc. 2018, 81, 94–101. [Google Scholar]
- Crompton, R.; Williams, H.; Ansell, D.; Campbell, L.; Holden, K.; Cruickshank, S.; Hardman, M.J. Oestrogen Promotes Healing in a Bacterial LPS Model of Delayed Cutaneous Wound Repair. Lab. Investig. 2016, 96, 439–449. [Google Scholar] [CrossRef]
- Farhana, A.; Khan, Y.S. Biochemistry, Lipopolysaccharide. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2024. [Google Scholar]
- Chan, Y.H.; Harith, H.H.; Israf, D.A.; Tham, C.L. Differential Regulation of LPS-Mediated VE-Cadherin Disruption in Human Endothelial Cells and the Underlying Signaling Pathways: A Mini Review. Front. Cell Dev. Biol. 2020, 7, 280. [Google Scholar]
- Murphy, M.P.; Holmgren, A.; Larsson, N.; Halliwell, B.; Chang, C.J.; Kalyanaraman, B.; Rhee, S.G.; Thornalley, P.J.; Partridge, L.; Gems, D.; et al. Unraveling the Biological Roles of Reactive Oxygen Species. Cell Metab. 2011, 13, 361–366. [Google Scholar]
- Panieri, E.; Santoro, M.M. ROS Signaling and Redox Biology in Endothelial Cells. Cell. Mol. Life Sci. 2015, 72, 3281–3303. [Google Scholar]
- Tian, M.; Ma, Y.; Lin, W. Fluorescent Probes for the Visualization of Cell Viability. Acc. Chem. Res. 2019, 52, 2147–2157. [Google Scholar]
- Natarajan, V.; Chawla, R.; Mah, T.; Vivekanandan, R.; Tan, S.Y.; Sato, P.Y.; Mallilankaraman, K. Mitochondrial Dysfunction in Age-Related Metabolic Disorders. Proteomics 2020, 20, e1800404. [Google Scholar] [PubMed]
- Rastogi, S.; Haldar, C. Comparative Effect of Melatonin and Quercetin in Counteracting LPS Induced Oxidative Stress in Bone Marrow Mononuclear Cells and Spleen of Funambulus Pennanti. Food Chem. Toxicol. 2018, 120, 243–252. [Google Scholar] [PubMed]
- Fock, E.M.; Parnova, R.G. Protective Effect of Mitochondria-Targeted Antioxidants Against Inflammatory Response to Lipopolysaccharide Challenge: A Review. Pharmaceutics 2021, 13, 144. [Google Scholar] [CrossRef] [PubMed]
- Perelman, A.; Wachtel, C.; Cohen, M.; Haupt, S.; Shapiro, H.; Tzur, A. JC-1: Alternative Excitation Wavelengths Facilitate Mitochondrial Membrane Potential Cytometry. Cell Death Dis. 2012, 3, e430. [Google Scholar]
- Yang, R.; Gao, W.; Wang, Z.; Jian, H.; Peng, L.; Yu, X.; Xue, P.; Peng, W.; Li, K.; Zeng, P. Polyphyllin I Induced Ferroptosis to Suppress the Progression of Hepatocellular Carcinoma through Activation of the Mitochondrial Dysfunction Via Nrf2/HO-1/GPX4 Axis. Phytomedicine 2024, 122, 155135. [Google Scholar]
- Liu, S.; Chen, Q.; Liu, J.; Yang, X.; Zhang, Y.; Huang, F. Sinomenine Protects Against E.Coli-Induced Acute Lung Injury in Mice through Nrf2-NF-kappaB Pathway. Biomed. Pharmacother. 2018, 107, 696–702. [Google Scholar]
- Zhang, P.; Yang, M.; Chen, C.; Liu, L.; Wei, X.; Zeng, S. Toll-Like Receptor 4 (TLR4)/Opioid Receptor Pathway Crosstalk and Impact on Opioid Analgesia, Immune Function, and Gastrointestinal Motility. Front. Immunol. 2020, 11, 1455. [Google Scholar]
- Zhao, Z.; Sun, Y.; Ruan, X. Bornyl Acetate: A Promising Agent in Phytomedicine for Inflammation and Immune Modulation. Phytomedicine 2023, 114, 154781. [Google Scholar]
- Tang, J.; Xu, L.; Zeng, Y.; Gong, F. Effect of Gut Microbiota on LPS-Induced Acute Lung Injury by Regulating the TLR4/NF-kB Signaling Pathway. Int. Immunopharmacol. 2021, 91, 107272. [Google Scholar]
