In Vitro and In Silico Pharmacological Study of Three Combined Lamiaceae Essential Oils: Cytotoxicity and Antiviral Potential
Abstract
1. Introduction
2. Results
2.1. Chemical Profiling
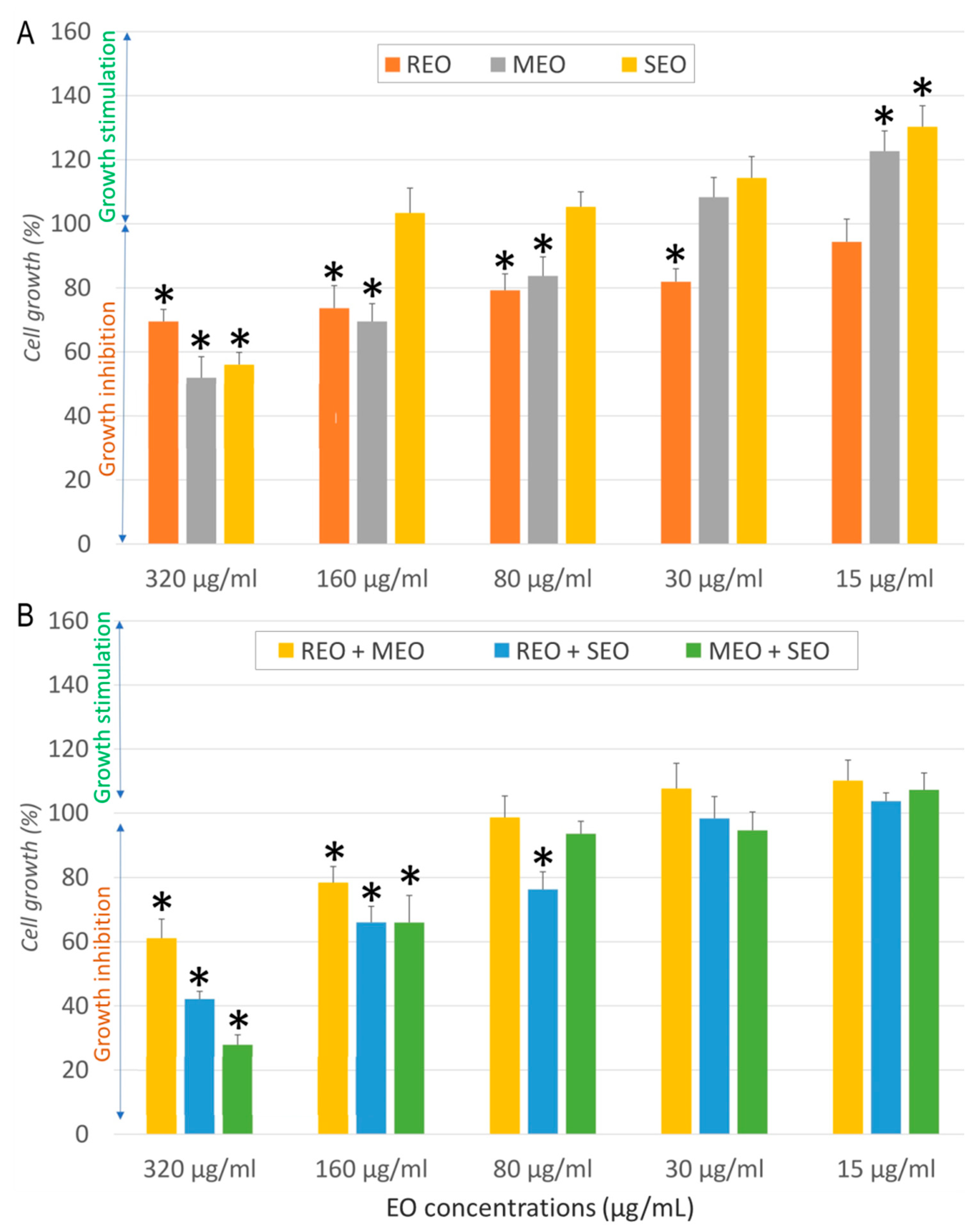
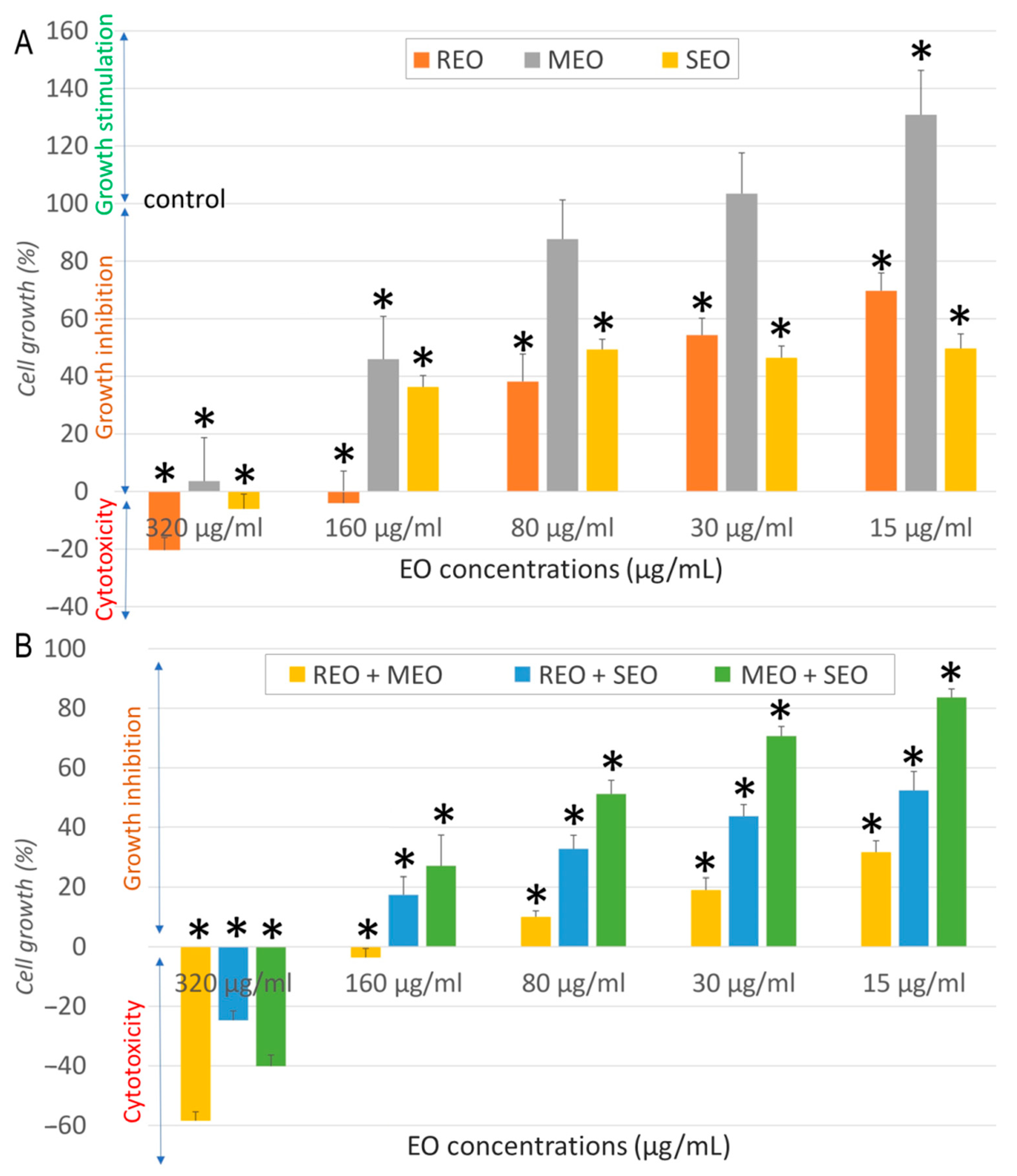
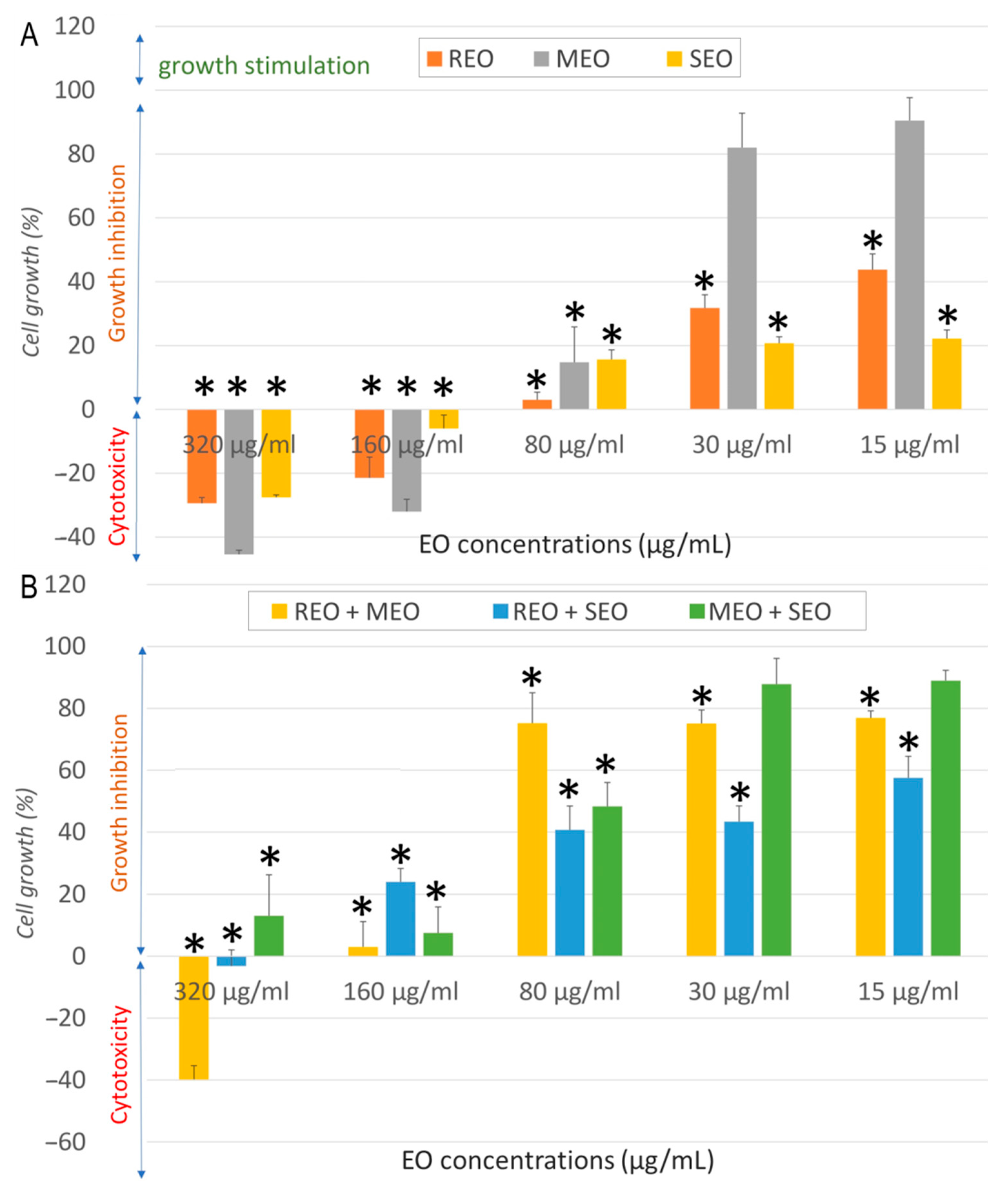
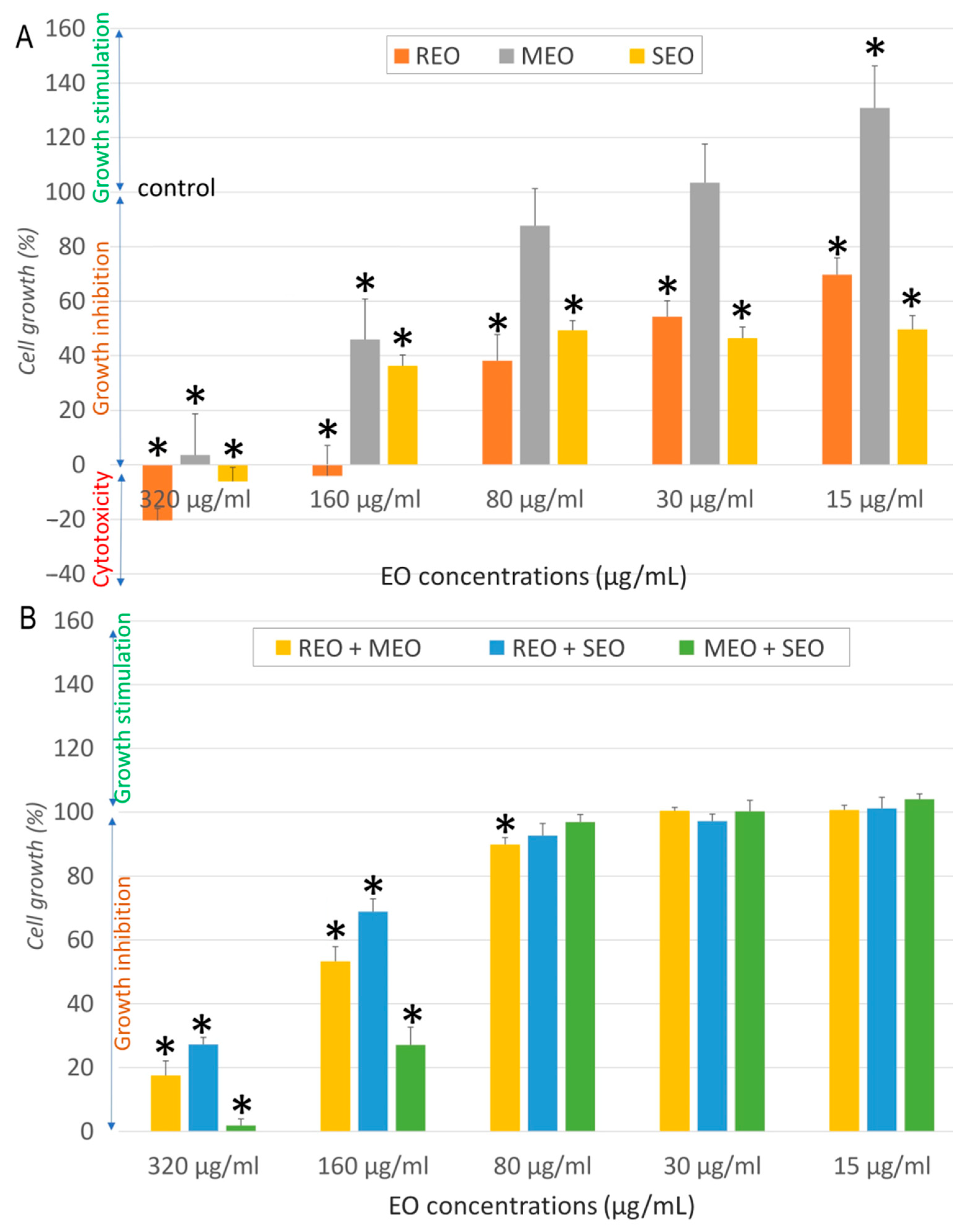
2.2. In Vitro Anticancer Activity
2.3. In Vitro Antiviral Activity
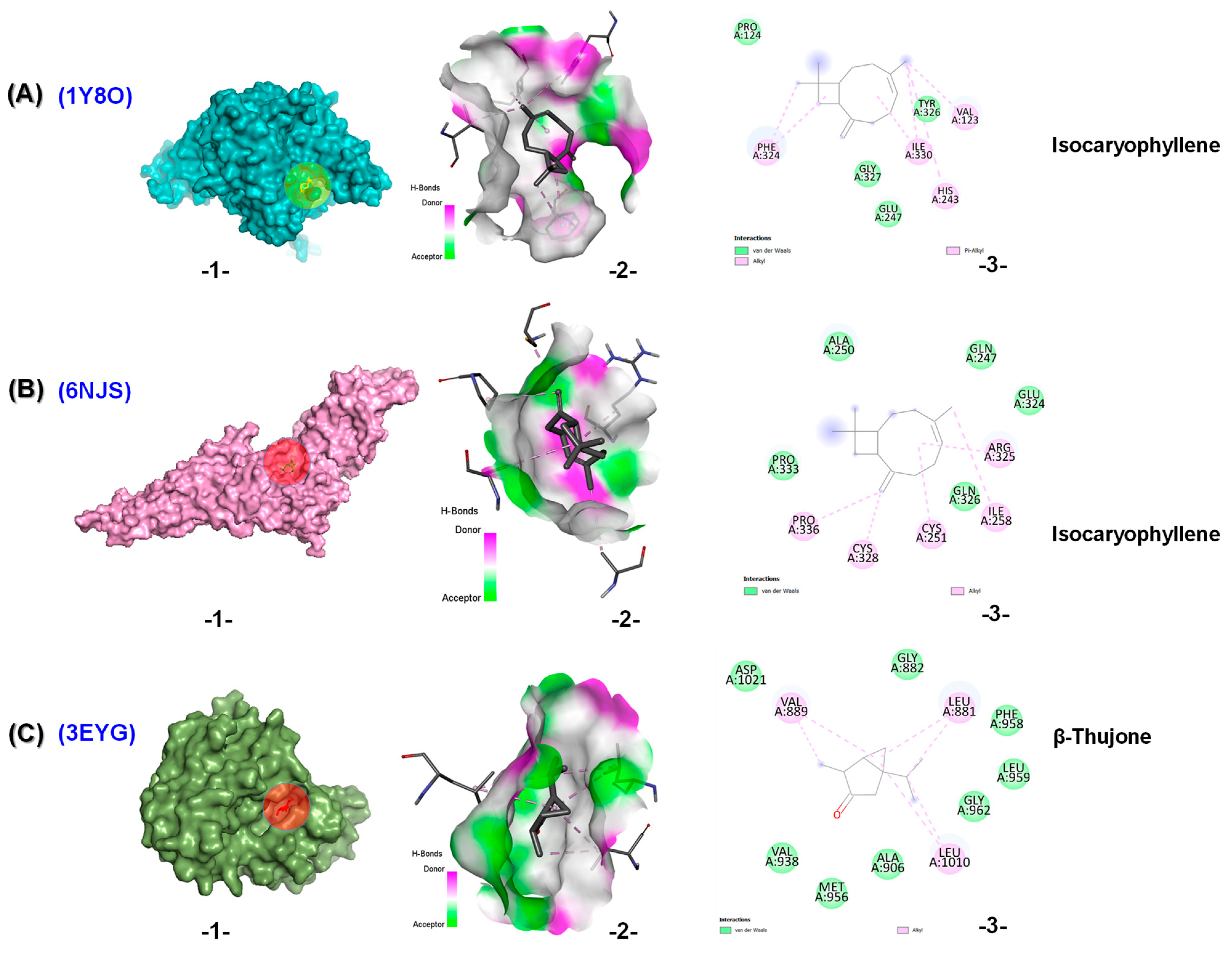
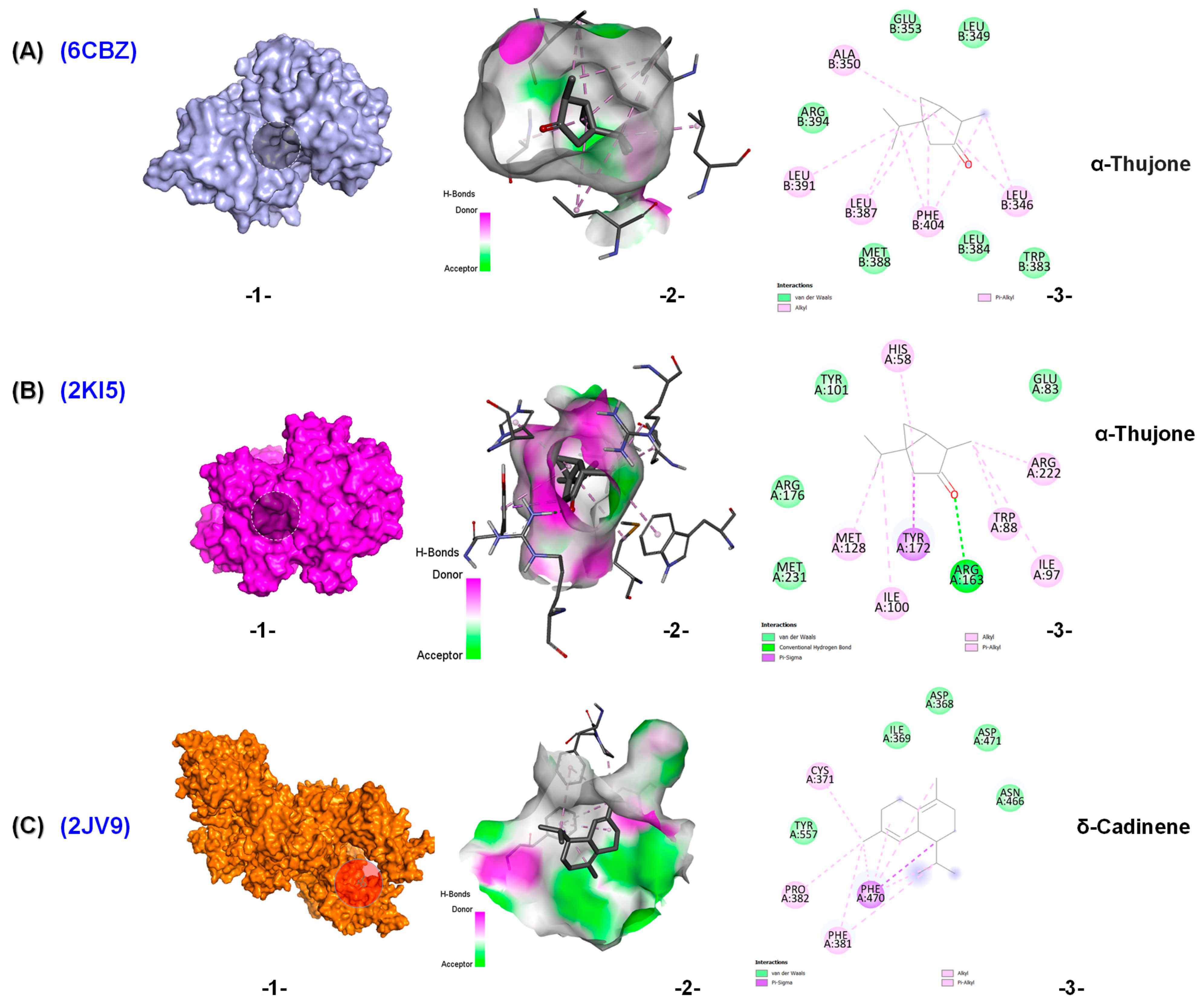
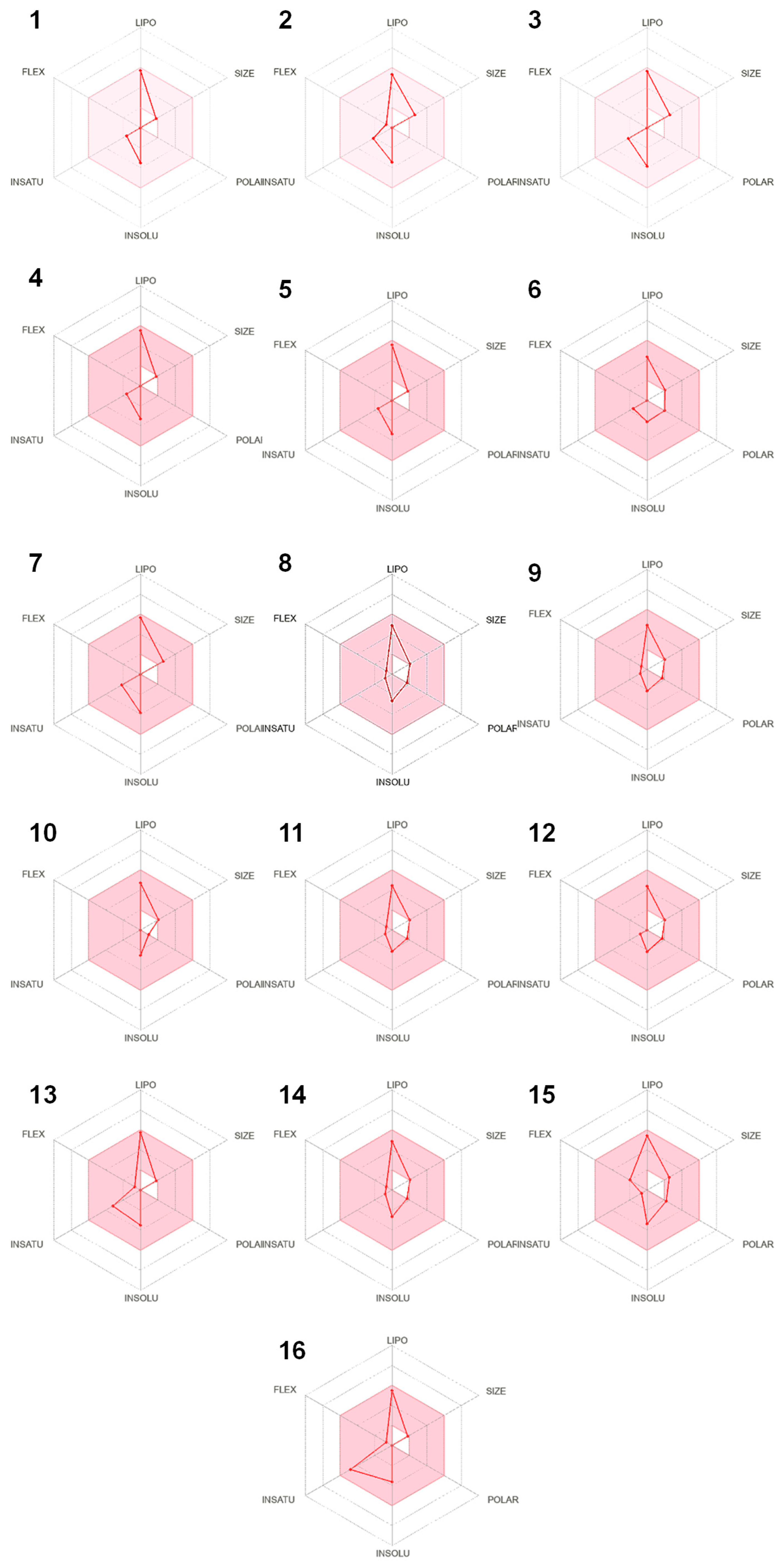
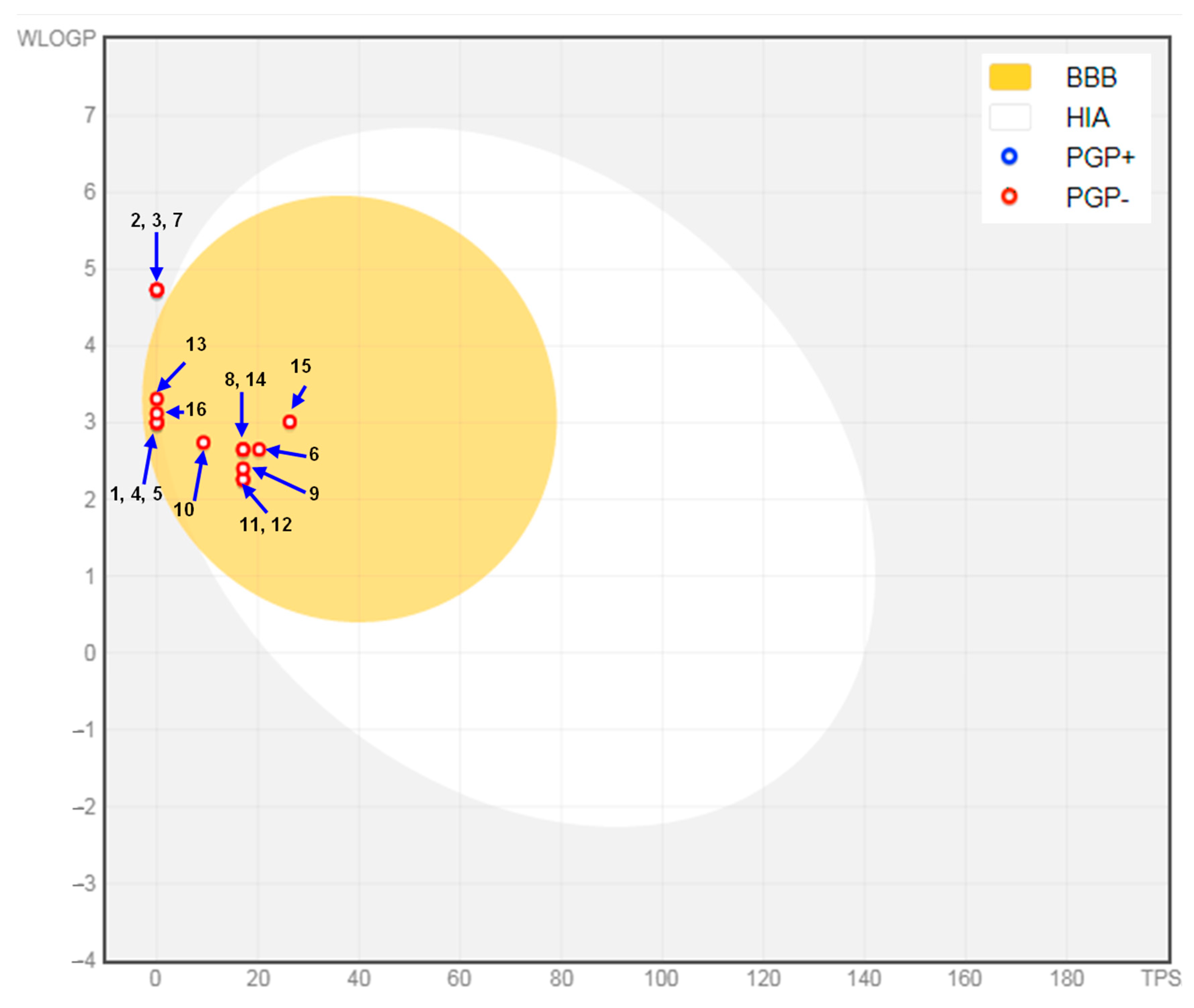
2.4. In Silico Results
3. Discussion
4. Materials and Methods
4.1. Botanical Material
4.2. Essential Oil Analysis
4.3. In Vitro Cytotoxic Activity
4.3.1. Cell Lines
4.3.2. Cell Culture Preparation
4.3.3. Cytotoxicity Assay
4.4. In Vitro Antiviral Assay
4.4.1. Viruses
4.4.2. Virus Inoculum
4.4.3. Antiviral Assay
4.5. Determination of Interaction Effects for Essential Oil Combinations
4.6. In Silico Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A549 | Human Lung Carcinoma Cell Line |
| ADMET | Absorption, Distribution, Metabolism, Excretion, and Toxicity |
| AdV5 | Human Adenovirus Type 5 |
| AMES | Bacterial Reverse Mutation Assay |
| ATCC | American Type Culture Collection |
| BBB | Blood–Brain Barrier |
| C | Control Growth |
| CC | Cell Control |
| CG% | Cell Growth Percentage |
| CPE | Cytopathic Effect |
| CYP | Cytochrome P450 enzymes |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DNA | Deoxyribonucleic Acid |
| DPBS | Dulbecco’s Phosphate-Buffered Saline |
| ECACC | European Collection of Authenticated Cell Cultures |
| EMEM | Eagle’s Minimum Essential Medium |
| EO(s) | Essential Oil(s) |
| ERαLBD | Estrogen Receptor Alpha Ligand Binding Domain |
| FBS | Fetal Bovine Serum |
| FFNSC | Flavors and Fragrances of Natural and Synthetic Compounds |
| FIC | Fractional Inhibitory Concentration |
| GC-MS | Gas Chromatography-Mass Spectroscopy |
| GI | Gastrointestinal |
| HeLa | Human Cervix Carcinoma Cell Line |
| HSV-1 | Human Herpes Simplex Virus Type 1 |
| IC50 | 50% Inhibitory Concentration |
| JAK1 | Tyrosine-Protein Kinase |
| KI | Kovats Retention Index |
| LGA | Lamarckian Genetic Algorithm |
| Log Kp | Logarithm of Skin Permeability Coefficient |
| LoVo | Human Colorectal Cancer Cell line |
| MCF7 | Human Breast Cancer Cell Line |
| MD | Molecular Docking |
| MEO | Essential Oil of Mentha × piperita L. |
| MNTC | Maximum Non-Toxic Concentration |
| NCI60 | National Cancer Institute 60 Human Tumor Cell Line Panel |
| NHDF | Normal Human Dermal Fibroblasts |
| NIST | National Institute of Standards and Technology |
| OCT2 | Organic Cation Transporter 2 |
| PBS | Phosphate-Buffered Saline |
| PDB | Protein Data Bank |
| PDK3 | Pyruvate Dehydrogenase Kinase 3 |
| P-gp | P-Glycoprotein |
| REO | Essential Oil of Salvia rosmarinus Spenn. (REO), |
| RT | Retention Time |
| SD | Standard Deviation |
