Natural Tyrosinase Inhibitors from Lycopodium japonicum
Abstract
1. Introduction
2. Results
2.1. Structure Elucidation of the Isolates
2.2. Tyrosinase Inhibitory Bioassays
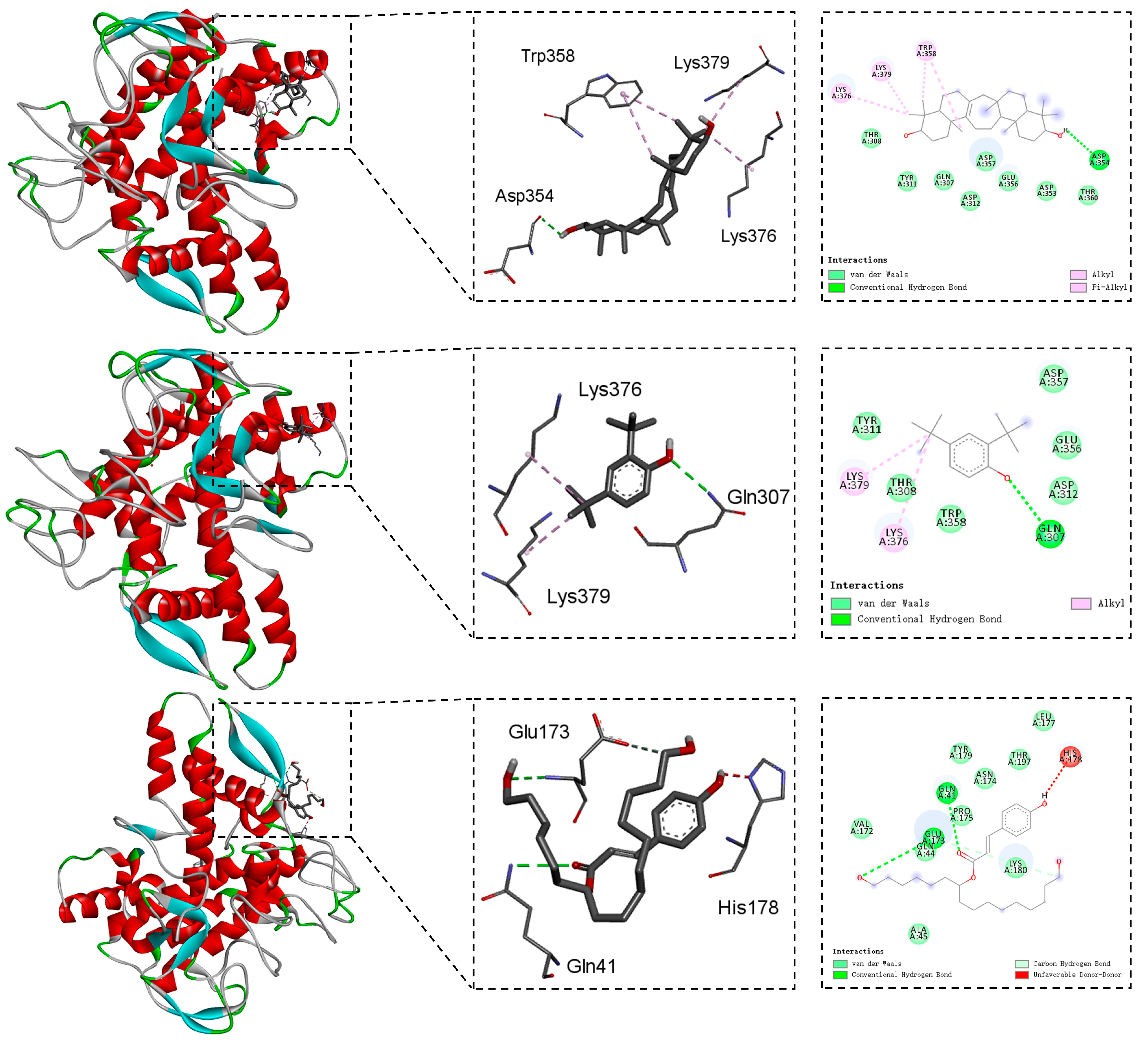
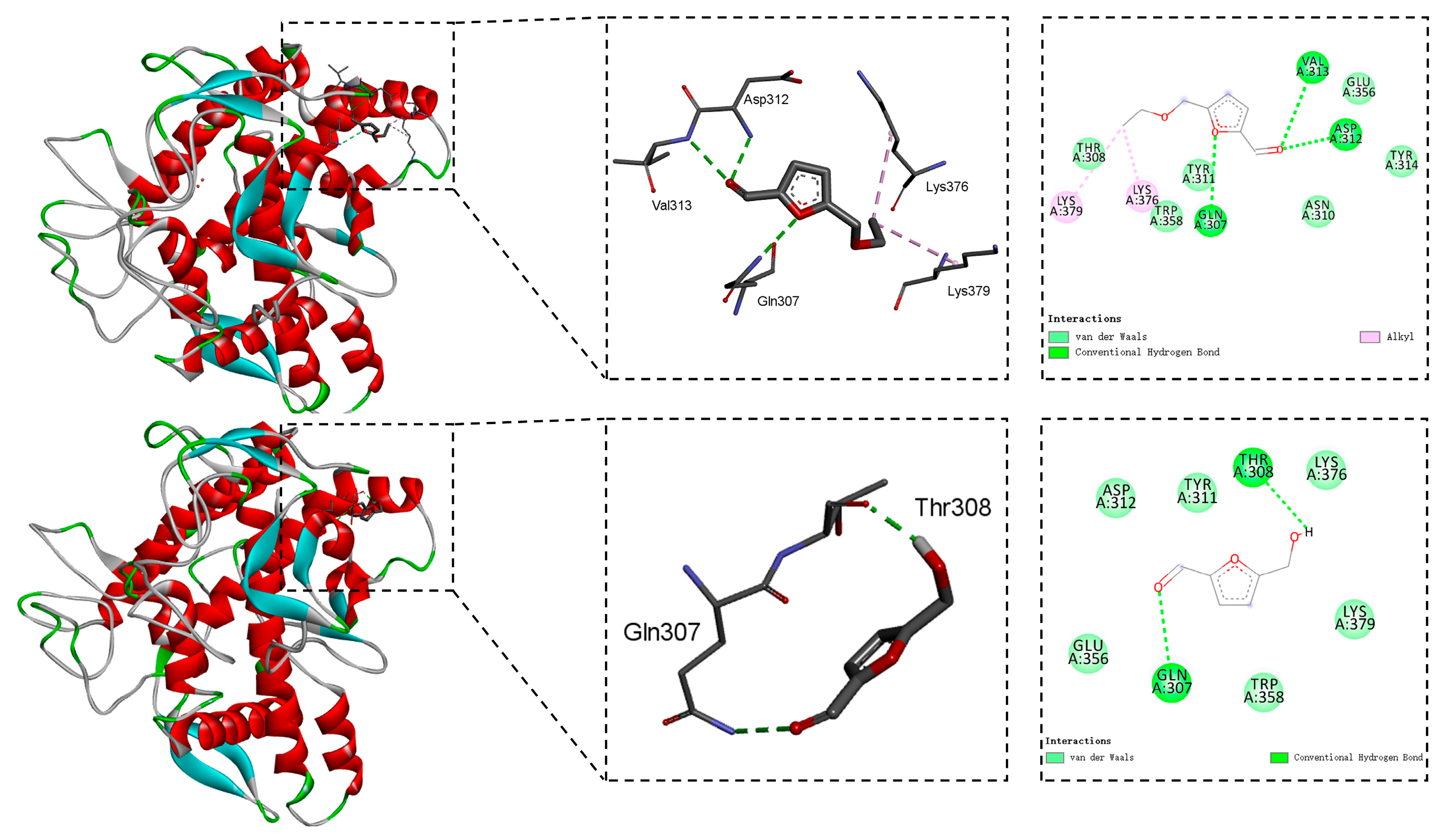
2.3. Molecular Docking Studies
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Characteristic 1H and 13C NMR Spectral Data of Isolates
3.5. In Vitro Tyrosinase Inhibitory Bioassay
3.6. Molecular Docking Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chang, T.-S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef]
- Arrowitz, C.; Schoelermann, A.M.; Mann, T.; Jiang, L.I.; Weber, T.; Kolbe, L. Effective tyrosinase inhibition by Thiamidol results in significant improvement of mild to moderate melasma. J. Investig. Dermatol. 2019, 139, 1691–1698. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Labay, C.; Hosta-Rigau, L. Tyrosinase-loaded multicompartment microreactor toward melanoma depletion. ACS Appl. Mater. Interfaces 2019, 11, 5862–5876. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef] [PubMed]
- Boonya-udtayan, S.; Thasana, N.; Jarussophon, N.; Ruchirawat, S. Serratene triterpenoids and their biological activities from Lycopodiaceae plants. Fitoterapia 2019, 136, 104181. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, Q.; Zhang, Y. Lycopodium japonicum: A comprehensive review on its phytochemicals and biological activities. Arab. J. Chem. 2020, 13, 5438–5450. [Google Scholar] [CrossRef]
- Xie, Z.; Liu, Y.; Luo, Q.; Wang, Q.; Min, Y.-B.; Liu, L.; Zheng, D.-K. New Lycopodium alkaloids with neuroprotective activities from Lycopodium japonicum Thunb. Nat. Prod. Res. 2025, 39, 718–724. [Google Scholar] [CrossRef]
