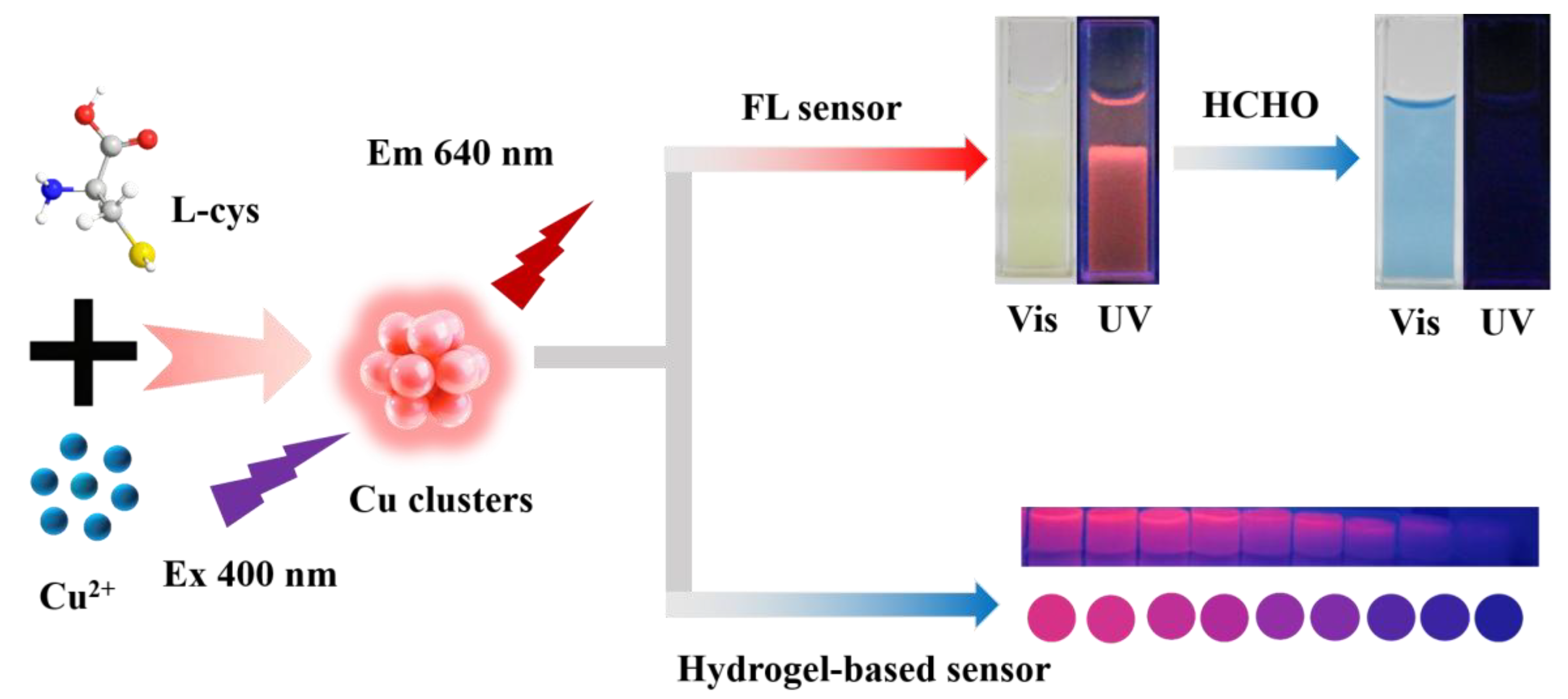
A Facile Fluorescent Visualization Method Based on Copper Clusters for Formaldehyde Detection
Abstract
1. Introduction
2. Results and Discussion
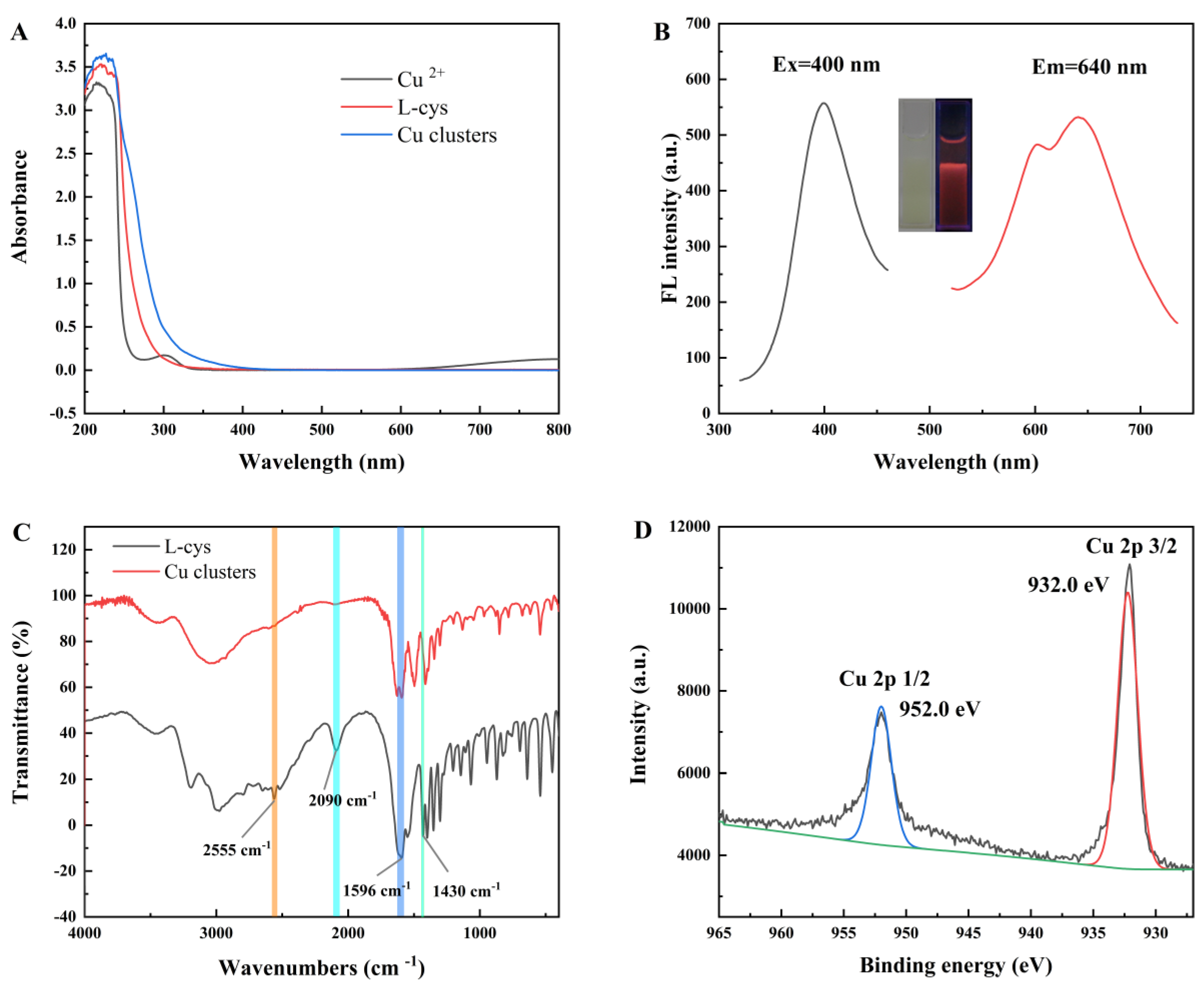
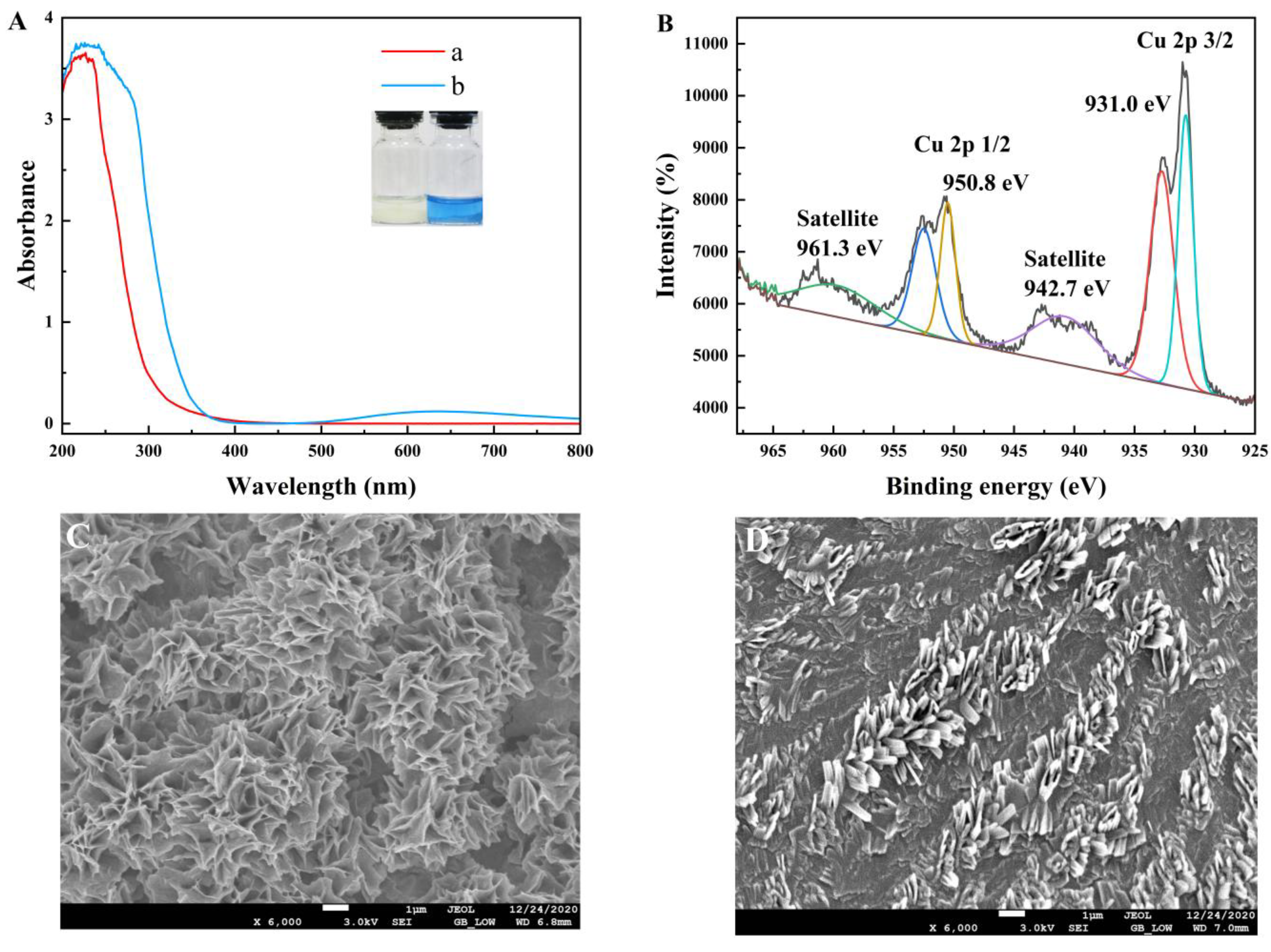
2.1. Characterization of Copper Clusters
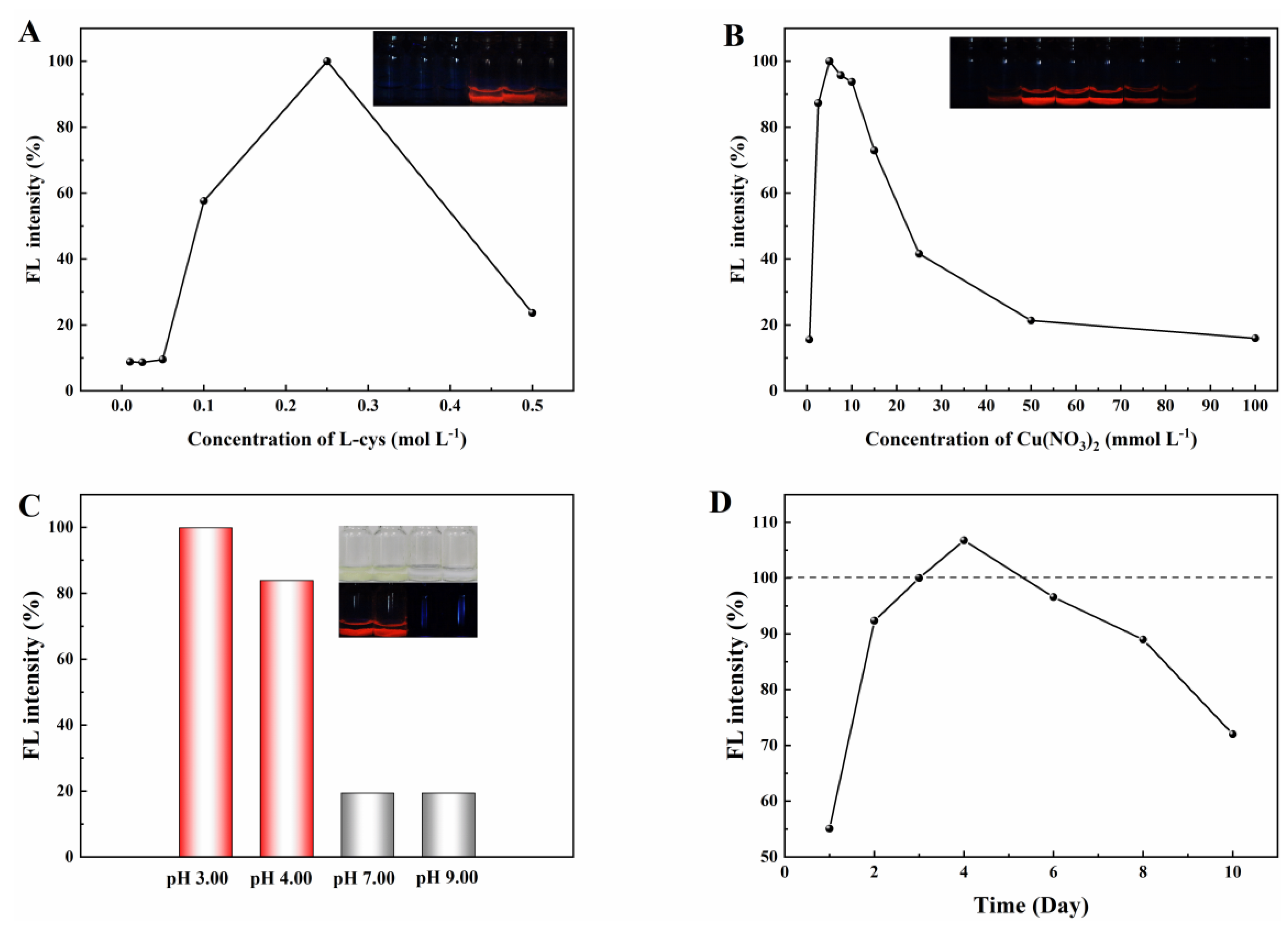
2.2. Optimization of Synthesis Conditions
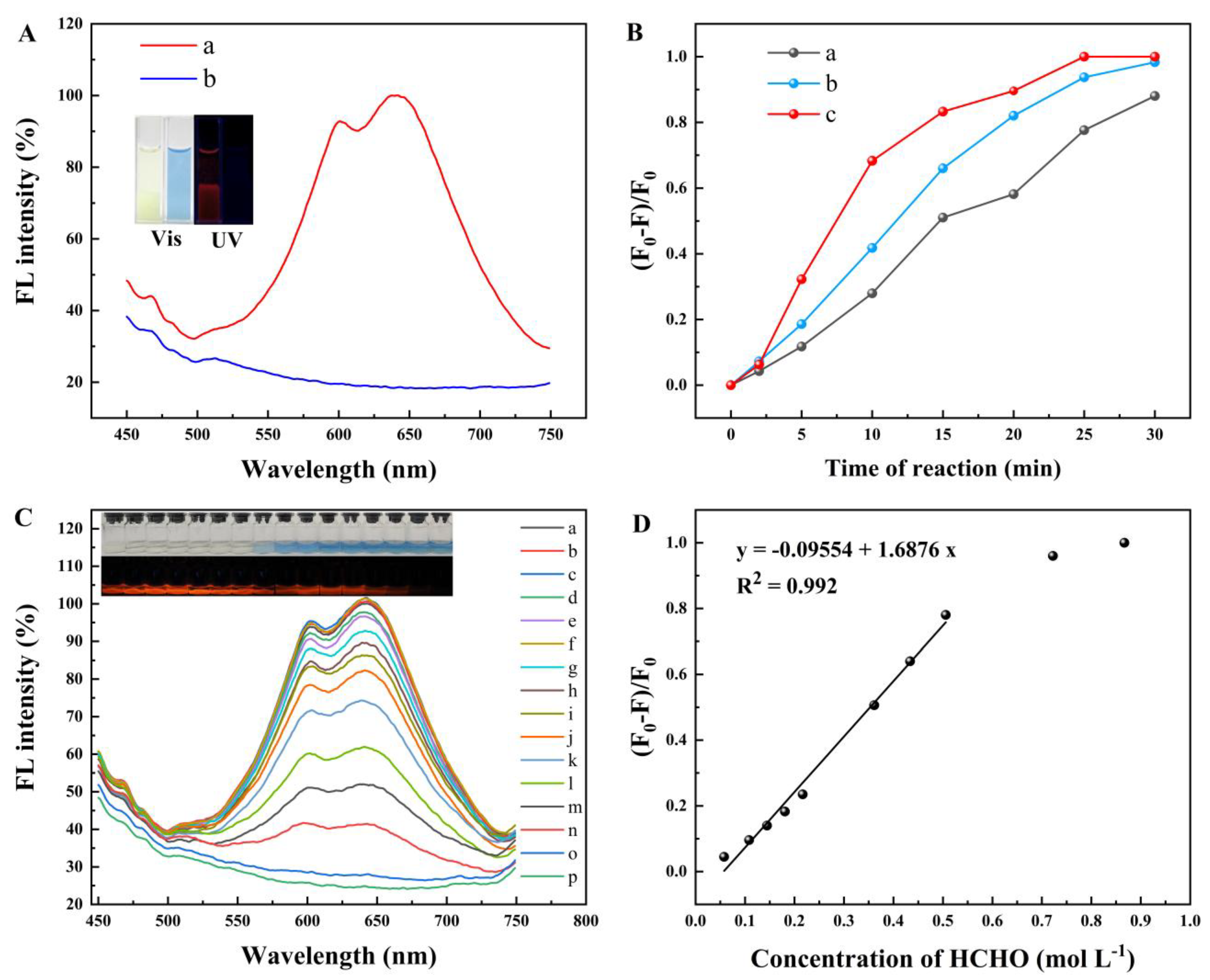
2.3. Detection of Formaldehyde
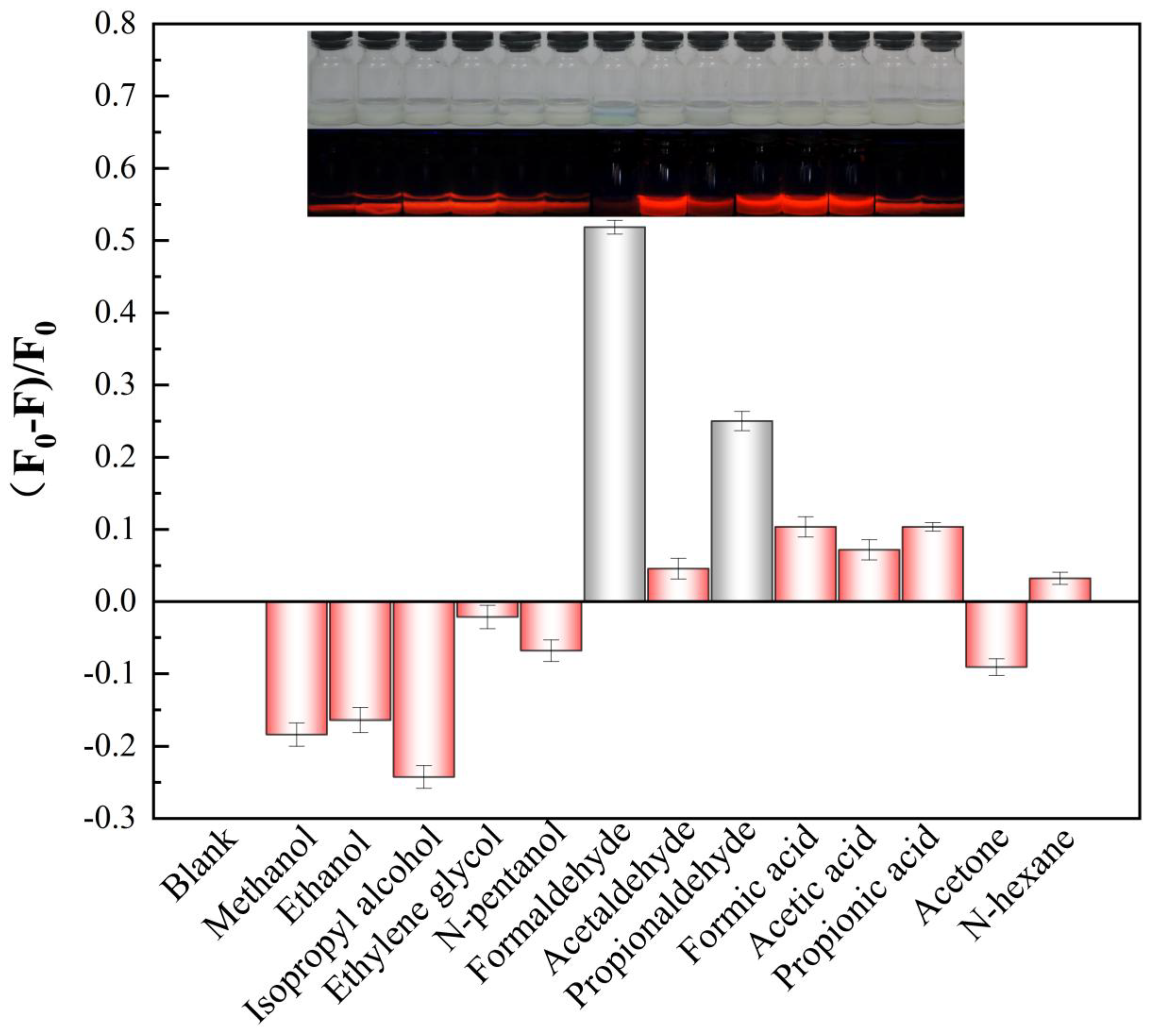
2.4. Selectivity
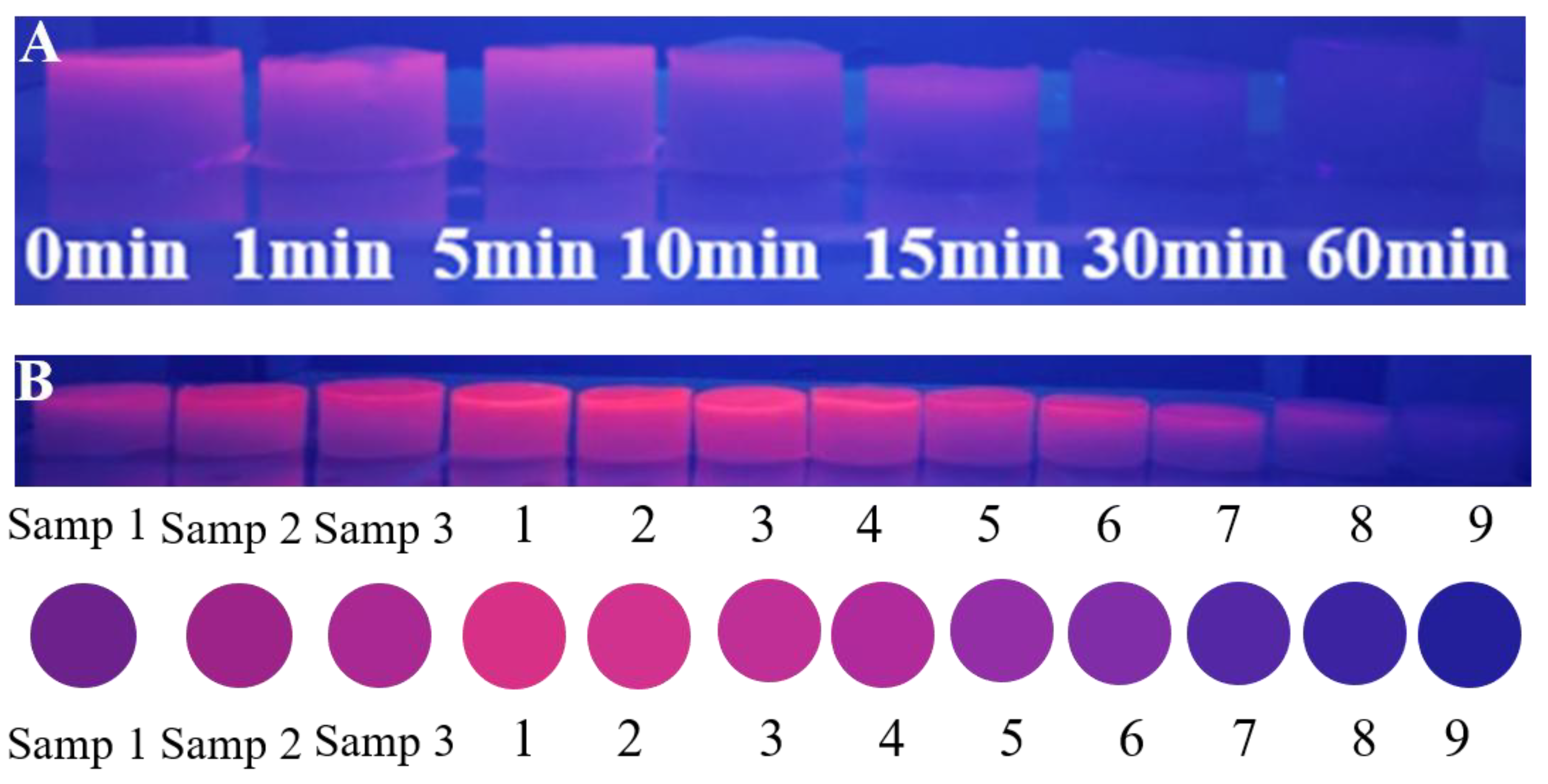
2.5. Detection of Formaldehyde in Air Samples
2.6. Visual Detection of Formaldehyde
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Instrumentation
3.3. Synthesis of Copper Clusters
3.4. Procedures for Fluorescence Measurements of Formaldehyde
3.5. Preparation Process for Real Samples
3.6. Preparation of Hydrogel-Stabilized Copper Clusters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.M.; Lin, Y.T.; Chen, J.L.; Zhang, J.; Zhu, Z.Q.; Liu, Q.J. A high sensitivity gas sensor for formaldehyde based on silver doped lanthanum ferrite. Sens. Actuators B Chem. 2014, 190, 171–176. [Google Scholar] [CrossRef]
