Abstract
A novel artificial light-harvesting system (LHS) for the photooxidation reaction was constructed by the phenyl-bis-naphthyl derivative (PBN) and water-soluble phosphate-pillar[5]arene (WPP5). After host–guest interaction, WPP5 integrated with PBN to form WPP5-PBN amphiphiles, which self-assembled to WPP5-PBN nanoparticles. Based on the aggregate state of PBN in WPP5-PBN nanoparticles, WPP5-PBN nanoparticles emitted a significant yellow fluorescence as energy donors. Due to the yellow fluorescence fully covering the absorption of sulforhodamine 101 (SR101), SR101 was used as energy acceptors and loaded in WPP5-PBN nanoparticles for constructing the WPP5-PBN-SR101 LHS, whose energy transfer efficiency and antenna effect were 66.32% and 22.34. Notably, after the energy of the WPP5-PBN antenna transferred to SR101, more singlet oxygen (1O2) production was observed in the WPP5-PBN-SR101 LHS, which was successfully used as a photocatalyst to catalyze the oxidation reaction of 4-methoxythioanisole to 1-methoxy-4-(methylsulfinyl)benzene, imitating the solar energy conversion to chemical energy.
1. Introduction
Photosynthesis is one of the most important light-harvesting and energy transfer processes in nature, which converts solar energy to chemical energy for life activities [1,2]. In a natural light-harvesting system (LHS), solar energy is absorbed by a pigment-protein complex and transferred to the reaction center for photocatalysis, which achieve solar energy conversion and storage [3,4]. Currently, inspired by the solar energy utilization of natural LHS, more artificial light-harvesting systems are being developed to imitate the solar energy transfer and conversion behavior by fluorescence resonance energy transfer (FRET) [5,6,7,8]. Initially, artificial LHSs were usually constructed by aggregation-caused quenching (ACQ) donors, which underwent multi-step covalent synthesis in an organic environment and performed lower emission efficiency in an aqueous environment. Fortunately, Tang et al. revealed a novel emission mechanism of aggregation-induced emission (AIE) in an aggregate state, which provided an approach to designing aggregate state donors and constructing artificial LHSs in an aqueous environment [9,10]. Recently, supramolecular strategy has been widely used in the construction of AIE-based artificial LHSs in an aqueous environment because of the higher assembly efficiency and the lower synthesis limit [11,12]. Moreover, supramolecular AIE-based artificial LHSs can not only actualize the energy transfer in an aqueous environment, but also finish the solar energy output for photocatalysis [13,14,15]. For example, Hu et al. reported a tetraphenylethylene-embedded pillar[5]arene-based artificial LHS, which significantly achieved the dehalogenation of bromoacetophenone in an aqueous environment [13]. Yi et al. developed a tetraphenylethylene-based artificial light-harvesting metallacycle system, which was successfully utilized to catalyze the C-H alkylation reaction in an aqueous solution [14]. Xing et al. designed a triphenylamine-based supramolecular artificial light-harvesting organic framework, which exhibited the significant photocatalytic ability of C-P bond coupling [15]. However, the photocatalysis applications of artificial LHSs always focused on the traditional chemical reactions and photocatalytic oxidation to sulfoxide was rarely reported. Therefore, it was meaningful to design a novel artificial LHS for the photocatalytic oxidation reaction in water.
Herein, a novel artificial LHS for the photooxidation reaction to sulfoxide was constructed by the host–guest interaction of phenyl-bis-naphthyl derivative (PBN) and water-soluble phosphate-pillar[5]arene (WPP5). As shown in Scheme 1, after WPP5 binding with PBN, the supramolecular amphiphilic WPP5-PBN self-assembled to WPP5-PBN nanoparticles, which emitted significant yellow fluorescence as donors. Due to the yellow emission of WPP5-PBN nanoparticles fully covering the absorption of sulforhodamine 101 (SR101), SR101 was loaded as acceptors in WPP5-PBN nanoparticles. After tightly stacking in an assembly process, a prominent fluorescence resonance energy transfer (FRET) was constructed in WPP5-PBN-SR101 nanoparticles and the harvested energy of WPP5-PBN antennas was transferred to SR101, whose fluorescence significantly changed to a red emission, confirming the construction of WPP5-PBN-SR101 LHS. Moreover, benefitting from the energy transfer behavior, more energy was transferred to excite SR101, which achieved a significant singlet oxygen (1O2) production. Notably, 1O2 could be used as photocatalysts to catalyze the oxidation reaction of 4-methoxythioanisole to 1-methoxy-4-(methylsulfinyl)benzene, which successfully imitated the energy conversion of the solar energy to chemical energy, suggesting WPP5-PBN-SR101 potential in artificial light-harvesting energy storage.