- Xia, D.; Wang, Z.-H.; Jiang, J.-M.; Yang, X.-W.; Gao, Y.; Xu, Y.-Y.; Chang, L.-Y.; Zhu, D.; Zhao, B.-J.; Zhu, X.-L.; et al. Lycojapomines A–E: Lycopodium alkaloids with anti-renal fibrosis potential from Lycopodium japonicum. Org. Lett. 2022, 24, 4684–4688. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Dong, L.-B.; Zhang, Z.-J.; Fan, M.; Zhu, Q.-F.; Qi, Y.-Y.; Liu, Y.-C.; Peng, L.-Y.; Wu, X.-D.; Zhao, Q.-S. Three new Lycopodium alkaloids from Lycopodium japonicum. J. Asian Nat. Prod. Res. 2019, 21, 17–24. [Google Scholar] [CrossRef]
- Wang, X.-J.; Zhang, G.-J.; Zhuang, P.-Y.; Zhang, Y.; Yu, S.-S.; Bao, X.-Q.; Zhang, D.; Yuan, Y.-H.; Chen, N.-H.; Ma, S.-G.; et al. Lycojaponicumins A–C, three alkaloids with an unprecedented skeleton from Lycopodium japonicum. Org. Lett. 2012, 14, 2614–2617. [Google Scholar] [CrossRef]
- Sun, Z.-H.; Li, W.; Tang, G.-H.; Yin, S. A new serratene triterpenoid from Lycopodium japonicum. J. Asian Nat. Prod. Res. 2017, 19, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yi, P.; Chen, Y.; Mei, Z.-n.; Hu, X.; Yang, G.-Z. Lycojaponicuminol A–F: Cytotoxic serratene triterpenoids from Lycopodium japonicum. Fitoterapia 2014, 96, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, D.; Yu, S. Two new triterpenoids from Lycopodium japonicum Thunb. Chin. J. Chem. 2014, 32, 1007–1010. [Google Scholar] [CrossRef]
- Li, X.-L.; Zhao, Y.; Cheng, X.; Tu, L.; Peng, L.-Y.; Xu, G.; Zhao, Q.-S. Japonicumins A–D: Four new compounds from Lycopodium japonicum. Helv. Chim. Acta 2006, 89, 1467–1473. [Google Scholar] [CrossRef]
- Ren, Q.; Zou, Z.; Liu, Y.; Chen, X.; Xu, K.; Tan, G. Two new lignans from Lycopodium japonicum Thunb. Rec. Nat. Prod. 2019, 13, 468–474. [Google Scholar] [CrossRef]
- Li, M.-J.; Liu, J.; Zhang, Y.-B.; Chen, N.-H.; Wang, G.-C.; Li, Y.-L. Chemical constituents from whole herb of Lycopodium japonicum. Chin. Tradit. Herb. Drugs 2015, 46, 33–37. [Google Scholar] [CrossRef]
- Wang, X.; Wang, F.; Wu, J.; Chen, S.-Q.; Jiang, C.-S.; Yang, S.-P.; Wang, C.; Cai, Y.-S. Japonisine A, a fawcettimine-type Lycopodium alkaloid with an unusual skeleton from Lycopodium japonicum Thunb. Fitoterapia 2022, 156, 105069. [Google Scholar] [CrossRef]
