Interface Catalysts of In Situ-Grown TiO2/MXenes for High-Faraday-Efficiency CO2 Reduction
Abstract
1. Introduction
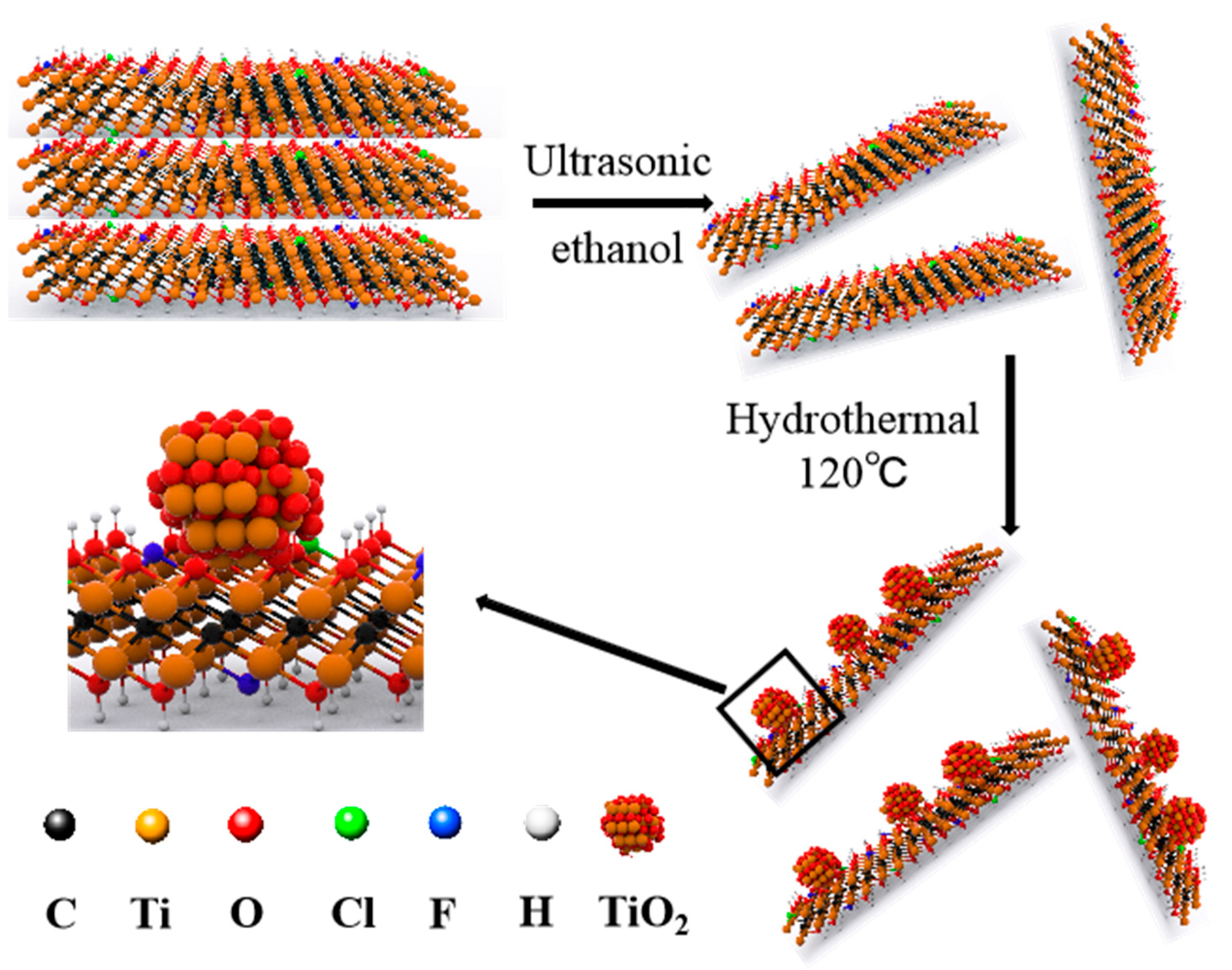
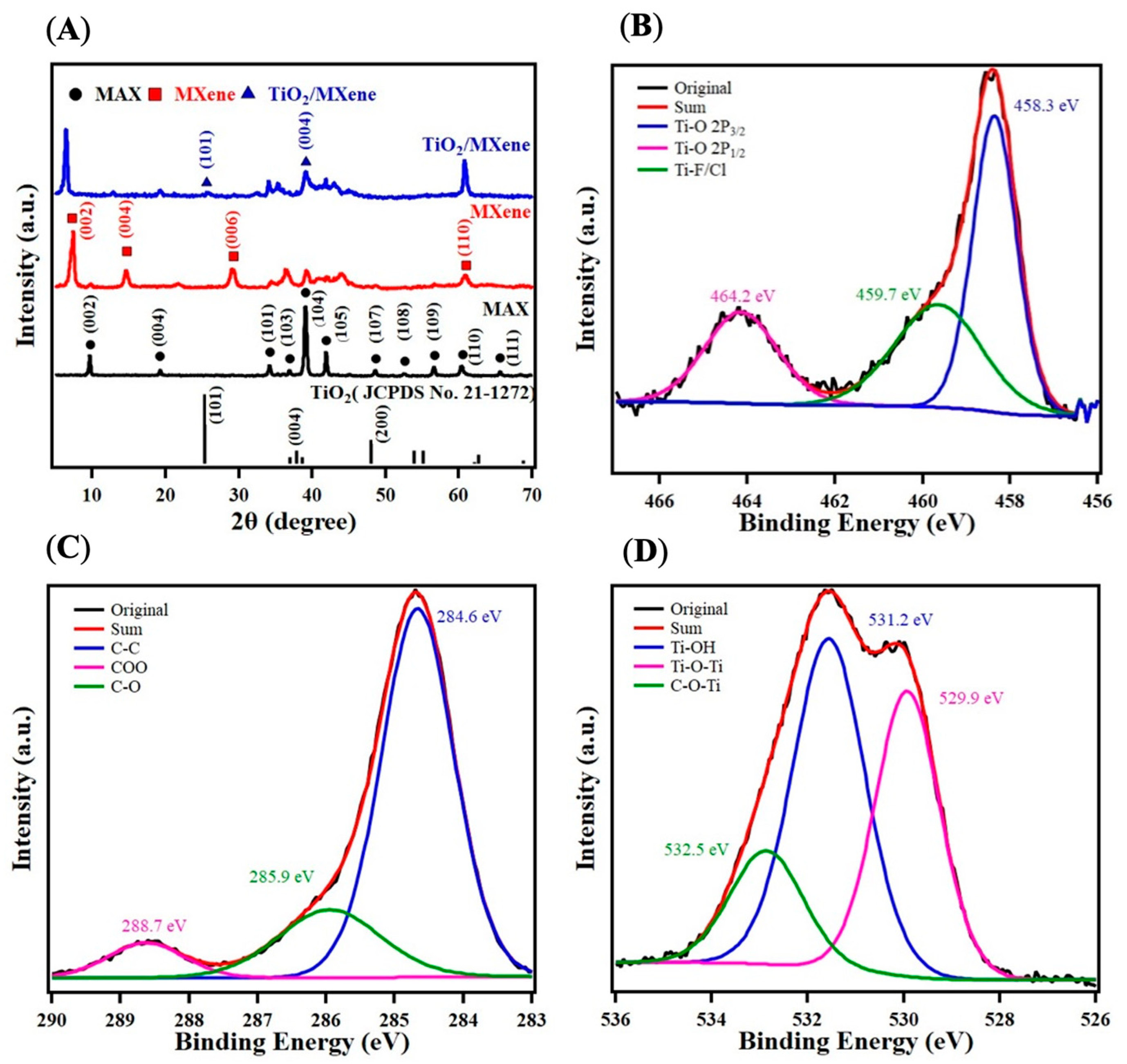
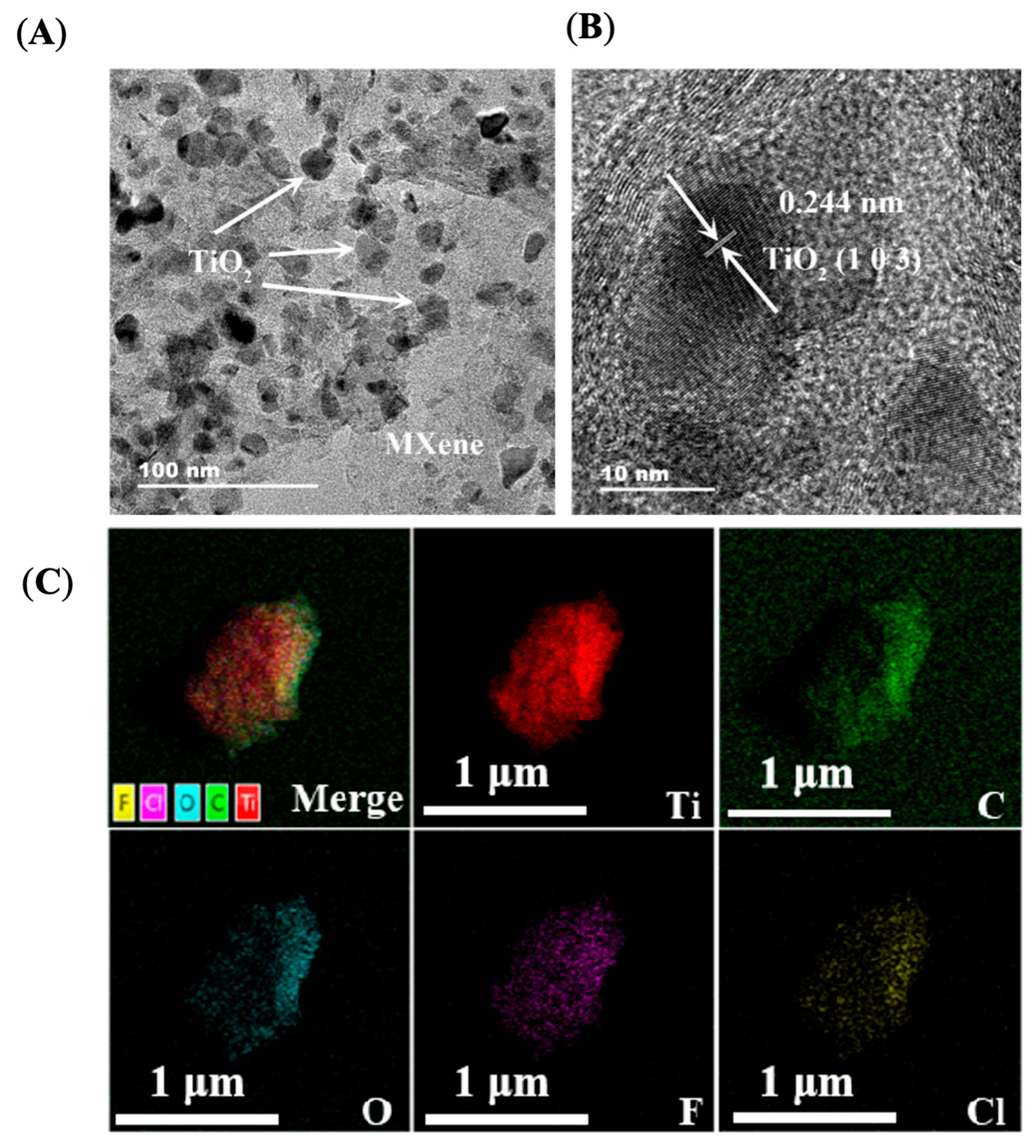
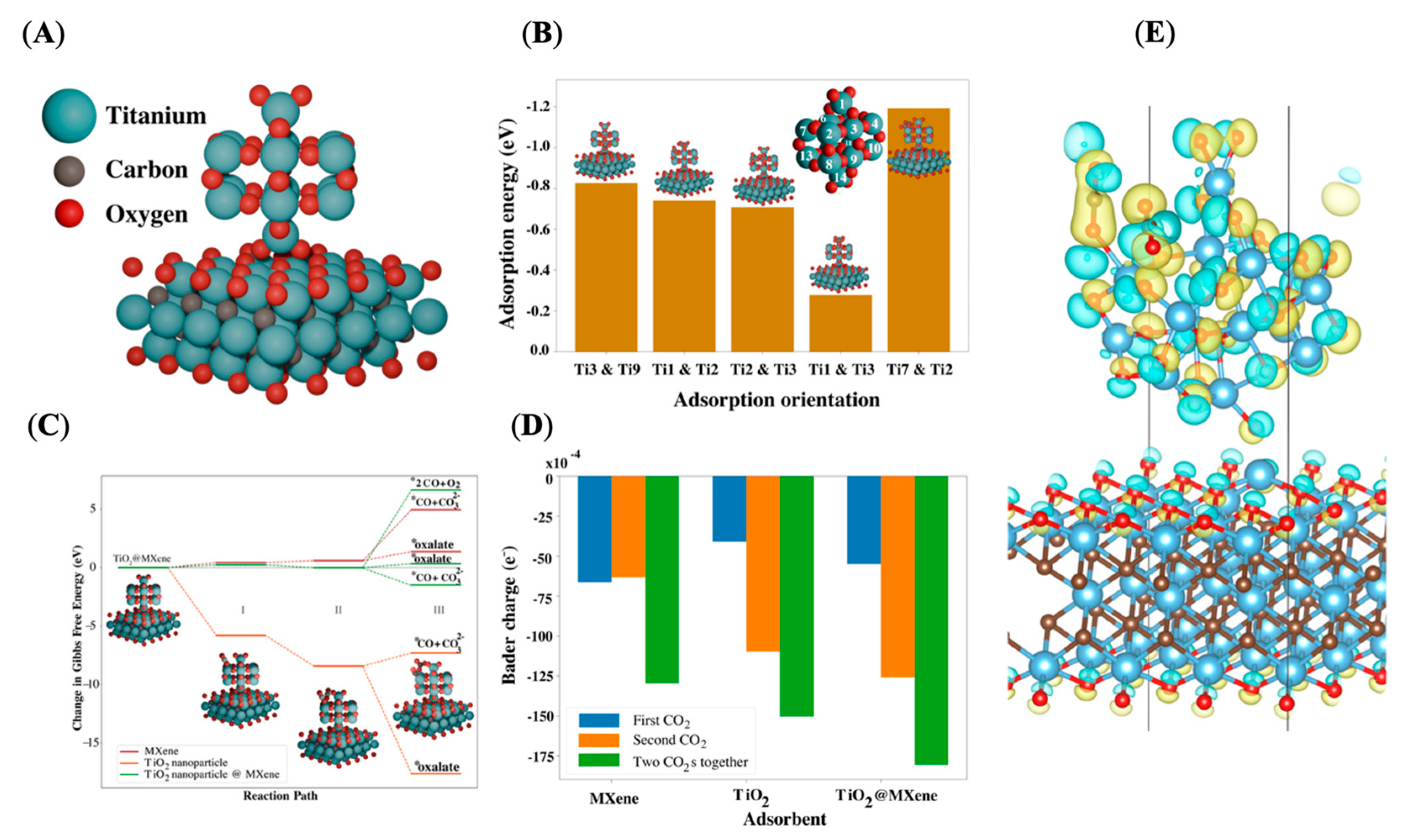
2. Results and Discussion
3. Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Meng, A.; Zhang, L.; Cheng, B.; Yu, J. Dual Cocatalysts in TiO2 Photocatalysis. Adv. Mater. 2019, 31, 1807660. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Peiris, S.; de Silva, H.B.; Ranasinghe, K.N.; Bandara, S.V.; Perera, I.R. Recent development and future prospects of TiO2 photocatalysis. J. Chin. Chem. Soc. 2021, 68, 738–769. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Park, S.-J. TiO2 photocatalyst for water treatment applications. J. Ind. Eng. Chem. 2013, 19, 1761–1769. [Google Scholar] [CrossRef]
- Park, H.; Park, Y.; Kim, W.; Choi, W. Surface modification of TiO2 photocatalyst for environmental applications. J. Photochem. Photobiol. C-Photochem. Rev. 2013, 15, 1–20. [Google Scholar] [CrossRef]
- Paz, Y. Application of TiO2 photocatalysis for air treatment: Patents’ overview. Appl. Catal. B-Environ. 2010, 99, 448–460. [Google Scholar] [CrossRef]
- Guo, Q.; Ma, Z.; Zhou, C.; Ren, Z.; Yang, X. Single Molecule Photocatalysis on TiO2 Surfaces. Chem. Rev. 2019, 119, 11020–11041. [Google Scholar] [CrossRef] [PubMed]
- Thompson, T.L.; Yates, J.T. Surface Science Studies of the Photoactivation of TiO2 New Photochemical Processes. Chem. Rev. 2006, 106, 4428–4453. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- He, Y.; Tilocca, A.; Dulub, O.; Selloni, A.; Diebold, U. Local ordering and electronic signatures of submonolayer water on anatase TiO2(101). Nat. Mater. 2009, 8, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Kershis, M.D.; Wilson, D.P.; White, M.G. Dynamics of acetone photooxidation on TiO2(110): State-resolved measurements of methyl photoproducts. J. Chem. Phys. 2013, 138, 204703. