Thiazolylcyanocyclopropanes: Novel Donor–Acceptor Cyclopropanes for Accessing Thiazole-Containing Targets
Abstract
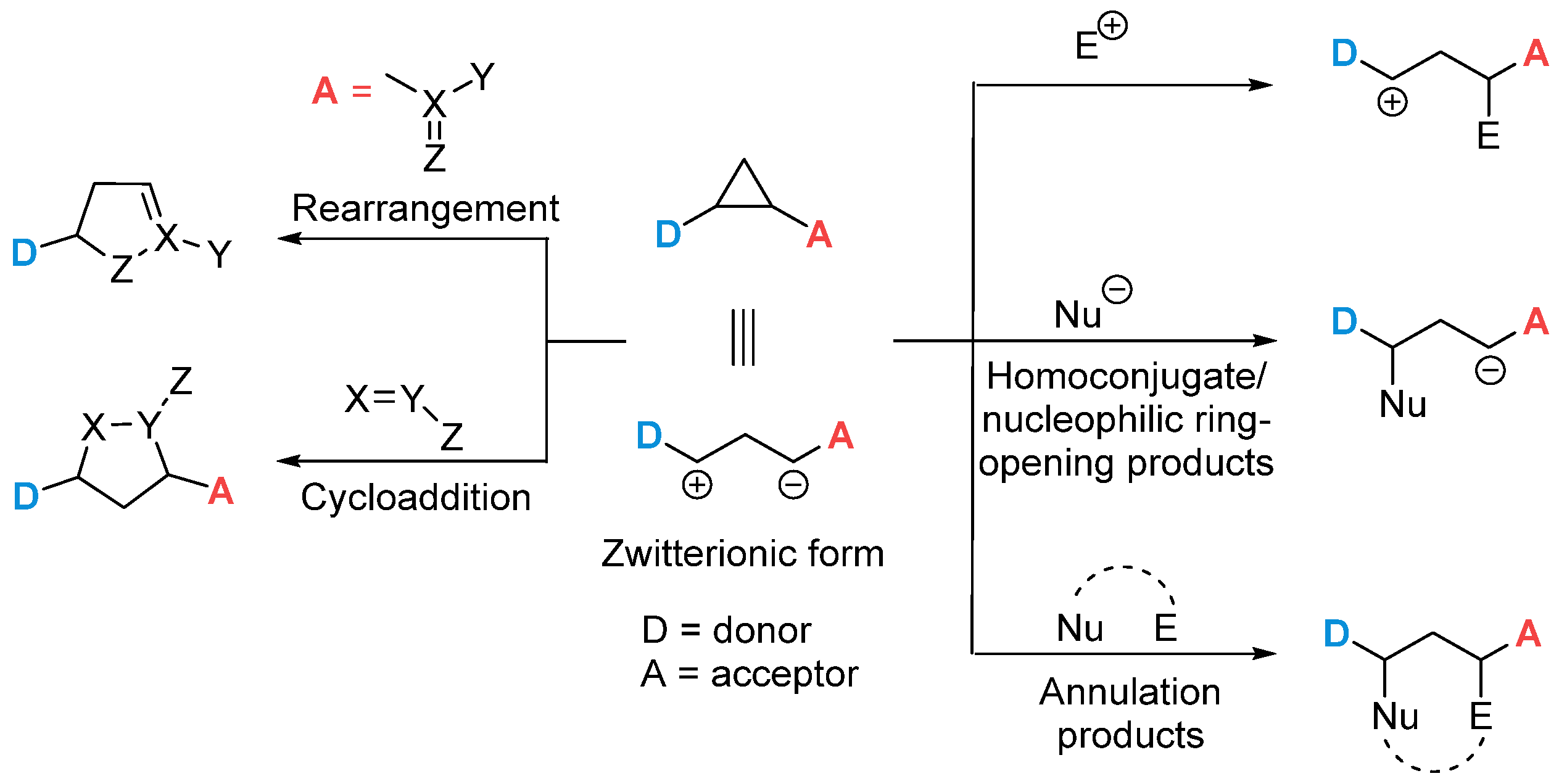
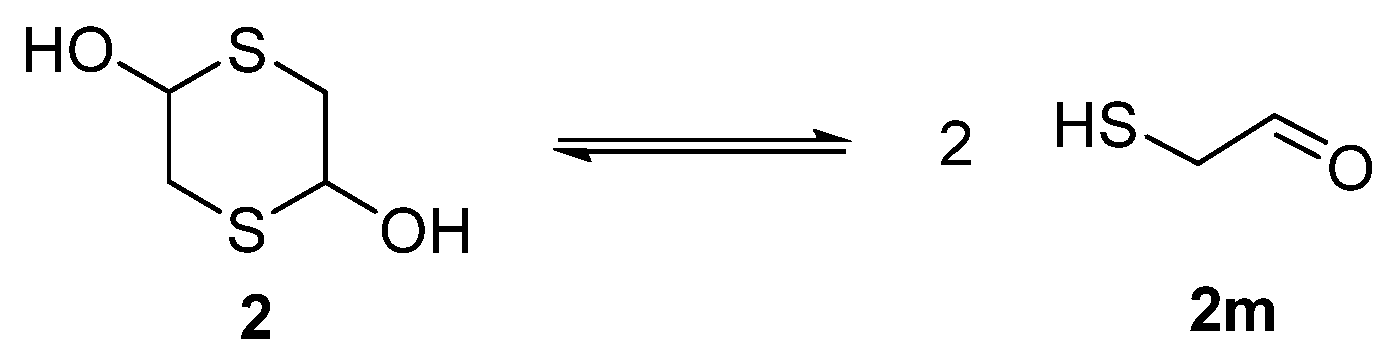
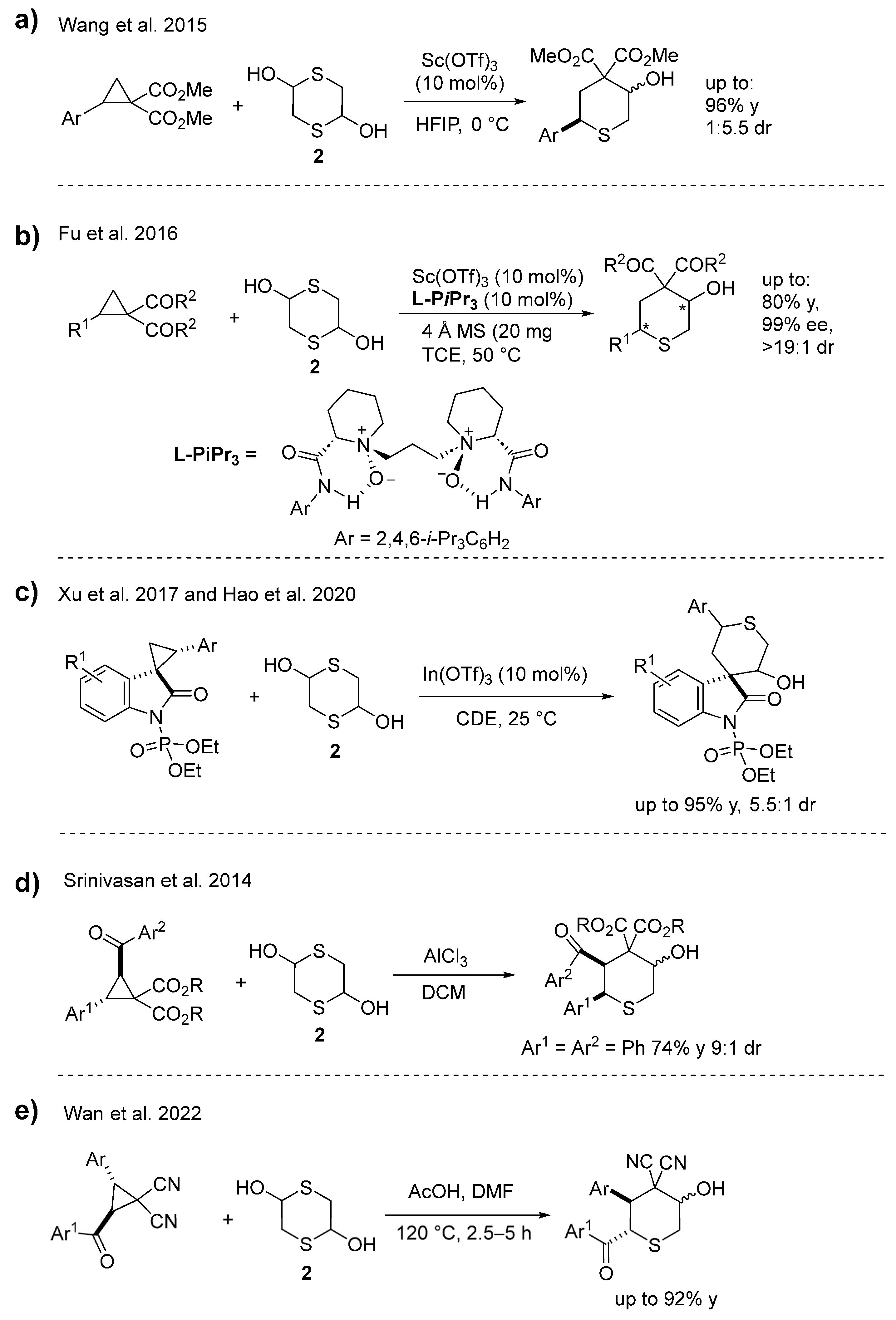
1. Introduction
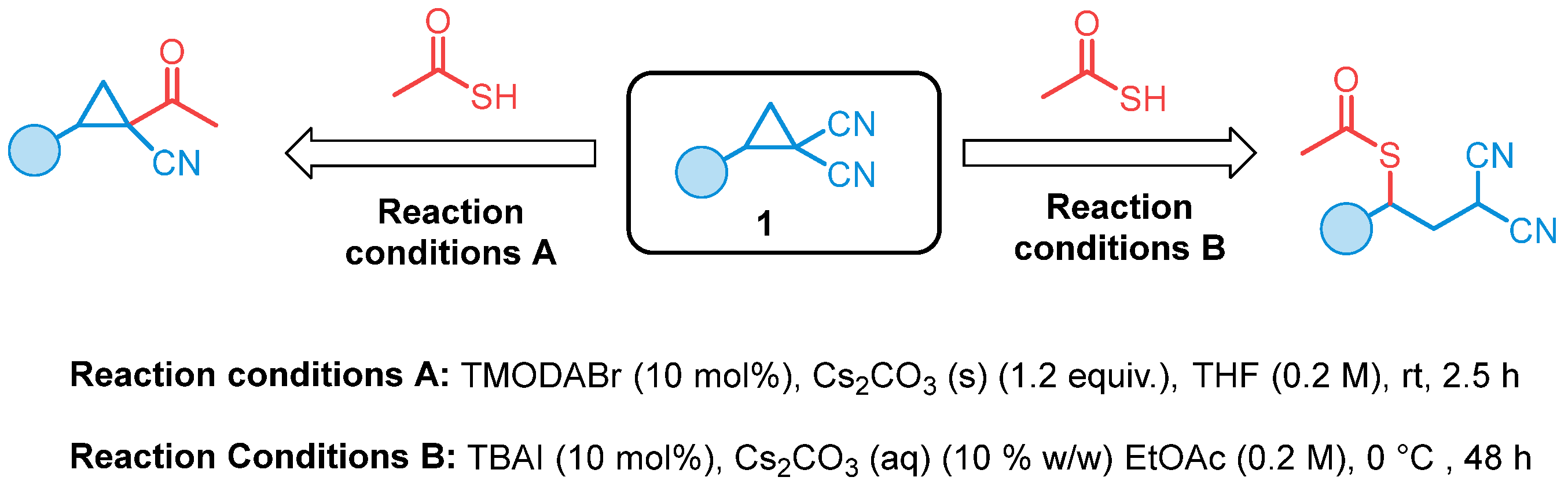
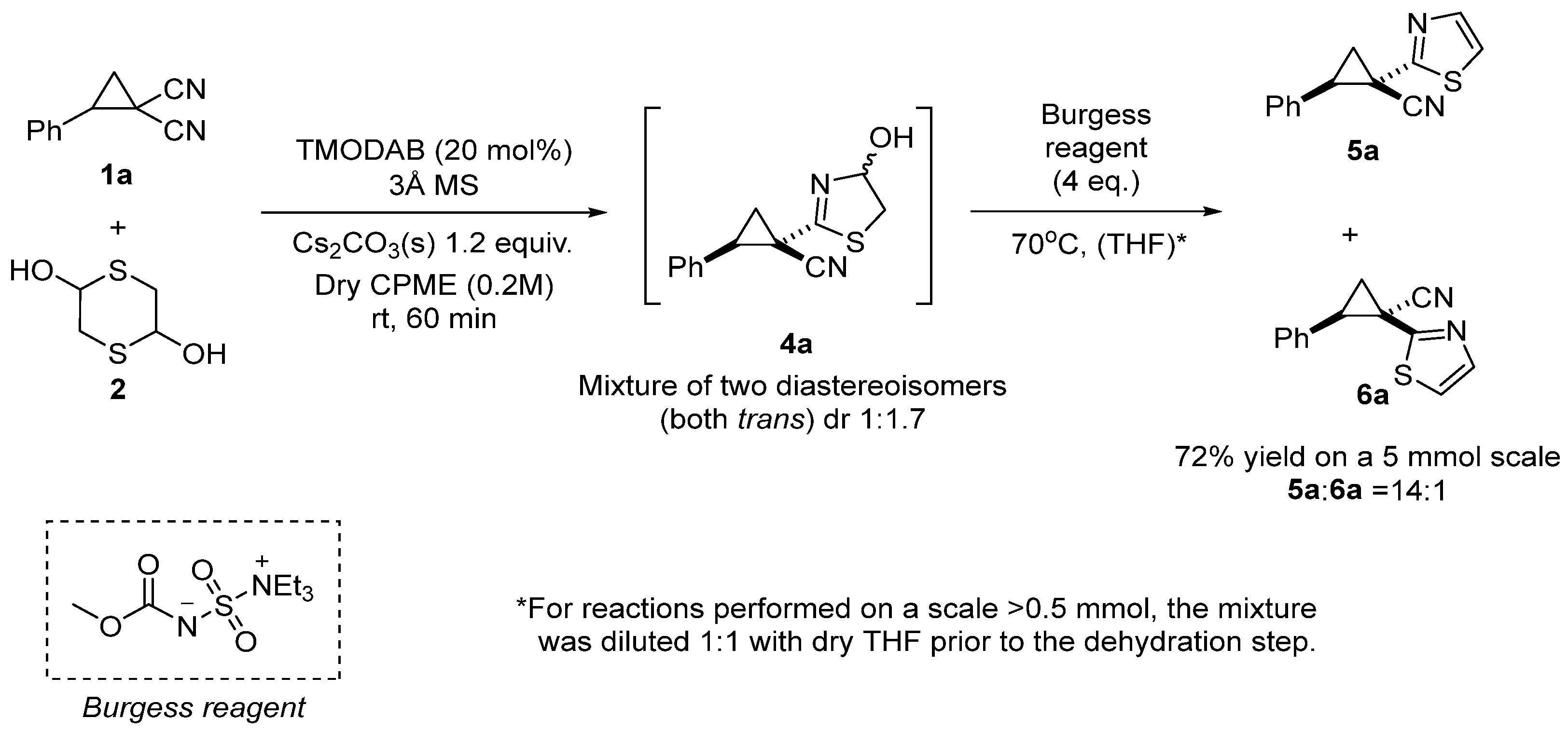
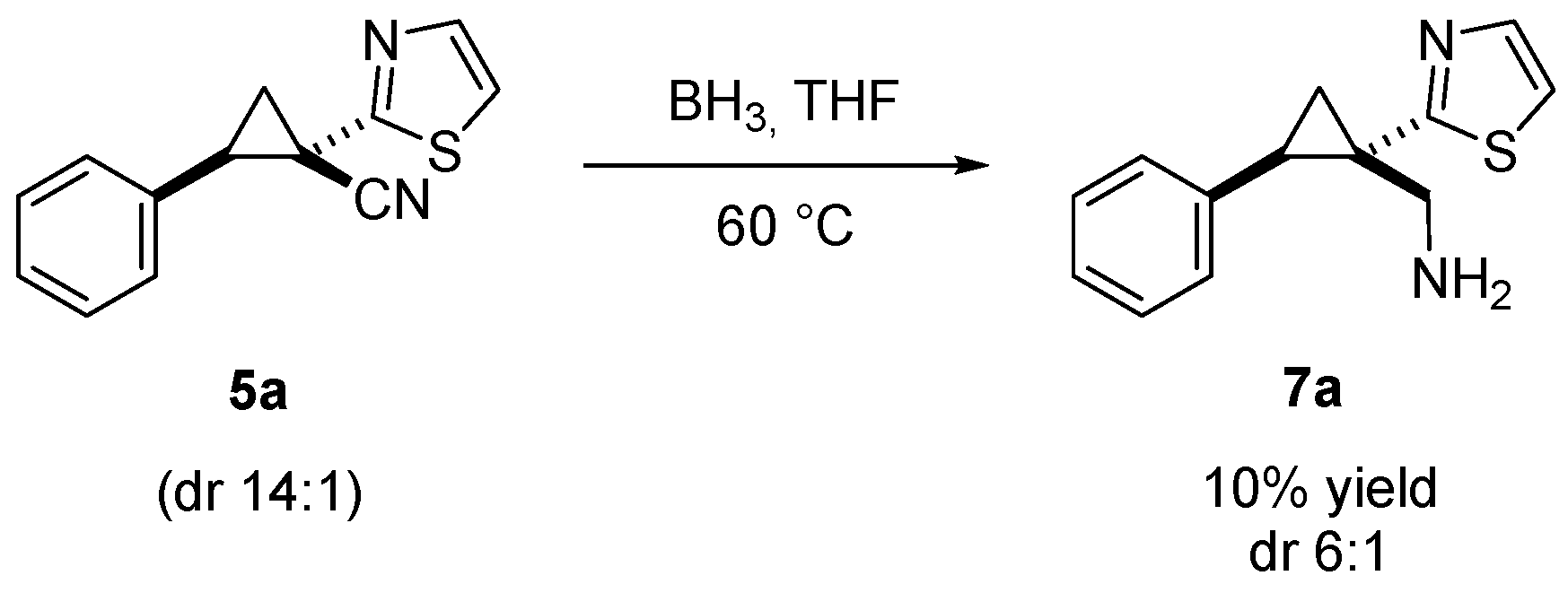
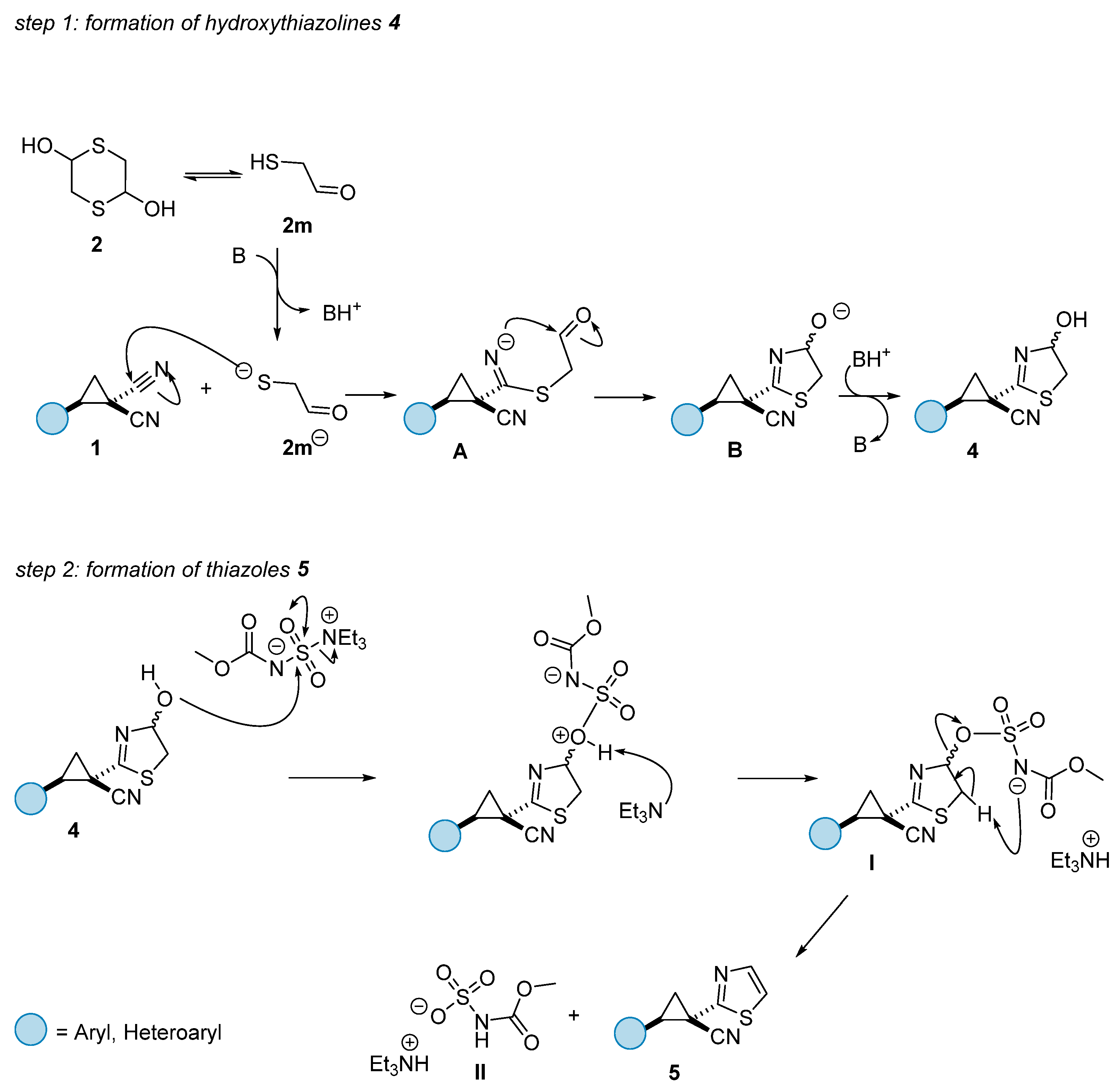
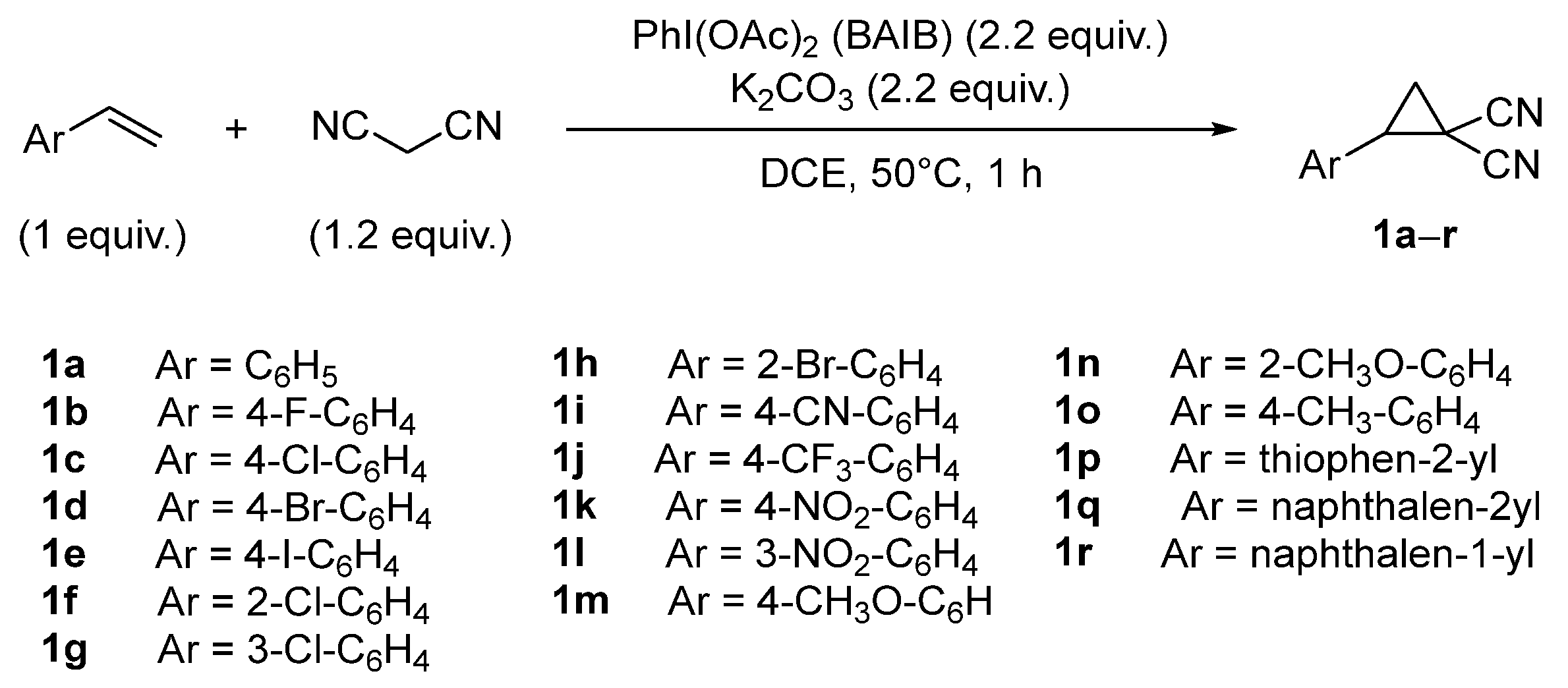
2. Results and Discussion
3. Materials and Methods
3.1. General Methods
3.2. Materials
3.3. Synthesis of 1-(4-Hydroxy-4,5-dihydrothiazol-2-yl)-2-phenylcyclopropane-1-carbonitrile (4a)
3.4. General One-Pot Procedure for the Synthesis of Thiazoles 5
- Trans-2-phenyl-1-(thiazol-2-yl)cyclopropane-1-carbonitrile (5a)
- Trans-(4-fluorophenyl)-1-(thiazol-2-yl)cyclopropane-1-carbonitrile (5b)
- Trans-2-(4-chlorophenyl)-1-(thiazol-2-yl)cyclopropane-1-carbonitrile (5c)
- Trans-2-(4-bromophenyl)-1-(thiazol-2-yl)cyclopropane-1-carbonitrile (5d)
- Trans-2-(4-iodophenyl)-1-(thiazol-2-yl)cyclopropane-1-carbonitrile (5e)
- Trans-2-(2-chlorophenyl)-1-(thiazol-2-yl)cyclopropane-1-carbonitrile (5f)
- Trans-2-(3-chlorophenyl)-1-(thiazol-2-yl)cyclopropane-1-carbonitrile (5g)
- Trans-2-(2-bromophenyl)-1-(thiazol-2-yl)cyclopropane-1-carbonitrile (5h)
- Trans-2-(4-cyanophenyl)-1-(thiazol-2-yl)cyclopropane-1-carbonitrile (5i)
- Trans-2-(4-trifluoromethylphenyl)-1-(thiazol-2-yl)cyclopropane-1-carbonitrile (5j)
- Trans-2-(4-nitrophenyl)-1-(thiazol-2-yl)cyclopropane-1-carbonitrile (5k)
- Trans-2-(3-nitrophenyl)-1-(thiazol-2-yl)cyclopropane-1-carbonitrile (5l)
- Trans-2-(4-methoxyphenyl)-1-(thiazol-2-yl)cyclopropane-1-carbonitrile (5m)
- Trans-2-(2-methoxyphenyl)-1-(thiazol-2-yl)cyclopropane-1-carbonitrile (5n)
- Trans-2-(2-methylphenyl)-1-(thiazol-2-yl)cyclopropane-1-carbonitrile (5o)
- Trans-1-(thiazol-2-yl)-2-(thiophen-2-yl)cyclopropane-1-carbonitrile (5p)
- Trans-2-(naphth-2-yl)-1-(thiazol-2-yl)cyclopropane-1-carbonitrile (5q)
- Trans-2-(naphth-1-yl)-1-(thiazol-2-yl)cyclopropane-1-carbonitrile (5r)
3.5. Synthesis of Trans-2-phenyl-1-(thiazol-2-yl)cyclopropyl)methanamine (7a)
3.6. Reactivity of Thiazolyl Derivatives 5
3.6.1. General Procedure for the Dichlorination of Compounds 5
- 2,4-Dichloro-4-phenyl-2-(thiazol-2-yl)butanenitrile (8a)
- 2,4-Dichloro-4-(4-cyanophenyl)-2-(thiazol-2-yl)butanenitrile (8b)
- 2,4-Dichloro-4-(4-nitrophenyl)-2-(thiazol-2-yl)butanenitrile (8c)
3.6.2. General Procedure for the Hydrogenation of Compounds 5
- 4-Phenyl-2-(thiazol-2-yl)butanenitrile (9a)
- 4-(2-Chlorophenyl)-2-(thiazol-2-yl)butanenitrile (9b)
- 2-(Thiazol-2-yl)-4-(4-(trifluoromethyl)phenyl)butanenitrile (9c)
3.6.3. General Procedure for the Arylation of Compounds 5
- 4-Phenyl-2-(thiazol-2-yl)-4-(2,4,6-trimethoxyphenyl)butanenitrile (10a)
- 4-(2-Chlorophenyl)-2-(thiazol-2-yl)-4-(2,4,6-trimethoxyphenyl)butanenitrile (10b)
- 4-(3-Cyano-3-(thiazol-2-yl)-1-(2,4,6-trimethoxyphenyl)propyl)benzonitrile (10c)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| D–A | Donor–acceptor |
