Abstract
Cistus ladanifer L., commonly known as gum rockrose, is a Mediterranean shrub of growing interest due to its valuable essential oils (EOs) and labdanum resin. This review synthesizes current knowledge on the chemical composition and biological activities of EOs and hydrolates from C. ladanifer across Mediterranean regions, with particular emphasis on Spain, Portugal, Morocco, and France. α-Pinene, viridiflorol, and camphene were found to be the major constituents in the EOs with antioxidant and antimicrobial properties. Additionally, the identified biological properties have prompted studies exploring innovative strategies such as nanoparticle encapsulation, the development of bioactive films, and the incorporation of EOs into food and pharmaceutical packaging. By-products from EO distillation, including lignocellulosic residues, the extraction of phenolic-rich compounds, and hydrolates, have shown potential for value-added applications. Altogether, C. ladanifer represents a versatile species with possible applications in cosmetics, pharmaceutical development, and the food industry.
1. Introduction
C. ladanifer is the most economically important species in the fragrance and cosmetics sectors among the Cistaceae family. This family comprises eight genera, with Hudsonia, Lechea, and Crocanthemum found in temperate regions of the Americas, while Cistus, Fumana, Helianthemum, Halimium, and Tuberaria are native to the Mediterranean region [1]. The family includes approximately 220 species, primarily heliophyte shrubs, subshrubs, and herbs that grow in open habitats with nutrient-poor soils [2]. The highest diversity of genera and species occurs within the Mediterranean floristic region. The genus Cistus is further divided into three subgenera: Cistus, Halimioides, and Leucocistus [1].
Within the subgenus Leucocistus, several species are recognized, including C. ladanifer and C. laurifolius (section Ladanium); C. salviifolius, C. sintenisii, C. monspeliensis, C. populifolius, and C. psilosepalus in the (section Ledonia); and C. pouzolzii (section Stephanocarpoidea) [3,4,5,6,7].
Three subspecies are reported for C. ladanifer: subsp. ladanifer (Figure 1A,B), which is mainly distributed in the Iberian Peninsula, northern Africa, southern France, and isolated patches in the Canary Islands (being considered an introduced species in the two latter places); subspecies sulcatus, which was first identified as Cistus palhinhae and is endemic to southwestern Portugal (Algarve region); and subspecies africanus, which is found in southern Spain (Cádiz, Málaga) but is more frequently found in northern Africa [3].

Figure 1.
Cistus ladanifer subsp. ladanifer var. maculatus (A) and Cistus ladanifer subsp. ladanifer var. albiflorus (B).
Cistus ladanifer is a perennial and woody shrub that can form dense populations mostly integrating poor siliceous and acidic soils that are formed of sandstone, granite, or shale [8]. There are two varieties of Cistus ladanifer subsp. ladanifer: var. maculatus, which has a red to maroon blotch at the base of each petal (Figure 1A), and var. albiflorus, which has five petals that may be completely white (Figure 1B). These two varieties may coexist in populations that are mixed or can occur separated in monomorphic populations [3].
A: Author: Juan Sevilla of Jardim Botânico UTAD, Flora Digital de Portugal.
<p>Imagem da espécie <i>Cistus ladanifer subesp. ladanifer</i> por Juan Sevilla do <a href="https://jb.utad.pt" target="_blank">Jardim Botânico UTAD, Flora Digital de Portugal</a>.</p>
B: Author: Isabel Queiroz Marcellot of Jardim Botânico UTAD, Flora Digital de Portugal.
<p>Imagem da espécie <i>Cistus ladanifer subesp. ladanifer</i> por Isabel Queiroz Marcellot do <a href="https://jb.utad.pt" target="_blank">Jardim Botânico UTAD, Flora Digital de Portugal</a>.</p>
Among the Cistaceae family, C. ladanifer is the most economically significant species in cosmetics and fragrance industries. It is used to produce a range of aromatic products such as Cistus oil (concrete and absolute), raw labdanum gum, labdanum gum (concrete and absolute), and labdanum oil. Raw gum labdanum is a resin extracted from the photosynthetic leaves and stems of C. ladanifer, with alkaline water to remove all the surface waxes, followed by acid neutralization (method used in Andaluzia, Spain). In Zamora, León y Salamanca (Spain), the raw gum is obtained using boiling water, with a lower yield than the procedure of Andaluzia. The resin obtained through these methods comprises terpenoids, mainly labdane-type diterpenes, and methylated flavonoids [9,10,11,12]. Concrete can be extracted using organic solvents from the crude resin or from the leaves and stems of C. ladanifer. The labdanum gum can be extracted with ethanol to yield the absolute. Diterpenoid and flavonoid fractions can then be extracted from labdanum absolute [9]. Essential oils (EOs) that are extracted by steam distillation or hydro-distillation can, likewise, be extracted from the labdanum or the aerial parts of C. ladanifer [9,10].
The trichomes found in the adaxial and abaxial parts of the leaf, primarily in young, developing leaves, secrete the labdanum of C. ladanifer. The fact that trichome density differs amongst C. ladanifer populations indicates that biotic and environmental factors influence exudate production. Summertime is when exudate production is at its greatest since it is triggered by ultraviolet (UV) radiation and water stress [13]. This is also strengthened by its much greater production in the field in comparison with greenhouse plants [14]. The number of flavonoids present in the exudate varies noticeably with the season, with the summer months having the highest concentration and the winter months having the lowest. The seasons also affect the amount of diterpenes but in a different way, that is, the highest production is in the winter and the lowest in the spring and summer [15].
The reduced plant diversity and richness in the presence of C. ladanifer is believed to be due to the allelochemicals aglycone flavonoids and diterpenes, released by its leaves and stems and present in the exudates. The allelopathic potential of C. ladanifer is due to the joint action of several secondary metabolites that, in turn, is dependent on the environmental factors (season, temperature, and photoperiod) [13,16].
The labdanum, essential oil (EO), and other extracts of C. ladanifer are used as fixatives in fragrance and cosmetics. Its traditional use as a medicinal plant is mostly associated with its ability to heal wounds. The EO is used to treat wounds, skin ulcers, hemorrhages, secondary infections, and other skin conditions like psoriasis and eczema. In addition to its use on the skin, the EOs and/or extracts have been shown to have anti-ulcerogenic, anti-acid, anti-diarrheal, antispasmodic, diuretic, analgesic, hypoglycemic, antihypertensive, and vasodilator properties in various target organs [17]. C. ladanifer branches have been employed to draw and catch houseflies (Musca domestica L., Diptera: Muscidae) due to their strong scent. Because its leaves are sticky, bunches of this species are hung up and serve as fly-capture tape [18]. The repellent activity of C. ladanifer EO using the mosquito Aedes aegypti was revealed to be poor when compared with those of Lavandula stoechas, Helichrysum italicum, and Laurus nobilis [19].
This review aims to synthesize the current knowledge on the chemical composition and biological properties of the EOs and volatile compounds derived from Cistus ladanifer.
2. Methods
The present work provides a brief review of the volatiles extracted from Cistus ladanifer as well as their properties and applications. The information in this review was taken from articles found on the Web of Science (WOS) platform, using the keywords “Cistus ladanifer essential oils”, “Cistus ladanifer volatiles”, “Rockrose essential oils”, “Rockrose volatiles”, “Labdanum essential oils”, “Labdanum volatiles”, up to 31 May 2025. A total of 211 results were obtained, distributed by the eight keywords (sections) as can be seen in the bottom left corner of Figure 2 (sum of all data). However, some articles appeared in several sections as depicted in the same Figure. In fact, instead of 211 articles, only 127 articles were really found (sum of all data over bars). For example, there is an article that appeared in all sections; it was a review article. After reading the articles, only 49 (Figure 3) addressed essential oils (46) or emission of volatiles (3 articles). Six of the forty-nine articles lacked the chemical composition of the essential oils. The biological properties of the rockrose EOs were the primary focus of these articles. The articles may appear in multiple sections, as expected, and the review is performed with the information provided by these articles (Figure 3).
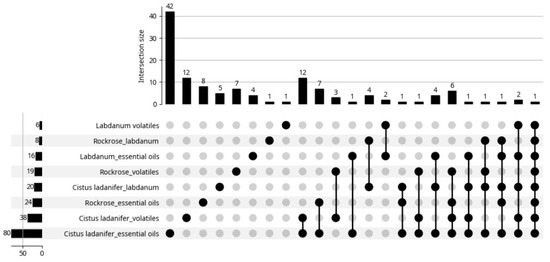
Figure 2.
Distribution of the articles by sections. Google Gemini. (2025). Generation of article distribution chart by sections [Computer software]. Available from https://gemini.google.com. Accessed 13 June 2025.
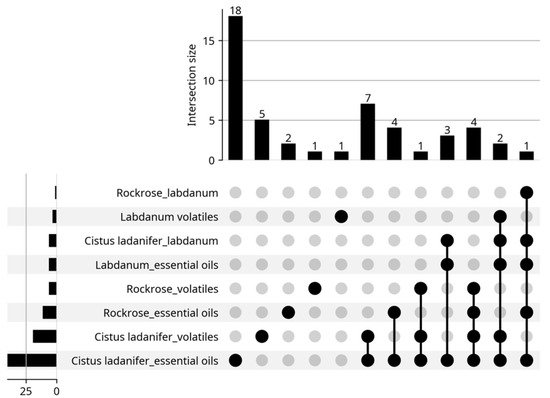
Figure 3.
Distribution of articles about essential oils by sections. Google Gemini. (2025). AI-generated figure for article distribution analysis [Computer software]. Available from https://gemini.google.com.
3. Chemical Composition of Essential Oils
Since ancient times, plants that produce volatile compounds (aromatic plants) have been utilized as spices, medical treatments, and in religious rituals due to their therapeutic qualities and appealing scents [20].
Essential oils (EOs) are complex mixtures of volatile compounds made by living organisms that are extracted solely by physical methods (pressing and distillation) from entire plants or plant parts that are recognized to have a specific taxonomic origin [21]. Other methods of extracting volatile fractions from aromatic plants do not fit the official definition of “essential oils” [22].
Essential oils (EOs) are composed of a wide variety of volatile compounds, including terpenes with regular carbon skeletons such as monoterpenes (C10), sesquiterpenes (C15), and diterpenes (C20) [23,24]. Terpenoids with irregular carbon skeletons, such as homoterpenes and norisoprenoids, also contribute to EO composition [23]. Phenolics derived from shikimic pathways can also be constituents of EOs such as methyl salicylate, which is the major component of wintergreen, or anethole in anise and fennel [23].
The chemical composition of C. ladanifer EO collected from different places in the Mediterranean area including Portugal is depicted in Table 1, since 1997. In the same Table, there is also the chemical composition in volatiles of mixtures obtained by methods other than hydro-distillation. It also described the composition of the volatile fraction of resinoids, concretes, and absolutes. In those cases where the authors had determined the biological properties (e.g., antimicrobial, antioxidant activities, among other ones), the results were also reported in the same Table. The structures of the main compounds (>4.5%) present in the C. ladanifer EOs are depicted in Figure 4.

Table 1.
Main compounds present in EO and volatiles of Cistus ladanifer and biological properties.
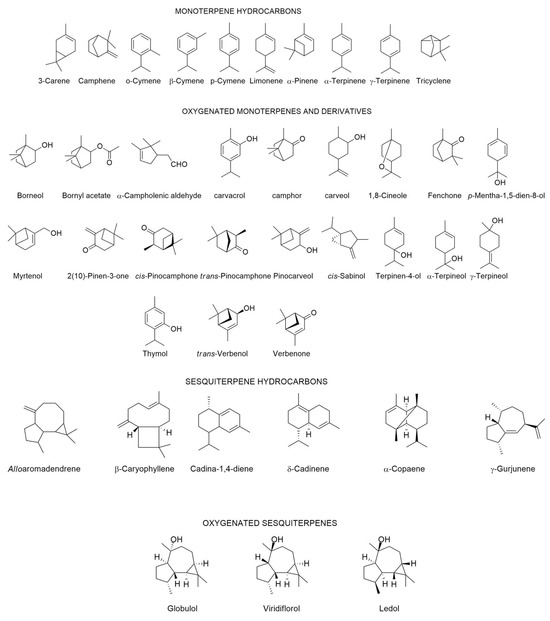
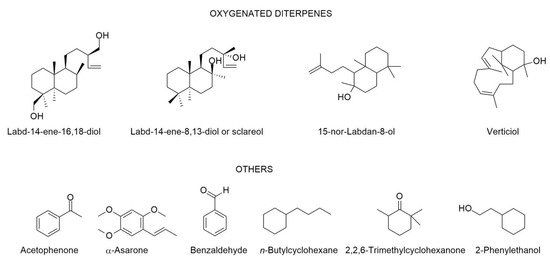
Figure 4.
Structures of the main compounds (>4.5%) found in the C. ladanifer EOs. Sections may be divided by subheadings.
3.1. Chemical Composition of Cistus ladanifer EOs from the Iberian Peninsula
Based on research published in the Web of Science (WOS) up to March 2025, Spain, Portugal, and Morocco have contributed the largest number of studies on the chemical composition of EOs or volatiles from C. ladanifer, with 13, 10, and 8 publications, respectively (Table 1).