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Yoshikawa, H.; Awaga, K.; Murayama, M.; Mori, T.; Sunada, K.; Bandow, S.; Iijima, S. Preparation, Photocatalytic Activities, and Dye-Sensitized Solar-Cell Performance of Submicron-Scale TiO2 Hollow Spheres. Langmuir 2008, 24, 547–550. [Google Scholar] [CrossRef]
- Chen, J.S.; Tan, Y.L.; Li, C.M.; Cheah, Y.L.; Luan, D.; Madhavi, S.; Boey, F.Y.C.; Archer, L.A.; Lou, X.W. Constructing hierarchical spheres from large ultrathin anatase TiO2 nanosheets with nearly 100% exposed (001) facets for fast reversible lithium storage. J. Am. Chem. Soc. 2010, 132, 6124–6130. [Google Scholar] [CrossRef]
- Zheng, Z.; Xie, W.; Lim, Z.S.; You, L.; Wang, J. CdS sensitized 3D hierarchical TiO2/ZnO heterostructure for efficient solar energy conversion. Sci. Rep. 2014, 4, 5721. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Direct Fabrication of Composite and Ceramic Hollow Nanofibers by Electrospinning. Nano Lett. 2004, 4, 933–938. [Google Scholar] [CrossRef]
- Habisreutinger, S.N.; Schmidt-Mende, L.; Stolarczyk, J.K. Photocatalytic Reduction of CO2 on TiO2 and Other Semiconductors. Angew. Chem. Int. Ed. 2013, 52, 7372–7408. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.S.; Zou, L.; Hu, E. Kinetic modelling for photosynthesis of hydrogen and methane through catalytic reduction of carbon dioxide with water vapour. Catal. Today 2008, 131, 125–129. [Google Scholar] [CrossRef]
- Kočí, K.; Obalová, L.; Šolcová, O. Kinetic study of photocatalytic reduction of CO2 over TIO2. Chem. Process Eng.-Inz. Chem. I Proces. 2010, 31, 395–407. [Google Scholar]
