Chemical, Bioactive, and Functional Characterization of a Protein Preparation from Prunus padus L. Flour
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Composition
2.2. Selected Bioactive Compounds and Antioxidant Capacity
2.3. Amino Acid Composition
2.4. Fatty Acid Composition
2.5. Functional Properties
2.5.1. Solubility, Water- and Oil-Absorption Capacity
2.5.2. Emulsifying Properties
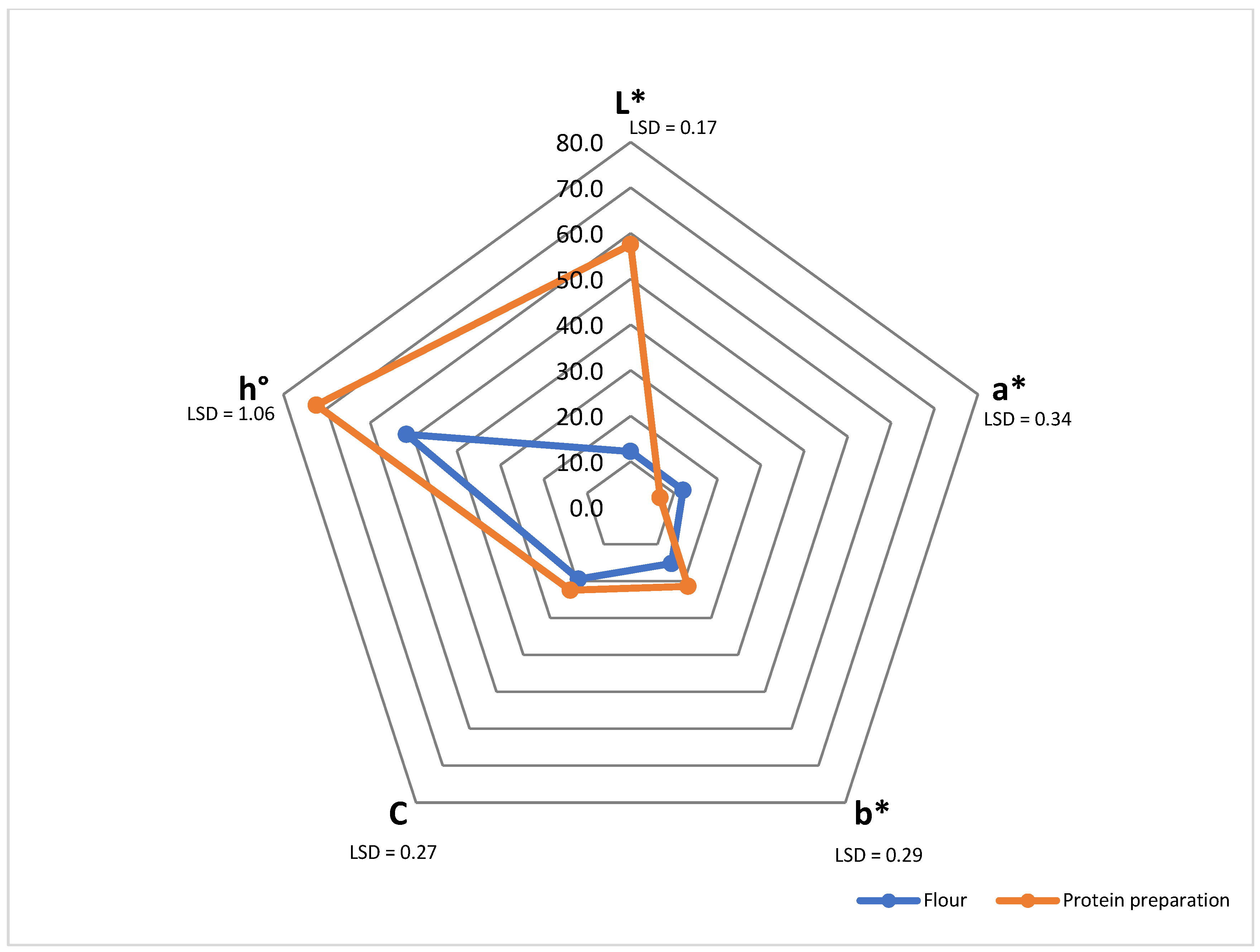
2.5.3. Colour
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Preparation of Protein Preparation
3.3. Chemical Composition
3.4. Total Polyphenolic Compounds and Antioxidant Activity
3.5. Amygdalin Content
3.6. Amino Acid Composition
3.7. Fatty Acid Profile
3.8. Functional Properties
3.8.1. Protein Solubility Index
3.8.2. Water- and Oil-Absorption Capacity
3.8.3. Emulsifying Properties
3.8.4. Colour Determination
3.9. Statistical Analysis
4. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Świderski, F. Żywność Wygodna I Żywność Funkcjonalna; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2018. [Google Scholar]
- Marta, O.; Nowak, R.; Los, R.; Rzymowska, J.; Malm, A.; Katarzyna, C. Biological activity and composition of teas and tinctures prepared from Rosa rugosa Thunb. Cent. Eur. J. Biol. 2012, 7, 172–182. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Hertog, M.G.L.; Hollman, P.C.H.; Katan, M.B. Content of potentially anticarcinogenic flavonoids of 28 vegetables and 9 fruits commonly consumed in the Netherlands. J. Agric. Food Chem. 1992, 40, 2379–2383. [Google Scholar] [CrossRef]
- Telichowska, A.; Kobus-Cisowska, J.; Szulc, P. Phytopharmacological Possibilities of Bird Cherry. Nutrients 2020, 12, 1966. [Google Scholar] [CrossRef] [PubMed]
- Donno, D.; Mellano, M.G.; De Biaggi, M.; Riondato, I.; Rakotoniaina, E.N.; Beccaro, G.L. New findings in Prunus padus L. Fruits as a source of natural compounds: Characterization of metabolite profiles and preliminary evaluation of antioxidant activity. Molecules 2018, 23, 725. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Langyan, S.; Sangwan, S.; Rohtagi, B.; Khandelwal, A.; Shrivastava, M. Protein for Human Consumption From Oilseed Cakes: A Review. Front. Sustain. Food Syst. 2022, 6, 856401. [Google Scholar] [CrossRef]
- Telichowska, A.; Kobus-Cisowska, J.; Stuper-Szablewska, K.; Ligaj, M.; Tichoniuk, M.; Szymanowska, D.; Szulc, P. Exploring antimicrobial and antioxidant properties of phytocomponents from different anatomical parts of Prunus padus L. Int. J. Food Prop. 2020, 23, 2097–2109. [Google Scholar] [CrossRef]
- Telichowska, A.; Kobus-Cisowska, J.; Cielecka-Piontek, J.; Sip, S.; Stuper-Szablewska, K.; Szulc, P. Prunus padus L. as a source of functional compounds—Antioxidant activity and antidiabetic effect. Emir. J. Food Agric. 2022, 34, 135–143. [Google Scholar] [CrossRef]
