Ultrasound-Assisted Extraction of Spirulina platensis Carotenoids: Effect of Drying Methods and Performance of the Emerging Biosolvents 2-Methyltetrahydrofuran and Ethyl Lactate
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. S. Platensis Samples
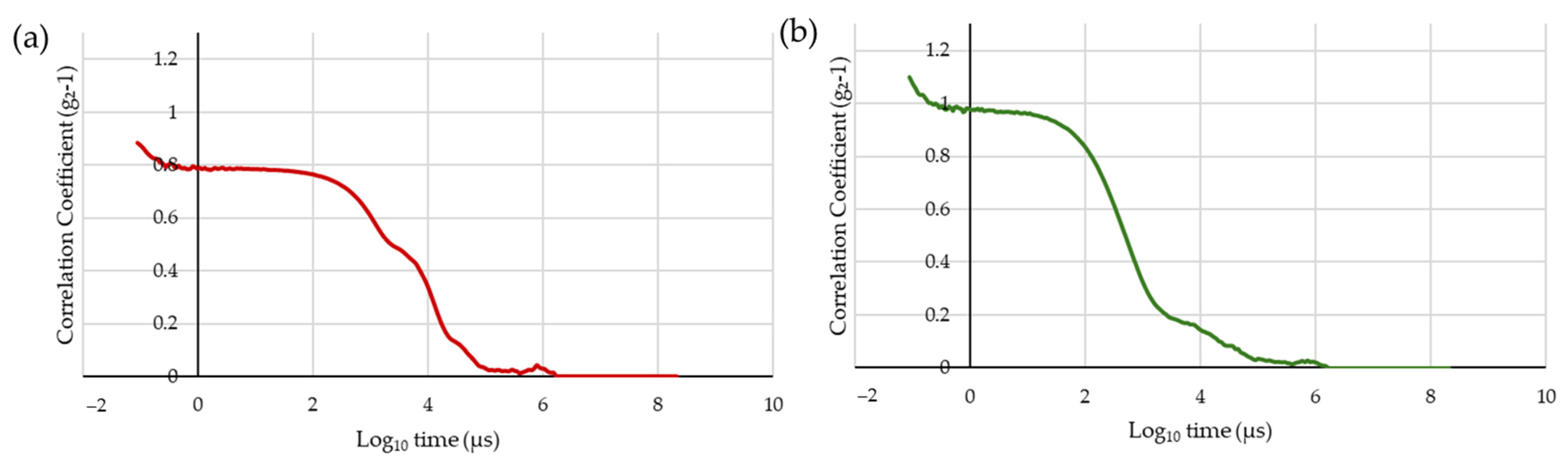
2.3. Particle Size Distribution Study in Solar-Dried and Spray-Dried Spirulina
2.4. COSMO-SAC Analysis
2.5. Carotenoids Extraction
2.5.1. Preliminary Solvent Screening
2.5.2. RSM Study
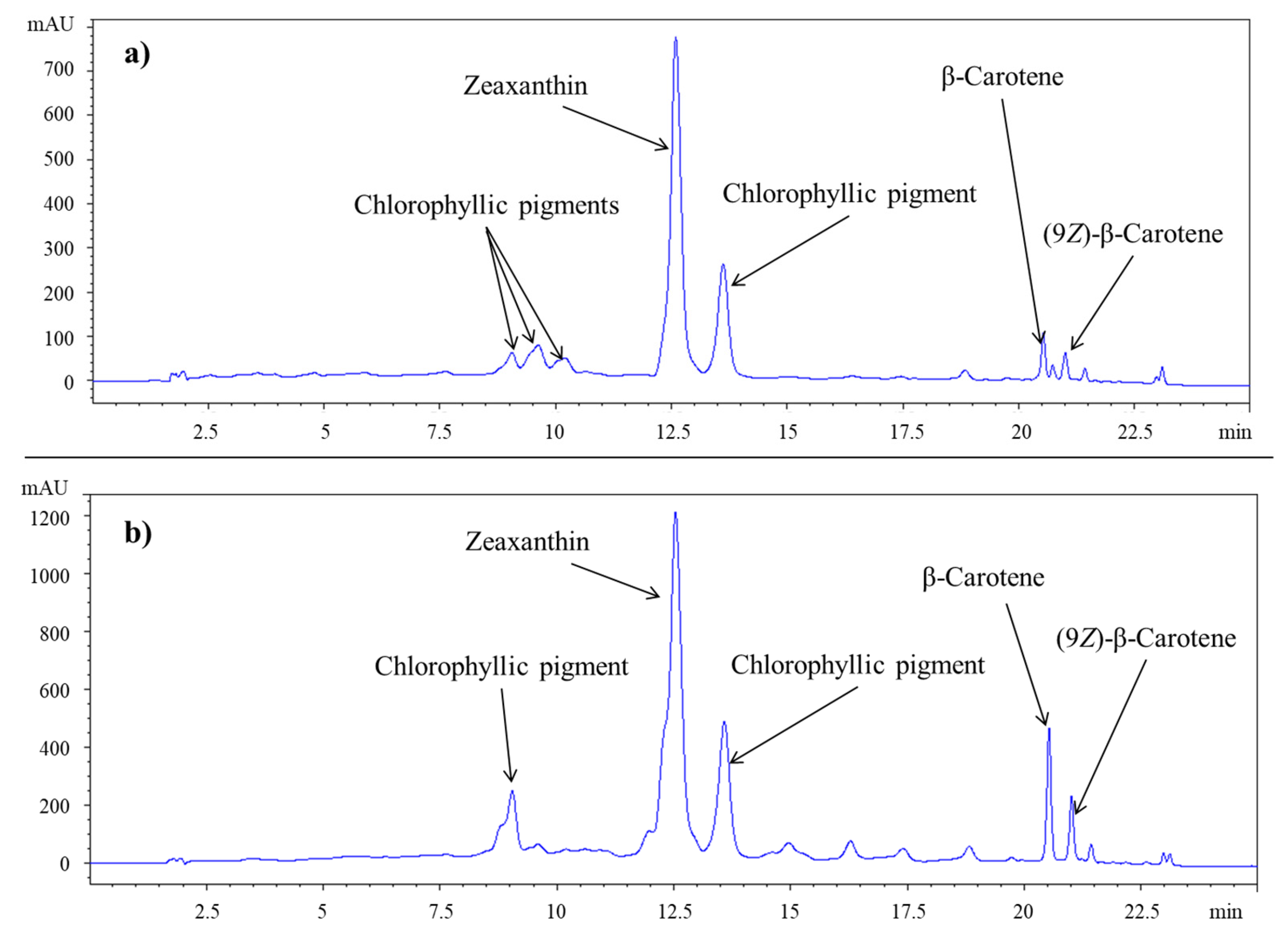
2.6. High-Performance Liquid Chromatography (HPLC) Analysis
2.7. Statistical Study
3. Results and Discussion
3.1. Particle Size Distribution Study in Solar-Dried and Spray-Dried Spirulina
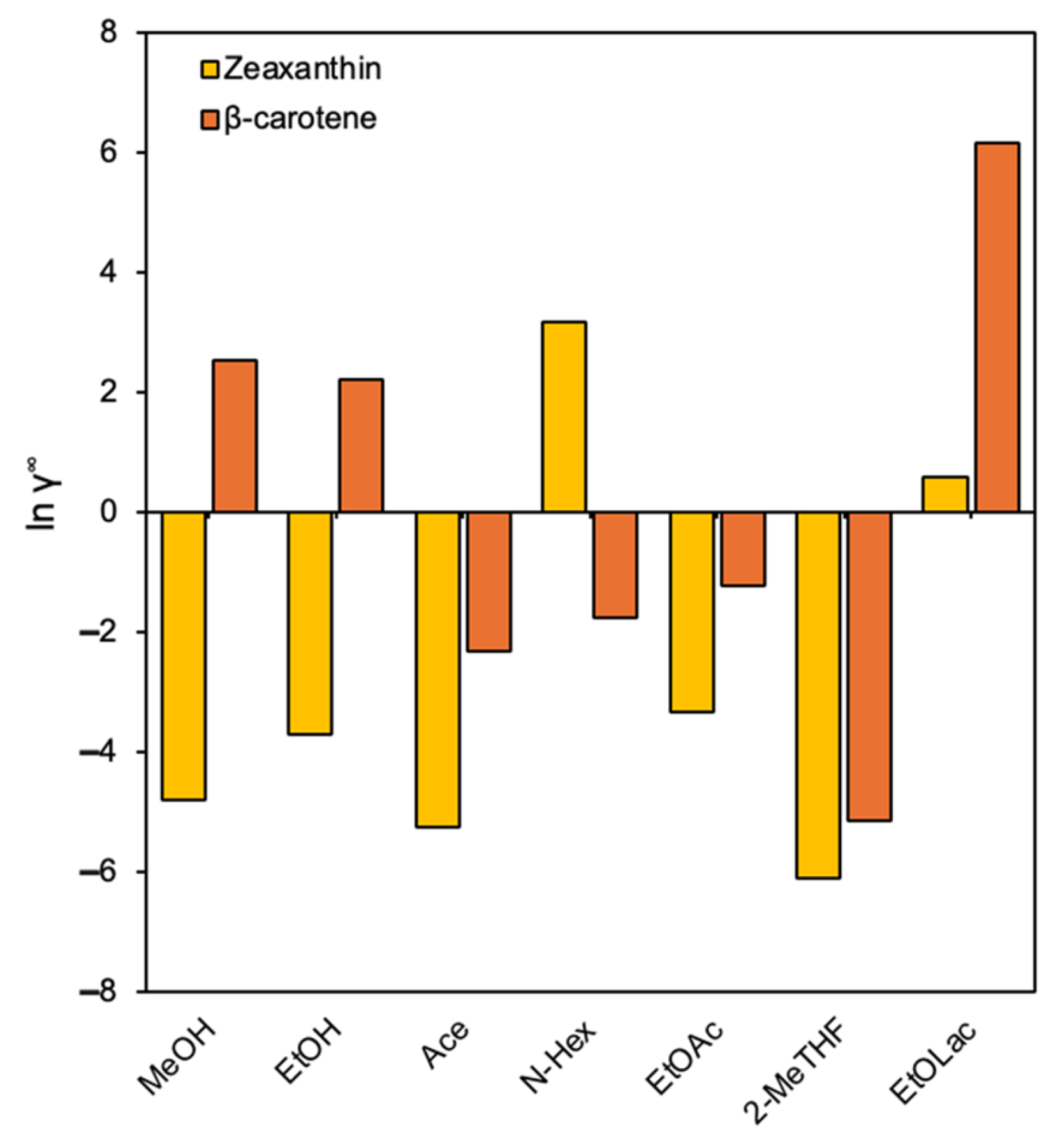
3.2. Results from the COSMO-SAC Study
3.3. Total Carotenoids Extracted as a Function of the Drying Method and the Solvent
3.4. Carotenes and Xanthophylls Extracted as a Function of the Drying Method and the Solvent
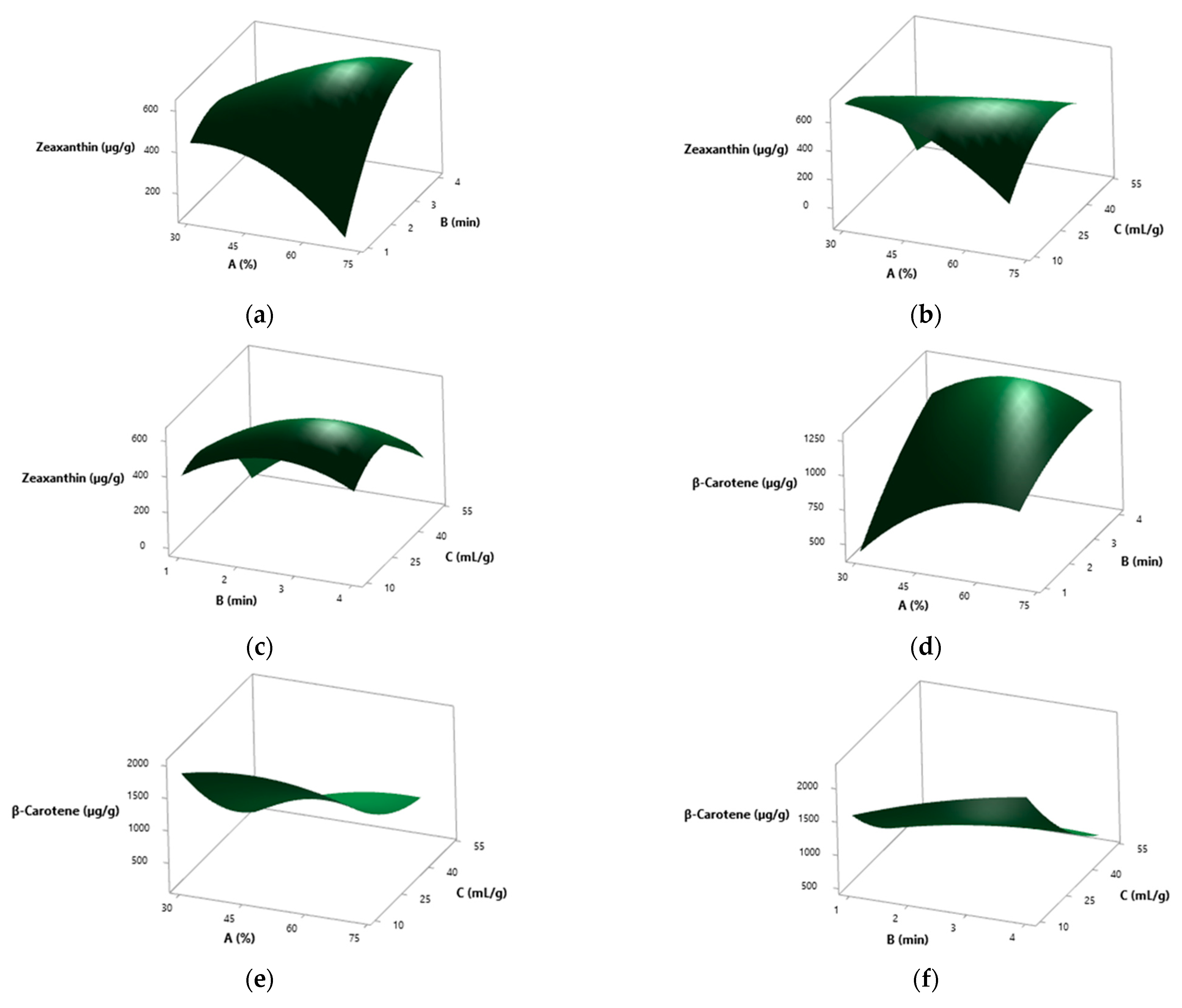
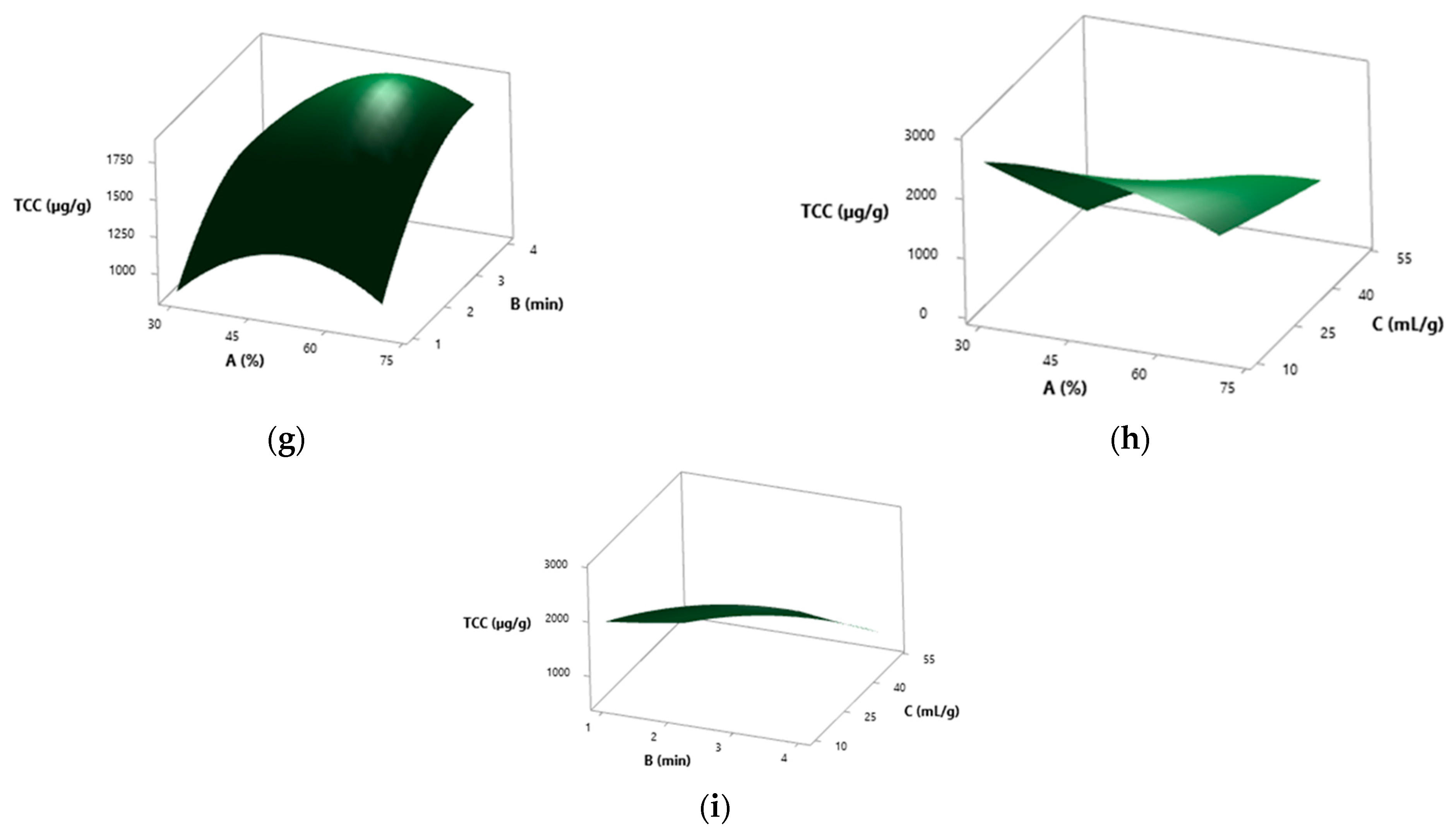
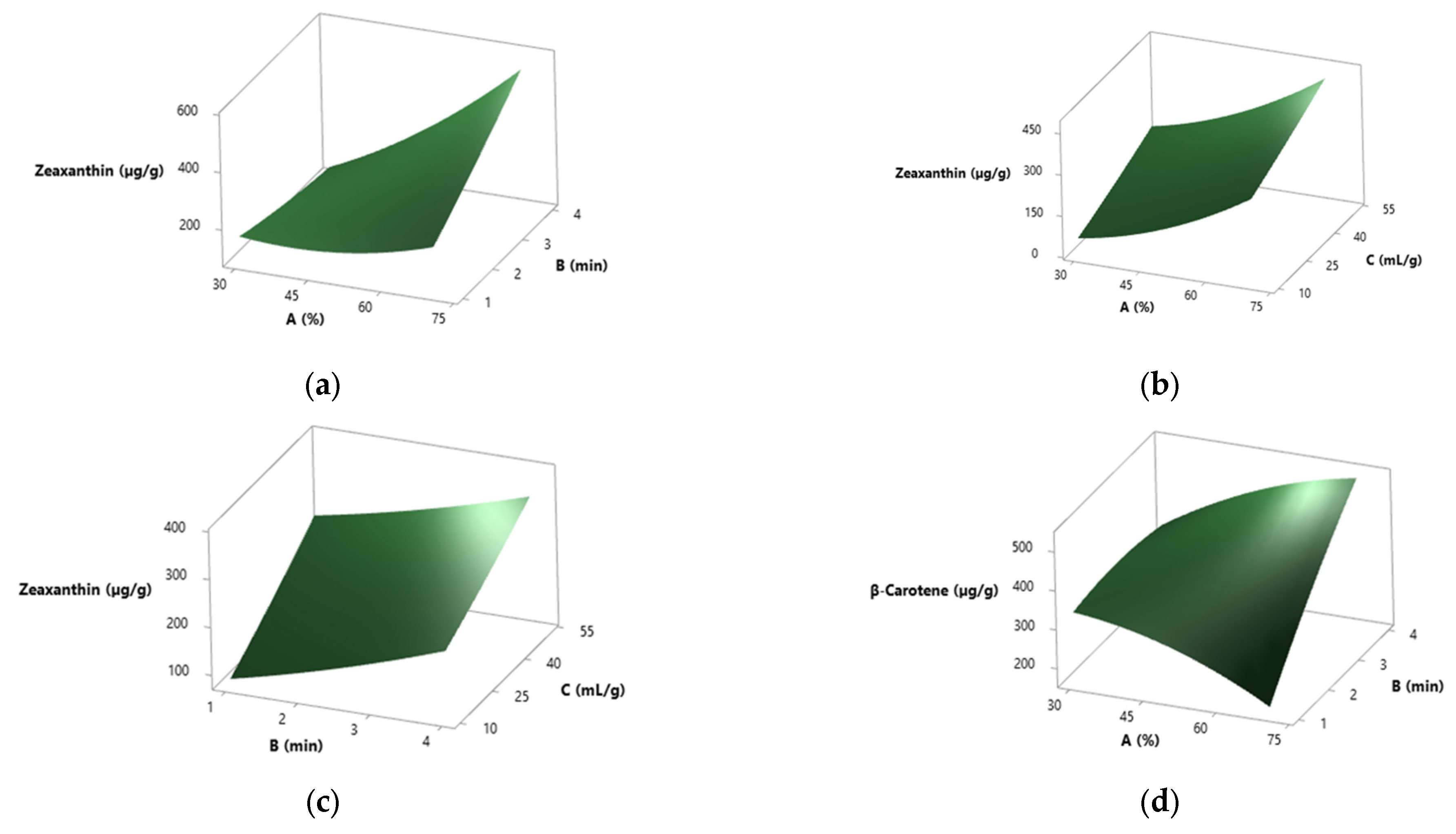
3.5. Results from the RSM Study
3.5.1. Spray-Dried S. platensis
3.5.2. Solar-Dried S. platensis
3.5.3. Solvent Effect
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT-Lancet Commission on Healthy Diets from Sustainable Food Systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Blue Bioeconomy and Blue Biotechnology. Available online: https://oceans-and-fisheries.ec.europa.eu/ocean/blue-economy/blue-bioeconomy-and-blue-biotechnology_en (accessed on 9 June 2025).
- Lafarga, T.; Fernández-Sevilla, J.M.; González-López, C.; Acién-Fernández, F.G. Spirulina for the Food and Functional Food Industries. Food Res. Int. 2020, 137, 109356. [Google Scholar] [CrossRef] [PubMed]
- Mapelli-Brahm, P.; Gómez-Villegas, P.; Gonda, M.L.; León-Vaz, A.; León, R.; Mildenberger, J.; Rebours, C.; Saravia, V.; Vero, S.; Vila, E.; et al. Microalgae, Seaweeds and Aquatic Bacteria, Archaea, and Yeasts: Sources of Carotenoids with Potential Antioxidant and Anti-Inflammatory Health-Promoting Actions in the Sustainability Era. Mar. Drugs 2023, 21, 340. [Google Scholar] [CrossRef]
- Raczyk, M.; Polanowska, K.; Kruszewski, B.; Grygier, A.; Michałowska, D. Effect of Spirulina (Arthrospira platensis) Supplementation on Physical and Chemical Properties of Semolina (Triticum durum) Based Fresh Pasta. Molecules 2022, 27, 355. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J. An Overview of Carotenoids, Apocarotenoids, and Vitamin A in Agro-Food, Nutrition, Health, and Disease. Mol. Nutr. Food Res. 2019, 63, e1801045. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Böhm, V.; Borge, G.I.A.; Cano, M.P.; Fikselová, M.; Gruskiene, R.; Lavelli, V.; Loizzo, M.R.; Mandić, A.I.; Brahm, P.M.; et al. Carotenoids: Considerations for Their Use in Functional Foods, Nutraceuticals, Nutricosmetics, Supplements, Botanicals, and Novel Foods in the Context of Sustainability, Circular Economy, and Climate Change. Annu. Rev. Food Sci. Technol. 2021, 12, 433–460. [Google Scholar] [CrossRef]
