Cistus ladanifer L.: Essential Oils, Volatiles, By-Products, and Their Biological Properties
Abstract
1. Introduction
2. Methods
3. Chemical Composition of Essential Oils
3.1. Chemical Composition of Cistus ladanifer EOs from the Iberian Peninsula
3.2. Chemical Composition of Cistus ladanifer EOs from Morocco
3.3. Chemical Composition of Cistus ladanifer EOs from France
3.4. Chemical Composition of Cistus ladanifer EOs from India and Germany
4. Biological Properties of Cistus ladanifer EOs or Volatiles
4.1. Biological Properties of Cistus ladanifer EOs Without Chemical Composition
4.2. Antioxidant Activity
4.3. Antimicrobial Activity
4.4. Phytotoxical Activity
4.5. Citotoxicity, Anti-Inflammatory, and Other Biological Activities
5. By-Products Obtained from Cistus ladanifer EOs Distilleries
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guzmán, B.; Vargas, P. Historical biogeography and character evolution of Cistaceae (Malvales) based on analysis of plastid rbcL and trnL-trnF sequences. Org. Divers. Evol. 2009, 9, 83–99. [Google Scholar] [CrossRef]
- Sánchez-Gómez, P.; Cánovas, J.L.; Lahora, A.; Catalán, A.E.; Jiménez, J.F. Disentangling the taxonomical uncertainties about the presence of Cistus pouzolzii (Cistaceae) in the Iberian Peninsula. Mediterr. Bot. 2024, 45, e90714. [Google Scholar] [CrossRef]
- Frazão, D.F.; Raimundo, J.R.; Domingues, J.L.; Quintela-Sabarís, C.; Gonçalves, J.C.; Delgado, F. Cistus ladanifer (Cistaceae): A natural resource in Mediterranean-type ecosystems. Planta 2018, 247, 289–300. [Google Scholar] [CrossRef]
- Civeyrel, L.; Leclercq, J.; Demoly, J.-P.; Agnan, Y.; Quèbre, N.; Pélissier, C.; Otto, T. Molecular systematics, character evolution, and pollen morphology of Cistus and Halimium (Cistaceae). Plant Syst. Evol. 2011, 295, 23–54. [Google Scholar] [CrossRef]
- Carlier, J.; Leitão, J.; Fonseca, F. Population genetic structure of Cistus ladanifer L. (Cistaceae) and genetic differentiation from co-occurring Cistus species. Plant Species Biol. 2008, 23, 141–151. [Google Scholar] [CrossRef]
- Demoly, J.-P.; Marrero, M.V.; Baudet, A.B. Contribution à la connaissance des cistes de la section Macrostylia Willk. (Cistus L., Ciataceae). J. Bot. 2006, 36, 13–38. [Google Scholar]
- Papaefthimiou, D.; Papanikolaou, A.; Falara, V.; Givanoudi, S.; Kostas, S.; Kanellis, A.K. Genus Cistus: A model for exploring labdane-type diterpenes’ biosynthesis and a natural source of high value products with biological, aromatic, and pharmacological properties. Front. Chem. 2014, 2, 45. [Google Scholar] [CrossRef]
- Ribeiro, S.; Delgado, F. Cistus ladanifer L. subsp. Ladanifer. In Cardo; Azevedo, L., Ed.; Norprint: Santo Tirso, Portugal, 2016; pp. 52–53. [Google Scholar]
- Frazão, D.F.; Martins-Gomes, C.; Steck, J.L.; Keller, J.; Delgado, F.; Gonçalves, J.C.; Bunzel, M.; Pintado, C.M.B.S.; Diaz, T.S.; Silva, A.M. Labdanum resin from Cistus ladanifer L.: A natural and sustainable ingredient for skin care cosmetics with relevant cosmeceutical bioactivities. Plants 2022, 11, 1477. [Google Scholar] [CrossRef] [PubMed]
- Weyerstahl, P.; Marschall, H.; Weirauch, M.; Thefeld, K.; Surburg, H. Constituents of commercial labdanum oil. Flavour Fragr. J. 1998, 13, 295–318. [Google Scholar] [CrossRef]
- Greche, H.; Mrabet, N.; Ismaili-Alaoui, M.; Hajjaji, N.; Bousta, D.; Dahchour, A.; Boukir, A.; Benjilali, B. Chemical composition, antibacterial and antifungal activities of Moroccan Cistus ladanifer L. leaves extracts. In Recherches sur les Plantes Aromatiques et Médicinales, Preceedings of the Congrès International, Mezraoua (Taounate) and Fes, Morocco, 22–24 March 2007; Grèche, H., Ennabili, A., Eds.; INPMA: Rabat, Morocco, 2008; pp. 201–213. [Google Scholar]
- Morgado, J.M.; Tapias, R.; Alesso, P. Producción de goma bruta de jara (Cistus ladanifer L.) en el suroeste de la Península Ibérica. In Proceedings of the Congresos Forestales 2005, Zaragoza, Spain, 26–30 September 2005. [Google Scholar]
- Chaves, N.; Alías, J.C.; Sosa, T. Phytotoxicity of Cistus ladanifer L.: Role of allelopathy. Allelopath. J. 2016, 38, 113–132. [Google Scholar]
- Dias, A.S.; Costa, C.T.; Dias, L.S. Allelopathic plants. XVII.: Cistus ladanifer L. Allelopath. J. 2005, 16, 1–30. [Google Scholar]
- Alías, J.C.; Sosa, T.; Valares, C.; Escudero, J.C.; Chaves, N. Seasonal variation of Cistus ladanifer L. diterpenes. Plants 2012, 1, 6–15. [Google Scholar] [CrossRef]
- Lobón, N.C.; Gallego, J.C.A.; Díaz, T.S.; García, J.C.E. Allelopathic potential of Cistus ladanifer chemicals in response to variations of light and temperature. Chemoecology 2002, 12, 139–145. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Rolo, J.; Gaspar, C.; Ramos, L.; Cavaleiro, C.; Salgueiro, L.; Palmeira-de-Oliveira, R.; Teixeira, J.P.; Martínez-de-Oliveira, J.; Palmeira-de-Oliveira, A. Thymus mastichina (L.) L. and Cistus ladanifer L. for skin application: Chemical characterization and in vitro bioactivity assessment. J. Ethnopharmacol. 2023, 302, 115830. [Google Scholar] [CrossRef] [PubMed]
- González, J.A.; García-Barriuso, M.; Gordaliza, M.; Amich, F. Traditional plant-based remedies to control insect vectors of disease in the Arribes del Duero (western Spain): An ethnobotanical study. J. Ethnopharmacol. 2011, 138, 595–601. [Google Scholar] [CrossRef]
- Drapeau, J.; Fröler, C.; Touraud, D.; Kröckel, U.; Geier, M.; Rose, A.; Kunz, W. Repellent studies with Aedes aegypti mosquitoes and human olfactory tests on 19 essential oils from Corsica, France. Flavour Fragr. J. 2009, 24, 160–169. [Google Scholar] [CrossRef]
- Kubeczka, K.-H. History and sources of essential oil research. In Handbook of Essential Oils, Science, Technology, and Applications, 3rd ed.; Başer, K.H.C., Buchbauer, G., Eds.; CRC Press, Taylor Francis Group, LLC: Boca Raton, FL, USA, 2020; Chapter 2; pp. 3–40. [Google Scholar]
- Franz, C.; Novak, J. Sources of essential oils. In Handbook of Essential Oils, Science, Technology, and Applications, 3rd ed.; Başer, K.H.C., Buchbauer, G., Eds.; CRC Press, Taylor Francis Group, LLC: Boca Raton, FL, USA, 2020; Chapter 3; pp. 41–84. [Google Scholar]
- Rubiolo, P.; Sgorbini, B.; Liberto, E.; Cordero, C.; Bicchi, C. Essential oils and volatiles: Sample preparation and analysis. A review. Flavour Fragr. J. 2010, 25, 282–290. [Google Scholar] [CrossRef]
