Bioactive Molecules from Tropical American Plants: Potential Anti-Inflammatory Agents for Cytokine Storm Management
Abstract
1. Introduction
1.1. Hyperinflammatory Response and Cytokine Storm
1.2. Plant-Derived Natural Products as a Source of Anti-Inflammatory Agents
1.3. Polyphenols
1.3.1. Flavonoids
1.3.2. Non-Flavonoid Polyphenols
1.4. Alkaloids
1.5. Terpenoids and Saponins
| Compound (Sub-Class) | Structural Formula | Model/Assay | Effects and Mechanism | Sources in Tropical Plants | Ref. |
|---|---|---|---|---|---|
| 1,8-cineole (monoterpenoid) |  | In vitro Human monocytes-LPS In vivo Guinea pigs | ↓ TNF-α production ↓ IL-1β, LTB4, TxB2 ↓ TNF-α and IL-1β | Eucalyptus spp. Salvia sp. Cinnamomun Laurus nobilis | [113,114] |
| Geraniol (monoterpenoid) | 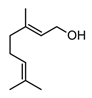 | In vitro RAW 264.7-LPS | ↓ NO, PGE2 ↓ iNOS and COX-2 expression ↓ NF-κB | Pelargonium spp. Citrus oils Cymbopogon spp. | [113] |
| Curcumol (sesquiterpenoid) | 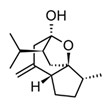 | In vitro RAW 264.7-LPS In vivo Mice-LPS | ↓ iNOS expression ↓ NO production ↓ TNF-α, IL-1β and IL-6 ↓ regulation JNK-AP-1 | Curcuma essential oils | [116] |
| Parthenolide (sesquiterpenoid) | 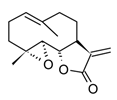 | In vitro PBMCs-LPS In vivo Balb/c female mice-LPS | ↓ iNOS and COX-2 expression ↓ TNF-α, IL-1, IL-4, IL-8 and IL-12 ↓ NF-κB | Tanacetum spp. | [117] |
| Epi-eudebeiolide C (sesquiterpenoid) | 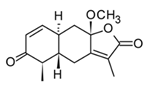 | In vitro RAW 264.7-LPS | ↓ iNOS expression ↓ NF-κB ↓ IkB phosphorylation | Salvia plebeia | [115] |
| Andalusol (diterpenoid) | 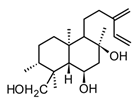 | In vitro J744 cells-LPS | ↓ iNOS expression ↓ NF-κB | Siderits foetens | [112] |
| Salofficinoid G (diterpenoid) | 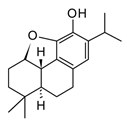 | In vitro RAW 264.7-LPS | ↓ iNOS and COX-2 expression ↓ NO | Salvia officinalis | [118] |
| Lupeol (triterpenoid) |  | In vitro Macrophages | Block Akt pathways ↓ NF-κB ↓ PGE2 production | Mangifera indica, Zanthoxylum spp. Tamarindus spp. Celastrus spp. | [119] |
| Ursolic acid (triterpenoid) | 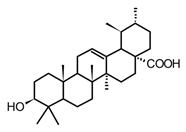 | In vitro RAW 264.7-LPS | ↓ iNOS and COX-2 expression ↓ PGE2 production ↓MAPK, TNF-α, IL-6, IL-1β, TLR4 | Ocimum sanctum Thymus vulgaris Lavandula spp. Nepeta sibthorpii Mentha piperita | [119] |
| Diosgenin (steroid from the saponin Dioscin) | 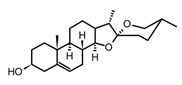 | In vitro Macrophages-LPS | ↓ ICAM-1 expression ↓ NF-κB ↓ MAPK, Akt, IKKβ | Solanum spp. Dioscorea spp. | [119] |
2. Conclusions
3. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bloom, D.E.; Cadarette, D. Infectious disease threats in the twenty-first century: Strengthening the global response. Front. Immunol. 2019, 10, 549. [Google Scholar] [CrossRef]
- World Health Organization. An R&D Blueprint for Action to Prevent Epidemics. In Plan of Action; World Health Organization: Geneva, Switzerland, 2016; Available online: https://cdn.who.int/media/docs/default-source/blue-print/an-randd-blueprint-for-action-to-prevent-epidemics.pdf (accessed on 7 November 2024).
- Mehand, M.S.; Al-Shorbaji, F.; Millett, P.; Murgue, B. The WHO R&D Blueprint: 2018 Review of emerging infectious diseases requiring urgent research and development efforts. Antivir. Res. 2018, 159, 63–67. [Google Scholar] [CrossRef]
- Liu, Y.C.; Kuo, R.L.; Shih, S.R. COVID-19: The first documented coronavirus pandemic in history. Biomed. J. 2020, 43, 328–333. [Google Scholar] [CrossRef]
- Andersen, K.G.; Rambaut, A.; Lipkin, W.I.; Holmes, E.C.; Garry, R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020, 26, 450–452. [Google Scholar]
- Chaplin, S. COVID-19: A brief history and treatments in development. Prescriber 2020, 31, 23–28. [Google Scholar]
- World Health Organization. Therapeutics and COVID-19. 2020. Available online: https://www.who.int/teams/health-care-readiness/covid-19/therapeutics (accessed on 5 May 2024).
- Alag, S. Analysis of COVID-19 clinical trials: A data-driven, ontology-based, and natural language. PLoS ONE 2020, 15, e0239694. [Google Scholar]
- Clinicaltrials.gov. Clinical Trials: A Data-Driven, Ontology-Based, and Natural Language Processing Approach. CovidResearchTrials. 2020. Available online: https://clinicaltrials.gov/ (accessed on 5 May 2024).
- GOV.UK. UK Medicines Regulator Gives Approval for First UK COVID-19 Vaccine. 2020. Available online: https://www.gov.uk/government/news/uk-medicines-regulator-gives-approval-for-first-uk-covid-19-vaccine (accessed on 5 October 2023).
- FDA. FDA Takes Key Action in Fight Against COVID-19 by Issuing Emergency Use Authorization for First COVID-19 Vaccine. 2020. Available online: https://www.fda.gov/news-events/press-announcements/fda-takes-key-action-fight-against-covid-19-issuing-emergency-use-authorization-first-covid-19 (accessed on 21 November 2024).