- Mapelli-Brahm, P.; Stinco, C.M.; Meléndez-Martínez, A.J. Comparative Study of the Bioaccessibility of the Colorless Carotenoids Phytoene and Phytofluene in Powders and Pulps of Tomato: Microstructural Analysis and Effect of Addition of Sunflower Oil. Food Funct. 2018, 9, 5016–5023. [Google Scholar] [CrossRef]
- Grace, M.H.; Hoskin, R.T.; Hayes, M.; Iorizzo, M.; Kay, C.; Ferruzzi, M.G.; Lila, M.A. Spray-Dried and Freeze-Dried Protein-Spinach Particles; Effect of Drying Technique and Protein Type on the Bioaccessibility of Carotenoids, Chlorophylls, and Phenolics. Food Chem. 2022, 388, 133017. [Google Scholar] [CrossRef]
- Lazzarini, C.; Casadei, E.; Valli, E.; Tura, M.; Ragni, L.; Bendini, A.; Toschi, T.G. Sustainable Drying and Green Deep Eutectic Extraction of Carotenoids from Tomato Pomace. Foods 2022, 11, 405. [Google Scholar] [CrossRef]
- Van De Walle, S.; Gifuni, I.; Coleman, B.; Baune, M.C.; Rodrigues, A.; Cardoso, H.; Fanari, F.; Muylaert, K.; Van Royen, G. Innovative vs classical methods for drying heterotrophic Chlorella vulgaris: Impact on protein quality and sensory properties. Food Res. Int. 2024, 182, 114142. [Google Scholar] [CrossRef]
- Kidane, H.; Farkas, I.; Buzás, J. Characterizing agricultural product drying in solar systems using thin-layer drying models: Comprehensive review. Discov. Food 2025, 5, 84. [Google Scholar] [CrossRef]
- Mary Leema, J.T.; Persia Jothy, T.; Dharani, G. Rapid Green Microwave Assisted Extraction of Lutein from Chlorella sorokiniana (NIOT-2)—Process Optimization. Food Chem. 2022, 372, 131151. [Google Scholar] [CrossRef]
- Wang, M.; Morón-Ortiz, Á.; Zhou, J.; Benítez-González, A.; Mapelli-Brahm, P.; Meléndez-Martínez, A.J.; Barba, F.J. Effects of Pressurized Liquid Extraction with Dimethyl Sulfoxide on the Recovery of Carotenoids and Other Dietary Valuable Compounds from the Microalgae Spirulina, Chlorella and Phaeodactylum tricornutum. Food Chem. 2023, 405, 134885. [Google Scholar] [CrossRef] [PubMed]
- Morón-Ortiz, Á.; Mapelli-Brahm, P.; León-Vaz, A.; Benitez-González, A.M.; León, R.; Meléndez-Martínez, A.J. Ultrasound-Assisted Extraction of Carotenoids from Phytoene-Accumulating Chlorella sorokiniana Microalgae: Effect of Milling and Performance of the Green Biosolvents 2-Methyltetrahydrofuran and Ethyl Lactate. Food Chem. 2024, 434, 137437. [Google Scholar] [CrossRef]
- Morón-Ortiz, Á.; Mapelli-Brahm, P.; Meléndez-Martínez, A.J. Sustainable Green Extraction of Carotenoid Pigments: Innovative Technologies and Bio-Based Solvents. Antioxidants 2024, 13, 239. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Legal Content (CELEX:32023L0175). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32023L0175 (accessed on 13 July 2025).
