Abstract
Ammonia borane (AB) has attracted much attention in the field of solid-state hydrogen storage due to its high hydrogen storage capacity. Nanoconfinement in UiO-66 can reduce the hydrogen release temperature. In particular, terephthalic acid was used as a linker to further improve the dehydrogenation properties through the modification of -NH2, -OH, -NO2, -Br, and -F groups. The hydrogen release content of 0.5AB/UiO-66 was 1.98 wt.%, whereas the hydrogen release content of UiO-66-2OH modified by -OH groups increased to 3.85 wt.%. The non-covalent interaction results show that -NH2 and -OH preferred adsorption with -BH3, and the H in -NH2 and -OH were able to interact directly with the H in AB to modify the dehydrogenation process of AB, whereas -NO2, -Br, and -F indirectly affected the charge density of hydrogen atoms in AB to alter the dehydrogenation property of AB. The modification of functional groups provides a theoretical basis for the design of high-performance MOF nanoconfinement AB composite hydrogen storage materials.
1. Introduction
Hydrogen is a very promising renewable energy source due to its high energy density and environmental friendliness [1]. However, the primary obstacle to the more widespread utilization of hydrogen is the question of its storage and delivery [2,3]. There is a pressing need to develop safe and efficient means of hydrogen storage that are superior to the current methods of high-pressure gaseous and low-temperature liquid storage [4,5]. One potential solution is to develop efficient materials for hydrogen storage. These include, for example, liquid organic hydrogen carriers [6,7], metal hydrides [8,9,10], alkali-metal coordinated hydrides [11,12,13], and boron and nitrogen hydrides [14,15,16,17,18]. In recent years, AB has received considerable attention as a promising solid hydrogen storage material, exhibiting an ultra-high theoretical gravimetric hydrogen storage density (19.6 wt.%) [19,20], along with a lower dehydrogenation temperature compared to other materials, such as NaBH4 [21,22] and MgH2 [9,23].
Nevertheless, pure ammonia borane (AB) is not the optimal choice for chemical hydrogen storage, because under heating conditions, complex BNHx components are formed and impurity gases are produced, including ammonia, diborane, and borazine [24,25]. Furthermore, AB exhibits a reduced capacity to dehydrogenate a single molecule of hydrogen at low temperature (200 °C), which presents a challenge to the effective use of AB within the hydrogen economy.
Due to their advantageous properties, including a uniform pore structure, structural diversity, adjustable pore size, and a high specific surface area [26,27,28], metal–organic framework (MOF) materials have demonstrated significant efficacy in the domain of nanoconfinement. Numerous studies have indicated that the nanoconfinement capabilities of MOF materials can substantially enhance the dehydrogenation performance of AB, such as AB@MOF with JUC-32-Y as a porous framework, as reported for the first time by Li et al. [29]. The analytical results showed that some of the Y3+ are unsaturated sites and JUC-32-Y has the uncoordinated functional group C=O. The presence of Y3+ and the functional group has the potential to alter the charge distribution in the AB, resulting in the chemical bonding in the AB becoming unstable [30,31,32,33]. This destabilization would reduce the peak dehydrogenation temperature of AB@JUC-32-Y to 84 °C without the generation of volatile impurities. However, the effect of unsaturated metal sites and functional groups on AB dehydrogenation was not analyzed by Li et al. Gao et al. [34] investigated the effect of different functional groups on the dehydrogenation properties of AB. The ligand was modified on the basis of MIL-101(Cr) by adding -NH2, -NO2, and -NHCOCH3 functional groups. The results indicated that the incorporation of various functional groups significantly lowered the dehydrogenation temperature and enhanced the dehydrogenation capacity of AB, with the -NH2 group exhibiting the most obvious effect. First-principles calculations demonstrated that the charge densities of the hydrogen atoms in AB were altered following the introduction of the -NH2 group into the ligand. The incorporation of functional groups resulted in a modification of the charge density of AB, subsequently enhancing its dehydrogenation performance. Peil et al. [35] further investigated the effect of functional groups on the dehydrogenation of AB with the help of solid-state NMR. Two functional groups, -NH2 and -OH, were added to Al-MIL-53, and a significant decrease in the dehydrogenation temperature of AB was observed with the addition of these functional groups. The decomposition pathways of the two materials are very similar, with Al-MIL-OH producing 31% cubic boron nitride and 69% boron oxide at 140 °C, while 23% cubic boron nitride and 77% boron oxide are detected for Al-MIL-NH2. It is speculated that the high percentage of boron oxide may be due to the presence of -OH. However, the interaction between AB and different functional groups remains unclear.
Consequently, this study investigates the impact of various functional groups, -NH2, -OH, -NO2, -Br, and -F, on the properties of AB when incorporated into UiO-66. The purity of hydrogen and the dehydrogenation content of AB/UiO-66-X (X = NH2, OH, 2OH, NO2, Br, F) were examined using temperature-programmed desorption mass spectrometry (TPD-MS) and temperature-programmed desorption gas chromatography (TPD-GC). Additionally, the interactions between AB and the functional groups were analyzed utilizing XPS and the first principles investigation. This study provides theoretical guidance for the design of high-performance MOF nanoconfined AB composite hydrogen storage materials.
2. Results and Discussion
The characterization of UiO-66 and UiO-66-X are shown in the Supplementary Materials. Figure 1a and Figure 1b show the PXRD patterns and the FT-IR spectra of 0.5AB/UiO-66 and 0.5AB/UiO-66-X, respectively. In Figure 1a, the PXRD patterns of 0.5AB/UiO-66 and 0.5AB/UiO-66-X do not show any peaks of pristine AB, suggesting that the AB particles are very small and well distributed. In Figure 1b, the peaks observed in the range of 3310–3210 cm−1 are associated with the antisymmetric stretching of H-N bonds. The absorption peaks at 2312 cm−1 and 2275 cm−1 are ascribed to the stretching of H-B bonds. Additionally, the peaks at 1597 cm−1, 1553 cm−1, and 1375 cm−1 correspond to the scissors modes of H-N. The stretching mode of B-N is identified at a peak of 778 cm−1, while the H wagging modes are detected at peaks of 1055 cm−1 and 728 cm−1 [36]. Additionally, a weak absorption peak associated with B-O is noted in the 0.5AB/UiO-66-2OH sample, suggesting the presence of O-H and the binding of AB.
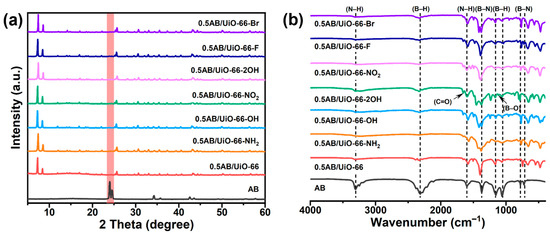
Figure 1.