- Khimani, F.; Wolf, A.J.; Yoon, B.; Blancke, A.; Gerhart, C.; Endsley, D.; Dougherty, A.; Ray, A.K.; Yango, A.F.; Flynn, S.D.; et al. Therapeutic considerations for prevention and treatment of thrombotic events in COVID-19. Thromb. Update 2023, 10, 100126. [Google Scholar]
- Kimura-Ohba, S.; Asaka, M.N.; Utsumi, D.; Takabatake, Y.; Takahashi, A.; Yasutomi, Y.; Isaka, Y.; Kimura, T. d-Alanine as a biomarker and a therapeutic option for severe influenza virus infection and COVID-19. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2023, 1869, 166584. [Google Scholar]
- Warrayat, A.; Ali, A.; Waked, J.; Tocci, D.; Speth, R.C. Assessment of the therapeutic potential of salubrinal for ME/CFS and long-COVID. Trends Mol. Med. 2024. ahead of print. [Google Scholar]
- Song, Y.; Lu, J.; Qin, P.; Chen, H.; Chen, L. Interferon-I modulation and natural products: Unraveling mechanisms and therapeutic potential in severe COVID-19. Cytokine Growth Factor Rev. 2024, 82, 18–30. [Google Scholar]
- Zhou, Q.; Zhang, L.; Dong, Y.; Wang, Y.; Zhang, B.; Zhou, S.; Huang, Q.; Wu, T.; Chen, G. The role of SARS-CoV-2-mediated NF-κB activation in COVID-19 patients. Hypertens. Res. 2024, 47, 375–384. [Google Scholar] [PubMed]
- Hiti, L.; Markovič, T.; Lainscak, M.; Lainščak, J.F.; Pal, E.; Mlinarič-Raščan, I. The Immunopathogenesis of a Cytokine Storm: The Key Mechanisms Underlying Severe COVID-19. Cytokine Growth Factor Rev. 2025, 82, 1–17. [Google Scholar] [PubMed]
- Yuki, K.; Fujiogi, M.; Koutsogiannaki, S. COVID-19 pathophysiology: A review. Clin. Immunol. 2020, 215, 108427. [Google Scholar]
- Vallamkondu, J.; John, A.; Wani, W.Y.; Ramadevi, S.P.; Jella, K.K.; Reddy, P.H.; Kandimalla, R. SARS-CoV-2 pathophysiology and assessment of coronaviruses in CNS diseases with a focus on therapeutic targets. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165889. [Google Scholar]
- Hojyo, S.; Uchida, M.; Tanaka, K.; Hasebe, R.; Tanaka, Y.; Murakami, M.; Hirano, T. How COVID-19 induces cytokine storm with high mortality. Inflamm. Regen. 2020, 40, 37. [Google Scholar]
- García, L.F. Immune response, inflammation, and the clinical spectrum of COVID-19. Front. Immunol. 2020, 11, 1441. [Google Scholar]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M.; Snader, K.M. The influence of natural products upon drug discovery. Nat. Prod. Rep. 2000, 17, 215–234. [Google Scholar]
- Azab, A.; Nassar, A.; Azab, A.N. Anti-inflammatory activity of natural products. Molecules 2016, 21, 1321. [Google Scholar] [CrossRef]
- Yuan, G.; Wahlqvist, M.L.; He, G.; Yang, M.; Li, D. Natural products and anti-inflammatory activity. Asia Pac. J. Clin. Nutr. 2006, 15, 143–152. [Google Scholar]
- Parasher, A. COVID-19: Current understanding of its pathophysiology, clinical presentation and treatment. Postgrad. Med. J. 2020, 97, 312–320. [Google Scholar] [CrossRef]
- Zhang, B.; Zhou, X.; Zhu, C.; Song, Y.; Feng, F.; Qiu, Y.; Feng, J.; Jia, Q.; Song, Q.; Zhu, B.; et al. Immune phenotyping based on neutrophil-to-lymphocyte ratio and IgG predicts disease severity and outcome for patients with COVID-19. Front. Mol. Biosci. 2020, 7, 157. [Google Scholar]
- Reyes, A.Z.; Hu, K.A.; Teperman, J.; Wampler Muskardin, T.L.; Tardif, J.C.; Shah, B.; Pillinger, M.H. Anti-inflammatory therapy for COVID-19 infection: The case for colchicine. Ann. Rheum. Dis. 2021, 80, 550–557. [Google Scholar] [CrossRef]
- Soy, M.; Keser, G.; Atagündüz, P.; Tabak, F.; Atagündüz, I.; Kayhan, S. Cytokine storm in COVID-19: Pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020, 39, 2085–2094. [Google Scholar] [PubMed]
- Hu, B.; Huang, S.; Yin, L. The cytokine storm and COVID-19. J. Med. Virol. 2021, 93, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef]
- Kandasamy, M. NF-ΚB signalling as a pharmacological target in COVID-19: Potential roles for IKKβ inhibitors. Naunyn Schmiedebergs Arch. Pharmacol. 2021, 394, 561–567. [Google Scholar] [CrossRef]
- Harvey, A.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 Years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar]
- Yongye, A.B.; Waddell, J.; Medina-Franco, J.L. Molecular scaffold analysis of natural products databases in the public domain. Chem. Biol. Drug Des. 2012, 80, 717–724. [Google Scholar] [CrossRef]
- Van Hattum, H.; Waldmann, H. Biology-oriented synthesis: Harnessing the power of evolution. J. Am. Chem. Soc. 2014, 136, 11853–11859. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Kuang, H.; Su, Y.; Sun, Y.; Feng, J.; Guo, R.; Chan, K. Naturally derived anti-inflammatory compounds from Chinese medicinal plants. J. Ethnopharmacol. 2013, 146, 9–39. [Google Scholar] [CrossRef] [PubMed]
- Maione, F.; Russo, R.; Khan, H.; Mascolo, N. Medicinal plants with anti-inflammatory activities. Nat. Prod. Res. 2016, 30, 1343–1352. [Google Scholar] [CrossRef]
- Napagoda, M.T.; Sundarapperuma, T.; Fonseka, D.; Amarasiri, S.; Gunaratna, P. An ethnobotanical study of the medicinal plants used as anti-inflammatory remedies in Gampaha District, Western Province, Sri Lanka. Scientifica 2018, 2018, 9395052. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Aggarwal, B.B. Turmeric, the Golden Spice: From Traditional Medicine to Modern Medicine. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011. [Google Scholar]
- Lantz, R.C.; Chen, G.J.; Solyom, A.M.; Jolad, S.D.; Timmermann, B.N. The effect of turmeric extracts on inflammatory mediator production. Phytomedicine 2005, 12, 445–452. [Google Scholar] [CrossRef]
