Phenolic Profiling of Albanian Honeys by LC–MS/MS: Gallic Acid as a Predictive Marker of Antioxidant Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Honey Samples
2.2. Characterization of the Floral Origin of Honey
2.3. Determination of Total Polyphenol Content (TPC) in Honey
2.4. Analysis of Phenolic Compounds
2.4.1. Reagents
2.4.2. Extraction Methods
2.4.3. LC–MS/MS Conditions
2.4.4. Calibration Curve and Quantification
2.5. Statistical Analysis
3. Results and Discussion
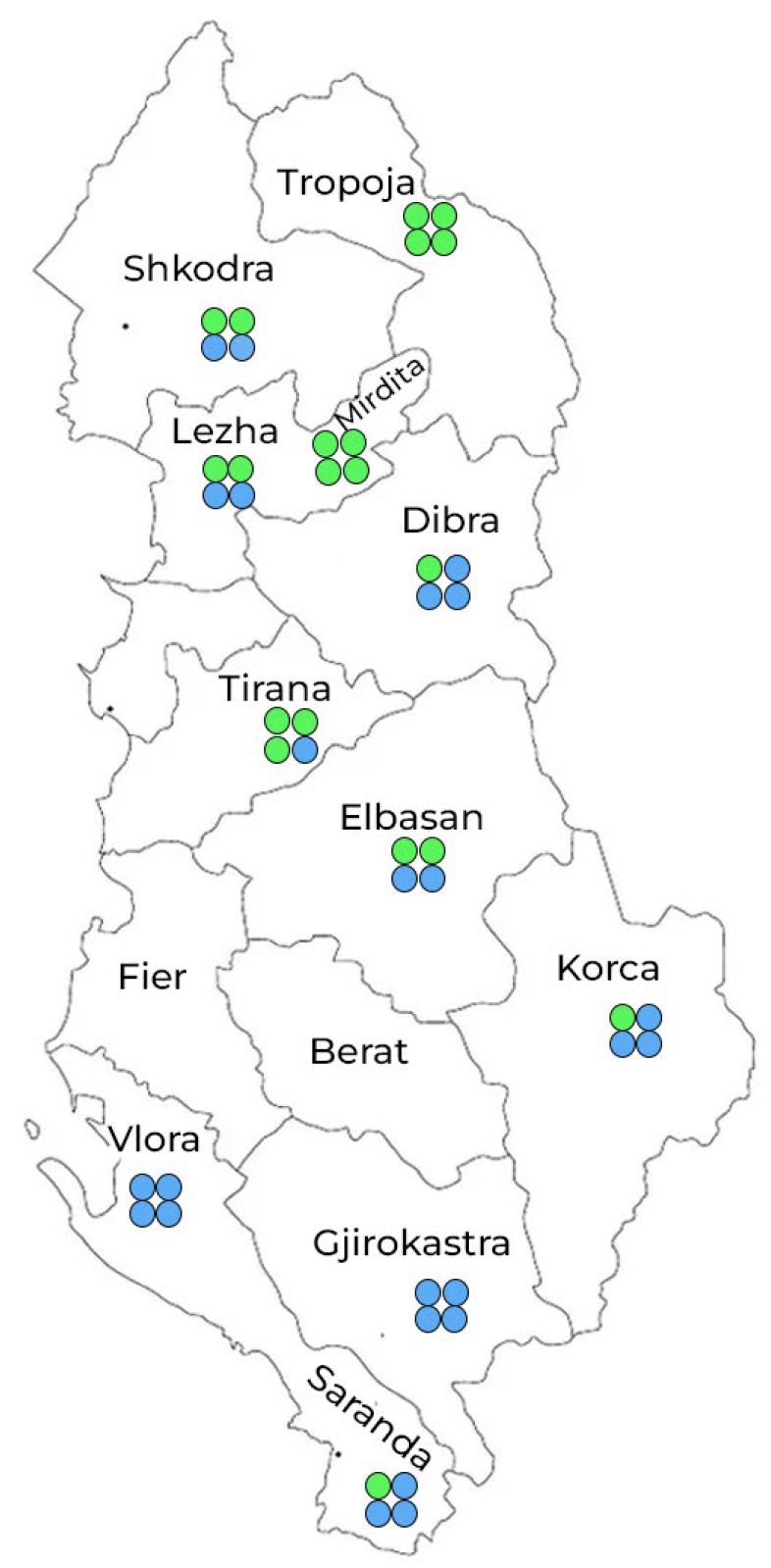
3.1. Floral Profiling of Honey Samples
3.2. TPC of Honey Samples by Region and Floral Types
3.3. Phenolic Compounds Content in Honey Samples
3.4. Chemometric Analysis of Phenolic Compounds in Honey Samples
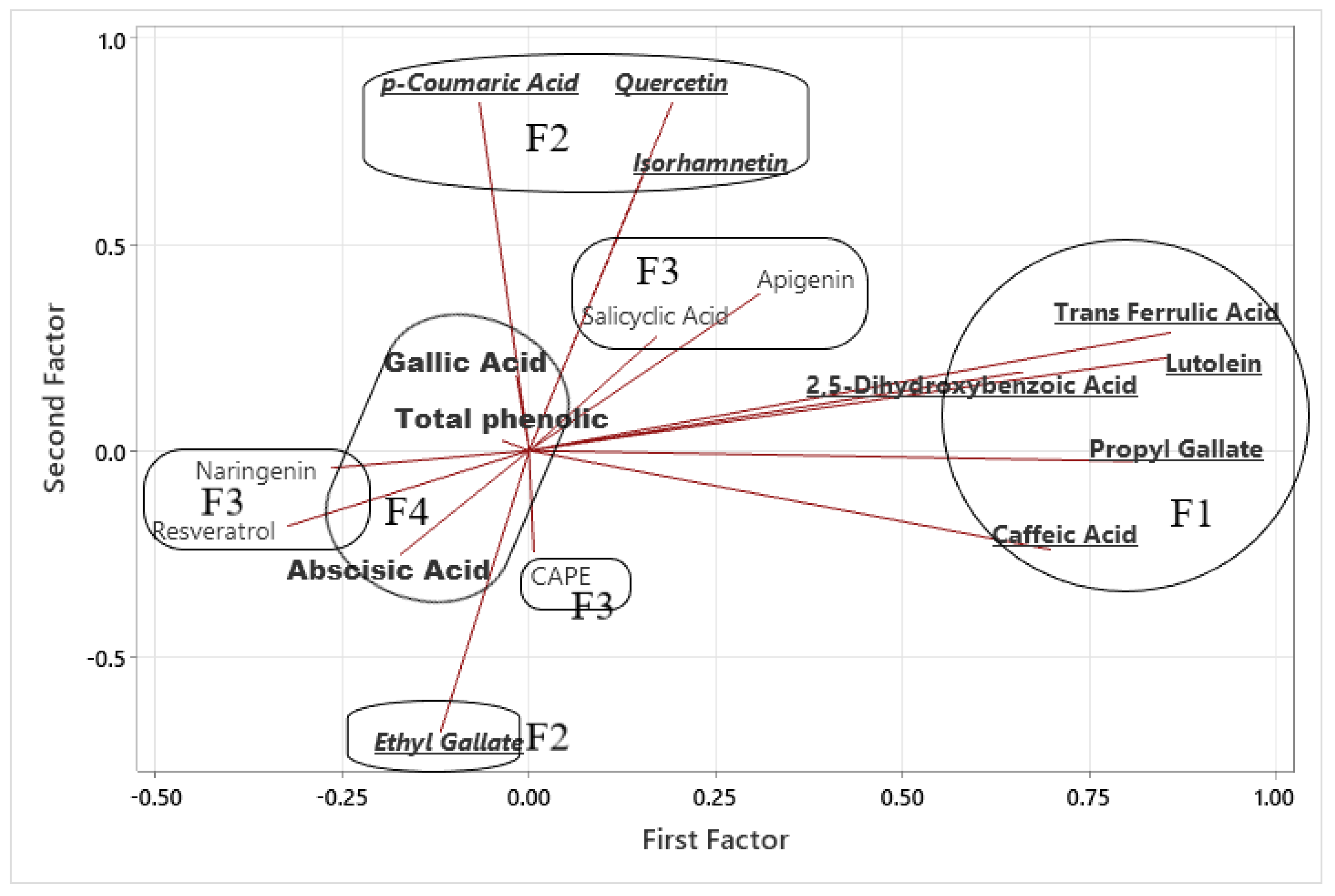
3.5. Multivariate Relationships Among Phenolic Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Compound | LOD (µg/L) | LOQ (µg/L) |
|---|---|---|
| 2,5-Dihydroxybenzoic Acid | 0.14 | 0.47 |
| 2-Hydroxycinnamic Acid | 0.48 | 1.58 |
| Abscisic Acid | 0.15 | 0.49 |
| Caffeic Acid | 0.05 | 0.17 |
| Catechin+Epicatechin | 0.22 | 0.75 |
| Chlorogenic Acid | 0.09 | 0.29 |
| Ethyl Gallate | 0.09 | 0.28 |
| Gallic Acid | 0.31 | 1.02 |
| Gibberellic Acid | 0.95 | 3.16 |
| Indole-3-acetic Acid | 0.11 | 0.36 |
| Isorhamnetin | 0.06 | 0.18 |
| Jasmonic Acid | 0.06 | 0.19 |
| Kaempferol | 0.31 | 1.08 |
| Luteolin | 0.04 | 0.14 |
| Myricetin | 0.05 | 0.18 |
| Naringin | 0.07 | 0.24 |
| p-Coumaric Acid | 0.62 | 2.05 |
| Phlorizin | 0.02 | 0.08 |
| Propyl Gallate | 0.07 | 0.24 |
| Protocatechuic Acid | 0.04 | 0.15 |
| Quercetin | 1.01 | 3.35 |
| Resveratrol | 0.27 | 0.89 |
| Rutin | 0.04 | 0.13 |
| Salicylic Acid | 0.08 | 0.26 |
| Sinapic Acid | 0.91 | 2.97 |
| Syringic Acid | 1.01 | 3.34 |
| Trans-Ferrulic Acid | 0.11 | 0.35 |
| Compound | LOD (µg/L) | LOQ (µg/L) | Ion Pair | RT * | % RSD ** (RT) | [M−H]− m/z | R2 (Linearity) |
|---|---|---|---|---|---|---|---|
| Abscisic Acid | 0.15 | 0.49 | 262.9/219.1; 262.9/153 | 4.338 | 3.6 | [262.9]− | 0.999 |
| Jasmonic Acid | 0.06 | 0.19 | 209.0/164.9; 209.0/59.1 | 4.423 | 1.7 | [209.0]− | 0.998 |
| Naringenin | 0.10 | 0.31 | 271.0/118.9; 271.0/150.9 | 4.370 | 0.9 | [271.0]− | 0.995 |
| Apigenin | 0.11 | 0.33 | 268.9/116.8; 268.9/150.7; 268.9/224.6 | 4.430 | 1.9 | [268.9]− | 0.999 |
| Verbascoside | 0.20 | 0.62 | 623.0/161.1; 623.0/461.2 | 3.952 | 2.1 | [623.0]− | 0.999 |
| Hesperidin | 0.27 | 0.83 | 609.0/301.0 | 4.062 | 3.9 | [609.0]− | 0.996 |
| Indole-3-Acetic acid | 0.22 | 0.68 | 173.9/128.0; 173.9/130.1 | 4.129 | 2.8 | [173.9]− | 0.998 |
| Oleuropein | 0.09 | 0.28 | 539.0/275.1; 539.0/307.2; 539.0/377.1 | 4.131 | 2.0 | [539.0]− | 0.995 |
| Aloin A | 0.05 | 0.17 | 417.0/297.1 | 4.207 | 3.4 | [417.0]− | 0.996 |
| Nr. | Region | No. | Honey Type | Melissopalynological Classification |