- Cargnello, M.; Roux, P.P. Activation and Function of the MAPKs and their Substrates, the MAPK-Activated Protein Kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar]
- Yu, J.; Ming, H.; Li, H.Y.; Yu, B.; Chu, M.; Zhu, H.; Zhu, X. IMM-H007, a Novel Small Molecule Inhibitor for Atherosclerosis, Represses Endothelium Inflammation by Regulating the Activity of NF-kappaB and JNK/AP1 Signaling. Toxicol. Appl. Pharmacol. 2019, 381, 114732. [Google Scholar] [PubMed]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-kappaB Pathway for the Therapy of Diseases: Mechanism and Clinical Study. Signal Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [PubMed]
- Wang, Z.; Fang, C.; Yao, M.; Wu, D.; Chen, M.; Guo, T.; Mo, J. Research Progress of NF-kappaB Signaling Pathway and Thrombosis. Front. Immunol. 2023, 14, 1257988. [Google Scholar]
- DiPietro, L.A. Angiogenesis and Scar Formation in Healing Wounds. Curr. Opin. Rheumatol. 2013, 25, 87–91. [Google Scholar]
- Arnaoutova, I.; Kleinman, H.K. In Vitro Angiogenesis: Endothelial Cell Tube Formation on Gelled Basement Membrane Extract. Nat. Protoc. 2010, 5, 628–635. [Google Scholar]
- Kelley, M.; Fierstein, S.; Purkey, L.; DeCicco-Skinner, K. Endothelial Cell Tube Formation Assay: An In Vitro Model for Angiogenesis. Methods Mol. Biol. 2022, 2475, 187–196. [Google Scholar]
- Kubota, Y.; Kleinman, H.K.; Martin, G.R.; Lawley, T.J. Role of Laminin and Basement Membrane in the Morphological Differentiation of Human Endothelial Cells into Capillary-Like Structures. J. Cell Biol. 1988, 107, 1589–1598. [Google Scholar]
- DeCicco-Skinner, K.L.; Henry, G.H.; Cataisson, C.; Tabib, T.; Gwilliam, J.C.; Watson, N.J.; Bullwinkle, E.M.; Falkenburg, L.; O’Neill, R.C.; Morin, A.; et al. Endothelial Cell Tube Formation Assay for the in Vitro Study of Angiogenesis. J. Vis. Exp. 2014, 91, e51312. [Google Scholar]
- Li, Y.; Zhu, H.; Wei, X.; Li, H.; Yu, Z.; Zhang, H.; Liu, W. LPS Induces HUVEC Angiogenesis in Vitro through miR-146a-Mediated TGF-Beta1 Inhibition. Am. J. Transl. Res. 2017, 9, 591–600. [Google Scholar]
- Kim, Y.; West, X.Z.; Byzova, T.V. Inflammation and Oxidative Stress in Angiogenesis and Vascular Disease. J. Mol. Med. 2013, 91, 323–328. [Google Scholar]
- Karl, E.; Zhang, Z.; Dong, Z.; Neiva, K.G.; Soengas, M.S.; Koch, A.E.; Polverini, P.J.; Núñez, G.; Nör, J.E. Unidirectional crosstalk between Bcl-xL and Bcl-2 enhances the angiogenic phenotype of endothelial cells. Cell Death Differ. 2007, 14, 1657–1666. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Tao, R.; Chen, L.; Xiong, Y.; Xue, H.; Hu, L.; Yan, C.; Xie, X.; Lin, Z.; Panayi, A.C.; et al. Exosomes derived from pioglitazone-pretreated MSCs accelerate diabetic wound healing through enhancing angiogenesis. Nanobiotechnology 2021, 19, 150. [Google Scholar] [CrossRef] [PubMed]
- Miyake, M.; Goodison, S.; Urquidi, V.; Gomes Giacoia, E.; Rosser, C.J. Expression of CXCL1 in human endothelial cells induces angiogenesis through the CXCR2 receptor and the ERK1/2 and EGF pathways. Lab. Investig. 2013, 93, 768–778. [Google Scholar] [CrossRef]
- O’Leary, A.P.; Fox, J.M.; Pullar, C.E. Beta-Adrenoceptor Activation Reduces Both Dermal Microvascular Endothelial Cell Migration via a cAMP-Dependent Mechanism and Wound Angiogenesis. J. Cell. Physiol. 2015, 230, 356–365. [Google Scholar] [CrossRef]