| PTC | Phase transfer catalysis |
| PT | Phase transfer |
| TBABr | Tetrabutylammonium bromide |
| BzTEABr | Benzyltrietilammonium bromide |
| TMODAB | Tetrametiloctadecylammonium bromide |
| CPME | Cyclopentyl methyl ether |
| TFT | α,α,α-Trifluorotoluene |
| DIPEA | Diisopropylethylamine |
| TFA | Trifluoroacetic acid |
| HFIP | Hexafluoroisopropanol |
References
- de Meijere, A. Bonding properties of cyclopropane and their chemical consequences. Angew. Chem. Int. Ed. Engl. 1979, 18, 809–826. [Google Scholar] [CrossRef]
- Kreft, A.; Lücht, A.; Grunenberg, J.; Jones, P.G.; Werz, D.B. Kinetic Studies of Donor–Acceptor Cyclopropanes: The Influence of Structural and Electronic Properties on the Reactivity. Angew. Chem. Int. Ed. 2019, 58, 1955–1959. [Google Scholar] [CrossRef] [PubMed]
- Tomilov, Y.V.; Menchikov, L.G.; Novikov, R.A.; Ivanova, O.A.; Trushkov, I.V. Methods for the synthesis of donor-acceptor cyclopropanes. Russ. Chem. Rev. 2018, 87, 201–250. [Google Scholar] [CrossRef]
- Banerjee, P.; Biju, A.T. Donor-Acceptor Cyclopropanes in Organic Synthesis, 1st ed.; Wiley-VCH GmbH: Weinheim, Germany, 2024; ISBN 978-3-527-34987-6. [Google Scholar]
- Reissig, H.-U.; Zimmer, R. Donor-Acceptor-substituted cyclopropane derivatives and their application in organic synthesis. Chem. Rev. 2003, 103, 1151–1196. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Pagenkopf, B.L. Recent advances in donor–acceptor (DA) cyclopropanes. Tetrahedron 2005, 61, 321–347. [Google Scholar] [CrossRef]
- Carson, C.A.; Kerr, M.A. Heterocycles from cyclopropanes: Applications in natural product synthesis. Chem. Soc. Rev. 2009, 38, 3051–3060. [Google Scholar] [CrossRef]
- Cavitt, M.A.; Phun, L.H.; France, S. Intramolecular donor-acceptor cyclopropane ring-opening cyclizations. Chem. Soc. Rev. 2014, 43, 804–818. [Google Scholar] [CrossRef]
- Schneider, T.F.; Kaschel, J.; Werz, D.B. A new golden age for donor-acceptor cyclopropanes. Angew. Chem. Int. Ed. 2014, 53, 5504–5523. [Google Scholar] [CrossRef]
- Budynina, E.; Ivanov, K.; Sorokin, I.; Melnikov, M. Ring Opening of Donor−Acceptor Cyclopropanes with N-Nucleo-philes. Synthesis 2017, 49, 3035–3068. [Google Scholar] [CrossRef]
- Pagenkopf, B.L.; Vemula, N. Cycloadditions of Donor–Acceptor Cyclopropanes and Nitriles. Eur. J. Org. Chem. 2017, 2017, 2561–2567. [Google Scholar] [CrossRef]
- Ivanova, O.A.; Trushkov, I.V. Donor-Acceptor Cyclopropanes in the Synthesis of Carbocycles. Chem. Rec. 2019, 19, 2189–2208. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.; Das, S. Recent advances in ring-opening of donor acceptor cyclopropanes using C-nucleophiles. Org. Biomol. Chem. 2021, 19, 965–982. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Liu, X.; Feng, X. Asymmetric catalytic reactions of Donor–Acceptor cyclopropanes. Angew. Chem. Int. Ed. 2021, 60, 9192–9204. [Google Scholar] [CrossRef] [PubMed]