- He, J.; Chen, X.-Q.; Li, M.-M.; Zhao, Y.; Xu, G.; Cheng, X.; Peng, L.-Y.; Xie, M.-J.; Zheng, Y.-T.; Wang, Y.-P.; et al. Lycojapodine A, a novel alkaloid from Lycopodium japonicum. Org. Lett. 2009, 11, 1397–1400. [Google Scholar] [CrossRef]
- Wang, X.-J.; Li, L.; Si, Y.-K.; Yu, S.-S.; Ma, S.-G.; Bao, X.-Q.; Zhang, D.; Qu, J.; Liu, Y.-B.; Li, Y. Nine new lycopodine-type alkaloids from Lycopodium japonicum Thunb. Tetrahedron 2013, 69, 6234–6240. [Google Scholar] [CrossRef]
- Wang, X.-J.; Liu, Y.-B.; Li, L.; Yu, S.-S.; Lv, H.-N.; Ma, S.-G.; Bao, X.-Q.; Zhang, D.; Qu, J.; Li, Y. Lycojaponicumins D and E: Two new alkaloids from Lycopodium japonicum. Org. Lett. 2012, 14, 5688–5691. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhu, Y.; Zhan, R.; Chen, Y.-g. A new fawcettimine-related alkaloid from Lycopodium japonicum. Chem. Nat. Compd. 2018, 54, 729–731. [Google Scholar] [CrossRef]
- Bu, Q.; Yang, Q.-B.; Ge, Z.-Y.; Shi, Z.-X.; Liang, L.-F. Phytochemicals and health-promoting evaluations of Ophiorrhiza puffii, a Chinese endemic plant. Nat. Prod. Res. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Bu, Q.; Ge, Z.-Y.; Liang, L.-F. Chemical and biological investigation on the potential ornamental plant Ophiorrhiza chinensis. Agronomy 2024, 14, 1872. [Google Scholar] [CrossRef]
- Yang, G.-X.; Zang, Y.; Hu, C.-L.; Xiong, J.; Hu, J.-F. Serratene triterpenoids in Lycopodium japonicum from Hubei province. Chin. Tradit. Herb. Drugs 2014, 45, 3524–3527. [Google Scholar] [CrossRef]
- Sa, N.H.; Tam, N.T.; Quan, T.D.; Tinh, B.X.; Thien, D.D.; Sung, T.V.; Adorisio, S.; Delfino, D.V. Chemical constituents and their biological activity of Pinus dalatensis. Part 1. Terpenoids from the leaves. Vietnam J. Chem. 2017, 55, 509–513. [Google Scholar] [CrossRef]
- Zheng, K.-K.; Zhao, Y.-Y.; Yuan, P.-L.; Wu, Y.-C.; Zhang, L.-Q.; Guo, F.-J.; Li, Y.-M. Chemical constituents of non-alkaloids from Huperzia serrata. Chin. Tradit. Herb. Drugs 2016, 47, 15–20. [Google Scholar] [CrossRef]