- Li, Y.; Xu, T.; Tu, Z.; Dai, W.; Xue, Y.; Tang, C.; Gao, W.; Mao, C.; Lei, B.; Lin, C. Bioactive antibacterial silica-based nanocomposites hydrogel scaffolds with high angiogenesis for promoting diabetic wound healing and skin repair. Theranostics 2020, 10, 4929–4943. [Google Scholar] [CrossRef]
- Laiva, A.L.; O’Brien, F.J.; Keogh, M.B. Innovations in gene and growth factor delivery systems for diabetic wound healing. J. Tissue Eng. Regen. Med. 2018, 12, e296–e312. [Google Scholar] [CrossRef]
- Shi, Y.; Vanhoutte, P.M. Macro- and Microvascular Endothelial Dysfunction in Diabetes. J. Diabetes 2017, 9, 434–449. [Google Scholar] [CrossRef]
- Fung, T.H.; Patel, B.; Wilmot, E.G.; Amoaku, W.M. Diabetic Retinopathy for the Non-Ophthalmologist. Clin. Med. 2022, 22, 112–116. [Google Scholar] [CrossRef]
- Guo, W.; Song, Y.; Sun, Y.; Du, H.; Cai, Y.; You, Q.; Fu, H.; Shao, L. Systemic Immune-Inflammation Index is Associated with Diabetic Kidney Disease in Type 2 Diabetes Mellitus Patients: Evidence from NHANES 2011–2018. Front. Endocrinol. 2022, 13, 1071465. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Tan, T.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers: A Review. JAMA 2023, 330, 62–75. [Google Scholar] [CrossRef]
- Laurent, S. Antihypertensive Drugs. Pharmacol. Res. 2017, 124, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Nath, M.; Bhattacharjee, K.; Choudhury, Y. Pleiotropic Effects of Anti-Diabetic Drugs: A Comprehensive Review. Eur. J. Pharmacol. 2020, 884, 173349. [Google Scholar] [PubMed]
- Attardo, S.; Musumeci, O.; Velardo, D.; Toscano, A. Statins Neuromuscular Adverse Effects. Int. J. Mol. Sci. 2022, 23, 8364. [Google Scholar] [CrossRef] [PubMed]
- Lui, J.L.; Shaw, N.M.; Abbasi, B.; Hakam, N.; Breyer, B.N. Adverse Reactions of PDE5 Inhibitors: An Analysis of the World Health Organization Pharmacovigilance Database. Andrology 2023, 11, 1408–1417. [Google Scholar]
- Dzidic-Krivic, A.; Sher, E.K.; Kusturica, J.; Farhat, E.K.; Nawaz, A.; Sher, F. Unveiling Drug Induced Nephrotoxicity using Novel Biomarkers and Cutting-Edge Preventive Strategies. Chem. Biol. Interact. 2024, 388, 110838. [Google Scholar]
- LaMoia, T.E.; Shulman, G.I. Cellular and Molecular Mechanisms of Metformin Action. Endocr. Rev. 2021, 42, 77–96. [Google Scholar]
- Triggle, C.R.; Marei, I.; Ye, K.; Ding, H.; Anderson, T.J.; Hollenberg, M.D.; Hill, M.A. Repurposing Metformin for Vascular Disease. Curr. Med. Chem. 2023, 30, 3955–3978. [Google Scholar] [CrossRef]
- Feng, J.; Wang, X.; Ye, X.; Ares, I.; Lopez-Torres, B.; Martinez, M.; Martinez-Larranaga, M.; Wang, X.; Anadon, A.; Martinez, M. Mitochondria as an Important Target of Metformin: The Mechanism of Action, Toxic and Side Effects, and New Therapeutic Applications. Pharmacol. Res. 2022, 177, 106114. [Google Scholar]
- Salvatore, T.; Pafundi, P.C.; Galiero, R.; Rinaldi, L.; Caturano, A.; Vetrano, E.; Aprea, C.; Albanese, G.; Di Martino, A.; Ricozzi, C.; et al. Can Metformin Exert as an Active Drug on Endothelial Dysfunction in Diabetic Subjects? Biomedicines 2020, 9, 3. [Google Scholar] [CrossRef]