- García-Aguilar, L.; Rojas-Molina, A.; Ibarra-Alvarado, C.; Rojas-Molina, J.I.; Vázquez-Landaverde, P.A.; Luna-Vázquez, F.J.; Zavala-Sánchez, M.A. Nutritional value and volatile compounds of black cherry (Prunus serotina) seeds. Molecules 2015, 20, 3479–3495. [Google Scholar] [CrossRef]
- López-Calabozo, R.; Martínez-Martín, I.; Rodríguez-Fernández, M.; Absi, Y.; Vivar-Quintana, A.M.; Revilla, I. The Influence of the Nutritional and Mineral Composition of Vegetable Protein Concentrates on Their Functional Properties. Foods 2025, 14, 509. [Google Scholar] [CrossRef]
- Jimenez-Pulido, I.J.; Rico, D.; Perez, J.; Martinez-Villaluenga, C.; Daniel De, L.; Martin Diana, A.B. Impact of Protein Content on the Antioxidants, Anti-Inflammatory Properties and Glycemic Index of Wheat and Wheat Bran. Foods 2022, 11, 2049. [Google Scholar] [CrossRef]
- Mustafa, M.A.M.; Sorour, M.A.H.; Mehanni, A.H.E.; Hussien, S.M. Amino acid profile, physico-chemical properties and fatty acids composition of some fruit seed kernels after detoxification. Chem. Biol. Technol. Agric. 2023, 10, 37. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The health benefits of dietary fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Te Morenga, L. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Whole grains and human health. Nutr. Res. Rev. 2004, 17, 99–110. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Drăghici-Popa, A.M.; Pârvulescu, O.C.; Stan, R.; Brezoiu, A.M. Optimization of Ultrasound-Assisted Extraction of Phenolic Compounds from Romanian Blackthorn (Prunus spinosa L.) Fruits. Antioxidants 2025, 14, 3–13. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Laskowski, P.; Oszmiański, J. Evaluation of sour cherry (Prunus cerasus L.) fruits for their polyphenol content, antioxidant properties, and nutritional components. J. Agric. Food Chem. 2014, 24, 12332–12345. [Google Scholar] [CrossRef]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef]
- Feng, Y.; Jin, C.; Lv, S.; Zhang, H.; Ren, F.; Wang, J. Molecular Mechanisms and Applications of Polyphenol-Protein Complexes with Antioxidant Properties: A Review. Antioxidants 2023, 12, 1577. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Lante, A.; Grossmann, L. Protein-polyphenol complexation vs. conjugation: A review on mechanisms, functional differences, and antioxidant-emulsifier roles. Food Hydrocoll. 2025, 169, 111590. [Google Scholar] [CrossRef]
- Bolarinwa, I.F.; Orfila, C.; Morgan, M.R.A. Determination of amygdalin in apple seeds, fresh apples and processed apple juices. Food Chem. 2015, 170, 437–442. [Google Scholar] [CrossRef]
