Solid-State Fermentation of Riceberry Rice with Mushroom Mycelium for Enhanced Beta-Glucan Production and Health Applications
Abstract
1. Introduction
2. Results
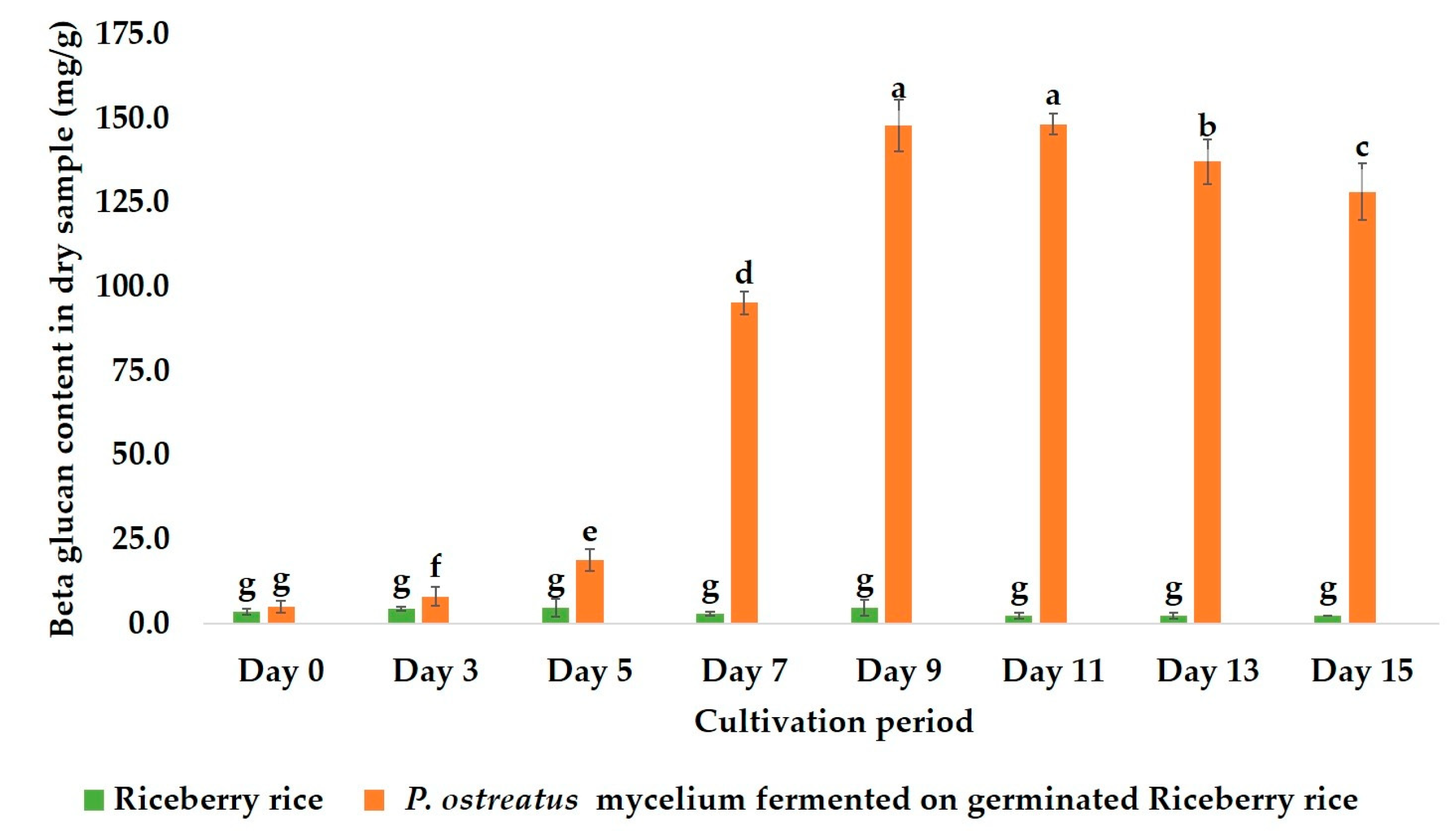
2.1. Optimal Fermentation Time for β-Glucan Production
2.2. Chemical and Physical Characterization of Extracted β-Glucan
2.2.1. Extraction Yield and Purity
2.2.2. Fourier-Transform Infrared (FTIR) Spectroscopy Analysis
2.3. Prebiotic Properties of Crude β-Glucan Extract
2.3.1. Prebiotic Index (PI)
2.3.2. Prebiotic Activity Score (PAS)
2.4. Anti-Colorectal Cancer Properties of Crude β-Glucan
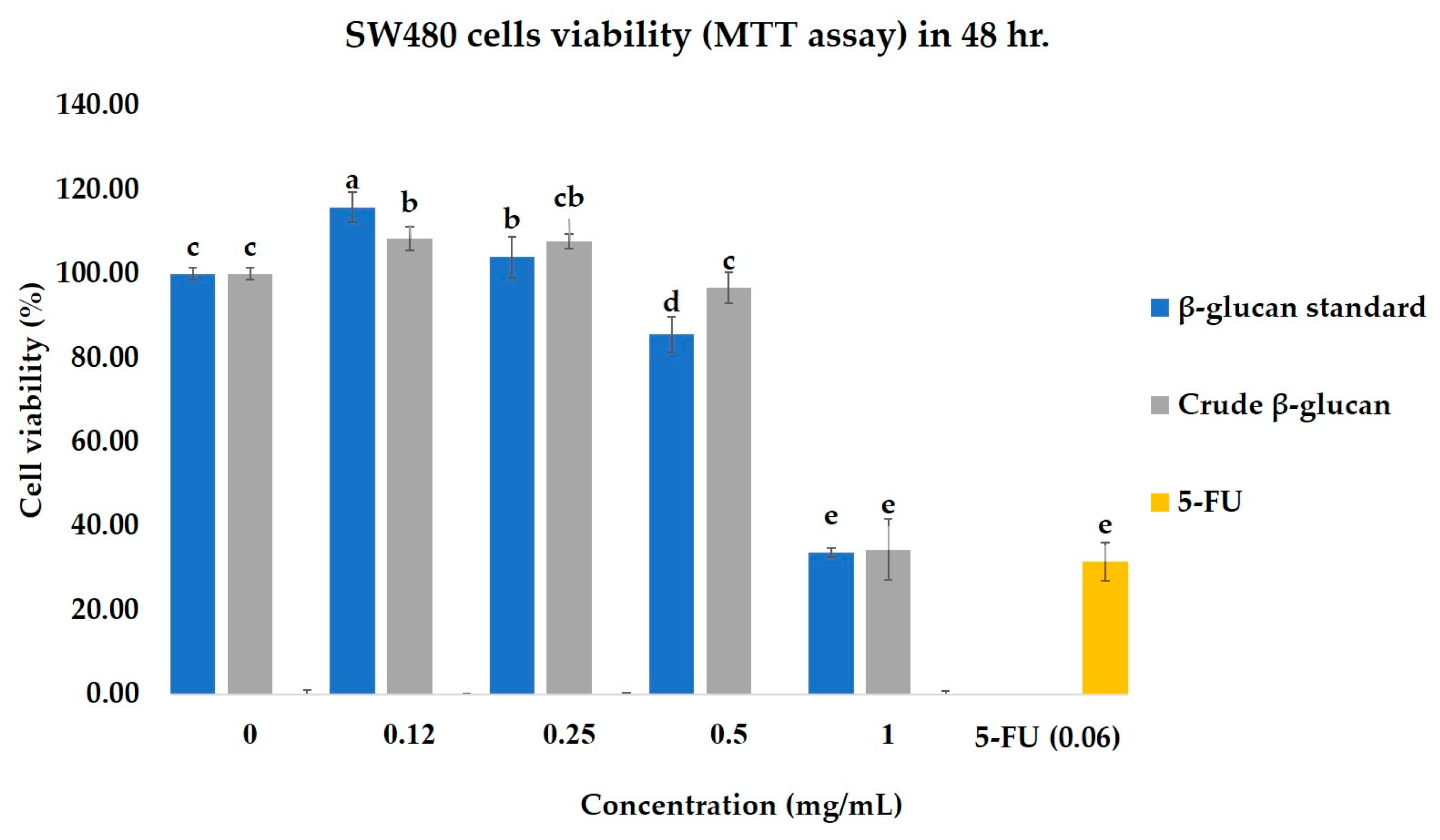
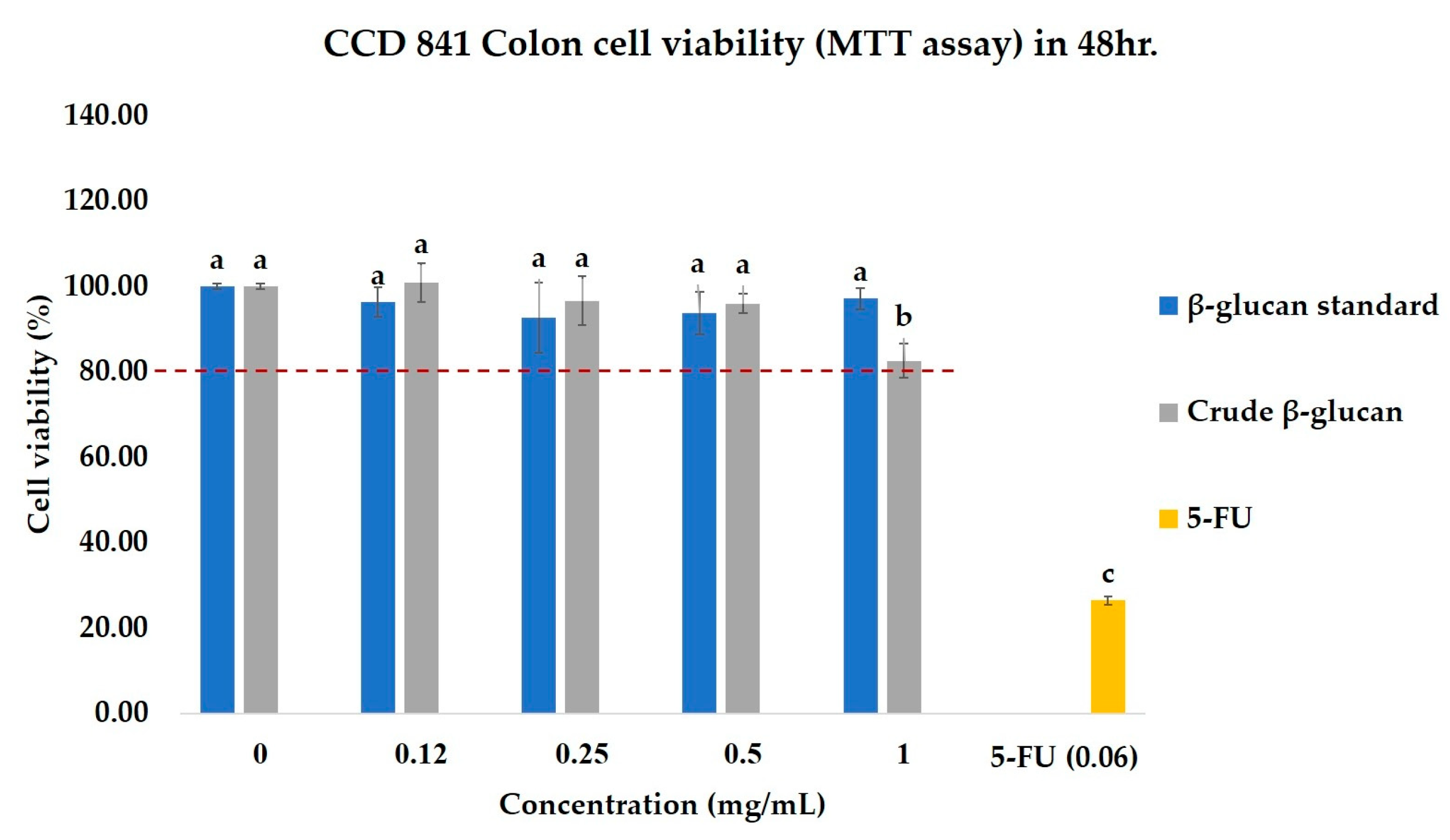
2.4.1. Effects of Crude β-Glucan on the Cell Viability of Colorectal Cancer Cell Lines (SW480) and Normal Colon Cell Lines (CCD841 CoN)
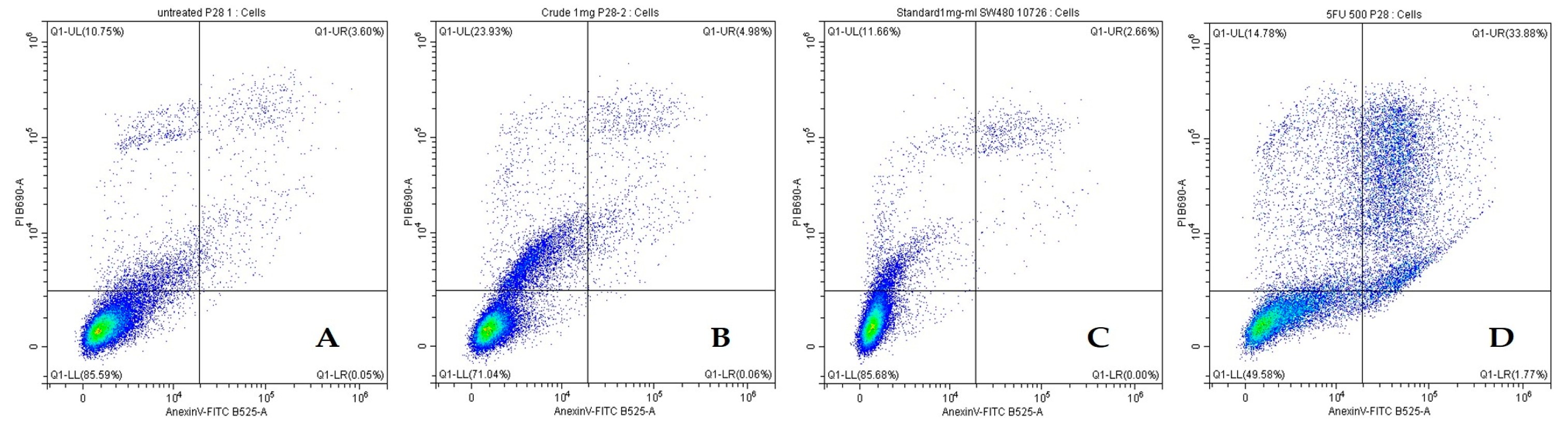
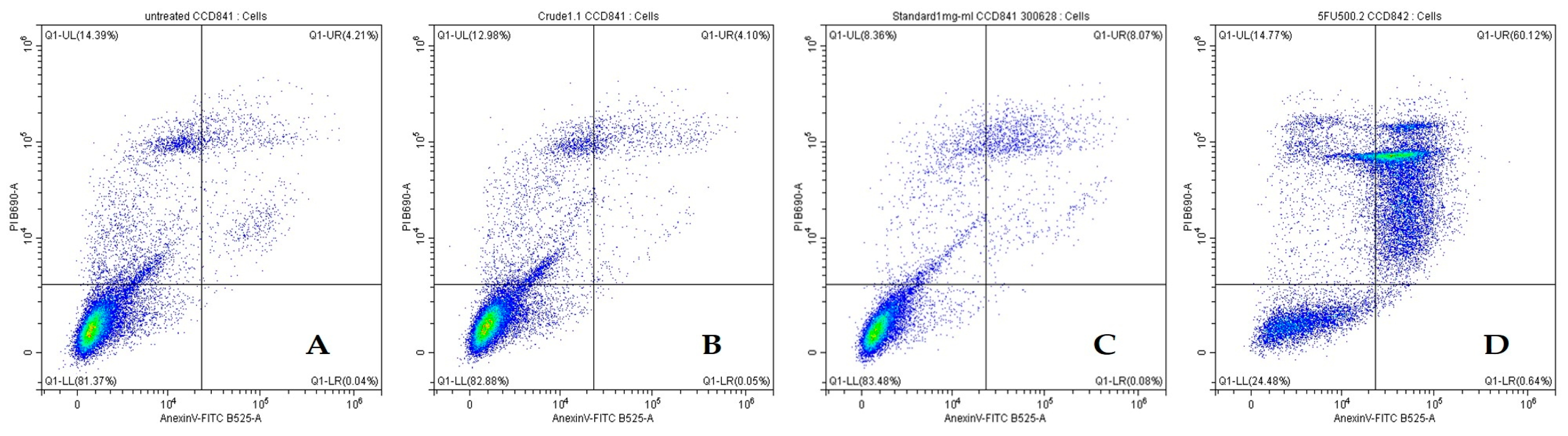
2.4.2. Effects of Crude β-Glucan to Induce Apoptosis in Colon Cancer Cells (SW480) and Normal Colon Cells (CCD841 CoN)
3. Discussion