(a) Characterization of 0.5AB/UiO-66 and 0.5AB/UiO-66-X. PXRD patterns, (b) FT-IR spectra.
The N2 adsorption–desorption isotherms of 0.5AB/UiO-66 and 0.5AB/UiO-66-X are shown in Figure S5. The specific surface area of the samples was found to be nearly negligible, suggesting that during the impregnation process, the AB component was incorporated into the pores of the MOF.
The dehydrogenation properties of 0.5AB/UiO-66 and UiO-66-X were investigated. As illustrated in Figure 2a, pure AB commenced the release of the first equivalent of hydrogen at approximately 109.1 °C, reaching a peak temperature of around 119.4 °C. The subsequent dehydrogenation event exhibited a peak temperature of approximately 157.5 °C. Concurrently, the dehydrogenation process of pure AB resulted in the generation of significant quantities of volatile gases, including ammonia, diborane, and borazine. Figure 2b shows the TPD-MS profiles of 0.5AB/UiO-66. Their initial and peak dehydrogenation temperatures were lower than those of pure AB. For the initial dehydrogenation temperature, the 0.5AB/UiO-66 is 68.9 °C and the dehydrogenation peak temperature is 90.5 °C. Meanwhile, no production of NH3, borazine, or diborane was detected for 0.5AB/UiO-66 during the dehydrogenation process. In addition, due to the nanoconfinement effect of UiO-66, the pore may be restricted to AB during dehydrogenation, resulting in only one step of dehydrogenation of AB.
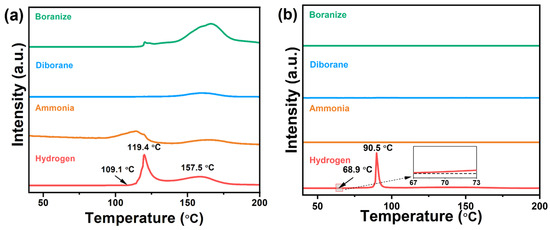
Figure 2.
TPD-MS profiles of (a) AB and (b) 0.5AB/UiO-66.
The TPD-MS profiles of 0.5AB/UiO-66-X are shown in Figure 3. The dehydrogenation temperatures of 0.5AB/UiO-66-X were significantly lower compared to those of pure AB. Among the samples, 0.5AB/UiO-66-NH2 was observed to produce minimal ammonia during the dehydrogenation process. In contrast, 0.5AB/UiO-66 did not generate any impurity gases during the same process. This observation has led to the hypothesis that the reaction between AB and -NH2 in UiO-66-NH2 during dehydrogenation with heating may be responsible for the ammonia production. The mechanism of action of the dehydrogenation reaction of -OH and -NH2 with AB may be different from that of -NO2, -Br, and -F, so that -NO2, -Br, and -F cause AB to release the second molecular weight of hydrogen. The mechanism of action for the dehydrogenation of AB by acting on different functional groups is discussed in detail in the latter part of this paper. The higher dehydrogenation temperature of 0.5AB/UiO-66-X compared to 0.5AB/UiO-66 may be due to the stronger interaction of functional groups within the pore directly with AB.
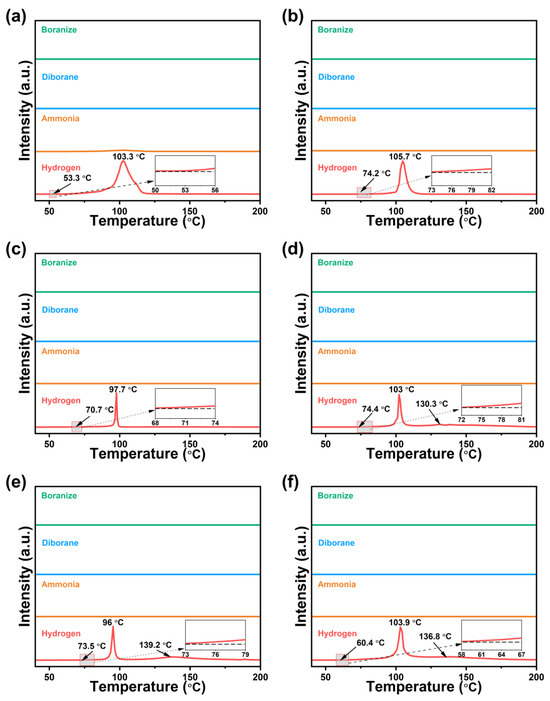
Figure 3.
TPD-MS profiles of (a) 0.5AB/UiO-66-NH2, (b) 0.5AB/UiO-66-OH, (c) 0.5AB/UiO-66-2OH, (d) 0.5AB/UiO-66-NO2, (e) 0.5AB/UiO-66-F, and (f) 0.5AB/UiO-66-Br.
Figure 4 shows the non-isothermal desorption profile of 0.5AB/UiO-66 and 0.5AB/UiO-66-X (X = NH2, OH, 2OH, NO2, Br, F). It is evident that the AB loaded with MOF exhibits hydrogen release at about 80 °C. The results are presented in Table 1; 0.5AB/UiO-66 can release 0.91 molecular equivalents of hydrogen. After modifying the functional groups, the hydrogen release of 0.5AB/UiO-66-X was higher than that of 0.5AB/UiO-66, and the dehydrogenation of 0.5AB/UiO-66-NH2, 0.5AB/UiO-66-OH, and 0.5AB/UiO-66-2OH was significantly higher than that of 0.5AB/UiO-66-NO2, 0.5AB/UiO-66-Br, and 0.5AB/UiO-66-F. This may be due to the binding of H in -NH2 and -OH with H in AB. Among the composites, the dehydrogenation amount of 0.5AB/UiO-66-2OH reaches 1.77 molecular equivalents, which is significantly higher than that of the other composites. Compared with the results reported in the literature, 0.5AB/UiO-66-2OH released a higher hydrogen content, and although AB-MIL-101-NH2 was able to release more hydrogen, its TPD-MS results showed that impurity gases were still released [34].