- Raven, P.H.; Gereau, R.E.; Phillipson, P.B.; Chatelain, C.; Jenkins, C.N.; Ulloa Ulloa, C. The distribution of biodiversity richness in the tropics. Sci. Adv. 2020, 6, eabc6228. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, V.P.; Arruda, C.; Abd El-Salam, M.; Bastos, J.K. Brazilian medicinal plants with corroborated anti- inflammatory activities: A review. Pharm. Biol. 2018, 56, 253–268. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; Juárez-Vázquez, M.D.C.; Campos-Xolalpa, N. Medicinal plants from Mexico, Central America, and the Caribbean used as immunostimulants. Evid. Based Complement. Alternat. Med. 2016, 2016, 4017676. [Google Scholar] [CrossRef]
- Garrido, G.; González, D.; Lemus, Y.; García, D.; Lodeiro, L.; Quintero, G.; Delporte, C.; Núñez-Sellés, A.J.; Delgado, R. In vivo and in vitro anti-inflammatory activity of Mangifera indica L. extract (VIMANG). Pharmacol. Res. 2004, 50, 143–149. [Google Scholar] [CrossRef]
- Cosentino, M.; Bombelli, R.; Carcano, E.; Luini, A.; Marino, F.; Crema, F.; Dajas, F.; Lecchini, S. Immunomodulatory properties of Achyrocline satureioides (Lam.) D.C. infusion: A study on human leukocytes. J. Ethnopharmacol. 2008, 116, 501–507. [Google Scholar] [CrossRef]
- Ovalle-Marin, A.; Parra-Ruiz, C.; Rivas, F.; Orellana, J.F.; Garcia-Diaz, D.; Jimenez, P. Characterization of Persea americana Mill. peels and leaves extracts and analysis of its potential in vitro anti-inflammatory properties. Boletín Latinoam. Caribe Plantas Med. Aromáticas 2020, 19, 395–407. [Google Scholar]
- Gutierrez, R.; Hoyo-Vadillo, C. Anti-inflammatory potential of Petiveria alliacea on activated RAW264.7 murine macrophages. Pharmacogn. Mag. 2017, 13, S174–S178. [Google Scholar] [CrossRef] [PubMed]
- Rivera, D.E.; Ocampo, Y.C.; Castro, J.P.; Barrios, L.; Diaz, F.; Franco, L.A. A screening of plants used in Colombian traditional medicine revealed the anti-inflammatory potential of Physalis angulata calyces. Saudi J. Biol. Sci. 2019, 26, 1758–1766. [Google Scholar] [CrossRef] [PubMed]
- De Morais Lima, G.R.; de Albuquerque Montenegro, C.; de Almeida, C.L.; de Athayde-Filho, P.F.; Barbosa-Filho, J.M.; Batista, L.M. Database survey of anti-inflammatory plants in South America: A Review. Int. J. Mol. Sci. 2011, 12, 2692–2749. [Google Scholar] [CrossRef]
- Abad, M.J.; Bessa, A.L.; Ballarin, B.; Aragón, O.; Gonzales, E.; Bermejo, P. Anti-inflammatory activity of four Bolivian Baccharis species (Compositae). J. Ethnopharmacol. 2006, 103, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Yao, H.; Song, J.; Liu, C.; Zhu, Y.; Ma, X.; Pang, X.; Xu, H.; Chen, S. Identification of medicinal plants in the family Fabaceae using a potential DNA barcode ITS2. J. Ethnopharmacol. 2010, 130, 116–121. [Google Scholar] [CrossRef]
- Veiga Junior, V.F.; Rosas, E.C.; Carvalho, M.V.; Henriques, M.G.; Pinto, A.C. Chemical composition and anti-inflammatory activity of copaiba oils from Copaifera cearensis Huber Ex Ducke, Copaifera reticulata Ducke and Copaifera multijuga Hayne—A comparative study. J. Ethnopharmacol. 2007, 112, 248–254. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Moccelini, S.K.; Da Silva, V.C.; Ndiaye, E.A.; De Sousa, P.T.; Vieira, P.C. Phytochemical study from root barks of Zanthoxylum rigidum Humb. & Bonpl. ex Willd (Rutaceae). Quimica Nova 2009, 32, 131–133. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food sources, bioavailability, metabolism, and bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Vinayagam, R.; Xu, B. Antidiabetic properties of dietary flavonoids: A cellular mechanism review. Nutr. Metab. 2015, 12, 60. [Google Scholar] [CrossRef]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Gaforio, J. Dietary flavonoids as cancer chemopreventive agents: An updated review of human studies. Antioxidants 2019, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, R.I.; Chircov, C.; Grumezescu, A.M.; Volceanov, A.; Teleanu, D.M. Antioxidant therapies for neuroprotection-A review. J. Clin. Med. 2019, 8, 1659. [Google Scholar] [CrossRef]
- González, J.; García, M.V.; González-Gallego, J.; Sánchez, S.; Tuñón, M.J. Anti-inflammatory and immunomodulatory properties of dietary flavonoids. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 435–452. [Google Scholar]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Comalada, M.; Camuesco, D.; Sierra, S.; Ballester, I.; Xaus, J.; Gµlvez, J.; Zarzuelo, A. In vivo quercitrin anti-inflammatory effect involves release of quercetin, which inhibits inflammation through down-regulation of the NF-KB pathway. Eur. J. Immunol. 2005, 35, 584–592. [Google Scholar] [CrossRef]
- Karuppagounder, V.; Arumugam, S.; Thandavarayan, R.A.; Sreedhar, R.; Giridharan, V.V.; Watanabe, K. Molecular targets of quercetin with anti-inflammatory properties in atopic dermatitis. Drug Discov. Today 2016, 21, 632–639. [Google Scholar] [CrossRef]
- Xianchu, L.; Lan, Z.; Ming, L.; Yanzhi, M. Protective effects of rutin on lipopolysaccharide-induced heart injury in Mice. J. Toxicol. Sci. 2018, 43, 329–337. [Google Scholar] [CrossRef]
- Miean, K.H.; Mohamed, S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef]
- Hema, K.; Sukumar, D. Isolation and phytochemical studies of quercetin and quercetin 3-O-rhamnoside. Int. J. Pharma. Biol. Sci. 2013, 4, 519–524. [Google Scholar]
- Comalada, M.; Ballester, I.; Bailon, E.; Sierra, S.; Xaus, J.; Gálvez, J.; Sánchez, F.; Zarzuelo, A. Inhibition of pro-inflammatory markers in primary bone marrow-derived mouse macrophages by naturally occurring flavonoids: Analysis of the structure–activity relationship. Biochem. Pharmacol. 2006, 72, 1010–1021. [Google Scholar]