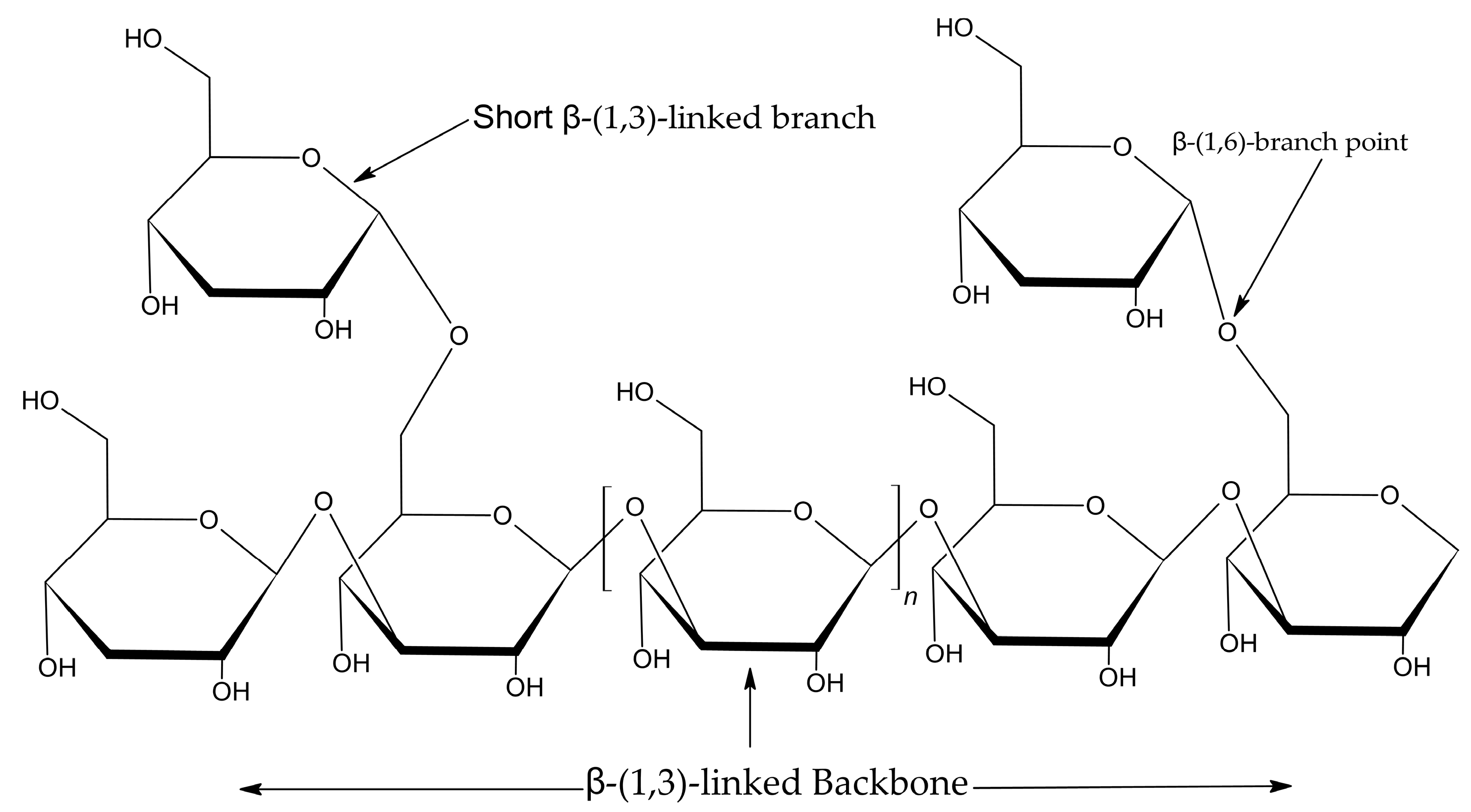
4. Materials and Methods
4.1. Materials
4.2. Fungal Culture and Solid-State Fermentation
4.2.1. Inoculum and Riceberry Rice Media Preparation
4.2.2. Solid-State Fermentation (SSF)
4.3. β-Glucan Extraction
4.4. Analytical Characterization
4.4.1. β-Glucan Quantification
4.4.2. Thermogravimetric Analysis (TGA)
4.4.3. Fourier-Transform Infrared (FTIR) Spectroscopy
4.5. Prebiotic Activity Assays
4.5.1. Probiotic Growth Stimulation
4.5.2. Prebiotic Index (PI) and Prebiotic Activity Score (PAS)
4.6. Anti-Cancer Activities
4.6.1. Cell Lines and Culture Conditions
4.6.2. Cell Viability (MTT) Assay
4.6.3. Apoptosis Assay by Flow Cytometry
4.7. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, C.; Du, M.; Han, Y.; Sun, W.; Chen, Z.; Liu, Q.; Zhu, H.; Zhao, L.; Li, S.; Wang, J. Microalgae in Health Care and Functional Foods: β-Glucan Applications, Innovations in Drug Delivery and Synthetic Biology. Front. Pharmacol. 2025, 16, 1557298. [Google Scholar] [CrossRef]