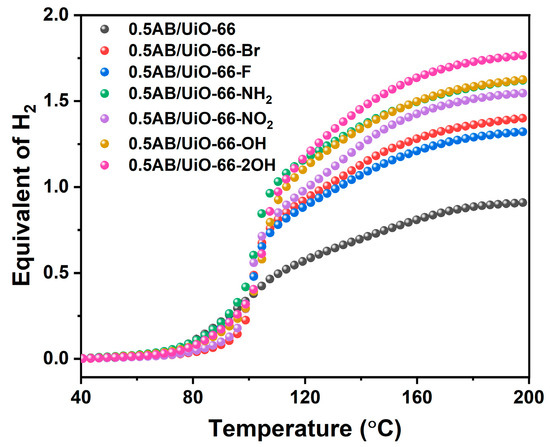
Figure 4.
Equivalent of H2 evolution during thermolysis of 0.5AB/UiO-66 and 0.5AB/UiO-66-X.

Table 1.
Content and equivalent of H2 evolution during thermolysis of 0.5AB/MOFs.
The XRD patterns of 0.5AB/UiO-66 and 0.5AB/UiO-66-X after complete dehydrogenation are shown in Figure S6a. UiO-66 and UiO-66-X demonstrate the maintenance of a more complete structure. The FT-IR spectra of 0.5AB/UiO-66 and 0.5AB/UiO-66-X after complete dehydrogenation are shown in Figure S6b. The formation of B-O bonds and the disappearance of C=O bonds were observed in all curves, suggesting that AB may react with C=O.
To investigate the role of different functional groups in the dehydrogenation process, XPS tests on MOFs, 0.5AB/MOFs, and the products after dehydrogenation were performed. As shown in Figure S7, Zr 3d was not significantly shifted before and after MOF loading of AB and after dehydrogenation of AB, indicating that there is no electron transfer between Zr4+ and AB. In Figure 5a,b and Figure S8, it can be observed that the N 1s of UiO-66, UiO-66-NH2, UiO-66-OH, UiO-66-2OH, UiO-66-Br, and UiO-66-F exhibit a shift towards higher binding energies upon loading AB, indicating an electron-loss state. In contrast, when AB is loaded into UiO-66-NO2 (Figure 5c), the binding energy of the N 1s becomes lower, suggesting an electron-gaining state. As illustrated in Figure 5 and Figure S8, the N 1s orbital of 0.5AB/UiO-66 and 0.5AB/UiO-66-X after dehydrogenation exhibited only two peaks near 400.5 eV and 402 eV, corresponding to the B-N bond and N-O bond, respectively. In comparison to the spectra of pure AB, the peak at 398.5 eV was absent, indicating that nano-restricted AB effectively prevents the formation of solid by-products during the dehydrogenation process.
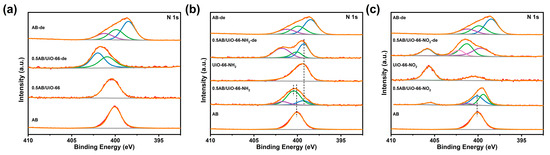
Figure 5.
XPS spectra of the N 1s orbitals of the materials. (a) 0.5AB/UiO-66 and 0.5AB/UiO-66-de, (b) 0.5AB/UiO-66-NH2, UiO-66-NH2 and 0.5AB/UiO-66-NH2-de, (c) 0.5AB/UiO-66-NO2, UiO-66-OH2 and 0.5AB/UiO-66-NO2-de.
As shown in Figure S9, there is no significant change in B 1s when AB is loaded onto the MOF compared to pure AB. For the 0.5AB/UiO-66 and 0.5AB/UiO-66-X after dehydrogenation, the B 1s peak reveals two peaks, corresponding to the B-N bond and the B-O bond, situated at approximately 192.3 and 193.1 eV, respectively, as previously discussed in the literature [30,38]. Furthermore, the FT-IR spectrum of 0.5AB/UiO-66 and 0.5AB/UiO-66-X after dehydrogenation (Figure S6) also exhibits peaks characteristic of B-N and B-O bonds.
As illustrated in Figure 6, the loading of AB into UiO-66-NO2, UiO-66-OH, and UiO-66-2OH cause a shift in the O 1s peaks of -NO2 and -OH to low binding energies, suggesting an electron-gaining state. Upon full dehydrogenation of AB, the formation of O-B bonds, due to the strong interactions between O and B, improves the dehydrogenation performance of AB. This is mentioned in the published works [35,39].
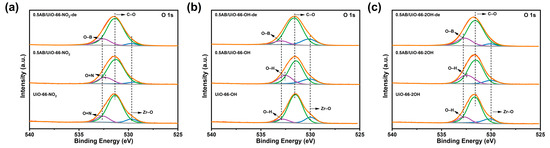
Figure 6.
XPS spectra of the O 1s orbitals of the materials. (a) 0.5AB/UiO-66-NO2, UiO-66-NO2 and 0.5AB/UiO-66-NO2-de, (b) 0.5AB/UiO-66-OH, UiO-66-OH and 0.5AB/UiO-66-OH-de, (c) 0.5AB/UiO-66-2OH, UiO-66-2OH and 0.5AB/UiO-66-2OH-de.
Furthermore, as illustrated in Figure 7, the Br 3d orbitals and F 1s orbitals of UiO-66-Br and UiO-66-F exhibited a shift towards lower binding energies following the loading of AB, indicating that an electron transfer occurs between AB and UiO-66-Br and UiO-66-F. However, after the dehydrogenation of AB, the peaks of Br 3d and F 1s orbitals were restored to be consistent with those of UiO-66-Br and UiO-66-F. This suggests that -Br and -F only have an effect on the electronic structure of AB, but are not involved in the reaction during the dehydrogenation of AB.
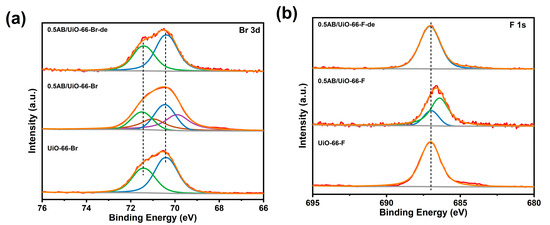
Figure 7.
XPS spectra of the (a) Br 3d and (b) F 1s orbitals of the materials.
Non-covalent interaction (NCI) scattering and reduced density gradient (RDG) analyses were conducted to examine and differentiate the interactions [40] between benzene with various functional groups and the compound AB. RDG isosurface mapping contains blue, green, and red regions, standing for the attraction, van der Waals, and steric repulsion, respectively [41].
The presence of green and a slight amount of blue coloration observed between H2BDC and AB, as illustrated in Figure 8a,b, suggests the occurrence of electrostatic interactions between N···H and B···H, respectively. Table S1 illustrates that there is a minimal variation in the charge density of H atoms in AB both before and after the optimization process.