- Tsai, Y.C.; Wang, S.L.; Wu, M.Y.; Liao, C.H.; Lin, C.H.; Chen, J.J.; Fu, S.L. Pilloin, a flavonoid isolated from Aquilaria sinensis, exhibits anti-inflammatory activity in vitro and in vivo. Molecules 2018, 23, 3177. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Liu, Y.; Luo, X.; Yang, Z. Advances in biosynthesis, pharmacology, and pharmacokinetics of pinocembrin, a promising natural small-molecule drug. Molecules 2019, 24, 2323. [Google Scholar] [CrossRef]
- Zhou, L.T.; Wang, K.J.; Li, L.; Li, H.; Geng, M. Pinocembrin inhibits lipopolysaccharide-induced inflammatory mediators production in BV2 microglial cells through suppression of PI3K/Akt/NF-κB Pathway. Eur. J. Pharmacol. 2015, 761, 211–216. [Google Scholar] [CrossRef]
- Alberca, R.W.; Teixeira, F.M.E.; Beserra, D.R.; De Oliveira, E.A.; Andrade, M.; Pietrobon, A.J.; Sato, M.N. Perspective: The potential effects of naringenin in COVID-19. Front. Immunol. 2020, 11, 570919. [Google Scholar]
- Li, Z.; Shi, Z.; Tang, S.; Yao, H.P.; Lin, X.; Wu, F. Epigallocatechin-3-gallate ameliorates LPS-induced inflammation by inhibiting the phosphorylation of Akt and ERK signaling molecules in Rat H9c2 cells. Exp. Ther. Med. 2020, 20, 1621–1629. [Google Scholar]
- Jang, S.; Kelley, K.W.; Johnson, R.W. Luteolin reduces IL-6 production in microglia by inhibiting JNK phosphorylation and activation of AP-1. Proc. Natl. Acad. Sci. USA. 2008, 105, 7534–7539. [Google Scholar] [CrossRef]
- Liu, Q.; Ci, X.; Wen, Z.; Peng, L. Diosmetin alleviates lipopolysaccharide-induced acute lung injury through activating the Nrf2 pathway and inhibiting the NLRP3 inflammasome. Biomol. Ther. 2018, 26, 157–166. [Google Scholar]
- Tutunchi, H.; Naeini, F.; Ostadrahimi, A.; Hosseinzadeh-Attar, M.J. Naringenin, a flavanone with antiviral and anti-inflammatory effects: A promising treatment strategy against COVID-19. Phytother. Res. 2020, 34, 3137–3147. [Google Scholar]
- Martínez, G.; Mijares, M.R.; De Sanctis, J.B. Effects of flavonoids and its derivatives on immune cell responses. Recent. Pat. Inflamm. Allergy Drug Discov. 2019, 13, 84–104. [Google Scholar]
- Zhang, P.; Mak, J.C.; Man, R.Y.; Leung, S.W. Flavonoids reduces lipopolysaccharide-induced release of inflammmatory mediators in human bronchial epithelial cells: Structure-activity relationship. Eur. J. Pharmacol. 2019, 865, 172731. [Google Scholar] [CrossRef]
- Watson, R.R.; Preedy, V.R.; Zibadi, S. Polyphenols: Prevention and Treatment of Human Disease, 2nd ed.; Watson, R., Preedy, V., Zibadi, S., Eds.; Academic Press: London, UK, 2018. [Google Scholar]
- Gavrilas, L.I.; Cruceriu, D.; Ionescu, C.; Miere, D.; Balacescu, O. Pro-apoptotic genes as new targets for single and combinatorial treatments with resveratrol and curcumin in colorectal cancer. Food Funct. 2019, 10, 3717–3726. [Google Scholar] [CrossRef]
- Rahaman, M.M.; Rakib, A.; Mitra, S.; Tareq, A.M.; Emran, T.B.; Shahid-Ud-Daula, A.; Amin, M.N.; Simal-Gandara, J. The genus curcuma and inflammation: Overview of the pharmacological perspectives. Plants 2021, 10, 63. [Google Scholar]
- Dvorakova, M.; Landa, P. Anti-inflammatory activity of natural stilbenoids: A review. Pharmacol. Res. 2017, 124, 126–145. [Google Scholar]
- Saqib, U.; Kelley, T.T.; Panguluri, S.K.; Liu, D.; Savai, R.; Baig, M.S.; Schürer, S.C. Polypharmacology or promiscuity? Structural interactions of resveratrol with its bandwagon of targets. Front. Pharmacol. 2018, 9, 1201. [Google Scholar] [CrossRef]
- Kwon, M.Y.; Kim, S.M.; Park, J.; Lee, J.; Cho, H.; Lee, H.; Jeon, C.; Park, J.H.; Han, I.O. A caffeic acid-ferulic acid hybrid compound attenuates lipopolysaccharide-mediated inflammation in BV2 and RAW264.7 cells. Biochem. Biophys. Res. Commun. 2019, 515, 565–571. [Google Scholar] [CrossRef]
- Luo, C.; Zou, L.; Sun, H.; Peng, J.; Gao, C.; Bao, L.; Ji, R.; Jin, Y.; Sun, S. A review of the anti-inflammatory effects of rosmarinic acid on inflammatory diseases. Front. Pharmacol. 2020, 11, 153. [Google Scholar] [CrossRef]
- Petersen, M. Rosmarinic acid: New aspects. Phytochem. Rev. 2013, 12, 207–227. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A review of its effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Zahedipour, F.; Hosseini, S.A.; Sathyapalan, T.; Majeed, M.; Jamialahmadi, T.; Al-rasadi, K.; Banach, M.; Sahebkar, A. Potential effects of curcumin in the treatment of COVID-19 infection. Phytoter. Res. 2020, 34, 2911–2920. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Hosseini, M.S.; Khalili, N.; Naimi, E.; Majeed, M.; Sahebkar, A. Antioxidant and anti-inflammatory effects of curcuminoid-piperine combination in subjects with metabolic syndrome: A randomized controlled trial and an updated meta-analysis. Clin. Nutr. 2015, 34, 1101–1108. [Google Scholar] [CrossRef]
- Ortiz, A.; Sansinenea, E. Phenylpropanoid Derivatives and Their Role in Plants’ Health and as antimicrobials. Curr. Microbiol. 2023, 80, 380. [Google Scholar] [CrossRef] [PubMed]
- Fattorusso, E.; Taglialatela-Scafati, O. Modern Alkaloids: Structure, Isolation, Synthesis, and Biology; Fattorusso, E., Taglialatela-Scafati, O., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Aniszewski, T. Alkaloids Chemistry, Biology, Ecology, and Applications; Elsevier: Amsterdam, The Netherlands, 2015; Available online: https://www.elsevier.com/books/alkaloids/aniszewski/978-0-444-59433-4 (accessed on 5 May 2024).