| SEO | Essential Oil of Salvia officinalis L. |
| SI | Selectivity Index |
| SRB | Sulforhodamine B |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| TCA | Trichloroacetic Acid Solution |
| TCID50 | Tissue Culture Infective Dose 50% |
| Ti | Treated Cells (at given concentration) |
| TPSA | Topological Polar Surface Area |
| Tz | Time Zero |
| VC | Virus Control |
References
- Bunse, M.; Daniels, R.; Gründemann, C.; Heilmann, J.; Kammerer, D.R.; Keusgen, M.; Lindequist, U.; Melzig, M.F.; Morlock, G.E.; Schulz, H.; et al. Essential oils as multicomponent mixtures and their potential for human health and well-being. Front. Pharmacol. 2022, 13, 956541. [Google Scholar] [CrossRef]
- Dolghi, A.; Coricovac, D.; Dinu, S.; Pinzaru, I.; Dehelean, C.A.; Grosu, C.; Chioran, D.; Merghes, P.E.; Sarau, C.A. Chemical and antimicrobial characterization of Mentha piperita L. and Rosmarinus officinalis L. essential oils and in vitro potential cytotoxic effect in human colorectal carcinoma cells. Molecules 2022, 27, 6106. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.Y.; Kim, M.J.; Kim, H.I.; Park, J.; Baek, S.H. Hyperthermia treatment as a promising anti-cancer strategy: Therapeutic targets, perspective mechanisms and synergistic combinations in experimental approaches. Antioxidants 2022, 11, 625. [Google Scholar] [CrossRef]
- Marrelli, M.; De Luca, M.; Toma, C.C.; Grande, F.; Occhiuzzi, M.A.; Caruso, R.; Conforti, F.; Statti, G. Enhancing the nitric oxide inhibitory activity using a combination of plant essential oils and mixture design approach. Heliyon 2024, 10, e31080. [Google Scholar] [CrossRef]
- Karthika, C.; Hari, B.; Rahman, M.H.; Akter, R.; Najda, A.; Albadrani, G.M.; Sayed, A.A.; Akhtar, M.F.; Abdel-Daim, M.M. Multiple strategies with the synergistic approach for addressing colorectal cancer. Biomed. Pharmacother. 2021, 140, 111704. [Google Scholar] [CrossRef]
- Mehalaine, S.; Belfadel, O.; Menasria, T.; Messaili, A. Chemical composition and antibacterial activity of essential oils of three medicinal plants from Algerian semi-arid climatic zone. Phytothérapie 2017, 1–9. [Google Scholar] [CrossRef]
- Gezici, S.; Turkmen, M.; Karahan, F. Exploring the anti-cancer properties of essential oils from some Lamiaceae species against human cancer cells with multivariate analysis. S. Afr. J. Bot. 2024, 166, 287–296. [Google Scholar] [CrossRef]
- Vora, L.K.; Gholap, A.D.; Hatvate, N.T.; Naren, P.; Khan, S.; Chavda, V.P.; Balar, P.C.; Jimil Gandhi, J.; Khatri, D.K. Essential oils for clinical aromatherapy: A comprehensive review. J. Ethnopharmacol. 2024, 330, 118180. [Google Scholar] [CrossRef]
- Siddiqui, T.; Khan, M.U.; Sharma, V.; Gupta, K. Terpenoids in essential oils: Chemistry, classification, and potential impact on human health and industry. Phytomed. Plus 2024, 4, 100549. [Google Scholar] [CrossRef]