- Tsuda, Y.; Isobe, K.; Sano, T. Triterpenoid chemistry. IX. Lycopodium triterpenoid. (6). The structures of three new tetra-ols, lycocryptol, 21-epilycocryptol, and diepilycocryptol, and two new acids, lycernuic acid-A and -B. Chem. Pharm. Bull. 1975, 23, 264–271. [Google Scholar] [CrossRef]
- Zhang, Z.; ElSohly, H.N.; Jacob, M.R.; Pasco, D.S.; Walker, L.A.; Clark, A.M. Natural products inhibiting Candida albicans secreted aspartic proteases from Lycopodium cernuum. J. Nat. Prod. 2002, 65, 979–985. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, W.-Y.; Chen, W.-J.; Wei, J.-H.; Liang, C.-Y.; Feng, X.; Lu, C.-S. Chemical constituents and antioxidant activity in vitro of Zhuang medicine Dalbergia rimosa. J. Chin. Med. Mater. 2024, 47, 1147–1152. [Google Scholar] [CrossRef]
- Teng, X.-F.; Liu, Y.-L.; Yang, F.; Wang, H.-J.; He, L. Chemical constituents from Lycopodium fargesii and their anti-osteoporotic activity. Nat. Prod. Res. Dev. 2022, 34, 226–231. [Google Scholar] [CrossRef]
- Pal, P.P.; Begum, S.A.; Basha, A.S.; Araya, H.; Fujimoto, Y. A new lignan (polonilignan) and inhibitors of nitric oxide production from Penicillium polonicum, an endophytic fungi of Piper nigrum. Chem. Biodivers. 2023, 20, e202200840. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.-X.; Sun, J.-Y.; Liu, C.; Zhang, J.-S.; Zhang, H. Antiradical aromatic constituents from Pleurotus eryngii. Rec. Nat. Prod. 2021, 15, 169–174. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, B.-P.; Zhang, W.-J.; Yang, G.-L.; Zhang, C.-L.; Cao, Z.-Y. Chemical constituents from aerial parts of Ribes mandshuricum. Chin. Tradit. Herb. Drugs 2018, 49, 772–779. [Google Scholar] [CrossRef]
- Cho, J.-Y.; Kim, M.-S.; Lee, Y.G.; Jeong, H.Y.; Lee, H.J.; Ham, K.-S.; Moon, J.-H. A phenyl lipid alkaloid and flavone C-diglucosides from Spergularia marina. Food Sci. Biotechnol. 2016, 25, 63–69. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, J.K.; Yu, J.S.; Jeong, S.Y.; Choi, J.H.; Kim, J.-C.; Ko, Y.-J.; Kim, S.-H.; Kim, K.H. Ginkwanghols A and B, osteogenic coumaric acid-aliphatic alcohol hybrids from the leaves of Ginkgo biloba. Arch. Pharm. Res. 2021, 44, 514–524. [Google Scholar] [CrossRef]