- Tang, X.; Bai, Y.; Duong, A.; Smith, M.T.; Li, L.; Zhang, L. Formaldehyde in China: Production, consumption, exposure levels, and health effects. Environ. Int. 2009, 35, 1210–1224. [Google Scholar] [CrossRef] [PubMed]
- Salthammer, T. Formaldehyde sources, formaldehyde concentrations and air exchange rates in European housings. Build. Environ. 2019, 150, 219–232. [Google Scholar] [CrossRef]
- Yuan, C.; Pu, J.; Fu, D.; Min, Y.; Wang, L.; Liu, J. UV–vis spectroscopic detection of formaldehyde and its analogs: A convenient and sensitive methodology. J. Hazard. Mater. 2022, 438, 129457. [Google Scholar] [CrossRef]
- Luong, J.; Yang, X.; Hua, Y.; Yang, P.; Gras, R. Gas Chromatography with In Situ Catalytic Hydrogenolysis and Flame Ionization Detection for the Direct Measurement of Formaldehyde and Acetaldehyde in Challenging Matrices. Anal. Chem. 2018, 90, 13855–13859. [Google Scholar] [CrossRef] [PubMed]
- Wahed, P.; Razzaq, M.A.; Dharmapuri, S.; Corrales, M. Determination of formaldehyde in food and feed by an in-house validated HPLC method. Food Chem. 2016, 202, 476–483. [Google Scholar] [CrossRef]
- Zhu, T.; Wang, H.; Zang, L.; Jin, S.; Guo, S.; Park, E.; Mao, Z.; Jung, Y.M. Flexible and Reusable Ag Coated TiO2 Nanotube Arrays for Highly Sensitive SERS Detection of Formaldehyde. Molecules 2020, 25, 1199. [Google Scholar] [CrossRef]
- Chung, P.-R.; Tzeng, C.-T.; Ke, M.-T.; Lee, C.-Y. Formaldehyde Gas Sensors: A Review. Sensors 2013, 13, 4468–4484. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Roy, S.; Choudhury, N.; Behera, P.P.; Sivaprakasam, K.; Ramakrishnan, L.; De, P. From small molecules to polymeric probes: Recent advancements of formaldehyde sensors. Sci. Technol. Adv. Mater. 2022, 23, 49–63. [Google Scholar] [CrossRef]
- Bruemmer, K.J.; Green, O.; Su, T.A.; Shabat, D.; Chang, C.J. Chemiluminescent Probes for Activity-Based Sensing of Formaldehyde Released from Folate Degradation in Living Mice. Angew. Chem. Int. Ed. 2018, 57, 7508–7512. [Google Scholar] [CrossRef]