- Al-Zereini, W.A.; Al-Trawneh, I.N.; Al-Qudah, M.A.; TumAllah, H.M.; Abudayeh, Z.H.; Hijazin, T. Antibacterial, antioxidant, and cytotoxic activities of Syzygium aromaticum (L.) Merr. & Perry essential oil with identification of its chemical constituents. Z. Naturforsch 2023, 78, 105–112. [Google Scholar] [CrossRef]
- Menasria, T.; Aguilera, M. Genomic diversity of SARS-CoV-2 in Algeria and North African countries: What we know so far and what we expect? Microorganisms 2022, 10, 467. [Google Scholar] [CrossRef]
- Boukoucha, M.; Menasria, T.; Bouguerra, N. Phenotypic characterization and genotypic subtyping of Salmonella enterica Serovars Enteritidis and Gallinarum isolated from human and poultry-related samples. Food Biotechnol. 2018, 32, 206–221. [Google Scholar] [CrossRef]
- Bekut, M.; Brkić, S.; Kladar, N.; Dragović, G.; Gavarić, N.; Božin, B. Potential of selected Lamiaceae plants in anti (retro) viral therapy. Pharmacol. Res. 2018, 133, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Zaatout, N.; Ayachi, A.; Kecha, M. Staphylococcus aureus persistence properties associated with bovine mastitis and alternative therapeutic modalities. J. Appl. Microbiol. 2020, 129, 1102–1119. [Google Scholar] [CrossRef] [PubMed]
- Zeljković, Ć.S.; Schadich, E.; Džubák, P.; Hajdúch, M.; Tarkowski, P. Antiviral activity of selected Lamiaceae essential oils and their monoterpenes against SARS-CoV-2. Front. Pharmacol. 2022, 13, 893634. [Google Scholar] [CrossRef]
- Schnitzler, P. Essential oils for the treatment of herpes simplex virus infections. Chemotherapy 2019, 64, 1–7. [Google Scholar] [CrossRef]
- Khemili, A.; Bensizerara, D.; Chenchouni, H.; Chaibi, R.; Aissani, N.; Tegegne, D.T.; El-Sayed, E.R.; Szumny, A. Biological potential and essential oil profile of two wild Apiaceae species from Algeria (Daucus carota L. and Foeniculum vulgare Mill.): Larvicidal and Antibacterial Effects. Molecules 2024, 29, 4614. [Google Scholar] [CrossRef]
- Battistini, R.; Rossini, I.; Ercolini, C.; Goria, M.; Callipo, M.R.; Maurella, C.; Pavoni, E.; Serracca, L. Antiviral activity of essential oils against hepatitis A virus in soft fruits. Food Environ. Virol. 2019, 11, 90–95. [Google Scholar] [CrossRef]
- Christopoulou, S.D.; Androutsopoulou, C.; Hahalis, P.; Kotsalou, C.; Vantarakis, A.; Lamari, F.N. Rosemary extract and essential oil as drink ingredients: An evaluation of their chemical composition, genotoxicity, antimicrobial, antiviral, and antioxidant properties. Foods 2021, 10, 3143. [Google Scholar] [CrossRef]
- Amri, M.; Jubinville, É.; Goulet-Beaulieu, V.; Fliss, I.; Jean, J. Evaluation of inhibitory activity of essential oils and natural extracts on foodborne viruses. J. Appl. Microbiol. 2024, 135, lxae221. [Google Scholar] [CrossRef]
- Lanave, G.; Catella, C.; Catalano, A.; Lucente, M.S.; Pellegrini, F.; Fracchiolla, G.; Diakoudi, G.; Palmisani, G.; Trombetta, C.M.; Martella, V.; et al. Assessing the virucidal activity of essential oils against feline calicivirus, a non-enveloped virus used as surrogate of norovirus. Heliyon 2024, 10, e30492. [Google Scholar] [CrossRef]