- Bredihhin, A.; Mäeorg, U.; Vares, L. Evaluation of carbohydrates and lignocellulosic biomass from different wood species as raw material for the synthesis of 5-bromomethyfurfural. Carbohydr. Res. 2013, 375, 63–67. [Google Scholar] [CrossRef]
- Miyazawa, M.; Anzai, J.; Fujioka, J.; Isikawa, Y. Insecticidal compounds against Drosophila melanogaster from Cornus officinalis Sieb. Et Zucc. Nat. Prod. Res. 2003, 17, 337–339. [Google Scholar] [CrossRef]
- Yang, C. On the nomenclature revision of the Chinese drug shisong and its resources. Zhongyao Tongbao 1981, 6, 12–15. [Google Scholar]
- Yan, J.; Zhang, X.-M.; Li, Z.-R.; Zhou, L.; Chen, J.-C.; Sun, L.-R.; Qiu, M.-H. Three new triterpenoids from Lycopodium japonicum Thunb. Helv. Chim. Acta 2005, 88, 240–244. [Google Scholar] [CrossRef]
- Teng, C.; He, Y.; Feng, J.; Ba, X.; Li, D. Chemical composition of Lycopodium japonicum. Chin. Tradit. Herb. Drugs 2010, 41, 1960–1963. [Google Scholar]
- Zhu, J.-Y.; Ge, Z.-Y.; Yang, Q.-B.; Jiang, C.-F.; Wu, L.; Jiang, X.-Y.; Liang, L.-F. Bioassay-guided isolation of chemical constituents from Lycopodiastrum casuarinoides and targeted evaluation of their potential efficacy in cosmetics. Cosmetics 2025, 12, 174. [Google Scholar] [CrossRef]
- Ashraf, Z.; Rafiq, M.; Nadeem, H.; Hassan, M.; Afzal, S.; Waseem, M.; Afzal, K.; Latip, J. Carvacrol derivatives as mushroom tyrosinase inhibitors; synthesis, kinetics mechanism and molecular docking studies. PLoS ONE 2017, 12, e0178069. [Google Scholar] [CrossRef] [PubMed]
- Ismaya, W.T.; Rozeboom, H.J.; Weijn, A.; Mes, J.J.; Fusetti, F.; Wichers, H.J.; Dijkstra, B.W. Crystal structure of Agaricus bisporus mushroom tyrosinase: Identity of the tetramer subunits and interaction with tropolone. Biochemistry 2011, 50, 5477–5486. [Google Scholar] [CrossRef] [PubMed]