- Sell, C. Chemistry of essential oils. In Handbook of Essential Oils, Science, Technology, and Applications, 3rd ed.; Başer, K.H.C., Buchbauer, G., Eds.; CRC Press, Taylor Francis Group, LLC: Boca Raton, FL, USA, 2020; Chapter 6; pp. 161–189. [Google Scholar]
- Mariotti, J.-P.; Tomi, F.; Casanova, J.; Costa, J.; Bernardini, A.F. Composition of the essential oil of Cistus ladanifer L. cultivated in Corsica (France). Flavour Fragr. J. 1997, 12, 147–151. [Google Scholar] [CrossRef]
- Robles, C.; Bousquet-Mélou, A.; Farzino, S.; Bonin, G. Comparison of essential oil composition of two varieties of Cistus ladanifer. Biochem. Syst. Ecol. 2003, 31, 339–343. [Google Scholar] [CrossRef]
- Rossi, P.-G.; Berti, L.; Panighi, J.; Luciani, A.; Maury, J.; Muselli, A.; Serra, D.R.; Gonny, M.; Bolla, J.-M. Antibacterial action of essential oils from Corsica. J. Essent. Oil Res. 2007, 19, 176–182. [Google Scholar] [CrossRef]
- Upadhyay, N.; Singh, V.K.; Dwivedy, A.K.; Das, S.; Chaudhari, A.K.; Dubey, N.K. Cistus ladanifer L. essential oil on as a plant based preservative against molds infesting oil seeds, aflatoxin B1 secretion, oxidative deterioration and methylglyoxal biosynthesis. LWT-Food Sci. Technol. 2018, 92, 395–403. [Google Scholar] [CrossRef]
- Oller-López, J.L.; Rodríguez, R.; Cueva, J.M.; Oltra, J.E.; Bazdi, B.; Dahdouh, A.; Lamarti, A.; Mansour, A.I. Composition of the essential oils of Cistus ladaniferus and C. monspeliensis from Morocco. J. Essent. Oil Res. 2005, 17, 553–555. [Google Scholar] [CrossRef]
- Greche, H.; Mrabet, N.; Zira, S.; Ismaïli-Alaoui, M.; Benjilali, B. The volatiles of the leaf oil of Cistus ladanifer L. var. albiflorus and labdanum extracts of Moroccan origin and their antimicrobial activities. J. Essent. Oil Res. 2009, 21, 166–173. [Google Scholar]
- Viuda-Martos, M.; Sendra, E.; Pérez-Alvarez, J.A.; Fernández-López, J.; Amensour, M.; Abrini, J. Identification of flavonoid content and chemical composition of the esential oils of Moroccan herbs: Myrtle (Myrtus communis L.), rockroase (Cistus ladanifer L.) and Montpellier cistus (Cistus monspeliensis L.). J. Essent. Oil Res. 2011, 23, 1–9. [Google Scholar] [CrossRef]
- Zidane, H.; Elmiz, M.; Aouinti, F.; Tahani, A.; Wathelet, J.; Sindic, M.; Elbachiri, A. Chemical composition and antioxidant activity of essential oil, various organic extracts of Cistus ladanifer and Cistus libanotis growing in Eastern Morocco. Afr. J. Biotechnol. 2013, 12, 5314–5320. [Google Scholar] [CrossRef]
- Mohammed, B.; Said, C.; Fouzia, F.R.; Kawtar, F.B.; Zoubida, H.; Abdelilah, O.; Mohammed, E.; Ghizlane, E. Chemical composition and antimicrobial activity of the essential oil of Cistus ladanifer var. maculatus Dun. J. Microbiol. Biotechnol. Food Sci. 2018, 8, 925–930. [Google Scholar] [CrossRef]
- Benali, T.; Bouyahya, A.; Habbadi, K.; Zengin, G.; Khabbach, A.; Achbani, E.H.; Hammani, K. Chemical composition and antibacterial activity of the essential oil and extracts of Cistus ladanifer subsp. ladanifer and Mentha suaveolens against phytopathogenic bacteria and their ecofriendly management of phytopathogenic bacteria. Biocat. Agric. Biotechnol. 2020, 28, 101696. [Google Scholar] [CrossRef]
- El Karkouri, J.; Bouhrim, M.; Al Kamaly, O.M.; Mechchate, H.; Kchibale, A.; Adadi, I.; Amine, S.; Ismaili, S.A.; Zair, T. Chemical composition, antibacterial and antifungal activity of the essential oil from Cistus ladanifer L. Plants 2021, 10, 2068. [Google Scholar] [CrossRef] [PubMed]
- El Hachlafi, N.; Kandsi, F.; Elbouzidi, A.; Lafdil, F.Z.; Nouioura, G.; Abdallah, E.M.; Abdnim, R.; Bnouham, M.; Al-Mijalli, S.M.; Mrabti, H.N.; et al. Extraction of bioactive compound-rich essential oil from Cistus ladanifer L. by microwave-assisted hydrodistillation: GC-MS characterization, in vitro pharmacological activities, and molecular docking. Separations 2024, 11, 199. [Google Scholar] [CrossRef]
- Ramalho, P.S.; de Freitas, V.A.P.; Macedo, A.; Silva, G.; Silva, A.M.S. Volatile components of Cistus ladanifer leaves. Flavour Fragr. J. 1999, 14, 300–302. [Google Scholar] [CrossRef]
- Gomes, P.B.; Mata, V.G.; Rodrigues, A.E. Characterization of the Portuguese-grown Cistus ladanifer essential oil. J. Essent. Oil Res. 2005, 17, 160–165. [Google Scholar] [CrossRef]
- Teixeira, S.; Mendes, A.; Alves, A.; Santos, L. Simultaneous distillation-extraction of high-value volatile compounds from Cistus ladanifer L. Anal. Chim. Acta 2007, 584, 439–446. [Google Scholar] [CrossRef]
- Falcão, S.I.; Freire, C.; Figueiredo, A.C.; Vilas-Boas, M. The volatile composition of Portuguese propolis towards its origin discrimination. Rec. Nat. Prod. 2016, 10, 176–188. [Google Scholar]
- Vieira, M.; Bessa, L.J.; Martins, M.R.; Arantes, S.; Teixeira, A.P.S.; Mendes, Â.; da Costa, P.M.; Belo, A.D.F. Chemical composition, antibacterial, antibiofilm and synergistic properties of essential oils from Eucalyptus globulus Labill. and seven Mediterranean aromatic plants. Chem. Biodivers. 2017, 14, e1700006. [Google Scholar] [CrossRef] [PubMed]
- Luis, A.; Ramos, A.; Domingues, F. Pullulan films containing rockrose essential oil for potential food packaging applications. Antibiotics 2020, 9, 681. [Google Scholar] [CrossRef]
- Tavares, C.S.; Martins, A.; Faleiro, M.L.; Miguel, M.G.; Duarte, L.C.; Gameiro, J.A.; Roseiro, L.B.; Figueiredo, A.C. Bioproducts from forest biomass: Essential oils and hydrolates from wastes of Cupressus lusitanica Mill. and Cistus ladanifer L. Ind. Crops Prod. 2020, 144, 112034. [Google Scholar] [CrossRef]
- Ferraz, C.A.; Sousa, A.C.A.; Caramelo, D.; Delgado, F.; de Oliveira, A.P.; Pastorinho, M.R. Chemical profile and eco-safety evaluation of essential oils and hydrolates from Cistus ladanifer, Helichrysum italicum, Ocimum basilicum and Thymbra capitate. Ind. Crops Prod. 2022, 175, 114232. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Gaspar, C.; Rolo, J.; Palmeira-de-Oliveira, R.; Teixeira, J.P.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, A. Comparative efficacy of essential oils against Cutibacterium acnes: Effect upon strains from phylotypes with different virulance patterns. Microb. Pathog. 2025, 199, 107159. [Google Scholar] [CrossRef]
- Costa, R.; de Fina, M.R.; Valentino, M.R.; Crupi, M.L.; Mondello, L. Application of a new GC-MS library, designed with a retention index filter tool, to the analysis of the essential oil of Cistus ladanifer. Acta Hort. 2009, 826, 271–276. [Google Scholar] [CrossRef]