- Cano-Lamadrid, M.; Martínez-Zamora, L.; Mozafari, L.; Bueso, M.C.; Kessler, M.; Artés-Hernández, F. Response Surface Methodology to Optimize the Extraction of Carotenoids from Horticultural By-Products—A Systematic Review. Foods 2023, 12, 4456. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.; Mouro, C.; Pontes, R.; Nunes, J.; Gouveia, I. Ultrasound-Assisted Extraction of Bioactive Pigments from Spirulina platensis in Natural Deep Eutectic Solvents. Bioresour. Bioprocess. 2023, 10, 88. [Google Scholar] [CrossRef]
- Bachchhav, M.B.; Kulkarni, M.V.; Ingale, A.G. Process-Intensified Extraction of Phycocyanin Followed by β-Carotene from Spirulina platensis Using Ultrasound-Assisted Extraction. Sep. Sci. Technol. 2019, 55, 932–944. [Google Scholar] [CrossRef]
- Gerber, R.P.; Soares, R.P. Prediction of Infinite-Dilution Activity Coefficients Using UNIFAC and COSMO-SAC Variants. Ind. Eng. Chem. Res. 2010, 49, 7488–7496. [Google Scholar] [CrossRef]
- Ferrarini, F.; Flôres, G.B.; Muniz, A.R.; Soares, R.P. An Open and Extensible Sigma-Profile Database for COSMO-Based Models. AIChE J. 2018, 64, 3443–3455. [Google Scholar] [CrossRef]
- Trentin, J.; Mussagy, C.U.; Arantes, M.S.T.; Pedro, A.C.; Mafra, M.R.; Farias, F.O. Antioxidant Ready-to-Use Grape Pomace Extracts Recovered with Natural Eutectic Mixtures for Formulation of Color-Rich Gummies. Foods 2024, 13, 2840. [Google Scholar] [CrossRef]
- Castejón, N.; Parailloux, M.; Izdebska, A.; Lobinski, R.; Fernandes, S.C.M. Valorization of the Red Algae Gelidium sesquipedale by Extracting a Broad Spectrum of Minor Compounds Using Green Approaches. Mar. Drugs 2021, 19, 574. [Google Scholar] [CrossRef] [PubMed]
- Morón-Ortiz, Á.; Mapelli-Brahm, P.; León-Vaz, A.; Benitez-González, A.M.; Martín-Gómez, A.N.; León, R.; Meléndez-Martínez, A.J. Assessment of Milling and the Green Biosolvents Ethyl Lactate and 2-Methyltetrahydrofuran (2-Methyloxolane) for the Ultrasound-Assisted Extraction of Carotenoids in Common and Phytoene-Rich Dunaliella bardawil Microalgae. LWT Food Sci. Technol. 2024, 213, 117007. [Google Scholar] [CrossRef]
- Estévez-Santiago, R.; Olmedilla-Alonso, B.; Fernández-Jalao, I. Bioaccessibility of Provitamin a Carotenoids from Fruits: Application of a Standardised Static in Vitro Digestion Method. Food Funct. 2016, 7, 1354–1366. [Google Scholar] [CrossRef]
- Stinco, C.M.; Benítez-González, A.M.; Meléndez-Martínez, A.J.; Hernanz, D.; Vicario, I.M. Simultaneous Determination of Dietary Isoprenoids (Carotenoids, Chlorophylls and Tocopherols) in Human Faeces by Rapid Resolution Liquid Chromatography. J. Chromatogr. A 2019, 1583, 63–72. [Google Scholar] [CrossRef]