- Susanti, I.; Wibowo, H.; Siregar, N.C.; Pawiroharsono, S.; Sujatna, F.D. Antitumor Activity of Beta Glucan Extract from Oyster Mushroom (Pleurotus ostreatus Jacq. P. Kum) on DMBA-Induced Breast Cancer in Vivo. Int. J. Pharmtech Res. 2018, 11, 190–197. [Google Scholar] [CrossRef]
- Soodpakdee, K.; Nacha, J.; Rattanachart, N.; Owatworakit, A.; Chamyuang, S. Fermentation With Pleurotus Ostreatus Enhances the Prebiotic Properties of Germinated Riceberry Rice. Front. Nutr. 2022, 9, 839145. [Google Scholar] [CrossRef]
- Ciecierska, A.; Drywień, M.E.; Hamulka, J.; Sadkowski, T. Nutraceutical functions of beta-glucans in human nutrition. Rocz. Panstw. Zakl. Hig./Ann. Natl. Inst. Hyg. 2019, 70, 315–324. [Google Scholar] [CrossRef]
- Varelas, V.; Liouni, M.; Calokerinos, A.C.; Nerantzis, E.T. An Evaluation Study of Different Methods for the Production of β-D-Glucan from Yeast Biomass. Drug Test. Anal. 2016, 8, 46–55. [Google Scholar] [CrossRef]