|---|---|---|---|---|
| 1 | Tropoja | H24 | MF | Castanea 61%(D), Trifolium(r), Allium (i), |
| H40 | MF | Castanea 39%(S), Allium (r), Arbutus (i), | ||
| H2 | MF | Castanea 45%(D), Quercus(s), Thymus (i) | ||
| H3 | MF | Castanea 72%(D), Allium (s), Trifolium(r), | ||
| 2 | Shkodra | H6 | MF | Castanea 54%(D), Cercis (r), Quercus(s) |
| H7 | MF | Castanea 67%(D), Cistus (r), Allium (i) | ||
| H8 | PF | Trifolium(r), Castanea (r), Mentha(r) | ||
| H27 | PF | Castanea (s), Trifolium(r), Cercis (r) | ||
| 3 | Lezha | H31 | MF | Accacie 34%(S), Arbutus (i), Lavandula (r) |
| H33 | MF | Salvia 28%(S), Quercus (s), Mentha(r) | ||
| H36 | PF | Galega (r), Castanea (r), Quercus (r) | ||
| H23 | PF | Quercus (s), Punica (r), Castanea (i) | ||
| 4 | Mirdita | H5 | MF | Erica 48%(D), Castanea(r), Trifolium(i) |
| H35 | MF | Castanea 31%(S), Allium (r), Castanea (i) | ||
| H11 | MF | Castanea 68%(D), Trifolium(r), Allium (i), | ||
| H12 | MF | Castanea 55%(D), Allium (s), Trifolium(r) | ||
| 5 | Dibra | H25 | MF | Castanea 53% (D), Trifolium(r), Allium (i), |
| H13 | PF | Melilotus (s), Prunus (r), Juniperus (i) | ||
| H14 | PF | Allium (s), Trifolium(s), Castanea (r) | ||
| H26 | PF | Quercus (s), Punica (r), Castanea (i) | ||
| 6 | Tirana | H13 | MF | Erica 58%(D), Arbutus (s), Cercis (r) |
| H15 | PF | Arbutus 59%(D), Helianthus (s), Castanea (i) | ||
| H28 | PF | Arbutus 63%(D), Allium (r), (S), Cercis (r) | ||
| H29 | PF | Mentha (s), Allium (r), Arbutus (i) | ||
| 7 | Elbasan | H39 | MF | Arbutus 46%(D), Allium(r), Castanea (i) |
| H32 | MF | Rosa 35%(S), Castanea (s), Tordylium(s) | ||
| H44 | PF | Rubus (s), Pimpinella (r), Cercis (r) | ||
| H15 | PF | Melilotus(r), Trifolium(r), Castanea (r), | ||
| 8 | Korça | H16 | MF | Staehelinauniflosculo 58%(D),Trifolium(r) |
| H1 | PF | Salvia (r), Medigago (r), Mentha(r) | ||
| H4 | PF | Trifolium(r), Castanea (r), Mentha(r) | ||
| H19 | PF | Quercus(s), Origanum (r), Trifolium(r) | ||
| 9 | Vlora | H30 | PF | Ononis (s), Plantago (r), Arbutus (i) |
| H37 | PF | Robinia (r), Castanea (r), Mentha(r) | ||
| H9 | PF | Trifolium(r), Platanus(r), Mentha(r) | ||
| H10 | PF | Tamarix(s), Tordylium(s), Trifolium(r) | ||
| 10 | Gjirokastra | H34 | PF | Trifolium(r), Platanus(r), Menth a(r) |
| H43 | PF | Thymus (r), Castanea (r), Helianthus (r) | ||
| H17 | PF | Medicago (r), Cercis (r), Mentha (r) | ||
| H18 | PF | Tamarix(s), Tordylium(s), Trifolium(r) | ||
| 11 | Saranda | H17 | MF | Citrus (S), Trifolium (r), Lavandula |
| H38 | PF | Medicago (r), Cercis (r), Mentha (r) | ||
| H41 | PF | Castanea (r), Arbutus (i), Trifolium(r) | ||
| H42 | PF | Medicago (r), Cercis (r), Mentha (r) |
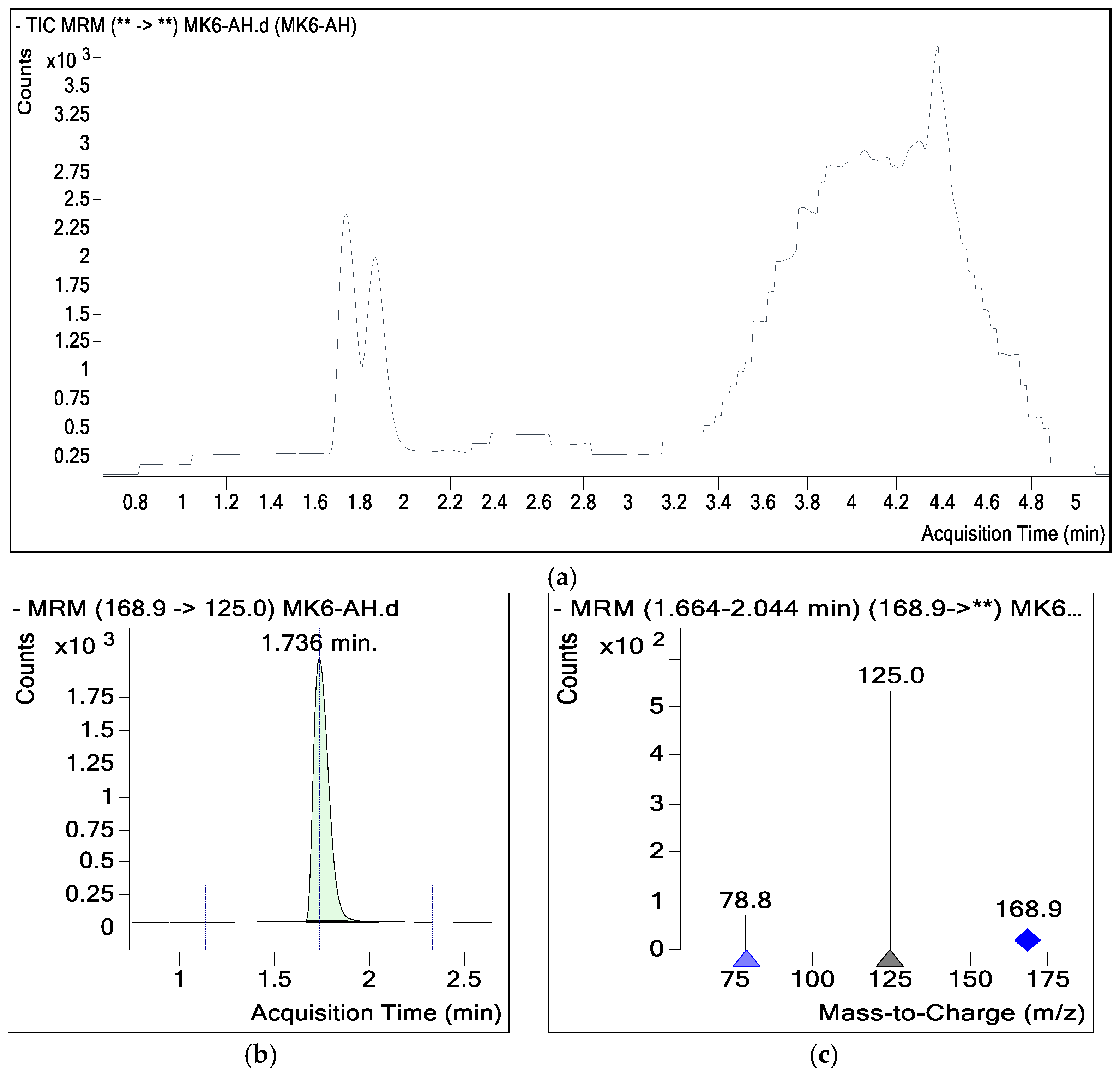
| Compound | RT (min) | Final Conc ng/g |
|---|---|---|
| Gallic Acid | 1.736 | 120,990.80 |