- Pirenne, V.; Muriel, B.; Waser, J. Catalytic Enantioselective Ring-Opening Reactions of Cyclopropane. Chem. Rev. 2021, 121, 227–263. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.; Doyle, M.P. Asymmetric [3+n]-Cycloaddition Reactions of Donor-Acceptor Cyclopropanes. ChemCatChem 2023, 15, e202301090. [Google Scholar] [CrossRef]
- Deepthi, A.; Meenakshy, C.B.; Mohan, M. Synthesis of Heterocycles from Donor-Acceptor Cyclopropanes: A Five-Year Recap. Synthesis 2023, 55, 3875–3894. [Google Scholar] [CrossRef]
- Doraghi, F.; Karimian, S.; Qareaghaj, O.H.; Karimi, M.J.; Larijani, B.; Mahdavi, M. Recent Advances in Ring-Opening Reactions of 2-Substituted Donor-Acceptor Cyclopropanes under Metal Catalysis. J. Organomet. Chem. 2024, 1005, 122963. [Google Scholar] [CrossRef]
- Lv, L.; Su, J.; Li, Z. Recent developments in the ring-opening transformations of gem-difluorocyclopropanes. Org. Chem. Front. 2024, 11, 6518–6533. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, C.; Huang, J.; Huang, L.; Feng, H. Palladium-Catalyzed Regioselective Monofluoroallylation of Indoles with gem-Difluorocyclopropanes. Org. Lett. 2024, 26, 6905–6909. [Google Scholar] [CrossRef]
- Halskov, K.S.; Kniep, F.; Lauridsen, V.H.; Iversen, E.H.; Donslund, B.S.; Jørgensen, K.A. Organocatalytic Enamine-Activation of Cyclopropanes for Highly Stereoselective Formation of Cyclobutanes. J. Am. Chem. Soc. 2015, 137, 1685–1691. [Google Scholar] [CrossRef]
- Blom, J.; Vidal-Albalat, A.; Jorgensen, J.; Barlose, C.L.; Jessen, K.S.; Iversen, M.V.; Jørgensen, K.A. Directing the Activation of Donor-Acceptor Cyclopropanes Towards Stereoselective 1,3-Dipolar Cycloaddition Reactions by Bronsted Base Catalysis. Angew. Chem. Int. Ed. 2017, 56, 11831–11835. [Google Scholar] [CrossRef] [PubMed]
- Ortega, A.; Manzano, R.; Uria, U.; Carrillo, L.; Reyes, E.; Tejero, T.; Merino, P.; Vicario, J.L. Catalytic Enantioselective Cloke–Wilson Rearrangement. Angew. Chem. Int. Ed. 2018, 57, 8225–8229. [Google Scholar] [CrossRef] [PubMed]
- McLeod, D.A.; Thøgersen, M.K.; Barløse, C.L.; Skipper, M.L.; Obregón, E.B.; Jørgensen, K.A. Enantioselective (8 + 3) Cycloadditions by Activation of Donor–Acceptor Cyclopropanes Employing Chiral Brønsted Base Catalysis. Angew. Chem. Int. Ed. 2022, 61, e202206096. [Google Scholar] [CrossRef] [PubMed]
- Obregon, E.B.; Rost, L.G.; Kocemba, I.R.; Kristensen, A.; McLeod, D.A.; Jørgensen, K.A. Enantioselective (3 + 2) Annulation of Donor-Acceptor Cyclopropanes with Aldehydes and Ketones Catalyzed by Bronsted Bases. Angew. Chem. Int. Ed. 2024, 63, e202410524. [Google Scholar] [CrossRef]
- Shintani, R.; Murakami, M.; Tsuji, T.; Tanno, H.; Hayashi, T. Palladium-catalyzed decarboxylative [4 + 3] cyclization of γ-methylidene-δ-valerolactones with 1,1-dicyanocyclopropanes. Org. Lett. 2009, 11, 5642–5645. [Google Scholar] [CrossRef]
- Dieskau, A.P.; Holzwarth, M.S.; Plietker, B. Fe-catalyzed allylic C-C-bond activation: Vinylcyclopropanes as versatile a1,a3,d5-synthons in traceless allylic substitutions and [3 + 2]-cycloadditions. J. Am. Chem. Soc. 2012, 134, 5048–5051. [Google Scholar] [CrossRef]