- Verdeguer, M.; Blázquez, M.A.; Boira, H. Chemical composition and herbicidal activity of the essential oil from a Cistus ladanifer L. population from Spain. Nat. Prod. Res. 2012, 26, 1602–1609. [Google Scholar] [CrossRef]
- Morales-Soto, A.; Oruna-Concha, M.J.; Elmore, J.S.; Barrajón-Catalán, E.; Micol, V.; Roldán, C.; Segura-Carretero, A. Volatile profile of Spanish Cistus plants as sources of antimicrobials for industrial applications. Ind. Crops Prod. 2015, 74, 425–433. [Google Scholar] [CrossRef]
- Najar, B.; Shortrede, J.E.; Pistelli, L.; Buhagiar, J. Chemical composition and in vitro cytotoxic screening of sixteen comercial essential oil on five cancer cell lines. Chem. Biodivers. 2020, 17, e1900478. [Google Scholar] [CrossRef] [PubMed]
- Xavier, V.; Finimundy, T.C.; Heleno, S.A.; Amaral, J.S.; Calhelha, R.C.; Vaz, J.; Pires, T.C.S.P.; Mediavilla, I.; Esteban, L.S.; Ferreira, I.C.F.R.; et al. Chemical and bioactive characterization of the essential oils obtained from three Mediterranean plants. Molecules 2021, 26, 7472. [Google Scholar] [CrossRef]
- Mediavilla, I.; Blázquez, M.A.; Ruiz, A.; Esteban, L.S. Influence of the storage of Cistus ladanifer L. bales from mechanized harvesting on the essential oil yield and qualitative composition. Molecules 2021, 26, 2379. [Google Scholar] [CrossRef]
- Mediavilla, I.; Guillamón, E.; Ruiz, A.; Esteban, L.S. Essential oils from residual foliage of forest tree and shrub species: Yield and antioxidant capacity. Molecules 2021, 26, 3257. [Google Scholar] [CrossRef]
- Pérez-Izquierdo, C.; Serrano-Pérez, P.; Rodríguez-Molina, C. Chemical composition, antifungal and phytotoxic activities of Cistus ladanifer L. essential oil and hydrolate. Biocatal. Agric. Biotechnol. 2022, 45, 102527. [Google Scholar] [CrossRef]
- Pérez-Izquierdo, C.; Buesco, M.J.J.; Rodríguez-Molina, M.C.; Pulido, F. Spatial variation in yield, chemical composition, and phytotoxic activity of Cistus ladanifer essential oils. Chem. Biodivers. 2023, 20, e20230095. [Google Scholar] [CrossRef] [PubMed]
- Chaloupková, V.; Mediavilla, I.; Bados, R.; Houdková, M.; Rondevaldová, J.; Esteban, L.S. Annual variation in yield, chemical composition, antioxidant and antibacterial activity of rockrose (Cistus ladanifer L.) essential oils. Biocatal. Agric. Biotechnol. 2024, 60, 103279. [Google Scholar] [CrossRef]
- Pérez-Izquierdo, C.; Buesco, M.J.J.; Serrano-Pérez, P.; Rodríguez-Molina, M.C.; Pulido, F. Unravelling the impact of plant ontogenic factors on the content and phytotoxic potential of Cistus ladanifer L. (rockrose) essential oils. Sci. Hort. 2024, 331, 113127. [Google Scholar] [CrossRef]
- Perez-Izquierdo, C.; Serrano-Pérez, P.; Osuna, M.D.; Rodríguez-Molina, M.C. Influence of the phenological stage of Cistus ladanifer L. on the bioherbicidal potential of its essential oil. Rev. Ciênc. Agrar. 2024, 47, 156–160. [Google Scholar]
- Rincón, J.; de Lucas, A.; Gracia, I. Isolation of rock rose essential oil using supercritical CO2 extraction. Sep. Sci. Technol. 2000, 35, 2745–2763. [Google Scholar] [CrossRef]
- Benali, T.; Laghmari, M.; Touhtouh, J.; Aanniz, T.; Lemhadri, A.; Daoudi, M.D.; Bouyahya, A.; Lee, L.-H.; Ullah, R.; Alotaibi, A.; et al. Chemical composition and bioactivity of essential oils from Cistus ladanifer L., Pistacia lentiscus L., and Matricaria chamomilla L. Biochem. Syst. Ecol. 2024, 116, 104880. [Google Scholar] [CrossRef]
- Garcia-Martin, D.; Garcia-Vallejo, C. Contribution à la connaissance de l’huile essentielle de Cistus ladaniferus L. var. maculatus Dun “ciste commun” (jara) d’Espagne. Parfum. Cosmét. Savons 1969, 12, 283–290. [Google Scholar]
- Simon-Fuentes, A.; Sendra, J.M.; Cuñat, P. Neutral volatiles of Cistus ladaniferus L. essential oil. An. Quim. 1987, 83, 201. [Google Scholar]
- Viciano-Tudela, S.; Sendra, S.; Parra, L.; Jimenez, J.M.; Lloret, J. Proposal of a gas sensor-based device for detecting adulteration in essential oil of Cistus ladanifer. Sustainability 2023, 15, 3357. [Google Scholar] [CrossRef]
- Viciano-Tudela, S.; Parra, L.; Navarro-Garcia, P.; Sendra, S.; Lloret, J. Proposal of a new system for essential oil classification based on low-cost gas sensor and machine learning techniques. Sensors 2023, 23, 5812. [Google Scholar] [CrossRef]
- Blasco, F.J.D.; Viciano-Tudela, S.; Parra, L.; Ahmad, A.; Chaloupková, V.; Bados, R.; Pascual, S.E.; Mediavilla, I.; Sendra, S.; Lloret, J. Employment of MQ gas sensors for the classification of Cistus ladanifer essential oils. Microchem. J. 2024, 206, 111585. [Google Scholar] [CrossRef]
- Carrión-Prieto, P.; Ramos, P.M.; Maria, T.M.R.; Hernández-Navarro, S.; Garrido-Laurnaga, F.; Eusébio, M.E.S.; Martin-Gil, J. Vibrational and termal of essential oils derived from Cistus ladanifer and Erica arborea shrubs. Nat. Prod. Commun. 2017, 12, 119–122. [Google Scholar]
- Mimaki, Y.; Ama, A.K.; Sashida, Y.; Miyata, Y.; Fujii, A. A novel hexahydrodibenzofuran derivative with potente inhibitory activity on melanin biosynthesis of cultured B-16 melanoma cells from Lindera umbellata bark. Chem. Pharmac. Bull. 1995, 43, 893–895. [Google Scholar] [CrossRef]
- Martin, D.G. Información sobre los productos de secrecion de la “jara pringosa” (C. ladaniferus L.): Aceite esencial. “gomo-resina” (ladano) y extractos diversos de la planta y del ladano. An. Inst. For. Investig. Exp. 1962, 34, 213–249. [Google Scholar]
- Peyron, L.; Alessandri, A. Huile essentielle de feuillage de Cistus ladaniferus L. cultivé en Corse. Parfum. Cosmét. Arômes 1986, 67, 59–67. [Google Scholar]
- Garnier, N.; Dodinet, E. An offering of Cistus (rock rose) found in a Carthaginian Tomb (6th–5th c. BC): An interdisciplinary approach combining archaeobotany and biomolecular archeology. Archeo Sci. 2013, 37, 51–66. [Google Scholar]
- Königs, R.; Gülz, P.-G. The monoterpenes in the essential leaf oil of Cistus ladanifer L. Z. Für Pflanzenphysiol. 1974, 72, 237–248. [Google Scholar] [CrossRef]
- Gülz, P.-G.; Kobold, U.; Michaelis, K.; Vostrowsky, O. The composition of terpene hydrocarbons in the essential oils from leaves of four Cistus species. Z. Für Naturforschung 1984, 39, 699–704. [Google Scholar] [CrossRef]
- Proksch, P.; Gülz, P.-G.; Budzikiewicz, H. Phenylpropanoic acid esters in the essential oil of Cistus ladanifer L. (Cistaceae). Z. Für Naturforschung 1980, 35, 201–203. [Google Scholar]
- Proksch, P.; Gülz, P.-G.; Budzikiewicz, H. Further oxygenated compounds in the essential oil of Cistus ladanifer L. (Cistaceae). Z. Für Naturforschung 1980, 3, 529–532. [Google Scholar] [CrossRef]