| Protocatechuic Acid | 1.872 | 65,331.69 |
| 2.5-Dihydroxybenzoic Acid | 2.183 | 974.35 |
| Syringic Acid | 3.586 | nd |
| Caffeic Acid | 3.714 | 200.09 |
| Chlorogenic Acid | 3.719 | nd |
| Salicyclic Acid | 3.783 | 437.51 |
| Catechin | 3.888 | 386.22 |
| Verbascoside | 4.062 | nd |
| Gibbarellic Acid | 4.157 | nd |
| Hesperidin | 3.944 | nd |
| Rutin | 4.037 | 204.48 |
| 2-Hydroxytranscinnamic Acid | 4.019 | nd |
| p-Coumaric Acid | 4.053 | nd |
| Naringin | 4.012 | nd |
| Trans Ferrulic Acid | 4.246 | 654.98 |
| Sinapic Acid | 4.194 | nd |
| Ethyl Gallate | 4.085 | 3.49 |
| Phlorizin | 4.156 | nd |
| Indole-3-Acetic Acid | 4.221 | nd |
| Oleuropein | 4.164 | nd |
| Myricetin | 4.123 | nd |
| Resveratrol | 4.169 | nd |
| Aloin A | 4.139 | nd |
| Propyl Gallate | 4.220 | nd |
| Quercetin | 4.275 | 260.25 |
| Lutolein | 4.302 | 64.78 |
| Abscisic Acid | 4.337 | 445.78 |
| Compound | RT | Final Conc. |
| Naringenin | 4.387 | 85.75 |
| Jasmonic Acid | 4.389 | nd |
| Genistein | 4.370 | 69.18 |
| Isorhamnetin | 4.385 | 558.33 |
| Kaempferol | 4.386 | 354.79 |
| Apigenin | 4.421 | 28.99 |
| Caffeic Acetyl Phenyl Ester | 4.580 | 7.01 |
| Parameter 1 | Parameter 2 | Correlation | 95% CI for ρ | p-Value |
|---|---|---|---|---|
| Total phenolic | Gallic Acid | 0.855 | (0.747, 0.918) | <0.001 |
| Trans-Ferrulic Acid | 2,5-Dihydroxybenzoic | 0.414 | (0.134, 0.633) | 0.005 |
| Propyl Gallate | 2,5-Dihydroxybenzoic | 0.483 | (0.218, 0.682) | 0.001 |
| Lutolein | 2,5-Dihydroxybenzoic | 0.611 | (0.381, 0.770) | <0.001 |
| Trans-Ferrulic Acid | Caffeic Acid | 0.548 | (0.300, 0.727) | <0.001 |
| Propyl Gallate | Caffeic Acid | 0.519 | (0.263, 0.707) | <0.001 |
| Lutolein | Caffeic Acid | 0.423 | (0.141, 0.642) | 0.005 |
| Naringenin | Salicylic Acid | 0.401 | (0.118, 0.624) | 0.007 |
| Isorhamnetin | Salicylic Acid | 0.568 | (0.326, 0.740) | <0.001 |
| Caffeic Acetyl Ester | Salicylic Acid | 0.439 | (0.163, 0.651) | 0.003 |
| Ethyl Gallate | p-Coumaric Acid | −0.461 | (−0.666, −0.190) | 0.002 |
| Quercetin | p-Coumaric Acid | 0.585 | (0.349, 0.751) | <0.001 |
| Isorhamnetin | p-Coumaric Acid | 0.392 | (0.108, 0.617) | 0.008 |
| Propyl Gallate | Trans-Ferrulic Acid | 0.607 | (0.378, 0.766) | <0.001 |
| Lutolein | Trans-Ferrulic Acid | 0.775 | (0.618, 0.872) | <0.001 |
| Quercetin | Ethyl Gallate | −0.522 | (−0.709, −0.266) | <0.001 |
| Naringenin | Resveratrol | −0.412 | (−0.632, −0.132) | 0.005 |
| Apigenin | Resveratrol | −0.435 | (−0.648, −0.158) | 0.003 |
| Caffeic Acetyl Ester | Resveratrol | −0.464 | (−0.669, −0.194) | 0.002 |
| Propyl Gallate | Resveratrol | −0.415 | (−0.634, −0.134) | 0.005 |
| Lutolein | Propyl Gallate | 0.64 | (0.420, 0.789) | <0.001 |
| Apigenin | Lutolein | 0.434 | (0.154, 0.650) | 0.004 |
| Total phenolic | Abscisic Acid | 0.725 | (0.546, 0.841) | <0.001 |