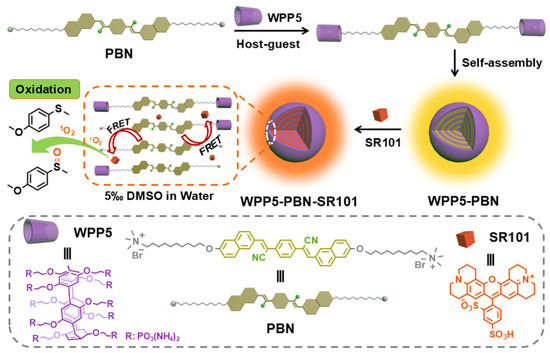
Scheme 1.
Schematic illustration of phenyl-bis-naphthyl derivative-based artificial light-harvesting system for singlet oxygen oxidation.
2. Results and Discussion
2.1. Construction of Supramolecular Nanoparticles
WPP5 was synthesized according to the reported procedures [16,17]. PBN was synthesized by using the 6-hydroxy-2-naphthaldehyde as starting materials, which suffered three-step reactions (Scheme 2 and Figures S1–S7). After obtaining WPP5 and PBN, their host–guest interaction was investigated by UV–Vis absorption spectrum [4,16]. As shown in Figure 1, the 320–500 nm absorption peak of PBN appeared a significant red shift after mixing with the WPP5 solution, confirming their host–guest interaction in water.

Scheme 2.
Synthesis route of PBN.
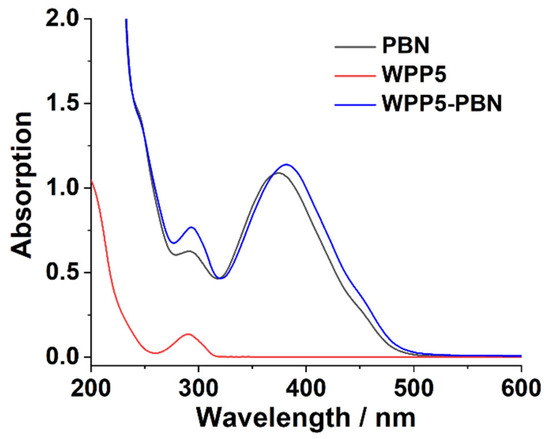
Figure 1.
The host–guest interaction absorption spectra of WPP5 and PBN in water: [PBN] = 50.00 μM, [WPP5] = 7.50 μM, and [PBN]:[WPP5] = 20:3.
Moreover, after adding WPP5 to PBN solution for a while, a significant Tyndall effect light-path was generated in the WPP5-PBN solution by laser pen irradiation, revealing the formation of WPP5-PBN nanoparticles (Figure 2a). Fortunately, benefitting from the formation of WPP5-PBN nanoparticles, a special space structure was generated in the WPP5-PBN nanoparticle. Due to the rich C-H and π bonds in the chemical structures of PBN, WPP5, and SR101, when SR101 was added to the solution of WPP5 and PBN, SR101 could effectively load in the layers of WPP5-PBN via C-H···π and π···π stacking interactions [18,19]. For further exploring the details of these supramolecular nanoparticles, their nano-size distribution and micromorphology were investigated by dynamic light scattering (DLS) and transmission electron microscope (TEM). As shown in Figure 2, the DLS result of WPP5-PBN nanoparticles displayed a small-scale distribution of 190–260 nm, whose average diameter was around 200 nm. The TEM image indicated WPP5-PBN nanoparticles to have a sphere shape. After loading SR101, the distribution of WPP5-PBN-SR101 nanoparticles significantly changed to 160–380 nm, but the morphology of WPP5-PBN-SR101 nanoparticles also exhibited a spherical shape (Figure 2b,d). Furthermore, the nanostructure of WPP5-PBN-SR101 was investigated by X-ray photoelectron spectroscopy (XPS). As shown in Figure S8, the significant C-S, S=O, and S-O signals of SR101 were observed, confirming the encapsulation of SR101 in WPP5-PBN-SR101 nanoparticles.