- Strack, D.; Proksch, V.; Gülz, P.-G. Reversed phase high performance liquid chromatography of essential oils. Z. Für Naturforschung 1980, 3, 675–681. [Google Scholar] [CrossRef]
- Barbosa, P.; Lima, A.S.; Vieira, P.; Dias, L.S.; Tinoco, M.T.; Barroso, J.G.; Pedro, L.G.; Figueiredo, A.C.; Mota, M. Nematicidal activity of essential oils and volatiles derived from Portuguese aromatic flora against the pinewood nematode, Bursapheelnchus xylophilus. J. Nematol. 2010, 42, 8–16. [Google Scholar] [PubMed]
- Guimarães, R.; Sousa, M.J.; Ferreira, I.C.F.R. Contribution of essential oils and phenolics to the antioxidant properties of aromatic plants. Ind. Crops Prod. 2010, 32, 152–156. [Google Scholar] [CrossRef]
- Kotba, I.; Bouaichi, A.; Lougraimzi, H.; Habbadi, K.; Errifi, A.; Touchami, A.O.; Douira, A.; Achbani, E.H. Effect of temperature, pH and essential oils on the mycelial growth of Rhizoctonia solani Kühn (Cantharellales: Ceratobasidiaceae) isolates. J. Microbiol. Biotechnol. Food Sci. 2020, 10, 461–466. [Google Scholar] [CrossRef]
- Thoma, J.L.; Cantrell, C.L.; Zheljazkov, V.D. Effects of essential oil fumigation on potato sprouting at room-temperature storage. Plants 2022, 11, 3109. [Google Scholar] [CrossRef]
- Miguel, M.G. Antioxidant and anti-inflammatory activities of essential oils: A short review. Molecules 2010, 15, 9252. [Google Scholar] [CrossRef]
- El Kharraf, S.; El-Guendouz, S.; Farah, A.; Mateus, M.C.; El Hadrami, E.M.; Miguel, M.G. Impact of fifteen combinations of the main components of rosemary, lavander and citrus essential oils on in vitro biological activities. S. Afr. J. Bot. 2023, 156, 162–168. [Google Scholar] [CrossRef]
- Brindhadevi, K.; Elesawy, B.H.; Elfasakhany, A.; Badruddin, I.A.; Kamangar, S. Wound dressings coated with silver nanoparticles and essential oil of labdanum. Appl. Nanosci. 2023, 13, 1345–1354. [Google Scholar] [CrossRef]
- Chaves, N.; Escudero, J.C. Allelopathic effect of Cistus ladanifer on seed germination. Funct. Ecol. 1997, 11, 432–440. [Google Scholar] [CrossRef]
- Chaves, N.; Sosa, T.; Escudero, J.C. Plant growth inhibiting flavonoids in exudate of Cistus ladanifer and in associated soils. J. Chem. Ecol. 2001, 27, 623–631. [Google Scholar] [CrossRef]
- Sosa, T.; Valares, C.; Aliás, J.C.; Lobón, N.C. Persistence of flavonoids in Cistus ladanifer soils. Plant Soil 2010, 337, 51–63. [Google Scholar] [CrossRef]
- Sosa, T.; Aliás, J.C.; Escudero, J.C.; Chaves, N. Interpopulational variation in the flavonoid composition of Cistus ladanifer L. exudate. Biochem. Syst. Ecol. 2005, 33, 353–364. [Google Scholar] [CrossRef]
- Chaves, N.; Sosa, T.; Valares, C.; Alías, J.C. Routes of incorporation of phytotoxic compounds of Cistus ladanifer L. into soil. Allelopath. J. 2015, 36, 25–36. [Google Scholar]
- Chaves, N.; Sosa, T.; Alías, J.C.; Escudero, J.C. Identification and effects of interaction phytotoxic compounds from exudate of Cistus ladanifer leaves. J. Chem. Ecol. 2001, 27, 611–621. [Google Scholar] [CrossRef]
- Han, C.; Shao, H.; Zhou, S.; Mei, Y.; Cheng, Z.; Huang, L.; Lv, G. Chemical composition and phytotoxicity of essential oil from invasive plant, Ambrosia artemisiifolia L. Ecotoxicol. Environ. Saf. 2021, 211, 111879. [Google Scholar] [CrossRef]
- Alves-Ferreira, J.; Miranda, I.; Duarte, L.C.; Roseiro, L.B.; Lourenço, A.; Quilhó, T.; Cardoso, S.; Fernandes, M.C.; Carvalheiro, F.; Pereira, H. Cistus ladanifer as a source of chemicals: Structural and chemical characterization. Biomass Convers. Biorefin. 2020, 10, 325–337. [Google Scholar] [CrossRef]
- Alves-Ferreira, J.; Lourenço, A.; Morgado, F.; Duarte, L.C.; Roseiro, L.B.; Fernandes, M.C.; Pereira, H.; Carvalheiro, F. Delignification of Cistus ladanifer biomass by organosolv and alkali processes. Energies 2021, 14, 1127. [Google Scholar] [CrossRef]
- Alves-Ferreira, J.; Duarte, L.C.; Lourenço, A.; Roseiro, L.B.; Fernandes, M.C.; Pereira, H.; Carvalheiro, F. Distillery residues from Cistus ladanifer (rockrose) as feedstock for the production of added-value phenolic compounds and hemicellulosic oligosaccharides. BioEnergy Res. 2019, 12, 347–358. [Google Scholar] [CrossRef]
- Alves-Ferreira, J.; Duarte, L.C.; Fernandes, M.C.; Pereira, H.; Carvalheiro, F. Hydrothermal treatments of Cistus ladanifer industrial residues obtained from essential oil distilleries. Waste Biomass Valoriz. 2019, 10, 1303–1310. [Google Scholar] [CrossRef]
- Tavares, C.S.; Martins, A.; Miguel, M.G.; Carvalheiro, F.; Duarte, L.C.; Gameiro, J.A.; Figueiredo, A.C.; Roseiro, L.B. Bioproducts from forest biomass II. Bioactive compounds from the steam-distillation by-products of Cupressus lusitanica Mill. and Cistus ladanifer L. wastes. Ind. Crops Prod. 2020, 158, 112991. [Google Scholar] [CrossRef]
- Sánchez-Vioque, R.; Polissiou, M.; Astraka, K.; de los Mozos-Pascual, M.; Tarantilis, P.; Herraiz-Peñaver, D.; Santana-Méridas, O. Polyphenol composition and antioxidant and metal chelating activities of the solid residues from the essential oil industry. Ind. Crops Prod. 2013, 49, 150–159. [Google Scholar] [CrossRef]
- Alves-Ferreira, J.; Carvalheiro, F.; Duarte, L.C.; Ferreira, A.R.P.; Martinez, A.; Pereira, H.; Fernandes, M.C. D-Lactic acid production from Cistus ladanifer residues: Co-fermentation of pentoses and hexoses by Escherichia coli JU15. Ind. Crops Prod. 2022, 177, 114519. [Google Scholar] [CrossRef]
- Álvaro, A.G.; Mediavilla, I.; Palomar, C.R.; Esteban, L.S.; Crespo, I.G. Energy valorization of solid residue from steam distillation of aromatic shrubs. Ind. Crops Prod. 2024, 222, 119485. [Google Scholar] [CrossRef]
- Köse, M.D.; Tekin, B.N.; Bayraktar, O. Simultaneous isolation and selective encapsulation of volatile compounds from essential oil during electrospraying of β-cyclodextrin. Carbohydr. Polym. 2021, 258, 117673. [Google Scholar] [CrossRef]

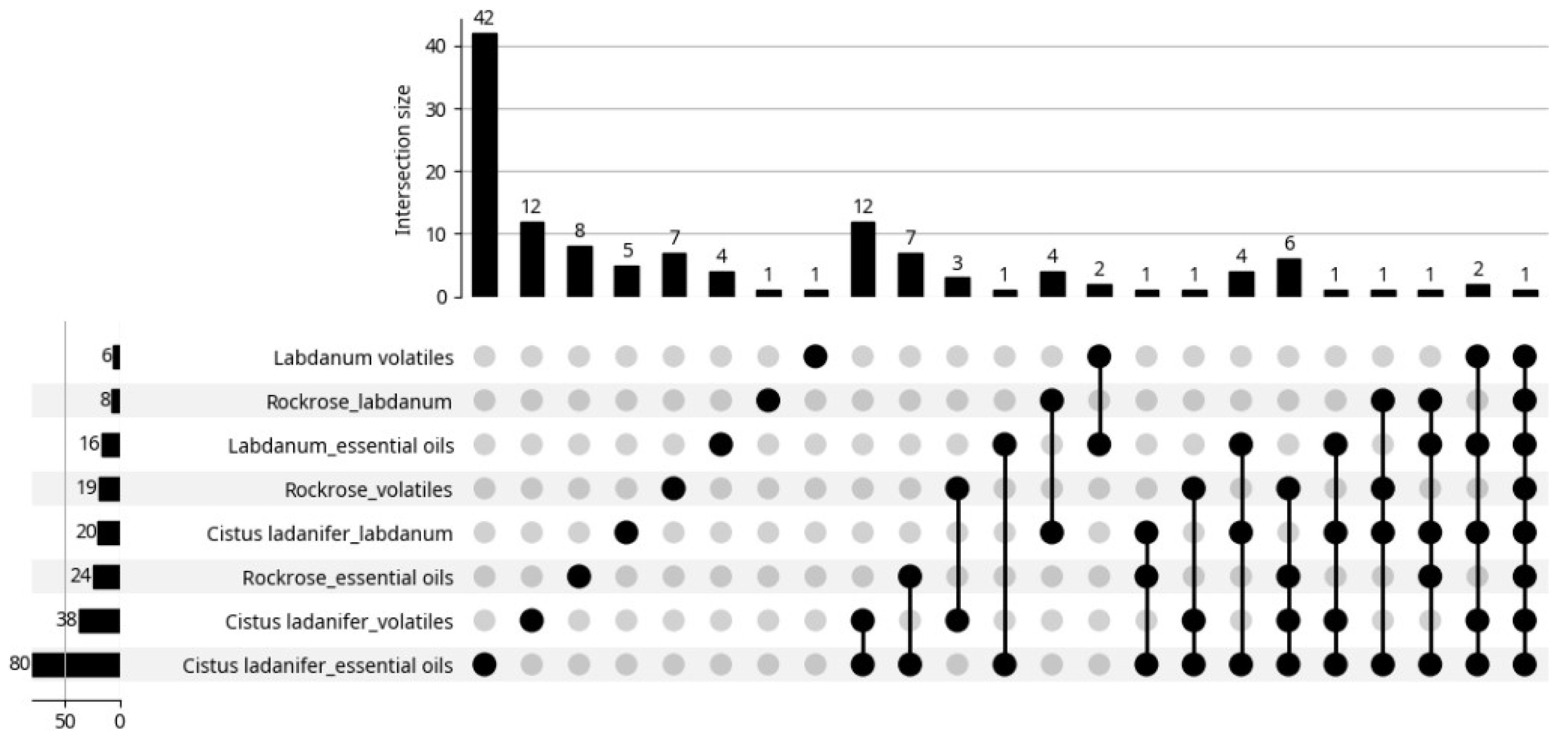
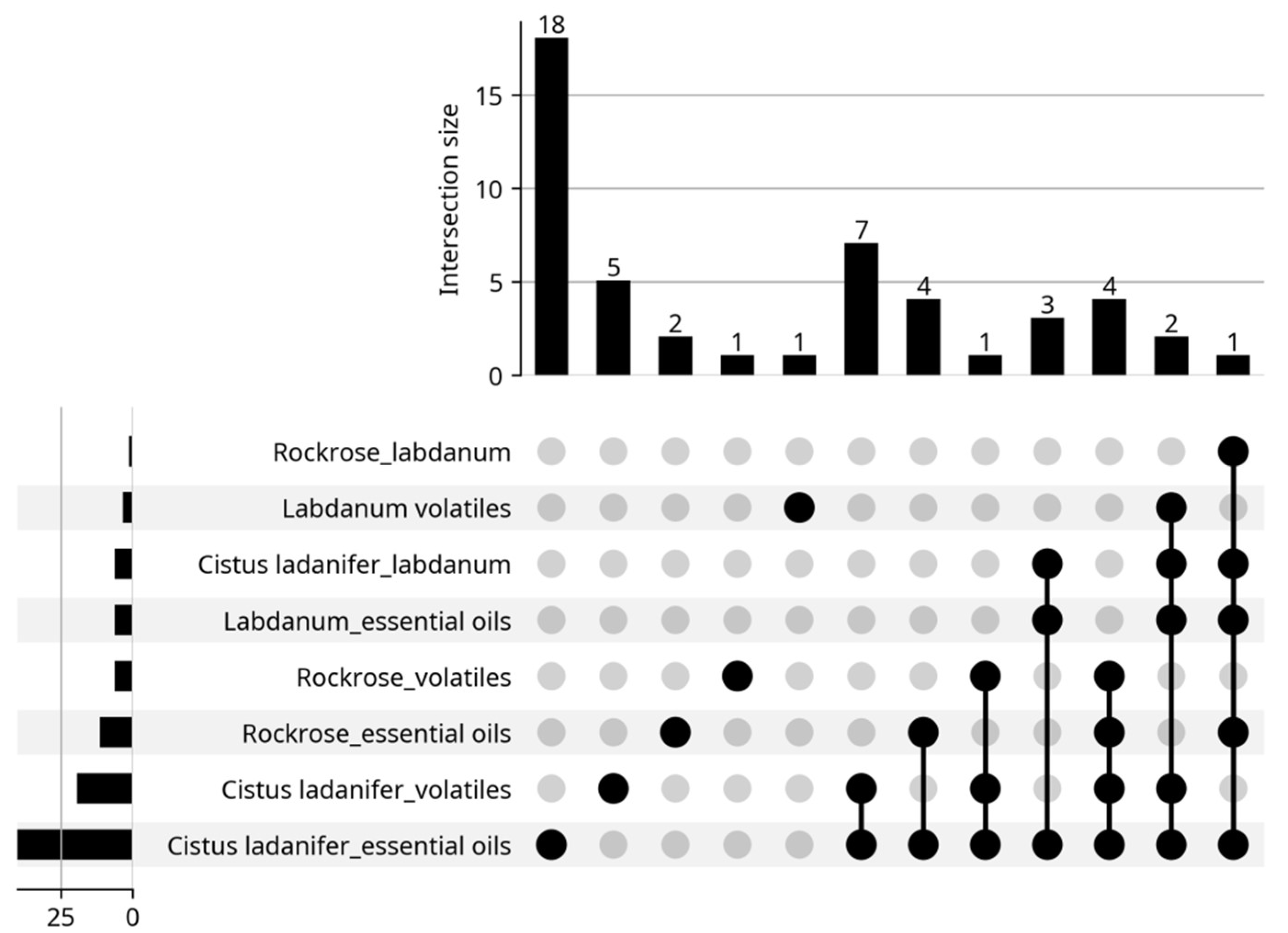
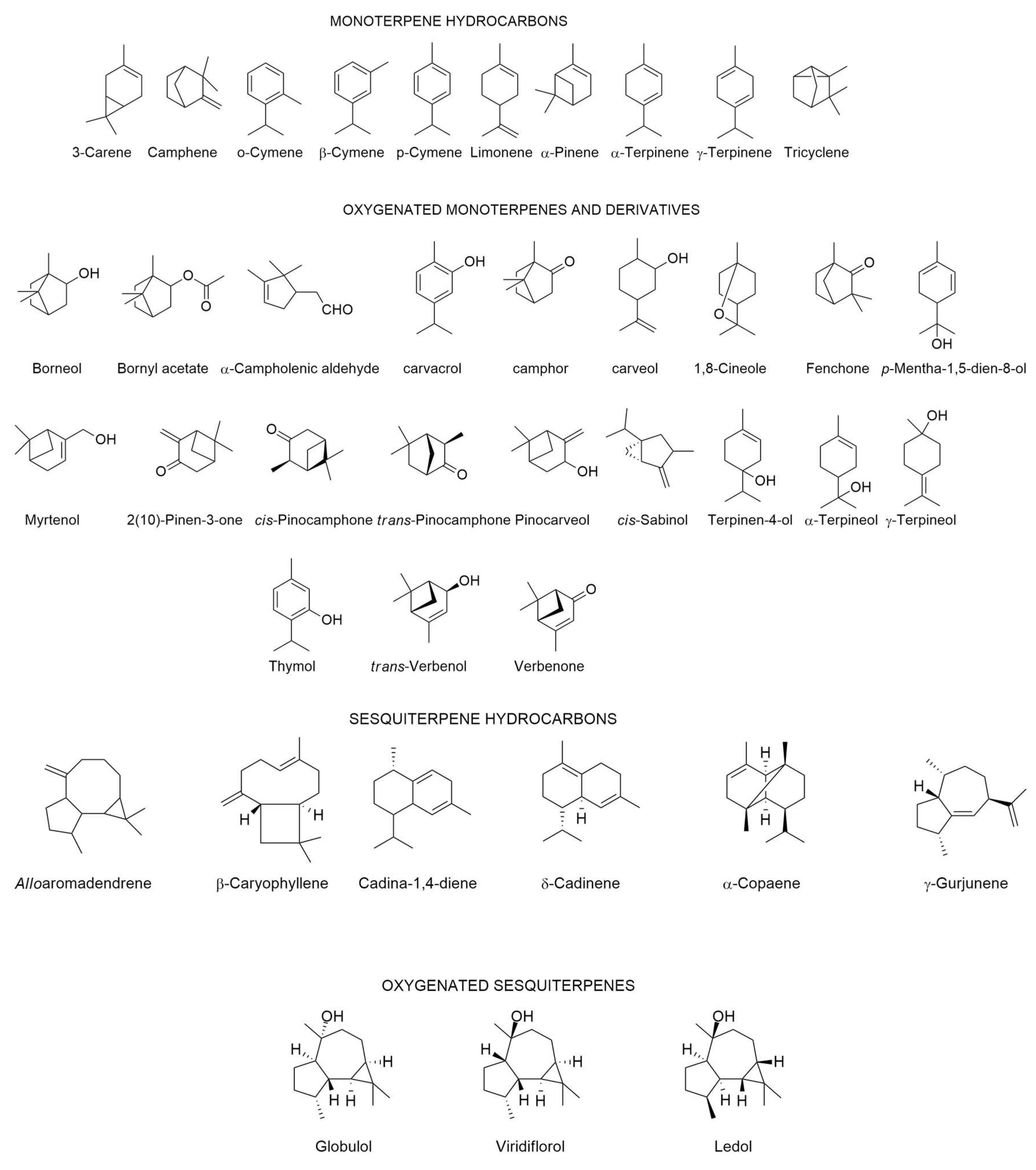
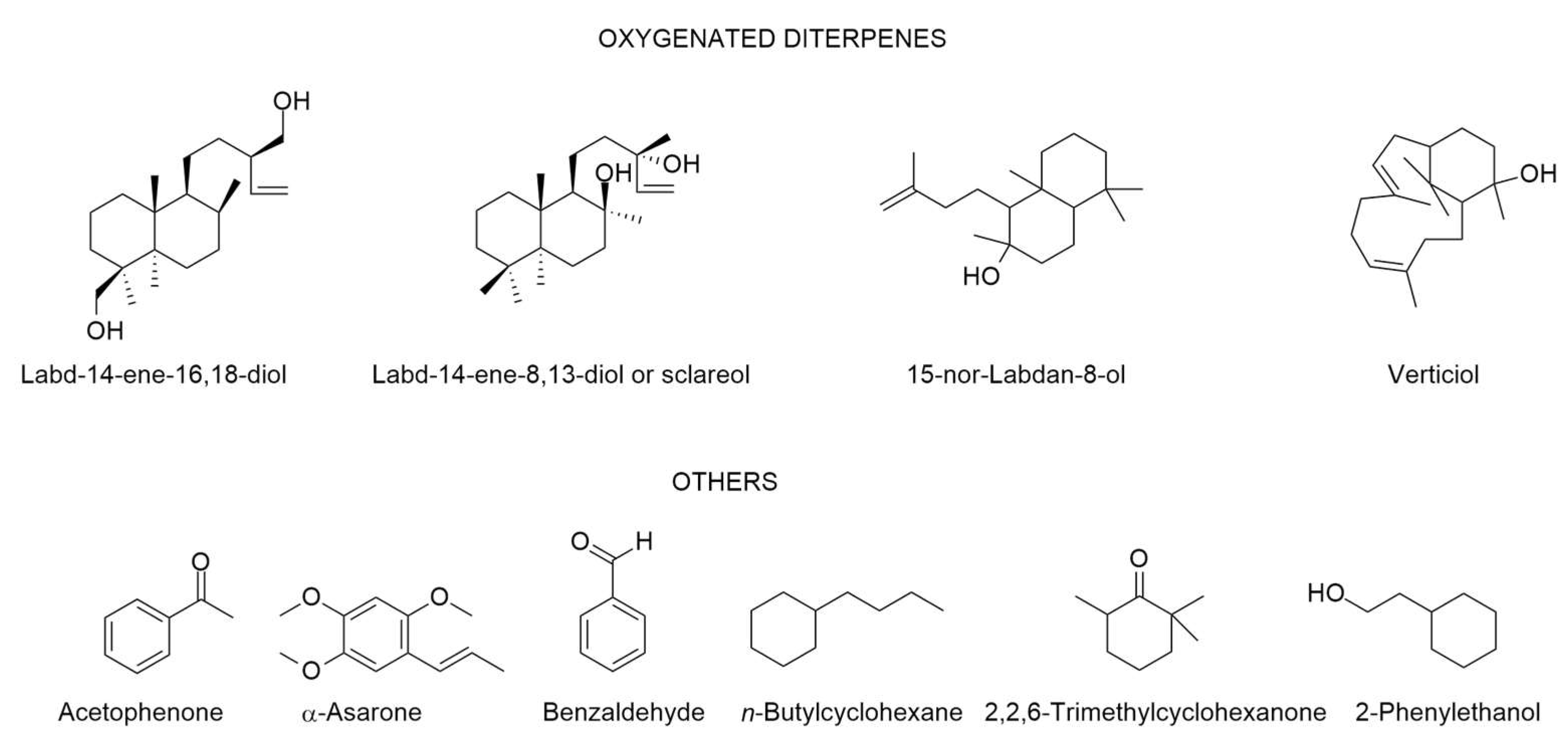




| Origin | Plant Part Used | Extraction Type/Identification/Yield | Main Identified Compounds (>5%) ● | Biological Properties | Reference |
|---|---|---|---|---|---|