- Liesche, J.; Marek, M.; Günther-Pomorski, T. Cell Wall Staining with Trypan Blue Enables Quantitative Analysis of Morphological Changes in Yeast Cells. Front. Microbiol. 2015, 6, 107. [Google Scholar] [CrossRef]
- Frioui, M.; Yimer, G.A.; Shamtsyan, M.; Barakova, N.; Dozortseva, A.; Kolesnikov, B.; Sadovoy, V.; Kiprushkina, E. Isolation of Bioactive Beta-Glucans from Mycelium of Pleurotus ostreatus Mushroom. Bioact. Compd. Health Dis. 2024, 7, 649–658. [Google Scholar] [CrossRef]
- Kupradit, C.; Ranok, A.; Mangkalanan, S.; Khongla, C.; Musika, S. β-Glucan and Antioxidant Activities of Four Edible Mushroom Extracts from Thailand. Asian J. Agric. Biol. 2023, 2023, 202107285. [Google Scholar] [CrossRef]
- Pérez-Bassart, Z.; Fabra, M.J.; Martínez-Abad, A.; López-Rubio, A. Compositional Differences of β-Glucan-Rich Extracts from Three Relevant Mushrooms Obtained through a Sequential Extraction Protocol. Food Chem. 2023, 402, 134207. [Google Scholar] [CrossRef] [PubMed]
- Valverde, M.E.; Hernández-Pérez, T.; Paredes-López, O. Edible Mushrooms: Improving Human Health and Promoting Quality Life. Int. J. Microbiol. 2015, 2015, 376387. [Google Scholar] [CrossRef]