- Qian, S.; Xie, Z.; Liu, J.; Li, M.; Wang, S.; Luo, N.; Wang, C. DBU-Promoted Cascade Annulation of Nitroarylcyclo-propane-1,1-dicarbonitriles and 3-Aryl-2-cyanoacrylates: An Access to Highly Functionalized Cyclopenta[b]furan Derivatives. J. Org. Chem. 2018, 83, 14768–14776. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Z.; Li, P.; Yang, T.; Ding, K.; Zhang, Z.M.; Tan, Y.; Li, Z. Proteome-wide Ligand and Target Discovery by Using Strain-Enabled Cyclopropane Electrophiles. J. Am. Chem. Soc. 2024, 146, 20823–20836. [Google Scholar] [CrossRef]
- Govaerts, S.; Mayer-Figge, J.L.; Chotia, M.; Kirsch, S.F.; Gómez-Suárez, A. Synthesis and Nucleophilic Ring-Opening of 1,1-Dicyanocyclopropanes: Accessing β-Aminocarbonyl Derivatives from Olefins. Org. Lett. 2025, 27, 5549–5554. [Google Scholar] [CrossRef]
- Liu, J.; Xue, Y.; Cheng, W.; Wang, C. DABCO-Promoted (3 + 2) Annulation of D−A Cyclopropanes with Alkynoates for the Synthesis of Cyclopentenol Derivatives. J. Org. Chem. 2024, 89, 18671–18678. [Google Scholar] [CrossRef]
- Li, H.; Cheng, W.; Wang, C. Annulation of 2-Aroyl D−A Cyclopropanes via Selectively Ring-Opening Process with o-Benzenediamines to Access Quinoxaline Derivatives. J. Org. Chem. 2024, 89, 10333–10337. [Google Scholar] [CrossRef] [PubMed]
- Augustin, A.U.; Werz, D.B. Exploiting Heavier Organochalcogen Compounds in Donor–Acceptor Cyclopropane Chemistry. Acc. Chem. Res. 2021, 54, 1528–1541. [Google Scholar] [CrossRef] [PubMed]
- Mlostoń, G.; Celeda, M.; Kowalczyk, M.; Oliver, G.A.; Werz, D.B. Ring-Opening Reactions of Donor-Acceptor Cyclopropanes with Enolizable 5-Mercapto-1H-tetrazoles. Eur. J. Org. Chem. 2024, 27, e202400831. [Google Scholar] [CrossRef]
- Li, L.; Li, Z.; Wang, Q. Organocatalytic Nucleophilic Ring Opening of Cyclopropanecarbaldehydes by Benzenethiols: Tandem Synthesis of Benzo[b]thiepines. Synlett 2009, 2009, 1830–1834. [Google Scholar] [CrossRef]
- Braun, C.M.; Shema, A.M.; Dulin, C.C.; Nolin, K.A. The homologous conjugate addition of thiols to electron-deficient cyclopropanes catalyzed by a calcium(II) complex. Tetrahedron Lett. 2013, 54, 5889–5891. [Google Scholar] [CrossRef]
- Xia, Y.; Lin, L.; Chang, F.; Fu, X.; Liu, X.; Feng, X. Asymmetric Ring-Opening of Cyclopropyl Ketones with Thiol, Alcohol, and Carboxylic Acid Nucleophiles Catalyzed by a Chiral N,N’-DioxideScandium(III) Complex. Angew. Chem. Int. Ed. 2015, 54, 13748–13752. [Google Scholar] [CrossRef]
- Wallbaum, J.; Garve, L.K.B.; Jones, P.G.; Werz, D.B. Ring Opening 1,3-Halochalcogenation of Cyclopropane Dicarboxylates. Org. Lett. 2017, 19, 98–101. [Google Scholar] [CrossRef]
- Guin, A.; Rathod, T.; Gaykar, R.N.; Roy, T.; Biju, A.T. Lewis Acid Catalyzed Ring-Opening 1,3-Aminothiolation of Donor-Acceptor Cyclopropanes Using Sulfenamides. Org. Lett. 2020, 22, 2276–2280. [Google Scholar] [CrossRef]