- Lanave, G.; Pellegrini, F.; Triggiano, F.; De Giglio, O.; Lucente, M.S.; Diakoudi, G.; Catella, C.; Gentile, A.; Tardugno, R.; Fracchiolla, G.; et al. In Vitro Virucidal Activity of Different Essential Oils against Bovine Viral Diarrhea Virus Used as Surrogate of Human Hepatitis C Virus. Antibiotics 2024, 13, 514. [Google Scholar] [CrossRef]
- Schuhmacher, A.; Reichling, J.; Schnitzler, P.J.P.V. Virucidal effect of peppermint oil on the enveloped viruses, herpes simplex virus type 1 and type 2 in vitro. Phytomedicine 2003, 10, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Şen, P.; Bolouri, P.; Şahin, F. Improvement of in vitro antimicrobial and antifungal activities of peppermint essential oil conjugated with chitosan and promising antiviral properties. TurkJAC 2023, 5, 77–82. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Saab, A.M.; Tundis, R.; Statti, G.A.; Menichini, F.; Lampronti, I.; Gambari, R.; Cinatl, J.; Doerr, H.W. Phytochemical analysis and in vitro antiviral activities of the essential oils of seven Lebanon species. Chem. Biodivers. 2008, 5, 461–470. [Google Scholar] [CrossRef]
- Santoyo, S.; Jaime, L.; García-Risco, M.R.; Ruiz-Rodríguez, A.; Reglero, G. Antiviral properties of supercritical CO2 extracts from oregano and sage. Int. J. Food Prop. 2014, 17, 1150–1161. [Google Scholar] [CrossRef]
- Abou Baker, D.H.; Amarowicz, R.; Kandeil, A.; Ali, M.A.; Ibrahim, E.A. Antiviral activity of Lavandula angustifolia L. and Salvia officinalis L. essential oils against avian influenza H5N1 virus. J. Agric. Food Res. 2021, 4, 100135. [Google Scholar] [CrossRef] [PubMed]
- Wróblewska-Łuczka, P.; Cabaj, J.; Bargieł, J.; Łuszczki, J.J. Anticancer effect of terpenes: Focus on malignant melanoma. Pharmacol. Rep. 2023, 75, 1115–1125. [Google Scholar] [CrossRef]
- European Medicines Agency. Final Assessment Report on Rosmarinus officinalis L., Aetheroleum and Rosmarinus officinalis L., Folium (Revision 1) [Internet]; EMA: London, UK, 2010; Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-rosmarinus-officinalis-l-aetheroleum-rosmarinus-officinalis-l-folium-revision-1_en.pdf (accessed on 15 April 2025).
- European Medicines Agency. Assessment Report on Salvia officinalis L., Folium and Salvia officinalis L., Aetheroleum (Revision 1) (Report EMA/HMPC/150801/2015). Committee on Herbal Medicinal Products (HMPC). Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-salvia-officinalis-l-folium-and-salvia-officinalis-l-aetheroleum-revision-1_en.pdf (accessed on 15 April 2025).
- European Medicines Agency. Assessment Report on Mentha × piperita L., Folium and Aetheroleum (Revision 1) [Internet]; EMA: London, UK, 2020; Available online: https://www.fitoterapia.net/archivos/202007/assessment-report-mentha-x-piperita-l-folium-aetheroleum-revision-1_en.pdf?1 (accessed on 15 April 2025).