In Spain, ledol [52], such as trans-pinocarveol [46], predominated in one sample, viridiflorol in three [53,55,56], and α-pinene in seven cases [45,47,48,49,50,51,54] (Table 1). Viridiflorol was found in six other samples even though it was not the main monoterpenoid. α-Pinene and viridiflorol appear to be typical of C. ladanifer volatiles or EOs from Spain (Table 1). Already in 1969, Garcia-Martin and Garcia-Vallejo [59] studied the C. ladanifer var. maculatus EO from Spain for the first time and reported that α-pinene was dominant (44%), followed by camphene, p-cymene, 1,8-cineole, borneol and linalyl, and bornyl acetate but at much lower percentages.
The chemical composition of the neutral volatile fraction of a commercial EO of C. ladanifer produced in Spain was evaluated by Simon-Fuentes et al. [60]. The fractionation of the EO was carried out by aqueous NaHCO3 and NaOH extraction and column chromatography that produced four fractions: carboxylic acids, phenols, mono-and sesquiterpene hydrocarbons, and oxygenated compounds. In the neutral volatile fraction, Simon-Fuentes et al. [60] found three main compounds, with concentrations of which percentages were >5%: α-pinene (35), camphene (10) and 2,2,6-trimethylcyclohexanone (6) [60].
The enantiomeric distribution of chiral compounds was determined for six pairs of the C. ladanifer EO from Spain by Costa et al. [45]. The enantiomeric distribution for the main compound, α-pinene, was 6.8 (−):93.2(+).
In six samples of C. ladanifer EOs from Portugal, α-pinene was the main monoterpene. One sample had 2,2,6-trimethylcyclohexanone, another viridiflorol [11,17,37,38,39,40,41,43,44], and one had acetophenone, which was obtained via sequential extraction using water and dichloromethane instead of steam distillation, possibly affecting the chemical composition [36] (Table 1).
In both Portugal and Spain, α-pinene is the main constituent of C. ladanifer EO, followed by viridiflorol in Spain and camphene in Portugal.
The variability in the chemical composition of the EOs can be attributed to several factors, including geographic location, seasonal variation, and plant age. For example, for plants collected in Andévalo (Huelva, Spain), α-pinene (42.50%) was the main constituent of the EO followed by viridiflorol (13.36%), whereas for the EO obtained from plants of Cerezal (Zamora, Spain), the concentrations of these two monoterpenes were much closer (19.27 and 24.13%, respectively) [49].
Chaloupková et al. [54] studied the variation in yield and chemical composition of C. ladanifer EOs obtained from two locations in central Spain over one year and obtained from steam distillation. The age of plants was also different in those two zones. The oil yield ranged from 0.03 to 0.19% (weight/dry weight), being higher during the autumn and early winter months. Regarding the chemical composition, the authors [54] observed that a variation also occurred over time in both samples. The pattern of the main compounds (>4.5%) is depicted in Figure 5A (7-year-old plants from Bustares) and 5B (12-year-old plants from Hiendelaencina).
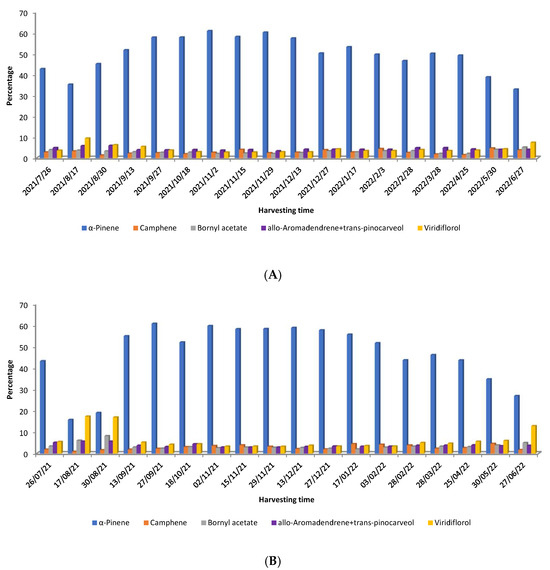
Figure 5.
Distribution of the main compounds (>4.5%) of C. ladanifer EO obtained from 7-year-old plants from Bustares (A) and from 12-year-old plants from Hiendelaencina (B) [54].
α-Pinene was predominant in both plant samples, but its biosynthesis varied seasonally (Figure 5). In August and May–June (Figure 5A,B), lower α-pinene and higher viridiflorol levels were observed, especially in older plants. These monoterpenes showed an inverse relationship. In 12-year-old plants, α-pinene and viridiflorol levels were nearly equal in August (Figure 5A,B). Despite differences in age and harvesting location (Hiendelaencina for older plants, Bustares for younger), the accumulation of α-pinene and viridiflorol followed a similar pattern across seasons (Figure 5A,B) [54].
Essential oils obtained from C. ladanifer waste materials in Beira Baixa (Portugal) during March and August revealed notable seasonal differences in composition: α-pinene levels were higher in March, whereas viridiflorol was more abundant in August (Figure 6) [42]. This finding is somehow similar to that observed in samples from Spain, using the same extraction procedure (steam distillation for 1 h in samples from Spain and 1 h 30 min in Portugal). It seems that α-pinene is preferentially biosynthesized in March (winter/spring) and viridiflorol in August (summer). Figure 6 also depicts that monoterpene hydrocarbons (tricyclene, α-pinene, camphene, and limonene) decreased from March to August, in contrast to viridiflorol, bornyl acetate, trans-pinocarveol, and borneol, as well as the ketone 2,2,6-trimethylcyclohexanone (Figure 6). In the same work, these authors also compared the chemical composition of the EOs obtained by steam distillation and hydro-distillation, and the results are presented in Figure 6. The EOs obtained by hydro-distillation had less amounts of α-pinene, camphene, limonene, bornyl acetate, and viridiflorol, and higher concentrations of trans-pinocarveol, borneol, verbenone, terpinen-4-ol, and 2,2,6-trimethylcyclohexanone than those oils obtained by steam distillation (Figure 6). These differences can be the result of two factors: type of extraction and extraction time because in the hydro-distillation the time of extraction was 3 h in contrast to 1 h or 1 h 30 min in steam distillation [42,54].
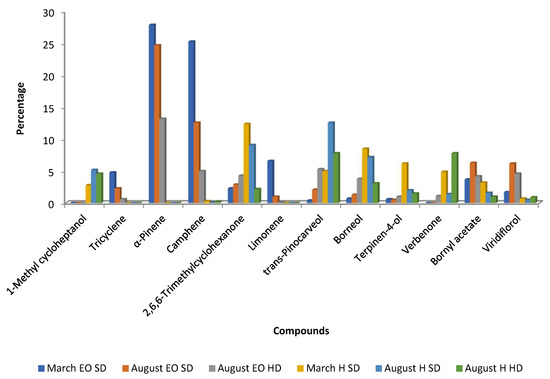
Figure 6.
Main compounds (>4.5%) of C. ladanifer essential oil (EO) and hydrolates (H) obtained by steam distillation (SD) or hydro-distillation (HD) from wastes collected in Beira Baixa (Portugal) in March and August [42].
The effects of collection zone considering the edaphic substrate, and the intra-population variability were studied by Pérez-Izquierdo et al. [53]. The results are summarized in Figure 7, in which only the compounds present at >4.5% are represented. Viridiflorol and ledol were the predominant compounds. The variability in the amounts of viridiflorol is higher in the populations growing in slate substrates because it is possible to have samples with the highest percentage (A3 PZ) and the lowest percentage (A2 PZ) (Figure 7). In this case, there is also intra-population variability. The granite substrate does not exhibit this diversity as much (Figure 7). The populations B1–B3 had higher percentages of ledol in a consistent form, while the remaining samples showed variations between the populations and substrates. The EOs isolated from the populations A3 and B1–B3 had the lowest amounts α-pinene and trans-pinocarveol (Figure 7). All samples of the granite substrates along with the samples A1 and A2 had the highest percentages of those compounds. Once more, compared with plants growing in substrate slate, the EO samples taken from plants growing in granite substrate were more uniform (Figure 7).
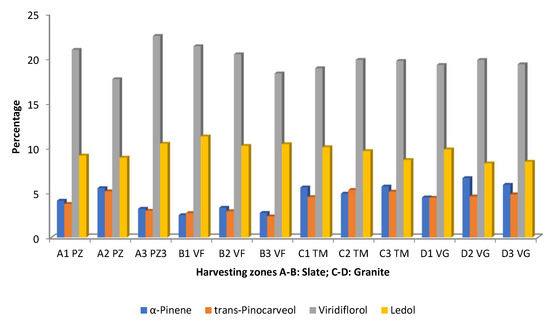
Figure 7.
Main compounds (>4.5%) of C. ladanifer (EO) obtained from plant populations (A1,B1,C1,D1; A2,B2,C2,D2; and A3,B3,C3,D3 growing in PZ: Pozuelo de Zarzón (slate substrate); VF: Valverde del Fresno (slate substrate); TM: Torre de Don Miguel (granite substrate); VG: VillasBuenas de Gata (granite substrate) [53].
In two studies, Pérez-Izquierdo and co-authors examined the impact of the phenological stage on the chemical composition of C. ladanifer EOs from northwest Extremadura. In the first study, viridiflorol and ledol were more prevalent in August (20.38% and 8.92%, respectively) than in October (15.97% and 7.26%), whereas α-pinene was greater in October (12.91%) than in August (3.38%) [56]. In the second study, EOs from plants harvested in May (flowering phase) and August (fruit maturation) at several ages (3–5, 9–11, and >15 years) were assessed. Viridiflorol and ledol levels were lower in EOs from plants older than 15 years during flowering than in those during the fruit maturation stage. During the fruit maturity period, there was a decrease in 2,2,6-trimethylcyclohexanone and an increase in trans-pinocarveol and α-pinene (Figure 8) [55]. Regarding EO yields, C. ladanifer EO yield values ranged from 0.11 to 0.42 mL/100 g of dry plant material. The average EO yield of plants harvested at the flowering phenological stage was substantially lower (0.17%) than that of plants picked at fruit maturation (0.27%). Moreover, plants between the ages of three and five produced noticeably less than those between the ages of 9 and 11 at the flowering stage. However, no significant differences in EO yield were observed for the three age ranges studied at the fruit maturation stage [55].
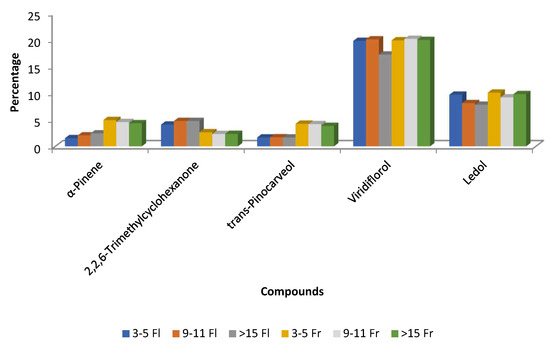
Figure 8.
Main compounds (>4.5%) of C. ladanifer (EO) obtained from different aged plants at two phenological stages in Extremadura (Spain). 3–5 Fl: plants of 3–5 years old at flowering phase; 9–11 Fl: plants of 9–11 years old at flowering phase; >15 Fl: plants of >15 years old at flowering phase; 3–5 Fr: plants of 3–5 years old at fruit maturation phase; 9–11 Fr: plants of 9–11 years old at fruit maturation phase; > 15 Fr: plants of >15 years old at fruit maturation phase [55].
Gomes et al. [37] reported that viridiflorol predominated in all EOs of C. ladanifer samples (wild plants collected in the center-interior of Portugal and cultivated plants in the north of Portugal that were propagated from a wild plant found in the dry plain region in the South of Portugal); nevertheless, 15-nor-labdan-8-ol was at higher concentrations in the EOs obtained from the cultivated plants (5.2%) than in the wild ones (1.7%). Small differences were also observed in the chemical composition of the EOs obtained from dried and fresh material (Table 1).
The effect of plant storage on the chemical composition of the essential oil of C. ladanifer was evaluated by Mediavilla et al. [50] in plants collected in Hiendelaencina—Guadalajara (Spain). The results showed that after 120 days of storage, a decrease in α-pinene (49.64–46.67%) and an increase in viridiflorol (10.03–12.50%) were observed. The boiling points of viridiflorol (293.0–294.0 °C) and α-pinene (155–156 °C) may help to explain these results. Despite the decrease in the yield of EO obtained from stored C. ladanifer plants over time, the authors [50] reported that this difference could not be considered significant at the 95% confidence level (Table 1). They considered a normal variability between bales, related to variations in the proportion of young twigs and wood, rather than because of the time elapsed after harvesting [50].
Using supercritical CO2, Rincón et al. [57] isolated volatiles from C. ladanifer leaves collected in July in Ciudad Real, Spain. They investigated how the chemical composition was affected by temperature, pressure, and extraction time. Regardless of extraction duration, camphor and α-pinene were the most abundant chemicals, followed by camphene, borneol, γ-terpineol, and thymol. The authors found that the yields dropped with temperature (40–60 °C), although there was no significant difference between 30 and 40 °C. Oil yields increased as pressure rose (8–10 MPa), with a significant increase between 8 and 9 MPa but less so between 9 and 10 MPa. The experimental data revealed a similar pattern at 30, 50, and 60 °C [57].