- Augustin, A.U.; Sensse, M.; Jones, P.G.; Werz, D.B. Stereospecific Reactions of Donor-Acceptor Cyclopropanes with Thioketones: Access to Highly Substituted Tetrahydrothiophenes. Angew. Chem. Int. Ed. 2017, 56, 14293–14296. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Nakatake, D.; Yazaki, R.; Ohshima, T. An Expeditious Route to trans-Configured Tetrahydrothiophenes Enabled by Fe(OTf)3-Catalyzed [3 + 2] Cycloaddition of Donor-Acceptor Cyclopropanes with Thionoesters. Chem. Eur. J. 2018, 24, 6062–6066. [Google Scholar] [CrossRef]
- Goldberg, A.F.G.; O’Connor, N.R.; Craig, R.A.; Stoltz, B.M. Lewis Acid Mediated (3 + 2) Cycloadditions of Donor-Acceptor Cyclopropanes with Heterocumulenes. Org. Lett. 2012, 14, 5314–5317. [Google Scholar] [CrossRef]
- Xie, M.-S.; Zhao, G.-F.; Qin, T.; Suo, Y.-B.; Qu, G.-R.; Guo, H.-M. Thiourea participation in [3+2] cycloaddition with donor-acceptor cyclopropanes: A domino process to 2-amino-dihydrothiophenes. Chem. Commun. 2019, 55, 1580–1583. [Google Scholar] [CrossRef] [PubMed]
- Garve, L.K.B.; Pawliczek, M.; Wallbaum, J.; Jones, P.G.; Werz, D.B. [4+3] Cycloaddition of Donor−Acceptor Cyclopropanes with Amphiphilic Benzodithioloimine as Surrogate for ortho-Bisthioquinone. Chem. Eur. J. 2016, 22, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Augustin, A.U.; Merz, J.L.; Jones, P.G.; Mloston, G.; Werz, D.B. (4+3)-Cycloaddition of Donor-Acceptor Cyclopropanes with Thiochalcones: A Diastereoselective Access to Tetrahydrothiepines. Org. Lett. 2019, 21, 9405–9409. [Google Scholar] [CrossRef]
- Zamberlan, F.; Fantinati, A.; Trapella, C. 1,4-Dithiane-2,5-diol: An Attractive Platform for the Synthesis of Sulfur-Containing Functionalized Heterocycles. Eur. J. Org. Chem. 2018, 2018, 3248–3264. [Google Scholar] [CrossRef]
- Wang, H.P.; Zhang, H.-H.; Hu, X.-Q.; Xu, P.-F.; Luo, Y.-C. Sc(OTf)3-Catalysed [3+3] Annulation of Cyclopropane 1,1-Diesters with Mercaptoacetaldehyde: A Facile Strategy for the Synthesis of Tetrahydrothiopyranols. Eur. J. Org. Chem. 2015, 2015, 3486–3494. [Google Scholar] [CrossRef]
- Fu, X.; Lin, L.; Xia, Y.; Zhou, P.; Liu, X.; Feng, X. Catalytic asymmetric [3 + 3] annulation of cyclopropanes with mercaptoacetaldehyde. Org. Biomol. Chem. 2016, 14, 5914–5917. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.-W.; Liu, J.-K.; Shen, L.; Cao, Z.-Y.; Zhao, X.-L.; Yan, J.; Zhou, J. Diastereo- and enantioselective [3 + 3] cycloaddition of spirocyclopropyl oxindoles using both aldonitrones and ketonitrones. Nat. Commun. 2017, 8, 1619. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Gong, Y.; Cao, Z.; Zhou, Y.; Zhou, J. A highly efficient In(OTf)3-catalyzed [3+3] annulation of spirocyclopropyl oxindoles with 1,4-dithiane-2,5-diol. Chin. Chem. Lett. 2020, 31, 681–684. [Google Scholar] [CrossRef]
- Srinivasan, K.; Sathishkannan, G. [3+3] Annulation of donor–acceptor cyclopropanes with mercaptoacetaldehyde: Application to the synthesis of tetrasubstituted thiophenes. Chem. Commun. 2014, 50, 4062–4064. [Google Scholar] [CrossRef]
- Wan, X.; Li, X.; Wang, S.; Wang, C. Acetic Acid Mediated Regioselective [3+3] Cycloaddition of Substituted Cyclopropane-1,1-dicarbonitriles with 1,4-Dithiane-2,5-diol. J. Org. Chem. 2022, 87, 13375–13382. [Google Scholar] [CrossRef]