- Shkrob, I.A.; Zhu, Y.; Marin, T.W.; Abraham, D.P. Reduction of Carbonate Electrolytes and the Formation of Solid-Electrolyte Interface (SEI) in Lithium-Ion Batteries. 1. Spectroscopic Observations of Radical Intermediates Generated in One-Electron Reduction of Carbonates. J. Phys. Chem. C 2013, 117, 19255–19269. [Google Scholar] [CrossRef]
- Kreft, S.; Wei, D.; Junge, H.; Beller, M. Recent advances on TiO2-based photocatalytic CO2 reduction. EnergyChem 2020, 2, 100044. [Google Scholar] [CrossRef]
- Low, J.; Zhang, L.; Tong, T.; Shen, B.; Yu, J. TiO2/MXene Ti3C2 composite with excellent photocatalytic CO2 reduction activity. J. Catal. 2018, 361, 255–266. [Google Scholar] [CrossRef]
- VahidMohammadi, A.; Rosen, J.; Gogotsi, Y. The world of two-dimensional carbides and nitrides (MXenes). Science 2021, 372, eabf1581. [Google Scholar] [CrossRef] [PubMed]
- Gogotsi, Y.; Anasori, B. The Rise of MXenes. ACS Nano 2019, 13, 8491–8494. [Google Scholar] [CrossRef] [PubMed]
- Gogotsi, Y. The Future of MXenes. Chem. Mater. 2023, 35, 8767–8770. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.R.G.; Shekhirev, M.; Wyatt, B.C.; Anasori, B.; Gogotsi, Y.; Seh, Z.W. Fundamentals of MXene synthesis. Nat. Synth. 2022, 1, 601–614. [Google Scholar] [CrossRef]
- Ahn, S.; Han, T.-H.; Maleski, K.; Song, J.; Kim, Y.-H.; Park, M.-H.; Zhou, H.; Yoo, S.; Gogotsi, Y.; Lee, T.-W. A 2D Titanium Carbide MXene Flexible Electrode for High-Efficiency Light-Emitting Diodes. Adv. Mater. 2020, 32, 2000919. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Lee, K.H.; Anjum, D.H.; Sougrat, R.; Jiang, Q.; Kim, H.; Alshareef, H.N. MXenes stretch hydrogel sensor performance to new limits. Sci. Adv. 2018, 4, eaat0098. [Google Scholar] [CrossRef]
- Driscoll, N.; Richardson, A.G.; Maleski, K.; Anasori, B.; Adewole, O.; Lelyukh, P.; Escobedo, L.; Cullen, D.K.; Lucas, T.H.; Gogotsi, Y.; et al. Two-Dimensional Ti3C2 MXene for High-Resolution Neural Interfaces. ACS Nano 2018, 12, 10419–10429. [Google Scholar] [CrossRef]