Figure 2.
DLS distributions of (a) WPP5-PBN and (b) WPP5-PBN-SR101 nanoparticles. TEM images of (c) WPP5-PBN and (d) WPP5-PBN-SR101 nanoparticles. Inset Tyndall effect photos of WPP5-PBN and WPP5-PBN-SR101: [PBN] = 50.00 μM, [WPP5] = 7.50 μM, [SR101] = 0.50 μM, and [PBN]:[WPP5]:[SR101] = 100:15:1.
2.2. Investigation of Energy Transfer Process
Based on the AIE feature of PBN guest, the aggregated PBN in WPP5-PBN nanoparticles emitted significant yellow fluorescence, which could conduct as energy donors (D) to construct an energy transfer process. As shown in Figure 3a, as WPP5-PBN nanoparticles’ yellow fluorescence entirely covered the absorption of SR101, SR101 was considered as energy acceptors (A) to receive the energy from the WPP5-PBN artificial light-harvesting antenna. After loading SR101 in WPP5-PBN nanoparticles, SR101 was tightly loaded in WPP5-PBN amphiphiles and the donor–acceptor distance was in the ideal state, arising a significant FRET process in WPP5-PBN-SR101 nanoparticles. With the molar ratio of SR101 loaded from 1000:1 to 100:1 (D:A), WPP5-PBN’s emission at 551 nm gradually decreased, but the characteristic emission of SR101 at 608 nm exhibited a rapid increase, displaying an efficient artificial energy transfer process in WPP5-PBN-SR101 nanoparticles (Figure 3b). In addition, according to the reported investigation in the energy transfer process [4,8], fluorescence color changes were significant signals for confirming the energy transfer process. The fluorescence spectra of the energy transfer process were often converted into fluorescence color coordinate points in a CIE chromaticity diagram, which clearly revealed the fluorescence color change trajectory, identifying the energy transfer process. Thus, when the fluorescence spectra of the above energy transfer process were converted into fluorescence color coordinate points in a CIE chromaticity diagram, the coordinate points in the CIE chromaticity diagram indicated varying amounts of additive and the energy transfer process was clearly observed according to the coordinate trajectory from yellow to red regions, corresponding to the fluorescence color changes from yellow emission in the WPP5-PBN solution to red emission in the WPP5-PBN-SR101 solution (Figure 3d). Moreover, the artificial light-harvesting behaviors were also verified by tracking the lifetime changes in donors (WPP5-PBN) in the energy transfer process [4,8,16]. According to the time-correlated single photon counting (TCSPC) curve of WPP5-PBN nanoparticles, the τ1 and τ2 of donors were determined to be 2.19 ns and 9.34 ns by the bis-exponential fitting calculation (Figure S9). However, after loading SR101 in WPP5-PBN nanoparticles, the τ1 and τ2 of the donors in WPP5-PBN-SR101 nanoparticles were decreased to 0.96 ns and 4.69 ns, attributing to WPP5-PBN’s energy transferred to SR101 (acceptors) (Figure S10). The τ of the donor was significantly changed from 6.48 ns in WPP5-PBN nanoparticles to 2.64 ns in WPP5-PBN-SR101 nanoparticles, suggesting a convincing energy transfer process in WPP5-PBN-SR101 nanoparticles. The energy transfer efficiency and antenna effect were calculated to be 66.32% and 22.34 in WPP5-PBN-SR101 nanoparticles, displaying the significant light-harvesting ability for energy transfer utilization (Figures S11 and S12).