- Marianacci, O.; Micheletti, G.; Bernardi, L.; Fini, F.; Fochi, F.; Pettersen, D.; Sgarzani, V.; Ricci, A. Organocatalytic asymmetric Mannich reactions with N-Boc and N-Cbz protected α-amido sulfones. Chem. Eur. J. 2007, 13, 8338–8351. [Google Scholar] [CrossRef] [PubMed]
- Fini, F.; Micheletti, G.; Bernardi, L.; Pettersen, D.; Fochi, M.; Ricci, A. An easy entry to optically active α-amino phosphonic acid derivatives using phase-transfer catalysis (PTC). Chem. Commun. 2008, 2008, 4345–4347. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, L.; Fini, F.; Fochi, M.; Ricci, A. Organocatalyzed Enantioselective Synthesis of Nitroalkanes Bearing All-Carbon Quaternary Stereogenic Centers through Conjugate Addition of Acetone Cyanohydrin. Synlett 2008, 2008, 1857–1861. [Google Scholar] [CrossRef]
- Gioia, C.; Fini, F.; Mazzanti, A.; Bernardi, L.; Ricci, A. Organocatalytic asymmetric formal [3+2] cycloaddition with in situ-generated N-carbamoyl nitrones. J. Am. Chem. Soc. 2009, 131, 9614–9615. [Google Scholar] [CrossRef] [PubMed]
- Cassani, C.; Bernardi, L.; Fini, F.; Ricci, A. Catalytic asymmetric Mannich reactions of sulfonylacetates. Angew. Chem. Int. Ed. 2009, 48, 5694–5697. [Google Scholar] [CrossRef]
- Mazzotta, S.; Gramigna, L.; Bernardi, L.; Ricci, A. One-Pot synthesis of optically active β-amino-α-methylene carbonyl derivatives from α-amidosulfones using quinine-based Phase-Transfer Catalysts. Org. Process Res. Dev. 2010, 14, 687–691. [Google Scholar] [CrossRef]
- Bernardi, L.; Fochi, M.; Carbone, R.; Martinelli, A.; Fox, M.E.; Cobley, C.J.; Kandagatla, B.; Oruganti, S.; Dahanukar, V.H.; Carlone, A. Organocatalytic Asymmetric Conjugate Additions to Cyclopent-1-enecarbaldehyde: A Critical Assessment of Organocatalytic Approaches towards the Telaprevir Bicyclic Core. Chem. Eur. J. 2015, 21, 19208–19222. [Google Scholar] [CrossRef]
- Bertuzzi, G.; Silvestrini, F.; Moimare, P.; Pecorari, D.; Mazzanti, A.; Bernardi, L.; Fochi, M. Chemodivergent Preparation of Various Heterocycles via Phase-Transfer Catalysis: Enantioselective Synthesis of Functionalized Piperidines. Adv. Synth. Catal. 2020, 362, 1167–1175. [Google Scholar] [CrossRef]
- Bisag, G.D.; Viola, P.; Bernardi, L.; Fochi, M. Divergent Reactivity of D-A Cyclopropanes under PTC Conditions, Ring-Opening vs. Decyanation Reaction. Catalysts 2023, 13, 760. [Google Scholar] [CrossRef]
- Singh, I.P.; Gupta, S.; Kumar, S. Thiazole Compounds as Antiviral Agents: An Update. Med. Chem. 2020, 16, 4–23. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.-X.; Wang, Y.-T.; Zhang, S.-N.; Li, Y.; Chen, X.-B.; Wang, S.-Q.; Liu, H.-M. Application and synthesis of thiazole ring in clinically approved drugs. Eur. J. Med. Chem. 2023, 250, 115172. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Sharma, V.; Sharma, A.; Goyal, R.; Tonk, R.K.; Thakur, V.K.; Sharma, P.C. Green chemistry approaches for thiazole containing compounds as a potential scaffold for cancer therapy. Sustain. Chem. Pharm. 2021, 23, 100496. [Google Scholar] [CrossRef]