| Isorhamnetin | Naringenin | 0.424 | (0.146, 0.640) | 0.004 |
| Apigenin | Naringenin | 0.401 | (0.118, 0.624) | 0.007 |
| Caffeic Acetyl Ester | Naringenin | 0.705 | (0.516, 0.828) | <0.001 |
| Apigenin | Isorhamnetin | 0.516 | (0.258, 0.705) | <0.001 |
References
- Beretta, G.; Granata, P.; Ferrero, M.; Orioli, M.; Facino, R.M. Standardization of antioxidant properties of honey by a combination of spectrophotometric/fluorometric assays and chemometrics. Anal. Chim. Acta 2005, 533, 185–191. [Google Scholar] [CrossRef]
- Bertoncelj, J.; Dobersek, U.; Jamnik, M.; Golob, T. Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chem. 2007, 105, 82–828. [Google Scholar] [CrossRef]
- Socha, R.; Juszczak, L.; Pietrzyk, S.; Fortuna, T. Antioxidant activity and phenolic composition of herb honeys. Food Chem. 2009, 113, 568–574. [Google Scholar] [CrossRef]
- Khalil, M.I.; Alam, N.; Moniruzzaman, M.; Sulaiman, S.A.; Gan, S.H. Phenolic Acid Composition and Antioxidant Properties of Malaysian Honeys. J. Food Sci. 2011, 76, C921–C928. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, S.; Jurendic, T.; Sieber, R.; Gallmann, P. Honey for nutrition and health: A review. Am. J. Clin. Nutr. 2008, 87, 1080–1085. [Google Scholar] [CrossRef]
- Al-Mamary, M.; Al-Meeri, A.; Al-Habori, M. Antioxidant activities and total phenolics of different types of honey. Nutr. Res. 2002, 22, 1041–1047. [Google Scholar] [CrossRef]
- Frankel, S.; Robinson, G.E.; Berenbaum, M.R. Antioxidant activity and correlated characteristic of 14 mono-floral honeys. J. Apic. Res. 1998, 37, 27–31. [Google Scholar] [CrossRef]
- Gheldof, N.; Wang, X.H.; Engeseth, N.J. Identification and quantification of antioxidant components of honeys from various floral sources. J. Agric. Food Chem. 2002, 50, 5870–5877. [Google Scholar] [CrossRef]
- Von der Ohe, W.; Persano Oddo, L.; Piana, M.L.; Morlot, M.; Martin, P. Harmonized methods of melissopalynology. Apidologie 2004, 35, 18–25. [Google Scholar] [CrossRef]
- Silici, S.; Sagdic, O.; Ekici, L. Total phenolic content, antiradical, antioxidant and antimicrobial activities of Rhododendron honeys. Food Chem. 2010, 121, 238–243. [Google Scholar] [CrossRef]
- Perna, A.; Intaglietta, I.; Simonetti, A.; Gambacorta, E. A comparative study on phenolic profile, vitamin C content and antioxidant activity of Italian honeys of different botanical origin. Int. J. Food Sci. Technol. 2013, 48, 1899–1908. [Google Scholar] [CrossRef]