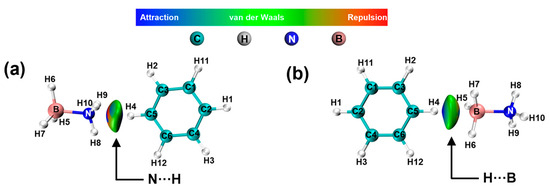
Figure 8.
NCI and RDG analysis of AB interactions with -H. (a) H···NH3-BH3, (b) H···BH3-NH3.
The interaction of -NH2 with AB is shown in Figure 9a,b. The results indicate the presence of N-H···H-B and N···H-N electrostatic interactions between -NH2 and -NH3, as well as B-H···H-N electrostatic interactions between -NH2 and -BH3. When the adsorption site is -NH3, the structure is optimized so that -BH3 is gradually adsorbed to -NH2, whereas when the adsorption site is -BH3, the structure is optimized so that -NH3 is not adsorbed by -NH2, which suggests that -NH2 prefers adsorption with -BH3 and thus interacts with AB. The same results are also reflected in Figure 9c,d. When the adsorption site is -NH3, the structure is optimized so that -BH3 is gradually adsorbed to -OH, whereas when the adsorption site is -BH3, the structure is optimized so that -NH3 is not adsorbed by -OH, which suggests that -OH prefers adsorption with -BH3. In addition, the H in both -NH2 and -OH can interact electrostatically with the H in AB.
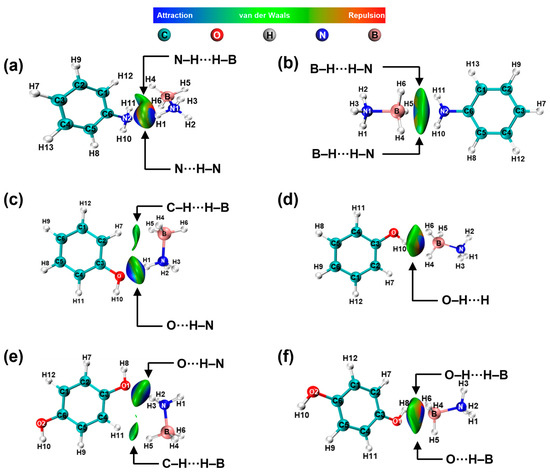
Figure 9.
NCI and RDG analysis of AB interactions with -NH2 (a,b) and -OH (c–f).
Tables S2–S4 show that in the optimized structure, there is a significant change in the charge density of the H atoms in AB that have electrostatic interactions with -NH2 and -OH compared to Table S1.
In Figure 10, the results indicate the presence of N···N and O···H-N electrostatic interactions between -NO2 and -NH3, as well as O···H-N and O···H-B electrostatic interactions between -NO2 and -BH3. In contrast to the -NH2 and -OH groups, the -NO2 group exerts a lesser influence on the charge density of hydrogen atoms in AB (Table S5).
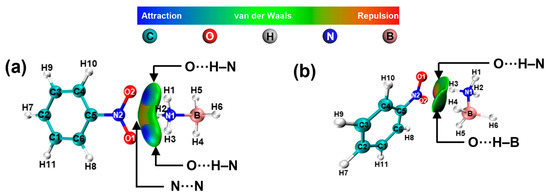
Figure 10.
NCI and RDG analysis of AB interactions with -NO2. (a) NO2···NH3-BH3, (b) NO2···BH3-NH3.
The interactions of the -NH2, -OH, and -NO2 groups differ from those of the -Br and -F groups. The latter typically engage in electrostatic interactions with N and boron B in AB, which subsequently influences the hydrogen atoms within AB. The results of these interactions are Figure 11a–d. It is observed from Tables S6 and S7 that the change in charge density of H atoms in -NH3 is essentially the same, and the change in charge density of H atoms in -BH3 is essentially the same.
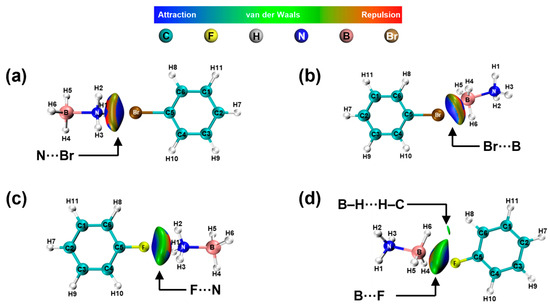
Figure 11.
NCI and RDG analysis of AB interactions with -Br (a,b) and -F (c,d).
The NCI results demonstrate that the electronic structure of AB is influenced to varying degrees by the presence of functional groups such as -NH2, -OH, -NO2, -Br, and -F. All of these functional groups are capable of disrupting the symmetry of the AB structure. It is possible that some of the H atoms in NH2-BDC, OH-BDC, and DHTA may interact electrostatically with the H atoms in AB. In light of the observed effect of different functional groups on the extent of AB dehydrogenation (Figure 5), it is postulated that in addition to the release of H atoms from AB, H atoms from some of the linkers may also be released. -NH2 and-OH can change the dehydrogenation process of AB in the pore, thus changing the multi-step dehydrogenation process of AB to one-step dehydrogenation. After the dehydrogenation of AB in UiO-66-NO2, the peak of the nitro group can still be found in FT-IR (Figure S6b), and it is presumed that the nitro group has not reacted with AB. Similar to -NO2, the orbitals of Br 3d and F 1s are consistent with UiO-66-Br and UiO-66-F (Figure 7), respectively, and it is presumed that Br and F also did not react with AB. But -NO2, -Br, and -F can activate the second step of the dehydrogenation reaction of AB within the UiO-66-X orifice, as can be illustrated in Figure 3d–f. In conclusion, the electronic structure of AB is affected by the addition of functional groups, which changes the dehydrogenation properties of AB.
3. Materials and Methods
3.1. Chemicals
Zirconium tetrachloride (ZrCl4, 98%) and acetic acid (99.8%) were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Ethanol absolute (EtOH, ≥99.5%), N,N-dimethylformamide (DMF, ≥99.5%), and tetrahydrofuran (THF, ≥99.5%) were purchased from Beijing Tong Guang Fine Chemicals Company (Beijing, China). The 1,4-dicarboxybenzene (H2BDC, 98%), 2-aminoterephthalic acid (NH2-BDC, 98%), 2-hydroxyterephthalic acid (OH-BDC, 98%), 2-bromoterephthalic acid (Br-BDC, 98%), 2-fluoroterephthalic acid (F-BDC, 98%), 2-nitroterephthalic acid (NO2-BDC, 98%) and 2,5-dihydroxyterephthalic acid (DHTA, 98%) were purchased from Energy Chemical Co., Ltd. (Shanghai, China). Ammonia borane (NH3BH3, AB, 97%) was purchased from 9 Ding Chemistry (Shanghai, China).