- Ayman, M.; Abdelmonsef, A.H.; Rashdan, H.R.M. Mini Review on The Synthesis and Biological Impact of Thiazoles. ChemistrySelect 2023, 8, e202300414. [Google Scholar] [CrossRef]
- Ramyashree, N.; Lokesh, K.S.; Tabassum, S.; Chundattu, S.J.; Govindaraju, S. Efficient Single-Pot Synthesis of Thiazole Derivatives: A Synoptic View. ChemistrySelect 2025, 10, e202404789. [Google Scholar] [CrossRef]
- Kempson, J. Name Reactions in Heterocyclic Chemistry II; Li, J.J., Corei, E.J., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 299–308. [Google Scholar] [CrossRef]
- Prieschl, M.; Sedelmeier, J.; Püntener, K.; Hildbrand, S.; Williams, J.D.; Kappe, C.O. Rediscovering Cyanogen Gas for Organic Synthesis: Formation of 2-Cyanothiazole Derivatives. J. Org. Chem. 2023, 88, 9594–9598. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, Y.; Yan, X.; Zhang, Y.; Ma, X. Synthesis of hydroxy-thiazoline substituted pyridine derivatives via [3+2] annulation of 1,4-dithiane-2,5-diol with cyanopyridine. Org. Biomol. Chem. 2024, 22, 8511–8515. [Google Scholar] [CrossRef]
- Chandra, A.; Cheekatla, S.R.; Vishwakarma, V.K.; Kumar, D. The Significant Role of Burgess Reagent and Its Analogues in Organic Synthesis. Asian J. Org. Chem. 2025, 14, e202500338. [Google Scholar] [CrossRef]
- Burgess, E.M.; Penton, H.R., Jr.; Taylor, E.A. Thermal reactions of alkyl N-carbomethoxysulfamate esters. J. Org. Chem. 1973, 38, 26–31. [Google Scholar] [CrossRef]
- Burgess, E.M.; Penton, H.R., Jr.; Taylor, E.A. Synthetic applications of N-carboalkoxysulfamate esters. J. Am. Chem. Soc. 1970, 92, 5224–5226. [Google Scholar] [CrossRef]
- Garve, L.K.B.; Barkawitz, P.; Jones, P.G.; Werz, D.B. Ring-Opening 1,3-Dichlorination of Donor-Acceptor Cyclopropanes by Iodobenzene Dichloride. Org. Lett. 2014, 16, 5804–5807. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, H.E.; Kottlyar, V.; Nudelman, A. NMR Chemical Shifts of Common Laboratory Solvents as Trace Impurities. J. Org. Chem. 1997, 62, 7512–7515. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Li, M.; Dong, Z.; Liang, F.; Zhang, J. Hypervalent iodine(iii)-mediated cyclopropa(e)nation of alkenes/alkynes under mild conditions. Org. Biomol. Chem. 2014, 12, 1341–1350. [Google Scholar] [CrossRef]
- Yoshimura, A.; Jones, T.N.; Yusubov, M.S.; Zhdankina, V.V. Hypoiodite-Mediated Catalytic Cyclopropanation of Alkenes with Malononitrile. Adv. Synth. Catal. 2014, 356, 3336–3340. [Google Scholar] [CrossRef]
- Yoshimura, A.; Koski, S.R.; Kastern, B.J.; Fuchs, J.M.; Jones, T.N.; Yusubova, R.Y.; Nemykin, V.N.; Zhdankin, V.V. Hypoiodite-Mediated Cyclopropanation of Alkenes. Chem. Eur. J. 2014, 20, 5895–5898. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Moku, B.; Li, F.; Ran, J.; Han, J.; Long, S.; Zha, G.-F.; Qin, H.-L. Stereoselective Construction of Nitrile-Substituted Cyclopropanes from 2-Substituted Ethenesulfonyl Fluorides via Carbon-Sulfur Bond Cleavage. Adv. Synth. Catal. 2019, 361, 4596–4601. [Google Scholar] [CrossRef]
