Abstract
Protein homeostasis is a dynamic process essential for cellular function and survival, tightly controlled by the ubiquitin–proteasome system. Within this system, ubiquitin-specific protease 7 (USP7) plays a key role as a deubiquitinating enzyme, thus modulating the stability, localization, and activity of a wide variety of substrates. USP7 is involved in critical cellular processes such as DNA repair, apoptosis, immune response, and epigenetic regulation. The dysregulation of USP7 expressions or activity has been linked to several pathological conditions, including cancer, neurodegenerative and inflammatory diseases, and viral infections. This enzyme exerts its biological functions through the stabilization of both oncogenic and tumor suppressor proteins, highlighting its sensitive role in tumorigenesis. Despite the identification of selective USP7 inhibitors with promising preclinical activity, the development of clinically effective compounds remains a major challenge. This review summarizes the current understanding of USP7 structure, function, and biological relevance, with a particular emphasis on its potential as a therapeutic target in oncology.
1. Introduction
Aggregated and/or misfolded proteins are constantly eliminated inside eukaryotic cells to preserve cellular functions. This continuous and complex process, called protein homeostasis, is mainly regulated by the ubiquitin–proteasome system (UPS) (Figure 1) [1,2]. A crucial mechanism in this turnover is represented by protein ubiquitination, a post-translation modification that contributes to cell homeostasis by regulating the stability, localization, and activities of different proteins [3,4,5]. Protein ubiquitination is controlled by deubiquitinating enzymes (DUBs) that selectively remove ubiquitin chains from proteins [6,7]. Among the nine DUB families, ubiquitin-specific proteases (USPs) are the predominant and most studied [8].
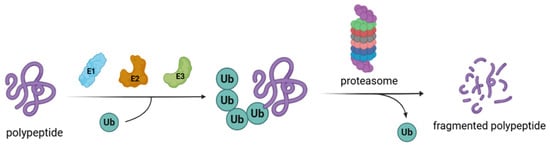
Figure 1.
Schematic activity of the ubiquitin proteosome system. Ub = ubiquitin, E1 = E1 ligase, E2 = E2 ligase, E3 = E3 ligase.
Ubiquitin-specific protease 7 (USP7), also known as herpesvirus-associated ubiquitin-specific protease (HAUSP), belongs to the USP family and is involved in the regulation of different pathways such as immune response, epigenetic control, viral replication, cell apoptosis, DNA damage response, and DNA replication and transcription [9,10]. Dysregulations of USP7 expression could lead to different pathological conditions including cancer, viral infections, inflammatory diseases, and neurodegenerative diseases [11,12,13,14]. Consequently, USP7 represents a relevant pharmacological target for the treatment of various pathological disorders; in particular, its aberrant expression in many cancers has been widely studied to explore novel antitumor strategies [15]. In fact, USP7 mediates the deubiquitination and stabilization of oncogenic proteins such as murine double minute 2 (MDM2), murine double minute X (MDMX), and the tumor suppressors p53 [16], phosphatase and tensin homolog (PTEN) [17], nuclear factor-kappa B (NF-κB) [18], β-catenin [19], and forkhead box P3 (FOXP3) [20]. However, despite several USP7 inhibitors having been reported in the scientific literature, the search for potent and selective compounds is still challenging and, unfortunately, to date, no USP7 inhibitors have been approved for clinical use [21].
1.1. Structure and Function of the Ubiquitin–Proteasome System
The UPS consists of an intricate and complex network which is mainly implicated in the regulation of ubiquitin conjugation, a multistep process associated with several biological functions including DNA repair, transcriptional regulation, endocytosis, intracellular signaling, and protein trafficking [22].
Ubiquitin is a small 76-amino-acid protein, widely found all over eukaryotic cells. Its covalent attachment to the target, mediated by UPS enzymes, regulates protein interaction, localization, and degradation [23]. The ubiquitination process is orchestrated by the sequential action of three different enzymes: E1 (ubiquitin-activating enzyme), E2 (ubiquitin-conjugating enzymes), and E3 (ubiquitin–protein ligase) [24]. The reaction begins with the formation of a thioester intermediate between E1 catalytic cysteine and ubiquitin C-terminal glycine through an ATP-dependent mechanism. In the second step, the activated ubiquitin is relocated to E2 by trans thiolation. Finally, E3 recruits the target protein and transfers ubiquitin from the E2 enzymes to the substrate (Figure 2). Usually, a lysine residue on the substrate attacks the ubiquitin-E2 thioester bond, leading to the acylation of the lysine ɛ-amino group. However, when there are no accessible lysine residues on the target, ubiquitin is conjugated to the substrate N-terminal amino group [25,26,27]. According to the number of ubiquitin moieties attached to the substrate, three different types of ubiquitination linkages have been described. The addition of a single ubiquitin unit on a single lysine residue on the substrate is called monoubiquitination. On the other hand, multi-monoubiquitination occurs when a single ubiquitin unit is conjugated to multiple lysine residues of the target protein. Finally, polyubiquitination refers to the attachment of more than two ubiquitin units to the same lysine residue of the target protein [3,28]. Polyubiquitination can occur on specific ubiquitin lysin residues (namely, K6, K11, K27, K29, K33, K48, and K63) or on the N-terminal methionine M1; these amino acids can be independently ubiquitinated, leading to the formation of different types of ubiquitin chains linked by isopeptide bonds to the substrate protein. K48-Linked polyubiquitin chains are a signal for the 26S proteasome-mediated degradation of the target, whereas the other chains have a non-degradation role [29,30]. 26S proteasome (present in both cytosol and the nucleus of eucaryotic cells) plays a pivotal role in the UPS. In detail, this multi-subunit proteolytic complex degrades a large variety of regulatory damaged and unfolded proteins, acting as a proteome modulator and controlling numerous cellular processes [31].
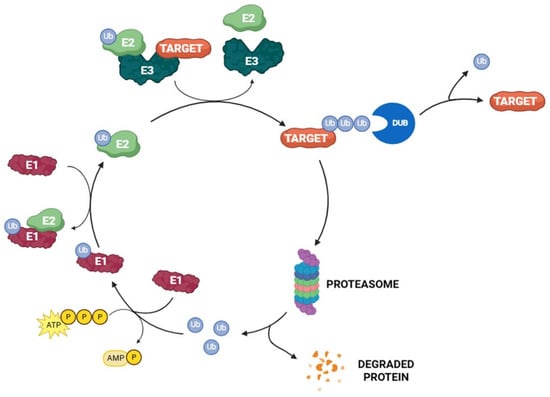
Figure 2.
The UPS pathway.
Protein ubiquitination can be reversed by DUBs, a special class of enzymes associated with 26S proteasome that remove the ubiquitin units from the target protein [32]. Up to now, about 100 DUBs have been identified in humans and are divided into two main categories: cysteine proteases and metalloproteases. According to their sequence and domain architecture, cysteine proteases are further subclassified into eight families, namely ubiquitin-specific proteases (USPs), ubiquitin C-terminal hydrolases (UCHs), Otubain domain ubiquitin-binding proteins (OTUs), Machado–Joseph disease protein domain proteases (MJDs), the MIU-containing novel DUB family (MINDY), monocyte chemotactic protein-induced proteins (MCPIPs), permuted papain fold peptidase of dsDNA viruses and eukaryotes (PPPDEs), and zinc finger with UFM1-specific peptidase domain proteins (ZUFSPs). On the other hand, the class of metalloproteases is only represented by the group of JAMM/MPN domain-associated metallopeptidases (JAMMs) [33,34].
USP is the largest and best characterized family among DUBs, containing approximately 60 members. This family is able to hydrolyze the ubiquitin amide bond through a catalytic triad composed of a cysteine, a histidine, and an asparagine or aspartate residue [35]. The catalytic domain of all USP enzymes shares a common structural arrangement resembling an extended right hand with palm, thumb, and finger subdomains. The catalytic pocket is located between the palm and the thumb, while the fingers could stabilize distal ubiquitin on the protein substrate (Figure 3) [35,36].
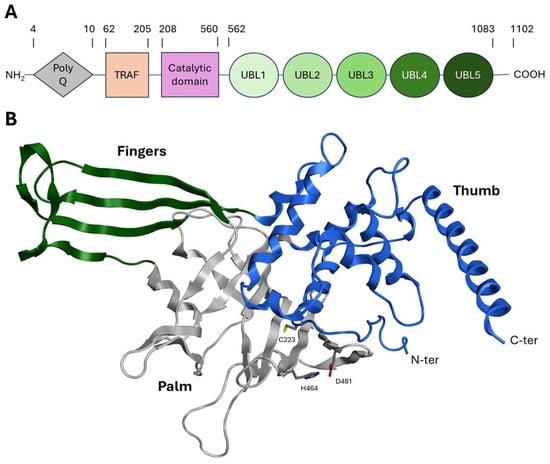
Figure 3.
(A) USP7 subdomains. (B) Crystal structure of the catalytic domain of USP7 (PDB: 59NT). The finger, thumb, and palm subdomains are colored green, blue, and gray, respectively. Catalytic residues are represented as stick models.
1.2. USP7 Structure
USP7 represents the best characterized and most studied deubiquitinating enzyme within the USP family. The gene encoding for this 135 kDa (1102 amino acids) cysteine protease is located on chromosome 16p13.2. The USP7 structure can be divided into the following subdomains: (i) an N-terminal polyglutamine (polyQ) stretch region (aa 1–50); (ii) a tumor necrosis receptor-associated factor (TRAF)-like structural domain (aa 53–205); (iii) a central catalytic region (aa 208–560); (iv) five ubiquitin-like domains (UBL1–5) (aa 562–1083); and (v) nineteen amino acid residues at the C-terminus (aa 1084–1102) (Figure 3A) [37,38]. These subdomains are described in detail below:
- The polyQ and TRAF-like domains are highly conserved in humans, mice, and rats and are responsible for the USP7 nuclear localization. Despite the lack of a proper localization signal in this region, it has been postulated that the binding of USP7 to its substrates (commonly nuclear proteins) could favor the translocation into the nucleus [39,40].
- The TRAF-like domain consists of eight β-sheets and one α-helix. This region contains several protein–protein interaction patterns that allow USP7 to recognize the different substrates involved in the ubiquitination pathway, such as MDM2, p53, Epstein–Barr nuclear antigen 1 (EBNA1), viral interferon regulatory factor 4 (IRF-4), latency-associated nuclear antigen (LANA), and testis-specific protein Y-encoded-like 5 (TSPYL5) [41,42].
- The catalytic domain is structurally arranged as an outstretched right hand consisting of thumb, finger, and palm domains. Its structure contains ten α-helices and fourteen β-sheets and includes a conserved catalytic triad composed of Cys223, His464, and Asp481 (Figure 3B) [43]. These three residues are usually in a non-reactive conformation and switch to an active state upon ubiquitin binding. The activation mechanism is facilitated by a region known as the “switching loop,” which brings the catalytic triad residues into proximity, enabling ubiquitin interaction [41].
- The UBL domain consists of five ubiquitin-like ββαβαβ-fold domains possibly involved in substrate recognition and protein–protein interactions [44]. Specifically, UBL 1, 2, and 3 domains interact with infected cellular polypeptide 0 (ICP0), ubiquitin-like with PHD and ring finger domain 1 (UHRF1), and DNA methyltransferase-1 (DNMT1), among others [45]. UBL structural domains 4 and 5 bind to forkhead box O4 (FoxO4) [46]. Notably, the UBL4 domain interacts with the UbcH5c E2 conjugating enzyme, facilitating the transfer of ubiquitin molecules from E1 to the substrate protein, thereby enhancing its stability and mediating various cellular processes [47,48].
UBL domains assume a central role in USP7 regulation, thus affecting several physiological functions such as genome stability, epigenetic regulation, immune response, and viral infection [1]. More specifically, the UBL4–5 tandem, together with the conserved C-terminal peptide, is essential for the allosteric stabilization of the catalytic “switching loop,” thereby enabling full enzymatic activity [43,44]. Additionally, UBL1–3 act as accessory regulatory modules that facilitate cofactor-dependent activation and contribute to substrate recruitment [45]. For these reasons, targeting the UBL domains or altering the stabilization of the inactive conformation via UBL-mediated mechanisms may be feasible strategies for a therapeutic approach [9,17,43].
The USP7 catalytic mechanism includes different sequential steps (Figure 4). At first, ubiquitin interacts with USP7, and the complex is stabilized by different interactions, including the hydrogen bond between the ubiquitin C-terminal carbonyl and Asn481 amide group. Then, His464 abstracts a proton from the Cys223 thiol group that can attack the isopeptide bond, leading to the substrate release and the thioester intermediate formation. Finally, the thioester group is hydrolyzed by a water molecule and free ubiquitin is released. In this context, Asp481 plays a key role in polarizing His464 and restricting its side-chain rotation [49,50].
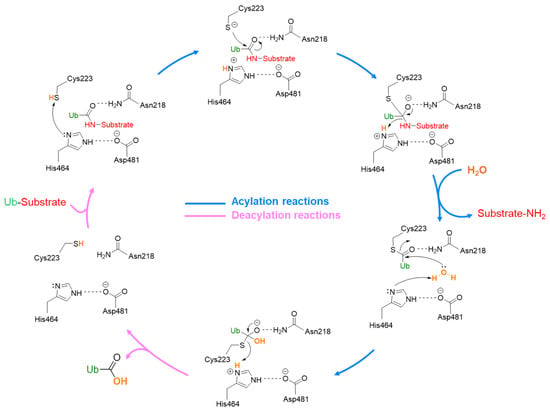
Figure 4.
USP7 catalytic mechanism.
Beyond the catalytic triad, various other critical residues within the USP7 catalytic domain influence enzyme specificity and activity. In detail, Ser18 regulates the conformational dynamics of the catalytic cleft, while Phe270 and Phe439 are essential for substrate binding and recognition [51]. Additionally, structural studies revealed that an allosteric site near the catalytic cleft is able to modulate USP7 activity by regulating its conformational changes [52]. Moreover, the hydrolytic activity of the catalytic domain is significantly influenced by the nature of the ubiquitin chains on the substrate protein [30,53].
1.3. USP7 Roles and Functions
USP7 has been implicated in the development and progression of various illnesses, including cancer, neurodegenerative disorders, viral infections, and inflammatory disease regression [13,54,55]. Among all the pathologies in which this enzyme is involved, human cancer stands out as the most studied. In this context, USP7 plays a dual role, acting as both a tumor promoter and a tumor suppressor depending on the cancer type [54].
USP7 is critically involved in cancer development, mediating the deubiquitylation (and therefore the stabilization) of oncogenic proteins such as MDM2 and c-Myc, and influencing growth factor TGF-β, which promotes cell survival and proliferation. These actions affect different processes, including cellular DNA damage repair, tumor-related signaling pathway regulation, and immune response, indicating USP7 as a promising target for the development of novel antitumor therapeutics [55,56].
USP7 overexpression has been reported in numerous malignant tumors as a predictive marker of tumor progression and poor prognosis [57]. More specifically, the dysregulation of USP7 expression has been linked to the onset of many malignancies, such as multiple myeloma [58], prostate cancer [16], ovarian cancer [59], cervical cancer [60], glioma [61], colorectal cancer (CRC) [19], liver and brain cancer [9], gastric cancer [2], and head and neck cancer [62]. Additionally, USP7 activity is related to angiogenesis that promotes tumor irroration [15]. Lastly, USP7 is deeply involved in immune system regulation [63]. Specifically, the enzyme can promote cancer immune evasion, enhancing the immunosuppressive functions of regulatory T (Treg) cells and suppressing the activity of effector T (Teff) cells [64]. In recent years, several studies demonstrated a correlation between antitumor drug resistance and USP7 overexpression. Indeed, USP7 pharmacological inhibition could represent a solution for highly aggressive tumors unaffected by drug treatments, indirectly modulating the immune system response [64]. Despite USP7 being mainly involved in cell cycle regulation and tumor progression, other biological pathways have been investigated. For instance, recent studies analyzed the interaction of the enzyme with melanoma-associated antigen D1 (Maged1), a protein specialized in H2A monoubiquitination in the paraventricular thalamus (PVT) that significantly contributes to cocaine adaptive behaviors and transcriptional repression induced by cocaine [65]. For all these reasons, USP7 emerged as a privileged target for the development of new pharmacologically active compounds.
2. USP7 Implications in Cancer
As previously discussed, USP7 plays a key role in tumor development and progression, as well as in the regulation of the immune response. In fact, through the deubiquitination process, this enzyme can control the expression of several targets involved in tumor growth and angiogenesis. As anticipated, depending on the substrate and the tumor type, USP7 can act as a tumor suppressor or oncogene [1]. In the next paragraphs, the major USP7 targets involved in cancer development will be discussed.
2.1. MDM2 and p53 Regulation
The tumor suppressor protein p53 is a pivotal factor in the regulation of cell growth, apoptosis, and DNA repair. However, it is highly prone to mutations that shift its function from a tumor suppressor to a tumor promoter due to changes in its spatial conformation [66]. In response to cellular stress, p53 undergoes ubiquitination (beside other post-translational modifications) and proteasomal degradation [67,68]. MDM2 plays a key role in this process, being a substrate of both USP7 and p53 E3 ubiquitin ligase. In normal conditions, MDM2 binds the USP7 TRAF domain even in the presence of an excess of p53 (Figure 5) [69]. Conversely, when the cells are exposed to DNA damage, MDM2 is phosphorylated by the ataxia-telangiectasia mutated kinase, causing its dissociation from USP7. This disconnection triggers MDM2 self-ubiquitination and a subsequent proteasomal degradation (Figure 5). Additionally, USP7 undergoes dephosphorylation by the protein phosphatase Mg2+/Mn2+-dependent 1G, altering its binding preference to p53. As a result, USP7-mediated p53 deubiquitination leads to cell cycle arrest and apoptosis [10,50]. USP7 helps to stabilize p53 directly by removing its ubiquitin tag or indirectly by deubiquitinating MDM2, a negative regulator of p53. MDM2 binds to the N-terminal deactivation domain of p53, facilitating its ubiquitination and subsequent degradation, thereby inhibiting its transcriptional activity [51]. Furthermore, USP7 interacts with other p53 regulators, such as the Alternate Reading Frame (ARF), which activates p53 [70]. The death domain-associated protein (DAXX) also plays a role in the MDM2/p53 pathway by forming a ternary complex with MDM2 and USP7 [71]. In breast cancer, USP7 cooperates with DAXX in regulating mitosis and taxane resistance, independently from p53 activity [72]. It is well-known that the tumor-suppressing functions of p53, which control cell growth arrest, apoptosis, and senescence, are impaired in about half of human cancers. Furthermore, the amplification and overexpression of p53 negative regulators MDM2 and MDMX are associated with the development of various cancer types [73]. Considering that USP7 is a crucial regulator of the MDM2/MDMX-p53 pathway, its impact on cancer progression and survival has been demonstrated to be linked to the stabilization of MDM2 and MDMX in different tumors such as neuroblastoma (NB) [74], glioma [61], multiple myeloma [58], epithelial ovarian cancer [59], and both lung squamous cell carcinoma and large cell carcinoma [75]. In particular, glioblastoma, one of the most common and aggressive malignant brain tumors, is a good example of the involvement of USP7 and MDM2 in the pathology onset. More specifically, USP7 overexpression leads to the degradation of the retinoblastoma protein, a regulator of the G1 phase of the cell cycle [76]. Furthermore, USP7 can bind to Lysine-specific histone demethylase 1A (LSD1), which further increases the proliferation of the tumor. In fact, when LSD1 is stabilized by USP7, the p53-dependent signaling is suppressed, thus allowing glioma proliferation [77].