- Mărgăoan, R.; Mărghitaș, L.A.; Dezmirean, D.S.; Dulf, F.V.; Bonta, V.; Cornea-Cipcigan, M.; Vodnar, D.C. Monofloral honeys as a potential source of natural antioxidants, minerals and medicine. Antioxidants 2021, 10, 1023. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.; Dias, L.G.; Moreira, L.L.; Rodrigues, P.; Estevinho, L. Physicochemical, microbiological and antimicrobial properties of commercial honeys from Portugal. Food Chem. Toxicol. 2010, 48, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Hoxha, F.; Lamçe, F.; Beqo, M.; Kongoli, R.; Malollari, I.; Kyçyk, O. Quality evaluation of commercialized honey in Tirana using physicochemical analysis. Albanian J. Agric. Sci. 2019, 18, 26–31. [Google Scholar]
- Sulejmani, E.; Musliu, H.Z.; Uzunov, R. North Macedonian Forest honey: A study of the hydroxymethylfurfural, sugar profile and physical quality parameters. Int. J. Food Technol. Nutr. 2021, 4, 35–43. [Google Scholar]
- Shahu, E.; Ninga, E.; Hoxhaj, F.; Mara, V. Physicochemical characteristics of honey produced in different district of Albania. J. Multidiscip. Eng. Sci. Technol. 2019, 6, 11108–11110. [Google Scholar]
- Zekaj, Z.; Dajçari, E.; Barçuni, D. Investigation of honey quality and its correlation with floral sources. Albanian J. Agric. Sci. 2021, 70, 153–161. [Google Scholar]
- Dajçari, E.; Barçuni, D. Impact of geographical and environmental factors on the quality of Albanian honey. Albanian J. Agric. Sci. 2016, 18, 3–15. [Google Scholar]
- Mărghitaș, L.A.; Dezmirean, D.S.; Moise, A. Physicochemical and bioactive properties of different floral origin honeys from Romania. Food Chem. 2009, 112, 863–867. [Google Scholar] [CrossRef]
- Muthusamy, P.; Prabakaran, K.; Aravindhan, R. Honey as a functional food: Antioxidant and anti-inflammatory properties. Int. J. Food Sci. 2019, 50, 391–398. [Google Scholar]
- Hadidi, M.; Liñán-Atero, R.; Tarahi, M.; Christodoulou, M.C.; Aghababaei, F. The Potential Health Benefits of Gallic Acid: Therapeutic and Food Applications. Antioxidants 2024, 13, 1001. [Google Scholar] [CrossRef] [PubMed]
- Olas, B. Honey and Its Phenolic Compounds as an Effective Natural Medicine for Cardiovascular diseases in Humans. Nutrients 2020, 12, 283. [Google Scholar] [CrossRef] [PubMed]
- Karlıdağ, S. Investigation of phenolic compounds and antioxidant properties of honey samples from different regions. Food Nutr. Res. 2025, 69, 12234. [Google Scholar] [CrossRef] [PubMed]
- Miorini, T.; Marques, P.M.; Lopes, A.R. Antioxidant effects of honey: An overview. Antioxidants 2021, 10, 1753. [Google Scholar]
- Tlak Gajger, I.; Dar, S.A.; Maček, J.; Papeš, R.; Glavaš, H.; Grozdanic, N. Antioxidant Capacity and Therapeutic Applications of Honey. Antioxidants 2025, 14, 959. [Google Scholar] [CrossRef]
- Bereksi-Reguig, Z.; Bensouici, C.; Djahoudi, A.; Derdour, A.; Reguig, A.; Boumerfeg, S.; Arrar, L. Bioactive Compounds, Antioxidant Properties, and Antimicrobial Profiles of 37 Algerian Honey Samples. Antioxidants 2024, 13, 933. [Google Scholar]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Lamas, L.B.; Flórez, S.M.; Toyos, P.A.; et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef]