- Ward, E.J.; Lacey, J.; Crua, C.; Dymond, M.K.; Maleski, K.; Hantanasirisakul, K.; Gogotsi, Y.; Sandeman, S. 2D Titanium Carbide (Ti3C2Tx) in Accommodating Intraocular Lens Design. Adv. Funct. Mater. 2020, 30, 2000841. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Li, X.; Huang, Z.; Shuck, C.E.; Liang, G.; Gogotsi, Y.; Zhi, C. MXene chemistry, electrochemistry and energy storage applications. Nat. Rev. Chem. 2022, 6, 389–404. [Google Scholar] [CrossRef]
- Nan, J.; Guo, X.; Xiao, J.; Li, X.; Chen, W.; Wu, W.; Liu, H.; Wang, Y.; Wu, M.; Wang, G. Nanoengineering of 2D MXene-Based Materials for Energy Storage Applications. Small 2021, 17, 1902085. [Google Scholar] [CrossRef]
- Xiong, D.; Li, X.; Bai, Z.; Lu, S. Recent Advances in Layered Ti3C2Tx MXene for Electrochemical Energy Storage. Small 2018, 14, 1703419. [Google Scholar] [CrossRef] [PubMed]
- Kajiyama, S.; Szabova, L.; Sodeyama, K.; Iinuma, H.; Morita, R.; Gotoh, K.; Tateyama, Y.; Okubo, M.; Yamada, A. Sodium-Ion Intercalation Mechanism in MXene Nanosheets. ACS Nano 2016, 10, 3334–3341. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Mashtalir, O.; Ren, C.E.; Dall’Agnese, Y.; Rozier, P.; Taberna, P.L.; Naguib, M.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. Cation Intercalation and High Volumetric Capacitance of Two-Dimensional Titanium Carbide. Science 2013, 341, 1502–1505. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Li, Z.; Li, G.; Hu, T.; Zhang, C.; Wang, X. All-Solid-State Flexible Fiber-Based MXene Supercapacitors. Adv. Mater. Technol. 2017, 2, 1700143. [Google Scholar] [CrossRef]
- Zhu, J.; Tang, Y.; Yang, C.; Wang, F.; Cao, M. Composites of TiO2 Nanoparticles Deposited on Ti3C2 MXene Nanosheets with Enhanced Electrochemical Performance. J. Electrochem. Soc. 2016, 163, A785. [Google Scholar] [CrossRef]