In order to distinguish between C. ladanifer, Pinus pinaster EOs, and C. ladanifer oil adulterated with P. pinaster, Melaleuca alternifolia, and red fruits, Viciano-Tudela et al. [61,62] tested MQ sensors with microcontrollers. They found that KNN (k-nearest neighbors) attained 100% accuracy with a precision and recall of 1 compared with support vector machine (SVM), naive Bayesian (NB), and neural network (NN) algorithms. Later on, Blasco et al. [63] reported 91.6% accuracy for terpenic hydrocarbons utilizing MQ sensors but limited accuracy for viridiflorol and α-pinene. This was the first time that metal oxide sensors were used to accurately determine the concentration of a chemical constituent in a complex EO matrix. The authors [61,62,63] working on this subject concluded that this technique provided an inexpensive means of evaluating EO quality and identifying adulteration.
Cistus ladanifer collected in Spain and its EO obtained by hydro-distillation was also chemically studied but using different approaches. The authors [64] used attenuated total reflectance Fourier-transform infrared (ATR-FTIR) vibrational spectroscopy, differential scanning calorimetry (DSC), and thermogravimetric (TG) analyses. The results showed that the specific location of the bands from unsaturated and α,β-unsaturated oxo groups had been related to the different contents of terpenoids of C. ladanifer EO (mono- and sesquiterpenoids). With regard to thermal behavior, C. ladanifer EO showed relatively high thermal stability, leading the authors [64] to conclude that EO constituents were conserved in the extraction process, in this case hydro-distillation.
3.2. Chemical Composition of Cistus ladanifer EOs from Morocco
The chemical profiles of C. ladanifer EOs from Morocco differed from those of the Iberian Peninsula. Three samples were dominated by viridiflorol [53,54,55], two by camphene [31,32], and one sample each by γ-terpinene [35], 1,8-cineole [30], and trans-pinocarveol [28] (Table 1).
The chemical composition of the varieties maculatus [28] and albiflorus [29] of C. ladanifer from Morocco collected in Chefchaounem and Tanger (north of Morocco) had distinct main compounds; nevertheless, this difference could not only be attributed to the different varieties because the harvesting places and extraction processes were different (Table 1). The lowest percentages of α-pinene and camphene in the var. maculatus can be attributed to the first extraction process (Soxhlet) using hexane. The monoterpene hydrocarbons, α-pinene and camphene, must have remained in the hexane. In other work, samples of C. ladanifer oil obtained from plants of Chefchaounem had, as a main component, 1,8-cineole [30]. In this case, it is not known which variety was analyzed.
The chemical compositions of the resinoid, concrete, and absolute were also assessed by Greche et al. [29] from C. ladanifer of Tanger (Table 1). Labdane-type diterpenes were constituents present in these products, as expected.
Along with Tanger, the samples collected in Taza [33] and El Harcha forest (province of Khemisset) [34] produced EOs with viridiflorol as the main compound (Table 1). Camphene at high relative percentages was found in samples of C. ladanifer from Oulmes (province of Khemisset) [32] and Tafoughalt [31]. The EO obtained from plants C. ladanifer var. maculatus of Oulmes presented the diterpene verticiol with the same percentage as that of camphene. Samples from the same province, Khemisset, had diverse chemical profiles (Table 1).
γ-Terpinene and linderol were the most representative compounds of C. ladanifer EO from Aknoul (Fès-Meknès region) [35]. Linderol A, a C26 compound (C26H30O5), was isolated for the first time in the bark of Lindera umbellata [65]. The presence of this unexpected compound can be attributed to the type of extraction process used (microwave-assisted hydro-distillation) or a mistake, because the molecular formula provided by the authors was C10H18O, not compatible with linderol. Linderol could also be considered a synonym for borneol, but, in the same EO, the authors cite the presence of borneol.
3.3. Chemical Composition of Cistus ladanifer EOs from France
In a 1962 review, Martin [66] compared the chemical composition of the EO of C. ladanifer from France (Estérel region) and Spain. In both, there were borneol. Acetophenone, benzoic aldehyde, 2,2,6-trimethylcyclohexanone, eugenol, free carboxylic acids, unidentified alcohols, and a mix of terpenes were detected in C. ladanifer from France, whereas, in that from Spain, there were 2,3-butadione, furfural, 1,8-cineole, phenols, and α-pinene, but free carboxylic acids were absent. According to the same review, the author claimed that fresh and dried French plants differed in their EO chemical compositions.
In France, α-pinene dominated in almost all three samples of C. ladanifer EOs [24,25,26] (Table 1). Previously, Peyron and Alessandri [67] had already reported, in a review work, the presence of α-pinene as a main component in the volatile mixtures of some C. ladanifer plants cultivated in Corse [67].
The analysis of the EOs of twenty plant populations of C. ladanifer cultivated in Corse revealed that there were two samples (samples 1 and 2) in which viridiflorol dominated, with very low amounts of α-pinene (2 and 2.5%) [24] (Table 1). In contrast, samples 19 and 20 had the highest percentages of α-pinene (44.1 and 47.4%, respectively) and lower amounts of viridiflorol (4.9 and 4.7%, respectively) (Figure 9). In addition to these two groups that are easily distinguished, there are two other groups. For example, in samples 3–9, α-pinene ranged from 11.1 to 24.2%, and viridiflorol ranged from 10.7 to 7.8% (Figure 9). In the third group (samples 10–18), the percentages of α-pinene are ≥30% up to 40% and the percentages of viridiflorol ranged from 8.1 to 5.2% (Figure 9).
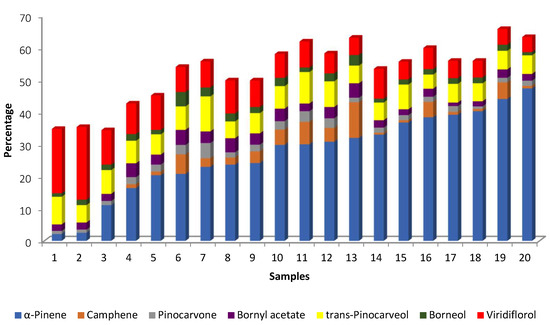
Figure 9.
Main compounds (>4.5%) of EOs obtained from 20 populations of C. ladanifer cultivated in Corse [24].
Robles et al. [25] examined the chemical composition of the two C. ladanifer varieties (maculatus and albiflorus) in three locations within the Massif de l’Estérel (south of France) (Figure 10). Only concentrations higher than 50 mg/mL were considered in this Figure, and only three compounds (viridiflorol, 2,2,6-trimethylcyclohexanone, and α-pinene) had concentrations above 50 mg/mL. The main component in all samples was α-pinene; nevertheless, the variety albiflorus had lower amounts than the variety of maculatus. The trend of viridiflorol was the opposite of that of α-pinene (Figure 10). The inverse correlation between α-pinene and viridiflorol, Figure 11, was also observed in the previous works [24,54].
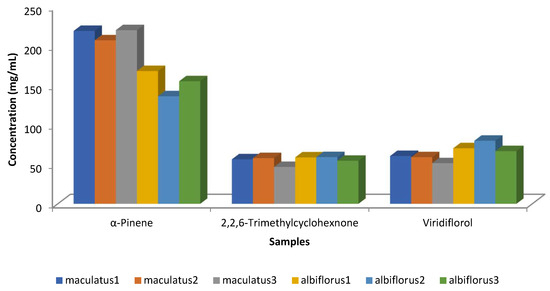
Figure 10.
Main compounds (>50 mg/mL) of the EOs isolated from C. ladanifer var. maculatus and var. albiflorus in their locations within the Massif de l’Estérel (south of France) [25].
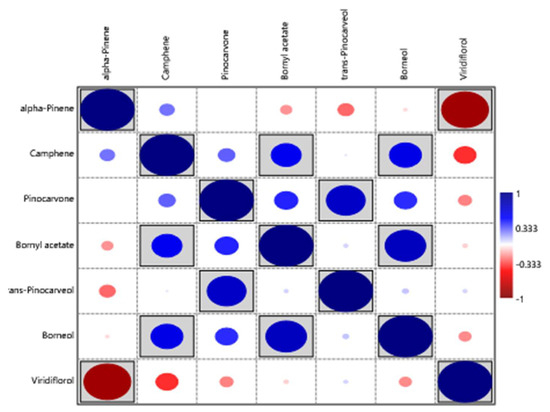
Figure 11.
Correlation between the main compounds (>50 mg/mL) of C. ladanifer EOs isolated from the var. maculatus and var. albiflorus in their locations within the Massif de l’Estérel (south of France) [24,54]. Grey background indicates that differences are significant (p < 0.05)
A resinous archeological sample from a tomb in Carthago (6th–5th century BC), housed in the Fragonard Museum (Grasse, France), was identified as labdanum, resin of the Cistus species, although no direct documentation supported this identification [68].
However, knowing through ancient documentation that populations in the Mediterranean area used Cistus spp, among other species, the authors [68] compared the chemical composition of a commercial cistus EO with that of the archeological sample. The old sample contained very few low-molecular-weight molecules, whereas the EO nearly entirely contains volatile chemicals known as mono- and sesquiterpenes. This suggests that it may be better to look for di- and triterpenes rather than mono- or sesquiterpenes in samples with archeologic origins [68].
3.4. Chemical Composition of Cistus ladanifer EOs from India and Germany
The commercial EO from India with the trade name “labdanum essential oil” had, as a main component, α-asarone [27], that is, completely different from the aforementioned C. ladanifer EOs. Also, from commercial origin of unknown country, camphene was the main compound followed by α-pinene [58] (Table 1). These findings indicate that some commercially available products labeled as Cistus ladanifer oil may have markedly different chemical profiles, raising concerns about authenticity or possible adulteration.
The EO of C. ladanifer leaves from the Botanical Institute of the University of Köln had more than 70 compounds present in low proportions of maximal 6.8% [69], in contrast to that verified later by Gülz et al. [70] that reported, as the main compounds of the EO, α-pinene (43%), camphene (12%), and δ-cadinene (7%). These compounds were detected in a fraction obtained from silica gel column chromatography by elution with pentane. In the former work, the authors observed fluctuations in the levels of the compounds over the year [70]. For example, alcohol decreased from spring to autumn, while hydrocarbons and carbonyl compounds increased during the same period. Some compounds reported by Königs and Gülz [69] in the EO extracted from the leaves of C. ladanifer include α-pinene, camphene β-pinene, sabinene, myrcene, α-phellandrene, α-terpinene, limonene, γ-terpinene, and p-cymene (hydrocarbons); 1,8-cineole (bicyclic ether); 2,2,6-trimethylcyclohexanone, fenchone, α-thujone, iso-menthone, benzaldehyde, bornyl acetate, acetophenone, cis-citral, trans-citral, and geranyl acetate (carbonyl compounds); as well as cis-hexen-3-ol-1, trans-hexen-3-ol-1, linalool, terpinen-4-ol, borneol, α-terpineol, nerol, geraniol, and eugenol (alcohols) [69].
The phenylpropanoic acid esters with monoterpene alcohols (geranyl phenylpropanoate) were for the first time reported by Proksch et al. [71] in the EO obtained by steam distillation of C. ladanifer cultivated in the field of the Botanical Institute of the University of Köln. These compounds were isolated by fractionation through SiO2-column and thin layer chromatography. The other compounds identified by these authors were 3-phenylpropanoic acid methyl ester, 2-phenyl-ethanol-1, 3-phenyl-propanol-1, geraniol, and dehydrogeraniol. In the same year, six other compounds (benzyl benzoate, cis-ocimene, 2-hydroxy-6-methylacetophenone, pinocarvone, campholene aldehyde, and tagetone) were also identified by the same authors [72]. These compounds were identified by techniques of gas-chromatography coupled with mass spectrometry (GC-MS), infrared spectroscopy (IR), and proton-nuclear magnetic resonance (1H-NMR). By reversed-phase high-performance liquid chromatography (RP-HPLC) using octyl and octadecyl silane (C8 and C18, respectively) bonded silica, water–acetonitrile mixtures as eluents, and ultraviolet (UV) detection at 200, 220, and 254 nm, Strack et al. [73] separated sesquiterpenes and oxygenated monoterpenes. The compounds were separated, and identified by this method, from C. ladanifer EO, were benzaldehyde, acetophenone, menthone, 1,8-cineole, fenchone, neral, geranial, bornyl acetate, geranyl acetate, phenylethyl phenylpropanoate, dehydrogeranyl phenylpropanoate, and geranyl phenylpropanoate. Despite this finding, further studies on the EO of C. ladanifer preferentially used GC-MS for the separation and identification of its compounds.
4. Biological Properties of Cistus ladanifer EOs or Volatiles
In addition to the chemical composition of C. ladanifer EOs and volatiles summarized in Table 1, numerous studies have reported their biological properties. These include antimicrobial, antioxidant activities, anti-inflammatory, cytotoxicity, and herbicidal effects, among others. The following sections provide an overview of these bioactivities as described by various authors.
