Dynamic Mechanical and Charlesby-Pinner Analyses of Radiation Cross-Linked Ethylene-Vinyl Acetate Copolymer (EVA)
Abstract
1. Introduction
2. Results and Discussion
2.1. WAXD Analysis
2.2. DSC Analysis
2.3. Frequency Sweep by Dynamic Mechanical Analysis (DMA)
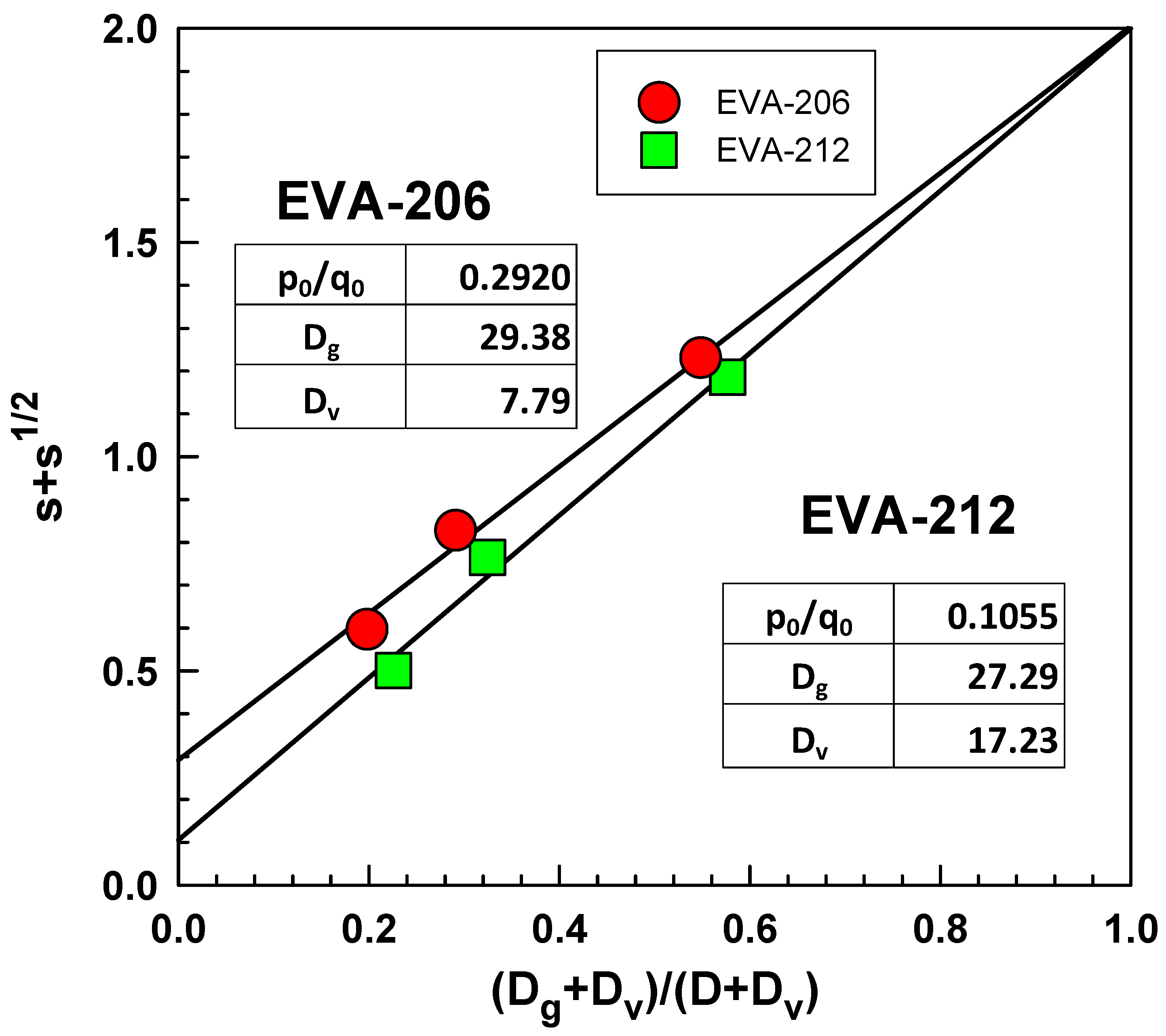
2.4. Gel Content Analysis
3. Materials and Methods
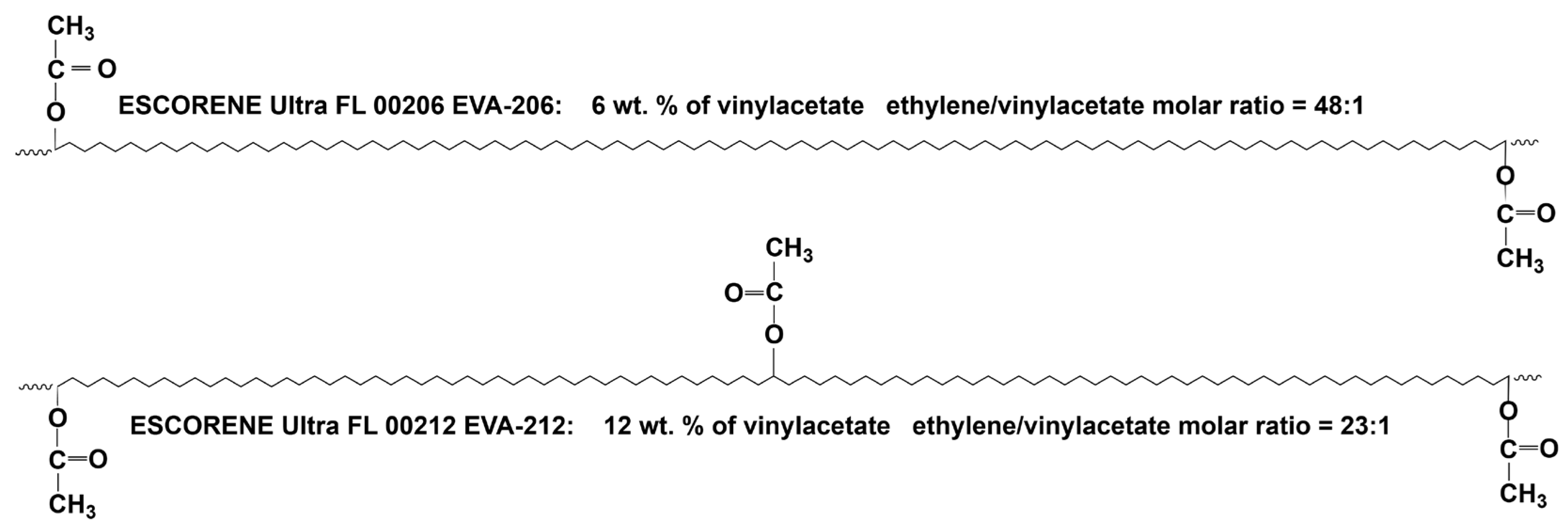
3.1. Materials
3.2. Compression Molding
3.3. Electron Beam Irradiation
3.4. DMA Tests
3.5. Gel Content
3.6. Size-Exclusion Chromatography
3.7. Differential Scanning Calorimetry (DSC)
3.8. Wide-Angle X-Ray Diffraction (WAXD)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El Fray, M.; Przybytniak, G.; Piatek-Hnat, M.; Kornacka, E.M. Physical effects of radiation processes in poly(aliphatic/aromatic-ester)s modified with e-beam radiation. Polymer 2010, 51, 1133–1139. [Google Scholar] [CrossRef]
- Kolhe, A.; Chauhan, A.; Dongre, A. A Review on various methods for the Cross-linking of Polymers. Res. J. Pharm. Dos. Forms Technol. 2022, 14, 183–188. [Google Scholar] [CrossRef]
- Ali, Z.I.; Legocka, I. Effect of metal salt of ethylene/methacrylic acid copolymer on electron beam crosslinking of low density polyethylene. Adv. Polym. Technol. 2005, 24, 103–113. [Google Scholar] [CrossRef]
- Manaila, E.; Craciun, G.; Ighigeanu, D.; Lungu, I.B.; Dumitru, M.; Stelescu, M.D. Electron Beam Irradiation: A Method for Degradation of Composites Based on Natural Rubber and Plasticized Starch. Polymers 2021, 13, 1950. [Google Scholar] [CrossRef]
- Wang, B.; Wang, M.H.; Xing, Z.; Zeng, H.Y.; Wu, G.Z. Preparation of radiation crosslinked foams from low-density polyethylene/ethylene-vinyl acetate (LDPE/EVA) copolymer blend with a supercritical carbon dioxide approach. J. Appl. Polym. Sci. 2013, 127, 912–918. [Google Scholar] [CrossRef]
- Sharif, J.; Aziz, S.H.S.A.; Hashim, K. Radiation effects on LDPE/EVA blends. Radiat. Phys. Chem. 2000, 58, 191–195. [Google Scholar] [CrossRef]
- Farias, G.M.G.; Agrawal, P.; Hanken, B.L.; de Araújo, J.P.; de Oliveira, A.D.B.; de Mélo, T.J.A. Effect of EVA copolymer containing different VA content on the thermal and rheological properties of bio-based high-density polyethylene/ethylene vinyl acetate blends. J. Therm. Anal. Calorim. 2021, 146, 2127–2139. [Google Scholar] [CrossRef]
- Alothman, O.Y. Processing and Characterization of High Density Polyethylene/Ethylene Vinyl Acetate Blends with Different VA Contents. Adv. Mater. Sci. Eng. 2012, 10, 635693. [Google Scholar] [CrossRef]