- Bai, R.; Yao, C.; Zhong, Z.; Ge, J.; Bai, Z.; Ye, X.; Xie, T.; Xie, Y. Discovery of natural anti-inflammatory alkaloids: Potential leads for the drug discovery for the treatment of inflammation. Eur. J. Med. Chem. 2021, 213, 113165. [Google Scholar] [CrossRef]
- Liang, J.H.; Wang, C.; Huo, X.K.; Tian, X.G.; Zhao, W.Y.; Wang, X.; Sun, C.-P.; Ma, X.C. The genus Uncaria: A review on phytochemical metabolites and biological aspects. Fitoterapia 2020, 147, 104772. [Google Scholar] [CrossRef]
- Rojas-Duran, R.; González-Aspajo, G.; Ruiz-Martel, C.; Bourdy, G.; Doroteo-Ortega, V.H.; Alban-Castillo, J.; Robert, G.; Auberger, P.; Deharo, E. Anti-inflammatory activity of mitraphylline isolated from Uncaria tomentosa Bark. J. Ethnopharmacol. 2012, 143, 801–804. [Google Scholar] [CrossRef]
- Nalli, Y.; Khajuria, V.; Gupta, S.; Arora, P.; Riyaz-Ul-Hassan, S.; Ahmed, Z.; Ali, A. Four new carbazole alkaloids from Murraya koenigii that display anti-inflammatory and anti-microbial activities. Org. Biomol Chem. 2016, 14, 3322–3332. [Google Scholar] [CrossRef]
- Chen, F.L.; Yang, Z.H.; Liu, Y.; Li, L.X.; Liang, W.C.; Wang, X.C.; Zhou, W.B.; Yang, Y.H.; Hu, R.M. Berberine inhibits the expression of TNFa, MCP-1, and IL-6 in AcLDL-stimulated macrophages through PPARc Pathway. Endocrine 2008, 33, 331–337. [Google Scholar] [CrossRef]
- Remichkova, M.; Dimitrova, P.; Philipov, S.; Ivanovska, N. Toll-like receptor-mediated anti-inflammatory action of glaucine and oxoglaucine. Fitoterapia 2009, 80, 411–414. [Google Scholar] [CrossRef]
- Cao, R.; Peng, W.; Wang, Z.; Xu, A. β-Carboline alkaloids: Biochemical and pharmacological functions. Curr. Med. Chem. 2007, 14, 479–500. [Google Scholar] [CrossRef] [PubMed]
- Liscombe, D.K.; MacLeod, B.P.; Loukanina, N.; Nandi, O.I.; Facchini, P.J. Evidence for the monophyletic evolution of benzylisoquinoline alkaloid biosynthesis in angiosperms. Phytochemistry 2005, 66, 2501–2520. [Google Scholar] [CrossRef] [PubMed]
- Diamond, A.; Desgagné-Penix, I. Metabolic engineering for the production of plant isoquinoline alkaloids. Plant Biotechnol. J. 2015, 14, 1319–1328. [Google Scholar] [CrossRef]
- Sandjo, L.P.; Kuete, V.; Tchangna, R.S.; Efferth, T.; Ngadjui, B.T. Cytotoxic benzophenanthridine and furoquinoline alkaloids from Zanthoxylum Buesgenii (Rutaceae). Chem. Cent. J. 2014, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Bräse, S. (Ed.) Privileged Scaffolds in Medicinal Chemistry; The Royal Society of Chemistry: London, UK, 2015. [Google Scholar] [CrossRef]
- Plazas, E.; Casoti, R.; Avila Murillo, M.; Batista Da Costa, F.; Cuca, L.E. Metabolomic profiling of Zanthoxylum species: Identification of anti-cholinesterase alkaloids candidates. Phytochemistry 2019, 168, 112128. [Google Scholar] [CrossRef]
- Chu, M.; Chen, X.; Wang, J.; Guo, L.; Wang, Q.; Gao, Z.; Kang, J.; Zhang, M.; Feng, J.; Guo, Q.; et al. Polypharmacology of berberine based on multi-target binding motifs. Front. Pharmacol. 2018, 9, 801. [Google Scholar] [CrossRef]
- Yin, J.; Xing, H.; Ye, J. Efficacy of berberine in patients with type 2 diabetes. Metabolism 2008, 57, 712–717. [Google Scholar] [CrossRef]
- Ehteshamfar, S.M.; Akhbari, M.; Afshari, J.T.; Seyedi, M.; Nikfar, B.; Shapouri-Moghaddam, A.; Ghanbarzadeh, E.; Momtazi-Borojeni, A. Anti-inflammatory and immune-modulatory impacts of berberine on activation of autoreactive T cells in autoimmune inflammation. J. Cell Mol. Med. 2020, 24, 13573–13588. [Google Scholar] [CrossRef] [PubMed]
- Di, G.; Xiang, L.D.; Yao, S.; Yu, L.J.; Da, M.Z. A new benzophenanthridine alkaloid from Zanthoxylum Nitidum. Chin. J. Nat. Med. 2009, 7, 274–277. [Google Scholar] [CrossRef]
- Slobodnick, A.; Shah, B.; Pillinger, M.H.; Krasnokutsky, S. Colchicine: Old and new. Am. J. Med. 2015, 128, 461–470. [Google Scholar] [CrossRef]