- Yuan, Z.; Yang, C.; Meng, F. Strategies for Improving the Sensing Performance of Semiconductor Gas Sensors for High-Performance Formaldehyde Detection: A Review. Chemosensors 2021, 9, 179. [Google Scholar] [CrossRef]
- Yang, Y.; Hao, Y.; Huang, L.; Luo, Y.; Chen, S.; Xu, M.; Chen, W. Recent Advances in Electrochemical Sensors for Formaldehyde. Molecules 2024, 29, 327. [Google Scholar] [CrossRef]
- Martínez-Aquino, C.; Costero, A.M.; Gil, S.; Gaviña, P. A New Environmentally-Friendly Colorimetric Probe for Formaldehyde Gas Detection under Real Conditions. Molecules 2018, 23, 2646. [Google Scholar] [CrossRef]
- Xia, H.; Hu, J.; Tang, J.; Xu, K.; Hou, X.; Wu, P. A RGB-Type Quantum Dot-based Sensor Array for Sensitive Visual Detection of Trace Formaldehyde in Air. Sci. Rep. 2016, 6, 36794. [Google Scholar] [CrossRef] [PubMed]
- Bruemmer, K.J.; Brewer, T.F.; Chang, C.J. Fluorescent probes for imaging formaldehyde in biological systems. Curr. Opin. Chem. Biol. 2017, 39, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Fortibui, M.M.; Lim, W.; Lee, S.; Park, S.; Kim, J. A Golgi