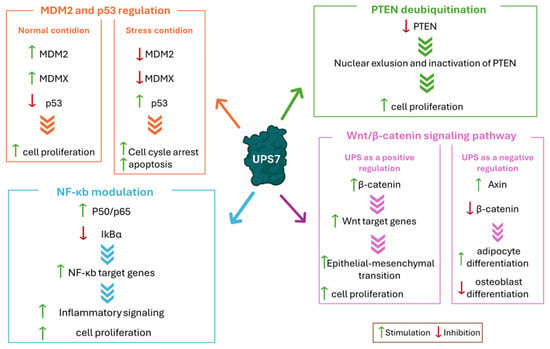
Figure 5.
USP7 interaction with the main substrates involved in cancer development. The most relevant pathways affected by USP7 are reported in different colored boxes. Green arrows indicate stimulation; red arrows indicate inhibition.
2.2. PTEN Deubiquitination
The lipoprotein phosphatase PTEN is a tumor suppressor gene frequently mutated in various cancer types. Together with p53, this protein is closely related to tumorigenesis and represents one of the evaluation indexes for tumor prognosis [78]. PTEN’s main function is carried out in the plasma membrane and consists of the inhibition of protein kinase B (Akt), causing the disconnection of the PI3K/Akt/mTOR signaling pathway and leading to cell cycle arrest and consequently to apoptosis [79]. Therefore, PTEN mutation, deletion, or silencing induces tumorigenesis [78,80]. PTEN cytosolic–nuclear translocation mainly occurs through post-translation modification. In detail, monoubiquitination leads to the transport inside the nucleus, while polyubiquitination causes PTEN proteosome-mediated degradation. Finally, USP7-mediated deubiquitination promotes PTEN deactivation through nuclear exclusion (Figure 5). In several malignancies, alterations in USP7 expression cause PTEN nuclear exclusion and lead to disease progression [81]. For example, in prostate cancer, USP7 is often upregulated, promoting PTEN localization in the cytoplasm and not in the nucleus. Additionally, USP7 can affect the stability of the zeste homolog 2 (EZH2) enhancer, a protein involved in the methylation of H3 histone on K27. The inhibition of PTEN nuclear translocation coupled with EZH2 overexpression leads to more aggressive tumors. For these reasons, USP7 inhibition could decrease prostate cancer cell migration, invasion, and proliferation [82]. Similarly, in acute myelogenous leukemia (AML), nucleophosmin 1 (NPM1) regulates PTEN nuclear exclusion by inhibiting its USP7-mediated deubiquitination. Therefore, the PTEN nuclear exclusion induces tumorigenesis and cancer spreading [83].
PTEN deubiquitination plays a pivotal role also in the growth and development of β-cell lymphomas. More specifically, the upregulation of thyroid hormone receptor interactor 13 (TRIP13) induces the intracellular post-translational modification of different proteins including NEK2, MDM2, and PTEN. Several studies reported that higher levels of TRIP13 are associated with poor survival and accelerate tumorigenesis [62]. Differently, in lung neuroendocrine tumors, the loss of nuclear USP7 leads to a reduction in PTEN deubiquitination and to an increase in polyubiquitination, which consequently tags the protein for proteasomal degradation [84].
2.3. Wnt/β-Catenin Signaling Pathway
The Wnt/β-catenin cascade is deeply involved in the regulation of embryonic development, apoptosis, tissue homeostasis, cell proliferation, differentiation, migration, and invasion. Thus, dysregulations of this signaling pathway are often associated with the progression of a wide variety of tumors [85]. The most important component of this pathway is β-catenin, a transcription factor which induces the expression of Wnt target genes. Among the different factors that regulate β-catenin levels, ubiquitination proves to be the most relevant one [86]. The complex composed of glycogen synthase kinase 3β (GSK3β), casein kinase 1α (CK1α), adenomatous polyposis coli (APC), and Axin is responsible for β-catenin phosphorylation in the absence of Wnt. The phosphorylated protein is polyubiquitinated by β-transducin repeat-containing protein (βTrCP) E3 ligase and sent to proteasomal degradation [86]. The Wnt/β-catenin cascade is also controlled by deubiquitination. In this pathway, USP7 can either work as a positive or negative regulator (Figure 5). In detail, USP7 acts as a negative regulator of the Wnt/β-catenin axis by deubiquitinating Axin, and therefore inducing the decrease in β-catenin and the suppression of the pathway [87]. Conversely, USP7 can interact with RNF220 E3 ligase to form a RNF220/USP7 β-catenin deubiquitinating complex that exerts positive regulator functions on the Wnt/β-catenin cascade [88]. It was recently demonstrated that CRCs are commonly characterized by a mutation of the β-catenin inhibitory domain (CID) that causes an alteration of the Wnt/β-catenin signaling pathway and consequent β-catenin accumulation [88]. Additionally, in CRCs, USP7-mediated deubiquitination seems to stabilize the DEAD-box helicase 3 X-linked (DDX3X), still promoting β-catenin accumulation. This increased activation of the Wnt/β-catenin axis induces epithelial–mesenchymal transition, a phenomenon strongly related to the advanced stages of the pathology [89]. USP7 pharmacological inhibition suppresses the proliferation of CRC by inducing apoptosis only on cells carrying a mutated CID, suggesting that USP7 acts as a tumor-specific drug target [88].
2.4. NF-κb Modulation
The NF-κb transcription factor regulates a series of biological processes involved in the development of multiple pathologies, including autoimmune diseases, inflammation, and hematologic and solid malignancies. NF-κB activates excessive innate immunity and abnormal cell growth, which directly influence tumor development and progression. Additionally, NF-κB also exerts direct anti-inflammatory activity by inhibiting inflammasomes [90]. In order to start gene transcription, NF-κB proteins can dimerize and form (among the others) the p65-p50 heterodimer that was proven to be involved in different pathologies [91]. In non-stimulated cells, the NF-κB dimers bind to NF-κB inhibitors (IκBs) in an inactive state. Upon stimulation, the IκBs subunit β is activated [92], inducing the expression of regulatory genes involved in the regulation of cellular stress, immune response, inflammation, and cell death, differentiation, and proliferation [91,92]. USP7 controls NF-κB through the direct deubiquitination of p65-NF-κB or by indirectly deubiquitinating NF-κB upstream factors (Figure 5) [18]. NF-κB activation seems to be a key mechanism in the bortezomib resistance developed by multiple myeloma. Indeed, recent evidence has demonstrated that USP7 inhibition can stabilize the IκBα inhibitor, causing the arrest of the NF-κB pathway and restoring tumor cell sensitivity to bortezomib. Moreover, USP7 plays a crucial role in multiple myeloma resistance development by binding the NIMA-related kinase 2 (NEK2). This interaction leads to an increase in NEK2 expression, further contributing to NF-κB pathway activation [93]. Additionally, the USP7-NF-κB complex stabilizes the Beclin-1 protein, promoting cellular autophagy and, consequently, contributing to bortezomib resistance [94]. Studies showed that the combination of USP7 inhibitors with bortezomib exhibited a synergistic activity against multiple myeloma, suggesting a promising therapeutic strategy for overcoming resistance to current treatments [95].
2.5. Interaction with Insulin Receptor Substrates
Insulin receptor substrates (IRSs) are signaling adapter proteins which mediate the bioactivities of insulin/insulin-like growth factor (IGF) signaling pathways [96]. Tyrosine kinase insulin receptors phosphorylate tyrosine residues of IRSs in response to IGF activation, allowing for the association of IRSs with SH2 domain-containing proteins (e.g., phosphatidylinositol 3-kinase (PI3K) and growth factor receptor-bound protein 2 (Grb2)). These mechanisms cause the activation of other downstream kinases, including Akt and extracellular signal-regulated kinase (ERK), that modulate a wide variety of biological and pathological processes [97]. USP7 is an IRS-associated protein that can protect both IRS-1 and IRS-2 from ubiquitination and consequent proteasomal degradation [15]. Insulin or IGF stimulation activates the PI3K pathway and causes the dissociation of USP7 from IRSs and subsequent IRS degradation [98]. It is well established that IRS-1 and IRS-2 control different functional outcomes in tumor cells. In detail, IRS-1 promotes proliferation through IGF-1R-dependent signaling, while IRS-2 interferes with cell migration, invasion, and glucose metabolism. To prevent excessive signal activation, USP7 inhibits the insulin/IGF signaling. However, the impact of this process on cancer progression is still not clear [99]. Indeed, tumor proliferation is also influenced by other USP7-mediated interactions. For example, recent studies demonstrated that USP7 promoted the spread of prostate cancer through an interaction with the 5-dihydrotestosterone-activated androgen receptor [100].
2.6. Cellular Energetics Deregulation
To generate energy for DNA replication and other cellular functions, tumor cells maintain glycolytic activity in both aerobic and anaerobic conditions while reducing the activity of the Krebs cycle and the mitochondrial electron transport system (ETS) [1,9]. Normal cells usually produce thirty-eight ATP molecules through complete aerobic respiration; on the other hand, in cancer cells, normal aerobic respiration is replaced by glycolysis, yielding only two ATP molecules. Hence, to meet their increased energy demand, tumors consume glucose at a significantly higher rate than healthy cells, a phenomenon known as the Warburg effect [101]. Normally, in healthy cells, glucagon triggers the cAMP/PKA signaling pathway, which induces the transcription of gluconeogenic genes. This process activates the expression of different transcription factors, including Forkhead box protein O1 (FOXO1). FOXO1 activity is further enhanced by an unidentified E3 enzyme through monoubiquitination, which promotes the binding of FOXO1 to the promoter regions of the G6Pc and Pck1 genes. Conversely, upon feeding, insulin is released, inhibiting hepatic gluconeogenesis through the PI3K/AKT-mediated phosphorylation and USP7-dependent deubiquitination of monoubiquitinated FOXO1, causing its nuclear exclusion [102].
Another USP7 substrate involved in glucose metabolism is sirtuin 7 (SIRT7), a NAD+-dependent class III deacetylase known for its ability to resist glycolytic stress [103]. The SIRT7 enzyme typically binds to the promoter of the G6Pc gene through the ETS-like transcription factor 4 (ELK4). Additionally, SIRT7 can deacetylate H3K1 histone, a key player in the activation of G6Pc transcription, thus starting gluconeogenesis. USP7 regulates SIRT7 by deubiquitinating a lysine residue (namely, K63) on the protein, thereby reducing its activity and suppressing gluconeogenesis [104]. However, despite extensive research, the exact role of USP7 in providing a metabolic advantage to tumor cells remains controversial. Indeed, experimental evidence suggests that the presence of USP7 might be disadvantageous for tumor cell survival. On the other hand, a reduced interaction between USP7 and SIRT7 has been observed in different malignancies, thus promoting gluconeogenesis and proliferation in cancer cells. Therefore, the role of USP7 in tumor cellular energetic deregulation needs further investigation [105].
2.7. Other USP7 Implications in Tumors
Breast cancer is commonly divided into three major categories based on hormone receptor expression, namely estrogen receptor (ER) positive, human epidermal growth factor receptor (HER2) positive, and triple-negative breast cancer (TNBC) [106]. In recent years, several studies have shown that the upregulation of Mitogen-inducible gene-6 (MIG-6) induces breast cancer progression with a poor prognosis. More specifically, MIG-6 is able to increase USP7-mediated Hypoxia-inducible factor 1-alpha (HIF-1α) deubiquitination, thus causing its overexpression. This process leads to an enhanced expression of glucose transporter 1 (GLUT1) and, consequently, to a promotion of glucose metabolism and TNBC growth [107]. Furthermore, USP7 can also increase the stability of ATP-binding cassette sub-family B member 1 (ABCB1), thus inducing chemoresistance in TNBC [108].
USP7 also plays a crucial role in the progression of various types of head and neck cancers. In particular, head and neck squamous cell carcinoma (HNSSC) progression was proven to be prompted by the stabilization of the Hippo signaling effector TAZ, induced by the USP7-mediated deubiquitination of its K48-linked polyubiquitin chain [109]. Moreover, in head and neck esophageal carcinoma, the migration and proliferation of tumor cells are mediated by a complex pathway that deeply involves USP7. In detail, Forkhead box protein O6 (FOXO6) upregulation causes an overexpression of USP7, which stabilizes Jumonji domain-containing protein-3 (JMJD3). JMJD3 is able to suppress the transcriptional activity of clusterin by competitively binding to its promoter region and indirectly promotes tumor growth [62].
Finally, in hepatocellular carcinoma (HCC), USP7 seems to increase the expression of the Tripartite Motif Containing 27 (TRIM27) gene by enhancing its stability through deubiqutination. In fact, the activation of the USP7-TRIM27 axis has been associated with an increase in cell proliferation and a poor prognosis [110,111].
3. USP7 Role in Angiogenesis and Metastasis
Angiogenesis is regulated by pro- and anti-angiogenic factors (e.g., vascular endothelial growth factor (VEGF) A, thrombospondin-1 (TSP-1)) present in the tumor microenvironment [112,113]. VEGF acts as the key regulator of the angiogenetic mechanism, and its secretion represents a rapid and efficient response to hypoxia in cancer cells. Hypoxia-inducible factors (HIFs) mediate VEGF transcription and consequently activate glycolysis, cell invasion, angiogenesis, and other survival responses [114]. In this context, USP7 plays a pivotal role by deubiquitinating HIFs and other downstream regulators involved in angiogenesis and metastasis processes.
3.1. HIF-1α Stabilization
As previously discussed, HIFs mediate angiogenesis by regulating VEGF transcription and can be deubiquitinaned by USP7. In particular, HIF-1α is deeply involved in several signaling pathways that lead to the vascularization of cancer cells [114]. Under normal oxygen conditions, HIF-1α is marked with prolyl hydroxylation at P402 and P564 residues and undergoes proteasomal degradation [115]. Conversely, under hypoxic conditions, USP7 is activated by the HECTH9 protein (polyubiquitination at K443 residue) and consequently stabilizes HIF-1α. The polyubiquitin chain on USP7 represents an anchor point for the CREB-binding protein (CBP), which promotes the acetylation of histone 3 on HIF-1α target genes at lysine 56 [116]. The activity of HIF-1α facilitates the disruption of nuclear export and the degradation of p53, and promotes p53-mediated transcription. As previously mentioned, USP7 binds to both p53 and MDM2 through its TRAF-like domain. The binding site for HIF-1α is also located on the TRAF-like domain, in proximity to the binding sites of p53 and MDM2. Therefore, it has been hypothesized that the HIF-1α/p53/MDM2/USP7 complex can contribute to the regulation of the cellular response to hypoxia [117]. Additionally, HIF-1α is actively implicated in epithelial–mesenchymal transition (EMT) and metastasis [118]. In this context, HIF-1α activity is regulated by FOXO in several ways [119,120,121]. USP7 is one of the antagonistic regulators of FOXO nuclear localization and transcriptional activation. In fact, USP7 plays a pivotal role in the regulation of FOXO4 activity, and for these reasons it has been currently under study as a therapeutic target for diseases associated with dysregulated FOXO4 signaling. However, the precise mechanisms behind the interaction between USP7, FOXO4, and HIF1α is still unclear, and future in-depth studies are needed to discover a specific therapeutic intervention [46,122,123].
3.2. EZH2 and Wnt/β-Catenin Pathways Activation
EZH2 is mainly expressed in tumor and vascular cells and is often associated with poor clinical outcomes. This enhancer modulates the expression of specific genes and promotes cell survival, proliferation, and invasion, EMT, and drug resistance in cancers [82]. Furthermore, EZH2 is responsible for the activation of interleukin 6 (IL-6) and tumor necrosis factor (TNF) activation, as well as the interferon gamma receptor 1 (IFNGR1) repression. These events initiate chronic inflammation, which may contribute to tumor development. Additionally, VEGF activation and the subsequent EZH2 high levels stimulate a paracrine circuit that in turn induces angiogenesis [114]. EZH2 is also able to interact with epigenetic modifiers such as LSD1, histone deacetylase 1 (HDAC1), DNA methyltransferase 1 (DNMT1), β-catenin, and the small mother against decapentaplegic (SMAD) proteins SMAD2 and SMAD4, all stabilized by the action of USP7 [124]. The EZH2-USP7 complex forms cytoplasmic chromatin fragments (CCF), which activate the cyclic GMP-AMP synthase-stimulator of interferon genes (cGAS-STING) pathway. This stimulation cascade supports interferon production and further contributes to metastasis formation [125].
As recently demonstrated, USP7 specifically targets EZH2 in prostate and ovarian cancers, thus affecting EZH2-mediated angiogenesis [82]. Therefore, further investigations are necessary to confirm USP7’s involvement in the angiogenetic progression of ovarian and prostate cancer cells and its potential as a therapeutic target [62]. On the contrary, the influence of USP7 on CRC through the activation of the Wnt/β-catenin pathway is well defined [126]. However, despite the large number of Wnt inhibitors identified, only a few drugs have been clinically approved for CRCs; this discrepancy can be ascribed to the inconsistency of the studies on USP7’s effects on the Wnt/β-catenin signaling pathway [127].
3.3. NOTCH1 and LSD1 Regulation
The Notch signaling pathway (NOTCH) and the Lysine-specific histone demethylase 1 (LSD1) are two critical ETM and metastasis mediators regulated by USP7 [77,128]. More specifically, USP7 and the histone lysine demethylase JMJD3 stabilize NOTCH1, causing a decrease in H3K27 trimethylation marks, a key point for leukemia initiation and progression. In this context, USP7, NOTCH1, and JMJD3 act in a positive feedback loop where the expression of USP7 is induced by NOTCH1/JMJD3 and turns into the stabilization of oncogenic complexes and target activation [128]. In recent years, several studies have demonstrated that USP7 inhibition can ameliorate the conditions of T cell acute lymphoblastic leukemia patients without toxic effects [129]. Furthermore, LSD1 possesses the ability to mediate gene repression and/or activation by selectively removing the methyl group from mono- or dimethylated histone H3 at lysine 4 (H3K4me1/2) and lysine 9 (H3K9me1/2). In vitro and in vivo studies suggest that USP7 deubiquitination leads to LSD1 overexpression in numerous cancer types (including breast cancer), consequently promoting cancer metastasis [77]. In fact, USP7 deubiquitination is favored by Coactivator Associated Arginine Methyltransferase 1 (CARM1), which stabilizes LSD1. This process causes E-cadherin suppression and the activation of vimentin transcription, which together prompt the invasion and metastasis of glioblastoma and breast cancer cells [130].