- Fazaeli, M.; Emam-Djomeh, Z.; Ashtari, A.K.; Omid, M. Effect of Spray Drying Conditions and Feed Composition on the Physical Properties of Black Mulberry Juice Powder. Food Bioprod. Process. 2012, 90, 667–675. [Google Scholar] [CrossRef]
- Jafari, S.M.; Ghalenoie, M.G.; Dehnad, D. Influence of Spray Drying on Water Solubility Index, Apparent Density, and Anthocyanin Content of Pomegranate Juice Powder. Powder Technol. 2017, 311, 59–65. [Google Scholar] [CrossRef]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic Light Scattering: A Practical Guide and Applications in Biomedical Sciences. Biophys. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef]
- Shishir, M.R.I.; Chen, W. Trends of Spray Drying: A Critical Review on Drying of Fruit and Vegetable Juices. Trends Food Sci. Technol. 2017, 65, 49–67. [Google Scholar] [CrossRef]
- Singh, R.; Saini, C.S. Influence of Different Drying Techniques on Dehydrated Tomato Powder. Int. J. Curr. Res. Rev. 2016, 8, 474–482. [Google Scholar]
- Varón, Y.E.; Fabiano-Tixier, A.-S.; Balcells, M.; Canela-Garayoa, R.; Bily, A.; Chemat, F. Is It Possible to Substitute Hexane with Green Solvents for Extraction of Carotenoids? Theoretical Versus Experimental Solubility Study. RSC Adv. 2016, 6, 105260–105267. [Google Scholar] [CrossRef]
- Valcareggi Morcelli, A.; da Silva Andrade, W.; Frankenberg, C.L.C.; Rech, R.; Marcílio, N.R. Extraction of Chlorophylls and Carotenoids from Microalgae: COSMO-SAC-Assisted Solvent Screening. Chem. Eng. Technol. 2021, 44, 1227–1232. [Google Scholar] [CrossRef]
- Kua, Y.L.; Gan, S.; Morris, A.; Ng, H.K. Ethyl Lactate as a Potential Green Solvent to Extract Hydrophilic (Polar) and Lipophilic (Non-Polar) Phytonutrients Simultaneously from Fruit and Vegetable By-Products. Sustain. Chem. Pharm. 2016, 4, 21–31. [Google Scholar] [CrossRef]
- Park, W.S.; Kim, H.-J.; Li, M.; Lim, D.H.; Kim, J.; Kwak, S.-S.; Kang, C.-M.; Ferruzzi, M.G.; Ahn, M.-J. Two Classes of Pigments, Carotenoids and C-Phycocyanin, in Spirulina Powder and Their Antioxidant Activities. Molecules 2018, 23, 2065. [Google Scholar] [CrossRef] [PubMed]
- Benucci, I.; Flore, M.; Esti, M. Partitioning Recovery of Natural Pigments from Spirulina platensis. Plant Foods Hum. Nutr. 2023, 78, 557–565. [Google Scholar] [CrossRef]
- Hynstova, V.; Sterbova, D.; Klejdus, B.; Hedbavny, J.; Huska, D.; Adam, V. Separation, Identification and Quantification of Carotenoids and Chlorophylls in Dietary Supplements Containing Chlorella vulgaris and Spirulina platensis Using High Performance Thin Layer Chromatography. J. Pharm. Biomed. Anal. 2018, 148, 108–118. [Google Scholar] [CrossRef]
- Mgoma, S.T.; Basitere, M.; Mshayisa, V.V.; De Jager, D. A Systematic Review on Sustainable Extraction, Preservation, and Enhancement in Food Processing: The Advancement from Conventional to Green Technology Through Ultrasound. Process 2025, 13, 965. [Google Scholar] [CrossRef]