4.1. Biological Properties of Cistus ladanifer EOs Without Chemical Composition
As aforementioned, the biological properties of C. ladanifer EOs were also evaluated by diverse authors even without the chemical profiles of the corresponding EOs provided in very few cases. This section is focused on the diverse biological properties of C. ladanifer EOs, in which the respective composition was not provided. For instance, Barbosa et al. [74] evaluated the nematicidal activity, against Bursaphelenchus xylophilus, of C. ladanifer EO obtained by distillation–extraction (D.-E.) using a Likens-Nickerson apparatus, but the chemical composition was not described. For the extraction of the volatiles, the aerial parts from the vegetative phase and collected in Portugal (Faro) were used. The percentage of inhibition was very low (2.49%). The best activities were found for the EO of Cymbopogon citratus (DC) Stapf. (98.12%) and Mentha pulegium L. (68.60%). Guimarães et al. [75] studied the antioxidant activity of C. ladanifer EO obtained by hydro-distillation from the fresh leaves collected in Portugal (Natural Park of Montesinho, Northeastern Portugal). The EO yield was 0.63%. The antioxidant activities were performed through three distinct methods and, in all cases, the antioxidant activity of C. ladanifer EO was higher than the remaining samples (Citrus latifolia, Cupressus lusitanica, and Eucalyptus gunnii). The methods used were the capacity for scavenging the 2,2-diphenyl-1-picrylhydrazyl free radicals (DPPH), reducing power, and inhibition of β-carotene bleaching that determined the capacity to inhibit lipid peroxidation. The results were expressed as concentrations, providing 50% of radical scavenging activity (EC50). The EC50 found for the DPPH, reducing power and inhibition of lipid peroxidation were 36.28, 4.00, and 0.12 mg/mL, respectively.
Kotba et al. [76] studied the effect of Thymus vulgaris, Origanum compactum, Rosmarinus officinalis, Eucalyptus sp., Salvia sp., Lavandula stoechas, and Cistus ladanifer EOs on the growth of Rhizoctonia solani strains, isolated from many crops in Morocco based on radial mycelial growth on potato dextrose agar at different pH and temperature levels. The EO obtained from the leaves of C. ladanifer showed the lowest level of growth inhibition rates on most of R. solani isolates, in contrast to those of O. compactum and T. vulgaris.
Biopesticides, including EOs, have a history of use as potato sprout suppressants. When potatoes are harvested, the many buds on their surfaces are in a natural state of dormancy and will not sprout. This natural dormancy is temporary, and the buds will start to sprout after several months of storage. Limiting potato sprouting during storage is necessary because it reduces tuber weight, alters the texture and nutritional content of the tuber, and produces the poisonous alkaloid solanine. Thoma et al. [77] studied the sprout suppressant effect of twenty-one EOs in cv. Ranger Russet potatoes at room-temperature storage. For shorter storage periods, the EOs of C. ladanifer, Ocimum basilicum, Ormenis mixta, and Salvia sclarea greatly decreased the length of the sprouts, while those of Laurus nobilis and Cinnamomum zeylanicum (bark) significantly decreased the number of sprouts. The chemical composition of C. ladanifer was not described; only that of Artemisia herba-alba was provided because this EO was the most effective at suppressing both sprout length and sprout number over 90 days.
4.2. Antioxidant Activity
Antioxidant activity of C. ladanifer EOs was determined by several methods (ferric reducing antioxidant activity power (FRAP), β-carotene bleaching assay, reducing power activity, oxygen radical absorbance capacity (ORAC), chelating activity, and the ability to scavenge DPPH and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) free radicals). The results were presented in different ways as can be seen in Table 1. The most used methods were those of DPPH and ABTS. The C. ladanifer EO from commercial origin of India possessed the ability for scavenging DPPH and ABTS free radicals with IC50 values of 7.3 and 1.13 μL/mL, respectively [27]. An EO of C. ladanifer from Morocco also showed ability for scavenging those free radicals but with IC50 values of 178.29 and 134.02 μg/mL for DPPH and ABTS, respectively, which is much better than that from India. The capacity for scavenging DPPH was also determined for Portuguese C. ladanifer EOs (IC50 between 0.691 and 0.9% (v/v) (Table 1). According to Oliveira et al. [17], such values can be considered as poor due to the low antioxidant activity index (0.0037). These authors also evaluated the antioxidant activity of the hydrolates and concluded that the activity (IC50 value = 43%, v/v) was much lower than that observed for the corresponding EO as expected, given the reduced levels of volatiles in the hydrolates. The capacity of the EOs of rockrose to scavenge DPPH free radicals were determined by other teams, but the results were not presented through IC50 values but by percentages of inhibition or Trolox equivalent/g, making it difficult to compare the results (Table 1).
The relationship between antioxidant activity and EO composition throughout the year was evaluated [54]. Regarding the correlation between ORAC values and EO composition, significant correlations in the EOs of the 7-year-old population were found for contents of verbenene, which demonstrated a strong negative correlation, and ledene that showed a moderate positive correlation. In the EOs of the older population (12 years old), negative correlations were found for contents of verbenene, myrtenal, and borneol; and positive correlations were found for limonene and α-pinene. Mediavilla et al. [51] found higher antioxidant activity of the C. ladanifer EOs, as measured by the ORAC assay, in October than in September, such as previously reported [54], although with lower differences between the harvesting periods than those reported [54]. However, there are authors [42] who did not find any correlation between antioxidant activity, measured through the ABTS method, and different harvesting periods of the year. This variability is not uncommon, and even occurs very frequently with other plants, because it seems to be related to the chemical composition of the EOs, which despite having a similar profile, the has main compounds that are present in different proportions [78]. Furthermore, EOs’ antioxidant properties can be ascribed to both their major molecules and their minor constituents, which may have antagonistic or synergistic effects [79].
The antioxidant activities of C. ladanifer EOs at 2 mg/mL, from Andévalo and Cerezal (Spain) with α-pinene and viridiflorol as main compounds, respectively, were also assessed using cellular murine macrophage cell line RAW 264.7 using azobis(2-methylpropionamidine) dihydrochloride (AAPH) and 2,7-dichlorofluorescein diacetate (DCFHDA) as a fluorescent marker. Both EOs had similar percentages of inhibition (83.24 and 81.13%, respectively) (Table 1) [49].
4.3. Antimicrobial Activity
Diverse microorganisms were tested, and the response differed as expected, not only due to the characteristic response of the microorganism to the natural product but also due to the chemical variability of the EOs. Diverse Gram-positive (Staphylococcus aureus, S. epidermidis, Listeria innocua, L. monocytogenes, Bacillus subtilis, B. cereus, Enterococcus faecalis, Cutibacterium acnes) and Gram-negative microorganisms (Escherichia coli, Campylobacter jejuni, Pseudomonas aeruginosa, Pseudomonas savastanoi, Salmonella typhy, Acinetobacter baumanni, Proteus mirabilis, Klebsiella pnuemoniae, Morganella morganii) were used. Some of the microorganisms were repeatedly used by several teams. As shown in Table 1, the effect against yeasts and fungi was also tested.
The most researched Gram-positive microorganism for C. ladanifer EOs was Staphylococcus aureus. There was activity in all studies, with inhibition zones measuring between 11 and 23 mm. Compared with the reference strain (11 mm), the EO containing 36% α-pinene was more efficient against methicillin-resistant Staphylococcus aureus (MRSA) (23 mm) [40]. The minimum inhibitory concentration (MIC) value found was 10 µL/mL in the Portugal and Morocco EOs and 16 µL/mL in the Portugal sample. Only the Moroccan EO had violiflorol as its primary constituent; other EOs had α-pinene (Table 1).
The anti-Listeria monocytogenes effect of the C. ladanifer EO revealed that Cerezal (Spain) EOs with more viridiflorol (24%) and lower α-pinene (19%) had lower MIC values (0.3 mg/mL) than Andévalo samples (0.6 mg/mL) [49]. Viridiflorol may be more successful than α-pinene, as evidenced by a similar pattern seen for Enterococcus faecalis. The activity against E. coli, however, was identical for both samples (0.6 mg/mL). MIC values for Listeria and E. faecalis in a sample from Mértola, Portugal, were 8 µL/mL [41], which were comparable to the Andévalo sample that was high in α-pinene. For E. faecalis, the Portuguese EO had a lower MIC (Table 1).
The impact of α-pinene-rich C. ladanifer EO on Cutibacterium acnes strains from several phylotypes that are linked to acne and healthy skin was studied [17,44]. MIC values varied with phylotype, ranging from 0.06% to 0.5% (v/v) (Table 1). Additionally, the EO inhibited the formation of biofilms; the half maximum effective concentration (EC50) values for biofilm biomass ranged from 0.06% to >0.5% (v/v). Nevertheless, Thymus x citriodorus EO, which mainly constitutes thymol, 1,8-cineole, and geraniol, show greater activity than C. ladanifer EO [44].
A study made by Chaloupková et al. [54], in which the annual variation in yield, chemical composition, and antibacterial activity against B. cereus of rockrose EO was evaluated in young plants (7 years old) and older ones (12 years old), permitted the conclusion that the older ones presented better activities than the youngest ones, although no correlation had been found between MIC values and EO composition. In the same work, the results of vapor phases referred to as agar (lid) showed weaker antibacterial activity compared with the liquid phase (broth) [54].
Escherichia coli and Pseudomonas aeruginosa were the most often employed species among Gram-negative bacteria to investigate the antibacterial activity of C. ladanifer EOs (Table 1). In all cases and regardless of the dominant compounds of the EOS, they possessed an antimicrobial effect. There are significant differences between the results that have the same unities and that are easy to compare. Regarding E. coli, a MIC value of 25 mg/mL was found for an EO of C. ladanifer from Morocco, and 0.6 mg/mL for an EO of Spain. This disparity may have been partially caused by the chemical compositions of the EOs, which differed significantly between Moroccan EO (camphene and verticiol) and Spanish EOs (α-pinene or viridiflorol) (Table 1). However, since the same EO of C. ladanifer inhibited the growth of E. coli SA/4 (an extended spectrum beta-lactamase producer) with an experimental inhibition zone of 20 mm, while the inhibition zone for the reference strain E. coli ATCC 25922 was smaller (10 mm) [40], other factors might affect the response.
α-Pinene and viridiflorol + α-pinene as main compounds of Spanish EOs of C. laddanifer collected in different places were able to prevent the growth of P. aeruginosa, Klebsiella pneumoniae, and Proteus mirabilis in the same way as they presented equal MIC values (2.5 mg/mL, >2.5 mg/mL, and >2.5 mg/mL, respectively) [49]. The C. ladanifer EO with viridiflorol + α-pinene had better activity against Morganella morganii (MIC = 0.6 mg/mL) than the EO in which α-pinene dominated (Table 1) [49].
Regardless of their chemical compositions, C. ladanifer EOs demonstrated the capacity to inhibit the growth of yeasts and fungi, albeit at varying intensities. When compared with the other C. ladanifer EOs, one EO had the lowest MIC values for Aspergillus niger, Tricophyton rubrum, and Candida albicans (0.001, 0.01, and 0.001 mg/mL, respectively), making it the most effective EO against those microorganisms. The MIC values differed from the others in a significant way. The main compounds in the EO were verticiol and camphene (Table 1) [32].
By assessing the impact on ergosterol biosynthesis, the mechanism of action of C. ladanifer EO against aflatoxigenic Aspergillus flavus AF-M-K5 was investigated [27]. Ergosterol content dramatically dropped as EO concentration rose; inhibition percentages ranged from 4% at 0.1 μL/mL to 84.52% at 0.5 μL/mL (Table 1). This inhibition implies that the fungal cell membrane was affected mainly by α-asarone, the main compound of the EO. Furthermore, C. ladanifer EO totally stopped A. flavus from producing aflatoxin B1 at 0.5 μL/mL [27].
Brindhadevi et al. [80] evaluated the antimicrobial activity of silver nanoparticles functionalized with labdanum oil. These functionalized nanoparticles were used to produce wound dressings to enhance the healing wounds. An increased antibacterial activity was observed when the produced wound dressings and silver nanoparticles were examined for antimicrobial activity. The data revealed that at the end of 24 h incubation at 37 °C, silver nanoparticles and silver nanoparticles functionalized with C. ladanifer oil exhibited inhibitory actions against Klebsiella pneumoniae, with inhibition zone diameters of 37 mm and 42 mm, respectively. Silver nanoparticles and silver nanoparticles functionalized with C. ladanifer oil had inhibitory zones of 40 and 41 mm for A. niger, 35 and 45 mm for S. aureus, 34 and 35 mm for B. subtilis, and 40 and 42 mm for E. coli, respectively. The chemical composition was not assessed, therefore it was difficult to attribute some of these abilities to some compounds, although the authors had discussed their results based on the chemical composition of the oil from other oil samples [80].