- Ludwiczuk, A.; Georgiev, M.I. Chapter outline, and learning objectives. Terpenoids. In Pharmacognosy; Academic Press: Cambridge, MA, USA, 2017; pp. 233–266. [Google Scholar] [CrossRef]
- De las Heras, B.; Hortelano, S. Molecular basis of the anti-inflammatory effects of terpenoids. Inflamm. Allergy Drug Targets 2009, 8, 28–39. [Google Scholar]
- De Cássia da Silveira e Sá, R.; Andrade, L.N.; De Sousa, D.P. A review on anti-inflammatory activity of monoterpenes. Molecules 2013, 18, 1227–1254. [Google Scholar] [CrossRef] [PubMed]
- Juergens, U.R. Anti-inflammatory properties of the monoterpene 1,8-cineole: Current evidence for co-medication in inflammatory airway diseases. Drug Res. 2014, 64, 638–646. [Google Scholar] [CrossRef]
- Jang, H.; Lee, S.; Lee, S.J.; Lim, H.; Jung, K.; Kim, Y.; Lee, S.; Rho, M.C. Anti-Inflammatory activity of eudesmane-type sesquiterpenoids from Salvia plebeia. J. Nat. Med. 2017, 80, 2666–2676. [Google Scholar]
- Chen, X.; Zong, C.; Gao, Y.; Cai, R.; Fang, L.; Lu, J.; Liu, F.; Qi, Y. Curcumol exhibits anti-inflammatory properties by interfering with the JNK-mediated AP-1 pathway in lipopolysaccharide-activated RAW264.7 cells. Eur. J. Pharmacol. 2014, 723, 339–345. [Google Scholar] [CrossRef]
- Mathema, V.B.; Koh, Y.S.; Thakuri, B.C.; Sillanpää, M. Parthenolide, a sesquiterpene lactone, expresses multiple anti-cancer and anti-Inflammatory activities. Inflammation 2012, 35, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wei, S.; Zhu, T.; Xue, G.; Xu, D.; Wang, W.; Wang, X.; Luo, J.; Kong, L. Anti-inflammatory norabietane diterpenoids from the leaves of Salvia officinalis L. J. Funct. Foods. 2019, 54, 154–163. [Google Scholar] [CrossRef]
- Jeong, G.S.; Bae, J.S. Anti-inflammatory effects of triterpenoids; naturally occurring and synthetic agents. Mini Rev. Org. Chem. 2014, 11, 316–329. [Google Scholar]
- Chadwick, M.; Trewin, H.; Gawthrop, F.; Wagstaff, C. Sesquiterpenoids lactones: Benefits to plants and people. Int. J. Mol. Sci. 2013, 14, 12780–12805. [Google Scholar] [CrossRef]
- Kwok, B.H.; Koh, B.; Ndubuisi, M.I.; Elofsson, M.; Crews, C.M. The anti-infammatory natural product parthenolide from the medicinal herb feverfew directly binds to and inhibits IkB kinase. Chem. Biol. 2001, 8, 759–766. [Google Scholar] [CrossRef]
- Bahrami, M.; Kamalinejad, M.; Latifi, S.A.; Seif, F.; Dadmehr, M. Cytokine storm in COVID-19 and parthenolide: Preclinical evidence. Phytother. Res. 2020, 34, 2429–2430. [Google Scholar] [CrossRef]
- Han, N.; Bakovic, M. Biologically active triterpenoids and their cardioprotective and anti-inflammatory effects. J. Bioanal. Biomed. 2015, S12, 5. [Google Scholar] [CrossRef]
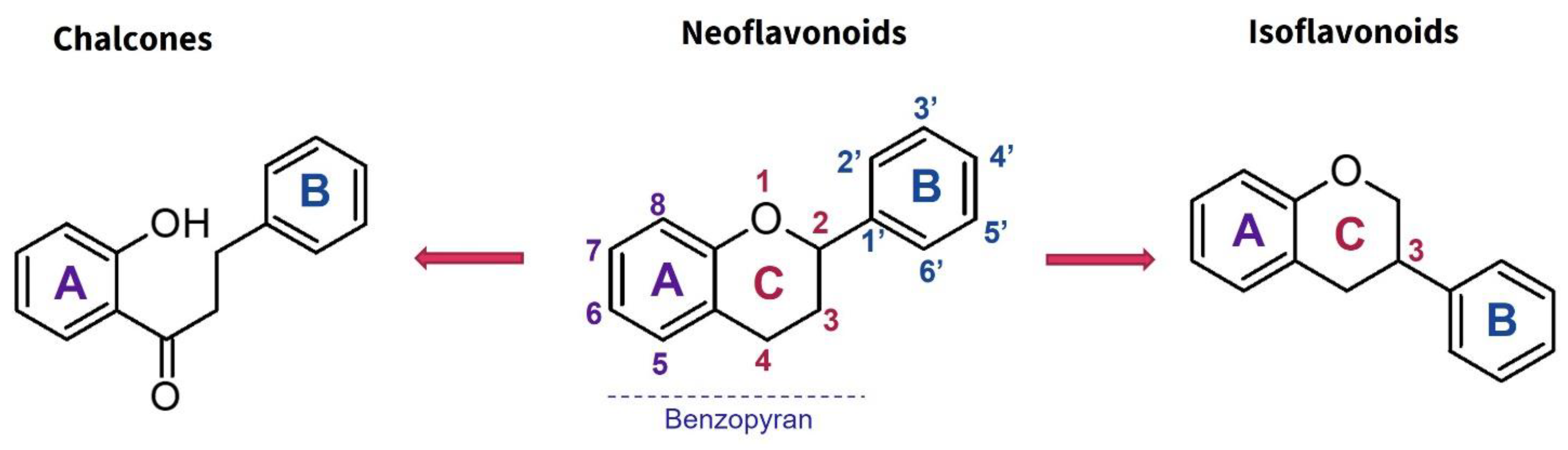
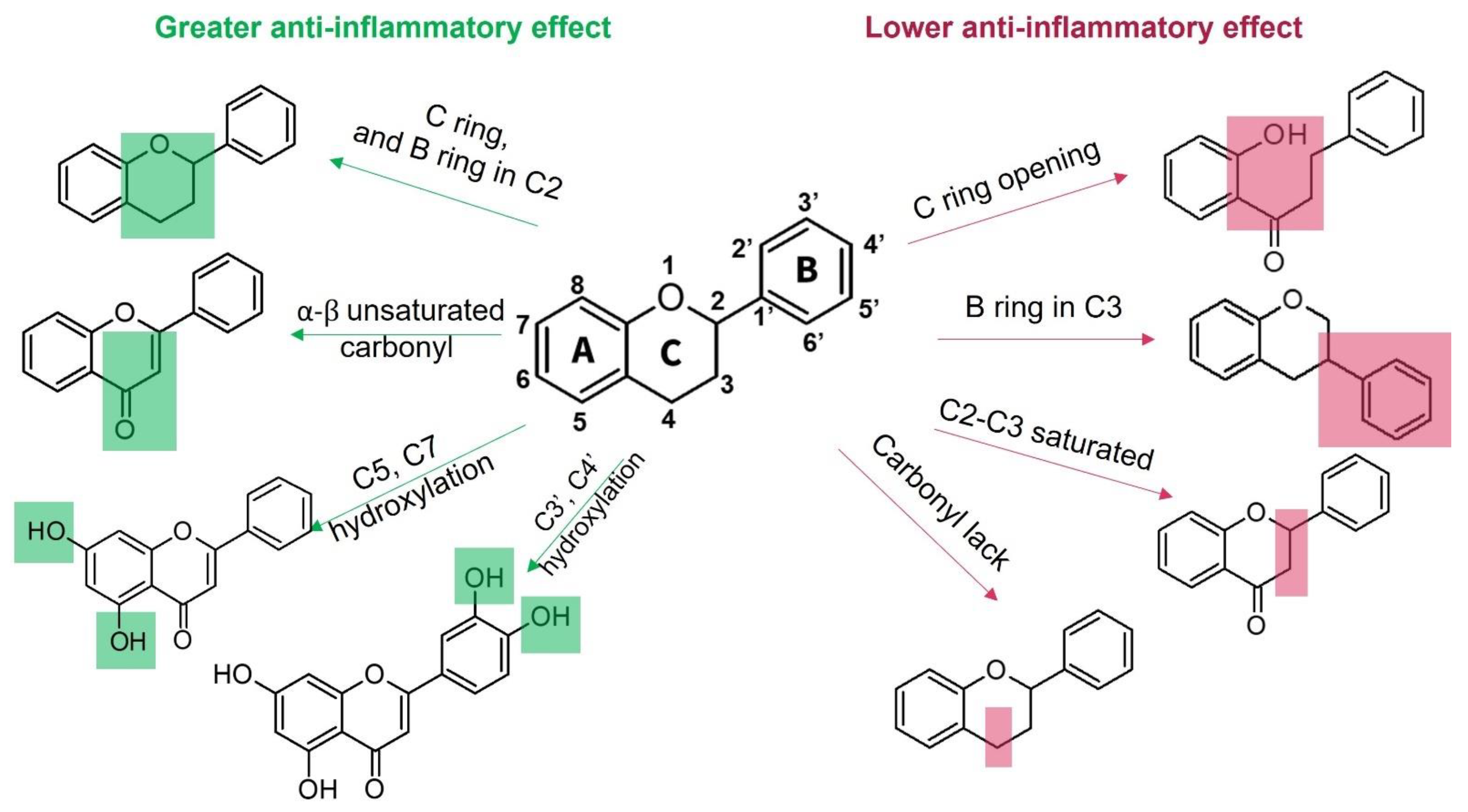

| Family | Botanical Name | Part Used | Extract | Model/Assay | Results | Ref. |
|---|---|---|---|---|---|---|
| Anacardiaceae | Anacardium occidentale L. | Leaves | Ethanol/water (40:60 v/v) | RAW 264.7-LPS | ↓ TNF-α, IL-1β, and IL6 ↓ Inducible NO and ROS | [43] |
| Mangifera indica | Stem, bark | Aqueous | RAW 264.7-LPS/IFN-γ and calcium ionophore A23187 | ↓ TNF-α Inhibition of PGE2 and LTB4 (IC50 < 25µM) | [45] | |
| Asteraceae | Achyrocline satureioides | Aerial parts | Aqueous | Human PMNs and PBMCs | Regulation of IFN-γ/IL-4 ratio ↓ ROS production | [46] |
| Baccharis dracunculifolia | Leaves | Ethanol/water (70:30 v/v) | Murine macrophages–LPS | NF-κB downregulation ↓ IL-1β, IL-6, and IL-10 ↓ ROS production | [43] | |
| Fabaceeae | Caesalpinia ferrea | Fruits (pods) | Aqueous | Different in vitro and in vivo | ↓ TNF-α, IL-1β, NO, and TGF-β | [43] |
| Copaifera multijuga | Oleoresin | Murine macrophages | ↓ TNF-α, IL-1, and IL-6 Inhibition of NO release | [43] | ||