- Zhong, Y.; Xu, T.; Ji, S.; Wu, X.; Zhao, T.; Li, S.; Zhang, P.; Li, K.; Lu, B. Effect of ultrasonic pretreatment on eliminating cyanogenic glycosides and hydrogen cyanide in cassava. Ultrason. Sonochem. 2021, 78, 105742. [Google Scholar] [CrossRef] [PubMed]
- Winiarska-Mieczan, A.; Kwiecień, M.; Jachimowicz-Rogowska, K.; Krusiński, R. Amigdalina-analiza jej toksycznego i antynowotwo rowego działania. J. Anim. Sci. Biol. Bioecon. 2024, 40, 5–13. [Google Scholar] [CrossRef]
- Bolarinwa, I.F.; Orfila, C.; Morgan, M.R.A. Amygdalin content of seeds, kernels and food products commercially-available in the UK. Food Chem. 2014, 152, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhao, B.; Cai, Z.; Yan, J.; Ma, R.; Yu, M. Amino Acid Profiles in Peach (Prunus persica L.) Fruit. Foods 2022, 11, 1718. [Google Scholar] [CrossRef]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; Hogstrand, C.; et al. Erucic acid in feed and food. EFSA J. 2016, 14, e04593. [Google Scholar] [CrossRef]
- Kersting, M.; Kalhoff, H.; Honermeier, B.; Sinningen, K.; Lücke, T. Erucic acid exposure during the first year of life—Scenarios with precise food-based dietary guidelines. Food Sci. Nutr. 2022, 10, 115–121. [Google Scholar] [CrossRef]
- Vetter, W.; Darwisch, V.; Lehnert, K. Erucic acid in Brassicaceae and salmon—An evaluation of the new proposed limits of erucic acid in food. NFS J. 2020, 19, 9–15. [Google Scholar] [CrossRef]
- Roslinsky, V.; Falk, K.C.; Gaebelein, R.; Mason, A.S.; Eynck, C. Development of B. carinata with super-high erucic acid content through interspecific hybridization. Theor. Appl. Genet. 2021, 134, 3167–3181. [Google Scholar] [CrossRef]
- Sharafi, Y.; Majidi, M.M.; Goli, S.A.H.; Rashidi, F. Oil content and fatty acids composition in Brassica Species. Int. J. Food Prop. 2015, 18, 2145–2154. [Google Scholar] [CrossRef]
- Pan, X.; Caldwell, C.D.; Falk, K.C.; Lada, R. The effect of cultivar, seeding rate and applied nitrogen on Brassica carinata seed yield and quality in contrasting environments. Can. J. Plant Sci. 2012, 92, 961–971. [Google Scholar] [CrossRef]
- Salejda, A.M.; Olender, K.; Zielińska-Dawidziak, M.; Mazur, M.; Szperlik, J.; Miedzianka, J.; Zawiślak, I.; Kolniak-Ostek, J.; Szmaja, A. Frankfurter-Type Sausage Enriched with Buckwheat By-Product as a Source of Bioactive Compounds. Foods 2022, 11, 674. [Google Scholar] [CrossRef] [PubMed]
- Oomah, B.D.; Mazza, G. Flaxseed proteins-a review. Food Chem. 1993, 48, 109–114. [Google Scholar] [CrossRef]
- Tang, C.H.; Ten, Z.; Wang, X.S.; Yang, X.Q. Physicochemical and functional properties of hemp (Cannabis sativa L.) protein isolate. J. Agric. Food Chem. 2006, 54, 8945–8950. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, N. Studies on functional, thermal and pasting properties of flours from different chickpea (Cicer arietinum L.) cultivars. Food Chem. 2005, 91, 403–411. [Google Scholar] [CrossRef]