4.4. Phytotoxical Activity
The reduced plant diversity and richness in the presence of C. ladanifer is believed to be due to the allelochemicals released by its leaves and stems. These compounds remain in the soil for a long time, breaking down very slowly in the soil. Despite having low concentrations (of the order of μg/g soil), aglycone flavonoids (apigenin, 3-O-methyl kaempferol, 4’-O-methylapigenin, 7-O-methylapigenin, 3,4’-di-O-methylkaempferol and 3,7-di-O-methylkaempferol) remaining on the soil for long periods may change its properties and inhibit the germination and growth of other species, explaining the phytotoxic activity attributed to C. ladanifer [81,82,83,84]. Diterpenes are also responsible for the phytotoxicity, and three were identified in C. ladanifer exudates (oxocativic acid, 7-oxo-8-labden-15-oic acid, and 6β-acetoxi-7-oxo-8-labden-15-oic acid) [15,85]. The allelopathic potential of C. ladanifer secondary metabolites, conjointly and not separately, is also dependent on the environmental factors, high temperatures, and long photoperiods. These three factors increase the phytotoxicity in inhibiting both germination and seedling development [16,86].
With very few exceptions, diterpenes are absent in the EOs of C. ladanifer, at least at concentrations >4.5% (Table 1), with monoterpenes, sesquiterpenes and other groups of compounds being the ones that are present in those EOs at relatively high concentrations. However, there are works showing that sesquiterpene-rich EOs present phytotoxic activity [87]. In this way, some authors [53] evaluated the effect of viridiflorol-rich EO of C. ladanifer from different locations in Spain on the seed germination and seedling length inhibition of Raphanus sativus (Figure 12 and Table 1). Both seed germination and seedling length were mostly affected by the EOs coming from the slate substrate zone, because lower ED50 meant higher inhibition. Comparing this Figure with that of Figure 7, it is possible to conclude that there is a negative correlation between the percentages of viridiflorol and ED50 values and a positive correlation between the percentages of α-pinene and the ED50 values. The authors [53] also found a negative correlation between these values and the percentages of the diterpene 15-nor-labdan-8-ol, not represented in Figure 7 or in Table 1 because the percentages were <4.5%. C. ladanifer EOs with high percentages of viridiflorol from Morocco also have the capacity to inhibit the germination of tomato seeds [33]. Raphanus sativus seed germination and seedling length were likewise inhibited by the C. ladanifer EO, which contained the sesquiterpene ledol at a rather high concentration (19%) [52], indicating that oxygenated sesquiterpenes had an inhibitory effect on weed seed germination [53]. Still using a Spanish rockrose EO, in which trans-pinocarveol (20%) and viridiflorol (14%) were main compounds, it was possible to particularly inhibit the seed germination of Amaranthus hybridus, Conyza canadensis, and Parietaria judaica, regardless of the concentrations of the EOs used (0.125 to 1 µL/mL) [46].
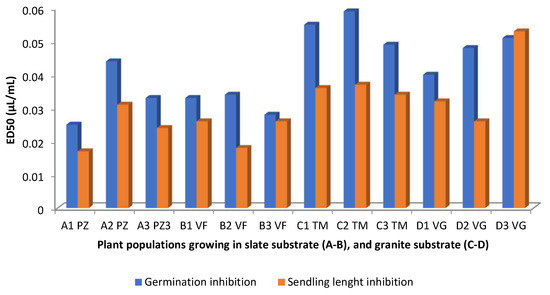
Figure 12.
Germination and seedling length inhibition of Raphanus sativus by C. ladanifer EOs obtained from plant populations (A1,B1,C1,D1; A2,B2,C2,D2; and A3,B3,C3,D3 growing in PZ: Pozuelo de Zarzón (slate substrate); VF: Valverde del Fresno (slate substrate); TM: Torre de Don Miguel (granite substrate); VG: VillasBuenas de Gata (granite substrate) [53].
With the exception of the concentration 0.44 µL/mL, the EOs obtained during the fruit maturation stage exhibited stronger inhibition of Raphanus sativus seedling length compared with those collected during flowering (Figure 13A). This indicates that the phytotoxic activity of C. ladanifer EOs varies according to the plant’s phenological stage. In contrast, this trend was less evident in seed germination inhibition (Figure 13B). When comparing these Figures to Figure 8 it seemed that higher percentages of α-pinene and lower concentrations of 2,2,6-trimethylcyclohexanone in the EOS obtained from plants at fruit maturation better inhibited the seedling length of R. sativus (Figure 8 and Figure 13A,B). This can be considered for compounds that are present in the C. ladanifer EOs at concentrations higher than 4.5%. Other correlations were made by the authors [55] between some other minor constituents of the EOs and the seedling length of R. sativus. For example, EO samples from the fruit maturation stage that are richer in monoterpenes and sesquiterpenes have higher phytotoxic activity on the early growth of R. sativus seedlings than the EO samples from the flowering stage, which have a higher percentage of diterpenes such as 15-nor-labdan-8-ol [55]. This is somehow distinct from what was previously reported [53]. Regarding the capacity to inhibit seed germination, there is no correlation between the phenological stage of the plant and phytotoxicity activity as found for the seedling growth, suggesting diverse inhibitory mechanisms involved in the activities. The highest percentages of monoterpene hydrocarbons in October than in August were also reported in other work [56]. In contrast to the aforementioned results, some other authors [27] demonstrated that the C. ladanifer EO with 79% of α-asarone did not influence seed germination and length of the radical and plumule of seedings of Brassica juncea, Arachis hypogaea, Sesamum indicum, Helianthus annuus, and B. campestris.
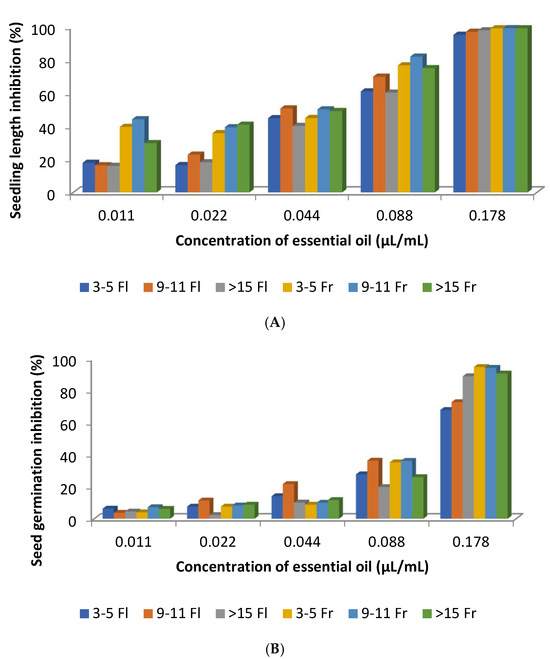
Figure 13.
Seedling length inhibition (A) and seed germination inhibition (B) by C. ladanifer EOs at different concentrations obtained from different aged plant at two phenological stages in Extremadura (Spain). 3–5 Fl: plants of 3–5 years old at flowering phase; 9–11 Fl: plants of 9–11 years old at flowering phase; >15 Fl: plants of >15 years old at flowering phase; 3–5 Fr: plants of 3–5 years old at fruit maturation phase; 9–11 Fr: plants of 9–11 years old at fruit maturation phase; >15 Fr: plants of >15 years old at fruit maturation phase [55].
4.5. Citotoxicity, Anti-Inflammatory, and Other Biological Activities
In vitro cytotoxicity of C. ladanifer EOs was evaluated using several cell lines (Table 1). Ecotoxicity was also checked through the determination of the acute toxicity performed through the model organism Daphnia magna. The result showed that the EO in which α-pinene dominated [39] was not toxic because the EC50 (concentration to immobilize 50 per cent of the D. magna after 48 h) was higher than 100 mg/L. The EC50 value was 199.7 mg/L.
The estimated half-maximal inhibitory concentrations for cellular viability (IC50) obtained for the two tested cell lines (mouse fibroblasts L929 and macrophages RAW264.7) and estimated half-maximal effective concentrations for nitric oxide (NO) production in macrophages (EC50) were 0.027, 0.012, and 0.002% (v/v) (Table 1) [17] for the α-pinene-rich EO of C. ladanifer from Portugal. The same assays were made with the corresponding hydrolates, and the values were significantly higher (Table 2). According to the authors [17], the cytotoxicity on L929 fibroblasts was followed to determine wound-healing potential, and the results showed that the hydrolates were more biocompatible than the EO due to the higher IC50 values. The anti-inflammatory activity of C. ladanifer EO measured through the NO production in macrophages after being exposed to lipopolysaccharide (LPS) at 1 µg/mL was also better than the respective hydrolates (lower EC50 values, higher activity) (Table 1 and Table 2).

Table 2.
Chemical composition and biological properties of C. ladanifer hydrolates.
The in vitro cytotoxic activity on human cancer cell lines (breast adenocarcinoma (MCF7, T47D, and MDA-MB-231), chronic myelogenous erythroleukemia (K562), and neuroblastoma cell lines (SH-SY5Y) of sixteen commercial EOs were evaluated [32], including that of Cistus ladanifer. The IC50 values in parts-per million (ppm) are depicted in Table 1. The K562 cells were the most sensitive to the C. ladanifer EO since they presented the lowest IC50 value (Table 1). However, the Cinnamomum zeylanicum and Litsea cubeba EOs were the most active with IC50 values <11.5 ppm, whereas for C. ladanifer EO, the IC50 value was >45 ppm. (E)-Cinnamaldehyde, and citral were the major compounds in C. zeylanicum and L. cubeba EOs, whereas α-pinene dominated the C. ladanifer EO [48]. In another study [49], C. ladanifer EO showed high potential on lung carcinoma NCl-H460 since presenting the concentration of the sample caused 50% of cell growth inhibition (GI50), a value lower (14.27 µg/mL) than those of the remaining tumor cell lines tested, particularly for the sample collected in Andévalo (Spain), in which α-pinene was the main component (Table 1). All of the samples in the non-tumor cell lines also showed cytotoxicity; nevertheless, with GI50 values higher than the tumor cell lines. These results might indicate that these C. ladanifer EOs can be used in some circumstances without being harmful [49].
The anti-inflammatory activity of C. ladanifer EOs from Spain (Andévalo and Cerezal) was also assayed [49] through the evaluation of the inhibition of the LPS-induced NO production on RAW264.7. The IC50 values were 19.27 and 21.00 µg/mL, respectively, that is, without significant difference between the EOS collected in both places.
The inhibitory activity of some enzymes (α-amylase, α-glucosidase) were also evaluated in C. ladanifer EOs from Morocco [32] and of commercial origin [58], with distinct chemical compositions (Table 1); nevertheless, the results could not be compared since the ways of presenting them were different. The inhibitory activities of tyrosinase, acetylcholinesterase, and butirylcholinesterase were also evaluated [58], as well as that of pancreatic lipase [32]. The inhibitory activities of the enzymes α-amylase, α-glucosidase, acetylcholinesterase, butirylcholinesterase, and tyrosinase, potently associated with diabetes, Alzheimer’s disease, and pigmentation disorders, respectively, were attributed to the presence of camphene, α-pinene, and bornyl acetate, the main compounds of the commercial EO of C. ladanifer [58].
5. By-Products Obtained from Cistus ladanifer EOs Distilleries
During the research for the current review on Cistus ladanifer EOs, some articles that did not focus on EOs were found. Rather, the authors focused on the use of the plant material that resulted from steam distillation as well as hydrolates. Therefore, a brief review of this topic was also deemed pertinent.