4. USP7 Influence on Immune Suppression
USP7 acts as an enhancer of the immunosuppressive functions of Treg cells and, at the same time, as a suppressor of Teff cells, promoting the tumor immune evasion response (Figure 6) [63]. Additionally, USP7 can also interact with specific immune checkpoint inhibitors and interfere with tumor proliferation due to its high expression in tumor-associated macrophages M2 [64,131]. Different pharmacological strategies have been explored to overcome resistance in several types of cancer, especially focusing on Treg inhibition. However, none of them proved to be Treg-specific, and in most of the analyzed cases the risk of inducing toxic inflammation and autoimmunity proved to be too high [132,133,134].
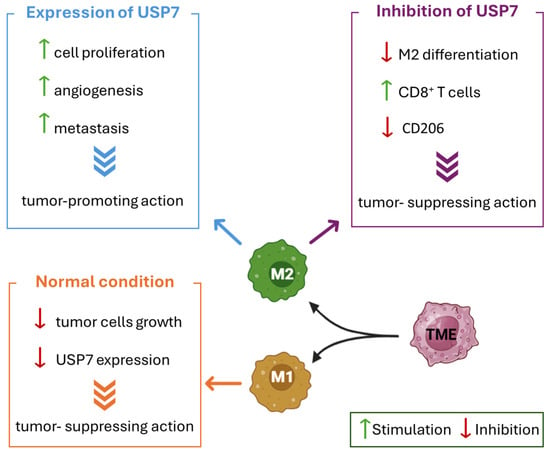
Figure 6.
The antitumor immunity suppression in TME mediated by USP7. The effects of USP7 inhibition and activation on M2 macrophages are reported in different colored boxes. The lack of USP7 expression in tumor-suppressing M1 macrophages is summarized in the orange box. Green arrows indicate stimulation. Red arrows indicate inhibition.
4.1. Treg Cells and FOXP3 Activation
Treg cells are the main regulators of immune system responses, being able to control the immunological tolerance to antigens and to maintain peripheral immune homeostasis. The high presence of Treg cells in the tumor microenvironment has been correlated with low survival rates and poor prognosis in several malignancies, including ovarian, breast, gastric, lung, and pancreatic cancers [135]. The FOXP3 transcription factor is the principal regulator of Treg cells, and its mutation causes immune dysregulation in humans [136]. The regulatory mechanism of FOXP3 and its influence on Treg functions have been extensively investigated to establish the role of this transcription factor in tumors’ escape from the immune response [63]. USP7 is critically involved in the regulation of FOXP3 expression [20], as it stabilizes the histone acetyltransferase Tip60 and consequently avoids FOXP3 degradation (Figure 6). Indeed, in Treg cells, Tip60 mediates FOXP3 transcriptional activity through its acetylation and dimerization. In the absence of Tip60, FOXP3 is ubiquitinated and subsequently degraded by the proteasome [137]. By removing polyubiquitin chains from both FOXP3 and Tip60, USP7 enhances FOXP3 activity and protects it from proteasomal degradation [138]. Recent studies suggested that, in FOXP3-expressing Treg cells, the transcriptional activity of the protein is also indirectly regulated by the USP7-p53 pathway [139]. Additionally, Khosravi and collaborators demonstrated that bone marrow-derived mesenchymal stem cells increase USP7 mRNA levels and induce stable FOXP3-expressing Treg cells, which in turn were protected from degradation by the overexpressed USP7 in a positive feedback loop [140]. Therefore, USP7 overexpression enhances the Treg cell-mediated immune suppression and finally leads to tumor growth and proliferation. On the contrary, USP7 inhibition activates antitumor immunity and prompts the effectiveness of conventional immunotherapy strategies [63].
4.2. The USP7 Impact on Immune Checkpoint Inhibitors
Immune checkpoint inhibitors are frequently used to improve the antitumor immune response. For example, anti-targeting programmed cell death 1 (PD-1) and programmed cell death ligand 1 (PD-L1) monoclonal antibodies enhance the stimulation of the immune system against cancer cells. Indeed, activated T cells expressed PD-1, which negatively modulates T cell-mediated immune responses [141]. On the contrary, these therapies usually present various issues, such as adverse side effects and resistance development. For these reasons, combination therapies, including the use of USP7 inhibitors, have been widely evaluated in recent years. In fact, the release of anti-inflammatory cytokine IL-10 is one of the common resistance mechanisms underlying anti-PD1 therapy in CRC, and it seems to be suppressed by the USP7 blockage. USP7 inhibition decreases PD-1 and PD-L1 interaction and increases the tumor cells’ sensitivity to the action of T cells, which in turn blocks the tumor growth [142]. Moreover, in gastric cancer, USP7-mediated deubiquitination is responsible for the interaction and stabilization of PD-L1, and the expression of the two proteins seems to be positively correlated. USP7 inhibition sensitized gastric cancer cells to T cell-mediated killing by increasing PD-L1 polyubiquitination and decreasing PD-1/PD-L1 interaction. Additionally, USP7 abrogation leads to tumor suppression through p53 stabilization [142]. In gastric cancer, USP7 is involved in ferroptosis, a programmed cell death mechanism induced by iron-depended lipid-reactive oxygen species. In this context, USP7 stabilizes Heterogeneous Nuclear Ribonucleoprotein A1 (hnRNPA1), which activates the secretion of miT-522 and consequently blocks ferroptosis [143,144]. Overall, these studies highlight the importance of USP7 inhibitors in combination therapies as potential novel therapeutic agents effective against various types of cancer.
4.3. USP7 Expression in Tumor-Associated Macrophages and ANXA1
Tumor-associated macrophages are part of the wide variety of cells with a high relevance in inflammation, immunity, and tumorigenesis [131]. These cells can differentiate into M1 and M2 macrophages depending on the different incoming inputs. In detail, M1 macrophages can suppress tumor cell growth, and for this reason they are more present in the early stages of cancer. On the contrary, M2 macrophages are involved in cell proliferation, angiogenesis, and metastasis, and they are abundant when the tumor environment is already established [145]. USP7 is highly expressed in tumor-promoting M2 macrophages, while it is absent in tumor-suppressing M1 macrophages (Figure 6). In recent studies, the effect of USP7 inhibition on M2 expression has been investigated. The arrest of USP7 causes a suppression of M2 macrophage differentiation and activates CD8+ T cells, with a consequent inhibition of Lewis lung carcinoma growth in vivo. Moreover, the block of USP7 reduces the levels of M2-associated marker CD206 and generally suppresses M2 macrophages, without notably cytotoxic effects [131].
Annexin A1 (ANXA1) is another protein-coding gene involved in the suppression of the innate immune system actors [146]; it plays a dual role in adaptive immunity, being able either to activate CD4+ Th cells (and consequently to block the tumor proliferation) or to inhibit their activity and help the cancer cells escape immune suppression [147]. It has been demonstrated that ANXA1 is stabilized by USP7 in HeLa cells; however, the impact of the USP7-ANXA1 axis on antitumor immunity is still unclear and needs further investigations.
To conclude, in recent years, a lot of effort has been spent to clarify the mechanisms through which USP7 modulates the immune system response against tumors. Nevertheless, more research is needed to provide novel cancer treatments which enhance the body’s natural defense [148].
5. USP7 Regulation of Genome Stability and Expression
Exposure to endogenous or exogenous agents such as DNA replication stresses, ultraviolet (UV) radiation, reactive oxygen species (ROS), ionizing radiation, and crosslinking agents could result in DNA damage, which represents a common feature in various cancer types. Usually, DNA damage consists of DNA adducts, DNA mismatch, or DNA strand breaks, which are commonly repaired by five major mechanisms: (i) base excision repair (BER); (ii) nucleotide excision repair (NER); (iii) mismatch repair (MMR); (iv) homologous recombination (HR); and (v) non-homologous end joining (NHEJ) [149].
In this context, USP7 preserves the integrity of genomic information by deubiquitinating proteins involved in the five major repair processes and consequently regulating the DNA response and repair system [41]. Moreover, USP7 controls gene expression through epigenetic effects such as DNA methylation or histone modifications that lead to changes in DNA expression patterns [150].
5.1. BER and NER
USP7 deubiquitinates and stabilizes specific proteins in the BER and NER excision repair systems, including Mule/ARF-BP1, UVSSA, CSB, and XPC [151]. More specifically, in response to DNA damage, p53 downregulates the Ser18-containing isoform of USP7 (USP7S), decreasing Mule/ARF-BP1 and p53 accumulation to allow for successful BER [51]. On the other hand, USP7 upregulation is necessary to guarantee the two NER mechanisms: transcription-coupled NER (TC-NER) and global genome NER (GG-NER).
Additionally, USP7 plays a pivotal role in UVSSA stabilization and consequently preserves RNA synthesis through successful TC-NER [152]. TC-NER initiation is carried out by the cocaine syndrome proteins CSA and CSB encoded by the cocaine syndrome gene. It has been demonstrated that USP7 inhibition blocks the recovery of CSB under DNA damage induced by UV radiation and, at the same time, reduces RNA synthesis. Conversely, GG-NER started thanks to the XPC-hRad23B/A complex, which can recognize DNA damage. The knockdown of USP7 promotes XPC complex degradation and leads to GG-NER impairment [153].
5.2. HR and NHEJ
DNA Double Strand Break (DSB) is the most cytotoxic type of DNA damage since, if not repaired, it may cause chromosomal aberrations [154]. HR and NHEJ pathways can recruit DNA damage mediator proteins, such as the p53-binding protein 1 (53BP1), receptor-associated protein 80 (RAP80), BRCA1, RAD51, and γH2AX, to the damaged chromatin, thus representing the main DNA repair mechanisms in response to DSBs [155,156]. Moreover, DNA damage response is regulated by several proteins that play a pivotal role in the repair processes by promoting the accumulation of BRCA1 and 53BP1, the major repair factors in the HR and NHEJ pathways [157]. In this context, USP7 acts as a crucial mediator of the HR and NHEJ repair mechanisms by directly regulating the stability of DNA repair proteins or by indirectly affecting their activities through deubiquitination [158]. In detail, USP7 down-expression leads to a decreased expression of RNF168, which ultimately arrests the accumulation of BRCA1 and 53BP1 [159].
Additionally, USP7 influences the activity of RNF169 E3-ligase and displaces the binding of 53BP1 and RAP80 at the DNA damage site. The entrance of the cells into senescence and apoptosis is bypassed when RNF169 accumulation occurred due to the modification of RAD-51-dependent homologous recombination [160].
Two other crucial players able to initiate and amplify the DNA damage response are the MRE11-RAD50-NBS1 (MRN) complex and the mediator of DNA damage checkpoint protein 1 (MDC1). In particular, MDC1 contributes to the accumulation at DNA lesions of 53BP1 and BRCA1 and induces the ubiquitination of γH2AX and H2A by recruiting RNF8 and RFN168 [161]. The interaction between the MRN complex and USP7 deubiquitinates and stabilizes MDC1. On the contrary, USP7 inhibition leads to the suppression of both HR and NHEJ repair pathways caused by the reduction in the MDC1-mediated accumulation of BRCA1 [162]. Increased USP7 levels seem to enhance the survival rate of cervical cancer cells through MDC1 stabilization and consequently improve resistance to DNA damage [163].
5.3. Cell Cycle Arrest
Cell cycle arrest and apoptosis represent other more drastic DNA damage response strategies. As previously discussed, USP7 plays a crucial role in cell cycle regulation, particularly through its interaction with p53 and MDM2. However, USP7 can also interact with several other substrates involved in this process. Despite its role appearing controversial in relation to its oncogenic activities, the enzyme can regulate cell cycle checkpoint proteins by altering their stability and therefore promoting cell cycle arrest [164].
The Ataxia Telangiectasia and Rad3-related protein Checkpoint kinase-1 (ATR-Chk1) pathway is one of the signal cascades involved in cell cycle arrest. More specifically, the phosphorylation of Chk1 (at serine residues 317 and 345) causes its dissociation from chromatin and the subsequent phosphorylation of other targets, such as Cell Division Cycle 25A (CDC25A). The process concludes with cell cycle arrest at the S and G2 phases [165]. USP7 stabilizes Chk1, claspin, and CDC25A, which distinctly affect CDK-dependent cell cycle progression. Indeed, the stabilization of CDC25A, and consequently its accumulation, may be exploited by cancer cells to continue cell cycle progression even in the presence of DNA damage. Additionally, by stabilizing CDC25A, USP7 ensures a constant protein level, which provides the cell the ability to resume its cell cycle after DNA repair [69,166].
Among the USP7 substrates involved in the cell cycle processes, Coiled-coil domain-containing protein 6 (CCDC6) proved to carry out an important role in non-small cell lung cancer (NSCLC). In particular, the overexpression of CCDC6 increases during the G2 phase and decreases during mitosis. Since USP7 is able to enhance CCDC6 stability, silencing the enzyme leads to the turnover of CCDC6 and cell cycle arrest [167]. In NSCLC tumors, USP7 can also deubiquitinate the TRAF-like domain of serine/threonine kinase Raf-1. By reducing Raf-1 activity, USP7 blocks the ERK signaling pathway, thereby regulating the tumor proliferation of NSCLC [84].
Moreover, USP7 appears to play a key role in lung cancers resistant to Paclitaxel through the regulation of two proteins involved in cell mitosis: Serine/threonine-protein kinase or polo-like kinase 1 (PLK1) and BUB3 Mitotic Checkpoint Protein (BUB3). In fact, recent studies have shown that a combined treatment with a PLK1 inhibitor (Volasertib) and a USP7 inhibitor (P22077, reported below) induces the death of Paclitaxel-resistant cancer cells [168].
6. USP7 Inhibitors
As reported above, several recent studies highlighted the attractiveness of USP7 as a valuable and innovative target for anticancer therapy and prompted extensive research efforts towards the development of potent and selective USP7 inhibitors. Up to now, nearly 160 small molecules able to pharmacologically inhibit USP7 have been discovered [54,169].
However, despite several USP7 inhibitors having been reported in the scientific literature, the search for potent and selective compounds is still challenging and, to date, no molecules have been approved as commercial drugs. Indeed, many molecules are not selective for USP7 and may also react with other DUB enzymes, making the substrate-specific identification difficult. In addition, most of the molecules exhibit a low inhibition potency, with only a few inhibitors presenting an IC50 value in the nanomolar range [15,170,171,172]. Another limiting factor is the difficulty in carrying out cellular assays due to unfavorable pharmacokinetic properties and the poor solubility of the molecules. For these reasons, only a small number of USP7 inhibitors have been tested in vivo [15].
Based on their interaction with the enzyme, three main categories of USP7 inhibitors can be distinguished, namely covalent inhibitors, noncovalent allosteric inhibitors, and covalent allosteric inhibitors [169]. According to the crystallographic data available so far, most allosteric inhibitors would interact with the enzyme in a region adjacent to the catalytic triad between the thumb and the palm (Site A, Figure 7). Additionally, two binding zones have been detected in the thumb subdomain above Site A and the catalytic triad; more specifically, Site B (Figure 7) hosts a couple of allosteric inhibitors, while Site C (Figure 7) is involved in the binding of allosteric activators (vide infra). Interestingly, in addition to the previous categories, a group of natural inhibitors extracted from sea sponges have been discovered [173,174,175].
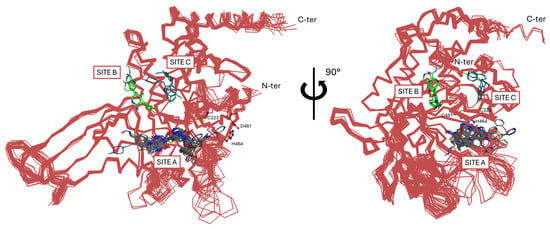
Figure 7.
Superimposition of the reported crystal structures of USP7 bind to allosteric inhibitors (Site A and Site B) and allosteric activators (Site C).
6.1. Covalent Inhibitors
Covalent inhibitors interact with the USP7 catalytic domain through the formation of a covalent bond with the catalytic Cys223 residue. This type of binding prevents the conformational transition of the enzyme, blocking its deubiquitinase activity irreversibly [169]. The amido-tetrahydro acridine derivatives HBX19818 and HBX28258 (Figure 8) act as USP7 covalent inhibitors, resulting in HBX19818 being effective against both CRC and chronic lymphoid leukemia (CLL), whereas HBX28258 is active only against CRC [15].
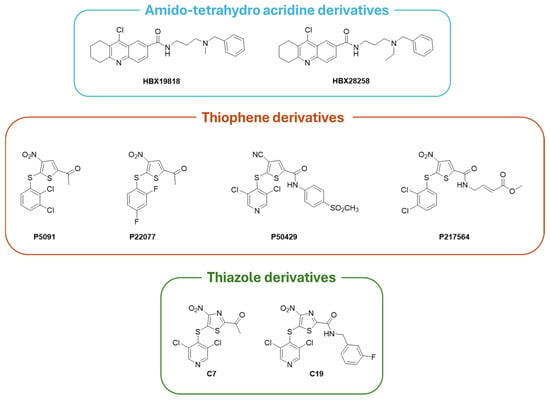
Figure 8.
Chemical structures of selected USP7 covalent inhibitors.
Tri-substituted thiophenes P5091, P22077, P50429, and P217564 (Figure 8) irreversibly block USP7 activity [58,171,176]. In detail, P5091 showed antitumor activity in vivo against multiple myeloma (MM.1S xenograft model), CRC, glioblastoma, and esophageal squamous cell carcinoma thanks to the activation of the p38 MAPK signaling pathway [74,177]. Conversely, P22077 and P50429 inhibitors act by modifying Cys223 and altering the orientation of the surrounding loops, thus preventing the alignment of the substrate towards the catalytic site; moreover, P22077 and P50429 are active on NB and CRC, respectively [178,179]. P217564 is a potent second-generation USP7 covalent inhibitor with enhanced suppressive action on Treg cells and, consequently, anticancer activity [180]. Based on the already known thiophene scaffold inhibitors, additional molecules active on USP7 have been developed as thiazole derivatives (i.e., derivatives C7 and C19, with IC50 of 0.67 and 1.35 µM, respectively, Figure 8). These USP7 thiazole inhibitors turned out to be more potent than their thiophene congeners, and both compounds showed a significant activity against CRC [178].