| France Cultivated in France (Corsica) from Spanish plant origin | Leaves and stems | Hydro-distillation/GC-FID, silica gel flash chromatography and 13C-NMR/0.07% (bulk sample; from 0.16 to 0.41% fresh material (individual samples) | Bulk sample: α-pinene 39, viridiflorol 12 (identification by 13C-NMR and quantification by GC) Individual 20 samples (minimal–maximal): α-pinene 2–47, viridiflorol 5–23, trans-pinocarveol 4–11, camphene not detected–11, pinocarvone 1–5, bornyl acetate 1–5, borneol 1–5 | Not determined | [24] |
| France (Massif de l’Estérel, South of France) | Young shoots, leaves, and stems of. C. ladanifer var. maculatus and C. ladanifer var. albiflorus | Steam distillation in a modified Dean and Stark apparatus/GC-MS/0.119% (w/w) fresh material (var. albiflorus); 0.081% (w/w) fresh material (var. maculatus) | Mean (μg/μL) > 10: albiflorus: α-pinene 153, viridiflorol 72, 2,2,6-trimethylcyclohexanone 58, terpinen-4-ol 35, verbenone 34, trans-pinocarveol 33, borneol 27, α-campholenic aldehyde 27, ledol 19, camphene 19, benzaldehyde 14, carveol 14, myrtenol 13, limonene 12, p-cymene 12 maculatus: α-pinene 216, viridiflorol 57, 2,2,6-trimethylcyclohexanone 54, verbenone 41, terpinen-4-ol 32, trans-pinocarveol 31, camphene 28, borneol 27, α-campholenic aldehyde 27, ledol 16, limonene 13, carveol 13, α-terpinene 12, p-cymene 11, benzaldehyde 11, myrtenol 11 | Not determined | [25] |
| France (Corse) Commercial from Huiles Essentielles et Hydrolats de Corse | Unknown | GC-MS/not determined | α-pinene 47, globulol 6, camphene 5 | Antimicrobial activity - MIC % (v/v) (Campylobacter jejuni) 0.125 | [26] |
| India (commercial obtained from Meena Perfumery Kannauj, India) | Labdanum essential oil | GC-MS/not determined | α-asarone * 79, camphene 6 | Antimicrobial activity - MIC = 0.6 μL/mL (Aspergillus flavus strain AF-M-K5 isolated from Brassica juncea seeds) - Absolute suppression of aflatoxin B1 production by A. flavus AF-M-K5 at 0.5 μL/mL. - Percentage mycelial inhibition at 0.6 μL/mL = 100% for Alternaria alternata, A. humicola, A. tenuis; Aspergillus chevalieri, A. luchuensis, A. minutus, A. niger, A. repens, A. terreus, A. versicolor; Cladospoium cladosporioides, C. humicola; Curvularia lunata; Mucor sp.; Peniclium luteum, P. purpuroger; Rhizopus stolonifer; Terbicularia sp. - Percentage inhibition of ergosterol content at 0.1, 0.2, 0.3, 0.4, 0.5 μL/mL: 4, 16, 19, 28.5, 84,52, respectively. - Decrease in methylglyoxal content with increasing concentration of EO: 0.6 and 1.0 μL/mL = 505.42 and 440.83 μM/g fresh weight. Antioxidant activity - IC50 = 7.3 μL/mL and 1.13 μL/mL for the DPPH and ABTS methods, respectively. - Phytotoxicity 100% germination and without reduction in the length of radical and plumule of seedlings: Brassica juncea (var. Varuna and Kranti), Arachis hypogaea (var. Chandra and Kaushal), Sesamum indicum (var. T-12 and Shekhar), Helianthus annuus (var. Modern and Surya), Brassica campestris (var. Pusa swarnim) fumigated with EO showed. | [27] |
| Morocco (Chefchaouen northern Morocco) | Leaves | Extraction with hexane (Soxhlet apparatus)/hydro-distillation/GC-FID/GC-MS/0.18% fresh material weigh | pinocarveol 8, viridiflorol 7, bornyl acetate 6 | Not determined | [28] |
| Morocco (Tanger, North Morocco) | Leaves and small branches | Hydro-distillation/GC-FID, GC-MS/0.3–0.4% (v/w) fresh material Resinoid obtained from the gum of labdanum using ethanol/2.9% of the dried material; 3.1% of the resinoid was the volatile fraction Concrete: extraction of dried material (17% moisture) with hexane and concentration under vacuum/5.0% from the dried material; 4.2% was the volatile fraction Absolute: obtained from the concrete using absolute ethanol with moderate heating followed by one night at −18 °C and the waxes eliminated by cold filtration/4.5% from the dried material; 4.6% was the volatile fraction | viridiflorol 19, bornyl acetate 17, camphene 12, ledol 8, α-pinene 5 labd-14-ene-16,18-diol 8, labd-14-ene-8,13-diol 5 pentyltricontane 18, labda-8,14-dienoic acid 10, labdanoic acid# 9, labda-7,8-dienoic acid 7, labd-14-ene-16,18-diol 7, labda-8,20-dienoic acid 5, 16-kaurene 5, labda-8(20),13(16),14-triene 5, octadecane 5 labdenoic acid # 13, labdanoic acid # 9, labd-14-ene-8,13-diol 7, labd-14-ene-16,18-diol 6 | Not determined | [29] |
| Morocco (Chefchaouen region (NW Morocco | Leaves | Hydro-distillation/GC-FID, GC-MS/1.4% (v/w) | 1,8-cineole 19, viridiflorol 16, γ-terpinene 6 | Not determined | [30] |
| Morocco (Tafoughalt, Eastern of Morocco) | Leaves | Hydro-distillation/GC-MS/0.14% (w/w) dried material | camphene 16, 2,2,6-trimethylcyclohexanone 7, borneol 11, terpinen-4-ol 6, δ-cadinene 6, | Antioxidant activity - Percentage of inhibition DPPH at 1–15 mg/mL: <60 and <80 (data from graphic) | [31] |
| Morocco (Oulmes) | Leaves | Hydro-distillation/GC/MS/0.1–0.2% (v/w) fresh material | camphene 18, verticiol 18, γ-gurjunene 7, n-butylcyclohexane § 6, bornyl acetate 6, 3-carene 5, cis-sabinol 5 | Antimicrobial activity - MIC–MBC (mg/mL) Escherichia coli 25–25, Pseudomonas aeruginosa 25–25, Staphylococcus epidermidis 12.5–12.5, Staphylococcus aureus 6.25–6.25 Antifungal activity - MIC–MFC (mg/mL) A niger 0.001–0.001, Trichophyton rubrum 0.01–0.01, C. albicans 0.001–0.001 | [32] |
| Morocco (Taza region) | Aerial parts | Hydro-distillation/GC-MS/not determined | viridiflorol 29, γ-gurjunene 15, cadina-1,4-diene 6, borneol 5 | Antimicrobial activity - MIC (mg/mL) for Clavibacter michiganensis subsp. michiganensis: 0.78, Pseudomonas savastanoi pv. savastanoi: 0.57 | [33] |
| Morocco (El Harcha forest, province of Khemisset, 150 km south-east of Rabat) | Unknown | Hydro-distillation/GC-FID/GC-MS/0.21% (v/w) dried material | viridiflorol 18, trans-pinocarveol 11, bornyl acetate 9, ledol 9, p-cymene 6, borneol 5, sclareol 5 | Antimicrobial activity - MIC and MBC = 10 µL/mL for S. aureus, Acinetobacter baumannii, E. coli, Salmonella typhi Antifungal activity - MIC–MFC (µL/mL): C. albicans: 32–32; C. tropicalis: 64–64; C. glabrata: 32–32; C. dubliniensis: 32–32; Candida sp.: 16–62; R. rubra: 32–32; C. neoformans: 64–64; Penicillium sp.: 64–64; Fusarium sp.: 64–64; A. niger: 32–32 | [34] |
| Morocco (Aknoul region) | Aerial parts | Microwave-assisted hydro-distillation/GC-MS/4.15% (v/w) | γ-terpinene 18, linderol * (C10H18O) 18, borneol 14, carvacrol 8, camphene 7, caryophyllene 7, β-cymene 5 | Antioxidant activity - IC50, µg/mL: DPPH 178.29; ABTS 134.02; FRAP 321.71; β-carotene 246.14 Antimicrobial activity - MIC–MFC (%, v/v): C. albicans 1–2; C. tropicalis 8–16 - MIC–MBC (%, v/v); Listeria innocua 0.25–0.5; E. coli O157:H7 4–4; Proteus mirabilis 0.25–0.25 Inhibition of α-amylase - IC50 = 0.41 mg/mL Inhibition of α-glucosidase IC50 = 0.49 mg/mL Inhibition of pancreatic lipase IC50 = 0.004 mg/mL | [35] |