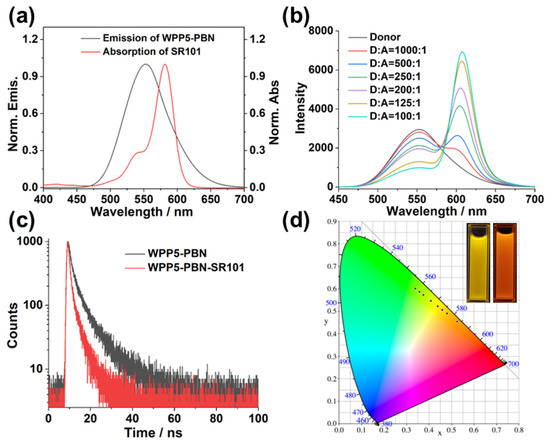
Figure 3.
(a) Emission of WPP5-PBN and absorption of SR101: [PBN] = 50.00 μM, [WPP5] = 7.50 μM, and [SR101] = 50.00 μM. (b) Fluorescence spectra of energy transfer in WPP5-PBN-SR101 from 1000:1 to 100:1 (D:A): [PBN] = 50.00 μM, [WPP5] = 7.50 μM, and [SR101] = 0.05 (1000:1), 0.10 (500:1), 0.20 (250:1), 0.25 (200:1), 0.40 (125:1), 0.50 (100:1) μM. (c) Lifetime curve spectra of WPP5-PBN and WPP5-PBN-SR101. The excitation wavelength was 365 nm and the observation wavelength was 551 nm. (d) CIE diagram of energy transfer process in WPP5-PBN-SR101. Inset fluorescence photos of WPP5-PBN (left) and WPP5-PBN-SR101 (right).
2.3. Investigation of Photooxidation Reaction
Subsequently, the WPP5-PBN-SR101 LHS was used as a photocatalyst to investigate the harvested energy output for utilization. As shown in Figure S13, 9,10-anthracenediylbis(methylene)dimalonic acid (ABDA) was applied as a singlet oxygen (1O2) indicator to explore the 1O2 production ability in the WPP5-PBN-SR101 LHS according to the absorbance quench at 376 nm [20]. No significant absorbance quench was observed in WPP5-PBN and SR101 groups, displaying WPP5-PBN and SR101 to have weak 1O2 production ability (Figure 4a). But, after the light-harvesting process in WPP5-PBN-SR101, a significant absorbance quench at 376 nm was found, which displayed more 1O2 produced in the WPP5-PBN-SR101 LHS due to more energy of WPP5-PBN (donors) transferred to excite SR101 (Figure 4a and Figure S14). In addition, the 1O2 production ability of the WPP5-PBN-SR101 LHS was directly measured by electron paramagnetic resonance (EPR), which used 2,2,6,6-tetramethylpiperidine (TEMP) as the 1O2 trapping agent. As shown in Figure 4b,c, the 1O2 signals were weak in WPP5-PBN and SR101 groups under 365 nm irradiation for 30 min, in accordance with their ABDA experiment results. However, a significant 1O2 signal peak was found in the WPP5-PBN-SR101 LHS, attributing to more energy of WPP5-PBN being transferred to excite SR101 for 1O2 production (Figure 4d). According to reported studies [21,22], 1O2 is usually used as an oxidant for the photocatalytic generation of sulfoxide, which were significant for the chemical-pharmaceutical products. Due to the excellent 1O2 production ability, the WPP5-PBN-SR101 LHS was used as the photocatalyst to oxidize the 4-methoxythioanisole to 1-methoxy-4-(methylsulfinyl)benzene, imitating the solar energy conversion to chemical energy of natural LHS (Scheme S1, Figures S15 and S16). As shown in Figure 4e,f, only 3% and 2% yields of the photooxidation products were observed in WPP5-PBN and SR101 groups because of the poor production of 1O2. Notably, after the artificial light-harvesting process in WPP5-PBN-SR101, the harvested energy of the WPP5-PBN antenna was efficiently transferred to SR101 for more 1O2 production, whose yield was significantly improved to 65%, displaying the WPP5-PBN-SR101 LHS potential in artificial photosynthesis.