6.2. Noncovalent Allosteric Inhibitors
Reversible allosteric noncovalent inhibitors bind regions adjacent to the catalytic triad and prevent the proper alignment of the three catalytic amino acids, blocking ubiquitin binding [169]. Among the different noncovalent allosteric inhibitors, pyrimidine-based compounds are the most studied. In particular, derivatives L55 and FT671 (Figure 9) are characterized by a pyrazole–pyrimidine structure, FT671 being a structural optimization of L55 [180,181]. The USP7-specific FT671 inhibitor (IC50 = 52 nM) is active against CRC and breast cancer, blocking the proliferation of MM.1S cells with an IC50 value of 0.33 µM, as determined by the CellTiter-Glo assay. The crystal structure of the USP7 catalytic domain complexed with FT671 (PDB code: 5NGE) showed the ability of the inhibitor to alter Phe409 orientation without affecting the positions of Tyr465 and Tyr514 [181].
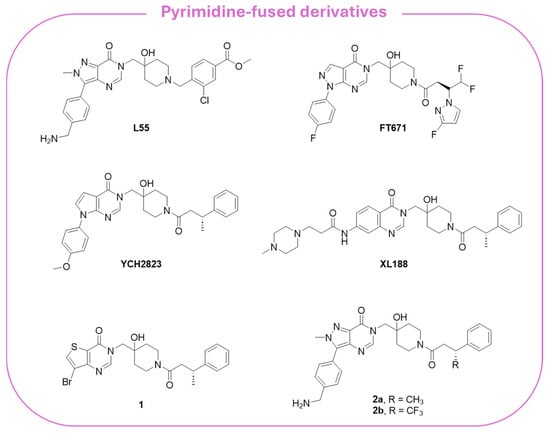
Figure 9.
Chemical structure of the pyrimidine-fused USP7 noncovalent allosteric inhibitors.
Taking FT671 as a lead compound, Cheng and collaborators synthesized a series of novel pyrrolo-pyrimidine derivatives and identified YCH2823 (Figure 9) as a potent USP7 inhibitor (IC50 = 0.50 µM) with a remarkable anti-proliferative impact on various tumor cells [182]. At the cellular level, YCH2823 modulates the turnover of multiple substrates, leading to p53 stabilization, p21 induction, and a decreased expression of Rad18 and DNMT1. These interactions collectively result in cell cycle arrest and apoptosis. Importantly, YCH2823 displays strong antitumor activity across a broad spectrum of cancer cell lines, including those harboring both wild-type and mutated TP53. Moreover, mTOR inhibitors enhanced YCH2823 efficacy, highlighting a promising therapeutic strategy to extend the clinical utility of USP7 inhibitors, particularly in MYCN-amplified cancers [182].
The quinazolin-4-one analog XL188 (Figure 9) is structurally related to the pyrazole–pyrimidine FT671, and the two inhibitors share amide groups on the piperidinol scaffold. More specifically, XL-188 emerged from a structure-guided optimization campaign that converged on a rigid quinazoline/heteroaromatic core [183]. A conserved multi-heteroatom motif on the core forms key hydrogen bonds with backbone and side-chain polar residues in the pocket; this hydrogen-bond network proved to be critical for potency. Additionally, a rigid core scaffold allowed the correct orientation of interacting groups, showing that flexible analogs reduced USP7 inhibition.
Peripheral substituents projecting into adjacent solvent channels were tuned to improve cellular potency and ADME properties without perturbing core contacts. As a result, modest increases in the polar surface area or polar solubilizing groups enhanced cell activity, whereas an excessive lipophilicity degraded free-drug exposure [183]. As emerged from the X-ray structure of XL188 complexed with the USP7 catalytic domain (PDB: 5VS6; Figure 10A), the inhibitor occupies the S4 and S5 subsites, about 5.5 Å away from the catalytic triad. The compound assumes an extended conformation, being the hydroxypiperidinyl-quinazolinone portion involved in multiple hydrogen bonds with Asp295 (side-chain carboxylate), Val296 (backbone NH), Gln297 (side-chain amide), Phe409 (backbone NH), and Tyr465 (OH side chain) (Figure 10B). The phenyl ring of XL188 is buried in the S4 pocket and is in contact with the aromatic rings of Tyr514, His456, and Phe409, and the aliphatic chains of Lys420 and Arg408. Notably, the side chain of Phe409 flips to reveal the hydrophobic pocket, a conformational rearrangement also observed upon binding of ubiquitin [43]. Interestingly, the XL188 inhibitor partially protrudes into the channel normally occupied by the ubiquitin C-terminal tail and protects the α-4/5 regions surrounding the S4-S5 pocket. This peculiar conformation made XL188 unable to bind other USP isoforms, therefore resulting in it being highly selective against USP7, with an inhibitory activity in the nanomolar range (IC50 = 90 nM) [184]. Furthermore, the proximity of XL-188 to the catalytic cysteine suggested a tractable vector for the design of covalent inhibitors. Thus, the decoration of the central core with appropriately positioned electrophile groups can convert a noncovalent lead into an irreversible analog with an improved potency [183,184].
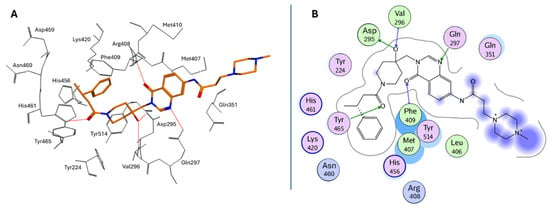
Figure 10.
(A) Bioactive conformation of XL188 in USP7 binding site; (B) Ligplot for the USP7/XL188 X-ray complex.
Through fragment-based methods, Gavory and coworkers identified thieno-pyrimidine 1 and pyrazolo-pyrimidines 2a,b (Figure 9) as selective USP7 allosteric inhibitors. The derivatives exhibited IC50 values in the sub-micromolar range and proved to significantly inhibit cell growth in p53-mutant cancer cells, being more active than several PROTAC congeners derived thereof [185].
GNE-6640 and GNE-6776 compounds (Figure 11A) represent an alternative class of USP7 allosteric inhibitors characterized by a highly substituted pyridine scaffold. The GNE series emerged from an NMR-fragment campaign and iterative structure-guided optimization that converged on a small, rigid heteroaromatic core that docks into an acidic pocket of the USP7 catalytic domain. Chemical elaboration around this core showed that a scaffold presenting an H-bond donor/acceptor triad was necessary for the potent attenuation of ubiquitin binding, whereas flexible linkers or over-polarization reduced biochemical potency and cell activity [171]. Peripheral substituents that projected into nearby solvent channels improved the cellular potency and ADME properties (solubility, plasma exposure) without perturbing the core pocket interactions; conversely, an excessive lipophilicity increased protein binding and worsened free-drug exposure in vivo. GNE-6776 embodies PK-balanced modifications that afforded superior cellular efficacy, oral bioavailability, and xenograft activity relative to earlier analogs (including GNE-6640), indicating that modest changes to terminal substituents and the polar surface area were fundamental for in vivo translation. These observations support a lead-optimization strategy that preserves the core H-bonding motif for the acidic pocket, rigidifies the scaffold to minimize entropy loss, and fine-tunes peripheral substituents to balance potency with favorable free-drug exposure for in vivo efficacy [171]. As emerged from crystallographic studies (PDB codes: 5UQV and 5UQX), these molecules bind USP7 in a pocket located at the interface of the palm, finger, and thumb subdomains, approximately 12 Å away from the catalytic triad (Site B; Figure 7). This binding site is not conserved in other classes of DUBs, and it was exploited to improve the selectivity against the USP7 isoform, where it seemed to play a role in the ubiquitin binding [183]. In both crystallographic complexes, the inhibitors assume an extended conformation (Figure 11B), with the phenol OH group hydrogen bonded to the His403 side chain (Figure 11C,D). The substituents at the 5-position of the aminopyridine scaffold (i.e., indazole for GNE-6640 and pyrimidine carboxamide for GNE-6776) point toward the solvent, protruding between α5 and α6 helices. Furthermore, the USP7-GNE-6640 complex is further stabilized by van der Waals interactions between the indazole and the amino acid residues on α5 and α6, while the NH atom of the GNE-6776 carboxamide-moiety is in contact with Asp305. Finally, the co-crystal structures of both molecules revealed their ability to sterically inhibit ubiquitin binding and prevent the transition of the USP7 catalytic domain α5 helix to the active conformation [171]. GNE-6640 shows an IC50 value of 0.75 µM on USP7, causing apoptosis and being more active than GNE-6776 (IC50 = 1.35 µM), which triggers cell cycle arrest [171].
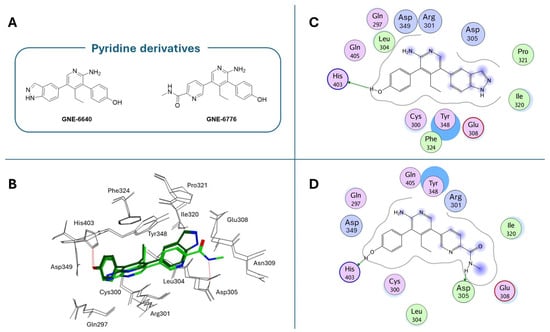
Figure 11.
(A) Chemical structures of pyridine-based USP7 noncovalent allosteric inhibitors; (B) binding poses of GNE-6640 (dark green) and GNE-6776 (light green) in USP7 binding site; (C) Ligplot for the USP7/GNE-6640 complex; (D) Ligplot for the USP7/GNE-6776 complex.
Thienopyridine USP7-797 (Figure 12) has been identified as a USP7 allosteric inhibitor endowed with an optimal balance between cellular potency and pharmacokinetic properties in vitro [186]. The inhibitor was able to reduce the viability of multiple TP53 wild-type cell lines, including several hematologic cancers, and MYCN amplified NB cell lines, as well as a subset of TP53 mutant cell lines [186].
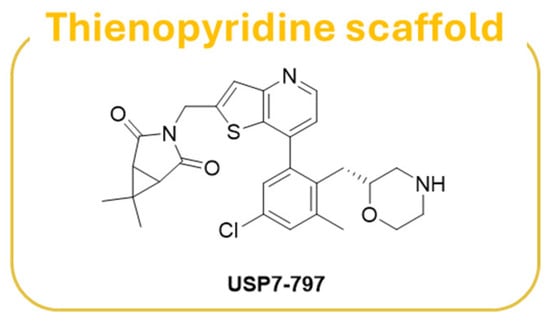
Figure 12.
Chemical structure of thienopyrimidne derivative USP7-797.
6.3. Covalent Allosteric Inhibitors
Covalent allosteric inhibitors bear reactive functionalities able to form covalent interactions with the target at a different site from the catalytic triad. In comparison with the other class of USP7 inhibitors, these compounds are poorly attractive due to the lack of selectivity, leading to off-target effects. For instance, the cyano-indenopyrazine HBX41108 inhibitor (Figure 13) can bind the enzyme–substrate complex in a pocket near the ubiquitin binding site [187]. However, HBX41108 seems to lack selectivity (e.g., IC50 (USP7) = 0.42 µM; IC50 (USP8) = 0.96 µM), as its 2,3-dicyano-pyrazine substructure is highly reactive and can covalently modify all DUBs at a 5 µM concentration [138,187,188,189].
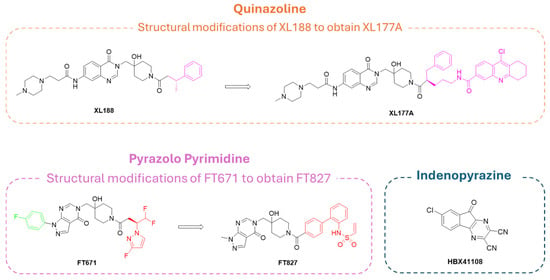
Figure 13.
Chemical structures of reported USP7 covalent allosteric inhibitors.
Differently, quinoline derivative XL177A (Figure 13), synthesized from its congener XL188, was more effective on USP7, being able to form both noncovalent allosteric and covalent orthosteric interactions. In fact, XL177A can covalently bind Cys223 residue, enhancing the exchange in the α2 to α4 region of USP7 [34].
Finally, pyrazolo-pyrimidine FT827 (Figure 13), prepared from the FT671 precursor, can play a dual role, acting as both a noncovalent and covalent allosteric inhibitor. However, the lack of specificity for the different USP isoforms reduces the pharmaceutical attractiveness of this compound [181]. In fact, despite the presence of the pyrazolo-pyrimidine core, designed to specifically target USP7 S4 and S5 sub-pockets, the pyrazole nitrogen substituents seemed to be highly interactive with different amino acids in the catalytic pocket of all USP isoforms, leading to a lack of selectivity [33].
6.4. USP7 PROTACs
Targeted protein degradation (TPD) is an emerging therapeutic approach with the potential to tackle disease-causing proteins that are proven to be highly challenging to target with conventional small molecules. Among TPD strategies, proteolysis-targeting chimera (PROTAC) degraders represent the most consolidated approach [190]. PROTAC heterobifunctional small molecules consist of two ligands joined by a linker; one ligand binds to a protein of interest, while the other warhead recruits an E3 ubiquitin ligase. Simultaneous binding of the protein of interest and ligase by the PROTAC induces the ubiquitylation and subsequent degradation of the protein through the UPS; after the proteolysis of the protein of interest, PROTAC is released and can mediate the degradation of another complex [191,192].
Based on XL188 noncovalent allosteric inhibitor, PROTAC 17 (Figure 14) has been developed. This compound is one of the most promising PROTACs for USP7 (IC50 = 1.6 ± 0.3 µM), consistently showing an activity in USP7-dependent cancer cells [193]. Similarly, U7D1 (Figure 14) has been designed from the potent non-competitive USP7 inhibitor 2a (Figure 9) and demonstrated relevant inhibitory activity (IC50 = 6 nM) against cancer cells bearing p53 mutations non-sensitive to other USP7 inhibitors [194]. Both PROTAC 17 and U7D1 were designed by linking well-known selective USP7 inhibitors to a Cereblon (CRBN) E3 ligase ligand through different decorated linkers. As demonstrated by proteomic studies, the nature of the CRBN ligand and the length of the linker directly influence USP7 degradation. Significantly, the PROTAC derivatives were more effective against cancer cells in comparison with the treatment of the sole USP7 inhibitor [193,194].
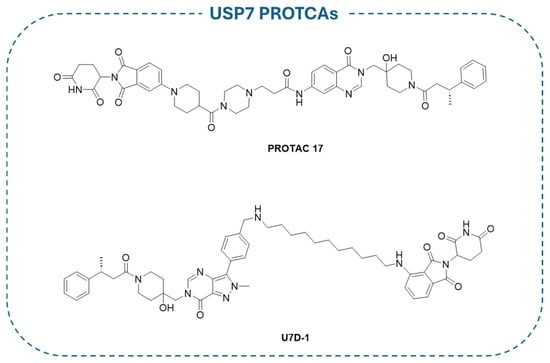
Figure 14.
Chemical structure of reported USP7 PROTACs.
6.5. Natural Inhibitors
Despite the majority of USP7-targeting derivatives being synthetic compounds, there are a few examples of natural inhibitors. In detail, pyrrole alkaloids isolated from marine sponges (i.e., Spongiacidin A, Spongiacidin C, Xestoquinone, Sulawesin A; Figure 15) showed USP7 inhibitory activity. Thus, Spongiacidin A was the first natural molecule extracted from a natural source with micromolar USP7 inhibitor activity (IC50 = 8.5 µM) [195]; then, Spongiacidin C was isolated from the marine sponge Stylissa massa and showed an improved potency against the enzyme (IC50 = 3.8 µM) [173]. The pentacyclic quinone Xestoquinone, isolated from the marine sponge Petrosia alfiani, showed multiple biological activities, including a significant inhibitory activity on USP7 (IC50 = 0.13 µM) [174]. Finally, Sulawesin A is a mixture of four diastereomers (namely, 5R,9R,18R, 5R,9R,18S, 5S,9S,18R, and 5S,9S,18S) isolated from a marine sponge, Psammocinia sp. The four isomers are furano-sesterterpene tetronic acids and are USP7 inhibitors with an IC50 value of 2.8 µM [175,196].
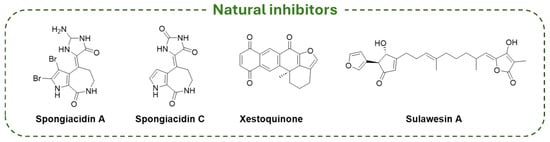
Figure 15.
Chemical structure of the USP7 natural inhibitors.
7. USP7 Activators
As previously discussed, USP7 can play several roles in cancer progression and metastasis, acting either as an oncogenic protein or tumor suppressor based on the targets involved and the cancer type. Although a wide variety of USP7 inhibitors have been developed over the last decade, USP7 activators may also represent useful molecules for anticancer therapy [44,52]. It has been demonstrated that the USP7 C-terminal portion can interact with the activation cleft in the catalytic domain, stabilizing the active conformation of the enzyme and thereby enhancing the enzyme’s deubiquitinase activity [43].
In this context, Shi and colleagues carried out a virtual screening on a panel of FDA-approved drugs and identified the antidepressant Sertraline (Figure 16) and the antihistamine drug Astemizole (Figure 16) as selective USP7 agonists. Further biochemical and biophysical experiments confirmed that the two derivatives could bind the switching loop region of the USP7 C-terminal domain, displaying an agonistic potency in the low micromolar range. Additionally, both Sertraline and Astemizole proved to enhance the deubiquitinase activity of selected USP7 pathogenic mutants, and the antihistamine compound was also able to upregulate the enzyme in mutants associated with Hao–Fountain Syndrome (HAFOUS) in a cell-based assay. Despite the side effects associated with the intrinsic action of these drugs, the obtained results highlighted the importance of the development of novel USP7 agonists for the treatment of diseases associated with the loss of function of the enzyme [197].
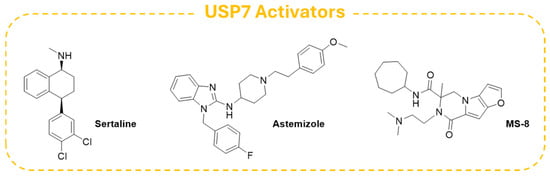
Figure 16.
Chemical structures of USP7 allosteric activators.
The crystal structure of the USP7/Sertraline complex revealed that the compound binds in a cleft composed by the switching loop (Phe283–Asp295) and α-helix α5 (Val296—Met311) (site C, Figure 7). Sertraline assumes a protruded bioactive conformation (Figure 17A, PDB: 9IJU) and interacts with hydrophobic residues such as Phe283, Trp285, His294, Leu299, and Val302 through van der Waals contacts, while the methylamino substituent is solvent exposed (Figure 17B). Additionally, the two aromatic structures of the derivative would occupy the S1 and S2 sub-pocket in the activation cleft, consequently stabilizing the active conformation of the enzyme and enhancing its deubiquitinase activity [197].