The residual material obtained after steam distillation of the aerial parts of C. ladanifer is usually used as firewood or fuel for further hydro-distillations after drying at room temperature (usually 1 day). These solid materials from C. ladanifer distilleries are lignocellulosic materials, which can be an income to produce biofuels and bioproducts. Alves-Ferreira et al. [88,89] investigated the utilization of mild autohydrolysis processes to selectively hydrolyze hemicelluloses from C. ladanifer residual material, producing hemicellulose-derived oligosaccharides and extracting water-soluble phenolic compounds (4-methylguaiacol, 4-ethylguaiacol, 4-vinylguaiacol, syringol, 4-methylsyringol, 4-vinylsyringol, trans-4-propenylsyringol, trans-coniferaldehyde) targeted to potential added-value applications and cellulose/lignin-enriched solids. The same authors also concluded in other research that the distillery residues with residues high concentrations of extractives, particularly polar extractives that were rich in phenolics (syringol derivatives such as 4-methylsyringol, 4-ethylsyringol, 4-vinyl syringol, 4-allylsyringol, cis-4-propenylsyringol, trans-4-propenyldyringol, syringaldehyde, homosyringaldehyde, acetosyringone, syringylacetone, and trans-sinapaldehyde; guaiacol derivatives such as 4-methylguaiacol, 4-ethylguaiacol, 4-vinylguaiacol, eugenol, trans-isoeugenol, vanillin, 1-(4-hydroxy-3-methoxyphenyl)-propyne, homovanillin, acetoguaiacone, guaiacylacetone, trans-coniferyl alcohol, and trans-coniferaldehyde; as well as p-hydroxyphenyl derivatives such as phenol, o-cresol, p-cresol, m-cresol, 2,3-dihydrobenzofuran, and 2,3-dimethyl-phenol), flavonoids (apigenin, isoquercetin, gallocatechins), and tannins (particularly in the stem extractives) were found, which demonstrated strong antioxidant activity, being a significant chemical characteristic of C. ladanifer compounds and their distillery residues [90]. Later on, Alves-Ferreira et al. [91] studied the delignification of extracted and hydrothermally pretreated biomass using two organosolv processes, ethanol/water mixtures, and alkali-catalyzed glycerol and by an alkali (sodium hydroxide) process under different reaction conditions, followed by the evaluation of the phenolic composition of soluble lignin, determined by capillary zone electrophoresis and pyrolysis-GC-MS. Low-molecular-weight phenolic compounds were identified in all the delignification liquors. Previously, Tavares et al. [92] also obtained phenolic-rich extracts from the remaining extracted solid residues of C. ladanifer using ultrasound-assisted extraction with ethanol (salicylic acid, 3-hydroxybenzoic acid, apigenin, quercetin, syringic acid) and 70% acetone (salicylic acid, 3-hydroxybenzoic acid, apigenin, gallocatechins, syringic acid). The waste distilled by-products remaining after steam distillation of the underutilized biomass from Cistus ladanifer can also be a natural source of other high value products with biological activities, namely, phenolic compounds (quercetins, gallocatechins, hydroxycinnamic acids derivatives, gallic acid), which were also studied by Tavares et al. [92]. Some of these compounds (gallic acid, ellagic acid, apigenin, kaempferol diglycoside, kaempferol methyl ether, kaempferol dimethylether) were also previously described by Sánchez-Vioque et al. [93] in C. ladanifer solid residues after steam distillation to obtain EOs. The extracts obtained by Soxhlet or ultrasound-assisted extraction containing those compounds showed significant antioxidant activity. Taking into account all of these findings, using C. ladanifer biomass for an extractive lignocellulosic-based biorefinery is a possible way that could raise profits for the current distilleries of essential oils [88].
Additional compounds can be produced from hemicellulose hydrolysates derived from C. ladanifer waste gathered from an EO distillery, such as D-lactic acid. Escherichia coli JU15 can effectively use both hemicellulose- and cellulose-derived sugars as the carbon source to produce D-lactic acid, with a high yield (92–99%). E. coli JU15 can also metabolize glucose and xylose while retaining high lactate yields when moderate concentrations of putative inhibitors, such as furans, formic acid, acetic acid, and phenolic compounds, are present in non-detoxified hydrolysates. Lactic acid is an organic acid that finds widespread use in the chemical, pharmaceutical, cosmetic, and food industries. The demand for polylactic acid, a biodegradable and thermostable semi-crystalline polymer used as a sustainable alternative to plastics made from petrochemicals, has increased dramatically due to its potential applications [94].
Álvaro et al. [95] demonstrated the potential of utilizing solid residues after EO extraction as energy resources. Biogas was produced by direct anaerobic digestion of the post-distillation biomass with a particle size of less than 1 mm and an inoculum obtained from the anaerobic digester facility of the Wastewater Treatment Plant of Soria (Spain). In comparison to the solid-fuel method, C. ladanifer exhibited noteworthy biomethane production with a total energy recovery of between 25% and 45%. According to the authors [95], distillation by-products of C. ladanifer can be potentially considered as co-substrate for biogas generation with a competitive methane yield, similar to other lignocellulosic wastes.
Hydrolates, hydrosols, or floral water correspond to the distilled water that remains after the hydro- or steam distillation and the separation from the corresponding EO. The hydrolate is usually rich in EO water-soluble compounds. This hydrolate can be considered a by-product that can be obtained within the biorefinery concept from forest biomass. It possesses antimicrobial, antioxidant, anti-inflammatory, antispasmodic, and relaxing properties, among others, beyond the pleasant odor and flavor, interesting for the perfumery, cosmetic, and flavor industries [42].
Table 2 depicts the chemical composition of hydrolates obtained from diverse regions and the corresponding biological properties found by the authors. Generally, the activities are poor or even absent as expected since the amounts of terpenes are lower than those found in the essential oils.
After steam distillation or hydro-distillation, Tavares et al. [42] also recovered the hydrolates and assessed their chemical composition (Table 2 and Figure 6 in Section 3.1). The hydrolates included very small percentages of viridiflorol and bornyl acetate and almost no hydrocarbon monoterpene molecules (tricyclene, α-pinene, camphene, and limonene) (Figure 6). In contrast, all hydrolates had higher percentages of verbenone, terpinen-4-ol, and trans-pinocarveol, whereas samples that were obtained by steam distillation had higher percentages of 2,2,6-trimethylcyclohexanone and borneol (Figure 6).
The EOs and hydrolates were also obtained by Pérez-Izquierdo et al. [52] from C. ladanifer collected in Spain (Guijo de Granadilla, north of Extremadura) by hydro-distillation. The hydrocarbons were absent in the hydrolate whereas pinocarvone (6.31%) and trans-pinocarveol (10.96%) were only present in this product. The percentage of viridiflorol was lower in the hydrolate (3.66%) than in the EO (7.11%) as reported for Portuguese samples (Table 2 and Figure 6 in Section 3.1) [42]. The same pattern was observed for 2,2,6-trimethylcyclohexanone (2.82% in the EO and 10.31% in the hydrolate) as observed in the Portuguese samples, mainly in those obtained by steam distillation. The percentages of bornyl acetate were similar in both the EO (4.80%) and the hydrolate (4.38%) (Table 1 and Table 2). In conclusion, in the two independent assays, there is a similar pattern.
C. ladanifer aerial parts collected in the central-west region of Portugal produced an EO rich in α-pinene (35.8%) that was absent in the hydrolate [43], as expected. However, the ketone 2,2,6-trimethylcyclohexanone that was at higher concentrations in hydrolates in the aforementioned works, in this case, was absent in the hydrolate, although it was at 6.7% in the EO. Other differences were also possible to detect: the presence of endo-borneol (8.4%), p-cymen-8-ol (10.7%), myrtenol (11.2%), verbenone (9.8%), and 4-hydroxy-3-methylacetophenone (21.6%) in the hydrolate, not reported by the other authors [42,52]. For commercial Portuguese-origin C. ladanifer hydrolates (Proentia® company), the lack of monoterpene hydrocarbons was also reported [17], although the EO was high in α-pinene.
6. Conclusions
Cistus ladanifer L., a widely recognized Mediterranean shrub, is a highly versatile species with significant potential for cosmetic and pharmaceutical applications. The chemical composition of its essential oils and volatiles varies across Mediterranean regions, with α-pinene, camphene, and viridiflorol commonly present, though in differing concentrations depending on geographic origin, season, plant age, storage conditions, and extraction methods.
The biological activities of C. ladanifer EOs and hydrolates, particularly their antibacterial, antioxidant, anti-inflammatory, cytotoxic, and phytotoxic properties, highlight the value of this species. To fully exploit its potential, it is crucial to develop improved preservation methods for EOs and hydrolates, enabling broader application in various industries.
Recent research has explored innovative approaches such as nanoencapsulation, bioactive film development, and the use of EOs in food and pharmaceutical packaging [38,96]. Additionally, the valorization of distillation by-products, including hydrolates, lignocellulosic residues, and phenolic-rich extracts, supports the sustainable utilization of C. ladanifer. Standardizing extraction processes and promoting the use of underexploited by-products are essential steps toward maximizing the benefits of this valuable yet underutilized species.
Moreover, the lignocellulosic residues left after essential oil extraction represent a valuable resource for biorefinery processes and the recovery of phenolic-rich compounds. Rather than viewing C. ladanifer as an invasive or undesirable species, especially in the increasingly arid and fire-prone Mediterranean basin, it should be recognized as a multifunctional natural resource that contributes to sustainable forestry, climate adaptation strategies, and circular bioeconomy initiatives.
Author Contributions
Conceptualization, M.d.G.M. and C.G.; writing—original draft preparation, M.d.G.M. and C.G.; writing—review and editing, M.d.G.M., C.G., B.B. and T.D. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are thankful to the Fundação para a Ciência e Tecnologia (FCT), Portugal, for the support through “Concurso Estímulo ao Emprego Científico na Modalidade de Apoio Institucional” CEECINST/00146/2018/CP1493/CT0003 (https://doi.org/10.54499/CEECINST/00146/2018/CP1493/CT0003) (Universidade do Algarve) and Project UIDB/05183 (MED strategic project). The Algerian authors also gratefully acknowledge the team of Laboratory of Biological and Agronomic Sciences (LBAS), University of Laghouat (Algeria).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Guzmán, B.; Vargas, P. Historical biogeography and character evolution of Cistaceae (Malvales) based on analysis of plastid rbcL and trnL-trnF sequences. Org. Divers. Evol. 2009, 9, 83–99. [Google Scholar] [CrossRef]
- Sánchez-Gómez, P.; Cánovas, J.L.; Lahora, A.; Catalán, A.E.; Jiménez, J.F. Disentangling the taxonomical uncertainties about the presence of Cistus pouzolzii (Cistaceae) in the Iberian Peninsula. Mediterr. Bot. 2024, 45, e90714. [Google Scholar] [CrossRef]
- Frazão, D.F.; Raimundo, J.R.; Domingues, J.L.; Quintela-Sabarís, C.; Gonçalves, J.C.; Delgado, F. Cistus ladanifer (Cistaceae): A natural resource in Mediterranean-type ecosystems. Planta 2018, 247, 289–300. [Google Scholar] [CrossRef]