| Lamiaceae | Hyptis pectinata | Leaves | Essential oil | Balb/C mice | ↓ TNF-α, PGE2, and IL-6 | [43] |
| Lauraceae | Persea americana Mill | Leaves | Ethanol/water (1:1) | RAW 264.7-LPS | ↓ TNF-α gene expression Inhibition of NO release | [47] |
| Petiveriaceae | Petiveria alliacea | Leaves | Ethanol | RAW 264.7-LPS | NF-κB downregulation ↓ PGE2, iNOS, and NO ↓ IL-1β, IL-6, and IL-10 | [48] |
| Plantaginaceae | Scoparia dulcis | Whole plant | Ethanol/water (70:30 v/v) | ICR mice | ↓ COX-2, NO, TNF-α, and IL-1β | [43] |
| Rubiaceae | Uncaria tomentosa | Root, bark | Aqueous | RAW 264.7-LPS | NF-κB downregulation ↓ IL-1, IL-17, and TNF-α | [43] |
| Solanaceae | Physalis angulata | Calyx | DCM | RAW 264.7-LPS ICR mice–SOZ | ↓ PGE2 and NO production ↓ IL-1β, IL-6, and TNF-α | [49] |
| Compound (Sub-Class) | Structural Formula | Model/Assay | Effects and Mechanism | Source in Tropical Plants | Ref. |
|---|---|---|---|---|---|
| Quercetin (flavonol) | 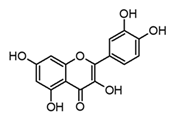 | In vitro BMDM-LPS | ↓ TNF-α and IL-1β ↓ iNOS expression ↓ IkB-α phosphorylation | Carica papaya Anacardium occidentale Capsicum annum Momordica charantia Moringa oleirfera Psidium guajava Amaranthus spp. Curcuma spp. | [64,67] |
| Quercitrin (flavonol glicoside) | 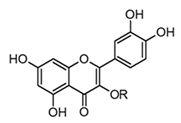 R = α-l-rhamnopyranoside | In vivo Rats–DSS | ↓ TNF-α and IL-1β ↓ iNOS expression | Allamanda cathartica Euphorbia spp. Myrtus spp. | [64,68] |
| Rutin (flavonol glicoside) | 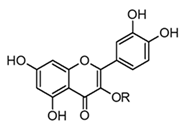 R = α-l-rhamnopyranosyl-(1→6)-β-d-glucopyranose | In vivo Mice–LPS | ↓ CK and LDH ↑ Antioxidant enzymes (SOD) and (CAT) ↓ TNF-α and IL-6 | Tephrosia purpurea Citrus spp. Malus spp. Rubus spp. | [57,66] |
| Luteolin (flavone) |  | In vitro BMDM-LPS | ↓ TNF-α and NF-κB ↓ iNOS expression ↓ IkB-α phosphorylation | Capsicum frutescens Apium graveolens Garcinia sp. | [67,69] |
| Diosmetin (flavone) | 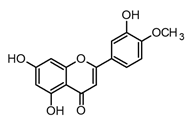 | In vitro BMDM-LPS | ↓ TNF-α and NF-κB ↓ iNOS expression ↓ IkB-α phosphorylation | Citrus spp. Rosmarinus officinalis | [58,69] |
| Pilloin (flavone) | 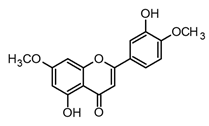 | In vitro RAW 264.7-LPS In vivo septic mice–LPS | ↓ TNF-α, IL-6, and COX-2 ↓ iNOS expression ↓ IkB-α phosphorylation JNK, ERK, and p38 inhibition | Piper auritum and Murraya panaculata | [70] |
| Pinocembrin (flavanone) |  | In vitro RAW 264.7-LPS BV2 microglia–LPS | ↓ TNF-α, IL-6, and COX-2 ↓ iNOS and COX-2 expression ↓ TNF- α, IL-1β, NO, and PGE2 ↓ PI3K/Akt phosphorylation | Piper spp. Peperomia spp. Asteraceae family | [71,72] |
| Naringenin (flavanone) |  | In vitro Murine macrophages–LPS In vivo Mouse–LPS | ↓ NF-κB, PI3K/Akt, MAPK ↓ IL-4 and IL-13 ↓ Neutrophils and oxidative stress ↓ TNF and IL-6 | Citrus spp. Solanum lycopersicum | [73] |
| Epigallocatechin-3-gallate EGCG (catechin) | 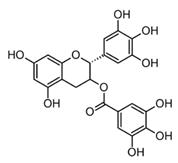 | In vitro Human neutrophils–SOZ Human corneal cells–LPS | MPO inhibition ↓ HOCl and O2−. ↓ TNF-α, IL-1β, IL-6 and IL-8 | Camellia sinensis L. | [74] (Li et al., 2020) |
| Compound (Class) | Structural Formula | Model/Assay | Effects and Mechanism | Sources in Tropical Plants | Ref. |
|---|---|---|---|---|---|
| Resveratrol (stilbene) |  | In vivo Balb-c mice Wistar rats | ↓ TNF-α ↓ IL-10, IL-18, IL-6, and IL-1β ↓ iNOS and NO levels ↓ COX-2 expression | Gnetaceae Fabaceae Dipterocarpaceae Vitaceae Families | [83] (Dvorakova and Landa 2017) |
| Curcumin (curcuminoid) | 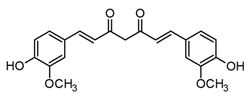 | In vitro Human macrophages–LPS | ↓ MMP1, MMP3, and MMP13 expression ↓ TNF-α inhibition of COX LOX production ↓ NO* and O2* levels ↓ iNOS expression | Curcuma genus | [82] (Rahaman et al., 2021) |
| Ferulic acid (phenylpropanoid) | 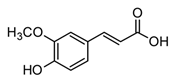 | In vitro RAW 264.7-LPS | ↓ TNF-α and IL-1β ↓ TNF-α and NF-κB | Beta vulgaris Hordeum vulgare Zea mays everta | [85] (Kwon et al., 2019) |