- Sethi, M.; Gupta, N.K.; Srivastava, A.K. Dynamic mechanical analysis of polyethylene and ethylene vinylacetate copolymer blends irradiated by electron beam. J. Appl. Polym. Sci. 2002, 86, 2429–2434. [Google Scholar] [CrossRef]
- Sabet, M.; Soleimani, H. The impact of electron beam irradiation on Low density polyethylene and Ethylene vinyl acetate. In Proceedings of the 5th International Conference on Nanomaterials and Materials Engineering (Icnme 2017), Bali, Indonesia, 1–3 April 2017; Volume 204. [Google Scholar] [CrossRef]
- Ramachandran, P.; Naskar, K.; Nando, G.B. Exploring the effect of radiation crosslinking on the physico-mechanical, dynamic mechanical and dielectric properties of EOC-PDMS blends for cable insulation applications. Polym. Adv. Technol. 2017, 28, 80–93. [Google Scholar] [CrossRef]
- Shin, B.Y.; Ha, M.H.; Han, D.H. Morphological, Rheological, and Mechanical Properties of Polyamide 6/Polypropylene Blends Compatibilized by Electron-Beam Irradiation in the Presence of a Reactive Agent. Materials 2016, 9, 342. [Google Scholar] [CrossRef]
- Rajawasam, C.W.H.; Dodo, O.J.; Weerasinghe, M.A.S.N.; Raji, I.O.; Wanasinghe, S.V.; Konkolewicz, D.; De Alwis Watuthanthrige, N. Educational series: Characterizing crosslinked polymer networks. Polym. Chem. 2024, 15, 219–247. [Google Scholar] [CrossRef]
- Carotenuto, C.; Paduano, L.; Grassia, L.; Minale, M. Viscoelasticity Evolution of Ethylene-Vinyl-Acetate Copolymers During Crystallization. Chem. Eng. Trans. 2018, 79, 1093–1098. [Google Scholar] [CrossRef]
- Sawatari, C.; Matsuo, M. Cross-Linking Effect of Polyethylene Polypropylene Blend Films Prepared by Gelation Crystallization from Solution. Polym. J. 1987, 19, 1365–1376. [Google Scholar] [CrossRef]
- Han, D.H.; Shin, S.H.; Petrov, S. Crosslinking and degradation of polypropylene by electron beam irradiation in the presence of trifunctional monomers. Radiat. Phys. Chem. 2004, 69, 239–244. [Google Scholar] [CrossRef]
- Ries, M.D.; Pruitt, L. Effect of cross-linking on the microstructure and mechanical properties of utra-high molecular weight polyethylene. Clin. Orthop. Relat. Res. 2005, 440, 149–156. [Google Scholar] [CrossRef]
- Dias, D.B.; Silva, L.G.D.A.E. Polyethylene foams cross-linked by electron beam. Radiat. Phys. Chem. 2007, 76, 1696–1697. [Google Scholar] [CrossRef]
- Koo, S.H.; Lee, K.Y.; Lee, H.G. Effect of cross-linking on the physicochemical and physiological properties of corn starch. Food Hydrocoll. 2010, 24, 619–625. [Google Scholar] [CrossRef]