- Wang, F.; Yang, C.; Duan, M.; Tang, Y.; Zhu, J. TiO2 nanoparticle modified organ-like Ti3C2 MXene nanocomposite encapsulating hemoglobin for a mediator-free biosensor with excellent performances. Biosens. Bioelectron. 2015, 74, 1022–1028. [Google Scholar] [CrossRef]
- Xiong, Z.-M.; O’Donovan, M.; Sun, L.; Choi, J.Y.; Ren, M.; Cao, K. Anti-Aging Potentials of Methylene Blue for Human Skin Longevity. Sci. Rep. 2017, 7, 2475. [Google Scholar] [CrossRef]
- Zhang, C.J.; Pinilla, S.; McEvoy, N.; Cullen, C.P.; Anasori, B.; Long, E.; Park, S.-H.; Seral-Ascaso, A.; Shmeliov, A.; Krishnan, D.; et al. Oxidation Stability of Colloidal Two-Dimensional Titanium Carbides (MXenes). Chem. Mater. 2017, 29, 4848–4856. [Google Scholar] [CrossRef]
- Shahzad, A.; Rasool, K.; Miran, W.; Nawaz, M.; Jang, J.; Mahmoud, K.A.; Lee, D.S. Mercuric ion capturing by recoverable titanium carbide magnetic nanocomposite. J. Hazard. Mater. 2018, 344, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, A.; Rasool, K.; Nawaz, M.; Miran, W.; Jang, J.; Moztahida, M.; Mahmoud, K.A.; Lee, D.S. Heterostructural TiO2/Ti3C2Tx (MXene) for photocatalytic degradation of antiepileptic drug carbamazepine. Chem. Eng. J. 2018, 349, 748–755. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, L.; Wang, H.; Zhu, G.; Wen, H.; Feng, H.; Sun, X.; Guan, X.; Wen, J.; Yao, Y. In Situ Hydrothermal Growth of TiO2 Nanoparticles on a Conductive Ti3C2Tx MXene Nanosheet: A Synergistically Active Ti-Based Nanohybrid Electrocatalyst for Enhanced N2 Reduction to NH3 at Ambient Conditions. Inorg. Chem. 2019, 58, 5414–5418. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.-H.; Li, X.; Zhang, Q.; Yu, H.; Peng, X.; Peng, F. Regulating Electron–Hole Separation to Promote Photocatalytic H2 Evolution Activity of Nanoconfined Ru/MXene/TiO2 Catalysts. ACS Nano 2020, 14, 14181–14189. [Google Scholar] [CrossRef]