Apparatus-Targeting, Naphthalimide-Based Fluorescent Molecular Probe for the Selective Sensing of Formaldehyde. Molecules 2021, 26, 4980. [Google Scholar] [CrossRef]
- Liu, X.; Li, N.; Li, M.; Chen, H.; Zhang, N.; Wang, Y.; Zheng, K. Recent progress in fluorescent probes for detection of carbonyl species: Formaldehyde, carbon monoxide and phosgene. Coord. Chem. Rev. 2020, 404, 213109. [Google Scholar] [CrossRef]
- Zhao, Q.; Shen, T.; Liu, Y.; Hu, X.; Zhao, W.; Ma, Z.; Li, P.; Zhu, X.; Zhang, Y.; Liu, M.; et al. Universal Nanoplatform for Formaldehyde Detection Based on the Oxidase-Mimicking Activity of MnO2 Nanosheets and the In Situ Catalysis-Produced Fluorescence Species. J. Agric. Food Chem. 2021, 69, 7303–7312. [Google Scholar] [CrossRef]
- Kim, J.; Hong, U.G.; Choi, Y.; Hong, S. Enhancing the evanescent field in TiO2/Au hybrid thin films creates a highly sensitive room-temperature formaldehyde gas biosensor. Colloids Surf. B Biointerfaces 2019, 182, 110303. [Google Scholar] [CrossRef]