- Kruszewski, B.; Boselli, E. Blackcurrant Pomace as a Rich Source of Anthocyanins: Ultrasound-Assisted Extraction under Different Parameters. Appl. Sci. 2024, 14, 821. [Google Scholar] [CrossRef]
- Saini, A.; Singh, J.; Kant, R. Comparative Study of Estimation Methods for Efficient Extraction of Chlorophyll a and Carotenoids Using Different Solvents. Bull. Pure Appl. Sci. Botany 2022, 41b, 79–86. [Google Scholar] [CrossRef]
- Rapinel, V.; Claux, O.; Abert-Vian, M.; McAlinden, C.; Bartier, M.; Patouillard, N.; Jacques, L.; Chemat, F. 2-Methyloxolane (2-MeOx) as Sustainable Lipophilic Solvent to Substitute Hexane for Green Extraction of Natural Products. Properties, Applications, and Perspectives. Molecules 2020, 25, 3417. [Google Scholar] [CrossRef]
- Drosaki, A.; Solomakou, N.; Kyriakoudi, A.; Mourtzinos, I.; Goula, A. Green Ultrasound-Assisted Extraction of Carotenoids from Peach Wastes Using Vegetable Oils. Waste Biomass Valorization 2025, 16, 3859–3872. [Google Scholar] [CrossRef]
- Martínez Girón, J.; Ordoñez Santos, L.E.; Rodríguez-Rodríguez, D.X. Extraction of Total Carotenoids from Peach Palm Fruit (Bactris gasipaes) Peel by Means of Ultrasound Application and Vegetable Oil. DYNA 2019, 86, 91–96. [Google Scholar] [CrossRef]
- Mozafari, L.; Cano-Lamadrid, M.; Martínez-Zamora, L.; Bueso, M.C.; Kessler, M.; Artés-Hernández, F. Pulsed Ultrasound-Assisted Extraction of Lycopene and β-Carotene from Industrial Grated Tomato By-Products. LWT Food Sci. Technol. 2024, 204, 116462. [Google Scholar] [CrossRef]
- Emam, H.A.; Abdel-Sattar, E.; Salama, M.M.; Salem, M.A.; Hashem, M.M. Spirulina platensis: Unveiling Phenotypic Plasticity Impact on Its Metabolic Profile and Bioactivity via Chemometric Analysis. Food Biosci. 2025, 63, 105605. [Google Scholar] [CrossRef]
- Eun, J.B.; Maruf, A.; Das, P.R.; Nam, S.H. A Review of Encapsulation of Carotenoids Using Spray Drying and Freeze Drying. Crit. Rev. Food Sci. Nutr. 2019, 60, 3547–3572. [Google Scholar] [CrossRef]
- Gandul-Rojas, B.; Roca, M.; Gallardo-Guerrero, L. Chlorophylls and Carotenoids in Food Products from Olive Tree. In Products from Olive Tree; InTechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Rebecca, L.J.; Sharmila, S.; Paul Das, M.; Seshiah, C. Extraction and Purification of Carotenoids from Vegetables. J. Chem. Pharm. Res. 2014, 6, 594–598. [Google Scholar]
- Podsędek, A.; Sosnowska, D.; Redzynia, M.; Anders, B. Antioxidant Capacity and Content of Brassica oleracea Dietary Antioxidants. Int. J. Food Sci. Technol. 2006, 41 (Suppl. S1), 49–58. [Google Scholar] [CrossRef]
- Veza, I.; Spraggon, M.; Fattah, I.M.R.; Idris, M. Response Surface Methodology (RSM) for Optimizing Engine Performance and Emissions Fueled with Biofuel: Review of RSM for Sustainability Energy Transition. Results Eng. 2023, 18, 101213. [Google Scholar] [CrossRef]