- Shaer, N.A.; Al-Abbas, N.S.; Mohamed, A.A.; Alqriqri, M.A. Cytotoxic effects of some essential oils on Mcf-7, Hfs and Hct116 cell lines. Afr. J. Biotechnol. 2020, 19, 392–399. [Google Scholar]
- Pachura, N.; Włodarczyk, M.; Bażanów, B.; Pogorzelska, A.; Gębarowski, T.; Kupczyński, R.; Szumny, A. Antiviral and Cytotoxic Activities of Ilex aquifolium Silver Queen in the Context of Chemical Profiling of Two Ilex Species. Molecules 2024, 29, 3231. [Google Scholar] [CrossRef]
- Jardak, M.; Elloumi-Mseddi, J.; Aifa, S.; Mnif, S. Chemical composition, anti-biofilm activity and potential cytotoxic effect on cancer cells of Rosmarinus officinalis L. essential oil from Tunisia. Lipids Health Dis. 2017, 16, 190. [Google Scholar] [CrossRef] [PubMed]
- Gezici, S.; Sekeroglu, N.; Kijjoa, A. In vitro anticancer activity and antioxidant properties of essential oils from Populus alba L. and Rosmarinus officinalis L. from South Eastern Anatolia of Turkey. Indian. J. Pharm. Educ. Res. 2017, 51, 498–503. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Chatha, S.A.S.; Jabbar, A.; Mahboob, S.; Nigam, P.S. Rosmarinus officinalis essential oil: Antiproliferative, antioxidant and antibacterial activities. Braz. J. Microbiol. 2010, 41, 1070–1078. [Google Scholar] [CrossRef]
- Powers, C.N.; Osier, J.L.; McFeeters, R.L.; Brazell, C.B.; Olsen, E.L.; Moriarity, D.M.; Satyal, P.; Setzer, W.N. Antifungal and cytotoxic activities of sixty commercially-available essential oils. Molecules 2018, 23, 1549. [Google Scholar] [CrossRef]
- Miladi, H.; Slama, R.B.; Mili, D.; Zouari, S.; Bakhrouf, A.; Ammar, E. Essential oil of Thymus vulgaris L. and Rosmarinus officinalis L.: Gas chromatography-mass spectrometry analysis, cytotoxicity and antioxidant properties and antibacterial activities against foodborne pathogens. Nat. Sci. 2013, 5, 729–739. [Google Scholar] [CrossRef]
- Al-Maharik, N.; Jaradat, N.; Hawash, M.; Al-Lahham, S.; Qadi, M.; Shoman, I.; Issa, L. Chemical composition, antioxidant, antimicrobial and anti-proliferative activities of essential oils of Rosmarinus officinalis from five different sites in Palestine. Separations 2022, 9, 339. [Google Scholar] [CrossRef]
- Guendouz, C.; Guenane, H.; Bakchiche, B.; Ascrizzi, R.; Flamini, G.; Bardaweel, S.K.; Sayed, A.M.; Ghareeb, M.A. Chemical composition and biological activities of nine essential oils obtained from Algerian plants. Nat. Prod. Res. 2024, 1–10. [Google Scholar] [CrossRef]
- Wang, W.; Li, N.; Luo, M.; Zu, Y.; Efferth, T. Antibacterial activity and anticancer activity of Rosmarinus officinalis L. essential oil compared to that of its main components. Molecules 2012, 17, 2704–2713. [Google Scholar] [CrossRef]
- Singh, H.; Kumar, R.; Mazumder, A.; Salahuddin, Y.R.K.; Chauhan, B.; Abdulah, M.M. Camphor and menthol as anticancer agents: Synthesis, structure-activity relationship and interaction with cancer cell lines. Anti-Cancer Agents Med. Chem. 2023, 23, 614–623. [Google Scholar] [CrossRef]
- Taibi, M.; Elbouzidi, A.; Haddou, M.; Baraich, A.; Gharsallaoui, A.; Mothana, R.A.; Alqahtani, A.M.; Asehraou, A.; Bellaouchi, R.; Addi, M.; et al. Evaluation of the Interaction Between Menthol and Camphor, Major Compounds of Clinopodium nepeta Essential Oil: Antioxidant, Anti-inflammatory and Anticancer Activities Against Breast Cancer Cell Lines. Chem. Biodivers. 2025, 22, e202403098. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.I.; Anwar, F.; Nigam, P.S.; Ashraf, M.; Gilani, A.H. Seasonal variation in content, chemical composition and antimicrobial and cytotoxic activities of essential oils from four Mentha species. J. Sci. Food Agric. 2010, 90, 1827–1836. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, M.; Jovanović, K.K.; Marković, T.; Marković, D.; Gligorijević, N.; Radulović, S.; Soković, M. Chemical composition, antimicrobial, and cytotoxic properties of five Lamiaceae essential oils. Ind. Crops Prod. 2014, 61, 225–232. [Google Scholar] [CrossRef]
- Abdel-Hameed, E.S.S.; Salman, M.S.; Fadl, M.A.; Elkhateeb, A.; El-Awady, M.A. Chemical composition of hydrodistillation and solvent free microwave extraction of essential oils from Mentha piperita L. growing in Taif, Kingdom of Saudi Arabia, and their anticancer and antimicrobial activity. Orient. J. Chem. 2018, 34, 222. [Google Scholar] [CrossRef]
- El makawy, A.I.; Ibrahim, F.M.; Abdel-Aziem, S.H. Assessment of Satureja montana L. and Mentha piperita L. antioxidant activity, cytotoxicity and pattern of apoptotic gene expression in hepatoma cells. Jordan. J. Biol. Sci. 2019, 12, 251–258. [Google Scholar]
- Abedinpour, N.; Ghanbariasad, A.; Taghinezhad, A.; Osanloo, M. Preparation of nanoemulsions of Mentha piperita essential oil and investigation of their cytotoxic effect on human breast cancer lines. BioNanoScience 2021, 11, 428–436. [Google Scholar] [CrossRef]
- Yücel, D.; Sezer, C.V.; Yücel, E.; Kutlu, H.M. Antiproliferative and cytotoxic activities of Mentha × piperita L. essential oil in non-small cell lung cancer cells. Indian J. Exp. Biol. 2022, 60, 753–758. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, H.; Wang, J.; Zhou, L.; Yang, P. Chemical composition and anti-inflammatory, cytotoxic and antioxidant activities of essential oil from leaves of Mentha piperita grown in China. PLoS ONE 2014, 9, e114767. [Google Scholar] [CrossRef] [PubMed]
- Mubarak, E.E.; Ali, L.Z.; Ahmed, I.F.A.; Ahmed, A.B.A.; Taha, R.M. Essential oil compositions and cytotoxicity from various organs of Eucalyptus camaldulensis. Int. J. Agric. Biol. 2015, 17, 320–326. [Google Scholar]
- Fitsiou, E.; Anestopoulos, I.; Chlichlia, K.; Galanis, A.; Kourkoutas, I.; Panayiotidis, M.I.; Pappa, A. Antioxidant and antiproliferative properties of the essential oils of Satureja thymbra and Satureja parnassica and their major constituents. Anticancer. Res. 2016, 36, 5757–5763. [Google Scholar] [CrossRef]
- Wu, Z.L.; Du, Y.H.; Guo, Z.F.; Lei, K.J.; Jia, Y.M.; Xie, M.; Kang, X.; Wei, Q.; He, L.; Wang, Y.; et al. Essential oil and its major compounds from oil camphor inhibit human lung and breast cancer cell growth by cell-cycle arresting. Int. J. Clin. Exp. Med. 2016, 9, 12852–12861. [Google Scholar]
- Mamur, S. Investigation of Cytotoxic Effect of Monoterpenes Beta-Citronellol and (-)-Menthone in Human Breast Cancer (MCF-7) Cell Line. Adnan Menderes Univ. J. Health Sci. 2019, 3, 111–119. [Google Scholar]
- Kallel, I.; Bayoudh, A.; Gargouri, B.; Khannous, L.; Elaguel, A.; Tarhouni, N.; Lassoued, S.; Ben Messaoud, E.; Hadrich, B. Modeling of antiproliferative effects of Salvia officinalis L. essential oil optimized using Box–Behnken design. J. Plant Biochem. Biotechnol. 2023, 32, 239–252. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Eldeeb, H.M.; Khan, R.A.; Al-Omar, M.S.; Mohammed, S.A.; Sajid, M.S.; Aly, M.S.A.; Ahmad, A.M.; Ahmed, A.H.; Abdellatif, A.A.H.; et al. Sage, Salvia officinalis L., constituents, hepatoprotective activity, and cytotoxicity evaluations of the essential oils obtained from fresh and differently timed dried herbs: A comparative analysis. Molecules 2021, 26, 5757. [Google Scholar] [CrossRef]
- El Hadri, A.; Del Río, M.G.; Sanz, J.; Coloma, A.G.; Idaomar, M.; Ozonas, B.R.; González, J.B.; Reus, M.I.S. Cytotoxic activity of α-humulene and transcaryophyllene from Salvia officinalis in animal and human tumor cells. An. R. Acad. Nac. Farm. 2010, 76, 343–356. [Google Scholar]
- Loizzo, M.R.; Tundis, R.; Menichini, F.; Saab, A.M.; Statti, G.A.; Menichini, F. Cytotoxic activity of essential oils from Labiatae and Lauraceae families against in vitro human tumor models. Anticancer. Res. 2007, 27, 3293–3299. [Google Scholar] [PubMed]
- Reichling, J. Antiviral and virucidal properties of essential oils and isolated compounds–a scientific approach. Planta Med. 2022, 88, 587–603. [Google Scholar] [CrossRef]
- Schnitzler, P.; Koch, C.; Reichling, J. Susceptibility of drug-resistant clinical herpes simplex virus type 1 strains to essential oils of ginger, thyme, hyssop, and sandalwood. Antimicrob. Agents Chemother. 2007, 51, 1859–1862. [Google Scholar] [CrossRef]