| Portugal (Douro region) | Leaves | Water followed by dichloromethane extraction/GC-MS-FID; NMR for 2,2,6-trimethylcyclohexanone/not determined | acetophenone 29, 2-phenylethanol 12, 2,2,6-trimethylcyclohexanone 12, borneol 8 | Not determined | [36] |
| Portugal (wild in the mountains of the center-interior; cultivated in the north after propagation from wild plant found in the South of Portugal | Leaves and small branches | Hydro-distillation/GC-MS, GC-FID/0.2–0.3% dried material | Wild (fresh material): viridiflorol 15, α-pinene 5; dried material: viridiflorol 17, C15H26O sesquiterpene alcohol 6, globulol 5 Cultivated (fresh material): viridiflorol 15; dried material: viridiflorol 14, 15-nor-labdan-8-ol 5 | Not determined | [37] |
| Portugal (Mirandela, Northern Portugal) | Leaves | Simultaneous distillation–extraction (SDE) (Likens-Nickerson): extraction solvent (pentane); extraction time (60 min)/headspace solid-phase microextraction (HS-SPME) for 60 min at 40 °C and with 20% NaCl using 85 µm polyacrylate fiber/GC-FID)/not determined | mg/g (>3%): α-pinene 22, 2,2,6-trimethylcyclohexanone 6, bornyl acetate 4, borneol 3 | Not determined | [38] |
| Portugal (Bragança region, northeast Portugal) | Branches, in the floral stage | Hydro-distillation/GC-FID, GC-MS/not determined | 2,2,6-trimethylcyclohexanone 30, viridiflorol 7 | Not determined | [39] |
| Portugal (Universidade de Évora, Évora) | Leaves | Hydro-distillation/GC-FID, 13C-NMR/not determined | α-pinene 36, camphene 12, fenchone 9, bornyl acetate 9, viridiflorol 8 | Antimicrobial activity - Inhibition zone [mm] S. aureus ATCC 25923 (11), Bacillus subtilis ATCC 6633 (11), E. coli ATCC 25922 (10), P. aeruginosa ATCC 27853 (9), S. aureus 42/60 (23), E. coli SA/4 (20) and S. pneumoniae ATCC 49619 (38) | [40] |
| Portugal (Mértola) | Obtained commercially from Herdade de Vale Covo; aerial parts particularly leaves | Steam distillation/GC-FID/GC-MS/not determined | α-pinene 39, camphene 8, trans-pinocarveol 6, bornyl acetate 5 | Antioxidant activity - DPPH IC50 (%) = 0.9 - β-carotene bleaching IC50 (%) = 0.48 Antimicrobial activity - MIC (μL/mL) S. aureus ATCC 25923 (16), L. monocytogenes LMG 16779 (8), E. faecalis ATCC 29212 (8), B. cereus ATCC 11778 (2), E. coli ATCC 25922 (32), S. Typhimurium ATCC 13311 (32), P. aeruginosa ATCC 27853 (32) | [41] |
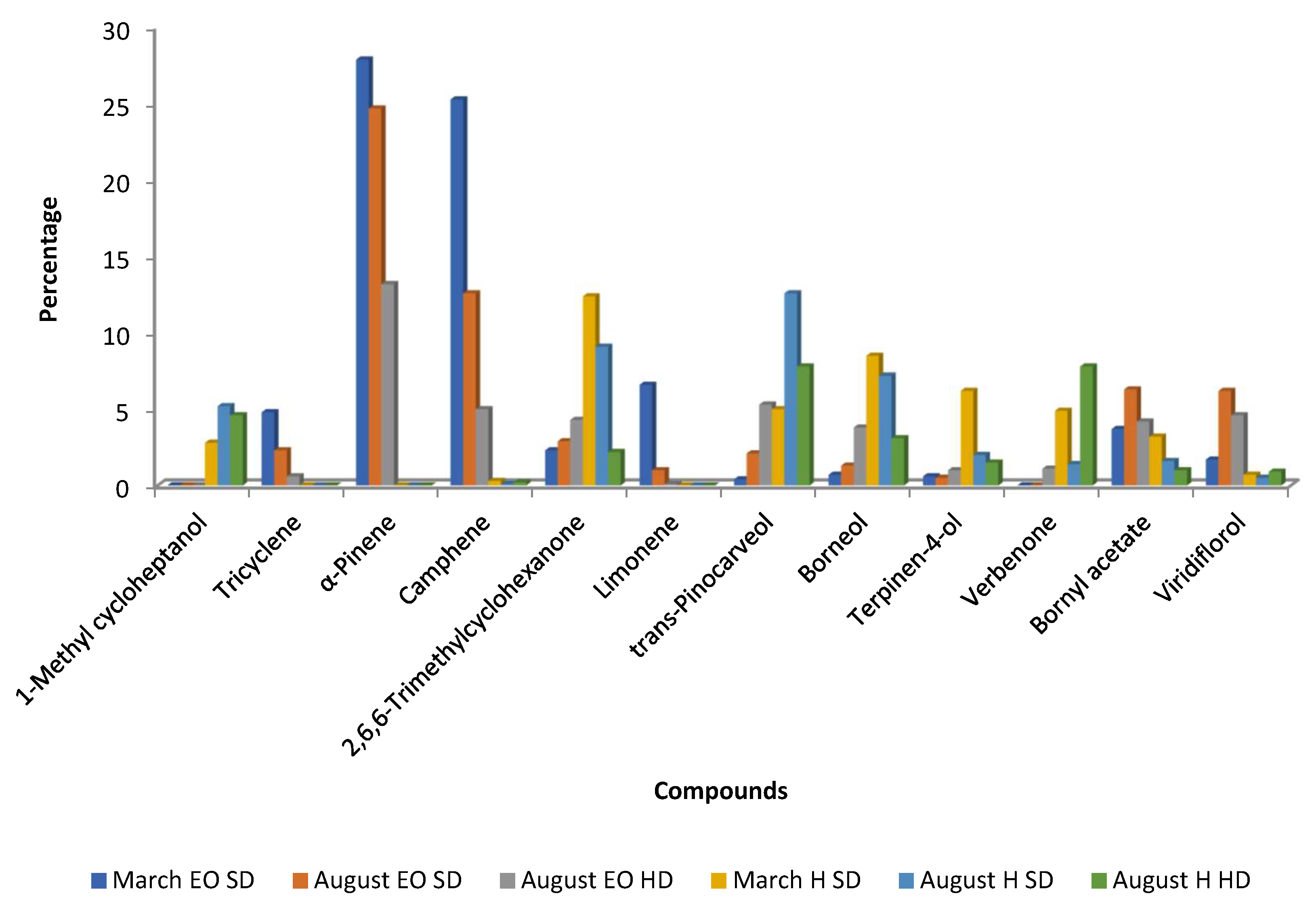
| Portugal (Beira Baixa) | Aerial parts of waste samples resulting from forest landscaping by Silvapor, Ambiente e Inovacao | Steam distillation using a stainless-steel distiller (1100 L, Vieirinox®, Aveiro, Portugal)/GC-FID, GC-MS/0.01% (March)–0.04% (v/w) (August) | March–August: α-pinene 28–25, camphene 25–13, limonene 7–1, tricyclene 5–2, bornyl acetate 4–6, viridiflorol 2–6 | Antimicrobial activity - Diameter of the inhibition zone (mm): March–August Escherichia coli DSM 1077 9.3–12.0; Staphylococcus aureus ATCC 6538 8.5–9.8; Candida albicans 8.8–9.0 Antioxidant activity March–August - Percentage of inhibition at 30 µL (ABTS) 68.1–69.7 - Percentage of inhibition at 50 µL (Xanthine oxidase) 9.9–98.5 | [42] |
| Aerial parts of waste samples resulting from forest landscaping by Silvapor, Ambiente e Inovacao | Hydro-distillation/GC-FID, GC-MS/0.15% (v/w) (August) | August: α-pinene 13, camphene 5, trans-pinocarveol 5, viridiflorol 5 | Not determined | ||
| Portugal (central-west region of Portugal) | Flowers, leaves, and stems (commercially purchased from Aromas do Valado, Portugal) | Steam distillation/GC-MS/not determined | α-pinene 36, camphene 7, 2,2,6-trimethylcyclohexanone 7, o-cymene 5, bornyl acetate 5 | Ecotoxicity Non-toxic because EC50 is >100 mg/L) | [43] |
| Portugal | Leaves, flowers, and thin branches, acquired from Proentia® company (Portugal) | Steam distillation/GC-FID, GC-MS/not determined | α-pinene 50, camphene 10 | Antioxidant activity - DPPH: IC50%, v/v) 0.691 In vitro cytotoxicity - Fibroblasts L929 IC50 (%, v/v) 0.027 Macrophages (RAW 264.7) IC50 (%, v/v) 0.012 NO production EC50 (%, v/v) 0.002 Antimicrobial activity - MIC-MLC (minimum lethal concentration) (%, v/v) S. aureus 1–2, Staphylococcus epidermidis 0.06–1, E. coli >2–>2, P. aeruginosa 2–2 Cutibacterium acnes 0.25–0.5 Antifungal activity - MIC–MLC (%, v/v) C. albicans 1–>2, Aspergillus brasiliensis 0.5–>2 C. acnes biofilm formation: Remarkable effect in inhibiting biofilm formation at all tested concentrations (1/4, ½, 1, 2 MIC) C. acnes biofilm disruption: Effect in inhibiting biofilm disruption at the concentrations (1/2, 1, 2 MIC) | [17] |
| Portugal | Leaves, flowers, and thin branches, acquired from Proentia® company (Portugal) | Steam distillation/GC-FID, GC-MS/not determined | α-pinene 50, camphene 10 | Antimicrobial activity - MIC–MBC (%, v/v) of C. acnes isolates of different phylotypes: 0.06–1.00 Half maximum effective concentration (EC50) for the effect on biofilm biomass—metabolic activity (%, v/v) of C. acnes isolates: 0.083–>0.938 | [44] |
| Spain | Leaves and stalks | Distillation/GC-FID, GC-MS/not determined Enantio-GC-MS using the stationary phase 2,3-diethyl-6-tert butyl-β-cyclodextrin | g/100 g: α-pinene 24, camphene 13, α-copaene 8, bornyl acetate 6, cis-pinocamphone 6, limonene 4, p-cymene 4, α-terpineol 4, (E)-caryophyllene 3, trans-pinocamphone 3 Enantiomeric ratio: β-pinene 7 (−):93 (+), sabinene 23 (−):77 (+), camphor 52 (−):48 (+), linalool (92):8 (+), terpinen-4-ol 22 (−):78 (+), α-terpineol 85 (−):15 (+) | Not determined | [45] |
| Spain (Guadarrama mountain range (San Lorenzo del Escorial | Aerial parts | Hydro-distillation/GC-FID, GC-MS/0.34% fresh material | trans-pinocarveol 20, viridiflorol 14, bornyl acetate 7, terpinen-4-ol 6, 2(10)-pinen-3-one 5, p-mentha-1,5-dien-8-ol 5, α-pinene 5 | Phytotoxicity - Weed seed germination percentages at 0.125–1 µL/mL: Amaranthus hybridus 0–0%, Portulaca oleracea 90.0–41.0%, Chenopodium album 23.0–33.0%, Conyza canadensis 1.0–1.0%, Parietaria judaica 1.0–1.0% - Seedling length (mm) at 0.125–1 µL/mL: A. hybridus (=no seedling length); C. canadensis 0.25–0.07; P. judaica 0.61–0.39 | [46] |
| Spain (mountainous area near Puertollano, Ciudad Real) | Aerial parts | HS-SPME using a 50/30 µm divinylbenzene (DVB)/polydimethylsiloxane (PDMS) fiber/GC-MS/not determined | α-pinene 17, bornyl acetate 15, camphene 13, 2,2,6-trimethylcyclohexanone 12, α-campholenal 5, trans-pinocarveol 5 | Not determined | [47] |