- Civeyrel, L.; Leclercq, J.; Demoly, J.-P.; Agnan, Y.; Quèbre, N.; Pélissier, C.; Otto, T. Molecular systematics, character evolution, and pollen morphology of Cistus and Halimium (Cistaceae). Plant Syst. Evol. 2011, 295, 23–54. [Google Scholar] [CrossRef]
- Carlier, J.; Leitão, J.; Fonseca, F. Population genetic structure of Cistus ladanifer L. (Cistaceae) and genetic differentiation from co-occurring Cistus species. Plant Species Biol. 2008, 23, 141–151. [Google Scholar] [CrossRef]
- Demoly, J.-P.; Marrero, M.V.; Baudet, A.B. Contribution à la connaissance des cistes de la section Macrostylia Willk. (Cistus L., Ciataceae). J. Bot. 2006, 36, 13–38. [Google Scholar]
- Papaefthimiou, D.; Papanikolaou, A.; Falara, V.; Givanoudi, S.; Kostas, S.; Kanellis, A.K. Genus Cistus: A model for exploring labdane-type diterpenes’ biosynthesis and a natural source of high value products with biological, aromatic, and pharmacological properties. Front. Chem. 2014, 2, 45. [Google Scholar] [CrossRef]
- Ribeiro, S.; Delgado, F. Cistus ladanifer L. subsp. Ladanifer. In Cardo; Azevedo, L., Ed.; Norprint: Santo Tirso, Portugal, 2016; pp. 52–53. [Google Scholar]
- Frazão, D.F.; Martins-Gomes, C.; Steck, J.L.; Keller, J.; Delgado, F.; Gonçalves, J.C.; Bunzel, M.; Pintado, C.M.B.S.; Diaz, T.S.; Silva, A.M. Labdanum resin from Cistus ladanifer L.: A natural and sustainable ingredient for skin care cosmetics with relevant cosmeceutical bioactivities. Plants 2022, 11, 1477. [Google Scholar] [CrossRef] [PubMed]
- Weyerstahl, P.; Marschall, H.; Weirauch, M.; Thefeld, K.; Surburg, H. Constituents of commercial labdanum oil. Flavour Fragr. J. 1998, 13, 295–318. [Google Scholar] [CrossRef]
- Greche, H.; Mrabet, N.; Ismaili-Alaoui, M.; Hajjaji, N.; Bousta, D.; Dahchour, A.; Boukir, A.; Benjilali, B. Chemical composition, antibacterial and antifungal activities of Moroccan Cistus ladanifer L. leaves extracts. In Recherches sur les Plantes Aromatiques et Médicinales, Preceedings of the Congrès International, Mezraoua (Taounate) and Fes, Morocco, 22–24 March 2007; Grèche, H., Ennabili, A., Eds.; INPMA: Rabat, Morocco, 2008; pp. 201–213. [Google Scholar]
- Morgado, J.M.; Tapias, R.; Alesso, P. Producción de goma bruta de jara (Cistus ladanifer L.) en el suroeste de la Península Ibérica. In Proceedings of the Congresos Forestales 2005, Zaragoza, Spain, 26–30 September 2005. [Google Scholar]
- Chaves, N.; Alías, J.C.; Sosa, T. Phytotoxicity of Cistus ladanifer L.: Role of allelopathy. Allelopath. J. 2016, 38, 113–132. [Google Scholar]
- Dias, A.S.; Costa, C.T.; Dias, L.S. Allelopathic plants. XVII.: Cistus ladanifer L. Allelopath. J. 2005, 16, 1–30. [Google Scholar]
- Alías, J.C.; Sosa, T.; Valares, C.; Escudero, J.C.; Chaves, N. Seasonal variation of Cistus ladanifer L. diterpenes. Plants 2012, 1, 6–15. [Google Scholar] [CrossRef]
- Lobón, N.C.; Gallego, J.C.A.; Díaz, T.S.; García, J.C.E. Allelopathic potential of Cistus ladanifer chemicals in response to variations of light and temperature. Chemoecology 2002, 12, 139–145. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Rolo, J.; Gaspar, C.; Ramos, L.; Cavaleiro, C.; Salgueiro, L.; Palmeira-de-Oliveira, R.; Teixeira, J.P.; Martínez-de-Oliveira, J.; Palmeira-de-Oliveira, A. Thymus mastichina (L.) L. and Cistus ladanifer L. for skin application: Chemical characterization and in vitro bioactivity assessment. J. Ethnopharmacol. 2023, 302, 115830. [Google Scholar] [CrossRef] [PubMed]
- González, J.A.; García-Barriuso, M.; Gordaliza, M.; Amich, F. Traditional plant-based remedies to control insect vectors of disease in the Arribes del Duero (western Spain): An ethnobotanical study. J. Ethnopharmacol. 2011, 138, 595–601. [Google Scholar] [CrossRef]
- Drapeau, J.; Fröler, C.; Touraud, D.; Kröckel, U.; Geier, M.; Rose, A.; Kunz, W. Repellent studies with Aedes aegypti mosquitoes and human olfactory tests on 19 essential oils from Corsica, France. Flavour Fragr. J. 2009, 24, 160–169. [Google Scholar] [CrossRef]
- Kubeczka, K.-H. History and sources of essential oil research. In Handbook of Essential Oils, Science, Technology, and Applications, 3rd ed.; Başer, K.H.C., Buchbauer, G., Eds.; CRC Press, Taylor Francis Group, LLC: Boca Raton, FL, USA, 2020; Chapter 2; pp. 3–40. [Google Scholar]
- Franz, C.; Novak, J. Sources of essential oils. In Handbook of Essential Oils, Science, Technology, and Applications, 3rd ed.; Başer, K.H.C., Buchbauer, G., Eds.; CRC Press, Taylor Francis Group, LLC: Boca Raton, FL, USA, 2020; Chapter 3; pp. 41–84. [Google Scholar]
- Rubiolo, P.; Sgorbini, B.; Liberto, E.; Cordero, C.; Bicchi, C. Essential oils and volatiles: Sample preparation and analysis. A review. Flavour Fragr. J. 2010, 25, 282–290. [Google Scholar] [CrossRef]
- Sell, C. Chemistry of essential oils. In Handbook of Essential Oils, Science, Technology, and Applications, 3rd ed.; Başer, K.H.C., Buchbauer, G., Eds.; CRC Press, Taylor Francis Group, LLC: Boca Raton, FL, USA, 2020; Chapter 6; pp. 161–189. [Google Scholar]
- Mariotti, J.-P.; Tomi, F.; Casanova, J.; Costa, J.; Bernardini, A.F. Composition of the essential oil of Cistus ladanifer L. cultivated in Corsica (France). Flavour Fragr. J. 1997, 12, 147–151. [Google Scholar] [CrossRef]
- Robles, C.; Bousquet-Mélou, A.; Farzino, S.; Bonin, G. Comparison of essential oil composition of two varieties of Cistus ladanifer. Biochem. Syst. Ecol. 2003, 31, 339–343. [Google Scholar] [CrossRef]
- Rossi, P.-G.; Berti, L.; Panighi, J.; Luciani, A.; Maury, J.; Muselli, A.; Serra, D.R.; Gonny, M.; Bolla, J.-M. Antibacterial action of essential oils from Corsica. J. Essent. Oil Res. 2007, 19, 176–182. [Google Scholar] [CrossRef]
- Upadhyay, N.; Singh, V.K.; Dwivedy, A.K.; Das, S.; Chaudhari, A.K.; Dubey, N.K. Cistus ladanifer L. essential oil on as a plant based preservative against molds infesting oil seeds, aflatoxin B1 secretion, oxidative deterioration and methylglyoxal biosynthesis. LWT-Food Sci. Technol. 2018, 92, 395–403. [Google Scholar] [CrossRef]
- Oller-López, J.L.; Rodríguez, R.; Cueva, J.M.; Oltra, J.E.; Bazdi, B.; Dahdouh, A.; Lamarti, A.; Mansour, A.I. Composition of the essential oils of Cistus ladaniferus and C. monspeliensis from Morocco. J. Essent. Oil Res. 2005, 17, 553–555. [Google Scholar] [CrossRef]
- Greche, H.; Mrabet, N.; Zira, S.; Ismaïli-Alaoui, M.; Benjilali, B. The volatiles of the leaf oil of Cistus ladanifer L. var. albiflorus and labdanum extracts of Moroccan origin and their antimicrobial activities. J. Essent. Oil Res. 2009, 21, 166–173. [Google Scholar]
- Viuda-Martos, M.; Sendra, E.; Pérez-Alvarez, J.A.; Fernández-López, J.; Amensour, M.; Abrini, J. Identification of flavonoid content and chemical composition of the esential oils of Moroccan herbs: Myrtle (Myrtus communis L.), rockroase (Cistus ladanifer L.) and Montpellier cistus (Cistus monspeliensis L.). J. Essent. Oil Res. 2011, 23, 1–9. [Google Scholar] [CrossRef]
- Zidane, H.; Elmiz, M.; Aouinti, F.; Tahani, A.; Wathelet, J.; Sindic, M.; Elbachiri, A. Chemical composition and antioxidant activity of essential oil, various organic extracts of Cistus ladanifer and Cistus libanotis growing in Eastern Morocco. Afr. J. Biotechnol. 2013, 12, 5314–5320. [Google Scholar] [CrossRef]
- Mohammed, B.; Said, C.; Fouzia, F.R.; Kawtar, F.B.; Zoubida, H.; Abdelilah, O.; Mohammed, E.; Ghizlane, E. Chemical composition and antimicrobial activity of the essential oil of Cistus ladanifer var. maculatus Dun. J. Microbiol. Biotechnol. Food Sci. 2018, 8, 925–930. [Google Scholar] [CrossRef]
- Benali, T.; Bouyahya, A.; Habbadi, K.; Zengin, G.; Khabbach, A.; Achbani, E.H.; Hammani, K. Chemical composition and antibacterial activity of the essential oil and extracts of Cistus ladanifer subsp. ladanifer and Mentha suaveolens against phytopathogenic bacteria and their ecofriendly management of phytopathogenic bacteria. Biocat. Agric. Biotechnol. 2020, 28, 101696. [Google Scholar] [CrossRef]
- El Karkouri, J.; Bouhrim, M.; Al Kamaly, O.M.; Mechchate, H.; Kchibale, A.; Adadi, I.; Amine, S.; Ismaili, S.A.; Zair, T. Chemical composition, antibacterial and antifungal activity of the essential oil from Cistus ladanifer L. Plants 2021, 10, 2068. [Google Scholar] [CrossRef] [PubMed]
- El Hachlafi, N.; Kandsi, F.; Elbouzidi, A.; Lafdil, F.Z.; Nouioura, G.; Abdallah, E.M.; Abdnim, R.; Bnouham, M.; Al-Mijalli, S.M.; Mrabti, H.N.; et al. Extraction of bioactive compound-rich essential oil from Cistus ladanifer L. by microwave-assisted hydrodistillation: GC-MS characterization, in vitro pharmacological activities, and molecular docking. Separations 2024, 11, 199. [Google Scholar] [CrossRef]
- Ramalho, P.S.; de Freitas, V.A.P.; Macedo, A.; Silva, G.; Silva, A.M.S. Volatile components of Cistus ladanifer leaves. Flavour Fragr. J. 1999, 14, 300–302. [Google Scholar] [CrossRef]
- Gomes, P.B.; Mata, V.G.; Rodrigues, A.E. Characterization of the Portuguese-grown Cistus ladanifer essential oil. J. Essent. Oil Res. 2005, 17, 160–165. [Google Scholar] [CrossRef]
- Teixeira, S.; Mendes, A.; Alves, A.; Santos, L. Simultaneous distillation-extraction of high-value volatile compounds from Cistus ladanifer L. Anal. Chim. Acta 2007, 584, 439–446. [Google Scholar] [CrossRef]
- Falcão, S.I.; Freire, C.; Figueiredo, A.C.; Vilas-Boas, M. The volatile composition of Portuguese propolis towards its origin discrimination. Rec. Nat. Prod. 2016, 10, 176–188. [Google Scholar]
- Vieira, M.; Bessa, L.J.; Martins, M.R.; Arantes, S.; Teixeira, A.P.S.; Mendes, Â.; da Costa, P.M.; Belo, A.D.F. Chemical composition, antibacterial, antibiofilm and synergistic properties of essential oils from Eucalyptus globulus Labill. and seven Mediterranean aromatic plants. Chem. Biodivers. 2017, 14, e1700006. [Google Scholar] [CrossRef] [PubMed]
- Luis, A.; Ramos, A.; Domingues, F. Pullulan films containing rockrose essential oil for potential food packaging applications. Antibiotics 2020, 9, 681. [Google Scholar] [CrossRef]
- Tavares, C.S.; Martins, A.; Faleiro, M.L.; Miguel, M.G.; Duarte, L.C.; Gameiro, J.A.; Roseiro, L.B.; Figueiredo, A.C. Bioproducts from forest biomass: Essential oils and hydrolates from wastes of Cupressus lusitanica Mill. and Cistus ladanifer L. Ind. Crops Prod. 2020, 144, 112034. [Google Scholar] [CrossRef]
- Ferraz, C.A.; Sousa, A.C.A.; Caramelo, D.; Delgado, F.; de Oliveira, A.P.; Pastorinho, M.R. Chemical profile and eco-safety evaluation of essential oils and hydrolates from Cistus ladanifer, Helichrysum italicum, Ocimum basilicum and Thymbra capitate. Ind. Crops Prod. 2022, 175, 114232. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Gaspar, C.; Rolo, J.; Palmeira-de-Oliveira, R.; Teixeira, J.P.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, A. Comparative efficacy of essential oils against Cutibacterium acnes: Effect upon strains from phylotypes with different virulance patterns. Microb. Pathog. 2025, 199, 107159. [Google Scholar] [CrossRef]
- Costa, R.; de Fina, M.R.; Valentino, M.R.; Crupi, M.L.; Mondello, L. Application of a new GC-MS library, designed with a retention index filter tool, to the analysis of the essential oil of Cistus ladanifer. Acta Hort. 2009, 826, 271–276. [Google Scholar] [CrossRef]
- Verdeguer, M.; Blázquez, M.A.; Boira, H. Chemical composition and herbicidal activity of the essential oil from a Cistus ladanifer L. population from Spain. Nat. Prod. Res. 2012, 26, 1602–1609. [Google Scholar] [CrossRef]
- Morales-Soto, A.; Oruna-Concha, M.J.; Elmore, J.S.; Barrajón-Catalán, E.; Micol, V.; Roldán, C.; Segura-Carretero, A. Volatile profile of Spanish Cistus plants as sources of antimicrobials for industrial applications. Ind. Crops Prod. 2015, 74, 425–433. [Google Scholar] [CrossRef]