3.2. Instruments and Methods
PXRD patterns were measured using a Rigaku MiniFlex 600 diffractometer (Rigaku Corporation, Tokyo, Japan) with Cu-Kα X-ray radiation (λ = 0.154056 nm). The PXRD patterns were recorded from 3° to 60° (2θ) with a step size of 0.02° and a scan rate of 10° min−1. FT-IR spectra were collected at a transmission range of 400–4000 cm−1 on a Bruker ALPHA spectrometer (Bruker Optik GmbH, Ettlingen, Germany). Nitrogen sorption isotherms were recorded to specific surface areas and pore size distributions by using a Quantachrome Instrument ASiQMVH002-5 analyzer (Quantachrome Instruments, Boynton Beach, FL, USA) at 77 K. The samples were pretreated under a vacuum for 12 h at 120 °C. The pore size distributions were determined by non-local density functional theory (NLDFT) mode. FE-SEM images were collected on a Zeiss SUPRATM 55 SAPPHIRE scanning electron microscope (Carl Zeiss AG, Oberkochen, Germany) with 15 kV voltage. TGA curves were recorded from 40 to 800 °C at a heating rate of 10 °C min−1 using a NETZSCH STA 449F5 (NETZSCH, Selb, Germany) under a N2 atmosphere (50 mL min−1). The samples were pretreated under a vacuum for 12 h at 120 °C. X-ray photoelectron spectroscopy (XPS) measurements were performed on a Thermo Scientific K-Alpha electron spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) using monochromatic Al-Ka radiation of 1486.6 eV. Adventitious carbon was used to calibrate the binding energy shifts of the samples (C 1s = 284.8 eV).
The samples were initially placed in the center of an argon gas flow system (Sevenstar, Beijing, China) (50 mL min−1) tube furnace with a ramping rate of 3 °C min−1 from room temperature to 200 °C. The decomposition products were analyzed by a Hiden HPR-20 R&D (Hiden Analytical, Warrington, UK). The content of evolved hydrogen was analyzed by an Agilent 7890B using Agilent J&W HP-PLOT Q (Agilent Technologies Inc., Santa Clara, CA, USA). The hydrogen was detected by thermal conductivity detector (TCD) (Agilent Technologies Inc., Santa Clara, CA, USA). GC sampling was performed every 1 min during heating to capture real-time H2 concentration. The H2 signal was calibrated using standard H2/Ar mixtures (1–10 vol%).
The calculation was performed using the Gaussian 09 software (Gaussian Inc., Wallingford, CT, USA) package, using b3lyp/6-31G* functional basis sets [42]. The binding energies of seven molecules, namely terephthalic acid, 2-aminoterephthalic acid, 2-hydroxyterephthalic acid, 2-bromoterephthalic acid, 2-fluoroterephthalic acid, 2-nitroterephthalic acid, and 2,5-dihydroxyterephthalic acid, with the B and N sites of ammonia borane, were calculated separately, and the Mulliken charges of these 14 different combinations were analyzed. Finally, Multiwfn software version 3.7 (Beijing Kein Research Center for Natural Sciences, Beijing, China), combined with the VMD program, was used to perform IGMH analysis on the interactions of these 14 structures and achieve visualization [43,44].
3.3. Materials Prepared
- Synthesis of UiO-66:
UiO-66 particles were prepared by the reported solvothermal method [45] with modifications. Typically, 63 mg (0.38 mmol) of H2BDC and 106 mg (0.45 mmol) of ZrCl4 were dissolved in 50 mL of DMF, followed by the addition of 5 mL of acetic acid and sonication. The above solutions were transferred to a Teflon-lined steel autoclave (Yzreactor, Shanghai, China) (100 mL) and heated at 120 °C for 12 h, then cooled naturally to room temperature. The solution was washed three times with DMF to remove unreacted precursors and then solvent exchanged with EtOH over 3 days (1 time per day). Finally, the resulting UiO-66 particles were dried overnight at room temperature in a vacuum oven and then activated under a high dynamic vacuum at 150 °C for 12 h.
- Synthesis of UiO-66-NH2:
UiO-66-NH2 particles were prepared by the reported solvothermal method [46] with modifications. Typically, 81.6 mg (0.45 mmol) of NH2-BDC and 105 mg (0.45 mmol) of ZrCl4 were dissolved in 45 mL of DMF, followed by the addition of 4.5 mL of acetic acid and sonication. The above solutions were transferred to a Teflon-lined steel autoclave (100 mL) and heated at 120 °C for 12 h, then cooled naturally to room temperature. The solution was washed three times with DMF to remove unreacted precursors and then solvent exchanged with EtOH over 3 days (1 time per day). Finally, the resulting UiO-66-NH2 particles were dried overnight at room temperature in a vacuum oven and then activated under a high dynamic vacuum at 150 °C for 12 h.
- Synthesis of UiO-66-NO2:
UiO-66-NO2 particles were prepared by the reported solvothermal method [46] with modifications. Typically, 105 mg (0.5 mmol) of NO2-BDC and 117 mg (0.5 mmol) of ZrCl4 were dissolved in 50 mL of DMF, followed by the addition of 3 mL of acetic acid and sonication. The above solutions were transferred to a Teflon-lined steel autoclave (100 mL) and heated at 120 °C for 12 h, then cooled naturally to room temperature. The solution was washed three times with DMF to remove unreacted precursors and then solvent exchanged with EtOH over 3 days (1 time per day). Finally, the resulting UiO-66-NO2 particles were dried overnight at room temperature in a vacuum oven and then activated under a high dynamic vacuum at 150 °C for 12 h.
- Synthesis of UiO-66-Br:
UiO-66-Br particles were prepared by the reported solvothermal method [46] with modifications. Typically, 123 mg (0.5 mmol) of Br-BDC and 117 mg (0.5 mmol) of ZrCl4 were dissolved in 50 mL of DMF, followed by the addition of 1 mL of acetic acid and sonication. The above solutions were transferred to a Teflon-lined steel autoclave (100 mL) and heated at 120 °C for 12 h, then cooled naturally to room temperature. The solution was washed three times with DMF to remove unreacted precursors and then solvent exchanged with EtOH over 3 days (1 time per day). Finally, the resulting UiO-66-Br particles were dried overnight at room temperature in a vacuum oven and then activated under a high dynamic vacuum at 150 °C for 12 h.