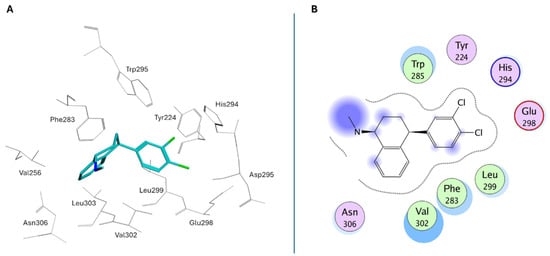
Figure 17.
(A) Binding pose of Sertraline in USP7 activation cleft; (B) Ligplot for the USP7/Sertraline crystallographic complex.
With the aim of discovering novel small molecules for the treatment of HAFOUS, Maisonet and coworkers carried out a large-scale screening and identified the compound MS-8 (Figure 16) as a potential USP7 activator. The molecule can mimic the USP7 mechanism of allosteric autoactivation by targeting the enzyme in the allosteric C-terminal binding pocket. Furthermore, MS-8 induced an increase in intracellular levels of the USP7 downstream substrates (e.g., TRIM27 and p21) in wildtype HCT116 cells without exhibiting cytotoxic effects. This evidence supported the use of small molecule activators for the treatment of HAFOUS and other related diseases. Interestingly, MS-8 is currently under study for the development of new DUB Targeting Chimeras (DUBTACs), heterobifunctional derivatives able to stabilize the levels of the protein of interest, preventing its proteasomal degradation [198].
The discovery of MS-8, together with the interesting properties shown by the clinically used molecules Sertraline and Astemizole, laid the groundwork for the development of novel anticancer therapies that will substitute the predominant inhibitory approach [199]. Additionally, cheminformatic and computational studies have discovered innovative druggable allosteric pockets that influence the dynamic switching loops and consequently increase the USP7 activity [200]. Overall, this preliminary evidence establishes a proof of concept for USP7 potentiation benefits, but medicinal chemistry optimization as well as more in vitro validation are still needed before pre-clinical application [198].
8. Conclusions
DUBs are fundamental regulators of the ubiquitin–proteasome system, playing a key role in maintaining protein homeostasis. Among the various families of DUBs, USPs represent the largest and the most studied group, with USP7 (also known as HAUSP) emerging as a crucial player in multiple cellular processes. Indeed, USP7 modulates a wide array of substrates by removing ubiquitin moieties, thereby influencing their stability, activity, and subcellular localization. Its regulatory functions span critical biological pathways, including the DNA damage response, immune signaling, epigenetic modifications, cell cycle progression, and apoptosis.
Interestingly, USP7 exerts its action by stabilizing both oncogenic and tumor suppressor proteins, such as MDM2, p53, PTEN, FOXP3, β-catenin, and NF-κB (Figure 18). This dual activity reflects its complex role in cancer biology, where USP7 can either promote or suppress tumor development depending on the cellular context. Aberrant expressions or activity of USP7 have been implicated in a broad range of pathological conditions, including cancer, neurodegeneration, viral infections, and autoimmune disorders, consequently positioning it as an attractive pharmacological target.
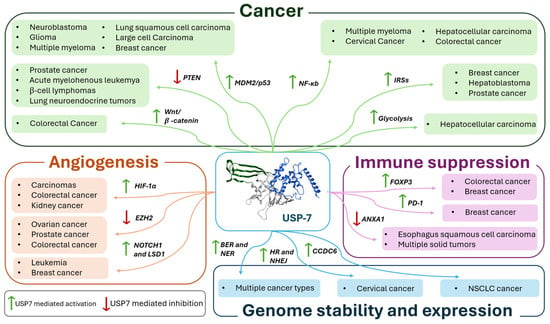
Figure 18.
Schematic representation of the pathways and related pathologies in which USP7 is involved.
As summarized in Table 1, numerous small-molecule USP7 inhibitors have been reported in recent years, targeting both catalytic and allosteric sites inside the catalytic region of the enzyme. Additionally, few allosteric activators have been synthesized, showing interesting biological results. Collectively, the reported compounds have demonstrated encouraging in vitro results, with some of them showing a high selectivity and potency. However, despite the growing interest and promising outcomes of in vitro and in vivo studies, no USP7 inhibitors have reached clinical approval yet. In fact, the dual activity of the enzyme and its ability to act on multiple targets within the same tumor make the pharmacological intervention very difficult, often leading to off-target or unwanted side effects [201].

Table 1.
List of reported USP7 inhibitors and activators.
Additionally, the role of UBL domains in the development of USP7-related diseases is still unclear. These domains are challenging to target with small molecules due to their shallow binding surfaces and dynamic conformational flexibility, which complicate drug design. Understanding UBL-mediated interactions remains crucial for developing effective therapeutics that modulate USP7 activity [109,202].
Finally, despite, on the one hand, the discovery of selective USP7 activators potentially being a game changer for the therapeutic approach to the enzyme, on the other hand, the collected results are still too weak to reach clinical application, and more SARs studies, as well as computational analysis, are needed to better understand the mechanisms of action of this class of compounds [199,200].
For these reasons, future research efforts should continue to focus on improving our understanding of USP7 substrate specificity and context-dependent roles in disease. In fact, a deeper comprehension of USP7 molecular mechanisms will be essential to guide the rational design of more effective and selective inhibitors.
The data reported here were collected from different databases (Scifinder, Web of Science, Scopus, Google Scholar, and Pubmed) using “Deubiquitinases” or “USP7” and “inhibitors” as keywords, and considering publications (i.e., patents, reviews, research articles) published in the 1990–2025 period.
Author Contributions
Conceptualization, M.L., F.R. and C.B.; writing—original draft preparation, M.L. and F.R.; bibliographic research, M.L., F.R., M.M., E.C. and A.S.; writing—review and editing, M.L., F.R., C.B. and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by European Union- Next Generation EU, PNRR M4.C2—Investimento 1.2 “Finanziamento di progetti presentati da giovani ricercatori” D.D. n. 47/2025, Project CUP D33C25000300001.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 53BP1 | p53-binding protein 1 |
| ABCB1 | ATP-binding cassette sub-family B member 1 |
| Akt | Protein kinase B |
| AML | Acute myelogenous leukemia |
| ANXA1 | Annexin A1 |
| APC | Adenomatous polyposis coli |
| ARF | Alternate Reading Frame |
| ATR-Chk1 | Ataxia Telangiectasia and Rad3-related protein Checkpoint kinase-1 |
| BER | Base excision repair |
| BUB3 | BUB3 Mitotic Checkpoint Protein |
| CARM1 | Coactivator Associated Arginine Methyltransferase 1 |
| CBP | CREB-binding protein |
| CCDC6 | Coiled-coil domain-containing protein 6 |
| CCF | Cytoplasmic chromatin fragments |
| CDC25A | Cell Division Cycle 25A |
| cGAS-STING | Cyclic GMP-AMP synthase-stimulator of interferon genes |
| CID | Catenin inhibitory domain |
| CK1α | Casein kinase 1α |
| CLL | Chronic lymphoid leukemia |
| CRBN | Cereblon |
| CRC | Colorectal cancer |
| DAXX | Death domain-associated protein |
| DDX3X | DEAD-box helicase 3 X-linked |
| DNMT1 | DNA methyltransferase-1 |
| DNMT1 | DNA methyltransferase 1 |
| DSB | Double Strand Break |
| DUB | Deubiquitinate |
| DUBTACs | DUB Targeting Chimeras |
| EBNA1 | Epstein–Barr nuclear antigen 1 |
| ELK4 | ETS-like transcription factor 4 |
| EMT | Epithelial–mesenchymal transition |
| ER | Estrogen receptor |
| ERK | Extracellular signal-regulated kinase |
| ETS | Electron transport system |
| EZH2 | Zeste homolog 2 enhancer |
| FOXO1 | Forkhead box protein O1 |
| FoxO4 | Forkhead box O4 |
| FOXO6 | Forkhead box protein O7 |
| FOXP3 | Forkhead box P3 |
| GG-NER | Global genome NER |
| GLUT1 | Glucose transporter 1 |
| Grb2 | Growth factor receptor-bound protein 2 |
| GSK3β | Glycogen synthase kinase 3β |
| H3K4me1/2 | Histone H3 at lysine 4 |
| H3K9me1/2 | Histone H3 at lysine 9 |
| HAFOUS | Hao–Fountain Syndrome |
| HAUSP | Herpesvirus-associated ubiquitin-specific protease |
| HCC | Hepatocellular carcinoma |
| HDAC1 | Histone deacetylase 1 |
| HER2 | Human epidermal growth factor receptor |
| HIF-1α | Hypoxia-inducible factor 1-alpha |
| HIFs | Hypoxia-inducible factors |
| hnRNPA1 | Heterogeneous Nuclear Ribonucleoprotein A1 |
| HNSSC | Head and neck squamous cell carcinoma |
| HR | Homologous recombination |
| ICP0 | Infected cellular polypeptide 0 |
| IFNGR1 | Interferon gamma receptor 1 |
| IGF | Insulin/insulin-like growth factor |
| IL-6 | Interleukin 6 |
| IRF-4 | Viral interferon regulatory factor 4 |
| IRS | Insulin receptor substrate |
| IκBs | NF-κB inhibitors |
| JAMM | JAMM/MPN domain-associated metallopeptidase |
| JMJD3 | Jumonji domain-containing protein-3 |
| K63 | Lysine residue |
| LANA | Latency-associated nuclear antigen |
| LSD1 | Lysine-specific histone demethylase 1 |
| HLSD1 | Histone lysine demethylase 1 |
| Maged1 | Melanoma-associated antigen D1 |
| MCPIP | Monocyte chemotactic protein-induced proteins |
| MDC1 | Mediator of DNA damage checkpoint protein 1 |
| MDM2 | Murine double minute 2 |
| MDMX | Murine double minute X |
| MIG-6 | Mitogen-inducible gene-6 |
| MINDY | MIU-containing novel DUB family |
| MJD | Machado–Joseph disease protein domain proteases |
| MM1.S | Multiple myeloma |
| MMR | Mismatch repair |
| MRN | MRE11-RAD50-NBS1 |
| NB | Neuroblastoma |
| NEK2 | NIMA-related kinase 2 |
| NER | Nucleotide excision repair |
| NF-κB | Nuclear factor-kappa B |
| NHEJ | Non-homologous end joining |
| NOTCH | Notch signaling pathway |
| NPM1 | Nucleophosmin 1 |
| NSCLC | Non-small cell lung cancer |
| OTU | Otubain domain ubiquitin-binding proteins |
| PD-1 | Programmed cell death 1 |
| PD-L1 | Programmed cell death ligand 1 |
| PI3K | Phosphatidylinositol 3-kinase |
| PLK1 | Polo-like kinase 1 |
| PPPDE | Permuted papain fold peptidase of dsDNA viruses and eukaryotes |
| PROTAC | Proteolysis-targeting chimera |
| PTEN | Phosphatase and tensin homolog |
| PVT | Paraventricular thalamus |
| RAP80 | Receptor-associated protein 80 |
| ROS | Reactive oxygen species |
| SIRT7 | Sirtuin 7 |
| SMAD | Small mother against decapentaplegic |
| TC-NER | Transcription coupled NER |
| Teff | Effector T cells |
| TNBC | Triple-negative breast cancer |
| TNF | Tumor necrosis factor |
| TPD | Targeted protein degradation |
| TRAF | Tumor necrosis receptor-associated factor |
| Treg | Regulatory T cells |
| TRIM27 | Tripartite Motif Containing 27 |
| TRIP13 | Thyroid hormone receptor interactor 13 |
| TSP-1 | Thrombospondin-1 |
| TSPYL5 | Testis-specific protein Y-encoded-like 5 |
| UBL1-5 | Ubiquitin-like domains 1-5 |
| UCH | Ubiquitin C-terminal hydrolases |
| UHRF1 | Ubiquitin-like with PHD and ring finger domain 1 |
| UPS | Ubiquitin–proteasome system |
| USP | Ubiquitin-specific proteases |
| USP7 | Ubiquitin-specific proteases 7 |
| USP7S | Ser18-containing isoform of USP7 |
| VEGF | Vascular endothelial growth factor |
| ZUFSP | Zinc finger with UFM1-specific peptidase domain protein |
| βTrCp | β-transducin repeat-containing protein |
References
- Carreira, L.D.; Oliveira, R.I.; Moreira, V.M.; Salvador, J.A.R. Ubiquitin-Specific Protease 7 (USP7): An Emerging Drug Target for Cancer Treatment. Expert Opin. Ther. Targets 2023, 27, 1043–1058. [Google Scholar] [CrossRef]
- Georges, A.; Marcon, E.; Greenblatt, J.; Frappier, L. Identification and Characterization of USP7 Targets in Cancer Cells. Sci. Rep. 2018, 8, 15833. [Google Scholar] [CrossRef]
- Selvaraju, K.; Mazurkiewicz, M.; Wang, X.; Gullbo, J.; Linder, S.; D’Arcy, P. Inhibition of Proteasome Deubiquitinase Activity: A Strategy to Overcome Resistance to Conventional Proteasome Inhibitors? Drug Resist. Updates 2015, 21–22, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Ouyang, T.; Li, M.; Hong, T.; Mhs, A.; Meng, W.; Zhang, N. Ubiquitin-Specific Peptidase 7: A Novel Deubiquitinase That Regulates Protein Homeostasis and Cancers. Front. Oncol. 2021, 11, 784672. [Google Scholar] [CrossRef] [PubMed]
- Ndubaku, C.; Tsui, V. Inhibiting the Deubiquitinating Enzymes (DUBs): Miniperspective. J. Med. Chem. 2015, 58, 1581–1595. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.-J.; Wang, B.; Zhang, Y.; Kang, H.-Q.; Nie, H.-Q.; Feng, M.-K.; Zhang, X.-Y.; Zhao, L.-J.; Wang, N.; Liu, H.-M.; et al. USP7 as an Emerging Therapeutic Target: A Key Regulator of Protein Homeostasis. Int. J. Biol. Macromol. 2024, 263, 130309. [Google Scholar] [CrossRef]
- Mevissen, T.E.T.; Komander, D. Mechanisms of Deubiquitinase Specificity and Regulation. Annu. Rev. Biochem. 2017, 86, 159–192. [Google Scholar] [CrossRef]
- Snyder, N.A.; Silva, G.M. Deubiquitinating Enzymes (DUBs): Regulation, Homeostasis, and Oxidative Stress Response. J. Biol. Chem. 2021, 297, 101077. [Google Scholar] [CrossRef]
- Park, H.-B.; Baek, K.-H. Current and Future Directions of USP7 Interactome in Cancer Study. Biochim. Biophys. Acta (BBA) Rev. Cancer 2023, 1878, 188992. [Google Scholar] [CrossRef]
- Yeasmin Khusbu, F.; Chen, F.; Chen, H. Targeting Ubiquitin Specific Protease 7 in Cancer: A Deubiquitinase with Great Prospects. Cell Biochem. Funct. 2018, 36, 244–254. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, T.; Dong, X.; Guo, Q.; Zheng, L.; Wang, X.; Zhang, N.; Li, D.; Ren, L.; Yi, F.; et al. De-Ubiquitination of SAMHD1 by USP7 Promotes DNA Damage Repair to Overcome Oncogenic Stress and Affect Chemotherapy Sensitivity. Oncogene 2023, 42, 1843–1856. [Google Scholar] [CrossRef]
- Pozhidaeva, A.; Bezsonova, I. USP7: Structure, Substrate Specificity, and Inhibition. DNA Repair 2019, 76, 30–39. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, F.; Zhang, G.; Zhang, Q.; Liu, Y.; Wang, Q.; Elsharkawy, M.S.; Zheng, M.; Wen, J.; Zhao, G.; et al. USP7 Promotes Deubiquitination and Stabilization of MyD88 to Enhance Immune Responses. Front. Immunol. 2022, 13, 900243. [Google Scholar] [CrossRef] [PubMed]
- Große, M.; Setz, C.; Rauch, P.; Auth, J.; Morokutti-Kurz, M.; Temchura, V.; Schubert, U. Inhibitors of Deubiquitinating Enzymes Interfere with the SARS-CoV-2 Papain-like Protease and Block Virus Replication In Vitro. Viruses 2022, 14, 1404. [Google Scholar] [CrossRef] [PubMed]
- Saha, G.; Roy, S.; Basu, M.; Ghosh, M.K. USP7—A Crucial Regulator of Cancer Hallmarks. Biochim. Biophys. Acta (BBA) Rev. Cancer 2023, 1878, 188903. [Google Scholar] [CrossRef]
- Qi, S.-M.; Cheng, G.; Cheng, X.-D.; Xu, Z.; Xu, B.; Zhang, W.-D.; Qin, J.-J. Targeting USP7-Mediated Deubiquitination of MDM2/MDMX-P53 Pathway for Cancer Therapy: Are We There Yet? Front. Cell Dev. Biol. 2020, 8, 233. [Google Scholar] [CrossRef] [PubMed]
- Song, M.S.; Salmena, L.; Carracedo, A.; Egia, A.; Lo-Coco, F.; Teruya-Feldstein, J.; Pandolfi, P.P. The Deubiquitinylation and Localization of PTEN Are Regulated by a HAUSP–PML Network. Nature 2008, 455, 813–817. [Google Scholar] [CrossRef]