- Kim, J.; Hong, S.; Choi, Y. Sensitive Detection of Formaldehyde Gas Using Modified Dandelion-Like SiO2/Au Film and Surface Plasmon Resonance System. J. Nanosci. Nanotechnol. 2019, 19, 4807–4811. [Google Scholar] [CrossRef]
- Chaiendoo, K.; Sooksin, S.; Kulchat, S.; Promarak, V.; Tuntulani, T.; Ngeontae, W. A new formaldehyde sensor from silver nanoclusters modified Tollens’ reagent. Food Chem. 2018, 255, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Chaiendoo, K.; Boonchiangma, S.; Promarak, V.; Ngeontae, W. New sensitive strategy for formaldehyde sensing by in situ generation of luminescent silver nanoclusters. Colloid Polym. Sci. 2018, 296, 1995–2004. [Google Scholar] [CrossRef]
- Liang, M.; Shao, C.; Zhang, Q.; Zhang, C.; Wang, Y.; Zheng, X.; Lu, S. High-Performance Formaldehyde Sensing Using Paper-Based Fluorescent Copper Nanoclusters. IEEE Sens. J. 2023, 23, 2076–2084. [Google Scholar] [CrossRef]
- Li, C.; Huang, J.; Zhu, H.; Liu, L.; Feng, Y.; Hu, G.; Yu, X. Dual-emitting fluorescence of Eu/Zr-MOF for ratiometric sensing formaldehyde. Sens. Actuators B Chem. 2017, 253, 275–282. [Google Scholar] [CrossRef]