| Spain (commercial provided by FLORAR s.r.l. (Pisa, Italy) | Aerial parts | GC-MS/not determined | α-pinene 45, p-cymene 6, unknown 6, trans-verbenol 5, bornyl acetate 5 | Cytotoxicity - IC50 (ppm) for the different cell lines: human chronic myelogenous erythroleukemia (K562 = 46.9), human breast adenocarcinomas (MCF7 = 90, MDA-MB-231, T47D > 300), human neuroblastoma cell lines derived from a highly malignant tumor cells (SH-SY5Y = 92.8), endometrial stromal cells (ESCs = 264.7) | [48] |
| Spain (Andévalo in Huelva and Cerezal in Zamora) | Branches with a maximum stem diameter of 50 mm that included twigs, leaves, and fruits | Steam distillation/GC-MS/<0.1% (w/w) dried material | Andévalo–Cerezal: α-pinene 43–19, viridiflorol 13–24, ledol 4–7, bornyl acetate 4–5, camphene 2–7 | Antimicrobial activity - MIC–MBC (mg/mL) (Andévalo) Escherichia coli 0.6–0.6, Klebsiella pneumoniae >2.5–> 2.5, Morganella morganii 2.5–2.5, Proteus mirabilis >2.5–>2.5, Pseudomonas aeruginosa 2.5–> 2.5, Enterococcus faecalis 1.25–1.25, Listeria monocytogenes 0.6–0.6 - MIC-MBC (mg/mL) (Cerezal) E. coli 0.6-0.6, K. pneumoniae >2.5–> 2.5, M. morganii 0.6–0.6, P. mirabilis >2.5–>2.5, P. aeruginosa 2.5–> 2.5, Enterococcus faecalis 0.6–0.6, L. monocytogenes 0.3–0.3 Antioxidant activity - Reducing power assay (effective concentration at which the absorbance is 0.5 and achieving 50% of antioxidant potential) (EC50) (mg/mL) Andévao–Cerezal 1.64–1.30 Antioxidant activity in cell cultures - Cellular murine macrophage cell line (RAW 264.7) antioxidant activity in % oxidation inhibition at the maximum concentration of 2 mg/mL: Andévao–Cerezal: 83.24–81.13 Cytotoxicity - activity in GI50 (concentration of the extract causing 50% of cell growth inhibition (µg/mL): Andévalo–Cerezal NCI-H460 (lung carcinoma): 14.27–53.80, MCF-7 (breast carcinoma): 27.80–58.45, AGS (gastric carcinoma): 78.41–46.59, CaCo2 (colon carcinoma): 75.31–48.78, Two normal cell lines: PLP2 (porcine liver cells): 207.64–142.08, VERO (monkey kidney cells): 70.77–46.03 using the sulforhodamine B (SRB) assay - Anti-inflammatory activity Inhibition of the lipopolysaccharide (LPS)-induced NO (nitric oxide) production on RAW264.7 (IC50) (µg/mL): Andévalo–Cerezal 19.27–21.00 | [49] |
| Spain (Guadalajara, 1030 m above sea level)) | Aerial parts in bales at different storage times (1–120 days) | Steam distillation/GC-FID, GC-MS/0.075% (0–7 days of storage); 0.066% (15–30 days of storage); 0.052% (w/w) (100–120 days of storage) | - 0–7 days of storage: α-pinene 50, viridiflorol 10 - 15–30 days of storage: α-pinene 47, viridiflorol 12 - 100–120 days of storage: α-pinene 47, viridiflorol 13 | Not determined | [50] |
| Spain (central or northern locations) | Twigs | Steam distillation/GC-FID, GC-MS/0.036% (September 2018); 0.037% (w/w) (October 2019) | - September 2018–October 2019: α-pinene 52–39, viridiflorol 6–10 | Antioxidant activity - Oxygen radical absorbance capacity (ORAC) assay expressed as µmoL Trolox/g EO: 209.36 (October 2019); <200 (September 2018) | [51] |
| Spain (Extremadura) | Aerial parts | Hydro-distillation/GC-MS/0.349% (v/w) dried material | ledol 19, α-pinene 15, viridiflorol 7, bornyl acetate 5 | Antifungal activity - Effective dose 50 in logit analysis (ED50) µL/mL): Cryphhonectria parasitica 0.027, Fusarium oxysporum 0.033, Phytophthora cinnamomi 0.024, Rhizoctonia solani 0.017 Phytotoxicity - on Raphanus sativus (Germination index, GI = 1 µL dose) and Lupinus luteus seeds (GI = 4 µL dose) | [52] |
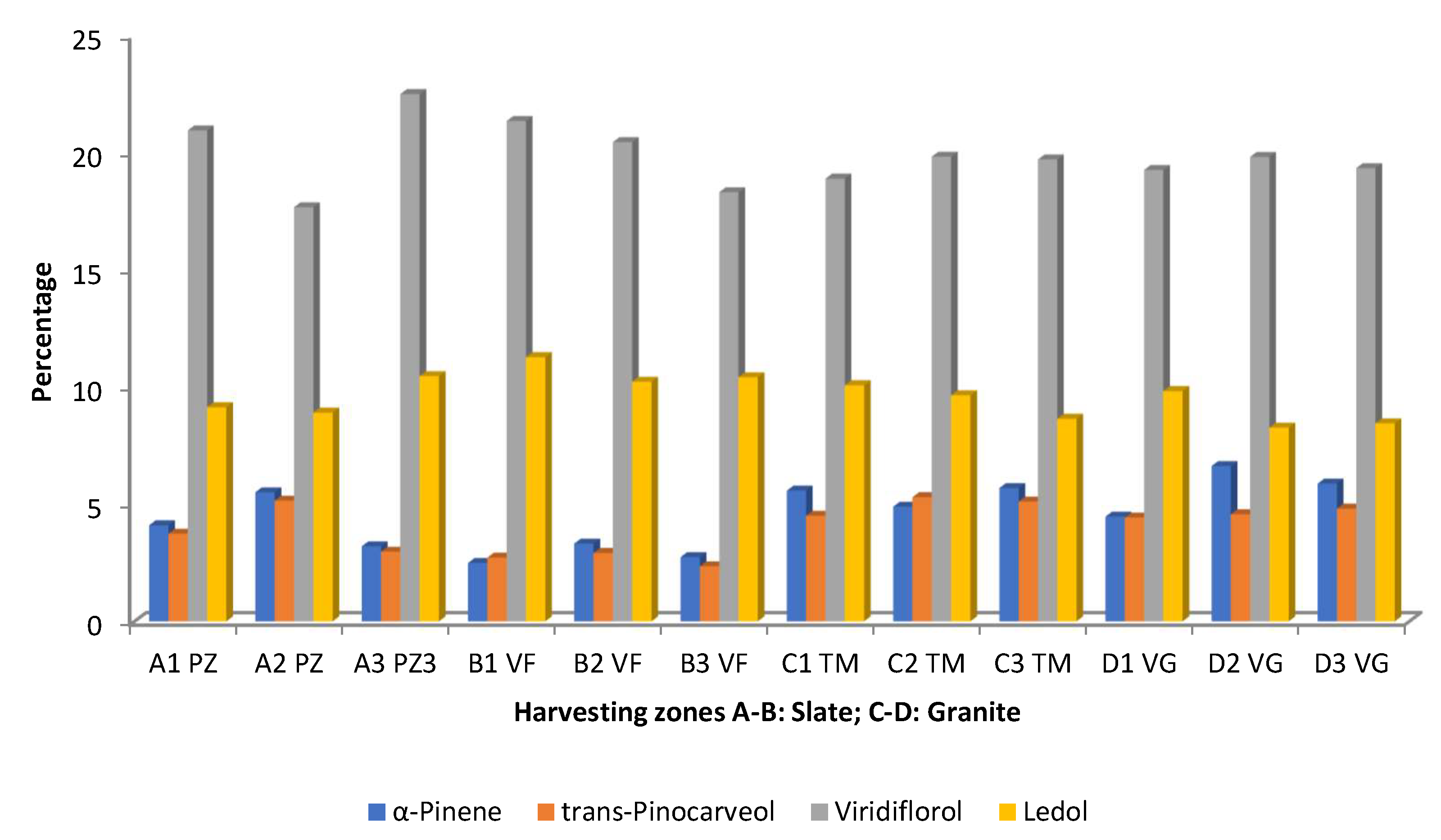
| Spain (Extremadura, different places) | Aerial parts | Hydro-distillation/GC-MS/0.19-0.42% (v/w) dried material | Pozuelo de Zarzón (slate) A1, A2, A3 viridiflorol 21, 18, 23; ledol 9, 9, 10; α-pinene 4, 6, 3; trans-pinocarveol 4, 5, 3 Valverde del Fresno (slate) B1, B23, B3 viridiflorol 21, 22, 21; ledol 11, 10, 10 Torre de Don Miguel (granite) C1, C2, C3 viridiflorol 18, 19, 20; ledol 10, 9, 9; α-pinene 6, 5, 6; trans-pinocarveol 5, 5, 5 Villasbuenas de Gata (granite) D1, D2, D3 viridiflorol 19, 20, 19; ledol 10, 8, 8; α-pinene 5, 7, 6; (E)-pinocarveol 4, 5, 5 | Phytotoxicity Effect on seed germination and seedling length of Raphanus sativus L. - Germination inhibition ED50 (µL/mL) A1 0.025, A2 0.044, A3 0.033, B1 0.033, B2 0.034, B3 0.028, C1 0.055, C2 0.059, C3 0.049, D1 0.040, D2 0.048, D3 0.051 - Seedling length inhibition ED50 (µL/mL): 0.017–0.053 | [53] |
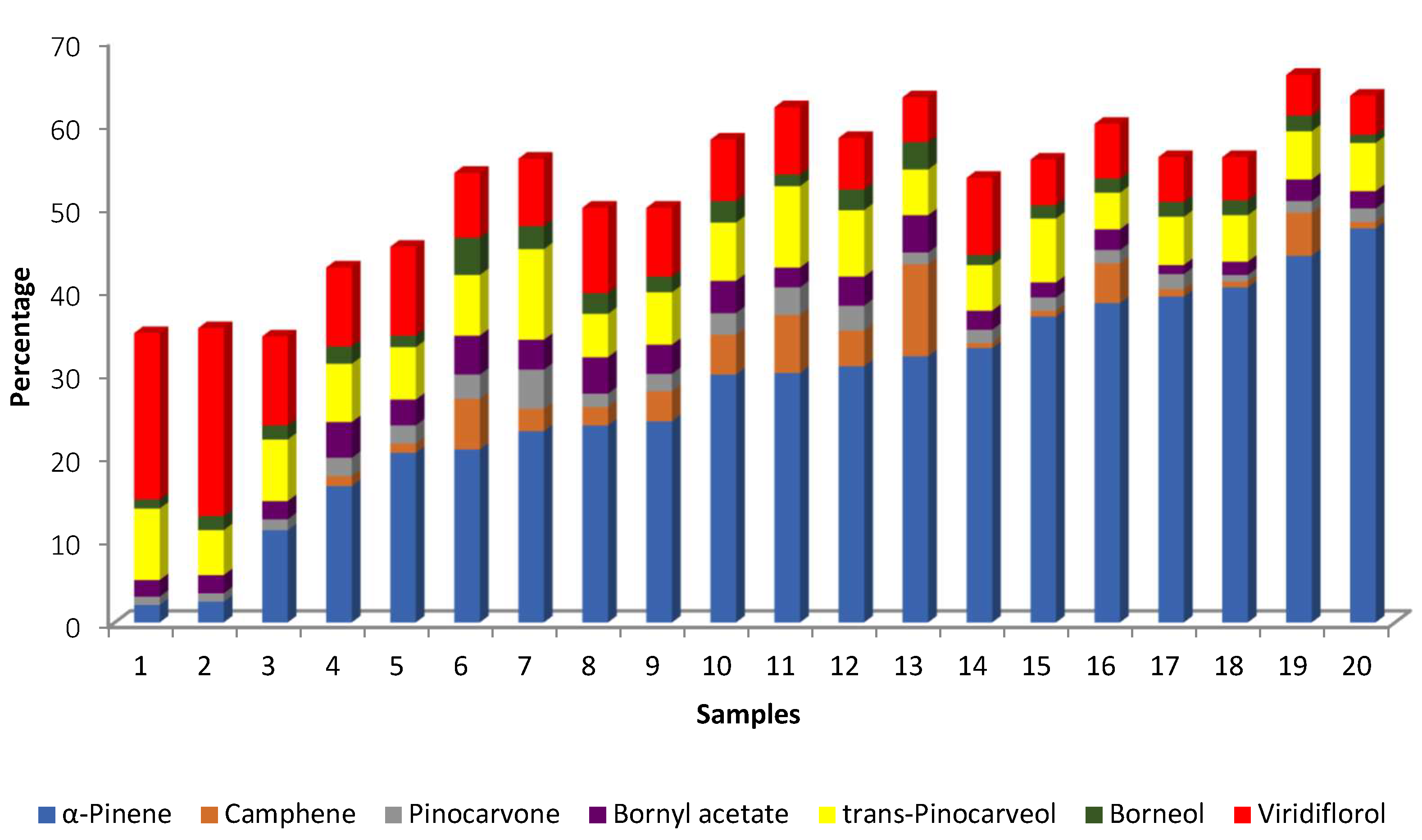
| Spain | Hydro-distillation/GC-FID/GC-MS/0.03–0.19% (7-year-old populations; 0.001–0.12% (w/w) (12-year-old populations) dried material | 7-year-old populations 2021 26/07, 17/08, 30/08, 13/09, 27/09, 18/10, 02/11, 15/11, 29/11, 13/12, 27/12 2022 17/01, 03/02, 28/02, 28/03, 24/04, 30/05, 27/06 α-pinene 43, 35, 45, 52, 58, 58, 61, 58, 60, 58, 50, 53, 50, 47, 50, 49, 39, 33 viridiflorol 4, 10, 6, 5, 4, 3, 3, 3, 3, 3, 4, 4, 4, 4, 4, 4, 4, 7 allo-aromadendrene + trans-pinocarveol 5, 6, 6, 4, 4, 4, 4, 4, 3, 4, 4, 4, 4, 5, 5, 4, 4, 4 bornyl acetate 4, 4, 3, 3, 3, 3, 2, 2, 2, 3, 3, 3, 3, 4, 2, 2, 4, 5 12-year-old populations 2021 26/07, 17/08, 30/08, 13/09, 27/09, 18/10, 02/11, 15/11, 29/11, 13/12, 27/12 2022 17/01, 03/02, 28/02, 28/03, 24/04, 30/05, 27/06 α-pinene 43, 16, 19, 55, 61, 52, 60, 58, 58, 59, 58, 56, 52, 44, 46, 44, 35, 27 viridiflorol 6, 17, 17, 5, 4, 4, 3, 3, 3, 4, 3, 4, 3, 5, 5, 6, 6, 13 allo-aromadendrene + trans-pinocarveol 5, 6, 6, 4, 3, 4, 3, 3, 3, 3, 3, 3, 3, 4, 4, 4, 4, 4 bornyl acetate 3, 6, 8, 3, 2, 3, 3, 3, 3, 3, 2, 2, 3, 3, 3, 3, 4, 5 | Antioxidant activity - ORAC method, IC50 µg/mL 117.47–>256.00 Antimicrobial activity against Bacillus cereus: - MIC Broth–MIC agar (µg/mL) 512–>1024 | [54] | |
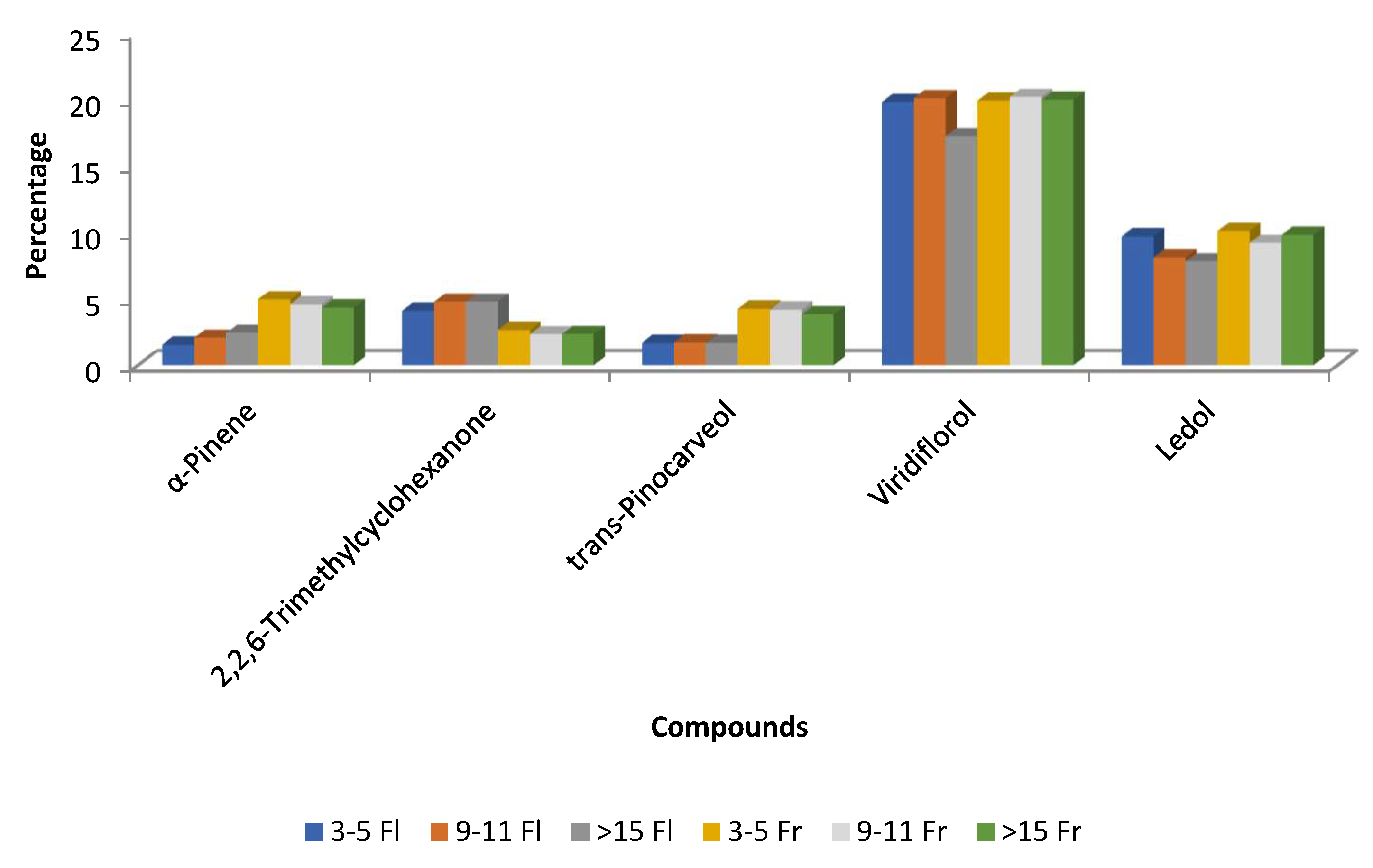
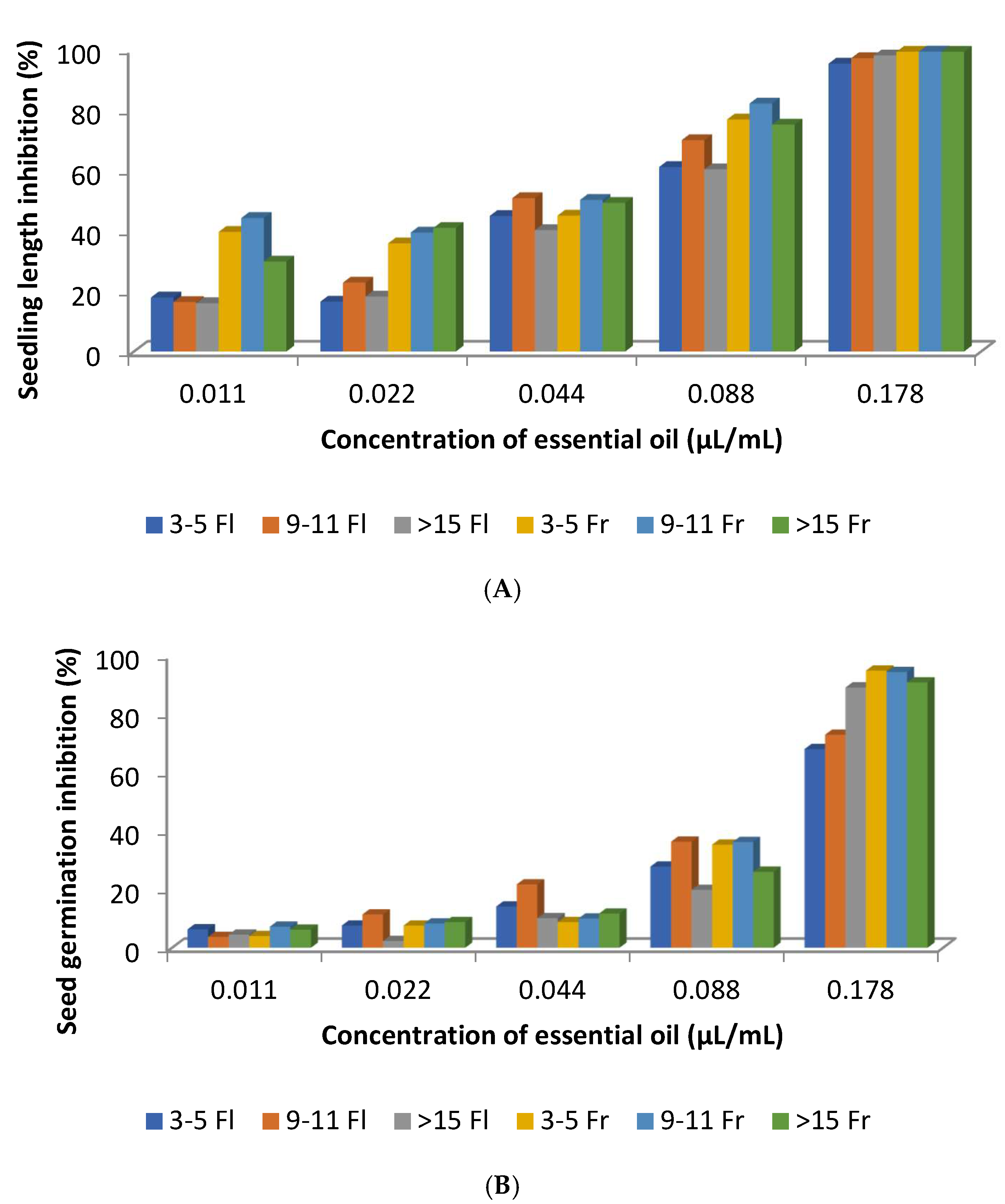
| Spain (Extremadura) | Aerial parts (stems, leaves, flowers, and fruits) | Hydro-distillation/GC-MS/0.17% (v/w) dried material (plants in flowering phase); 0.27% (v/w) dried material (plants in fruit maturation) | Plants in flowering phase of 3–5 years; 9–11 years; >15 years viridiflorol 20, 20, 17; ledol 10, 8, 8; 2,2,6-trimethylcyclohexanone 4, 5,5 Plants in fruit maturation of 3–5 years; 9–11 years; >15 years viridiflorol 20, 20, 20; ledol 10, 9, 10; α-pinene 5, 5, 4 | Phytotoxicity - Seedling length inhibition (%) of R. sativus for EO doses 0.011, 0.022, 0.044, 0.088, 0.178 µL/mL: 16.4–98.0 - Seed germination inhibition (%) of R. sativus for EO doses 0.011, 0.022, 0.044, 0.088, 0.178 µL/mL: 6.3–89.1 - Seedling length inhibition (%) of R. sativus for EO doses 0.011, 0.022, 0.044, 0.088, 0.178 µL/mL: 35.8–99.2 - Seed germination inhibition (%) of R. sativus for EO doses 0.011, 0.022, 0.044, 0.088, 0.178 µL/mL: 4.0–94.9 | [55] |