- Colleran, A.; Collins, P.E.; O’Carroll, C.; Ahmed, A.; Mao, X.; McManus, B.; Kiely, P.A.; Burstein, E.; Carmody, R.J. Deubiquitination of NF-κB by Ubiquitin-Specific Protease-7 Promotes Transcription. Proc. Natl. Acad. Sci. USA 2013, 110, 618–623. [Google Scholar] [CrossRef]
- An, T.; Gong, Y.; Li, X.; Kong, L.; Ma, P.; Gong, L.; Zhu, H.; Yu, C.; Liu, J.; Zhou, H.; et al. USP7 Inhibitor P5091 Inhibits Wnt Signaling and Colorectal Tumor Growth. Biochem. Pharmacol. 2017, 131, 29–39. [Google Scholar] [CrossRef]
- van Loosdregt, J.; Fleskens, V.; Fu, J.; Brenkman, A.B.; Bekker, C.P.J.; Pals, C.E.G.M.; Meerding, J.; Berkers, C.R.; Barbi, J.; Gröne, A.; et al. Stabilization of the Transcription Factor Foxp3 by the Deubiquitinase USP7 Increases Treg-Cell-Suppressive Capacity. Immunity 2013, 39, 259–271. [Google Scholar] [CrossRef]
- Oliveira, R.I.; Guedes, R.A.; Salvador, J.A.R. Highlights in USP7 Inhibitors for Cancer Treatment. Front. Chem. 2022, 10, 1005727. [Google Scholar] [CrossRef] [PubMed]
- Chondrogianni, N.; Gonos, E.S. Structure and Function of the Ubiquitin–Proteasome System. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2012; Volume 109, pp. 41–74. ISBN 978-0-12-397863-9. [Google Scholar]
- Chen, S.; Liu, Y.; Zhou, H. Advances in the Development Ubiquitin-Specific Peptidase (USP) Inhibitors. Int. J. Mol. Sci. 2021, 22, 4546. [Google Scholar] [CrossRef] [PubMed]
- Hochstrasser, M. Lingering Mysteries of Ubiquitin-Chain Assembly. Cell 2006, 124, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Nininahazwe, L.; Liu, B.; He, C.; Zhang, H.; Chen, Z.-S. The Emerging Nature of Ubiquitin-Specific Protease 7 (USP7): A New Target in Cancer Therapy. Drug Discov. Today 2021, 26, 490–502. [Google Scholar] [CrossRef]
- Deng, L.; Meng, T.; Chen, L.; Wei, W.; Wang, P. The Role of Ubiquitination in Tumorigenesis and Targeted Drug Discovery. Signal Transduct. Target. Ther. 2020, 5, 11. [Google Scholar] [CrossRef]
- Ravid, T.; Hochstrasser, M. Diversity of Degradation Signals in the Ubiquitin–Proteasome System. Nat. Rev. Mol. Cell Biol. 2008, 9, 679–689. [Google Scholar] [CrossRef]
- Pickart, C.M.; Fushman, D. Polyubiquitin Chains: Polymeric Protein Signals. Curr. Opin. Chem. Biol. 2004, 8, 610–616. [Google Scholar] [CrossRef]
- Sewduth, R.N.; Baietti, M.F.; Sablina, A.A. Cracking the Monoubiquitin Code of Genetic Diseases. Int. J. Mol. Sci. 2020, 21, 3036. [Google Scholar] [CrossRef]
- Chen, Z.J.; Sun, L.J. Nonproteolytic Functions of Ubiquitin in Cell Signaling. Mol. Cell 2009, 33, 275–286. [Google Scholar] [CrossRef]
- Bard, J.A.M.; Goodall, E.A.; Greene, E.R.; Jonsson, E.; Dong, K.C.; Martin, A. Structure and Function of the 26S Proteasome. Annu. Rev. Biochem. 2018, 87, 697–724. [Google Scholar] [CrossRef]
- Clague, M.J.; Urbé, S.; Komander, D. Breaking the Chains: Deubiquitylating Enzyme Specificity Begets Function. Nat. Rev. Mol. Cell Biol. 2019, 20, 338–352. [Google Scholar] [CrossRef] [PubMed]
- Nijman, S.M.B.; Luna-Vargas, M.P.A.; Velds, A.; Brummelkamp, T.R.; Dirac, A.M.G.; Sixma, T.K.; Bernards, R. A Genomic and Functional Inventory of Deubiquitinating Enzymes. Cell 2005, 123, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Schauer, N.J.; Liu, X.; Magin, R.S.; Doherty, L.M.; Chan, W.C.; Ficarro, S.B.; Hu, W.; Roberts, R.M.; Iacob, R.E.; Stolte, B.; et al. Selective USP7 Inhibition Elicits Cancer Cell Killing through a P53-Dependent Mechanism. Sci. Rep. 2020, 10, 5324. [Google Scholar] [CrossRef] [PubMed]
- Lange, S.M.; Armstrong, L.A.; Kulathu, Y. Deubiquitinases: From Mechanisms to Their Inhibition by Small Molecules. Mol. Cell 2022, 82, 15–29. [Google Scholar] [CrossRef]
- Komander, D.; Clague, M.J.; Urbé, S. Breaking the Chains: Structure and Function of the Deubiquitinases. Nat. Rev. Mol. Cell Biol. 2009, 10, 550–563. [Google Scholar] [CrossRef]
- Tavana, O.; Gu, W. Modulation of the P53/MDM2 Interplay by HAUSP Inhibitors. J. Mol. Cell Biol. 2017, 9, 45–52. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Chakraborty, D.; Basu, M.; Ghosh, M.K. Emerging Insights into HAUSP (USP7) in Physiology, Cancer and Other Diseases. Signal Transduct. Target Ther. 2018, 3, 17. [Google Scholar] [CrossRef]
- Fernández-Montalván, A.; Bouwmeester, T.; Joberty, G.; Mader, R.; Mahnke, M.; Pierrat, B.; Schlaeppi, J.; Worpenberg, S.; Gerhartz, B. Biochemical Characterization of USP7 Reveals Post-translational Modification Sites and Structural Requirements for Substrate Processing and Subcellular Localization. FEBS J. 2007, 274, 4256–4270. [Google Scholar] [CrossRef]
- Zapata, J.M.; Pawlowski, K.; Haas, E.; Ware, C.F.; Godzik, A.; Reed, J.C. A Diverse Family of Proteins Containing Tumor Necrosis Factor Receptor-Associated Factor Domains. J. Biol. Chem. 2001, 276, 24242–24252. [Google Scholar] [CrossRef]
- Valles, G.J.; Bezsonova, I.; Woodgate, R.; Ashton, N.W. USP7 Is a Master Regulator of Genome Stability. Front. Cell Dev. Biol. 2020, 8, 717. [Google Scholar] [CrossRef]
- Li, P.; Liu, H.-M. Recent Advances in the Development of Ubiquitin-Specific-Processing Protease 7 (USP7) Inhibitors. Eur. J. Med. Chem. 2020, 191, 112107. [Google Scholar] [CrossRef]
- Hu, M.; Li, P.; Li, M.; Li, W.; Yao, T.; Wu, J.-W.; Gu, W.; Cohen, R.E.; Shi, Y. Crystal Structure of a UBP-Family Deubiquitinating Enzyme in Isolation and in Complex with Ubiquitin Aldehyde. Cell 2002, 111, 1041–1054. [Google Scholar] [CrossRef]
- Faesen, A.C.; Dirac, A.M.G.; Shanmugham, A.; Ovaa, H.; Perrakis, A.; Sixma, T.K. Mechanism of USP7/HAUSP Activation by Its C-Terminal Ubiquitin-like Domain and Allosteric Regulation by GMP-Synthetase. Mol. Cell 2011, 44, 147–159. [Google Scholar] [CrossRef]
- Cheng, J.; Yang, H.; Fang, J.; Ma, L.; Gong, R.; Wang, P.; Li, Z.; Xu, Y. Molecular Mechanism for USP7-Mediated DNMT1 Stabilization by Acetylation. Nat. Commun. 2015, 6, 7023. [Google Scholar] [CrossRef] [PubMed]
- Van Der Horst, A.; De Vries-Smits, A.M.M.; Brenkman, A.B.; Van Triest, M.H.; Van Den Broek, N.; Colland, F.; Maurice, M.M.; Burgering, B.M.T. FOXO4 Transcriptional Activity Is Regulated by Monoubiquitination and USP7/HAUSP. Nat. Cell Biol. 2006, 8, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Sarkari, F.; Wheaton, K.; La Delfa, A.; Mohamed, M.; Shaikh, F.; Khatun, R.; Arrowsmith, C.H.; Frappier, L.; Saridakis, V.; Sheng, Y. Ubiquitin-Specific Protease 7 Is a Regulator of Ubiquitin-Conjugating Enzyme UbE2E1. J. Biol. Chem. 2013, 288, 16975–16985. [Google Scholar] [CrossRef] [PubMed]
- Steger, M.; Demichev, V.; Backman, M.; Ohmayer, U.; Ihmor, P.; Müller, S.; Ralser, M.; Daub, H. Time-Resolved in Vivo Ubiquitinome Profiling by DIA-MS Reveals USP7 Targets on a Proteome-Wide Scale. Nat. Commun. 2021, 12, 5399. [Google Scholar] [CrossRef]
- Cstorer, A.; Ménard, R. Catalytic Mechanism in Papain Family of Cysteine Peptidases. In Methods in Enzymology; Elsevier: San Diego, CA, USA, 1994; Volume 244, pp. 486–500. ISBN 978-0-12-182145-6. [Google Scholar]
- Zhou, J.; Wang, J.; Chen, C.; Yuan, H.; Wen, X.; Sun, H. USP7: Target Validation and Drug Discovery for Cancer Therapy. Med. Chem. 2018, 14, 3–18. [Google Scholar] [CrossRef]
- Khoronenkova, S.V.; Dianov, G.L. USP7S-Dependent Inactivation of Mule Regulates DNA Damage Signalling and Repair. Nucleic Acids Res. 2013, 41, 1750–1756. [Google Scholar] [CrossRef]
- Rougé, L.; Bainbridge, T.W.; Kwok, M.; Tong, R.; Di Lello, P.; Wertz, I.E.; Maurer, T.; Ernst, J.A.; Murray, J. Molecular Understanding of USP7 Substrate Recognition and C-Terminal Activation. Structure 2016, 24, 1335–1345. [Google Scholar] [CrossRef]
- Swatek, K.N.; Komander, D. Ubiquitin Modifications. Cell Res. 2016, 26, 399–422. [Google Scholar] [CrossRef]
- Wang, Z.; Kang, W.; You, Y.; Pang, J.; Ren, H.; Suo, Z.; Liu, H.; Zheng, Y. USP7: Novel Drug Target in Cancer Therapy. Front. Pharmacol. 2019, 10, 427. [Google Scholar] [CrossRef]
- Bojagora, A.; Saridakis, V. USP7 Manipulation by Viral Proteins. Virus Res. 2020, 286, 198076. [Google Scholar] [CrossRef]
- Lusardi, M.; Morretta, E.; Spallarossa, A.; Monti, M.C.; Rosano, C.; Iervasi, E.; Ponassi, M.; Mori, M.; Meneghetti, F.; Brullo, C. Targeting Ubiquitin-Specific Protease 7 with Novel 5-Amino-Pyrazole Inhibitors: Design, Synthesis, and Biological Evaluation. ChemMedChem 2025, 20, e202500185. [Google Scholar] [CrossRef] [PubMed]
- Collaud, S.; Tischler, V.; Atanassoff, A.; Wiedl, T.; Komminoth, P.; Oehlschlegel, C.; Weder, W.; Soltermann, A. Lung Neuroendocrine Tumors: Correlation of Ubiquitinylation and Sumoylation with Nucleo-Cytosolic Partitioning of PTEN. BMC Cancer 2015, 15, 74. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, D.; Tian, Z.; Nicholson, B.; Kumar, K.G.S.; Zhou, B.; Carrasco, R.; McDermott, J.L.; Leach, C.A.; Fulcinniti, M.; Kodrasov, M.P.; et al. A Small Molecule Inhibitor of Ubiquitin-Specific Protease-7 Induces Apoptosis in Multiple Myeloma Cells and Overcomes Bortezomib Resistance. Cancer Cell 2012, 22, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Ma, M. Ubiquitin-Specific Protease 7 Expression Is a Prognostic Factor in Epithelial Ovarian Cancer and Correlates with Lymph Node Metastasis. OncoTargets Ther. 2016, 9, 1559–1569. [Google Scholar] [CrossRef]
- Das, S.; Chandrasekaran, A.P.; Jo, K.-S.; Ko, N.R.; Oh, S.J.; Kim, K.-S.; Ramakrishna, S. HAUSP Stabilizes Cdc25A and Protects Cervical Cancer Cells from DNA Damage Response. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2020, 1867, 118835. [Google Scholar] [CrossRef]
- Cheng, C.; Niu, C.; Yang, Y.; Wang, Y.; Lu, M. Expression of HAUSP in Gliomas Correlates with Disease Progression and Survival of Patients. Oncol. Rep. 2013, 29, 1730–1736. [Google Scholar] [CrossRef]
- Li, N.; Zhao, Z.; Liu, P.; Zheng, Y.; Cai, S.; Sun, Y.; Wang, B. Upregulation of Deubiquitinase USP7 by Transcription Factor FOXO6 Promotes EC Progression via Targeting the JMJD3/CLU Axis. Mol. Ther. Oncolytics 2021, 20, 583–595. [Google Scholar] [CrossRef]
- Wang, L.; Kumar, S.; Dahiya, S.; Wang, F.; Wu, J.; Newick, K.; Han, R.; Samanta, A.; Beier, U.H.; Akimova, T.; et al. Ubiquitin-Specific Protease-7 Inhibition Impairs Tip60-Dependent Foxp3 + T-Regulatory Cell Function and Promotes Antitumor Immunity. eBioMedicine 2016, 13, 99–112. [Google Scholar] [CrossRef]
- Fu, C.; Zhu, X.; Xu, P.; Li, Y. Pharmacological Inhibition of USP7 Promotes Antitumor Immunity and Contributes to Colon Cancer Therapy. Onco Targets Ther. 2019, 12, 609–617. [Google Scholar] [CrossRef]
- Cheron, J.; Beccari, L.; Hagué, P.; Icick, R.; Despontin, C.; Carusone, T.; Defrance, M.; Bhogaraju, S.; Martin-Garcia, E.; Capellan, R.; et al. USP7/Maged1-Mediated H2A Monoubiquitination in the Paraventricular Thalamus: An Epigenetic Mechanism Involved in Cocaine Use Disorder. Nat. Commun. 2023, 14, 8481. [Google Scholar] [CrossRef]
- Fischer, M. Census and Evaluation of P53 Target Genes. Oncogene 2017, 36, 3943–3956. [Google Scholar] [CrossRef] [PubMed]
- Marei, H.E.; Althani, A.; Afifi, N.; Hasan, A.; Caceci, T.; Pozzoli, G.; Morrione, A.; Giordano, A.; Cenciarelli, C. P53 Signaling in Cancer Progression and Therapy. Cancer Cell Int. 2021, 21, 703. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, T.; Nakagawara, A. Role of P53 in Cell Death and Human Cancers. Cancers 2011, 3, 994–1013. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cao, M.; Dong, J.; Li, C.; Xu, W.; Zhan, Y.; Wang, X.; Yu, M.; Ge, C.; Ge, Z.; et al. ABRO1 Suppresses Tumourigenesis and Regulates the DNA Damage Response by Stabilizing P53. Nat. Commun. 2014, 5, 5059. [Google Scholar] [CrossRef]
- Hu, M.; Gu, L.; Li, M.; Jeffrey, P.D.; Gu, W.; Shi, Y. Structural Basis of Competitive Recognition of P53 and MDM2 by HAUSP/USP7: Implications for the Regulation of the P53–MDM2 Pathway. PLoS Biol. 2006, 4, e27. [Google Scholar] [CrossRef]
- Tang, J.; Qu, L.; Pang, M.; Yang, X. Daxx Is Reciprocally Regulated by Mdm2 and Hausp. Biochem. Biophys. Res. Commun. 2010, 393, 542–545. [Google Scholar] [CrossRef]
- Giovinazzi, S.; Morozov, V.M.; Summers, M.K.; Reinhold, W.C.; Ishov, A.M. USP7 and Daxx Regulate Mitosis Progression and Taxane Sensitivity by Affecting Stability of Aurora-A Kinase. Cell Death Differ. 2013, 20, 721–731. [Google Scholar] [CrossRef]
- Zhu, H.; Gao, H.; Ji, Y.; Zhou, Q.; Du, Z.; Tian, L.; Jiang, Y.; Yao, K.; Zhou, Z. Targeting P53–MDM2 Interaction by Small-Molecule Inhibitors: Learning from MDM2 Inhibitors in Clinical Trials. J. Hematol. Oncol. 2022, 15, 91. [Google Scholar] [CrossRef]
- Fan, Y.-H.; Cheng, J.; Vasudevan, S.A.; Dou, J.; Zhang, H.; Patel, R.H.; Ma, I.T.; Rojas, Y.; Zhao, Y.; Yu, Y.; et al. USP7 Inhibitor P22077 Inhibits Neuroblastoma Growth via Inducing P53-Mediated Apoptosis. Cell Death Dis. 2013, 4, e867. [Google Scholar] [CrossRef]
- Zhao, G.-Y.; Lin, Z.-W.; Lu, C.-L.; Gu, J.; Yuan, Y.-F.; Xu, F.-K.; Liu, R.-H.; Ge, D.; Ding, J.-Y. USP7 Overexpression Predicts a Poor Prognosis in Lung Squamous Cell Carcinoma and Large Cell Carcinoma. Tumor Biol. 2015, 36, 1721–1729. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Ghosh, M.K. HAUSP, a Novel Deubiquitinase for Rb–MDM 2 the Critical Regulator. FEBS J. 2014, 281, 3061–3078. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Cui, Y.; Xu, Q.; Jiang, Y. Stabilization of LSD1 by Deubiquitinating Enzyme USP7 Promotes Glioblastoma Cell Tumorigenesis and Metastasis through Suppression of the P53 Signaling Pathway. Oncol. Rep. 2016, 36, 2935–2945. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Garcia, V.; Tawil, Y.; Wise, H.M.; Leslie, N.R. Mechanisms of PTEN Loss in Cancer: It’s All about Diversity. Semin. Cancer Biol. 2019, 59, 66–79. [Google Scholar] [CrossRef]
- Lee, Y.-R.; Chen, M.; Pandolfi, P.P. The Functions and Regulation of the PTEN Tumour Suppressor: New Modes and Prospects. Nat. Rev. Mol. Cell Biol. 2018, 19, 547–562. [Google Scholar] [CrossRef]
- Fedorova, O.; Parfenyev, S.; Daks, A.; Shuvalov, O.; Barlev, N.A. The Role of PTEN in Epithelial–Mesenchymal Transition. Cancers 2022, 14, 3786. [Google Scholar] [CrossRef]
- Morotti, A.; Panuzzo, C.; Crivellaro, S.; Pergolizzi, B.; Familiari, U.; Berger, A.H.; Saglio, G.; Pandolfi, P.P. BCR-ABL Disrupts PTEN Nuclear-Cytoplasmic Shuttling through Phosphorylation-Dependent Activation of HAUSP. Leukemia 2014, 28, 1326–1333. [Google Scholar] [CrossRef]
- Lee, J.E.; Park, C.M.; Kim, J.H. USP7 Deubiquitinates and Stabilizes EZH2 in Prostate Cancer Cells. Genet. Mol. Biol. 2020, 43, e20190338. [Google Scholar] [CrossRef]