- Dicks, L.; Ellinger, S. Effect of the Intake of Oyster Mushrooms (Pleurotus Ostreatus) on Cardiometabolic Parameters—A Systematic Review of Clinical Trials. Nutrients 2020, 12, 1134. [Google Scholar] [CrossRef]
- Plahlevanloo, A.; Sarabi-Jamab, M.; Kaveh, M.; Ziaratnia, S.M. Production of β-Glucans from Oyster Mushroom Pleurotus ostreatus by Submerged Culture Method and Evaluation of Its Extraction Techniques. J. Res. Innov. Food Sci. Technol. 2023, 12, 231–244. [Google Scholar] [CrossRef]
- Brandon Rich the Mushroom Supplement Conundrum: Fruiting Bodies vs. Mycelium. Available online: https://www.shroomer.com/mushroom-fruiting-bodies-vs-mycelium-grain/ (accessed on 21 August 2023).
- Gaia Herbs Mycelium vs Fruiting Body Extract in Mushrooms: Which Is Better? Available online: https://www.gaiaherbs.com/blogs/seeds-of-knowledge/mushroom-mycelium-vs-fruiting-bodies?srsltid=AfmBOor_cr2AwTHfzWnUVQ90V1RT3tOJkaxX4ZVYdgMIq_kPO3NnH-Km (accessed on 15 March 2024).