- Zhou, Y.; Chai, Y.; Li, X.; Wu, Z.; Lin, J.; Han, Y.; Li, L.; Qi, H.; Gu, Y.; Kang, L.; et al. Defect-Rich TiO2 In Situ Evolved from MXene for the Enhanced Oxidative Dehydrogenation of Ethane to Ethylene. ACS Catal. 2021, 11, 15223–15233. [Google Scholar] [CrossRef]
- Tahir, M.; Tahir, B. In-situ growth of TiO2 imbedded Ti3C2TA nanosheets to construct PCN/Ti3C2TA MXenes 2D/3D heterojunction for efficient solar driven photocatalytic CO2 reduction towards CO and CH4 production. J. Colloid Interface Sci. 2021, 591, 20–37. [Google Scholar] [CrossRef]
- He, F.; Zhu, B.; Cheng, B.; Yu, J.; Ho, W.; Macyk, W. 2D/2D/0D TiO2/C3N4/Ti3C2 MXene composite S-scheme photocatalyst with enhanced CO2 reduction activity. Appl. Catal. B: Environ. 2020, 272, 119006. [Google Scholar] [CrossRef]
- Tang, Q.; Xiong, P.; Wang, H.; Wu, Z. Boosted CO2 photoreduction performance on Ru-Ti3CN MXene-TiO2 photocatalyst synthesized by non-HF Lewis acidic etching method. J. Colloid Interface Sci. 2022, 619, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, F.; Lei, S.; Wei, Y.; Zhao, D.; Gao, Y.; Ma, X.; Li, S.; Chang, S.; Wang, M.; et al. In situ grown two-dimensional TiO2/Ti3CN MXene heterojunction rich in Ti3+ species for highly efficient photoelectrocatalytic CO2 reduction. Chem. Eng. J. 2023, 452, 139392. [Google Scholar] [CrossRef]
- Khan, A.A.; Tahir, M.; Zakaria, Z.Y. Synergistic effect of anatase/rutile TiO2 with exfoliated Ti3C2TR MXene multilayers composite for enhanced CO2 photoreduction via dry and bi-reforming of methane under UV–visible light. J. Environ. Chem. Eng. 2021, 9, 105244. [Google Scholar] [CrossRef]
- Song, F.; Debow, S.; Zhang, T.; Qian, Y.; Huang-Fu, Z.-C.; Munns, K.; Schmidt, S.; Fisher, H.; Brown, J.B.; Su, Y.; et al. Interface Catalysts of Ni3Fe1 Layered Double Hydroxide and Titanium Carbide for High-Performance Water Oxidation in Alkaline and Natural Conditions. J. Phys. Chem. Lett. 2023, 14, 5692–5700. [Google Scholar] [CrossRef]