- Butt, M.S.; Batool, R. The nutritional and functional properties of wheat. Pak. J. Nutr. 2010, 9, 373–379. [Google Scholar] [CrossRef]
- Sosulski, F.W. The centrifuge method for determining flour absorption in hard red spring wheats. Cereal Chem. 1962, 39, 344–349. [Google Scholar]
- Embaby, H.E.; Rayan, A.M. Chemical composition and nutritional evaluation of the seeds of Acacia tortilis (Forssk.) Hayne ssp. raddiana. Food Chem. 2016, 200, 62–68. [Google Scholar] [CrossRef]
- Hrncic, M.K.; Ivanovski, M.; Cor, D.; Knez, Z. Chia Seeds (Salvia Hispanica L.): An Overview—Phytochemical Profile, Isolation Methods, and Application. Molecules 2020, 25, 11. [Google Scholar] [CrossRef]
- Barać, M.B.; Pešić, M.B.; Stanojević, S.P.; Kostić, A.Z.; Čabrilo, S.B. Techno-functional properties of pea (Pisum sativum) protein isolates-a review. Acta Period. Technol. 2015, 46, 1–18. [Google Scholar] [CrossRef]
- Zheng, J.; Sun, D.; Li, X.; Liu, D.; Li, C.; Zheng, Y.; Yue, X.; Shao, J.-H. The effect of fatty acid chain length and saturation on the emulsification properties of pork myofibrillar proteins. LWT-Food Sci. Technol. 2021, 139, 110242. [Google Scholar] [CrossRef]
- Tang, Y.R.; Ghosh, S. A Review of the Utilization of Canola Protein as an Emulsifier in the Development of Food Emulsions. Molecules 2023, 28, 8086. [Google Scholar] [CrossRef] [PubMed]
- Can Karaca, A.; Tan, C.; Assadpour, E.; Jafari, S.M. Recent advances in the plant protein-polyphenol interactions for the stabilization of emulsions. Adv. Colloid Interface Sci. 2025, 335, 103339. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Ovando, A.; Betancur-Ancona, D.; Chel-Guerrero, L. Physicochemical and functional properties of a protein-rich fraction produced by dry fractionation of chia seeds (Salvia hispanica L.). CYTA-J. Food 2013, 11, 75–80. [Google Scholar] [CrossRef]
- Stone, A.K.; Karalash, A.; Tyler, R.T.; Warkentin, T.D.; Nickerson, M.T. Functional attributes of pea protein isolates prepared using different extraction methods and cultivars. Food Res. Int. 2015, 76, 31–38. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Kaur, A.; Rana, J.C. Structural and functional characterization of kidney bean and field pea protein isolates: A comparative study. Food Hydrocoll. 2015, 43, 679–689. [Google Scholar] [CrossRef]
- Hahn, A.; Liszka, J.; Maksym, J.; Nemś, A.; Miedzianka, J. Preliminary Data of the Nutritive, Antioxidative, and Functional Properties of Watermelon (Citrullus lanatus L.) Flour and Seed Protein Concentrate. Molecules 2025, 30, 181. [Google Scholar] [CrossRef]
- Al-mentafji, H.N. Official Methods of Analysis of AOAC International; Association of Officiating Analytical Chemists: Washington, DC, USA, 2005. [Google Scholar]