- Palframan, R.; Gibson, G.R.; Rastall, R.A. Development of a Quantitative Tool for the Comparison of the Prebiotic Effect of Dietary Oligosaccharides. Lett. Appl. Microbiol. 2003, 37, 281–284. [Google Scholar] [CrossRef]
- Figueroa-González, I.; Rodríguez-Serrano, G.; Gómez-Ruiz, L.; García-Garibay, M.; Cruz-Guerrero, A. Prebiotic Effect of Commercial Saccharides on Probiotic Bacteria Isolated from Commercial Products. Food Sci. Technol. 2019, 39, 747–753. [Google Scholar] [CrossRef]
- Fehlbaum, S.; Prudence, K.; Kieboom, J.; Heerikhuisen, M.; van den Broek, T.; Schuren, F.H.J.; Steinert, R.E.; Raederstorff, D. In Vitro Fermentation of Selected Prebiotics and Their Effects on the Composition and Activity of the Adult Gut Microbiota. Int. J. Mol. Sci. 2018, 19, 3097. [Google Scholar] [CrossRef]
- Veni, B.K. Nutrition Profiles of Different Colored Rice: A Review. J. Pharmacogn. Phytochem. 2019, 8, 303–305. [Google Scholar]
- Bualoi, S.; Eamprasong, P. Suitable conditions for production of beta-glucan in oyster mushroom fruiting body. J. Sci. Technol. MSU 2021, 40, 352. [Google Scholar]
- Jantaramanant, P.; Sermwittayawong, D.; Noipha, K.; Hutadilok-Towatana, N.; Wititsuwannakul, R. β-Glucan-Containing Polysaccharide Extract from the Grey Oyster Mushroom [Pleurotus Sajor-Caju (Fr.) Sing.] Stimulates Glucose Uptake by the L6 Myotubes. Int. Food Res. J. 2014, 21, 779–784. [Google Scholar]
- Sermwittayawong, D.; Patninan, K.; Phothiphiphit, S.; Boonyarattanakalin, S.; Sermwittayawong, N.; Hutadilok-Towatana, N. Purification, Characterization, and Biological Activities of Purified Polysaccharides Extracted from the Gray Oyster Mushroom [Pleurotus Sajor-Caju (Fr.) Sing.]. J. Food Biochem. 2018, 42, e12606. [Google Scholar] [CrossRef]
- Synytsya, A.; Novak, M. Structural Analysis of Glucans. Ann. Transl. Med. 2014, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Yu, J.; Li, Y.; Luo, J.; Zhang, C.; Ou, S.; Zhang, G.; Yang, X.; Peng, X. Alternating Consumption of β-Glucan and Quercetin Reduces Mortality in Mice with Colorectal Cancer. Food Sci. Nutr. 2019, 7, 3273–3285. [Google Scholar] [CrossRef]
- Carlson, J.L.; Erickson, J.M.; Hess, J.M.; Gould, T.J.; Slavin, J.L. Prebiotic Dietary Fiber and Gut Health: Comparing the in Vitro Fermentations of Beta-Glucan, Inulin and Xylooligosaccharide. Nutrients 2017, 9, 1361. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Redondo-Blanco, S.; Miguélez, E.M.; Villar, C.J.; Clemente, A.; Lombó, F. Healthy Effects of Prebiotics and Their Metabolites against Intestinal Diseases and Colorectal Cancer. AIMS Microbiol. 2015, 1, 48–71. [Google Scholar] [CrossRef]
- Huebner, J.; Wehling, R.L.; Hutkins, R.W. Functional Activity of Commercial Prebiotics. Int. Dairy. J. 2007, 17, 770–775. [Google Scholar] [CrossRef]
- Morris, C.; Morris, G.A. The Effect of Inulin and Fructo-Oligosaccharide Supplementation on the Textural, Rheological and Sensory Properties of Bread and Their Role in Weight Management: A Review. Food Chem. 2012, 133, 237–248. [Google Scholar] [CrossRef]
- Fath El-Bab, A.F.; Majrashi, K.A.; Sheikh, H.M.; Shafi, M.E.; El-Ratel, I.T.; Neamat-Allah, A.N.F.; El-Raghi, A.A.; Elazem, A.Y.A.; Abd-Elghany, M.F.; Abdelnour, S.A.; et al. Dietary Supplementation of Nile Tilapia (Oreochromis niloticus) with β-Glucan and/or Bacillus Coagulans: Synergistic Impacts on Performance, Immune Responses, Redox Status and Expression of Some Related Genes. Front. Vet. Sci. 2022, 9, 1011715. [Google Scholar] [CrossRef]
- Aulitto, M.; Strazzulli, A.; Sansone, F.; Cozzolino, F.; Monti, M.; Moracci, M.; Fiorentino, G.; Limauro, D.; Bartolucci, S.; Contursi, P. Prebiotic Properties of Bacillus coagulans MA-13: Production of Galactoside Hydrolyzing Enzymes and Characterization of the Transglycosylation Properties of a GH42 β-Galactosidase. Microb. Cell Fact. 2021, 20, 71. [Google Scholar] [CrossRef] [PubMed]