- Synthesis of UiO-66-F:
UiO-66-F particles were prepared by the reported solvothermal method [46] with modifications. Typically, 92 mg (0.5 mmol) of F-BDC and 117 mg (0.5 mmol) of ZrCl4 were dissolved in 50 mL of DMF, followed by the addition of 5 mL of acetic acid and sonication. The above solutions were transferred to a Teflon-lined steel autoclave (100 mL) and heated at 120 °C for 12 h, then cooled naturally to room temperature. The solution was washed three times with DMF to remove unreacted precursors and then solvent exchanged with EtOH over 3 days (1 time per day). Finally, the resulting UiO-66-F particles were dried overnight at room temperature in a vacuum oven and then activated under a high dynamic vacuum at 150 °C for 12 h.
- Synthesis of UiO-66-OH:
UiO-66-OH particles were prepared by the reported solvothermal method [47] with modifications. Typically, 781 mg (4.3 mmol) of OH-BDC and 1 g (4.3 mmol) of ZrCl4 were dissolved in 70 mL of DMF, followed by the addition of 61 mL of acetic acid and 5 mL of H2O. The solution was transferred to a three-necked flask and then condensed and refluxed at 120 °C for 15 min, then cooled naturally to room temperature. The solution was washed three times with DMF to remove unreacted precursors and then solvent exchanged with EtOH over 3 days (1 time per day). Finally, the resulting UiO-66-OH particles were dried overnight at room temperature in a vacuum oven and then activated under a high dynamic vacuum at 150 °C for 12 h.
- Synthesis of UiO-66-2OH:
UiO-66-2OH particles were prepared by the reported solvothermal method [47] with modifications. Typically, 850 mg (4.3 mmol) of DHTA and 1 g (4.3 mmol) of ZrCl4 were dissolved in 70 mL of DMF, followed by the addition of 61 mL of acetic acid and 5 mL of H2O. The solution was transferred to a three-necked flask and then condensed and refluxed at 120 °C for 15 min, then cooled naturally to room temperature. The solution was washed three times with DMF to remove unreacted precursors and then solvent exchanged with EtOH over 3 days (1 time per day). Finally, the resulting UiO-66-2OH particles were dried overnight at room temperature in a vacuum oven and then activated under a high dynamic vacuum at 150 °C for 12 h.
- Synthesis of AB/MOF:
Here, 100 mg of AB was dissolved in 1 mL THF. Then, 200 mg of MOF was dispersed in 1 mL of THF by ultrasonication for 20 min. The prepared THF solution of AB was added to the above MOF dispersion. Next, the dispersion was continuously stirred for 12 h at room temperature. The sample was put in a vacuum dryer oven at 40 °C overnight for ensuring complete drying. In fact, the mass ratio of AB:MOF was 0.5:1, giving the sample a designation of 0.5AB/MOF, and it was stored in a desiccator for further characterization.
4. Conclusions
In this study, the effect of modifying various functional groups (-NH2, -OH, -NO2, -Br, and -F) in UiO-66 on the thermal dehydrogenation process of AB was investigated. All the functional groups had an effect on the dehydrogenation properties of AB, and the TPD-GC analysis showed that the dehydrogenation ratio of AB was significantly increased by the addition of functional groups compared to UiO-66. Among them, the introduction of -NH2 and -OH was able to bring the dehydrogenation of two AB to 3.53 wt.%, which was higher than that of UiO-66 at 1.98 wt.%. The modification of two -OH on terephthalic acid was able to further enhance the dehydrogenation ratio of AB, and UiO-66-2OH increased the dehydrogenation of AB to 3.85 wt.%. The XPS results showed that electron transfer occurred between the modified functional groups and a part of AB. The interactions between these functional groups and AB were investigated by NCI analysis. Overall, for functional groups containing H atoms, they can directly participate in the pyrolytic dehydrogenation reaction of AB by directly interacting with the H in AB, while changing the electronic structure of AB. For functional groups that do not contain H atoms, the dehydrogenation properties of AB can be improved by changing the electronic structure of AB. In this study, the functional group modification of UiO-66 and the effect of different functional groups on AB were analyzed to support the future design of high-performance MOF nanoconfined AB composite hydrogen storage materials.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30071487/s1, Figure S1: Characterization of UiO-66 and UiO-66-X. PXRD patterns (a), FT-IR spectra (b), N2 adsorption-desorption isotherms (at 77 K) (c) and pore size distributions (d); Figure S2: The SEM image of UiO-66; Figure S3: The SEM images of UiO-66-NH2 (a), UiO-66-OH (b), UiO-66-2OH (c), UiO-66-NO2 (d), UiO-66-Br (e), and UiO-66-F (f); Figure S4: TGA plots for UiO-66 and UiO-66-X; Figure S5: N2 adsorption-desorption isotherms (at 77 K) of 0.5AB/UiO-66 and 0.5AB/UiO-66-X; Figure S6: Characterization of 0.5AB/UiO-66-de and 0.5AB/UiO-66-X-de. PXRD patterns (a), FT-IR spectra (b); Figure S7: XPS spectra of the Zr 3d orbitals of the materials; Figure S8: XPS spectra of the N 1s orbitals of the materials; Figure S9: XPS spectra of the B 1s orbitals of the materials; Table S1: Muliken population analysis of charge densities of AB, H2BDC, H2BDC-AB; Table S2: Muliken population analysis of charge densities of AB, NH2-BDC, NH2-BDC-AB; Table S3: Muliken population analysis of charge densities of AB, OH-BDC, OH-BDC-AB; Table S4: Muliken population analysis of charge densities of AB, DHTA, DHTA-AB; Table S5: Muliken population analysis of charge densities of AB, NO2-BDC, NO2-BDC-AB; Table S6: Muliken population analysis of charge densities of AB, Br-BDC, Br-BDC-AB; Table S7: Muliken population analysis of charge densities of AB, F-BDC, F-BDC-AB; Table S8: The X,Y, and Z coordinates used for computational analysis.
Author Contributions
Conceptualization, T.Z. and L.Y.; methodology, S.X. and D.X.; software, S.X. and R.C.; validation, S.X., D.X., T.Z. and L.Y.; formal analysis, S.X. and W.W.; investigation, S.X. and W.Y.; data curation, S.X.; writing—original draft preparation, S.X. and D.X.; writing—review and editing, S.X., T.Z. and L.Y.; funding acquisition, T.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, Grant No. 22102008.
Data Availability Statement
The data presented in this study are available upon request from the corresponding authors.