- Makuuchi, K.; Cheng, S. Radiation Processing of Polymer Materials and Its Industrial Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Gao, L.; Hu, B.L.; Wang, L.P.; Cao, J.W.; He, R.; Zhang, F.Y.; Wang, Z.M.; Xue, W.H.; Yang, H.L.; Li, R.W. Intrinsically elastic polymer ferroelectric by precise slight cross-linking. Science 2023, 381, 540–544. [Google Scholar] [CrossRef]
- Yuan, Z.; Liu, Y.; Zhou, H. Chemical crosslinking enabling ferroelectric polymers for new memory applications. Innov. Mater. 2023, 1, 100025. [Google Scholar] [CrossRef]
- Datta, S.; Naskar, K.; Bhardwaj, Y.; Sabharwal, S. A study on dynamic rheological characterisation of electron beam crosslinked high vinyl styrene butadiene styrene block copolymer. Polym. Bull. 2011, 66, 637–647. [Google Scholar] [CrossRef]
- Hui, S.; Mushtaq, S.; Chaki, T.K.; Chattopadhyay, S. Effect of Controlled Electron Beam Irradiation on the Rheological Properties of Nanosilica-Filled LDPE-EVA Based Thermoplastic Elastomer. J. Appl. Polym. Sci. 2011, 119, 2153–2166. [Google Scholar] [CrossRef]
- Dutta, J.; Ramachandran, P.; Ismail, S.M.R.S.; Naskar, K. Melt Rheological Behavior and Creep Response of EVA/TPU Blends: Exploring the Effect of Electron Beam Irradiation and Peroxide Cross-linking. Polym. Plast. Technol. Eng. 2017, 56, 421–434. [Google Scholar] [CrossRef]
- Babu, R.; Singha, N.; Naskar, K. Melt Viscoelastic Properties of Peroxide Cured Polypropylene-Ethylene Octene Copolymer Thermoplastic Vulcanizates. Polym. Eng. Sci. 2010, 50, 455–467. [Google Scholar] [CrossRef]
- Durmuş, A.; Woo, M.; Kaşgöz, A.; Macosko, C.W.; Tsapatsis, M. Intercalated linear low density polyethylene (LLDPE)/clay nanocomposites prepared with oxidized polyethylene as a new type compatibilizer: Structural, mechanical and barrier properties. Eur. Polym. J. 2007, 43, 3737–3749. [Google Scholar] [CrossRef]
- Sung, Y.T.; Kum, C.K.; Lee, H.S.; Kim, J.S.; Yoon, H.G.; Kim, W.N. Effects of crystallinity and crosslinking on the thermal and rheological properties of ethylene vinyl acetate copolymer. Polymer 2005, 46, 11844–11848. [Google Scholar] [CrossRef]
- Ansari, I.A.; Gupta, G.A.; Ramkumar, J.; Kar, K.K. Handbook of Fly Ash; Elsevier Inc.: Oxford, UK, 2022. [Google Scholar]
- Goodarzi, V.; Kokabi, M.; Kashani, M.R.; Bahramian, A.R. Prediction of Long-Term Mechanical Properties of PVDF/BaTiO3 Nanocomposite. J. Appl. Polym. Sci. 2014, 131, 40596. [Google Scholar] [CrossRef]
- Luo, Y.; Li, P.; Pan, H.; Yu, M. The effect of vinyl content on the enhancement of the radiation induced cross-linking in siloxane copolymer. J. Radiat. Res. Radiat. Process. 1989, 7, 5–11. [Google Scholar]