- Yan, B. Lanthanide-Functionalized Metal–Organic Framework Hybrid Systems to Create Multiple Luminescent Centers for Chemical Sensing. Acc. Chem. Res. 2017, 50, 2789–2798. [Google Scholar] [CrossRef]
- Zou, J.; Xu, F.; Zheng, J.; Xiang, Y.; Li, M.; Zhou, Q.; Xia, H. Recyclable fluorescence sensing based on copper clusters for simultaneous determination of copper ions and ammonia. Analyst 2023, 148, 1068–1074. [Google Scholar] [CrossRef]
- Mu, G.; Zou, J.; Ma, M.; Yan, P.; Li, M.; Xia, H.; Xu, F. Ligand-modulated aqueous synthesis of fluorescent copper nanoclusters for wide-range linear detection of nitrite in foods. Microchem. J. 2025, 213, 113806. [Google Scholar] [CrossRef]
- Wei, W.; Lu, Y.; Chen, W.; Chen, S. One-Pot Synthesis, Photoluminescence, and Electrocatalytic Properties of Subnanometer-Sized Copper Clusters. J. Am. Chem. Soc. 2011, 133, 2060–2063. [Google Scholar] [CrossRef]
| Sample | Added (mmol L−1) | Detected a (mmol L−1) | Visualize |
|---|---|---|---|
| 1 | 0 | nd |  |
| 2 | 72.25 | 70.08 ± 0.01 |  |
| 3 | 144.50 | 133.90 ± 0.01 |  |