- Scarpelli, F.; Mastropietro, T.F.; Poerio, T.; Godbert, N. Mesoporous TiO2 Thin Films: State of the Art; InTech: Rijeka, Croatia, 2018. [Google Scholar]
- Gu, H.; Zhang, H.; Wang, X.; Li, Q.; Chang, S.; Huang, Y.; Gao, L.; Cui, Y.; Liu, R.; Dai, W.-L. Robust construction of CdSe nanorods@Ti3C2 MXene nanosheet for superior photocatalytic H2 evolution. Appl. Catal. B Environ. 2023, 328, 122537. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Chi, M.F.; Veith, G.M.; Zhang, P.F.; Lutterman, D.A.; Rosenthal, J.; Overbury, S.H.; Dai, S.; Zhu, H.Y. Rational Design of Bi Nanoparticles for Efficient Electrochemical CO2 Reduction: The Elucidation of Size and Surface Condition Effects. ACS Catal. 2016, 6, 6255–6264. [Google Scholar] [CrossRef]
- Allabour, A.; Coskun, H.; Zheng, X.L.; Kibria, M.G.; Strobel, M.; Hild, S.; Kehrer, M.; Stifter, D.; Sargent, E.H.; Stadler, P. Active Sulfur Sites in Semimetallic Titanium Disulfide Enable CO2 Electroreduction. ACS Catal. 2020, 10, 66–72. [Google Scholar] [CrossRef]
- Mendieta-Reyes, N.E.; Cheuquepán, W.; Rodes, A.; Gómez, R. Spectroelectrochemical Study of CO2 Reduction on TiO2 Electrodes in Acetonitrile. ACS Catal. 2020, 10, 103–113. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865, Erratum in Phys. Rev. Lett. 1997, 78, 1396. [Google Scholar] [CrossRef] [PubMed]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D.P. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Chávez-Rocha, R.; Mercado-Sánchez, I.; Vargas-Rodriguez, I.; Hernández-Lima, J.; Bazán-Jiménez, A.; Robles, J.; García-Revilla, M.A. Modeling Adsorption of CO2 in Rutile Metallic Oxide Surfaces: Implications in CO2 Catalysis. Molecules 2023, 28, 1776. [Google Scholar] [CrossRef]
- Liu, C.; Cundari, T.R.; Wilson, A.K. CO2 Reduction on Transition Metal (Fe, Co, Ni, and Cu) Surfaces: In Comparison with Homogeneous Catalysis. J. Phys. Chem. C 2012, 116, 5681–5688. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, C.; Jiao, X. Recent Advances of In Situ Insights into CO2 Reduction toward Fuels. ChemCatChem 2025, 17, e202401388. [Google Scholar] [CrossRef]