- Stack, H.M.; Kearney, N.; Stanton, C.; Fitzgerald, G.F.; Ross, R.P. Association of Beta-Glucan Endogenous Production with Increased Stress Tolerance of Intestinal Lactobacilli. Appl. Environ. Microbiol. 2010, 76, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Brust, H.; Orzechowski, S.; Fettke, J. Starch and Glycogen Analyses: Methods and Techniques. Biomolecules 2020, 10, 1020. [Google Scholar] [CrossRef]
- Tan, L.L.; Ngiam, J.J.; Sim, E.S.Z.; Conway, P.L.; Loo, S.C.J. Liquorilactobacillus satsumensis from Water Kefir Yields α-Glucan Polysaccharides with Prebiotic and Synbiotic Qualities. Carbohydr. Polym. 2022, 290, 119515. [Google Scholar] [CrossRef]
- Nowotarska, P.; Janeczek, M.; Wiatrak, B. Cytotoxic Activity of Fomitopsis betulina against Normal and Cancer Cells—A Comprehensive Literature Review. Wspolczesna Onkol. 2024, 28, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Shamekhi, S.; Abdolalizadeh, J.; Ostadrahimi, A.; Mohammadi, S.A.; Barzegari, A.; Lotfi, H.; Bonabi, E.; Zarghami, N. Apoptotic Effect of Saccharomyces cerevisiae on Human Colon Cancer SW480 Cells by Regulation of Akt/NF-ĸB Signaling Pathway. Probiotics Antimicrob. Proteins 2020, 12, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zou, S.; Xie, C.; Meng, Y.; Xu, X. Effect of the β-Glucan from Lentinus edodes on Colitis-Associated Colorectal Cancer and Gut Microbiota. Carbohydr. Polym. 2023, 316, 121069. [Google Scholar] [CrossRef]
- de Jesus, L.I.; Smiderle, F.R.; Ruthes, A.C.; Vilaplana, F.; Dal’Lin, F.T.; Maria-Ferreira, D.; Werner, M.F.; Van Griensven, L.J.L.D.; Iacomini, M. Chemical Characterization and Wound Healing Property of a β-D-Glucan from Edible Mushroom Piptoporus betulinus. Int. J. Biol. Macromol. 2018, 117, 1361–1366. [Google Scholar] [CrossRef]
- Zade, S.; Upadhyay, T.K.; Rab, S.O.; Sharangi, A.B.; Lakhanpal, S.; Alabdallah, N.M.; Saeed, M. Mushroom-Derived Bioactive Compounds Pharmacological Properties and Cancer Targeting: A Holistic Assessment. Discov. Oncol. 2025, 16, 654. [Google Scholar] [CrossRef] [PubMed]
- Hanashima, S.; Ikeda, R.; Matsubara, Y.; Yasuda, T.; Tsuchikawa, H.; Slotte, J.P.; Murata, M. Effect of Cholesterol on the Lactosylceramide Domains in Phospholipid Bilayers. Biophys. J. 2022, 121, 1143–1155. [Google Scholar] [CrossRef]
- Varnosfaderani, S.M.N.; Ebrahimzadeh, F.; Oryani, M.A.; Khalili, S.; Almasi, F.; Heris, R.M.; Payandeh, Z.; Li, C.; Afjadi, M.N.; Bahrami, A.A. Potential Promising Anticancer Applications of β-Glucans: A Review. Biosci. Rep. 2024, 44, BSR20231686. [Google Scholar] [CrossRef]
- Chatterjee, S.B.; Hou, J.; Ratnam Bandaru, V.V.; Pezhouh, M.K.; Syed Rifat Mannan, A.A.; Sharma, R. Lactosylceramide Synthase β-1,4-GalT-V: A Novel Target for the Diagnosis and Therapy of Human Colorectal Cancer. Biochem. Biophys. Res. Commun. 2019, 508, 380–386. [Google Scholar] [CrossRef]
- Han, B.; Baruah, K.; Cox, E.; Vanrompay, D.; Bossier, P. Structure-Functional Activity Relationship of β-Glucans From the Perspective of Immunomodulation: A Mini-Review. Front. Immunol. 2020, 11, 658. [Google Scholar] [CrossRef]
- Lavi, I.; Friesem, D.; Geresh, S.; Hadar, Y.; Schwartz, B. An Aqueous Polysaccharide Extract from the Edible Mushroom Pleurotus Ostreatus Induces Anti-Proliferative and pro-Apoptotic Effects on HT-29 Colon Cancer Cells. Cancer Lett. 2006, 244, 61–70. [Google Scholar] [CrossRef]
- Waszkiewicz-Robak, B. Spent Brewer’s Yeast and Beta-Glucans Isolated from Them as Diet Components Modifying Blood Lipid Metabolism Disturbed by an Atherogenic Diet. In Lipid Metabolism; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef]