- Minami, M.; Kita, M.; Nakaya, T.; Yamamoto, T.; Kuriyama, H.; Imanishi, J. The inhibitory effect of essential oils on herpes simplex virus type-1 replication in vitro. Microbiol. Immunol. 2003, 47, 681–684. [Google Scholar] [CrossRef]
- Orhan, İ.E.; Özçelik, B.E.R.R.İ.N.; Kartal, M.; Kan, Y. Antimicrobial and antiviral effects of essential oils from selected Umbelliferae and Labiatae plants and individual essential oil components. Turk. J. Biol. 2012, 36, 239–246. [Google Scholar] [CrossRef]
- Gavanji, S.; Sayedipour, S.S.; Larki, B.; Bakhtari, A. Antiviral activity of some plant oils against Herpes simplex virus type 1 in Vero cell culture. J. Acute Med. 2015, 5, 62–68. [Google Scholar] [CrossRef]
- Sokolova, A.S.; Yarovaya, O.I.; Shernyukov, A.V.; Gatilov, Y.V.; Razumova, Y.V.; Zarubaev, V.V.; Tretiak, T.S.; Pokrovsky, A.G.; Kiselev, O.I.; Salakhutdinov, N.F. Discovery of a new class of antiviral compounds: Camphor imine derivatives. Eur. J. Med. Chem. 2015, 105, 263–273. [Google Scholar] [CrossRef]
- Brand, Y.M.; Roa-Linares, V.C.; Betancur-Galvis, L.A.; Durán-García, D.C.; Stashenko, E. Antiviral activity of Colombian Labiatae and Verbenaceae family essential oils and monoterpenes on Human Herpes viruses. J. Essent. Oil Res. 2016, 28, 130–137. [Google Scholar] [CrossRef]
- Kovaleva, K.S.; Zubkov, F.I.; Bormotov, N.I.; Novikov, R.A.; Dorovatovskii, P.V.; Khrustalev, V.N.; Gatilov, Y.V.; Zarubaev, V.V.; Yarovaya, O.I.; Shishkina, L.N.; et al. Synthesis of D-(+)-camphor-based N-acylhydrazones and their antiviral activity. MedChemComm 2018, 9, 2072–2082. [Google Scholar] [CrossRef]
- Sokolova, A.S.; Putilova, V.P.; Yarovaya, O.I.; Zybkina, A.V.; Mordvinova, E.D.; Zaykovskaya, A.V.; Shcherbakov, D.N.; Orshanskaya, I.R.; Sinegubova, E.O.; Esaulkova, I.L.; et al. Synthesis and antiviral activity of camphene derivatives against different types of viruses. Molecules 2021, 26, 2235. [Google Scholar] [CrossRef]
- Astani, A.; Reichling, J.; Schnitzler, P. Comparative study on the antiviral activity of selected monoterpenes derived from essential oils. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2010, 24, 673–679. [Google Scholar] [CrossRef]
- Vimalanathan, S.; Hudson, J. Anti-influenza virus activity of essential oils and vapors. Am. J. Essent. Oil. Nat. Prod. 2014, 2, 47–53. [Google Scholar]
- Bassolé, I.H.N.; Juliani, H.R. Essential oils in combination and their antimicrobial properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef] [PubMed]
- Bouakkaz, H.; Neşetoğlu, N.; Benarous, K.; Bou-Salah, L.; Serseg, T.; Linani, A.; Unal, D.Ö.; Gölcü, A.; Khemili, A. The evaluation of Hertia cheirifolia L. extract by GC-MS coupled with in silico study as potent inhibitors of human pancreatic lipase. J. Biomol. Struct. Dyn. 2024, 1–12. [Google Scholar] [CrossRef]
- Ulutürk, M.; Karabacak Atay, Ç.; Dede, B.; Tilki, T. Potentially Bioactive Novel Isophthalic Acid Based Azo Molecules: Synthesis, Characterization, Quantum Chemical Calculations, ADMET Properties, Molecular Docking and Molecular Dynamics Simulations. Polycycl. Aromat. Compd. 2024, 44, 6765–6786. [Google Scholar] [CrossRef]
- Jairajpuri, D.S.; Khan, S.; Anwar, S.; Hussain, A.; Alajmi, M.F.; Hassan, I. Investigating the role of thymol as a promising inhibitor of pyruvate dehydrogenase kinase 3 for targeted cancer therapy. Int. J. Biol. Macromol. 2024, 259, 129314. [Google Scholar] [CrossRef] [PubMed]
- Petrović, M.; Petrović, V.; Mlinar, Z.; Babić, S.; Jukić, J.; Prebeg, T.; Kremer, D. Duration of Steam Distillation Affects Essential Oil Fractions in Immortelle (Helichrysum italicum). Horticulturae 2024, 10, 183. [Google Scholar] [CrossRef]
- Lucero, M.; Estell, R.; Tellez, M.; Fredrickson, E. A retention index calculator simplifies identification of plant volatile organic compounds. Phytochem. Anal. 2009, 20, 378–384. [Google Scholar] [CrossRef]
- Strober, W. Trypan blue exclusion test of cell viability. Curr. Protoc. Immunol. 1997, 21, A-3B. [Google Scholar] [CrossRef]
- NCI60. The US National Cancer Institute 60 Human Tumour Cell Line Anticancer Drug Screen. 2021. Available online: https://dtp.cancer.gov/discovery_development/nci-60/methodology.htm (accessed on 20 January 2025).
- Suffness, M.; Pezzuto, J.M. Methods in plant biochemistry: Assays for bioactivity. In Methods in Plant Biochemistry, 6th ed.; Hostettmann, K., Ed.; Academic Press Ltd.: London, UK, 1990; pp. 33–71. [Google Scholar]
- EN 14476; Chemical Disinfectants and Antiseptics. Quantitative Suspension Test for the Evaluation of Virucidal Activity in the Medical Area. Test Method and Requirements (Phase 2/Step 1). European Committee for Standardization (CEN): Brussels, Belgium, 2013. Available online: https://standards.globalspec.com/std/13386173/en-14476 (accessed on 1 February 2025).
- Ramakrishnan, M.A. Determination of 50% endpoint titer using a simple formula. World J. Virol. 2016, 5, 85. [Google Scholar] [CrossRef] [PubMed]
- Kamel, N.A.; Tohamy, S.T.; Alshahrani, M.Y.; Aboshanab, K.M. Evaluation of fortimicin antibiotic combinations against MDR Pseudomonas aeruginosa and resistome analysis of a whole genome sequenced pan-drug resistant isolate. BMC Microbiol. 2024, 24, 164. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2012, 64, 4–17. [Google Scholar] [CrossRef]
- Sharma, P.; Joshi, T.; Joshi, T.; Chandra, S.; Tamta, S. In silico screening of potential antidiabetic phytochemicals from Phyllanthus emblica against therapeutic targets of type 2 diabetes. J. Ethnopharmacol. 2020, 248, 112268. [Google Scholar] [CrossRef]
| N° | Compounds | Identif | Cf | KI Lit | REO | MEO | SEO | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | KI Exp | RT (min) | % | KI Exp | RT (min) | % | KI Exp | RT (min) | |||||
| 1 | trans-2-Hexenal | MS, KI, S | Ad | 854 | - | - | - | - | - | - | 0.12 | 6.25 | |
| 2 | 1-Hexanol | MS, KI, S | Al | 868 | - | - | - | 0.107 | 872 | 5.808 | - | - | - |
| 3 | Furan, 2,5-diethyltetrahydro- | MS, KI | Td | 897 | - | - | - | 0.047 | 898 | 6.608 | - | - | - |
| 4 | Bornylene | MS, KI | Hm | 908 | 0.017 | 907 | 6.883 | - | - | - | - | - | - |
| 5 | Tricyclene | MS, KI | Hm | 925 | 1.01 | 926 | 7.45 | - | - | - | 0.08 | 922 | 9.319 |
| 6 | α-Thujene | MS, KI, S | Hm | 929 | 0.081 | 932 | 7.617 | 0.037 | 931 | 7.608 | 0.31 | 929 | 9.655 |
| 7 | α-Pinene | MS, KI, S | Hm | 937 | 21.202 | 939 | 7.858 | 1.22 | 938 | 7.842 | 2.13 | 935 | 10 |
| 8 | Camphene | MS, KI, S | Hm | 952 | 18.943 | 954 | 8.375 | - | - | - | 4.17 | 949 | 10.853 |
| 9 | Dehydrosabinene | MS, KI | Hm | 956 | 0.014 | 959 | 8.567 | - | - | - | - | - | - |
| 10 | Sabinene | MS, KI, S | Hm | 974 | 0.012 | 977 | 9.283 | 0.268 | 977 | 9.275 | 0.33 | 974 | 12.561 |
| 11 | β-Pinene | MS, KI, S | Hm | 979 | 3.275 | 980 | 9.4 | 0.844 | 980 | 9.392 | 3.1 | 976 | 12.704 |
| 12 | Vinyl amyl carbinol | MS, KI, S | Al | 980 | - | - | - | - | - | - | 0.28 | 982 | 13.158 |
| 13 | β-Myrcene | MS, KI, S | Hm | 991 | 0.839 | 993 | 9.958 | 0.046 | 993 | 9.958 | 2.2 | 992 | 14.001 |
| 14 | 3-Octanol | MS, KI, S | Al | 994 | - | - | - | 0.089 | 997 | 10.125 | - | - | - |
| 15 | α-Phellandrene | MS, KI, S | Hm | 1005 | 0.357 | 1006 | 10.483 | - | - | - | - | - | - |
| 16 | 3-Carene | MS, KI, S | Hm | 1011 | 0.042 | 1012 | 10.717 | - | - | - | - | - | - |
| 17 | α-Terpinene | MS, KI, S | Hm | 1017 | 0.544 | 1019 | 10.975 | 0.044 | 1019 | 10.967 | 0.22 | 1016 | 15.869 |
| 18 | o-Cymene | MS, KI, S | Hm | 1022 | - | - | - | 0.169 | 1028 | 11.292 | - | - | - |
| 19 | p-Cymene | MS, KI | Hm | 1025 | 2.661 | 1028 | 11.3 | - | - | - | 0.59 | 1024 | 16.541 |
| 20 | Limonene | MS, KI, S | Hm | 1030 | 3.265 | 1032 | 11.475 | 1.456 | 1032 | 11.467 | - | - | - |
| 21 | Eucalyptol | MS, KI, S | Om | 1032 | 5.023 | 1034 | 11.558 | 4.985 | 1034 | 11.55 | 23.57 | 1030 | 17.034 |
| 22 | cis-β-Ocimene | MS, KI, S | Hm | 1038 | 0.051 | 1042 | 11.875 | - | - | - | - | - | - |