- Ding, Y.; Zhou, Y.; Ling, P.; Feng, X.; Luo, S.; Zheng, X.; Little, P.J.; Xu, S.; Weng, J. Metformin in Cardiovascular Diabetology: A Focused Review of its Impact on Endothelial Function. Theranostics 2021, 11, 9376–9396. [Google Scholar] [CrossRef]
- Dutta, S.; Shah, R.B.; Singhal, S.; Dutta, S.B.; Bansal, S.; Sinha, S.; Haque, M. Metformin: A Review of Potential Mechanism and Therapeutic Utility Beyond Diabetes. Drug Des. Devel. Ther. 2023, 17, 1907–1932. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Ji, J.Y. Progerin-Induced Impairment in Wound Healing and Proliferation in Vascular Endothelial Cells. Front. Aging 2022, 3, 844885. [Google Scholar]
- Meng, M.; Jiang, Y.; Wang, Y.; Huo, R.; Ma, N.; Shen, X.; Chang, G. Beta-Carotene Targets IP3R/GRP75/VDAC1-MCU Axis to Renovate LPS-Induced Mitochondrial Oxidative Damage by Regulating STIM1. Free Radic. Biol. Med. 2023, 205, 25–46. [Google Scholar] [PubMed]
- Zheng, D.; Liu, J.; Piao, H.; Zhu, Z.; Wei, R.; Liu, K. ROS-Triggered Endothelial Cell Death Mechanisms: Focus on Pyroptosis, Parthanatos, and Ferroptosis. Front. Immunol. 2022, 13, 1039241. [Google Scholar]
- Chen, H.; Chong, I.; Lee, Y.; Tsai, J.; Yuan, S.F.; Wang, H.; Liu, W.; Liu, P. Kruppel-Like Factor 5 Mediates Proinflammatory Cytokine Expression in Lipopolysaccharide-Induced Acute Lung Injury through Upregulation of Nuclear Factor-kappaB Phosphorylation in Vitro and in Vivo. Mediat. Inflamm. 2014, 2014, 281984. [Google Scholar]
- Hu, T.; Ju, J.; Mo, L.; Ma, L.; Hu, W.; You, R.; Chen, X.; Chen, Y.; Liu, Z.; Qiu, S.; et al. Anti-Inflammation Action of Xanthones from Swertia Chirayita by Regulating COX-2/NF-kappaB/MAPKs/Akt Signaling Pathways in RAW 264.7 Macrophage Cells. Phytomedicine 2019, 55, 214–221. [Google Scholar]
- Medina-Vera, D.; Navarro, J.A.; Tovar, R.; Rosell-Valle, C.; Gutiérrez-Adan, A.; Ledesma, J.C.; Sanjuan, C.; Pavón, F.J.; Baixeras, E.; Rodríguez de Fonseca, F.; et al. Activation of PI3K/Akt Signaling Pathway in Rat Hypothalamus Induced by an Acute Oral Administration of D-Pinitol. Nutrients 2021, 13, 2268. [Google Scholar] [CrossRef]
- Li, X.L.; Xu, M.; Yu, F.; Fu, C.L.; Yu, X.; Cheng, M.; Gao, H.Q. Effects of D-pinitol on myocardial apoptosis and fibrosis in streptozocin-induced aging-accelerated mice. J. Food Biochem. 2021, 45, e13669. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Go, M.Y.; Kim, J.; Jeon, C.Y.; Kim, M.; Shin, D.W. Pinitol Improves Lipopolysaccharide-Induced Cellular Damage in Human Dermal Microvascular Endothelial Cells. Molecules 2025, 30, 1513. https://doi.org/10.3390/molecules30071513
Go MY, Kim J, Jeon CY, Kim M, Shin DW. Pinitol Improves Lipopolysaccharide-Induced Cellular Damage in Human Dermal Microvascular Endothelial Cells. Molecules. 2025; 30(7):1513. https://doi.org/10.3390/molecules30071513
Chicago/Turabian StyleGo, Min Young, Jinsick Kim, Chae Young Jeon, Mujun Kim, and Dong Wook Shin. 2025. "Pinitol Improves Lipopolysaccharide-Induced Cellular Damage in Human Dermal Microvascular Endothelial Cells" Molecules 30, no. 7: 1513. https://doi.org/10.3390/molecules30071513
APA StyleGo, M. Y., Kim, J., Jeon, C. Y., Kim, M., & Shin, D. W. (2025). Pinitol Improves Lipopolysaccharide-Induced Cellular Damage in Human Dermal Microvascular Endothelial Cells. Molecules, 30(7), 1513. https://doi.org/10.3390/molecules30071513