- Wang, L.; Lu, W.; Li, J.; Hu, J.; Ding, R.; Lv, M.; Wang, Q. Optimization of Ultrasonic-Assisted Extraction and Purification of Zeaxanthin and Lutein in Corn Gluten Meal. Molecules 2019, 24, 2994. [Google Scholar] [CrossRef]
- Janepinich, P.; Satirapipathkul, C.; Kasetsomboon, N.; Yongphet, P. Enhancing Lutein Extraction from Marigolds Through Ultrasound-Assisted Optimization Using Response Surface Methodology. Maejo Int. J. Energy Environ. Commun. 2023, 5, 248359. [Google Scholar] [CrossRef]
- Priyanka, S.; Kirubagaran, R.; Mary Leema, J.T. Optimization of Ultrasound-Assisted Extraction (UAE) of Zeaxanthin from Marine Microalgae Dunaliella tertiolecta (NIOT 141) Using Response Surface Methodology. Res. J. Pharm. Technol. 2021, 14, 1729–1735. [Google Scholar] [CrossRef]
- Singh, Y.; Mazumder, A.; Giri, A.; Mishra, H.N. Optimization of Ultrasound-Assisted Extraction of β-Carotene from Chlorella Biomass (MCC7) and Its Use in Fortification of Apple Jam. J. Food Process. Eng. 2017, 40, e12321. [Google Scholar] [CrossRef]
- Singh, D.; Barrow, C.J.; Mathur, A.S.; Tuli, D.K.; Puri, M. Optimization of Zeaxanthin and β-Carotene Extraction from Chlorella saccharophila Isolated from New Zealand Marine Waters. Biocatal. Agric. Biotechnol. 2015, 4, 316–320. [Google Scholar] [CrossRef]
- Yang, D.; Qiu, W.; Xu, Y.; Hu, Z.; Wang, L. Optimisation and Modelling of Ultrasonic-Assisted Extraction of Canthaxanthin from Chromochloris zofingiensis Using Eutectic Solvents. Ind. Crops Prod. 2023, 202, 117002. [Google Scholar] [CrossRef]
- Şahin, S.; Nasir, N.T.B.M.; Erken, I.; Çakmak, Z.E.; Çakmak, T. Antioxidant Composite Films with Chitosan and Carotenoid Extract from Chlorella vulgaris: Optimization of Ultrasonic-Assisted Extraction of Carotenoids and Surface Characterization of Chitosan Films. Mater. Res. Express 2019, 6, 115024. [Google Scholar] [CrossRef]
- ElGamal, R.; Song, C.; Rayan, A.M.; Liu, C.; Al-Rejaie, S.; ElMasry, G. Thermal Degradation of Bioactive Compounds During Drying Process of Horticultural and Agronomic Products: A Comprehensive Overview. Agronomy 2023, 13, 1580. [Google Scholar] [CrossRef]
- Show, K.Y.; Lee, D.J.; Tay, J.H.; Lee, T.M.; Chang, J.S. Microalgal Drying and Cell Disruption—Recent Advances. Bioresour. Technol. 2015, 184, 3–12. [Google Scholar] [CrossRef]
- Chen, C.L.; Chang, J.S.; Lee, D.J. Dewatering and Drying Methods for Microalgae. Dry. Technol. 2015, 33, 443–454. [Google Scholar] [CrossRef]
- Stenutz, C. Solvent Properties: 2-Methyltetrahydrofuran. Available online: https://www.stenutz.eu/chem/solv6.php?name=2-methyltetrahydrofuran (accessed on 13 July 2025).