- Noguera, N.I.; Song, M.S.; Divona, M.; Catalano, G.; Calvo, K.L.; García, F.; Ottone, T.; Florenzano, F.; Faraoni, I.; Battistini, L.; et al. Nucleophosmin/B26 Regulates PTEN through Interaction with HAUSP in Acute Myeloid Leukemia. Leukemia 2013, 27, 1037–1043. [Google Scholar] [CrossRef]
- Park, H.-B.; Hwang, S.; Baek, K.-H. USP7 Regulates the ERK1/2 Signaling Pathway through Deubiquitinating Raf-1 in Lung Adenocarcinoma. Cell Death Dis. 2022, 13, 698. [Google Scholar] [CrossRef]
- Park, H.-B.; Kim, J.-W.; Baek, K.-H. Regulation of Wnt Signaling through Ubiquitination and Deubiquitination in Cancers. Int. J. Mol. Sci. 2020, 21, 3904. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X. Targeting the Wnt/β-Catenin Signaling Pathway in Cancer. J. Hematol. Oncol. 2020, 13, 165. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Lu, B.; Zamponi, R.; Charlat, O.; Aversa, R.; Yang, Z.; Sigoillot, F.; Zhu, X.; Hu, T.; Reece-Hoyes, J.S.; et al. USP7 Inhibits Wnt/β-Catenin Signaling through Promoting Stabilization of Axin. Nat. Commun. 2019, 10, 4184. [Google Scholar] [CrossRef] [PubMed]
- Novellasdemunt, L.; Foglizzo, V.; Cuadrado, L.; Antas, P.; Kucharska, A.; Encheva, V.; Snijders, A.P.; Li, V.S.W. USP7 Is a Tumor-Specific WNT Activator for APC -Mutated Colorectal Cancer by Mediating β-Catenin Deubiquitination. Cell Rep. 2017, 21, 612–627. [Google Scholar] [CrossRef]
- Basu, B.; Karmakar, S.; Basu, M.; Ghosh, M.K. USP7 Imparts Partial EMT State in Colorectal Cancer by Stabilizing the RNA Helicase DDX3X and Augmenting Wnt/β-Catenin Signaling. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2023, 1870, 119446. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Shared Principles in NF-κB Signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef]
- Xia, L.; Tan, S.; Zhou, Y.; Lin, J.; Wang, H.; Oyang, L.; Tian, Y.; Liu, L.; Su, M.; Wang, H.; et al. Role of the NFκB-Signaling Pathway in Cancer. OncoTargets Ther. 2018, 11, 2063–2073. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, C.; Zhang, Z.; Zhang, H.; Hu, H. NF-κB Signaling in Inflammation and Cancer. MedComm 2021, 2, 618–653. [Google Scholar] [CrossRef]
- Franqui-Machin, R.; Hao, M.; Bai, H.; Gu, Z.; Zhan, X.; Habelhah, H.; Jethava, Y.; Qiu, L.; Frech, I.; Tricot, G.; et al. Destabilizing NEK2 Overcomes Resistance to Proteasome Inhibition in Multiple Myeloma. J. Clin. Investig. 2018, 128, 2877–2893. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; He, Y.; Meng, B.; Chen, S.; Zhang, J.; Wu, X.; Zhu, Y.; Shen, Y.; Feng, X.; Guan, Y.; et al. NEK2 Induces Autophagy-mediated Bortezomib Resistance by Stabilizing Beclin-1 in Multiple Myeloma. Mol. Oncol. 2020, 14, 763–778. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhang, Y.; Shi, M.; Sun, Y.; Chen, C.; Niu, M.; Zhang, Q.; Zeng, L.; Yao, R.; Li, H.; et al. Blockade of Deubiquitinase USP7 Overcomes Bortezomib Resistance by Suppressing NF-κB Signaling Pathway in Multiple Myeloma. J. Leukoc. Biol. 2018, 104, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Rui, L.; Fisher, T.L.; Thomas, J.; White, M.F. Regulation of Insulin/Insulin-like Growth Factor-1 Signaling by Proteasome-Mediated Degradation of Insulin Receptor Substrate-2. J. Biol. Chem. 2001, 276, 40362–40367. [Google Scholar] [CrossRef]
- Taniguchi, C.M.; Emanuelli, B.; Kahn, C.R. Critical Nodes in Signalling Pathways: Insights into Insulin Action. Nat. Rev. Mol. Cell Biol. 2006, 7, 85–96. [Google Scholar] [CrossRef]
- Jones, J.I. Insulin-like Growth Factors and Their Binding Proteins: Biological Actions. Endocr. Rev. 1995, 16, 3–34. [Google Scholar] [CrossRef]
- Yoshihara, H.; Fukushima, T.; Hakuno, F.; Saeki, Y.; Tanaka, K.; Ito, A.; Yoshida, M.; Iemura, S.; Natsume, T.; Asano, T.; et al. Insulin/Insulin-like Growth Factor (IGF) Stimulation Abrogates an Association between a Deubiquitinating Enzyme USP7 and Insulin Receptor Substrates (IRSs) Followed by Proteasomal Degradation of IRSs. Biochem. Biophys. Res. Commun. 2012, 423, 122–127. [Google Scholar] [CrossRef]
- Chen, S.-T.; Okada, M.; Nakato, R.; Izumi, K.; Bando, M.; Shirahige, K. The Deubiquitinating Enzyme USP7 Regulates Androgen Receptor Activity by Modulating Its Binding to Chromatin. J. Biol. Chem. 2015, 290, 21713–21723. [Google Scholar] [CrossRef]
- Kim, S.-H.; Baek, K.-H. Regulation of Cancer Metabolism by Deubiquitinating Enzymes: The Warburg Effect. Int. J. Mol. Sci. 2021, 22, 6173. [Google Scholar] [CrossRef]
- Hall, J.A.; Tabata, M.; Rodgers, J.T.; Puigserver, P. USP7 Attenuates Hepatic Gluconeogenesis Through Modulation of FoxO1 Gene Promoter Occupancy. Mol. Endocrinol. 2014, 28, 912–924. [Google Scholar] [CrossRef]
- Kiran, S.; Anwar, T.; Kiran, M.; Ramakrishna, G. Sirtuin 7 in Cell Proliferation, Stress and Disease: Rise of the Seventh Sirtuin! Cell. Signal. 2015, 27, 673–682. [Google Scholar] [CrossRef]
- Wu, D.; Li, Y.; Zhu, K.S.; Wang, H.; Zhu, W.-G. Advances in Cellular Characterization of the Sirtuin Isoform, SIRT7. Front. Endocrinol. 2018, 9, 652. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Xiong, J.; Zhan, J.; Yuan, F.; Tang, M.; Zhang, C.; Cao, Z.; Chen, Y.; Lu, X.; Li, Y.; et al. Ubiquitin-Specific Peptidase 7 (USP7)-Mediated Deubiquitination of the Histone Deacetylase SIRT7 Regulates Gluconeogenesis. J. Biol. Chem. 2017, 292, 13296–13311. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Duan, J.-J.; Bian, X.-W.; Yu, S. Triple-Negative Breast Cancer Molecular Subtyping and Treatment Progress. Breast Cancer Res. 2020, 22, 61. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Li, C.; Lee, H.; Shin, D.; Chern, Y.; Pereira De Carvalho, B.; Chan, C. MIG-6 Is Essential for Promoting Glucose Metabolic Reprogramming and Tumor Growth in Triple-negative Breast Cancer. EMBO Rep. 2021, 22, e50781. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Lin, J.; Liu, Y.-E.; Chen, Y.-C.; Liu, S.-T.; Hsu, K.-W.; Chen, D.-R.; Wu, H.-T. USP7 Induces Chemoresistance in Triple-Negative Breast Cancer via Deubiquitination and Stabilization of ABCB1. Cells 2022, 11, 3294. [Google Scholar] [CrossRef]
- Li, J.; Feng, X.; Liu, Z.; Deng, Y.; Sun, Z.; Chen, B.; Wu, L.; Wang, X.; Miao, L.; Zeng, L.; et al. USP7 Promotes Temozolomide Resistance by Stabilizing MGMT in Glioblastoma. Cell Death Dis. 2025, 16, 631. [Google Scholar] [CrossRef]
- Sun, X.; Ding, Y.; Zhan, M.; Li, Y.; Gao, D.; Wang, G.; Gao, Y.; Li, Y.; Wu, S.; Lu, L.; et al. Usp7 Regulates Hippo Pathway through Deubiquitinating the Transcriptional Coactivator Yorkie. Nat. Commun. 2019, 10, 411. [Google Scholar] [CrossRef]
- Sakamoto, T.; Kuboki, S.; Furukawa, K.; Takayashiki, T.; Takano, S.; Yoshizumi, A.; Ohtsuka, M. TRIM27-USP7 Complex Promotes Tumour Progression via STAT3 Activation in Human Hepatocellular Carcinoma. Liver Int. 2023, 43, 194–207. [Google Scholar] [CrossRef]
- Olive, K.P.; Jacobetz, M.A.; Davidson, C.J.; Gopinathan, A.; McIntyre, D.; Honess, D.; Madhu, B.; Goldgraben, M.A.; Caldwell, M.E.; Allard, D.; et al. Inhibition of Hedgehog Signaling Enhances Delivery of Chemotherapy in a Mouse Model of Pancreatic Cancer. Science 2009, 324, 1457–1461. [Google Scholar] [CrossRef]
- Zee, Y.-K.; O’Connor, J.P.B.; Parker, G.J.M.; Jackson, A.; Clamp, A.R.; Taylor, M.B.; Clarke, N.W.; Jayson, G.C. Imaging Angiogenesis of Genitourinary Tumors. Nat. Rev. Urol. 2010, 7, 69–82. [Google Scholar] [CrossRef]
- Lu, X.; Kang, Y. Hypoxia and Hypoxia-Inducible Factors: Master Regulators of Metastasis. Clin. Cancer Res. 2010, 16, 5928–5935. [Google Scholar] [CrossRef]
- Episkopou, H.; Diman, A.; Claude, E.; Viceconte, N.; Decottignies, A. TSPYL5 Depletion Induces Specific Death of ALT Cells through USP7-Dependent Proteasomal Degradation of POT1. Mol. Cell 2019, 75, 469–482.e6. [Google Scholar] [CrossRef] [PubMed]
- Draskovic, I.; Arnoult, N.; Steiner, V.; Bacchetti, S.; Lomonte, P.; Londoño-Vallejo, A. Probing PML Body Function in ALT Cells Reveals Spatiotemporal Requirements for Telomere Recombination. Proc. Natl. Acad. Sci. USA 2009, 106, 15726–15731. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, M.; Luo, J.; Gu, W. Direct Interactions between HIF-1α and Mdm2 Modulate P53 Function. J. Biol. Chem. 2003, 278, 13595–13598. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Sun, H.; Luo, D.; Gan, L.; Mo, S.; Dai, W.; Liang, L.; Yang, Y.; Xu, M.; Li, J.; et al. Lnc-RP11-536 K7.3/SOX2/HIF-1α Signaling Axis Regulates Oxaliplatin Resistance in Patient-Derived Colorectal Cancer Organoids. J. Exp. Clin. Cancer Res. 2021, 40, 348. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Li, C.-L.; Chen, W.-J.; Liu, J.; Wu, H.-T. Diverse Roles of FOXO Family Members in Gastric Cancer. World J. Gastrointest. Oncol. 2021, 13, 1367–1382. [Google Scholar] [CrossRef]
- Jiramongkol, Y.; Lam, E.W.-F. FOXO Transcription Factor Family in Cancer and Metastasis. Cancer Metastasis Rev. 2020, 39, 681–709. [Google Scholar] [CrossRef]
- Dansen, T.B.; Burgering, B.M.T. Unravelling the Tumor-Suppressive Functions of FOXO Proteins. Trends Cell Biol. 2008, 18, 421–429. [Google Scholar] [CrossRef]
- Kim, C.G.; Lee, H.; Gupta, N.; Ramachandran, S.; Kaushik, I.; Srivastava, S.; Kim, S.-H.; Srivastava, S.K. Role of Forkhead Box Class O Proteins in Cancer Progression and Metastasis. Semin. Cancer Biol. 2018, 50, 142–151. [Google Scholar] [CrossRef]
- Brenkman, A.B.; De Keizer, P.L.J.; Van Den Broek, N.J.F.; Jochemsen, A.G.; Burgering, B.M. Mdm2 Induces Mono-Ubiquitination of FOXO4. PLoS ONE 2008, 3, e2819. [Google Scholar] [CrossRef]
- Gagarina, V.; Bojagora, A.; Lacdao, I.K.; Luthra, N.; Pfoh, R.; Mohseni, S.; Chaharlangi, D.; Tan, N.; Saridakis, V. Structural Basis of the Interaction Between Ubiquitin Specific Protease 7 and Enhancer of Zeste Homolog 2. J. Mol. Biol. 2020, 432, 897–912. [Google Scholar] [CrossRef]
- Duan, D.; Shang, M.; Han, Y.; Liu, J.; Liu, J.; Kong, S.H.; Hou, J.; Huang, B.; Lu, J.; Zhang, Y. EZH2–CCF–cGAS Axis Promotes Breast Cancer Metastasis. Int. J. Mol. Sci. 2022, 23, 1788. [Google Scholar] [CrossRef]
- Yilmaz, M.; Christofori, G. EMT, the Cytoskeleton, and Cancer Cell Invasion. Cancer Metastasis Rev. 2009, 28, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Tauriello, D.V.F.; Maurice, M.M. The Various Roles of Ubiquitin in Wnt Pathway Regulation. Cell Cycle 2010, 9, 3724–3733. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Y.; Kong, D.; Sarkar, H.F. The Role of Notch Signaling Pathway in Epithelial-Mesenchymal Transition (EMT) During Development and Tumor Aggressiveness. Curr. Drug Targets 2010, 11, 745–751. [Google Scholar] [CrossRef]
- Jin, Q.; Martinez, C.A.; Arcipowski, K.M.; Zhu, Y.; Gutierrez-Diaz, B.T.; Wang, K.K.; Johnson, M.R.; Volk, A.G.; Wang, F.; Wu, J.; et al. USP7 Cooperates with NOTCH1 to Drive the Oncogenic Transcriptional Program in T-Cell Leukemia. Clin. Cancer Res. 2019, 25, 222–239. [Google Scholar] [CrossRef]
- Liu, J.; Feng, J.; Li, L.; Lin, L.; Ji, J.; Lin, C.; Liu, L.; Zhang, N.; Duan, D.; Li, Z.; et al. Arginine Methylation-dependent LSD1 Stability Promotes Invasion and Metastasis of Breast Cancer. EMBO Rep. 2020, 21, e48597. [Google Scholar] [CrossRef]
- Dai, X.; Lu, L.; Deng, S.; Meng, J.; Wan, C.; Huang, J.; Sun, Y.; Hu, Y.; Wu, B.; Wu, G.; et al. USP7 Targeting Modulates Anti-Tumor Immune Response by Reprogramming Tumor-Associated Macrophages in Lung Cancer. Theranostics 2020, 10, 9332–9347. [Google Scholar] [CrossRef]
- Powell, D.J.; Felipe-Silva, A.; Merino, M.J.; Ahmadzadeh, M.; Allen, T.; Levy, C.; White, D.E.; Mavroukakis, S.; Kreitman, R.J.; Rosenberg, S.A.; et al. Administration of a CD25-Directed Immunotoxin, LMB-2, to Patients with Metastatic Melanoma Induces a Selective Partial Reduction in Regulatory T Cells In Vivo. J. Immunol. 2007, 179, 4919–4928. [Google Scholar] [CrossRef]
- Sharma, P.; Wagner, K.; Wolchok, J.D.; Allison, J.P. Novel Cancer Immunotherapy Agents with Survival Benefit: Recent Successes and next Steps. Nat. Rev. Cancer 2011, 11, 805–812. [Google Scholar] [CrossRef]
- Pardoll, D.M. The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Saleh, R.; Elkord, E. FoxP3+ T Regulatory Cells in Cancer: Prognostic Biomarkers and Therapeutic Targets. Cancer Lett. 2020, 490, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Yang, C.; Pan, F. Post-Translational Regulations of Foxp3 in Treg Cells and Their Therapeutic Applications. Front. Immunol. 2021, 12, 626172. [Google Scholar] [CrossRef] [PubMed]
- De Ceuninck, L.; Wauman, J.; Masschaele, D.; Peelman, F.; Tavernier, J. Reciprocal Cross-Regulation between RNF41 and USP8 Controls Cytokine Receptor Sorting and Processing. J. Cell Sci. 2013, 126, jcs.131250. [Google Scholar] [CrossRef]
- Colombo, M.; Vallese, S.; Peretto, I.; Jacq, X.; Rain, J.; Colland, F.; Guedat, P. Synthesis and Biological Evaluation of 9-Oxo-9 H-indeno[1,2-b]Pyrazine-2,3-dicarbonitrile Analogues as Potential Inhibitors of Deubiquitinating Enzymes. ChemMedChem 2010, 5, 552–558. [Google Scholar] [CrossRef]
- Kim, J.-E.; Shin, J.-S.; Moon, J.-H.; Hong, S.-W.; Jung, D.-J.; Kim, J.H.; Hwang, I.-Y.; Shin, Y.J.; Gong, E.-Y.; Lee, D.H.; et al. Foxp3 Is a Key Downstream Regulator of P53-Mediated Cellular Senescence. Oncogene 2017, 36, 219–230. [Google Scholar] [CrossRef]
- Khosravi, M.; Bidmeshkipour, A.; Cohen, J.L.; Moravej, A.; Hojjat-Assari, S.; Naserian, S.; Karimi, M.H. Induction of CD4+CD25+FOXP3+ Regulatory T Cells by Mesenchymal Stem Cells Is Associated with Modulation of Ubiquitination Factors and TSDR Demethylation. Stem Cell Res. Ther. 2018, 9, 273. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, X.; Fu, J.; Wang, H. Progress and Challenges in Precise Treatment of Tumors with PD-1/PD-L1 Blockade. Front. Immunol. 2020, 11, 339. [Google Scholar] [CrossRef]
- Wang, Z.; Kang, W.; Li, O.; Qi, F.; Wang, J.; You, Y.; He, P.; Suo, Z.; Zheng, Y.; Liu, H.-M. Abrogation of USP7 Is an Alternative Strategy to Downregulate PD-L1 and Sensitize Gastric Cancer Cells to T Cells Killing. Acta Pharm. Sin. B 2021, 11, 694–707. [Google Scholar] [CrossRef]
- Li, J.; Cao, F.; Yin, H.; Huang, Z.; Lin, Z.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, Present and Future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef]
- Tang, Z.; Huang, Z.; Huang, Y.; Chen, Y.; Huang, M.; Liu, H.; Ye, Q.A.; Zhao, J.; Jia, B. Ferroptosis: The Silver Lining of Cancer Therapy. Front. Cell Dev. Biol. 2021, 9, 765859. [Google Scholar] [CrossRef] [PubMed]