- Najar, B.; Shortrede, J.E.; Pistelli, L.; Buhagiar, J. Chemical composition and in vitro cytotoxic screening of sixteen comercial essential oil on five cancer cell lines. Chem. Biodivers. 2020, 17, e1900478. [Google Scholar] [CrossRef] [PubMed]
- Xavier, V.; Finimundy, T.C.; Heleno, S.A.; Amaral, J.S.; Calhelha, R.C.; Vaz, J.; Pires, T.C.S.P.; Mediavilla, I.; Esteban, L.S.; Ferreira, I.C.F.R.; et al. Chemical and bioactive characterization of the essential oils obtained from three Mediterranean plants. Molecules 2021, 26, 7472. [Google Scholar] [CrossRef]
- Mediavilla, I.; Blázquez, M.A.; Ruiz, A.; Esteban, L.S. Influence of the storage of Cistus ladanifer L. bales from mechanized harvesting on the essential oil yield and qualitative composition. Molecules 2021, 26, 2379. [Google Scholar] [CrossRef]
- Mediavilla, I.; Guillamón, E.; Ruiz, A.; Esteban, L.S. Essential oils from residual foliage of forest tree and shrub species: Yield and antioxidant capacity. Molecules 2021, 26, 3257. [Google Scholar] [CrossRef]
- Pérez-Izquierdo, C.; Serrano-Pérez, P.; Rodríguez-Molina, C. Chemical composition, antifungal and phytotoxic activities of Cistus ladanifer L. essential oil and hydrolate. Biocatal. Agric. Biotechnol. 2022, 45, 102527. [Google Scholar] [CrossRef]
- Pérez-Izquierdo, C.; Buesco, M.J.J.; Rodríguez-Molina, M.C.; Pulido, F. Spatial variation in yield, chemical composition, and phytotoxic activity of Cistus ladanifer essential oils. Chem. Biodivers. 2023, 20, e20230095. [Google Scholar] [CrossRef] [PubMed]
- Chaloupková, V.; Mediavilla, I.; Bados, R.; Houdková, M.; Rondevaldová, J.; Esteban, L.S. Annual variation in yield, chemical composition, antioxidant and antibacterial activity of rockrose (Cistus ladanifer L.) essential oils. Biocatal. Agric. Biotechnol. 2024, 60, 103279. [Google Scholar] [CrossRef]
- Pérez-Izquierdo, C.; Buesco, M.J.J.; Serrano-Pérez, P.; Rodríguez-Molina, M.C.; Pulido, F. Unravelling the impact of plant ontogenic factors on the content and phytotoxic potential of Cistus ladanifer L. (rockrose) essential oils. Sci. Hort. 2024, 331, 113127. [Google Scholar] [CrossRef]
- Perez-Izquierdo, C.; Serrano-Pérez, P.; Osuna, M.D.; Rodríguez-Molina, M.C. Influence of the phenological stage of Cistus ladanifer L. on the bioherbicidal potential of its essential oil. Rev. Ciênc. Agrar. 2024, 47, 156–160. [Google Scholar]
- Rincón, J.; de Lucas, A.; Gracia, I. Isolation of rock rose essential oil using supercritical CO2 extraction. Sep. Sci. Technol. 2000, 35, 2745–2763. [Google Scholar] [CrossRef]
- Benali, T.; Laghmari, M.; Touhtouh, J.; Aanniz, T.; Lemhadri, A.; Daoudi, M.D.; Bouyahya, A.; Lee, L.-H.; Ullah, R.; Alotaibi, A.; et al. Chemical composition and bioactivity of essential oils from Cistus ladanifer L., Pistacia lentiscus L., and Matricaria chamomilla L. Biochem. Syst. Ecol. 2024, 116, 104880. [Google Scholar] [CrossRef]
- Garcia-Martin, D.; Garcia-Vallejo, C. Contribution à la connaissance de l’huile essentielle de Cistus ladaniferus L. var. maculatus Dun “ciste commun” (jara) d’Espagne. Parfum. Cosmét. Savons 1969, 12, 283–290. [Google Scholar]
- Simon-Fuentes, A.; Sendra, J.M.; Cuñat, P. Neutral volatiles of Cistus ladaniferus L. essential oil. An. Quim. 1987, 83, 201. [Google Scholar]
- Viciano-Tudela, S.; Sendra, S.; Parra, L.; Jimenez, J.M.; Lloret, J. Proposal of a gas sensor-based device for detecting adulteration in essential oil of Cistus ladanifer. Sustainability 2023, 15, 3357. [Google Scholar] [CrossRef]
- Viciano-Tudela, S.; Parra, L.; Navarro-Garcia, P.; Sendra, S.; Lloret, J. Proposal of a new system for essential oil classification based on low-cost gas sensor and machine learning techniques. Sensors 2023, 23, 5812. [Google Scholar] [CrossRef]
- Blasco, F.J.D.; Viciano-Tudela, S.; Parra, L.; Ahmad, A.; Chaloupková, V.; Bados, R.; Pascual, S.E.; Mediavilla, I.; Sendra, S.; Lloret, J. Employment of MQ gas sensors for the classification of Cistus ladanifer essential oils. Microchem. J. 2024, 206, 111585. [Google Scholar] [CrossRef]
- Carrión-Prieto, P.; Ramos, P.M.; Maria, T.M.R.; Hernández-Navarro, S.; Garrido-Laurnaga, F.; Eusébio, M.E.S.; Martin-Gil, J. Vibrational and termal of essential oils derived from Cistus ladanifer and Erica arborea shrubs. Nat. Prod. Commun. 2017, 12, 119–122. [Google Scholar]
- Mimaki, Y.; Ama, A.K.; Sashida, Y.; Miyata, Y.; Fujii, A. A novel hexahydrodibenzofuran derivative with potente inhibitory activity on melanin biosynthesis of cultured B-16 melanoma cells from Lindera umbellata bark. Chem. Pharmac. Bull. 1995, 43, 893–895. [Google Scholar] [CrossRef]
- Martin, D.G. Información sobre los productos de secrecion de la “jara pringosa” (C. ladaniferus L.): Aceite esencial. “gomo-resina” (ladano) y extractos diversos de la planta y del ladano. An. Inst. For. Investig. Exp. 1962, 34, 213–249. [Google Scholar]
- Peyron, L.; Alessandri, A. Huile essentielle de feuillage de Cistus ladaniferus L. cultivé en Corse. Parfum. Cosmét. Arômes 1986, 67, 59–67. [Google Scholar]
- Garnier, N.; Dodinet, E. An offering of Cistus (rock rose) found in a Carthaginian Tomb (6th–5th c. BC): An interdisciplinary approach combining archaeobotany and biomolecular archeology. Archeo Sci. 2013, 37, 51–66. [Google Scholar]
- Königs, R.; Gülz, P.-G. The monoterpenes in the essential leaf oil of Cistus ladanifer L. Z. Für Pflanzenphysiol. 1974, 72, 237–248. [Google Scholar] [CrossRef]
- Gülz, P.-G.; Kobold, U.; Michaelis, K.; Vostrowsky, O. The composition of terpene hydrocarbons in the essential oils from leaves of four Cistus species. Z. Für Naturforschung 1984, 39, 699–704. [Google Scholar] [CrossRef]
- Proksch, P.; Gülz, P.-G.; Budzikiewicz, H. Phenylpropanoic acid esters in the essential oil of Cistus ladanifer L. (Cistaceae). Z. Für Naturforschung 1980, 35, 201–203. [Google Scholar]
- Proksch, P.; Gülz, P.-G.; Budzikiewicz, H. Further oxygenated compounds in the essential oil of Cistus ladanifer L. (Cistaceae). Z. Für Naturforschung 1980, 3, 529–532. [Google Scholar] [CrossRef]
- Strack, D.; Proksch, V.; Gülz, P.-G. Reversed phase high performance liquid chromatography of essential oils. Z. Für Naturforschung 1980, 3, 675–681. [Google Scholar] [CrossRef]
- Barbosa, P.; Lima, A.S.; Vieira, P.; Dias, L.S.; Tinoco, M.T.; Barroso, J.G.; Pedro, L.G.; Figueiredo, A.C.; Mota, M. Nematicidal activity of essential oils and volatiles derived from Portuguese aromatic flora against the pinewood nematode, Bursapheelnchus xylophilus. J. Nematol. 2010, 42, 8–16. [Google Scholar] [PubMed]
- Guimarães, R.; Sousa, M.J.; Ferreira, I.C.F.R. Contribution of essential oils and phenolics to the antioxidant properties of aromatic plants. Ind. Crops Prod. 2010, 32, 152–156. [Google Scholar] [CrossRef]
- Kotba, I.; Bouaichi, A.; Lougraimzi, H.; Habbadi, K.; Errifi, A.; Touchami, A.O.; Douira, A.; Achbani, E.H. Effect of temperature, pH and essential oils on the mycelial growth of Rhizoctonia solani Kühn (Cantharellales: Ceratobasidiaceae) isolates. J. Microbiol. Biotechnol. Food Sci. 2020, 10, 461–466. [Google Scholar] [CrossRef]
- Thoma, J.L.; Cantrell, C.L.; Zheljazkov, V.D. Effects of essential oil fumigation on potato sprouting at room-temperature storage. Plants 2022, 11, 3109. [Google Scholar] [CrossRef]
- Miguel, M.G. Antioxidant and anti-inflammatory activities of essential oils: A short review. Molecules 2010, 15, 9252. [Google Scholar] [CrossRef]
- El Kharraf, S.; El-Guendouz, S.; Farah, A.; Mateus, M.C.; El Hadrami, E.M.; Miguel, M.G. Impact of fifteen combinations of the main components of rosemary, lavander and citrus essential oils on in vitro biological activities. S. Afr. J. Bot. 2023, 156, 162–168. [Google Scholar] [CrossRef]
- Brindhadevi, K.; Elesawy, B.H.; Elfasakhany, A.; Badruddin, I.A.; Kamangar, S. Wound dressings coated with silver nanoparticles and essential oil of labdanum. Appl. Nanosci. 2023, 13, 1345–1354. [Google Scholar] [CrossRef]
- Chaves, N.; Escudero, J.C. Allelopathic effect of Cistus ladanifer on seed germination. Funct. Ecol. 1997, 11, 432–440. [Google Scholar] [CrossRef]
- Chaves, N.; Sosa, T.; Escudero, J.C. Plant growth inhibiting flavonoids in exudate of Cistus ladanifer and in associated soils. J. Chem. Ecol. 2001, 27, 623–631. [Google Scholar] [CrossRef]
- Sosa, T.; Valares, C.; Aliás, J.C.; Lobón, N.C. Persistence of flavonoids in Cistus ladanifer soils. Plant Soil 2010, 337, 51–63. [Google Scholar] [CrossRef]
- Sosa, T.; Aliás, J.C.; Escudero, J.C.; Chaves, N. Interpopulational variation in the flavonoid composition of Cistus ladanifer L. exudate. Biochem. Syst. Ecol. 2005, 33, 353–364. [Google Scholar] [CrossRef]
- Chaves, N.; Sosa, T.; Valares, C.; Alías, J.C. Routes of incorporation of phytotoxic compounds of Cistus ladanifer L. into soil. Allelopath. J. 2015, 36, 25–36. [Google Scholar]
- Chaves, N.; Sosa, T.; Alías, J.C.; Escudero, J.C. Identification and effects of interaction phytotoxic compounds from exudate of Cistus ladanifer leaves. J. Chem. Ecol. 2001, 27, 611–621. [Google Scholar] [CrossRef]
- Han, C.; Shao, H.; Zhou, S.; Mei, Y.; Cheng, Z.; Huang, L.; Lv, G. Chemical composition and phytotoxicity of essential oil from invasive plant, Ambrosia artemisiifolia L. Ecotoxicol. Environ. Saf. 2021, 211, 111879. [Google Scholar] [CrossRef]
- Alves-Ferreira, J.; Miranda, I.; Duarte, L.C.; Roseiro, L.B.; Lourenço, A.; Quilhó, T.; Cardoso, S.; Fernandes, M.C.; Carvalheiro, F.; Pereira, H. Cistus ladanifer as a source of chemicals: Structural and chemical characterization. Biomass Convers. Biorefin. 2020, 10, 325–337. [Google Scholar] [CrossRef]
- Alves-Ferreira, J.; Lourenço, A.; Morgado, F.; Duarte, L.C.; Roseiro, L.B.; Fernandes, M.C.; Pereira, H.; Carvalheiro, F. Delignification of Cistus ladanifer biomass by organosolv and alkali processes. Energies 2021, 14, 1127. [Google Scholar] [CrossRef]
- Alves-Ferreira, J.; Duarte, L.C.; Lourenço, A.; Roseiro, L.B.; Fernandes, M.C.; Pereira, H.; Carvalheiro, F. Distillery residues from Cistus ladanifer (rockrose) as feedstock for the production of added-value phenolic compounds and hemicellulosic oligosaccharides. BioEnergy Res. 2019, 12, 347–358. [Google Scholar] [CrossRef]
- Alves-Ferreira, J.; Duarte, L.C.; Fernandes, M.C.; Pereira, H.; Carvalheiro, F. Hydrothermal treatments of Cistus ladanifer industrial residues obtained from essential oil distilleries. Waste Biomass Valoriz. 2019, 10, 1303–1310. [Google Scholar] [CrossRef]
- Tavares, C.S.; Martins, A.; Miguel, M.G.; Carvalheiro, F.; Duarte, L.C.; Gameiro, J.A.; Figueiredo, A.C.; Roseiro, L.B. Bioproducts from forest biomass II. Bioactive compounds from the steam-distillation by-products of Cupressus lusitanica Mill. and Cistus ladanifer L. wastes. Ind. Crops Prod. 2020, 158, 112991. [Google Scholar] [CrossRef]
- Sánchez-Vioque, R.; Polissiou, M.; Astraka, K.; de los Mozos-Pascual, M.; Tarantilis, P.; Herraiz-Peñaver, D.; Santana-Méridas, O. Polyphenol composition and antioxidant and metal chelating activities of the solid residues from the essential oil industry. Ind. Crops Prod. 2013, 49, 150–159. [Google Scholar] [CrossRef]
- Alves-Ferreira, J.; Carvalheiro, F.; Duarte, L.C.; Ferreira, A.R.P.; Martinez, A.; Pereira, H.; Fernandes, M.C. D-Lactic acid production from Cistus ladanifer residues: Co-fermentation of pentoses and hexoses by Escherichia coli JU15. Ind. Crops Prod. 2022, 177, 114519. [Google Scholar] [CrossRef]
- Álvaro, A.G.; Mediavilla, I.; Palomar, C.R.; Esteban, L.S.; Crespo, I.G. Energy valorization of solid residue from steam distillation of aromatic shrubs. Ind. Crops Prod. 2024, 222, 119485. [Google Scholar] [CrossRef]
- Köse, M.D.; Tekin, B.N.; Bayraktar, O. Simultaneous isolation and selective encapsulation of volatile compounds from essential oil during electrospraying of β-cyclodextrin. Carbohydr. Polym. 2021, 258, 117673. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).