- Louveaux, J.; Maurizio, A.; Orwoll, G. Methods of melissopalynology. Bee World 1978, 59, 139–154. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; LamuelaRaventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Fischer, U.A.; Carle, R.; Kammerer, D.R. Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD–ESI/MSn. Food Chem. 2011, 127, 807–821. [Google Scholar] [CrossRef]
- Ecem Bayram, N.; Canli, D.; Gerçek, Y.C.; Bayram, S.; Çelik, S.; Güzel, F.; Morgil, H.; Cevahir Öz, G. Macronutrient and micronutrient levels and phenolic compound characteristics of mono-floral honey samples. J. Food Nutr. Res. 2020, 59, 311–322. [Google Scholar]
- Field, A. Discovering Statistics Using IBM SPSS Statistics, 5th ed.; Sage Publishing: Thousand Oaks, CA, USA, 2018; ISBN 9781526440310. [Google Scholar]
- Shrestha, N. Factor Analysis as a Tool for Survey Analysis. Am. J. Appl. Math. Stat. 2021, 9, 4–11. [Google Scholar] [CrossRef]
- Uçurum, Ö.; Gül, M.K.; Feyzioğlu, P.; Şanlier, N.; Göncüoğlu, A.; Üçdağ, O.; Güneş, M.M.; Karacaoğlu, P. Distinctive Properties of Pine, Oak, Chestnut and Multifloral Honeys: Total Phenolic Content, Color, and Potential in Apitherapy. Eur. Food Res. Technol. 2024, 250, 109–120. [Google Scholar] [CrossRef]
- Kuś, P.M.; Congiu, F.; Teper, D.; Sroka, Z. Antioxidant activity, color characteristics, total phenol content and general HPLC fingerprints of six Polish unifloral honey types. LWT—Food Sci. Technol. 2014, 55, 124–130. [Google Scholar] [CrossRef]
- Bešlo, D.; Bešlo, K.; Agić, D.; Vikić-Topić, D.; Lučić, B. Variations of Total Phenolic Content in Honey Samples Caused by Different Calibration Lines. Separations 2021, 8, 14. [Google Scholar] [CrossRef]
- Becerril-Sánchez, A.L.; Quintero-Salazar, B.; Dublán-García, O.; Escalona-Buendía, H.B. Phenolic Compounds in Honey and Their Relationship with Antioxidant Activity, Botanical Origin, and Color. Antioxidants 2021, 10, 1700. [Google Scholar] [CrossRef]
- Koulis, G.A.; Tsagkaris, A.S.; Katsianou, P.A.; Gialouris, P.-L.P.; Martakos, I.; Stergiou, F.; Fiore, A.; Panagopoulou, E.I.; Karabournioti, S.; Baessmann, C.; et al. Thorough Investigation of the Phenolic Profile of Reputable Greek Honey Varieties: Varietal Discrimination and Floral Markers Identification Using Liquid Chromatography–High-Resolution Mass Spectrometry. Molecules 2022, 27, 4444. [Google Scholar] [CrossRef]
- Vela, L.; De Lorenzo, C.; Perez, A. Antioxidant capacity of Spanish honeys and its correlation with polyphenol content and other physicochemical properties. J. Sci. Food Agric. 2007, 87, 1069–1075. [Google Scholar] [CrossRef]
- Di Marco, G.; Arfelli, G.; Gianfranceschi, G.; Guadalupi, L.; Nardi, L.; Romani, A.; Morozzi, G. Botanical Influence on Phenolic Profile and Antioxidant Activity of Italian Monofloral Honeys (Castanea, Erica, Honeydew, Eucalyptus). Molecules 2018, 23, 2643. [Google Scholar] [CrossRef]