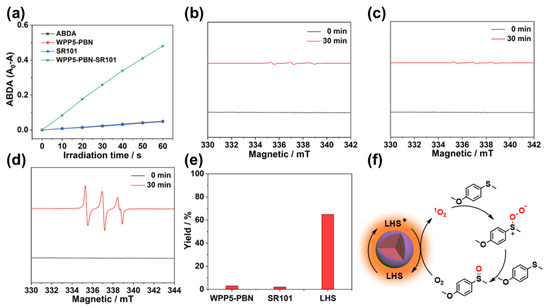
Figure 4.
(a) Absorbance quench spectra of ABDA, WPP5-PBN-ABDA, SR101-ABDA, and WPP5-PBN-SR101-ABDA solution at 376 nm. EPR spectra of (b) WPP5-PBN, (c) SR101, and (d) WPP5-PBN-SR101 solution. (e) The photooxidation yields of WPP5-PBN, SR101 and WPP5-PBN-SR101 LHS. (f) The proposed 1O2 oxidation mechanism. * indicate the excited state.
3. Materials and Methods
3.1. Synthesis of PBN
Compound 1 was synthesized according to our reported method [23].
Synthesis of Compound 2: Compound 1 (1.17 g, 3.00 mmol), 1,4-phenylenediacetonitrile (0.23 g, 1.47 mmol), and tetrabutylammonium hydroxide (8 drop, 50%, TBAH) were added in 40 mL ethanol and stirred for 24 h. The solid was separated and washed with ethyl ether to afford Compound 2 (1.16 g, 1.29 mmol, 86%). 1H NMR (CDCl3, 400 MHz) δ (ppm): 8.24 (s, 2H), 8.13 (d, J = 8.8 Hz, 2H), 7.83–7.78 (m, 8H), 7.72 (s, 2H), 7.22 (dd, J = 8.8, 2.4 Hz, 2H), 7.15 (d, J = 2.0 Hz, 2H), 4.12 (t, J = 6.4 Hz, 4H), 3.43 (t, J = 6.8 Hz, 4H), 1.90–1.83 (m, 8H), 1.53–1.33 (m, 24H). 13C NMR (CDCl3, 100 MHz) δ (ppm): 158.95, 142.90, 135.94, 135.29, 130.95, 130.43, 128.80, 128.40, 127.55, 126.43, 125.77, 120.15, 118.27, 108.98, 106.57, 68.19, 34.12, 32.83, 29.47, 29.39, 29.36, 29.18, 28.77, 28.18, 26.09. HR-ESI-MS: m/z [M + H]+ calcd for [C52H59Br2N2O2]+ 901.2938, 903.2917, 904.2951, found 901.2936, 903.2893, 904.2961.
Synthesis of Compound PBN: Compound 2 (0.90 g, 1.00 mmol) and trimethylamine (5 mL, 2 M in THF) were added in 20 mL CHCl3 and refluxed overnight. The solvent was concentrated and washed with ethyl ether to afford Compound PBN (1.01 g, 0.99 mmol, 99%). 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 8.40 (s, 2H), 8.28–8.26 (m, 2H), 8.16 (d, J = 8.8 Hz, 2H), 7.97–7.92 (m, 8H), 7.41 (s, 2H), 7.26 (d, J = 9.2 Hz, 2H), 4.15 (t, J = 6.4 Hz, 4H), 3.29–3.24 (m, 4H), 3.04 (s, 18H), 1.84–1.77 (m, 4H), 1.71–1.63 (m, 4H), 1.49–1.44 (m, 4H), 1.32–1.27 (m, 20H). 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 158.83, 143.95, 135.96, 134.96, 131.39, 130.87, 129.36, 128.33, 127.88, 126.80, 125.83, 120.35, 118.60, 108.42, 107.32, 68.22, 65.74, 52.59, 29.39, 29.24, 29.08, 28.98, 26.23, 26.02, 22.50. HR-ESI-MS: m/z [M − 2Br]2+ calcd for [C58H76N4O2]2+ 430.2979, found 430.2973.