- Calina, I.; Demeter, M.; Scarisoreanu, A.; Satulu, V.; Mitu, B. One Step e-Beam Radiation Cross-Linking of Quaternary Hydrogels Dressings Based on Chitosan-Poly(Vinyl-Pyrrolidone)-Poly(Ethylene Glycol)-Poly(Acrylic Acid). Int. J. Mol. Sci. 2020, 21, 9236. [Google Scholar] [CrossRef]
- Vargas, M.A.; Manero, O. Rheological characterization of the gel point in polymer-modified asphalts. J. Appl. Polym. Sci. 2011, 119, 2422–2430. [Google Scholar] [CrossRef]
- Vallés, E.M.; Carella, J.M.; Winter, H.H.; Baumgaertel, M. Gelation of a radiation crosslinked model polyethylene. Rheol. Acta 1990, 29, 535–542. [Google Scholar] [CrossRef]
- Reinitz, S.D.; Carlson, E.M.; Levine, R.A.C.; Franklin, K.J.; Van Citters, D.W. Dynamical mechanical analysis as an assay of cross-link density of orthopaedic ultra high molecular weight polyethylene. Polym. Test. 2015, 45, 174–178. [Google Scholar] [CrossRef]
- Bandzierz, K.S.; Reuvekamp, L.A.E.M.; Przybytniak, G.; Dierkes, W.K.; Blume, A.; Bielinski, D.M. Effect of electron beam irradiation on structure and properties of styrene-butadiene rubber. Radiat. Phys. Chem. 2018, 149, 14–25. [Google Scholar] [CrossRef]
- Wang, S.F.; Zhang, Y.; Zhang, Y.X.; Zhang, C.M.; Li, E.J. Crosslinking of polyvinyl chloride by electron beam irradiation in the presence of ethylene-vinyl acetate copolymer. J. Appl. Polym. Sci. 2004, 91, 1571–1575. [Google Scholar] [CrossRef]
- Datta, S.K.; Bhowmick, A.K.; Tripathy, D.K.; Chaki, T.K. Effect of electron beam radiation on structural changes of trimethylol propane trimethacrylate, ethylene vinyl acetate, and their blends. J. Appl. Polym. Sci. 1996, 60, 1329–1341. [Google Scholar] [CrossRef]
- Olejniczak, J.; Rosiak, J.; Charlesby, A. Gel Dose Curves for Polymers Undergoing Simultaneous Cross-Linking and Scission. Radiat. Phys. Chem. 1991, 37, 499–504. [Google Scholar] [CrossRef]
- Rosiak, J.M. Gel/sol analysis of irradiated polymers. Radiat. Phys. Chem. 1998, 51, 13–17. [Google Scholar] [CrossRef]
- Furusawa, K.; Dobashi, T.; Morishita, S.; Oyama, M.; Hashimoto, T.; Shinyashiki, N.; Yagihara, S.; Nagasawa, N. Structural and kinetic modification of aqueous hydroxypropylmethylcellulose (HPMC) induced by electron beam irradiation. Phys. A-Stat. Mech. Its Appl. 2005, 353, 9–20. [Google Scholar] [CrossRef]