- Bursch, M.; Mewes, J.-M.; Hansen, A.; Grimme, S. Best-Practice DFT Protocols for Basic Molecular Computational Chemistry. Angew. Chem. Int. Ed. 2022, 61, e202205735. [Google Scholar] [CrossRef] [PubMed]

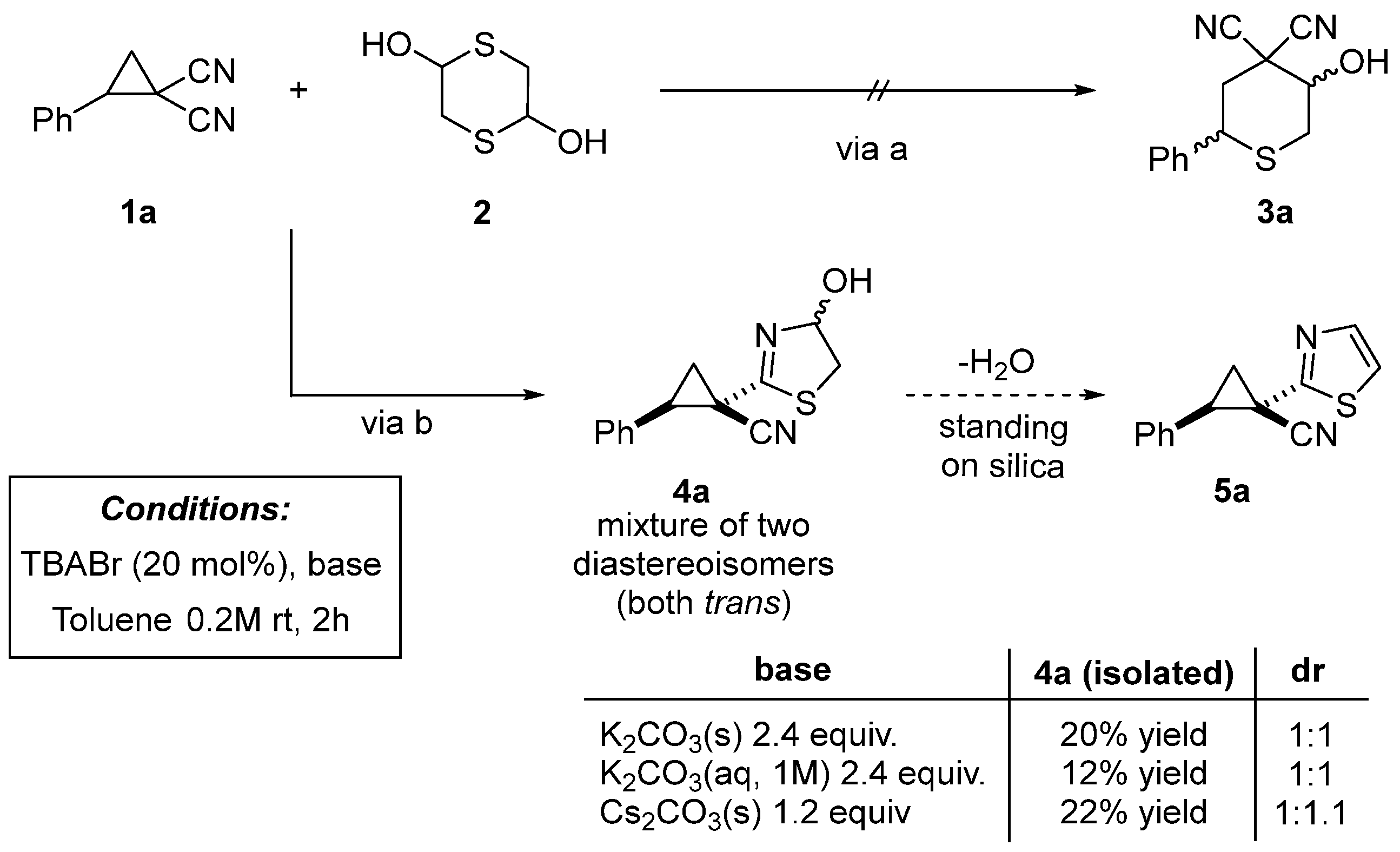


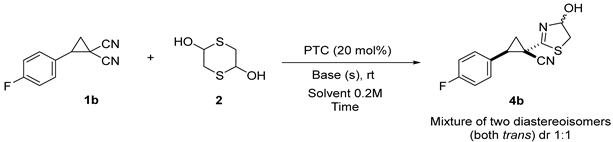 | |||||
| Entry | Ammonium Salt | Base (equiv.) | Solvent | Reaction Time (min) | Yield 4b (%) 2 |
|---|---|---|---|---|---|
| 1 | TBABr | K2CO3 (2.4 equiv.) | PhMe | 60 | 20 |
| 2 | BzTEABr | K2CO3 (2.4 equiv.) | PhMe | 60 | 34 |
| 3 | TMODAB | K2CO3 (2.4 equiv.) | PhMe | 60 | 44 |
| 4 | TMODAB | K2CO3 (1.2 equiv.) | PhMe | 60 | 53 |
| 5 | TMODAB | K2CO3 (1.2 equiv.) | EtOH | 60 | 65 |
| 6 | TMODAB | K2CO3 (1.2 equiv.) | nBuOH | 60 | 62 |
| 7 | TMODAB | K2CO3 (1.2 equiv.) | EtOAc | 60 | 55 |
| 8 | TMODAB | K2CO3 (1.2 equiv.) | CH3CN | 60 | 34 |
| 9 | TMODAB | K2CO3 (1.2 equiv.) | DCM | 60 | 46 |
| 10 | TMODAB | K2CO3 (1.2 equiv.) | 1,2-DCE | 60 | 52 |
| 11 | TMODAB | K2CO3 (1.2 equiv.) | Et2O | 60 | 42 |
| 12 | TMODAB | K2CO3 (1.2 equiv.) | THF | 60 | 40 |
| 13 | TMODAB | K2CO3 (1.2 equiv.) | tBuOMe | 60 | 48 |
| 14 | TMODAB | K2CO3 (1.2 equiv.) | CPME | 60 | 72 |
| 15 | TMODAB | Cs2CO3 (1.2 equiv.) | CPME | 60 | 75 |
| 16 3 | TMODAB | Cs2CO3 (1.2 equiv.) | CPME + 3 Å MS | 60 | 78 (71) |
| 17 3 | TMODAB | Cs2CO3 (1.2 equiv.) | CPME + 3 Å MS | 240 | 68 (62) |
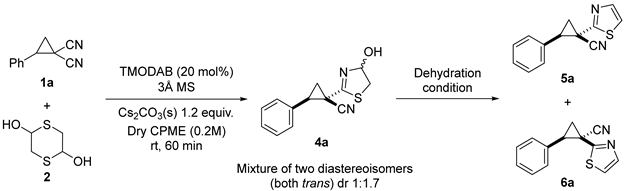 | |||
| Entry | Dehydration Condition 1 | Yield (%) 2 | 5a:6a 3 |
|---|---|---|---|
| 1 | TsCl/DIPEA | 37 | 4:1 |
| 2 | MsCl/Et3N | 34 | 12:1 |
| 3 | TsOH | 34 | 3:1 |
| 4 | TFA | 17 | 3:1 |
| 5 | Burgess reagent (2 equiv.) | 50 | 20:1 |
| 6 | Burgess reagent (3 equiv.) | 69 | 13:1 |
| 7 | Burgess reagent (4 equiv.) | 78 | 14:1 |
| 8 | Burgess reagent (6 equiv.) | 38 | 16:1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savini, E.B.; Bandieri, E.; Pecchini, P.; Santarelli, N.; Bernardi, L.; Fochi, M. Thiazolylcyanocyclopropanes: Novel Donor–Acceptor Cyclopropanes for Accessing Thiazole-Containing Targets. Molecules 2025, 30, 3767. https://doi.org/10.3390/molecules30183767
Savini EB, Bandieri E, Pecchini P, Santarelli N, Bernardi L, Fochi M. Thiazolylcyanocyclopropanes: Novel Donor–Acceptor Cyclopropanes for Accessing Thiazole-Containing Targets. Molecules. 2025; 30(18):3767. https://doi.org/10.3390/molecules30183767
Chicago/Turabian StyleSavini, Emanuèl Bruno, Edoardo Bandieri, Pietro Pecchini, Nicolò Santarelli, Luca Bernardi, and Mariafrancesca Fochi. 2025. "Thiazolylcyanocyclopropanes: Novel Donor–Acceptor Cyclopropanes for Accessing Thiazole-Containing Targets" Molecules 30, no. 18: 3767. https://doi.org/10.3390/molecules30183767
APA StyleSavini, E. B., Bandieri, E., Pecchini, P., Santarelli, N., Bernardi, L., & Fochi, M. (2025). Thiazolylcyanocyclopropanes: Novel Donor–Acceptor Cyclopropanes for Accessing Thiazole-Containing Targets. Molecules, 30(18), 3767. https://doi.org/10.3390/molecules30183767