Acknowledgments
The authors thank the Analysis and Testing Center of BIT for technical support and Huasuan Technology for theoretical calculation services.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Winter, C.-J. Hydrogen energy-Abundant, efficient, clean: A debate over the energy-system-of-change. Int. J. Hydrogen Energy 2009, 34, S1–S52. [Google Scholar] [CrossRef]
- Guan, D.; Wang, B.; Zhang, J.; Shi, R.; Jiao, K.; Li, L.; Wang, Y.; Xie, B.; Zhang, Q.; Yu, J.; et al. Hydrogen society: From present to future. Energy Environ. Sci. 2023, 16, 4926–4943. [Google Scholar]
- Staffell, I.; Scamman, D.; Velazquez Abad, A.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar]
- Wang, Y.; Xue, Y.; Züttel, A. Nanoscale engineering of solid-state materials for boosting hydrogen storage. Chem. Soc. Rev. 2024, 53, 972–1003. [Google Scholar] [CrossRef] [PubMed]
- Sattar, M.A.; Rasul, M.G.; Jahirul, M.I.; Hasan, M.M. An up-to-date review on the progress and challenges of hydrogen storage, and its safety and economic analysis. Sustain. Energy Fuels 2024, 8, 3545–3573. [Google Scholar]
- Rao, P.C.; Kim, Y.; Kim, H.; Son, Y.; Choi, Y.; Na, K.; Yoon, M. Methylbenzyl Naphthalene: Liquid Organic Hydrogen Carrier for Facile Hydrogen Storage and Release. ACS Sustain. Chem. Eng. 2023, 11, 12656–12666. [Google Scholar] [CrossRef]
- Deng, Y.; Guo, Y.; Jia, Z.; Liu, J.C.; Guo, J.; Cai, X.; Dong, C.; Wang, M.; Li, C.; Diao, J.; et al. Few-Atom Pt Ensembles Enable Efficient Catalytic Cyclohexane Dehydrogenation for Hydrogen Production. J. Am. Chem. Soc. 2022, 144, 3535–3542. [Google Scholar]
- Xi, S.; Wang, X.; Tome, K.C.; Zhang, T.; Han, Z.; Gao, M.; Zhou, S.; Yu, H. Effect of Ni and SAPO−34 co−additive on enhancing hydrogen storage performance of MgH2. Int. J. Hydrogen Energy 2021, 46, 23748–23756. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Zhang, H.; Xia, G.; Sun, D.; Yu, X. Heterostructures Built in Metal Hydrides for Advanced Hydrogen Storage Reversibility. Adv. Mater. 2020, 32, 2002647. [Google Scholar] [CrossRef]
- Liu, P.; Lian, J.; Chen, H.; Liu, X.; Chen, Y.; Zhang, T.; Yu, H.; Lu, G.; Zhou, S. In−situ synthesis of Mg2Ni-Ce6O11 catalyst for improvement of hydrogen storage in magnesium. Chem. Eng. J. 2020, 385, 123448. [Google Scholar] [CrossRef]
- Chong, L.; Zeng, X.; Ding, W.; Liu, D.J.; Zou, J. NaBH4 in “Graphene Wrapper:” Significantly Enhanced Hydrogen Storage Capacity and Regenerability through Nanoencapsulation. Adv. Mater. 2015, 27, 5070–5074. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Ju, X.; Wan, C.; Li, S. First-principles study of transition metal (Ti, Nb)-doped NaAlH4. Int. J. Hydrogen Energy 2016, 41, 3517–3526. [Google Scholar] [CrossRef]
- Ouyang, L.; Chen, W.; Liu, J.; Felderhoff, M.; Wang, H.; Zhu, M. Enhancing the Regeneration Process of Consumed NaBH4 for Hydrogen Storage. Adv. Energy Mater. 2017, 7, 1700299. [Google Scholar] [CrossRef]
- Xiong, Z.; Yong, C.K.; Wu, G.; Chen, P.; Shaw, W.; Karkamkar, A.; Autrey, T.; Jones, M.O.; Johnson, S.R.; Edwards, P.P.; et al. High-capacity hydrogen storage in lithium and sodium amidoboranes. Nat. Mater. 2008, 7, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Aichouche, A.; Bouhadda, Y.; Bououdina, M.; Benyelloul, K.; Bentria, B. The destabilising effect of alkali metal (Na and K) of hydrazine-borane N2H4BH3 for hydrogen storage: Ab-initio study. Int. J. Hydrogen Energy 2018, 43, 14520–14531. [Google Scholar] [CrossRef]
- Kumar, R.; Karkamkar, A.; Bowden, M.; Autrey, T. Solid-state hydrogen rich boron-nitrogen compounds for energy storage. Chem. Soc. Rev. 2019, 48, 5350–5380. [Google Scholar] [CrossRef]
- Hamilton, C.W.; Baker, R.T.; Staubitz, A.; Manners, I. B-N compounds for chemical hydrogen storage. Chem. Soc. Rev. 2009, 38, 279–293. [Google Scholar] [CrossRef]
- Guan, S.; Yuan, Z.; Zhao, S.; Zhuang, Z.; Zhang, H.; Shen, R.; Fan, Y.; Li, B.; Wang, D.; Liu, B. Efficient Hydrogen Generation from Ammonia Borane Hydrolysis on a Tandem Ruthenium-Platinum-Titanium Catalyst. Angew. Chem. Int. Ed. 2024, 63, e202408193. [Google Scholar] [CrossRef]
- Shen, R.; Liu, Y.; Wen, H.; Wu, X.; Han, G.; Yue, X.; Mehdi, S.; Liu, T.; Cao, H.; Liang, E.; et al. Engineering Bimodal Oxygen Vacancies and Pt to Boost the Activity Toward Water Dissociation. Small 2022, 18, e2105588. [Google Scholar] [CrossRef]
- Luo, J.; Wang, J.; Feng, X.; Cai, J.; Yao, W.; Song, J.; Chen, C.; Luo, D. Mechanistic insight into the promoting effect of magnesium nickel hydride on the dehydrogenation of ammonia borane. Int. J. Hydrogen Energy 2018, 43, 1681–1690. [Google Scholar]
- Huang, T.; Zou, J.; Zeng, X.; Wang, J.; Liu, H.; Ding, W. Reversible hydrogen sorption behaviors of the 3NaBH4-(x)YF3-(1-x)GdF3 system: The effect of double rare earth metal cations. Int. J. Hydrogen Energy 2019, 44, 4868–4877. [Google Scholar]
- Yang, H.; Lombardo, L.; Luo, W.; Kim, W.; Züttel, A. Hydrogen storage properties of various carbon supported NaBH4 prepared via metathesis. Int. J. Hydrogen Energy 2018, 43, 7108–7116. [Google Scholar]