- Hidiroglu, M.; Aksüt, D.; Serçe, O.; Karabulut, H.; Sen, M. Reducing the hydrocarbon gas diffusion and increasing the pressure-impact strength of fuel transfer pipelines for use in the automotive industry using radiation crosslinked polyamide 12. Radiat. Phys. Chem. 2019, 159, 118–123. [Google Scholar] [CrossRef]
- D4703-24; Standard Practice for Compression Molding Thermoplastic Materials into Test Specimens, Plaques, or Sheets. ASTM International: West Conshohocken, PA, USA, 2024.
- ASTM D2765-16; Standard Test Methods for Determination of Gel Content and Swell Ratio of Crosslinked Ethylene Plastics. ASTM International: West Conshohocken, PA, USA, 2024.
- Salehi, S.M.A.; Mirjalili, G.; Amrollahi, J. Effects of high-energy electron beam on low-density polyethylene materials containing EVA. J. Appl. Polym. Sci. 2004, 92, 1049–1052. [Google Scholar] [CrossRef]
- Westerman, B.; Stringfellow, P.M.; Eccleston, J.A.; Harbrow, D.J. Effect of ethylene vinyl acetate (EVA) closed cell foam on transmitted forces in mouthguard material. Br. J. Sports Med. 2002, 36, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Clegg, D.W. Irradiation Effects on Polymers; Elsevier Applied Science: London, UK, 1991. [Google Scholar]
- Svoboda, P. High-temperature study of radiation cross-linked ethylene-octene copolymers. Polymer Bulletin. 2017, 74, 121–144. [Google Scholar] [CrossRef]
- Ghobashy, M. Ionizing Radiation-Induced Polymerization; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar]
- Chmielewski, A.G. Applications of Ionizing Radiation in Materials Processing; Institute of Nuclear Chemistry and Technology: Warszawa, Poland, 2017. [Google Scholar]
- Hill, D.J.T.; Whittaker, A.K. Radiation Chemistry of Polymers. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Charlesby, A.; Pinner, S.H. Analysis of the Solubility Behaviour of Irradiated Polyethylene and Other Polymers. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1959, 249, 367–386. [Google Scholar] [CrossRef]
- Thomas, J.; Thomas, S.; Ahmad, Z. Crosslinkable Polyethylene: Manufacture, Properties, Recycling, and Applications; Springer Nature: Singapore, 2021. [Google Scholar]
- Turgis, J.D.; Coqueret, X. Electron beam sensitivity of butyl acrylate copolymers: Effects of composition on reactivity. Macromol. Chem. Phys. 1999, 200, 652–660. [Google Scholar] [CrossRef]
- Balmforth, N.; Craster, R. Geophysical Aspects of Non-Newtonian Fluid Mechanics; Springer-Verlag: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
















| Sample | Crystallinity (%) |
|---|---|
| EVA 206–0 kGy | 39.07 |
| EVA 206–60 kGy | 39.05 |
| EVA 206–120 kGy | 39.01 |
| EVA 206–180 kGy | 38.48 |