| Spain (Extremadura) | Aerial parts (stems, leaves, flowers, and fruits) | Hydro-distillation/GC-MS/not determined | August–October: Viridiflorol 20–16, α-pinene 3–13, ledol 9–7 | Germination index (GI) of seeds of rice and tomato (0%): 0.08 µL/mL GI of seeds of Echinochloa crus-galli (0%): 0.08 µL/mL | [56] |
| Spain (Ciudad Real) | Mature leaves nascent in the spring and exuding high levels of labdanum during the summer | Supercritical carbon dioxide (8–10 MPa and 30–60 °C/GC-FID/between 40 and 60 °C at a fixed pressure of 8 MPa; extraction yields decreased as temperature increased, but there was no significant change between 30 and 40 °C. Oil extraction yields rise as pressure increases at a constant temperature of 40 °C. Similar pattern at 30, 50, and 60 °C. The best conditions for obtaining the profile reported were temperature (T) = 40 °C; pressure (P) = 9 MPa; flow rate = 0.7 hg/h; diameter of particle = 0.30 mm | Extraction times (15, 30, 60, 90, 180 min): Terpenes: camphor 25, 23, 22, 22, 22; α-pinene 20, 20, 20, 20, 19; camphene 5, 4, 4, 4, 4; borneol 5, 5, 5, 4, 4; γ-terpineol 5, 5, 5, 5, 5; thymol 5, 4, 4, 4, 4. Waxes: nonacosane 21, heptacosane 14, tricosane 12, pentacosane 9, methyleicosane 7, entriacontane 7, triacontane 6, hexacosane 5 | Not determined | [57] |
| Unknown | Commercially provided by Lemondarome Co., Std. Aerial parts | Steam distillation/GC-MS-MS/not determined | camphene 32, α-pinene 16, bornyl acetate 16, tricyclene 7, 2,2,6-trimethylcyclohexanone 7 | Antioxidant activity - mg Trolox equivalent (TE)/g: DPPH, ABTS, Cuprac, FRAP: 6.82, 12.10, 57.20, 38.30 Antioxidant activity phosphomolybdenum (mmolTE)/g): 13.42 Antioxidant activity chelating activity (mg EDTA/g): 24.31 Antimicrobial activity - MIC (mg/mL). Proteus mirabilis 50, B. subtilis >50, S. aureus 50, C. albicans 12.5 Enzyme inhibitory activity - α-glucosidase (µg acarbose equivalent/g) 1.39; -α-amylase (µg acarbose equivalent/g) 1.07; tyrosinase (mg kojic acid equivalent/g) 10.22; butirylcolinesterase (mg galantamine equivalent/g) 2.45; acetylcholinesterase (mg galantamine equivalent/g) 4.71 | [58] |
| Origin | Plant Part Used | Extraction Type/Identification | Main Compounds (>5%) | Biological Properties | Reference |
|---|---|---|---|---|---|
| Portugal (Beira Baixa | Aerial parts of waste samples resulting from forest landscaping by Silvapor, Ambiente e Inovacao | Steam distillation using a stainless-steel distiller (1100 L, Vieirinox®, Aveiro, Portugal)/GC-FID, GC-MS Hydro-distillation/GC-FID, GC-MS | March–August: trans-pinocarveol 5–13, 2,6,6-trimethyl cyclohexanone 12–9, borneol 9–7, terpinen-4-ol 6–2, 1-methyl cycloheptanol § 3–5, verbenone 5–1, August: trans-Pinocarveol 8, verbenone 8, 1-methyl cycloheptanol § 5 | Antimicrobial activity -Diameter of the inhibition zone (mm): March–August; E. coli DSM: no activity; S. aureus ATCC 6538: no activity Antioxidant activity March–August: Percentage of inhibition at 30 µL (ABTS) 8.2–8.1; percentage of inhibition at 60 µL (superoxide) 14.3–13.4; percentage of inhibition at 50 µL (xanthine oxidase): 25.3–25.7; percentage of inhibition at 200 µL (chelating activity): 24.1–25.1 Anti-inflammatory activity Albumin denaturation assay: Percentage of inhibition at 1 mL hydrolate: 94% for both samples Not determined | [42] |
| Portugal (central-west region of Portugal) | Flowers, leaves and stems (commercial purchased to Aromas do Valado, Portugal) | Steam distillation/GC-MS | 4-hydroxy-3-methylacetophenone 22, p-cymen-8-ol 11, myrtenol 11, D-verbenone 10, endo-borneol 8 | Ecotoxicity: No observable effect on Daphnia magna after 48 h of exposure up to 2000 mg/L. | [43] |
| Spain (Extremadura) | Aerial parts | Hydro-distillation/GC-MS | trans-pinocarveol 11, 2,2,6-trimethylcyclohexanone 10, pinocarvone 6, ledol 5 | Antifungal activity Effective dose 50 in logit analysis (ED50) µL/mL): C. parasitica 141.9; F. oxysporum 235.2; P. cinnamomi 144.4; R. solani 88.1 Seedlings mortality (%) of the Lupinus luteus caused by three strains of P. cinnamomi (CA-4, CA-9, MYC-18) at the end of phase at 2 at 0, 30, 62.5, 125, 250, 500 µL/mL of hydrolate: (0): 100, 100, 100; (30): 100, 100, 90; (62.5): 100, 100, 90; (125): 90, 100, 100; (250): 50, 30, 10; (500): 0, 0, 0 ED50 (µL/mL): 234.1, 238.1, 165.5 | [52] |
| Portugal | Leaves, flowers, and thin branches acquired from Proentia® company (Portugal) | Steam distillation/GC-FID, GC-MS | trans-pinocarveol 25, borneol 14, terpinen-4-ol 10, 2,6-trimethylcyclohexanol * 8, 1,8-cineole 6, myrtenol 6, acetophenone 5, verbenone 5 | Antioxidant activity DPPH: IC50%, v/v) 43.00 (poor due to the low antioxidant activity index 0.0037) In vitro cytotoxicity (%, v/v) Fibroblasts L929: 5.71; Macrophages: 8.57; NO production (EC50): 0.79 Antimicrobial activity MIC-MLC (minimum lethal concentration) (%, v/v): S. aureus Staphylococcus epidermidis, E. coli P. aeruginosa: Does not present relevant antimicrobial activity Cutibacterium acnes: 25 | [17] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gago, C.; Bakchiche, B.; Djekhioua, T.; da Graça Miguel, M. Cistus ladanifer L.: Essential Oils, Volatiles, By-Products, and Their Biological Properties. Molecules 2025, 30, 4425. https://doi.org/10.3390/molecules30224425
Gago C, Bakchiche B, Djekhioua T, da Graça Miguel M. Cistus ladanifer L.: Essential Oils, Volatiles, By-Products, and Their Biological Properties. Molecules. 2025; 30(22):4425. https://doi.org/10.3390/molecules30224425
Chicago/Turabian StyleGago, Custódia, Boulanouar Bakchiche, Tahar Djekhioua, and Maria da Graça Miguel. 2025. "Cistus ladanifer L.: Essential Oils, Volatiles, By-Products, and Their Biological Properties" Molecules 30, no. 22: 4425. https://doi.org/10.3390/molecules30224425
APA StyleGago, C., Bakchiche, B., Djekhioua, T., & da Graça Miguel, M. (2025). Cistus ladanifer L.: Essential Oils, Volatiles, By-Products, and Their Biological Properties. Molecules, 30(22), 4425. https://doi.org/10.3390/molecules30224425