- Jaśkiewicz, K.; Szczesna, T.; Jachuła, J. How Phenolic Compounds Profile and Antioxidant Activity Depend on Botanical Origin of Honey—A Case of Polish Varietal Honeys. Molecules 2025, 30, 360. [Google Scholar] [CrossRef]
- Kędzierska-Matysek, M.; Stryjecka, M.; Teter, A.; Skałecki, P.; Domaradzki, P.; Florek, M. Relationships between the content of phenolic compounds and the antioxidant activity of Polish honey varieties as a tool for botanical discrimination. Molecules 2021, 26, 1810. [Google Scholar] [CrossRef]
- Nedić, N.; Nešović, M.; Radišić, P.; Gašić, U.; Baošić, R.; Joksimović, K.; Pezo, L.; Tešić, Ž.; Vovk, I. Polyphenolic and chemical profiles of honey from the Tara Mountain in Serbia. Front. Nutr. 2022, 9, 941463. [Google Scholar] [CrossRef]
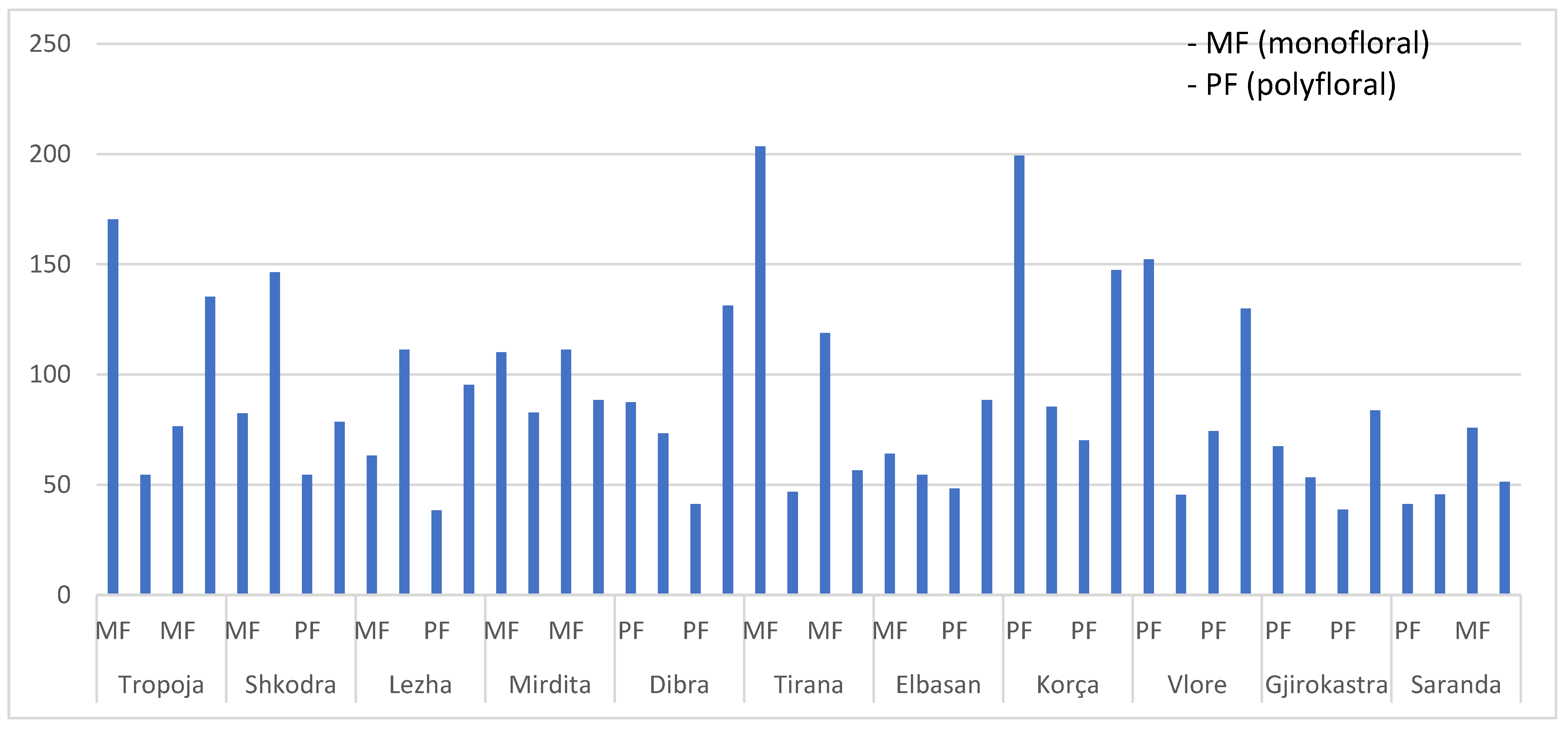

| Botanical Origin | Total Phenolic Contents mg GAE */100 g | Country/Region |
|---|---|---|
| Robinia pseudoacacia L. | 6.32 | Albania |
| 28.2–52.0 | Croatia | |
| Chestnut (Castanea spp.) | 54.43–170.34 | Albania/Tropoja |
| 82.65–111.21 | Albania/Mirdita | |
| 1.83 | Korea | |
| 0.12 | Turkey | |
| 487–1134 | Portugal | |
| 129.2–212.7 | Croatia | |
| Citrus | 4.12 | Albania |
| 14 | Greece | |
| 167.8 | Italy | |
| 83.85 | India | |
| Poly-floral | 3.84–19.93 | Albania |
| 236.94–1021.62 | Poland | |
| 744–1277 | Portugal | |
| 1199 | Greece | |
| 170 | Mexico | |
| 141 | Poland | |
| 81.22–983.04 | Poland | |
| 140.83 | India/Shillong | |
| 126.07 | India/Mawsynram | |
| 74.42 | India Tezpur | |
| 40.18–118.82 | Argentine | |
| 60.5 | Algeria/Babors | |
| 26.2–68.6 | Estonia | |
| 0.26 | Turkey/Mesudiye |
| Phenolic Compounds | N | Mean | CV% | Min. | Q1 | Median | Q3 | Max. |
|---|---|---|---|---|---|---|---|---|
| Gallic Acid | 44 | 38.29 | 71 | 4.54 | 16.77 | 30.2 | 53.94 | 121 |
| Protocatechuic Acid | 44 | 12.38 | 93 | 1.83 | 4.88 | 9.44 | 14.92 | 65.3 |
| 2,5-Dihydroxybenzoic | 44 | 0.543 | 72 | 0.07 | 0.22 | 0.43 | 0.949 | 1.43 |
| Caffeic Acid | 44 | 0.589 | 115 | 0.05 | 0.21 | 0.36 | 0.672 | 3.72 |
| Chlorogenic Acid | 44 | 0.055 | 138 | 0.00 | 0.00 | 0.00 | 0.131 | 0.21 |
| Salicylic Acid | 44 | 1.020 | 82 | 0.13 | 0.55 | 0.76 | 1.242 | 5.16 |
| Rutin | 44 | 0.242 | 58 | 0.06 | 0.13 | 0.22 | 0.335 | 0.70 |
| p-Coumaric Acid | 44 | 0.331 | 138 | 0.00 | 0.00 | 0.14 | 0.501 | 1.55 |
| Trans-Ferrulic Acid | 44 | 1.620 | 137 | 0.00 | 0.33 | 0.54 | 2.337 | 7.90 |
| Ethyl Gallate | 44 | 0.010 | 96 | 0.00 | 0.01 | 0.01 | 0.012 | 0.03 |
| Resveratrol | 44 | 0.087 | 239 | 0.00 | 0.00 | 0.00 | 0.000 | 0.76 |
| Propyl Gallate | 44 | 0.002 | 78 | 0.00 | 0.01 | 0.00 | 0.038 | 0.01 |
| Quercetin | 44 | 0.327 | 83 | 0.03 | 0.09 | 0.21 | 0.561 | 0.97 |