| 23 | trans-β-Ocimene | MS, KI, S | Hm | 1049 | 0.023 | 1053 | 12.317 | 0.027 | 1042 | 11.867 | - | - | - |
| 24 | γ-Terpinene | MS, KI, S | Hm | 1060 | 0.594 | 1063 | 12.758 | 0.083 | 1063 | 12.758 | 0.48 | 1060 | 19.691 |
| 25 | trans-Sabinene hydrate | MS, KI, S | Om | 1070 | - | - | - | 0.114 | 1070 | 13.1 | 0.18 | 1067 | 20.432 |
| 26 | Terpinolene | MS, KI, S | Hm | 1088 | 0.423 | 1090 | 14.05 | 0.071 | 1090 | 14.042 | 0.37 | 1087 | 22.549 |
| 27 | cis-Sabinene hydrate | MS, KI, S | Om | 1093 | - | - | - | - | - | - | 0.11 | 1095 | 23.518 |
| 28 | Linalool | MS, KI, S | Om | 1099 | 0.084 | 1101 | 14.575 | 0.092 | 1101 | 14.567 | - | - | - |
| 29 | α-Thujone | MS, KI, S | Om | 1103 | - | - | - | - | - | - | 22.02 | 1102 | 24.287 |
| 30 | Fenchol | MS, KI, S | Om | 1113 | 0.14 | 1115 | 15.15 | - | - | - | - | - | - |
| 31 | β-Thujone | MS, KI, S | Om | 1114 | - | - | - | - | - | - | 4.37 | 1113 | 25.389 |
| 32 | cis-2-p-Menthen-1-ol | MS, KI | Om | 1122 | - | - | - | 0.025 | 1124 | 15.508 | - | - | - |
| 33 | α-Campholenal | MS, KI | Om | 1125 | 0.044 | 1129 | 15.733 | - | - | - | - | - | - |
| 34 | trans-Pinocarveol | MS, KI | Om | 1139 | 0.042 | 1142 | 16.3 | - | - | - | 0.12 | 1136 | 27.674 |
| 35 | Camphor | MS, KI | Om | 1142 | 23.517 | 1148 | 16.55 | - | - | - | 20.5 | 1140 | 28.189 |
| 36 | cis-Sabinol | MS, KI, S | Om | 1143 | - | - | - | 0.033 | 1143 | 16.325 | - | - | - |
| 37 | Isopulegol | MS, KI, S | Om | 1146 | - | - | - | 0.134 | 1149 | 16.575 | - | - | - |
| 38 | Camphene hydrate | MS, KI, S | Om | 1148 | 0.068 | 1152 | 16.708 | - | - | - | - | - | - |
| 39 | Menthone | MS, KI | Om | 1154 | - | - | - | 25.968 | 1158 | 16.992 | - | - | - |
| 40 | 5-Methylundecane | MS, KI | Hy | 1156 | - | - | - | 0.085 | 1163 | 17.225 | - | - | - |
| 41 | Pinocarvone | MS, KI, S | Om | 1164 | 0.028 | 1166 | 17.375 | - | - | - | - | - | - |
| 42 | Isomenthone | MS, KI, S | Om | 1164 | - | - | - | 9.613 | 1168 | 17.45 | - | - | - |
| 43 | trans-Pinocamphone | MS, KI | Om | 1160 | - | - | - | - | - | - | 0.14 | 1157 | 30.049 |
| 44 | endo-Borneol | MS, KI | Om | 1167 | 1.574 | 1169 | 17.508 | - | - | - | 0.8 | 1162 | 30.721 |
| 45 | δ-Terpineol | MS, KI | Om | 1166 | - | - | - | - | - | - | 0.58 | 1165 | 31.05 |
| 46 | Isopinocamphone | MS, KI | Om | 1173 | - | - | - | - | - | - | 0.08 | 1169 | 31.563 |
| 47 | Menthol | MS, KI, S | Om | 1174 | - | - | - | 0.283 | 1176 | 17.858 | - | - | - |
| 48 | γ-Terpineol, dihydro- | MS, KI, S | Om | 1178 | - | - | - | 43.502 | 1177 | 17.908 | - | - | - |
| 49 | Terpinen-4-ol | MS, KI, S | Om | 1182 | 0.967 | 1180 | 18.05 | 0.212 | 1180 | 18.058 | 0.5 | 1174 | 32.157 |
| 50 | p-Cymen-8-ol | MS, KI, S | Om | 1183 | 0.028 | 1188 | 18.433 | - | - | - | 0.08 | 1184 | 33.35 |
| 51 | Isoneomenthol | MS, KI | Om | 1188 | - | - | - | 0.673 | 1191 | 18.583 | - | - | - |
| 52 | α-Terpineol | MS, KI, S | Om | 1189 | 0.645 | 1192 | 18.667 | 0.063 | 1192 | 18.675 | 0.5 | 1187 | 33.84 |
| 53 | Myrtenal | MS, KI, S | Om | 1193 | 0.031 | 1197 | 18.925 | - | - | - | 0.11 | 1190 | 34.21 |
| 54 | Pulegone | MS, KI, S | Om | 1237 | - | - | - | 1.035 | 1242 | 20.842 | - | - | - |
| 55 | Cuminaldehyde | MS, KI, S | Ad | 1239 | - | - | - | - | - | - | 0.14 | 1236 | 39.527 |
| 56 | Carvone | MS, KI, S | Om | 1246 | - | - | - | 0.052 | 1247 | 21.067 | - | - | - |
| 57 | Piperitone | MS, KI, S | Om | 1253 | - | - | - | 0.166 | 1258 | 21.533 | - | - | - |
| 58 | Neomenthyl acetate | MS, KI | Om | 1274 | - | - | - | 0.145 | 1278 | 22.458 | - | - | - |
| 59 | Bornyl acetate | MS, KI | Om | 1285 | 0.314 | 1288 | 22.933 | - | - | - | 0.15 | 1282 | 45.302 |
| 60 | Dihydroedulan | MS, KI, S | Bz | 1293 | - | - | - | 0.029 | 1290 | 23.033 | - | - | - |
| 61 | Menthyl acetate | MS, KI, S | Om | 1295 | - | - | - | 5.981 | 1295 | 23.292 | - | - | - |
| 62 | Carvacrol | MS, KI, S | Om | 1299 | 0.018 | 1305 | 23.733 | - | - | - | - | - | - |
| 63 | Isomenthol acetate | MS, KI | Om | 1305 | - | - | - | 0.065 | 1309 | 23.925 | - | - | - |
| 64 | α-Cubebene | MS, KI | Hs | 1351 | 0.034 | 1353 | 25.742 | - | - | - | - | - | - |
| 65 | Ylangene | MS, KI | Hs | 1372 | 0.24 | 1374 | 26.683 | 0.021 | 1374 | 26.667 | - | - | - |
| 66 | α-Copaene | MS, KI | Hs | 1376 | 1.061 | 1379 | 26.875 | 0.026 | 1379 | 26.875 | - | - | - |
| 67 | β-Bourbonene | MS, KI | Hs | 1384 | 0.055 | 1387 | 27.258 | 0.056 | 1387 | 27.258 | - | - | - |
| 68 | β-Elemene | MS, KI | Hs | 1391 | - | - | - | 0.031 | 1394 | 27.575 | - | - | - |
| 69 | Isocaryophyllene | MS, KI | Hs | 1406 | - | - | - | - | - | - | 3.21 | 1406 | 60.811 |
| 70 | β-Caryophyllene | MS, KI, S | Hs | 1419 | 4.169 | 1422 | 28.725 | 0.726 | 1422 | 28.717 | - | - | - |
| 71 | β-Copaene | MS, KI | Hs | 1432 | 0.098 | 1432 | 29.125 | - | - | - | - | - | - |
| 72 | α-Caryophyllene | MS, KI | Hs | 1454 | - | - | - | 0.02 | 1457 | 30.133 | 1.73 | 1452 | 64.835 |
| 73 | trans-α-Bergamotene | MS, KI | Hs | 1435 | 0.029 | 1439 | 29.392 | - | - | - | - | - | - |
| 74 | Aromadendrene | MS, KI | Hs | 1440 | 0.051 | 1442 | 29.533 | - | - | - | 0.1 | 1449 | 65.683 |
| 75 | trans-β-Farnesene | MS, KI | Hs | 1457 | 0.131 | 1460 | 30.267 | 0.054 | 1460 | 30.275 | - | - | - |
| 76 | cis-Muurola-4(14),5-diene | MS, KI | Hs | 1463 | 0.035 | 1465 | 30.458 | - | - | - | - | - | - |
| 77 | Cadina-1(6),4-diene | MS, KI, S | Hs | 1481 | 0.038 | 1477 | 30.967 | - | - | - | - | - | - |
| 78 | γ-Muurolene | MS, KI | Hs | 1477 | 1.207 | 1480 | 31.092 | - | - | - | 0.15 | 1469 | 68.023 |
| 79 | Germacrene D | MS, KI, S | Hs | 1481 | - | - | - | 0.224 | 1484 | 31.267 | - | - | - |
| 80 | α-Amorphene | MS, KI | Hs | 1482 | 0.048 | 1483 | 31.225 | - | - | - | - | - | - |
| 81 | α-Curcumene | MS, KI | Hs | 1483 | 0.072 | 1486 | 31.342 | - | - | - | - | - | - |
| 82 | β-Selinene | MS, KI | Hs | 1486 | 0.108 | 1489 | 31.483 | - | - | - | - | - | - |
| 83 | Bicyclogermacrene | MS, KI | Hs | 1495 | - | - | - | 0.105 | 1499 | 31.933 | - | - | - |
| 84 | γ-Amorphene | MS, KI | Hs | 1496 | 0.374 | 1497 | 31.842 | - | - | - | - | - | - |
| 85 | α-Muurolene | MS, KI | Hs | 1499 | 0.502 | 1502 | 32.058 | - | - | - | - | - | - |
| 86 | β-Alaskene | MS, KI | Hs | 1499 | - | - | - | - | - | - | 0.29 | 1502 | 72.169 |
| 87 | β-Bisabolene | MS, KI, S | Hs | 1509 | 0.369 | 1511 | 32.383 | - | - | - | - | - | - |
| 88 | γ-Cadinene | MS, KI | Hs | 1513 | 0.923 | 1517 | 32.608 | - | - | - | - | - | - |
| 89 | Calamenene | MS, KI | Hs | 1523 | - | - | - | - | - | - | 0.12 | 1512 | 73.184 |
| 90 | β-Cadinene | MS, KI | Hs | 1518 | - | - | - | - | - | - | 0.13 | 1516 | 73.608 |
| 91 | δ-Cadinene | MS, KI | Hs | 1524 | 2.38 | 1527 | 32.975 | 0.048 | 1526 | 32.967 | - | - | - |
| 92 | Cubenene | MS, KI | Hs | 1532 | 0.129 | 1536 | 33.325 | - | - | - | - | - | - |
| 93 | α-Cadinene | MS, KI | Hs | 1538 | 0.124 | 1541 | 33.542 | - | - | - | - | - | - |
| 94 | α-Calacorene | MS, KI | Hs | 1542 | 0.211 | 1547 | 33.75 | - | - | - | - | - | - |
| 95 | Caryophyllene oxide | MS, KI, S | Os | 1581 | 0.334 | 1586 | 35.317 | 0.081 | 1586 | 35.317 | 0.45 | 1569 | 79.588 |
| 96 | Viridiflorol | MS, KI, S | Os | 1591 | - | - | - | 0.04 | 1594 | 35.658 | 0.91 | 1578 | 80.674 |
| 97 | α-Humulene epoxide II | MS, KI | Os | 1606 | 0.049 | 1612 | 36.333 | - | - | - | 0.12 | 1594 | 82.57 |
| 98 | Epicubenol | MS, KI | Os | 1627 | 0.051 | 1632 | 37.042 | - | - | - | - | - | - |
| 99 | Methyl jasmonate | MS, KI | Es | 1638 | 0.04 | 1652 | 37.775 | - | - | - | - | - | - |
| 100 | α-Bisabolol | MS, KI, S | Os | 1684 | 0.184 | 1687 | 39.092 | - | - | - | 0.45 | 1677 | 91.559 |
| 101 | Epi-13-Manool | MS, KI | Od | 2056 | - | - | - | - | - | - | 0.15 | 2056 | 115.15 |
| Number of compounds | 61 | 47 | 42 | ||||||||||
| Hydrocarbon monoterpenes (%) | 53.35 | 4.27 | 13.98 | ||||||||||
| Oxygenated monoterpenes (%) | 32.52 | 93.14 | 73.81 | ||||||||||
| Hydrocarbon sesquiterpenes (%) | 12.39 | 1.31 | 5.73 | ||||||||||
| Oxygenated sesquiterpenes (%) | 0.62 | 0.12 | 1.93 | ||||||||||
| Oxygenated diterpenes (%) | - | - | 0.15 | ||||||||||