3.2. Construction of Supramolecular Nanoparticles
Firstly, the PBN solution in DMSO (0.02 M), WPP5 solution in water (0.42 mM), and SR101 solution in DMSO (0.20 mM) were prepared, respectively. Then, 10 μL PBN solution and 72 μL WPP5 solution were mixed in water and diluted to 4 mL by ultrapure water for constructing WPP5-PBN nanoparticles. Next, 10 μL PBN solution, 72 μL WPP5 solution, and (0–10) μL SR101 solution were mixed in water and diluted to 4 mL by ultrapure water for constructing WPP5-PBN-SR101 nanoparticles. The nanoparticles solution was prepared by ultrapure water under room temperature. The molar ratio of [PBN]:[WPP5]:[SR101] in artificial LHS for photocatalysis was 100:15:1.
3.3. Photooxidation of 4-Methoxythioanisole
4-Methoxythioanisole (80 μL) was added in the freshly prepared LHS solution (4 mL) in a 10 mL glass vial. The mixture was bubbled by air, and then irradiated by 9 W 365 nm LED at room temperature to obtain the photooxidation product.
4. Conclusions
In summary, we have successfully constructed a new artificial light-harvesting system (LHS) by the supramolecular interaction of water-soluble phosphate-pillar[5]arene (WPP5) host molecule and phenyl-bis-naphthyl derivative (PBN) guest molecule. Based on the aggregate emission mechanism in the AIE effect, WPP5-PBN nanoparticles emitted a significant yellow fluorescence after the self-assembly process, displaying prospective donor ability in artificial LHSs. Due to WPP5-PBN’s yellow fluorescence fully covering the absorption of sulforhodamine 101 (SR101, acceptor), WPP5-PBN conducted as a light-harvesting antenna and transferred energy to SR101 after loading SR101 in WPP5-PBN nanoparticles, whose energy transfer efficiency and antenna effect were 66.32% and 22.34. Benefitting from the efficient energy transfer process in the WPP5-PBN-SR101 LHS, more energy was transferred to excite SR101 and produce a significant singlet oxygen (1O2), which had potential for the photooxidation reaction. Subsequently, the WPP5-PBN-SR101 LHS was used as a photocatalyst to oxidize the 4-methoxythioanisole to 1-methoxy-4-(methylsulfinyl)benzene, whose yield was significantly improved to 65%, imitating the solar energy conversion to chemical energy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30224424/s1. Figure S1. 1H NMR spectrum (400 MHz, CDCl3) of Compound 1. Figure S2. 1H NMR spectrum (400 MHz, CDCl3) of Compound 2. Figure S3. 13C NMR spectrum (100 MHz, CDCl3) of Compound 2. Figure S4. HR-ESI-MS spectrum of Compound 2. Figure S5. 1H NMR spectrum (400 MHz, DMSO-d6, 298 K) of PBN. Figure S6. 13C NMR spectrum (100 MHz, DMSO-d6, 298 K) of PBN. Figure S7. HR-ESI-MS spectrum of PBN. Figure S8. The XPS spectrum of WPP5-PBN-SR101. Figure S9. Fluorescence lifetime of WPP5-PBN nanoparticles. Figure S10. Fluorescence lifetime of WPP5-PBN-SR101 nanoparticles. Figure S11. Fluorescence spectra of WPP5-PBN and WPP5-PBN-SR101 under 365 nm excitation. Figure S12. Fluorescence spectrum of WPP5-PBN (black line), which was normalized with WPP5-PBN-SR101 (red line) at 551 nm; Fluorescence spectra of WPP5-PBN-SR101 (red line under 365 nm excitation and blue line under 535 nm excitation). Figure S13. The 1O2 oxidation mechanism of ABDA. Figure S14. The UV–Vis absorbance spectra of (a) free ABDA, (b) ABDA+WPP5-PBN, (c) ABDA+SR101, and (d) ABDA+WPP5-PBN-SR101 after 365 nm irradiation (9 W) for different times. Scheme S1. The photooxidation reaction. Figure S15. 1H NMR spectrum (400 MHz, CDCl3, 298 K) of photooxidation product. Figure S16. HR-ESI-MS spectrum of photooxidation product. Reference [24] is cited in the supplementary materials.