- Stenutz, C. Solvent Properties: Ethanol. Available online: https://www.stenutz.eu/chem/solv6.php?name=ethanol (accessed on 13 July 2025).
- Meléndez-Martínez, A.J.; Mandić, A.I.; Bantis, F.; Böhm, V.; Borge, G.I.A.; Brnčić, M.; Bysted, A.; Cano, M.P.; Dias, M.G.; Elgersma, A.; et al. A comprehensive review on carotenoids in foods and feeds: Status quo, applications, patents, and research needs. Crit. Rev. Food Sci. Nutr. 2022, 62, 1999–2049. [Google Scholar] [CrossRef]
- Maddiboyina, B.; Vanamamalai, H.K.; Roy, H.; Ramaiah; Gandhi, S.; Kavisri, M.; Moovendhan, M. Food and Drug Industry Applications of Microalgae Spirulina platensis: A Review. J. Basic Microbiol. 2023, 63, e202200704. [Google Scholar] [CrossRef]





| Run | A (%) | B (min) | C (mL/g) |
|---|---|---|---|
| 1 | 38.1 | 1.6 | 18.1 |
| 2 | 50.0 | 2.5 | 30.0 |
| 3 | 50.0 | 1.0 | 30.0 |
| 4 | 61.9 | 1.6 | 18.1 |
| 5 | 50.0 | 2.5 | 30.0 |
| 6 | 61.9 | 3.4 | 18.1 |
| 7 | 50.0 | 2.5 | 30.0 |
| 8 | 38.1 | 1.6 | 41.9 |
| 9 | 50.0 | 2.5 | 10.0 |
| 10 | 70.0 | 2.5 | 30.0 |
| 11 | 38.1 | 3.4 | 18.1 |
| 12 | 61.9 | 3.4 | 41.9 |
| 13 | 38.1 | 3.4 | 41.9 |
| 14 | 30.0 | 2.5 | 30.0 |
| 15 | 50.0 | 2.5 | 30.0 |
| 16 | 61.9 | 1.6 | 41.9 |
| 17 | 50.0 | 2.5 | 30.0 |
| 18 | 50.0 | 4.0 | 30.0 |
| 19 | 50.0 | 2.5 | 30.0 |
| 20 | 50.0 | 2.5 | 50.0 |
| Run | A | B | C | SD | SolD | ||||
|---|---|---|---|---|---|---|---|---|---|
| Zeaxanthin | β-Carotene | TCC | Zeaxanthin | β-Carotene | TCC | ||||
| 1 | 38.1 | 1.6 | 18.1 | 670.24 | 1249.27 | 1919.51 | 110.54 | 215.93 | 326.47 |
| 2 | 50.0 | 2.5 | 30.0 | 602.89 | 1106.14 | 1709.02 | 230.55 | 381.37 | 611.92 |
| 3 | 50.0 | 1.0 | 30.0 | 363.24 | 866.23 | 1229.47 | 166.33 | 275.30 | 441.63 |
| 4 | 61.9 | 1.6 | 18.1 | 347.50 | 1338.45 | 1685.95 | 191.04 | 230.41 | 421.45 |
| 5 | 50.0 | 2.5 | 30.0 | 631.50 | 1140.87 | 1772.37 | 212.52 | 385.44 | 597.96 |
| 6 | 61.9 | 3.4 | 18.1 | 532.55 | 1627.99 | 2160.54 | 339.49 | 529.87 | 869.36 |
| 7 | 50.0 | 2.5 | 30.0 | 571.99 | 1106.14 | 1678.13 | 219.64 | 394.19 | 613.83 |
| 8 | 38.1 | 1.6 | 41.9 | 258.20 | 442.29 | 700.49 | 186.05 | 455.35 | 641.40 |
| 9 | 50.0 | 2.5 | 10.0 | 566.78 | 2048.67 | 2615.44 | 142.47 | 337.68 | 480.15 |
| 10 | 70.0 | 2.5 | 30.0 | 492.65 | 1094.56 | 1587.20 | 380.94 | 357.12 | 738.06 |
| 11 | 38.1 | 3.4 | 18.1 | 588.36 | 1644.27 | 2232.63 | 109.93 | 388.14 | 498.07 |
| 12 | 61.9 | 3.4 | 41.9 | 557.96 | 876.77 | 1434.73 | 419.22 | 375.73 | 794.95 |
| 13 | 38.1 | 3.4 | 41.9 | 277.25 | 676.60 | 953.85 | 194.73 | 358.13 | 552.86 |
| 14 | 30.0 | 2.5 | 30.0 | 486.35 | 832.88 | 1319.23 | 136.85 | 352.46 | 489.31 |
| 15 | 50.0 | 2.5 | 30.0 | 602.51 | 1048.45 | 1650.95 | 204.16 | 405.48 | 609.64 |
| 16 | 61.9 | 1.6 | 41.9 | 329.58 | 829.00 | 1158.58 | 293.21 | 344.67 | 637.88 |
| 17 | 50.0 | 2.5 | 30.0 | 605.65 | 1219.43 | 1825.09 | 210.31 | 382.34 | 592.65 |