- Pylnev, M.; Su, T.-S.; Wei, T.-C. Titania augmented with TiI4 as electron transporting layer for perovskite solar cells. Appl. Surf. Sci. 2021, 549, 149224. [Google Scholar] [CrossRef]
- Sang, X.; Xie, Y.; Lin, M.-W.; Alhabeb, M.; Van Aken, K.L.; Gogotsi, Y.; Kent, P.R.C.; Xiao, K.; Unocic, R.R. Atomic Defects in Monolayer Titanium Carbide (Ti3C2Tx) MXene. ACS Nano 2016, 10, 9193–9200. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Debow, S.; Shen, Z.; Kulathuvayal, A.S.; Song, F.; Zhang, T.; Fisher, H.; Brown, J.B.; Qian, Y.; Huang-Fu, Z.-C.; Wang, H.; et al. Interface Catalysts of In Situ-Grown TiO2/MXenes for High-Faraday-Efficiency CO2 Reduction. Molecules 2025, 30, 4025. https://doi.org/10.3390/molecules30194025
Debow S, Shen Z, Kulathuvayal AS, Song F, Zhang T, Fisher H, Brown JB, Qian Y, Huang-Fu Z-C, Wang H, et al. Interface Catalysts of In Situ-Grown TiO2/MXenes for High-Faraday-Efficiency CO2 Reduction. Molecules. 2025; 30(19):4025. https://doi.org/10.3390/molecules30194025
Chicago/Turabian StyleDebow, Shaun, Zichen Shen, Arjun Sathyan Kulathuvayal, Fuzhan Song, Tong Zhang, Haley Fisher, Jesse B. Brown, Yuqin Qian, Zhi-Chao Huang-Fu, Hui Wang, and et al. 2025. "Interface Catalysts of In Situ-Grown TiO2/MXenes for High-Faraday-Efficiency CO2 Reduction" Molecules 30, no. 19: 4025. https://doi.org/10.3390/molecules30194025
APA StyleDebow, S., Shen, Z., Kulathuvayal, A. S., Song, F., Zhang, T., Fisher, H., Brown, J. B., Qian, Y., Huang-Fu, Z.-C., Wang, H., Zander, Z., Mirotznik, M. S., Opila, R. L., Su, Y., & Rao, Y. (2025). Interface Catalysts of In Situ-Grown TiO2/MXenes for High-Faraday-Efficiency CO2 Reduction. Molecules, 30(19), 4025. https://doi.org/10.3390/molecules30194025