- Boutilier, A.J.; Elsawa, S.F. Macrophage Polarization States in the Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 6995. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cai, L.; Wang, H.; Wu, P.; Gu, W.; Chen, Y.; Hao, H.; Tang, K.; Yi, P.; Liu, M.; et al. Pleiotropic Regulation of Macrophage Polarization and Tumorigenesis by Formyl Peptide Receptor-2. Oncogene 2011, 30, 3887–3899. [Google Scholar] [CrossRef] [PubMed]
- Henrique, T.; Zanon, C.D.F.; Girol, A.P.; Stefanini, A.C.B.; Contessoto, N.S.D.A.; Da Silveira, N.J.F.; Bezerra, D.P.; Silveira, E.R.; Barbosa-Filho, J.M.; Cornélio, M.L.; et al. Biological and Physical Approaches on the Role of Piplartine (Piperlongumine) in Cancer. Sci. Rep. 2020, 10, 22283. [Google Scholar] [CrossRef]
- Park, J.-J.; Lim, K.-H.; Baek, K.-H. Annexin-1 Regulated by HAUSP Is Essential for UV-Induced Damage Response. Cell Death Dis. 2015, 6, e1654. [Google Scholar] [CrossRef]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA Damage, Repair, and Mutagenesis. Environ. Mol. Mutagen. 2017, 58, 235–263. [Google Scholar] [CrossRef]
- Jaenisch, R.; Bird, A. Epigenetic Regulation of Gene Expression: How the Genome Integrates Intrinsic and Environmental Signals. Nat. Genet. 2003, 33, 245–254. [Google Scholar] [CrossRef]
- Higa, M.; Zhang, X.; Tanaka, K.; Saijo, M. Stabilization of Ultraviolet (UV)-Stimulated Scaffold Protein A by Interaction with Ubiquitin-Specific Peptidase 7 Is Essential for Transcription-Coupled Nucleotide Excision Repair. J. Biol. Chem. 2016, 291, 13771–13779. [Google Scholar] [CrossRef]
- Krasikova, Y.; Rechkunova, N.; Lavrik, O. Nucleotide Excision Repair: From Molecular Defects to Neurological Abnormalities. Int. J. Mol. Sci. 2021, 22, 6220. [Google Scholar] [CrossRef]
- Zhu, Q.; Ding, N.; Wei, S.; Li, P.; Wani, G.; He, J.; Wani, A.A. USP7-Mediated Deubiquitination Differentially Regulates CSB but Not UVSSA upon UV Radiation-Induced DNA Damage. Cell Cycle 2020, 19, 124–141. [Google Scholar] [CrossRef]
- Jeggo, P.A.; Pearl, L.H.; Carr, A.M. DNA Repair, Genome Stability and Cancer: A Historical Perspective. Nat. Rev. Cancer 2016, 16, 35–42. [Google Scholar] [CrossRef]
- Löbrich, M.; Shibata, A.; Beucher, A.; Fisher, A.; Ensminger, M.; Goodarzi, A.A.; Barton, O.; Jeggo, P.A. γH2AX Foci Analysis for Monitoring DNA Double-Strand Break Repair: Strengths, Limitations and Optimization. Cell Cycle 2010, 9, 662–669. [Google Scholar] [CrossRef]
- Lane, D.P. P53, Guardian of the Genome. Nature 1992, 358, 15–16. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhu, Q.; Wani, G.; Sharma, N.; Han, C.; Qian, J.; Pentz, K.; Wang, Q.; Wani, A.A. Ubiquitin-Specific Protease 7 Regulates Nucleotide Excision Repair through Deubiquitinating XPC Protein and Preventing XPC Protein from Undergoing Ultraviolet Light-Induced and VCP/P97 Protein-Regulated Proteolysis. J. Biol. Chem. 2014, 289, 27278–27289. [Google Scholar] [CrossRef] [PubMed]
- Haupt, Y.; Maya, R.; Kazaz, A.; Oren, M. Mdm2 Promotes the Rapid Degradation of P53. Nature 1997, 387, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Sharma, N.; He, J.; Wani, G.; Wani, A.A. USP7 Deubiquitinase Promotes Ubiquitin-Dependent DNA Damage Signaling by Stabilizing RNF168*. Cell Cycle 2015, 14, 1413–1425. [Google Scholar] [CrossRef]
- Poulsen, M.; Lukas, C.; Lukas, J.; Bekker-Jensen, S.; Mailand, N. Human RNF169 Is a Negative Regulator of the Ubiquitin-Dependent Response to DNA Double-Strand Breaks. J. Cell Biol. 2012, 197, 189–199. [Google Scholar] [CrossRef]
- Bartocci, C.; Denchi, E.L. Put a RING on It: Regulation and Inhibition of RNF8 and RNF168 RING Finger E3 Ligases at DNA Damage Sites. Front. Genet. 2013, 4, 128. [Google Scholar] [CrossRef]
- Noubissi, F.K.; McBride, A.A.; Leppert, H.G.; Millet, L.J.; Wang, X.; Davern, S.M. Detection and Quantification of γ-H2AX Using a Dissociation Enhanced Lanthanide Fluorescence Immunoassay. Sci. Rep. 2021, 11, 8945. [Google Scholar] [CrossRef]
- Su, D.; Ma, S.; Shan, L.; Wang, Y.; Wang, Y.; Cao, C.; Liu, B.; Yang, C.; Wang, L.; Tian, S.; et al. Ubiquitin-Specific Protease 7 Sustains DNA Damage Response and Promotes Cervical Carcinogenesis. J. Clin. Investig. 2018, 128, 4280–4296. [Google Scholar] [CrossRef]
- Mailand, N.; Falck, J.; Lukas, C.; Syljuåsen, R.G.; Welcker, M.; Bartek, J.; Lukas, J. Rapid Destruction of Human Cdc25A in Response to DNA Damage. Science 2000, 288, 1425–1429. [Google Scholar] [CrossRef]
- Smith, J.; Mun Tho, L.; Xu, N.; Gillespie, D.A. The ATM–Chk2 and ATR–Chk1 Pathways in DNA Damage Signaling and Cancer. In Advances in Cancer Research; Academic Press: San Diego, CA, USA, 2010; Volume 108, pp. 73–112. ISBN 978-0-12-380888-2. [Google Scholar]
- Alonso-de Vega, I.; Martín, Y.; Smits, V.A. USP7 Controls Chk1 Protein Stability by Direct Deubiquitination. Cell Cycle 2014, 13, 3921–3926. [Google Scholar] [CrossRef] [PubMed]
- Morra, F.; Luise, C.; Visconti, R.; Staibano, S.; Merolla, F.; Ilardi, G.; Guggino, G.; Paladino, S.; Sarnataro, D.; Franco, R.; et al. New Therapeutic Perspectives in CCDC 6 Deficient Lung Cancer Cells. Int. J. Cancer 2015, 136, 2146–2157. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Liu, Y.; Gao, Y.; Yuan, B.; Qi, X.; Fu, Y.; Zhu, Q.; Cao, T.; Zhang, S.; Yin, L.; et al. USP7 Is a Novel Deubiquitinase Sustaining PLK1 Protein Stability and Regulating Chromosome Alignment in Mitosis. J. Exp. Clin. Cancer Res. 2019, 38, 468. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhao, H.; Yu, C.; Kang, Y.; Yang, X. Targeting Ubiquitin-Specific Protease 7 (USP7) in Cancer: A New Insight to Overcome Drug Resistance. Front. Pharmacol. 2021, 12, 648491. [Google Scholar] [CrossRef]
- Bushman, J.W.; Donovan, K.A.; Schauer, N.J.; Liu, X.; Hu, W.; Varca, A.C.; Buhrlage, S.J.; Fischer, E.S. Proteomics-Based Identification of DUB Substrates Using Selective Inhibitors. Cell Chem. Biol. 2021, 28, 78–87.e3. [Google Scholar] [CrossRef]
- Altun, M.; Kramer, H.B.; Willems, L.I.; McDermott, J.L.; Leach, C.A.; Goldenberg, S.J.; Kumar, K.G.S.; Konietzny, R.; Fischer, R.; Kogan, E.; et al. Activity-Based Chemical Proteomics Accelerates Inhibitor Development for Deubiquitylating Enzymes. Chem. Biol. 2011, 18, 1401–1412. [Google Scholar] [CrossRef]
- Kategaya, L.; Di Lello, P.; Rougé, L.; Pastor, R.; Clark, K.R.; Drummond, J.; Kleinheinz, T.; Lin, E.; Upton, J.-P.; Prakash, S.; et al. USP7 Small-Molecule Inhibitors Interfere with Ubiquitin Binding. Nature 2017, 550, 534–538. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Miyazaki, M.; Kodrasov, M.P.; Rotinsulu, H.; Losung, F.; Mangindaan, R.E.P.; De Voogd, N.J.; Yokosawa, H.; Nicholson, B.; Tsukamoto, S. Spongiacidin C, a Pyrrole Alkaloid from the Marine Sponge Stylissa Massa, Functions as a USP7 Inhibitor. Bioorganic Med. Chem. Lett. 2013, 23, 3884–3886. [Google Scholar] [CrossRef]
- Tanokashira, N.; Kukita, S.; Kato, H.; Nehira, T.; Angkouw, E.D.; Mangindaan, R.E.P.; De Voogd, N.J.; Tsukamoto, S. Petroquinones: Trimeric and Dimeric Xestoquinone Derivatives Isolated from the Marine Sponge Petrosia alfiani. Tetrahedron 2016, 72, 5530–5540. [Google Scholar] [CrossRef]
- Afifi, A.H.; Kagiyama, I.; El-Desoky, A.H.; Kato, H.; Mangindaan, R.E.P.; De Voogd, N.J.; Ammar, N.M.; Hifnawy, M.S.; Tsukamoto, S. Sulawesins A–C, Furanosesterterpene Tetronic Acids That Inhibit USP7, from a Psammocinia sp. Marine Sponge. J. Nat. Prod. 2017, 80, 2045–2050. [Google Scholar] [CrossRef]
- Weinstock, J.; Wu, J.; Cao, P.; Kingsbury, W.D.; McDermott, J.L.; Kodrasov, M.P.; McKelvey, D.M.; Suresh Kumar, K.G.; Goldenberg, S.J.; Mattern, M.R.; et al. Selective Dual Inhibitors of the Cancer-Related Deubiquitylating Proteases USP7 and USP47. ACS Med. Chem. Lett. 2012, 3, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Leonhardt, H.; Spada, F. Usp7 and Uhrf1 Control Ubiquitination and Stability of the Maintenance DNA Methyltransferase Dnmt1. J. Cell. Biochem. 2011, 112, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Song, J.; Wang, J.; Xu, C.; Chen, C.; Gu, W.; Sun, H.; Wen, X. Synthesis and Biological Evaluation of Thiazole Derivatives as Novel USP7 Inhibitors. Bioorganic Med. Chem. Lett. 2017, 27, 845–849. [Google Scholar] [CrossRef] [PubMed]
- Pozhidaeva, A.; Valles, G.; Wang, F.; Wu, J.; Sterner, D.E.; Nguyen, P.; Weinstock, J.; Kumar, K.G.S.; Kanyo, J.; Wright, D.; et al. USP7-Specific Inhibitors Target and Modify the Enzyme’s Active Site via Distinct Chemical Mechanisms. Cell Chem. Biol. 2017, 24, 1501–1512.e5. [Google Scholar] [CrossRef]
- Wang, F.; Wang, L.; Wu, J.; Sokirniy, I.; Nguyen, P.; Bregnard, T.; Weinstock, J.; Mattern, M.; Bezsonova, I.; Hancock, W.W.; et al. Active Site-Targeted Covalent Irreversible Inhibitors of USP7 Impair the Functions of Foxp3+ T-Regulatory Cells by Promoting Ubiquitination of Tip60. PLoS ONE 2017, 12, e0189744. [Google Scholar] [CrossRef]
- Turnbull, A.P.; Ioannidis, S.; Krajewski, W.W.; Pinto-Fernandez, A.; Heride, C.; Martin, A.C.L.; Tonkin, L.M.; Townsend, E.C.; Buker, S.M.; Lancia, D.R.; et al. Molecular Basis of USP7 Inhibition by Selective Small-Molecule Inhibitors. Nature 2017, 550, 481–486. [Google Scholar] [CrossRef]
- Li, M.; Liu, S.; Chen, H.; Zhou, X.; Zhou, J.; Zhou, S.; Yuan, H.; Xu, Q.-L.; Liu, J.; Cheng, K.; et al. N-Benzylpiperidinol Derivatives as Novel USP7 Inhibitors: Structure–Activity Relationships and X-Ray Crystallographic Studies. Eur. J. Med. Chem. 2020, 199, 112279. [Google Scholar] [CrossRef]
- Cheng, Y.-J.; Zhuang, Z.; Miao, Y.-L.; Song, S.-S.; Bao, X.-B.; Yang, C.-H.; He, J.-X. Identification of YCH2823 as a Novel USP7 Inhibitor for Cancer Therapy. Biochem. Pharmacol. 2024, 222, 116071. [Google Scholar] [CrossRef]
- Lamberto, I.; Liu, X.; Seo, H.-S.; Schauer, N.J.; Iacob, R.E.; Hu, W.; Das, D.; Mikhailova, T.; Weisberg, E.L.; Engen, J.R.; et al. Structure-Guided Development of a Potent and Selective Non-Covalent Active-Site Inhibitor of USP7. Cell Chem. Biol. 2017, 24, 1490–1500.e11. [Google Scholar] [CrossRef]
- Gavory, G.; O’Dowd, C.R.; Helm, M.D.; Flasz, J.; Arkoudis, E.; Dossang, A.; Hughes, C.; Cassidy, E.; McClelland, K.; Odrzywol, E.; et al. Discovery and Characterization of Highly Potent and Selective Allosteric USP7 Inhibitors. Nat. Chem. Biol. 2018, 14, 118–125. [Google Scholar] [CrossRef]
- Ohol, Y.M.; Sun, M.T.; Cutler, G.; Leger, P.R.; Hu, D.X.; Biannic, B.; Rana, P.; Cho, C.; Jacobson, S.; Wong, S.T.; et al. Novel, Selective Inhibitors of USP7 Uncover Multiple Mechanisms of Antitumor Activity In Vitro and In Vivo. Mol. Cancer Ther. 2020, 19, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- Colland, F.; Formstecher, E.; Jacq, X.; Reverdy, C.; Planquette, C.; Conrath, S.; Trouplin, V.; Bianchi, J.; Aushev, V.N.; Camonis, J.; et al. Small-Molecule Inhibitor of USP7/HAUSP Ubiquitin Protease Stabilizes and Activates P53 in Cells. Mol. Cancer Ther. 2009, 8, 2286–2295. [Google Scholar] [CrossRef] [PubMed]
- Reverdy, C.; Conrath, S.; Lopez, R.; Planquette, C.; Atmanene, C.; Collura, V.; Harpon, J.; Battaglia, V.; Vivat, V.; Sippl, W.; et al. Discovery of Specific Inhibitors of Human USP7/HAUSP Deubiquitinating Enzyme. Chem. Biol. 2012, 19, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Wertz, I.E.; Murray, J.M. Structurally-Defined Deubiquitinase Inhibitors Provide Opportunities to Investigate Disease Mechanisms. Drug Discov. Today: Technol. 2019, 31, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Hu, M.; Yang, Y.; Du, C.; Zhou, H.; Liu, C.; Chen, Y.; Fan, L.; Ma, H.; Gong, Y.; et al. An Overview of PROTACs: A Promising Drug Discovery Paradigm. Mol. Biomed. 2022, 3, 46. [Google Scholar] [CrossRef] [PubMed]
- Békés, M.; Langley, D.R.; Crews, C.M. PROTAC Targeted Protein Degraders: The Past Is Prologue. Nat. Rev. Drug Discov. 2022, 21, 181–200. [Google Scholar] [CrossRef]
- Chamberlain, P.P.; Hamann, L.G. Development of Targeted Protein Degradation Therapeutics. Nat. Chem. Biol. 2019, 15, 937–944. [Google Scholar] [CrossRef]
- Murgai, A.; Sosič, I.; Gobec, M.; Lemnitzer, P.; Proj, M.; Wittenburg, S.; Voget, R.; Gütschow, M.; Krönke, J.; Steinebach, C. Targeting the Deubiquitinase USP7 for Degradation with PROTACs. Chem. Commun. 2022, 58, 8858–8861. [Google Scholar] [CrossRef]
- Pei, Y.; Fu, J.; Shi, Y.; Zhang, M.; Luo, G.; Luo, X.; Song, N.; Mi, T.; Yang, Y.; Li, J.; et al. Discovery of a Potent and Selective Degrader for USP7. Angew. Chem. Int. Ed. 2022, 61, e202204395. [Google Scholar] [CrossRef]
- Inaba, K.; Sato, H.; Tsuda, M.; Kobayashi, J. Spongiacidins A−D, New Bromopyrrole Alkaloids from Hymeniacidon Sponge. J. Nat. Prod. 1998, 61, 693–695. [Google Scholar] [CrossRef] [PubMed]
- Kato, H. Structural Analysis of Previously Unknown Natural Products Using Computational Methods. J. Nat. Med. 2022, 76, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Xu, Z.; Chen, X.; Meng, Q.; Zhou, H.; Xiong, B.; Zhang, N. Sertraline and Astemizole Enhance the Deubiquitinase Activity of USP7 by Binding to Its Switching Loop Region. J. Med. Chem. 2025, 68, 5874–5890. [Google Scholar] [CrossRef] [PubMed]
- Jaen Maisonet, I.; Sharafi, M.; Korchak, E.J.; Salazar-Chaparro, A.; Bratt, A.; Parikh, T.; Varca, A.C.; Shah, B.; Darnowski, M.; Chung, M.; et al. Small-Molecule Allosteric Activator of Ubiquitin-Specific Protease 7 (USP7). bioRxiv 2025. [Google Scholar] [CrossRef]
- Korchak, E.J.; Sharafi, M.; Jaen Maisonet, I.; Salazar-Chaparro, A.; Semenova, I.V.; Khan, H.; O’Neil, A.L.; Caro, P.; Schaaf, C.P.; Buhrlage, S.J.; et al. Functional Spectrum of USP7 Pathogenic Variants in Hao–Fountain Syndrome: Insights into the Enzyme’s Activity, Stability, and Allosteric Modulation. Proc. Natl. Acad. Sci. USA 2025, 122, e2510252122. [Google Scholar] [CrossRef]
- Ojedele, O.A.; Umar, H.I.; Baammi, S.; Metouekel, A.; Mengistie, A.A.; Bin Jardan, Y.A.; Shazly, G.A.; Victor, O. Cheminformatics-Aided Discovery of Potential Allosteric Site Modulators of Ubiquitin-Specific Protease 7. Sci. Rep. 2024, 14, 24995. [Google Scholar] [CrossRef]
- Javaid, S.; Zadi, S.; Awais, M.; Wahab, A.; Zafar, H.; Maslennikov, I.; Choudhary, M.I. Identification of New Leads against Ubiquitin Specific Protease-7 (USP7): A Step towards the Potential Treatment of Cancers. RSC Adv. 2024, 14, 33080–33093. [Google Scholar] [CrossRef]
- Song, Y.; Ren, X.; Xiong, J.; Wang, W.; Zhao, Q.; Chang, J.; Yu, B. Ubiquitin-Specific Protease 7 (USP7) as a Promising Therapeutic Target for Drug Discovery: From Mechanisms to Therapies. J. Med. Chem. 2025, 68, 7914–7931. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).