| 4 | 289.00 | 301.12 ± 0.02 |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, J.; Chen, Q.; Mu, G.; Ma, M.; Yang, F.; Li, M.; Xu, F.; Xia, H. A Facile Fluorescent Visualization Method Based on Copper Clusters for Formaldehyde Detection. Molecules 2025, 30, 4022. https://doi.org/10.3390/molecules30194022
Zou J, Chen Q, Mu G, Ma M, Yang F, Li M, Xu F, Xia H. A Facile Fluorescent Visualization Method Based on Copper Clusters for Formaldehyde Detection. Molecules. 2025; 30(19):4022. https://doi.org/10.3390/molecules30194022
Chicago/Turabian StyleZou, Jie, Qing Chen, Guimin Mu, Miao Ma, Fang Yang, Mengtian Li, Fujian Xu, and Hui Xia. 2025. "A Facile Fluorescent Visualization Method Based on Copper Clusters for Formaldehyde Detection" Molecules 30, no. 19: 4022. https://doi.org/10.3390/molecules30194022
APA StyleZou, J., Chen, Q., Mu, G., Ma, M., Yang, F., Li, M., Xu, F., & Xia, H. (2025). A Facile Fluorescent Visualization Method Based on Copper Clusters for Formaldehyde Detection. Molecules, 30(19), 4022. https://doi.org/10.3390/molecules30194022