| EVA 212–0 kGy | 35.10 |
| EVA 212–60 kGy | 34.90 |
| EVA 212–120 kGy | 34.51 |
| EVA 212–180 kGy | 33.44 |
| Sample | Melting Temperature Tm (°C) | Degree of Crystallinity X (%) | Melting Temperature Tm (°C) | Degree of Crystallinity X (%) |
|---|---|---|---|---|
| EVA 206–0 kGy | 102.38 | 43.75 | 102.09 | 43.64 |
| EVA 206–60 kGy | 99.19 | 41.46 | 98.78 | 42.00 |
| EVA 206–120 kGy | 98.95 | 40.15 | 97.82 | 38.60 |
| EVA 206–180 kGy | 98.60 | 39.54 | 96.80 | 36.76 |
| EVA 212–0 kGy | 95.93 | 35.58 | 96.11 | 37.73 |
| EVA 212–60 kGy | 93.63 | 33.93 | 92.87 | 34.17 |
| EVA 212–120 kGy | 92.95 | 33.65 | 90.92 | 33.67 |
| EVA 212–180 kGy | 92.75 | 33.41 | 89.92 | 33.01 |
| Material | Dose (kGy) | n | k |
| EVA 206 | 60 120 180 | −0.75095 −0.83870 −0.89553 | 15,005 29,897 41,159 |
| Material | Dose (kGy) | n | k |
| EVA 212 | 60 120 180 | −0.77479 −0.85485 −0.91285 | 21,188 41,934 43,688 |
| Dose (kGy) | G′ (Pa) | Mc (kg/mol) | (mol/m3) |
|---|---|---|---|
| 60 | 49,800 | 65.42 | 14.16 |
| 120 | 130,000 | 25.06 | 36.95 |
| 180 | 218,000 | 14.94 | 61.97 |
| Dose (kGy) | G′ (Pa) | Mc (kg/mol) | (mol/m3) |
|---|---|---|---|
| 60 | 73,600 | 44.65 | 20.92 |
| 120 | 185,000 | 17.76 | 52.59 |
| 180 | 227,000 | 14.48 | 64.52 |
| Abbreviation | Trade Name | Vinylacetate (wt%) | Ethylene (wt%) | Vinylacetate (mol%) | Ethylene (mol%) | ET/VA (Molar Ratio) | Density (g/cm3) |
|---|---|---|---|---|---|---|---|
| EVA 206 | Escorene Ultra FL 00206 | 6 | 94 | 2.038 | 97.962 | 48.1 | 0.926 |
| EVA 212 | Escorene Ultra FL00212 | 12 | 88 | 4.255 | 95.745 | 22.5 | 0.934 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Svarcova, A.; Svoboda, P. Dynamic Mechanical and Charlesby-Pinner Analyses of Radiation Cross-Linked Ethylene-Vinyl Acetate Copolymer (EVA). Molecules 2025, 30, 1485. https://doi.org/10.3390/molecules30071485
Svarcova A, Svoboda P. Dynamic Mechanical and Charlesby-Pinner Analyses of Radiation Cross-Linked Ethylene-Vinyl Acetate Copolymer (EVA). Molecules. 2025; 30(7):1485. https://doi.org/10.3390/molecules30071485
Chicago/Turabian StyleSvarcova, Anna, and Petr Svoboda. 2025. "Dynamic Mechanical and Charlesby-Pinner Analyses of Radiation Cross-Linked Ethylene-Vinyl Acetate Copolymer (EVA)" Molecules 30, no. 7: 1485. https://doi.org/10.3390/molecules30071485
APA StyleSvarcova, A., & Svoboda, P. (2025). Dynamic Mechanical and Charlesby-Pinner Analyses of Radiation Cross-Linked Ethylene-Vinyl Acetate Copolymer (EVA). Molecules, 30(7), 1485. https://doi.org/10.3390/molecules30071485