- Lindsay, H. A colorimetric estimation of reducing sugars in potatoes with 3,5-dinitrosalicylic acid. Potato Res. 1973, 16, 176–179. [Google Scholar] [CrossRef]
- Gao, X.; Ohlander, M.; Jeppsson, N.; Bjork, L.; Trajkovski, V. Changes in antioxidant effects and their relationship to phytonutrients in fruits of sea buckthorn (Hippophae rhamnoides L.) during maturation. J. Agric. Food Chem. 2000, 48, 1485–1490. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 236, 1231–1237. [Google Scholar] [CrossRef]
- Miedzianka, J.; Zambrowicz, A.; Zielińska-Dawidziak, M.; Drożdż, W.; Nemś, A. Effect of Acetylation on Physicochemical and Functional Properties of Commercial Pumpkin Protein Concentrate. Molecules 2021, 26, 1575. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Adhikari, R.; Barrow, C.J.; Adhikari, B. Physicochemical and functional properties of protein isolate produced from Australian chia seeds. Food Chem. 2016, 212, 648–656. [Google Scholar] [CrossRef]
- Pęksa, A.; Miedzianka, J.; Szumny, A.; Łyczko, J.; Nemś, A.; Kita, A. Colour and flavour of potato protein preparations, depending on the antioxidants and coagulants used. Int. J. Food Sci. Technol. 2020, 55, 2323–2334. [Google Scholar] [CrossRef]

| Flour | Protein Preparation | |
|---|---|---|
| g/100 g | ||
| Dry matter | 91.26 ± 0.02 b | 96.58 ± 0.19 a |
| Total protein | 15.44 ± 0.21 b | 39.72 ± 0.21 a |
| Fat | 11.76 ± 0.01 a | 8.94 ± 0.12 b |
| Ash | 4.78 ± 0.01 b | 8.53 ± 0.01 a |
| Carbohydrates | 59.28 ± 0.11 a | 39.39 ± 0.16 b |
| Fibre | 9.9 ± 0.01 b | 36.32 ± 0.01 a |
| Total sugars | 16.88 ± 0.01 a | 1.09 ±0.03 b |
| Reducing sugars | 12.69 ± 0.02 a | 0.82 ±0.02 b |
| Analysed Material | TPC (mg GAE/g DM) | ABTS (µmol Trolox/g DM) | Amygdalin (mg/g) |
|---|---|---|---|
| Flour | 15.38 ± 0.07 a | 26.36 ± 2.27 a | 1.72 ± 0.01 a |
| Protein preparation | 15.31 ± 0.02 a | 29.26 ± 2.48 a | 0.15 ± 0.01 b |
| Amino Acid | Flour | Protein Preparation |
|---|---|---|
| mg/g | ||
| IAA * | ||
| Leucine | 9.80 ± 0.06 b | 35.12 ± 0.53 a |
| Isoleucine | 5.97 ± 0.04 b | 19.01 ± 0.36 a |
| Methionine | 2.88 ± 0.05 a | 2.57 ± 0.10 a |
| Cysteine | 0.33 ± 0.05 a | 0.18 ± 0.17 a |
| Phenylalanine | 5.32 ± 0.01 b | 22.10 ± 0.42 a |
| Threonine | 4.55 ± 0.28 b | 13.89 ± 0.38 a |
| Lysine | 10.07 ± 0.06 b | 14.84 ± 0.24 a |
| Tyrosine | 2.54 ± 0.01 b | 12.07 ± 0.26 a |
| Valine | 5.86 ± 0.20 b | 22.40 ± 0.50 a |
| DAA ** | ||
| Aspartic acid | 21.14 ± 0.89 b | 36.43 ± 0.82 a |
| Glutamic acid | 24.96 ± 0.66 b | 74.41 ± 1.25 a |
| Serine | 5.92 ± 0.35 b | 14.63 ± 0.48 a |
| Glycine | 9.11 ± 0.23 b | 22.55 ± 0.42 a |
| Alanine | 4.50 ± 0.08 b | 16.19 ± 0.45 a |
| Histidine | 3.51 ± 0.44 a | 2.71 ± 0.07 b |