- Souza, P.M.; Elias, S.T.; Simeoni, L.A.; de Paula, J.E.; Gomes, S.M.; Guerra, E.N.S.; Fonseca, Y.M.; Silva, E.C.; Silveira, D.; Magalhães, P.O. Plants from Brazilian Cerrado with potent tyrosinase inhibitory activity. PLoS ONE 2012, 7, e48589. [Google Scholar] [CrossRef]


| Compounds | Names | References |
|---|---|---|
| 1 | 16-oxo-3α-hydroxyserrat-14-en-21β-ol | [16] |
| 2 | 21-epi-serratenediol | [24] |
| 3 | 3β-hydroxy-14-serraten-21-one | [25] |
| 4 | serratenediol | [24,26] |
| 5 | methyl lycernuate A | [27,28] |
| 6 | α-onocerin | [29] |
| 7 | 26-nor-8-oxo-α-onocerin | [30] |
| 8 | β-stiosterol | [23] |
| 9 | trans-ethyl ferulate | [31] |
| 10 | cis-ethyl ferulate | [31,32] |
| 11 | ethyl 4-hydroxy-3-methoxybenzoate | [33] |
| 12 | 2,4-di-tert-butylphenol | [34] |
| 13 | ginkwanghol A | [35] |
| 14 | 5-ethoxymethylfurfural | [36] |
| 15 | 5-hydroxymethyl furfural | [37] |
| Compounds | IC50 (mM) |
|---|---|
| 4 | 2.16 ± 0.03 |
| 12 | 3.28 ± 0.03 |
| 13 | 1.46 ± 0.03 |
| 14 | 5.92 ± 0.06 |
| 15 | 6.82 ± 0.09 |
| Kojic acid 1 | 0.17 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, Z.-Y.; Wang, Y.-Q.; Yang, Q.-B.; Yan, X.-Y.; Wu, L.; Zhang, M.; Liang, L.-F. Natural Tyrosinase Inhibitors from Lycopodium japonicum. Molecules 2025, 30, 4024. https://doi.org/10.3390/molecules30194024
Ge Z-Y, Wang Y-Q, Yang Q-B, Yan X-Y, Wu L, Zhang M, Liang L-F. Natural Tyrosinase Inhibitors from Lycopodium japonicum. Molecules. 2025; 30(19):4024. https://doi.org/10.3390/molecules30194024
Chicago/Turabian StyleGe, Zeng-Yue, Ya-Qing Wang, Qi-Bin Yang, Xian-Yun Yan, Lei Wu, Min Zhang, and Lin-Fu Liang. 2025. "Natural Tyrosinase Inhibitors from Lycopodium japonicum" Molecules 30, no. 19: 4024. https://doi.org/10.3390/molecules30194024
APA StyleGe, Z.-Y., Wang, Y.-Q., Yang, Q.-B., Yan, X.-Y., Wu, L., Zhang, M., & Liang, L.-F. (2025). Natural Tyrosinase Inhibitors from Lycopodium japonicum. Molecules, 30(19), 4024. https://doi.org/10.3390/molecules30194024