| Lutolein | 44 | 0.111 | 77 | 0.01 | 0.05 | 0.09 | 0.167 | 0.33 |
| Abscisic Acid | 44 | 20.34 | 107 | 0.34 | 6.01 | 14.9 | 23.01 | 88.0 |
| Naringenin | 44 | 0.306 | 70 | 0.06 | 0.14 | 0.22 | 0.447 | 0.87 |
| Genistein | 44 | 0.064 | 147 | 0.01 | 0.01 | 0.02 | 0.075 | 0.33 |
| Isorhamnetin | 44 | 0.574 | 64 | 0.11 | 0.28 | 0.43 | 0.837 | 1.44 |
| Kaempferol | 44 | 0.660 | 68 | 0.17 | 0.28 | 0.55 | 0.948 | 1.88 |
| Apigenin | 44 | 0.040 | 64 | 0.00 | 0.02 | 0.04 | 0.059 | 0.09 |
| Caffeic Acid Phenyl Ester | 44 | 0.064 | 65 | 0.01 | 0.02 | 0.07 | 0.086 | 0.18 |
| Total Phenolic Compounds | 44 | 88.05 | 53 | 38.35 | 45.96 | 67.9 | 100.1 | 204 |
| Variable | F1 | F2 | F3 | F4 | Communality |
|---|---|---|---|---|---|
| Lutolein | 0.856 | 0 | 0 | 0 | 0.787 |
| Trans-Ferrulic Acid | 0.854 | 0 | 0 | 0 | 0.822 |
| Propyl Gallate | 0.808 | 0 | 0 | 0 | 0.752 |
| Caffeic Acid | 0.697 | 0 | 0 | 0 | 0.573 |
| 2,5-Dihydroxybenzoic Acid | 0.666 | 0 | 0 | 0 | 0.498 |
| Quercetin | 0 | 0.843 | 0 | 0 | 0.776 |
| p-Coumaric Acid | 0 | 0.843 | 0 | 0 | 0.731 |
| Isorhamnetin | 0 | 0.693 | −0.485 | 0 | 0.758 |
| Ethyl Gallate | 0 | −0.685 | 0 | 0 | 0.494 |
| Naringenin | 0 | 0 | −0.88 | 0 | 0.871 |
| Caffeic Acetyl Phenyl Ester | 0 | 0 | −0.878 | 0 | 0.833 |
| Resveratrol | 0 | 0 | 0.63 | 0 | 0.56 |
| Salicylic Acid | 0 | 0 | −0.57 | 0 | 0.467 |
| Apigenin | 0 | 0 | −0.565 | 0 | 0.559 |
| Total phenolic | 0 | 0 | 0 | 0.975 | 0.958 |
| Gallic Acid | 0 | 0 | 0 | 0.858 | 0.769 |
| Abscisic Acid | 0 | 0 | 0 | 0.677 | 0.579 |
| Variance | 3.461 | 3.013 | 3.007 | 2.307 | 11.787 |
| % Variance | 0.204 | 0.177 | 0.177 | 0.136 | 0.693 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamiti, X.; Shallari, G.; Pupuleku, B.; Yücel, A.; Çelìk, S.; Sulejmani, E.; Lazo, P. Phenolic Profiling of Albanian Honeys by LC–MS/MS: Gallic Acid as a Predictive Marker of Antioxidant Potential. Molecules 2025, 30, 4037. https://doi.org/10.3390/molecules30204037
Hamiti X, Shallari G, Pupuleku B, Yücel A, Çelìk S, Sulejmani E, Lazo P. Phenolic Profiling of Albanian Honeys by LC–MS/MS: Gallic Acid as a Predictive Marker of Antioxidant Potential. Molecules. 2025; 30(20):4037. https://doi.org/10.3390/molecules30204037
Chicago/Turabian StyleHamiti, Xhulieta, Gjyliza Shallari, Blerina Pupuleku, Alp Yücel, Saffet Çelìk, Erhan Sulejmani, and Pranvera Lazo. 2025. "Phenolic Profiling of Albanian Honeys by LC–MS/MS: Gallic Acid as a Predictive Marker of Antioxidant Potential" Molecules 30, no. 20: 4037. https://doi.org/10.3390/molecules30204037
APA StyleHamiti, X., Shallari, G., Pupuleku, B., Yücel, A., Çelìk, S., Sulejmani, E., & Lazo, P. (2025). Phenolic Profiling of Albanian Honeys by LC–MS/MS: Gallic Acid as a Predictive Marker of Antioxidant Potential. Molecules, 30(20), 4037. https://doi.org/10.3390/molecules30204037