Author Contributions
Conceptualization, G.S. and L.P.; Methodology, G.S.; L.P. and Y.C.; Validation, G.S.; L.P. and Y.C.; Formal analysis, G.S.; L.P. and Y.C.; Writing—original draft preparation, G.S. and L.P.; Writing—review and editing, G.S. and L.P.; Supervision, G.S. and L.P. All authors have read and agreed to the published version of the manuscript.
Funding
L.P. and G.S. acknowledge the National Natural Science Foundation of China (Nos. 52500067 and 22401160). G.S. acknowledges the Natural Science Foundation of Jiangsu Province (No. BK20220601). L.P. acknowledges the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (No. 25KJB610001) and the Changzhou Leading Innovative Talents Introduction and Cultivation Project (No. CQ20240127).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article and Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Croce, R.; Amerongen, H.V. Light harvesting in oxygenic photosynthesis: Structural biology meets spectroscopy. Science 2020, 369, eaay2058. [Google Scholar] [CrossRef]
- Wei, X.; Su, X.; Cao, P.; Liu, X.; Chang, W.; Li, M.; Zhang, X.; Liu, Z. Structure of spinach photosystem II–LHCII supercomplex at 3.2 Å resolution. Nature 2016, 534, 69–74. [Google Scholar] [CrossRef]
- Zhang, R.; Tian, X.; Zuo, M.; Zhang, T.; Pangannaya, S.; Hu, X.-Y. Bionic artificial leaves based on AIE-active supramolecular hydrogel for efficient photocatalysis. Adv. Sci. 2025, 12, 2504993. [Google Scholar] [CrossRef]
- Sun, G.; Li, M.; Li, J.; Feng, J.; Yan, Z.; Sun, Y.; Pu, L.; Zhu, J.; Tang, Y.; Yao, Y. Enhanced emission in a supramolecular artificial light-harvesting system for a photocatalytic thiol-ene reaction. Chem. Commun. 2025, 61, 6360–6363. [Google Scholar] [CrossRef]
- Duan, Q.; Zhang, Q.; Zhang, J.; Lin, S.; Xiao, T.; Wang, L. Artificial light-harvesting systems based on supramolecular polymers. Chin. Chem. Lett. 2025, 36, 111421. [Google Scholar] [CrossRef]
- Xu, W.-W.; Qin, Y.-X.; Yu, X.-Y.; Jiang, L.-N.; Zhang, H.-Y.; Chen, Y.; Liu, Y. Multipath cascade light harvesting for multicolor luminescence based on macrocyclic sulfonatocalix[4]arene. Chin. Chem. Lett. 2025, 36, 111068. [Google Scholar] [CrossRef]
- Zhang, L.; Qian, H.; Wu, Z.; Zhang, Q.; Li, S.; Cheng, M.; Xiao, T. Non-covalent dimer as donor chromophore for constructing artificial light-harvesting system in water. Molecules 2022, 27, 8876. [Google Scholar] [CrossRef]
- Sun, G.; Li, M.; Cai, L.; Zhu, J.; Tang, Y.; Yao, Y. Carbazole-based artificial light-harvesting system for photocatalytic cross-coupling dehydrogenation reaction. Chem. Commun. 2024, 60, 1412–1415. [Google Scholar] [CrossRef]
- Liu, M.-X.; Su, X.-L.; Chen, P.; Liu, Y.-Y.; Li, J.-P.; Zou, L.; Tang, B.Z.; Feng, H.-T. Sequential FRET system based on macrocyclic AIEgens for versatile photocatalysis. Chin. Chem. Lett. 2025, 111516. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 18, 1740. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Velmurugan, K.; Li, B.; Hu, X.Y. Artificial light-harvesting systems based on macrocycle-assisted supramolecular assembly in aqueous media. Chem. Commun. 2021, 57, 13641–13654. [Google Scholar] [CrossRef]