| 18 | 50.0 | 4.0 | 30.0 | 559.40 | 1279.41 | 1838.81 | 290.51 | 459.98 | 750.49 |
| 19 | 50.0 | 2.5 | 30.0 | 588.01 | 1106.14 | 1694.15 | 218.44 | 387.33 | 605.77 |
| 20 | 50.0 | 2.5 | 50.0 | 275.76 | 631.32 | 907.07 | 308.43 | 352.31 | 660.74 |
| Response | Regression Model (Coded Variables) | R2 | |
|---|---|---|---|
| SD | Zeaxanthin | 980 − 5.25 A + 38.0 B − 16.78 C − 0.2825 A×A − 62.75 B × B − 0.4531 C × C + 5.613 A × B + 0.6458 A × C + 1.700 B × C | 0.9905 |
| β-Carotene | 443 + 47.1 A + 612 B − 73.45 C − 0.4537 A × A − 32.2 B × B + 0.4870 C × C − 3.44 A × B + 0.454 A × C − 4.74 B × C | 0.9896 | |
| TCC | 1423 + 41.9 A + 650 B − 90.2 C − 0.736 A × A − 94.9 B × B + 0.034 C × C + 2.17 A × B + 1.100 A × C − 3.04 B × C | 0.9915 | |
| SolD | Zeaxanthin | 354.3 − 11.43 A − 128.6 B + 2.58 C + 0.0949 A × A + 3.33 B × B + 0.0113 C × C + 3.139 A × B + 0.0191 A × C − 0.155 B × C | 0.9921 |
| β-Carotene | −564 + 6.89 A + 135.4 B + 33.95 C − 0.0737 A × A − 7.40 B × B − 0.0982 C × C + 3.011 A × B − 0.2203 A × C − 6.338 B × C | 0.9819 | |
| TCC | −210 − 4.54 A + 6.7 B + 36.53 C + 0.0212 A × A − 4.07 B × B − 0.0869 C × C + 6.151 A × B − 0.2013 A × C − 6.493 B × C | 0.9952 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Rodríguez, E.; Morón-Ortiz, Á.; Mapelli-Brahm, P.; Mussagy, C.U.; Farias, F.O.; Olmedilla-Alonso, B.; Meléndez-Martínez, A.J. Ultrasound-Assisted Extraction of Spirulina platensis Carotenoids: Effect of Drying Methods and Performance of the Emerging Biosolvents 2-Methyltetrahydrofuran and Ethyl Lactate. Molecules 2025, 30, 3881. https://doi.org/10.3390/molecules30193881
Rodríguez-Rodríguez E, Morón-Ortiz Á, Mapelli-Brahm P, Mussagy CU, Farias FO, Olmedilla-Alonso B, Meléndez-Martínez AJ. Ultrasound-Assisted Extraction of Spirulina platensis Carotenoids: Effect of Drying Methods and Performance of the Emerging Biosolvents 2-Methyltetrahydrofuran and Ethyl Lactate. Molecules. 2025; 30(19):3881. https://doi.org/10.3390/molecules30193881
Chicago/Turabian StyleRodríguez-Rodríguez, Elena, Ángeles Morón-Ortiz, Paula Mapelli-Brahm, Cassamo U. Mussagy, Fabiane O. Farias, Begoña Olmedilla-Alonso, and Antonio J. Meléndez-Martínez. 2025. "Ultrasound-Assisted Extraction of Spirulina platensis Carotenoids: Effect of Drying Methods and Performance of the Emerging Biosolvents 2-Methyltetrahydrofuran and Ethyl Lactate" Molecules 30, no. 19: 3881. https://doi.org/10.3390/molecules30193881
APA StyleRodríguez-Rodríguez, E., Morón-Ortiz, Á., Mapelli-Brahm, P., Mussagy, C. U., Farias, F. O., Olmedilla-Alonso, B., & Meléndez-Martínez, A. J. (2025). Ultrasound-Assisted Extraction of Spirulina platensis Carotenoids: Effect of Drying Methods and Performance of the Emerging Biosolvents 2-Methyltetrahydrofuran and Ethyl Lactate. Molecules, 30(19), 3881. https://doi.org/10.3390/molecules30193881