- Ma, Z.; Panda, S.; Zhang, Q.; Sun, F.; Khan, D.; Ding, W.; Zou, J. Improving hydrogen sorption performances of MgH2 through nanoconfinement in a mesoporous CoS nano−boxes scaffold. Chem. Eng. J. 2021, 406, 126790. [Google Scholar]
- Demirci, U.B. Mechanistic insights into the thermal decomposition of ammonia borane, a material studied for chemical hydrogen storage. Inorg. Chem. Front. 2021, 8, 1900–1930. [Google Scholar]
- Champet, S.; van den Berg, J.; Szczesny, R.; Godula-Jopek, A.; Gregory, D.H. Nano-inclusion in one step: Spontaneous ice-templating of porous hierarchical nanocomposites for selective hydrogen release. Sustain. Energy Fuels 2019, 3, 396–400. [Google Scholar]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar]
- Wang, Q.; Astruc, D. State of the art and prospects in metal−organic framework (MOF)−based and MOF−derived nanocatalysis. Chem. Rev. 2020, 120, 1438–1511. [Google Scholar]
- Freund, R.; Zaremba, O.; Arnauts, G.; Ameloot, R.; Skorupskii, G.; Dinca, M.; Bavykina, A.; Gascon, J.; Ejsmont, A.; Goscianska, J.; et al. The current status of MOF and COF applications. Angew. Chem. Int. Ed. 2021, 60, 23975–24001. [Google Scholar]
- Li, Z.; Zhu, G.; Lu, G.; Qiu, S.; Yao, X. Ammonia borane confined by a metal−organic framework for chemical hydrogen storage: Enhancing kinetics and eliminating ammonia. J. Am. Chem. Soc. 2010, 132, 1490–1491. [Google Scholar] [CrossRef]
- Wu, Y.J.; Wang, C.Y. Insight into the catalytic effects of open metal sites in metal−organic frameworks on hydride dehydrogenation via nanoconfinement. ACS Sustain. Chem. Eng. 2019, 7, 16013–16025. [Google Scholar]
- Li, Z.; Liu, W.; Yang, H.; Sun, T.; Liu, K.; Wang, Z.; Niu, C. Improved thermal dehydrogenation of ammonia borane by MOF-5. RSC Adv. 2015, 5, 10746–10750. [Google Scholar]
- Srinivas, G.; Travis, W.; Ford, J.; Wu, H.; Guo, Z.-X.; Yildirim, T. Nanoconfined ammonia borane in a flexible metal–organic framework Fe-MIL-53: Clean hydrogen release with fast kinetics. J. Mater. Chem. A 2013, 1, 4167–4172. [Google Scholar] [CrossRef]
- Srinivas, G.; Ford, J.; Zhou, W.; Yildirim, T. Zn-MOF assisted dehydrogenation of ammonia borane: Enhanced kinetics and clean hydrogen generation. Int. J. Hydrogen Energy 2012, 37, 3633–3638. [Google Scholar]
- Gao, L.; Li, C.Y.; Yung, H.; Chan, K.Y. A functionalized MIL-101(Cr) metal-organic framework for enhanced hydrogen release from ammonia borane at low temperature. Chem. Commun. 2013, 49, 10629–10631. [Google Scholar]
- Peil, S.; Wisser, D.; Stähle, M.; Roßmann, P.K.; Avadhut, Y.S.; Hartmann, M. Hydrogen Release from Ammonia Borane Nanoconfined in Metal−Organic Frameworks with MIL−53 Topology. J. Phys. Chem. C 2021, 125, 9990–10000. [Google Scholar]
- Valero-Pedraza, M.-J.; Cot, D.; Petit, E.; Aguey-Zinsou, K.-F.; Alauzun, J.G.; Demirci, U.B. Ammonia Borane Nanospheres for Hydrogen Storage. ACS Appl. Nano Mater. 2019, 2, 1129–1138. [Google Scholar]
- Yang, H.; Li, Z.; Liu, K.; Meng, F.; Niu, C. Clean Hydrogen Release from Ammonia Borane in a Metal-Organic Framework with Unsaturated Coordinated Tm3+. J. Phys. Chem. C 2015, 119, 2260–2265. [Google Scholar]
- Mishra, S.; Kang, P.-C.; Guo, R.-F.; Wang, C.-Y.; Nebhani, L. Combined Effect of Functionality and Pore Size on Dehydrogenation of Ammonia Borane via Its Nanoconfinement in Polyacrylamide-Grafted Organically Modified Mesoporous Silica. ACS Appl. Energy Mater. 2021, 4, 6585–6598. [Google Scholar] [CrossRef]
- Kim, G.J.; Hunt, S.G.; Hwang, H.T. Effect of maleic acid on onset temperature and H2 release kinetics for thermal dehydrogenation of ammonia borane. Int. J. Hydrogen Energy 2020, 45, 33751–33758. [Google Scholar]
- Chen, W.; Chen, Z.; Chi, Y.; Tian, W. Double Cation—π Directed Two-Dimensional Metallacycle-Based Hierarchical Self-Assemblies for Dual-Mode Catalysis. J. Am. Chem. Soc. 2023, 145, 19746–19758. [Google Scholar]
- Wang, Z.; Hao, A.; Xing, P. Halogen Interaction Effects on Chiral Self-Assemblies on Cyclodipeptide Scaffolds Across Hierarchy. Small 2023, 19, 2302517. [Google Scholar]
- Frisch, M.J.; Trucks, G.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.; et al. Gaussian 09 Revision A.1; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [PubMed]
- Chen, L.W.; Hao, Y.C.; Guo, Y.; Zhang, Q.; Li, J.; Gao, W.Y.; Ren, L.; Su, X.; Hu, L.; Zhang, N.; et al. Metal-organic framework membranes encapsulating gold nanoparticles for direct plasmonic photocatalytic nitrogen fixation. J. Am. Chem. Soc. 2021, 143, 5727–5736. [Google Scholar]
- Zhang, J.; Bai, T.; Huang, H.; Yu, M.H.; Fan, X.; Chang, Z.; Bu, X.H. Metal-organic-framework-based photocatalysts optimized by spatially separated cocatalysts for overall water splitting. Adv. Mater. 2020, 32, 2004747. [Google Scholar]
- He, T.; Xu, X.; Ni, B.; Wang, H.; Long, Y.; Hu, W.; Wang, X. Fast and scalable synthesis of uniform zirconium-, hafnium-based metal-organic framework nanocrystals. Nanoscale 2017, 9, 19209–19215. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).