| Rosmarinic acid (phenylpropanoid ester) |  | In vitro RAW 264.7-LPS In vivo Mice–LPS Lung injury | ↓ TNF-α, IL-6, and IL-1β ↓ iNOS and NO levels ↓ TNF-α, IL-6, and IL-1β Inhibition of iNOS mRNA | Lamiacaeae family Salvia Rosmarinus Mentha Occinmun genus | [86] (Luo et al., 2020) [87] (Petersen 2013) |
| Compound (Sub-Class) | Structural Formula | Model/Assay | Effects and Mechanism | Sources in Tropical Plants | Ref. |
|---|---|---|---|---|---|
| Strictosidine (Indole) | 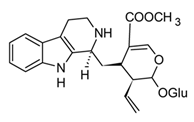 | In vitro N9 cells–LPS | Inhibition of NO production ↓ COX-2 and iNOS expression | Uncaria genus Catharanthus spp. | [95] (Liang et al., 2020) |
| Mitraphylline (Oxindolic) | 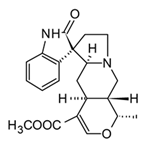 | In vivo Balb/c female mice–LPS | ↓ TNF-α, IL-1β, and IL-1α IL-17 and IL-4 | Uncaria tomentosa | [96] (Rojas-Duran et al., 2012) |
| Mukolidine (Carbazole derivative) | 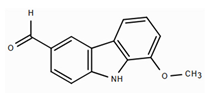 | In vitro PBMCs-LPS In vivo Balb/c female mice–LPS | ↓ TNF-α and IL-6 ↓ TNF-α and NF-κB | Murraya spp. | [97] (Nalli et al., 2016) |
| O-methylmurrayamine (Carbazole derivative) | 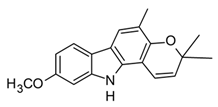 | In vitro PBMCs-LPS In vivo Balb/c female mice–LPS | ↓ TNF-α and IL-6 ↓ TNF-α and NF-κB | Murraya spp. | [97] (Nalli et al., 2016) |
| 4-Methoxy-5-hydroxycanthin-6-one (β-carboline) | 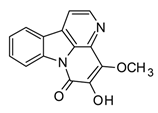 | In vitro RAW 264.7-LPS | ↓ TNF-α Inhibition of NO production ↓ iNOS expression | Picrasma spp. | [94] (Bai et al., 2021) |
| Harmine (β-carboline) | 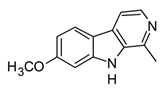 | In vitro RAW 264.7-LPS In vivo Mice–LPS | ↓ TNF-α, IL-1β and IL-6 NF-κB regulation | Peganum spp. | [94] (Bai et al., 2021) |
| Berberine (Protoberberine) | 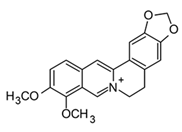 | In vitro Macrophages–AcLDL | ↓ TNF-α and IL-6 ↓ COX-2 expression ↓ NF-κB and MAPK signaling | Berberis spp. Coptis spp. Cordalis spp. Zanthoxylum spp. | [98] (Chen et al., 2008) |
| Glaucine (Aporphine) |  | In vitro Macrophages–LPS Macrophages–zymosan | ↓ TNF-α and IL-6 ↑ IL-10 | Glaucium flavum | [99] (Remichkova et al., 2009) |
| Nitidine nornitidine norchelerythrine (benzophenanthridines) | 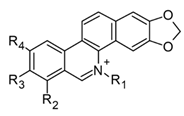 Nitidine: R1 = CH3, R2 = H, R3 = R4 = OCH3 Nornitidine: R1 = R2 = H, R3 = R4 = OCH3 Norchelerythrine: R1 = H, R2 = R3 = OCH3, R4 = H | In vitro 293 T cells RAW 264.7-LPS | ↓ NF-κB ↓ TNF-α, IL-1β, and IL-6 ↓ iNOS expression | Zanthoxylum spp. | [94] (Bai et al., 2021) |
| Colchicine | 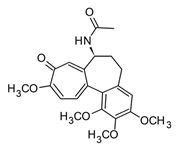 | In vitro Macrophages–CPPD In vivo | ↓ IL-1β Prevent TNF-α and IL-6 | Colchicum spp. | [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plazas, E.; Sierra-Marquez, L.; Olivero-Verbel, J. Bioactive Molecules from Tropical American Plants: Potential Anti-Inflammatory Agents for Cytokine Storm Management. Molecules 2025, 30, 1486. https://doi.org/10.3390/molecules30071486
Plazas E, Sierra-Marquez L, Olivero-Verbel J. Bioactive Molecules from Tropical American Plants: Potential Anti-Inflammatory Agents for Cytokine Storm Management. Molecules. 2025; 30(7):1486. https://doi.org/10.3390/molecules30071486
Chicago/Turabian StylePlazas, Erika, Lucellys Sierra-Marquez, and Jesus Olivero-Verbel. 2025. "Bioactive Molecules from Tropical American Plants: Potential Anti-Inflammatory Agents for Cytokine Storm Management" Molecules 30, no. 7: 1486. https://doi.org/10.3390/molecules30071486
APA StylePlazas, E., Sierra-Marquez, L., & Olivero-Verbel, J. (2025). Bioactive Molecules from Tropical American Plants: Potential Anti-Inflammatory Agents for Cytokine Storm Management. Molecules, 30(7), 1486. https://doi.org/10.3390/molecules30071486