- Suraiya, S.; Jang, W.J.; Haq, M.; Kong, I.-S. Isolation and Characterization of β-Glucan Containing Polysaccharides from Monascus Spp. Using Saccharina Japonica as Submerged Fermented Substrate. Polysaccharides 2024, 5, 435–449. [Google Scholar] [CrossRef]
- Zargarnezhad, M.; Mirbahari, S.N.; Ahmadi, R. The Cytotoxic Effects of Zinc Oxide Nanoparticles on SW480 Cell Lines and Measurement of Nitric Oxide in Cell Culture Medium. Jentashapir J. Cell. Mol. Biol. 2022, 13, e122530. [Google Scholar] [CrossRef]


| Sample | Moisture (% w/w) | β-Glucan (% w/w) | Residue (% w/w) | Purity (%) * | Total of β-Glucan (% w/w) |
|---|---|---|---|---|---|
| Crude β-glucan | 14.24 | 68.59 | 17.17 | 79.98 | 37.69 |
| β-glucan standard | 8.79 | 70.17 | 21.07 | 76.93 | - |
| Sample | Prebiotic Index | ||
|---|---|---|---|
| L. rhamnosus | B. coagulans | B. longum | |
| No sugar supplement | 0.37 ± 0.06 c | 0.45 ± 0.07 d | 1.48 ± 0.07 c |
| Inulin | 0.41 ± 0.09 c | 90.53 ± 2.28 c | 6.23 ± 0.32 b |
| β-glucan standard | 1.84 ± 0.15 b | 102.49 ± 2.17 b | 7.84 ± 0.19 a |
| Riceberry rice | 0.04 ± 0.00 c | 5.23 ± 0.36 d | 0.43 ± 0.01 d |
| Mycelium | 0.02 ± 0.01 c | 0.17 ± 0.14 d | 0.85 ± 0.59 d |
| Crude β-glucan | 6.36 ± 0.72 a | 115.70 ± 10.19 a | 1.43 ± 0.02 c |
| Sample | Prebiotic Activity Score (PAS) | ||
|---|---|---|---|
| L. rhamnosus | B. coagulans | B. longum | |
| No sugar supplement | 0.09 ± 0.04 b | 0.06 ± 0.07 d | 0.48 ± 0.10 c |
| Inulin | 0.14 ± 0.02 b | 1.41 ± 0.03 a | 1.22 ± 0.22 a |
| β-glucan standard | 0.16 ± 0.03 b | 1.13 ± 0.06 b | 0.91 ± 0.12 b |
| Riceberry rice | −0.09 ± 0.02 c | 0.87 ± 0.05 c | −0.12 ± 0.06 e |
| Mycelium | −0.54 ± 0.07 d | −0.15 ± 0.08 e | 0.29 ± 0.08 cd |
| Crude β-glucan | 0.56 ± 0.03 a | 1.39 ± 0.06 a | 0.21 ± 0.10 d |
| Treatments | Live Cell | Early Apoptosis Cell | Late Apoptosis Cell | Total Cell Death |
|---|---|---|---|---|
| Untreated | 86.20 ± 0.71 a | 0.04 ± 0.01 b | 3.75 ± 0.22 b | 13.77 ± 0.73 c |
| Crude β-glucan 1 mg/mL | 71.80 ± 0.66 b | 0.07 ± 0.02 b | 4.93 ± 0.05 b | 27.94 ± 0.93 b |
| β-glucan standard 1 mg/mL | 85.58 ± 0.71 a | 0.01 ± 0.01 b | 2.76 ± 0.10 b | 14.26 ± 2.08 c |
| 5-FU 0.06 mg/mL | 49.73 ± 1.07 c | 1.92 ± 0.39 a | 34.77 ± 2.42 a | 49.99 ± 1.54 a |
| Treatments | Live Cell | Early Apoptosis Cell | Late Apoptosis Cell | Total Cell Death |
|---|---|---|---|---|
| Untreated | 81.30 ± 0.61 b | 0.07 ± 0.04 b | 4.89 ± 1.21 c | 18.70 ± 0.62 b |
| Crude β-glucan 1 mg/mL | 83.85 ± 1.81 a | 0.08 ± 0.05 b | 4.83 ± 1.49 c | 15.95 ± 2.18 c |
| β-glucan standard 1 mg/mL | 83.68 ± 0.76 a | 0.08 ± 0.02 b | 7.44 ± 1.34 b | 16.04 ± 0.56 c |
| 5FU 0.06 mg/mL | 25.44 ± 1.23 c | 0.66 ± 0.08 a | 60.54 ± 0.47 a | 74.56 ± 1.23 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nacha, J.; Chen, H.; Owatworakit, A.; Saharat, K.; Makeudom, A.; Chamyuang, S. Solid-State Fermentation of Riceberry Rice with Mushroom Mycelium for Enhanced Beta-Glucan Production and Health Applications. Molecules 2025, 30, 3879. https://doi.org/10.3390/molecules30193879
Nacha J, Chen H, Owatworakit A, Saharat K, Makeudom A, Chamyuang S. Solid-State Fermentation of Riceberry Rice with Mushroom Mycelium for Enhanced Beta-Glucan Production and Health Applications. Molecules. 2025; 30(19):3879. https://doi.org/10.3390/molecules30193879
Chicago/Turabian StyleNacha, Jutamat, Hongyu Chen, Amorn Owatworakit, Kittirat Saharat, Anupong Makeudom, and Sunita Chamyuang. 2025. "Solid-State Fermentation of Riceberry Rice with Mushroom Mycelium for Enhanced Beta-Glucan Production and Health Applications" Molecules 30, no. 19: 3879. https://doi.org/10.3390/molecules30193879
APA StyleNacha, J., Chen, H., Owatworakit, A., Saharat, K., Makeudom, A., & Chamyuang, S. (2025). Solid-State Fermentation of Riceberry Rice with Mushroom Mycelium for Enhanced Beta-Glucan Production and Health Applications. Molecules, 30(19), 3879. https://doi.org/10.3390/molecules30193879