| Arginine | 5.88 ± 0.91 b | 17.90 ± 0.72 a |
| Proline | 5.64 ± 0.57 a | 18.41 ± 0.15 a |
| Total amino acids | 122.05 ± 0.26 b | 345.40 ± 6.97 a |
| Fatty Acid | Flour | Protein Preparation |
|---|---|---|
| % of Total Fatty Acid Profile | ||
| Palmitic acid (C16:0) | 1.42 ± 0.01 b | 2.24 ± 0.01 a |
| Stearic acid (C18:0) | 0.22 ± 0.01 b | 2.72 ± 0.01 a |
| Oleic acid (C18:1) | 5.35 ± 0.01 b | 6.65± 0.01 a |
| Linoleic acid (C18:2) | 0.79 ± 0.01 b | 1.66 ± 0.01 a |
| α-linolenic acid (C18:3n3) | 0.91 ± 0.01 b | 2.17 ± 0.01 a |
| Arachidic acid (C20:0) | 0.15 ± 0.01 a | 0.16 ± 0.01 a |
| Eicosenoic acid (C20:1) | 15.51 ± 0.01 b | 16.61 ± 0.01 a |
| Eicosadienoic acid (C20:2) | 0.06 ± 0.01 a | 0.07 ± 0.00 a |
| Eicosatrienoic acid (C20:3n3) | 0.06 ± 0.01 a | 0.06 ± 0.01 a |
| Arachidonic acid (C20:4) | 0.07 ± 0.00 b | 0.10 ± 0.00 a |
| Erucic acid (C22:1) | 74.55 ± 0.01 a | 68.16 ± 0.01 b |
| Docosadienoic acid (C22:2) | 0.10 ± 0.00 a | 0.12 ± 0.01 a |
| Lignoceric acid (C24:0) | 0.22 ± 0.01 a | 0.23 ± 0.01 a |
| Nervonic acid (C24:1) | 1.54 ± 0.01 a | 1.51 ± 0.01 a |
| ∑ SFA | 2.01 | 5.34 |
| ∑ MUFA | 96.94 | 92.92 |
| ∑ PUFA | 1.98 | 4.16 |
| Functional Property | Flour | Protein Preparation |
|---|---|---|
| PSI (%) | 28.4 ± 1.20 b | 76.8 ± 2.10 a |
| WAC (g water/g preparation) | 2.72 ± 0.11 a | 2.15 ± 0.07 b |
| WAC (g water/g protein) | 0.42 ± 0.11 b | 0.85 ± 0.07 a |
| OAC (ml oil/g preparation) | 5.42 ± 0.07 a | 1.67 ± 0.05 b |
| OAC (ml oil/g protein) | 0.84 ± 0.07 a | 0.66 ± 0.05 a |
| Emulsifying Properties | EA | ES |
|---|---|---|
| % | ||
| Flour | 82.22 ± 0.12 a | 35.56 ± 0.07 b |
| Protein preparation | 47.53 ± 0.22 b | 63.22 ± 0.08 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kusak, I.; Miedzianka, J.; Nemś, A.; Kosmenda, A.; Wolny, S. Chemical, Bioactive, and Functional Characterization of a Protein Preparation from Prunus padus L. Flour. Molecules 2025, 30, 3766. https://doi.org/10.3390/molecules30183766
Kusak I, Miedzianka J, Nemś A, Kosmenda A, Wolny S. Chemical, Bioactive, and Functional Characterization of a Protein Preparation from Prunus padus L. Flour. Molecules. 2025; 30(18):3766. https://doi.org/10.3390/molecules30183766
Chicago/Turabian StyleKusak, Izabela, Joanna Miedzianka, Agnieszka Nemś, Alicja Kosmenda, and Szymon Wolny. 2025. "Chemical, Bioactive, and Functional Characterization of a Protein Preparation from Prunus padus L. Flour" Molecules 30, no. 18: 3766. https://doi.org/10.3390/molecules30183766
APA StyleKusak, I., Miedzianka, J., Nemś, A., Kosmenda, A., & Wolny, S. (2025). Chemical, Bioactive, and Functional Characterization of a Protein Preparation from Prunus padus L. Flour. Molecules, 30(18), 3766. https://doi.org/10.3390/molecules30183766