| Non-terpene derivatives (%) | - | 0.36 | 0.54 | ||||||||||
| Total (%) | 98.92 | 99.20 | 96.14 | ||||||||||
| Yield (%) | 1.8 | 1.1 | 1.4 | ||||||||||
| Sample | Cell Line | IC50 ± SD (µg/mL) | SI | FICA | FICB | FIC Index | Interaction |
|---|---|---|---|---|---|---|---|
| REO | NHDF | 537.7 ± 19.3 | - | - | |||
| A549 | 32.3 ± 6.6 | 16.6 | |||||
| MCF7 | 13.7 ± 2.6 | 39.0 | |||||
| LoVo | 968.53 ± 68.12 | 0.6 | |||||
| MEO | NHDF | 282.3 ± 19.9 | - | ||||
| A549 | 157.1 ± 6.6 | 1.8 | |||||
| MCF7 | 50.9 ± 15.7 | 5.5 | |||||
| LoVo | 196.38 ± 4.5 | 1.4 | |||||
| SEO | NHDF | 325.5 ± 42.1 | - | ||||
| A549 | 80.5 ± 10.9 | 4.0 | |||||
| MCF7 | 9.7 ± 0.8 | 33.6 | |||||
| LoVo | 829.31 ± 20.15 | 0.4 | |||||
| REO + MEO | NHDF | 410.5 ± 8.9 | - | 0.382 | 0.727 | 1.109 | Indifferent |
| A549 | 11.11 ± 1.26 | 36.9 | 0.172 | 0.035 | 0.207 | Synergistic | |
| MCF7 | 85.06 ± 15.5 | 4.8 | 3.104 | 0.836 | 3.940 | Indifferent | |
| LoVo | 178.60 ± 10.80 | 2.3 | 0.092 | 0.455 | 0.547 | Additive | |
| REO + SEO | NHDF | 278.3 ± 22.9 | - | 0.259 | 0.427 | 0.686 | Additive |
| A549 | 16.83 ± 4.32 | 16.5 | 0.261 | 0.105 | 0.365 | Synergistic | |
| MCF7 | 27.42 ± 5.14 | 10.2 | 1.001 | 1.413 | 2.414 | Indifferent | |
| LoVo | 294.02 ± 18.60 | 0.1 | 0.152 | 0.177 | 0.329 | Synergistic | |
| MEO + SEO | NHDF | 223.6 ± 19.9 | - | 0.396 | 0.343 | 0.740 | Additive |
| A549 | 84.46 ± 13.45 | 2.6 | 0.269 | 0.525 | 0.793 | Additive | |
| MCF7 | 77.88 ± 4.15 | 2.9 | 0.765 | 4.014 | 4.779 | Antagonistic | |
| LoVo | 112.83 ± 4.10 | 2 | 0.287 | 0.068 | 0.355 | Synergistic | |
| Samples | Virus | |||||||
|---|---|---|---|---|---|---|---|---|
| AdV5 15′ | AdV5 60′ | HSV-1 15′ | HSV-1 60′ | |||||
| log10 Reduction | Infectivity Reduction | log10 Reduction | Infectivity Reduction | log10 Reduction | Infectivity Reduction | log10 Reduction | Infectivity Reduction | |
| REO | >4 log10 | >99.99% | >4 log10 | >99.99% | >4 log10 | >99.99% | >4 log10 | >99.99% |
| MEO | 4 log10 | 99.99% | >4 log10 | >99.99% | 4 log10 | 99.99% | >4 log10 | >99.99% |
| SEO | >4 log10 | >99.99% | 4 log10 | 99.99% | 4 log10 | 99.99% | >4 log10 | >99.99% |
| REO + MEO | 4 log10 | 99.99% | >4 log10 | >99.99% | 4 log10 | 99.99% | >4 log10 | >99.99% |
| REO + SEO | 4 log10 | 99.99% | >4 log10 | >99.99% | >4 log10 | >99.99% | >4 log10 | >99.99% |
| MEO + SEO | 1 log10 | 90% | 4 log10 | 99.99% | 4 log10 | 99.99% | >4 log10 | >99.99% |
| Binding Energies (kcal/mol) | ||||||
|---|---|---|---|---|---|---|
| Ligand | 1Y8O | 6NJS | 3EYG | 6CBZ | 2KI5 | 2JV9 |
| Co-Crystalized ligand | −7.3 a | −8.9 b | −6.4 c | −6.9 d | −6.1 e | −5.8 f |
| α-Pinene | −5.2 | −4.7 | −5.5 | −6.1 | −4.6 | −5.2 |
| β-Caryophyllene | −6.0 | −5.5 | −5.5 | −5.1 | −5.5 | −7.0 |
| β-Pinene | −5.5 | −4.6 | −4.7 | −6.0 | −4.7 | −5.3 |
| β-Thujone | −5.3 | −5.4 | −6.3 | −6.2 | −4.8 | −5.3 |
| Camphene | −4.9 | −5.0 | −5.3 | −5.8 | −4.6 | −5.1 |
| δ-Cadinene | −5.8 | −5.5 | −5.2 | −5.3 | −5.8 | −7.2 |
| Eucalyptol | −5.0 | −5.1 | −4.5 | −6.2 | −4.7 | −5.4 |
| γ-Terpineol, dihydro- | −5.1 | −4.6 | −4.7 | −4.7 | −4.5 | −5.9 |
| Isocaryophyllene | −6.4 | −6.0 | −5.5 | −5.2 | −5.8 | −6.5 |
| Isomenthone | −4.8 | −4.8 | −4.6 | −5.7 | −4.9 | −5.1 |
| Camphor | −5.0 | −5.0 | −5.5 | −6.2 | −5.0 | −5.4 |
| Limonene | −5.1 | −4.8 | −4.9 | −4.8 | −4.5 | −5.2 |
| Menthone | −4.7 | −4.8 | −5.6 | −4.2 | −4.4 | −5.4 |
| Menthyl acetate | −4.9 | −5.5 | −5.5 | −4.6 | −4.7 | −5.5 |
| p-Cymene | −5.1 | −4.8 | −4.3 | −4.6 | −4.2 | −5.6 |
| α-Thujone | −5.0 | −4.9 | −5.0 | −6.5 | −6.5 | −5.3 |
| Molecule | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Physicochemical properties | ||||||||||||||||
| Molecular formula | C10H16 | C15H24 | C15H24 | C10H16 | C10H16 | C10H18O | C15H24 | C10H18O | C10H16O | C10H18O | C10H16O | C10H16O | C10H16 | C10H18O | C12H22O2 | C10H14 |
| Molecular weight (g/mol) | 136.23 | 204.35 | 204.35 | 136.23 | 136.23 | 154.25 | 204.35 | 154.25 | 152.23 | 154.25 | 152.23 | 152.23 | 136.23 | 154.25 | 198.30 | 134.22 |
| Rotatable bonds | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 3 | 1 |
| H-bond acceptors | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 2 | 0 |
| H-bond donors | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| TPSA (å2) | 0 | 0 | 0 | 0 | 0 | 20.23 | 0 | 17.07 | 17.07 | 9.23 | 17.07 | 17.07 | 0 | 17.07 | 26.3 | 0 |
| Lipophilicity | ||||||||||||||||
| Consensus Log P | 3.44 | 4.14 | 4.24 | 3.42 | 3.43 | 2.41 | 4.24 | 2.6 | 2.35 | 2.67 | 2.35 | 2.37 | 3.37 | 2.61 | 3 | 3.5 |
| Absorption | ||||||||||||||||
| GI absorption | Low | Low | Low | Low | Low | High | Low | High | High | High | High | High | Low | High | High | Low |
| Skin permeability a | −3.95 | −4.85 | −4.44 | −4.18 | −4.13 | −5.73 | −4.44 | −5.08 | −5.62 | −5.3 | −5.62 | −5.67 | −3.89 | −5.08 | −4.67 | −4.21 |
| Distribution | ||||||||||||||||
| BBB permeant b | Yes | No | No | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Pgb substrate | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| Metabolism | ||||||||||||||||
| CYP1A2 inhibitor | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| CYP2C19 inhibitor | No | Yes | Yes | No | No | No | Yes | No | No | No | No | No | No | No | No | No |
| CYP2C9 inhibitor | Yes | Yes | Yes | Yes | Yes | No | Yes | No | No | No | No | No | Yes | No | Yes | No |
| CYP2D6 inhibitor | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | Yes |
| CYP3A4 inhibitor | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| Drug-likeness | ||||||||||||||||
| Bioavailability score | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 |
| Excretion | ||||||||||||||||
| Total clearance c | 0.043 | 1.182 | 1.088 | 0.03 | 0.049 | 1.122 | 1.088 | 0.244 | 0.135 | 1.009 | 0.135 | 0.109 | 0.213 | 0.244 | 1.207 | 0.239 |
| Renal OCT2 substrate | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| Toxicity | ||||||||||||||||
| AMES toxicity | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| Hepatotoxicity | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| Features | Plant Species | ||
|---|---|---|---|
| Salvia rosmarinus | Mentha piperita | Salvia officinalis | |
| Vernacular name | Rosemary | Peppermint | Common Sage |
| Geographic origin | Chelia Forest, Khenchela, Algeria | Bouraoui Belhadef Forest, Jijel, Algeria | Aïn Beïda, Oum El Bouaghi, Algeria |
| Elevation (m) | 1320 | 612 | 924 |
| Longitude | 06°48′40″ E | 06°06′04″ E | 07°20′14″ E |
| Latitude | 35°24′34″ N | 36°41′43″ N | 35°47′20″ N |
| Voucher No. | 2023AKSR | 2023AKMP | 2023AKSO |
| Harvest period | June 2023 | October 2023 | October 2023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khemili, A.; Bensizerara, D.; Chenchouni, H.; Gębarowski, T.; Bażanów, B.; Menasria, T.; Tomańska, A.; Chwirot, A.; Szumny, A. In Vitro and In Silico Pharmacological Study of Three Combined Lamiaceae Essential Oils: Cytotoxicity and Antiviral Potential. Molecules 2025, 30, 4182. https://doi.org/10.3390/molecules30214182
Khemili A, Bensizerara D, Chenchouni H, Gębarowski T, Bażanów B, Menasria T, Tomańska A, Chwirot A, Szumny A. In Vitro and In Silico Pharmacological Study of Three Combined Lamiaceae Essential Oils: Cytotoxicity and Antiviral Potential. Molecules. 2025; 30(21):4182. https://doi.org/10.3390/molecules30214182
Chicago/Turabian StyleKhemili, Aicha, Djamel Bensizerara, Haroun Chenchouni, Tomasz Gębarowski, Barbara Bażanów, Taha Menasria, Anna Tomańska, Aleksandra Chwirot, and Antoni Szumny. 2025. "In Vitro and In Silico Pharmacological Study of Three Combined Lamiaceae Essential Oils: Cytotoxicity and Antiviral Potential" Molecules 30, no. 21: 4182. https://doi.org/10.3390/molecules30214182
APA StyleKhemili, A., Bensizerara, D., Chenchouni, H., Gębarowski, T., Bażanów, B., Menasria, T., Tomańska, A., Chwirot, A., & Szumny, A. (2025). In Vitro and In Silico Pharmacological Study of Three Combined Lamiaceae Essential Oils: Cytotoxicity and Antiviral Potential. Molecules, 30(21), 4182. https://doi.org/10.3390/molecules30214182