- Wu, Z.; Qian, H.; Li, X.; Xiao, T.; Wang, L. Recent advances in two-step energy transfer light-harvesting systems driven by non-covalent self-assembly. Chin. Chem. Lett. 2024, 35, 108829. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, R.; Song, Z.; Zhang, K.; Tian, X.; Pangannaya, S.; Zuo, M.; Hu, X.-Y. Dimeric pillar[5]arene as a novel fluorescent host for controllable fabrication of supramolecular assemblies and their photocatalytic applications. Adv. Sci. 2023, 10, 2206897. [Google Scholar] [CrossRef]
- Zhang, D.; Yu, W.; Li, S.; Xia, Y.; Li, X.; Li, Y.; Yi, T. Artificial light-harvesting metallacycle system with sequential energy transfer for photochemical catalysis. J. Am. Chem. Soc. 2021, 143, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-Z.; Zhang, Z.-G.; Xin, C.-L.; Liu, H.; Yu, S.; Xing, L.-B. Artificial light-harvesting system with sequential energy transfer in photocatalytic C-P coupling based on supramolecular organic framework of triphenylamine. J. Colloid Interface Sci. 2025, 680, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Li, M.; Cai, L.; Wang, D.; Cui, Y.; Hu, Y.; Sun, T.; Zhu, J.; Tang, Y. Water-soluble phosphate-pillar[5]arene (WPP5)-based artificial light-harvesting system for photocatalytic cross-coupling dehydrogenation. J. Colloid Interface Sci. 2023, 641, 803–811. [Google Scholar] [CrossRef]
- Hu, X.-Y.; Liu, X.; Zhang, W.; Qin, S.; Yao, C.; Li, Y.; Cao, D.; Peng, L.; Wang, L. Controllable construction of biocompatible supramolecular micelles and vesicles by water-soluble phosphate pillar[5,6]arenes for selective anti-cancer drug delivery. Chem. Mater. 2016, 28, 3778–3788. [Google Scholar] [CrossRef]
- Yang, Z.; Fu, K.; Yu, W.; Jia, A.; Chen, X.; Cai, Y.; Li, X.; Feng, W.; Yuan, L. A multi-stimuli responsive [3]rotaxane based on hydrogen-bonded aramide azo-macrocycles. Chin. Chem. Lett. 2025, 36, 110842. [Google Scholar] [CrossRef]
- Sun, G.; Hu, M.; Cao, X.; Wan, Z.; Shao, T.; Shen, Y.; Li, M.; Zhu, J.; Tang, Y. Triphenylamine-based artificial light-harvesting system for singlet oxygen production and photooxidation reaction. Mater. Today Chem. 2025, 50, 103185. [Google Scholar] [CrossRef]
- Qian, W.; Zuo, M.; Sun, G.; Chen, Y.; Han, T.; Hu, X.-Y.; Wang, R.; Wang, L. The construction of an AIE-based controllable singlet oxygen generation system directed by a supramolecular strategy. Chem. Commun. 2020, 56, 7301–7304. [Google Scholar] [CrossRef]
- Zhang, R.-Z.; Bi, Y.-S.; Niu, K.-K.; Yu, S.; Liu, H.; Xing, L.-B. Multilevel supramolecular assembly with three-step sequential energy transfer for selective generation of sulfides and sulfoxides by photoredox thiol-ene cross-coupling reactions. Adv. Opt. Mater. 2025, 13, 2402112. [Google Scholar] [CrossRef]
- Zhang, R.-Z.; Liu, H.; Xin, C.-L.; Han, N.; Ma, C.-Q.; Yu, S.; Wang, Y.-B.; Xing, L.-B. Construction of aggregation-induced emission photosensitizers through host-guest interactions for photooxidation reaction and light-harvesting. J. Colloid Interface Sci. 2023, 651, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Cai, L.; Cui, H.; Hu, Y.; Wang, J.; Wang, M.; Zhu, J.; Sun, T.; Tang, Y. Naphthalenyl-phenylacrylonitrile-based supramolecular aqueous artificial light-harvesting system for photochemical catalysis. Dye. Pigment. 2022, 201, 110257. [Google Scholar] [CrossRef]
- Li, X.-L.; Cheng, D.-L.; Niu, K.-K.; Liu, H.; Yu, S.-S.; Wang, Y.-B.; Xing, L.-B. Construction of supramolecular dimer photosensitizers based on triphenylamine derivatives and cucurbit[8]uril for photocatalysis. J. Mater. Chem. A 2023, 11, 24911–24917. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).