Gardeniae Fructus Enhances Skin Barrier Function via AHR-Mediated FLG/LOR/IVL Expression
Abstract
1. Introduction
2. Results
2.1. Analysis of GF by UPLC-MS/MS
2.2. Effects of GF on Migration of HaCaT Cells
2.3. Protective Effects of GF on SLS-Induced HaCaT Cells
2.4. Proteomics Assay of GF on HaCaT Cells
2.5. Molecular Docking
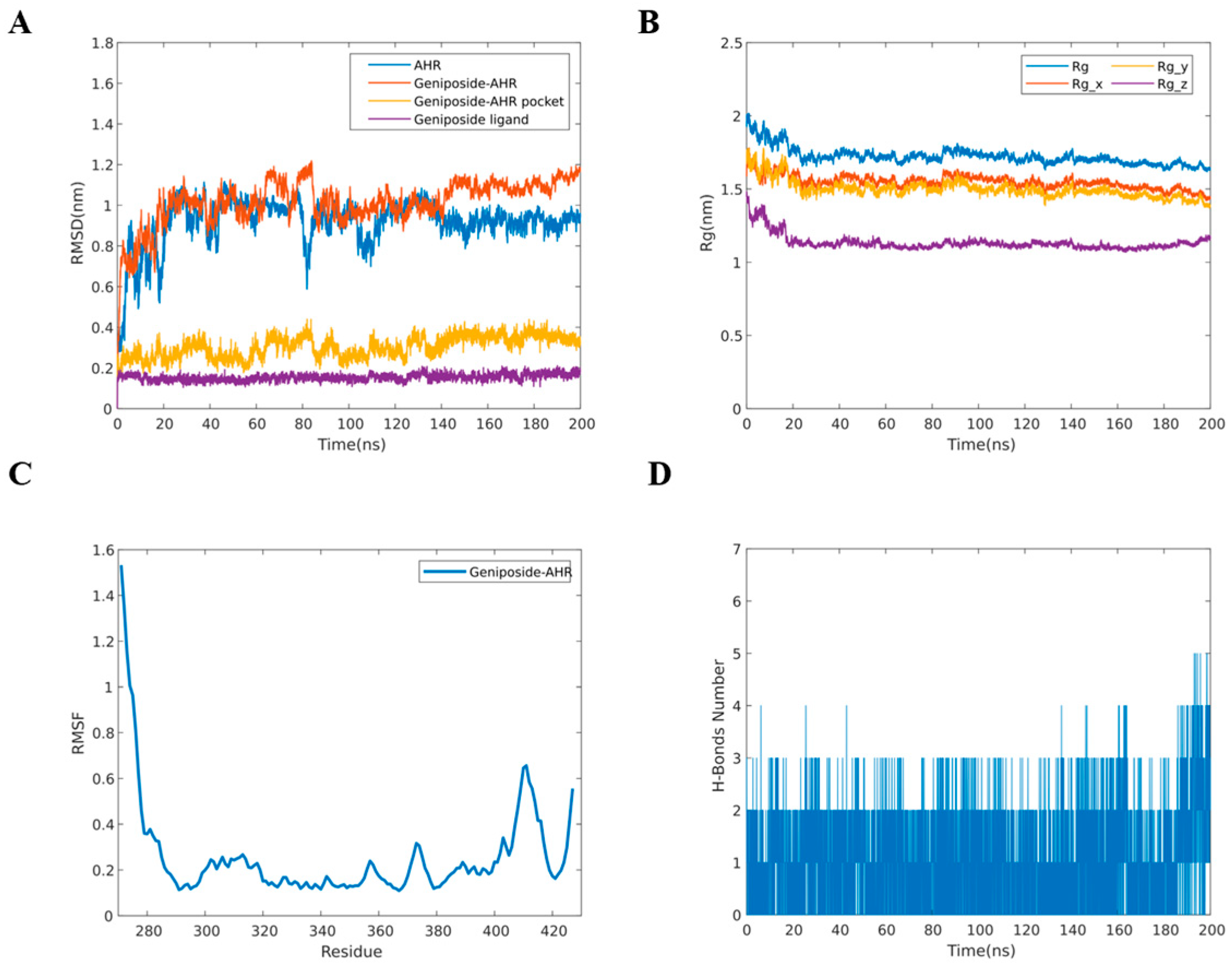
2.6. MD Simulation
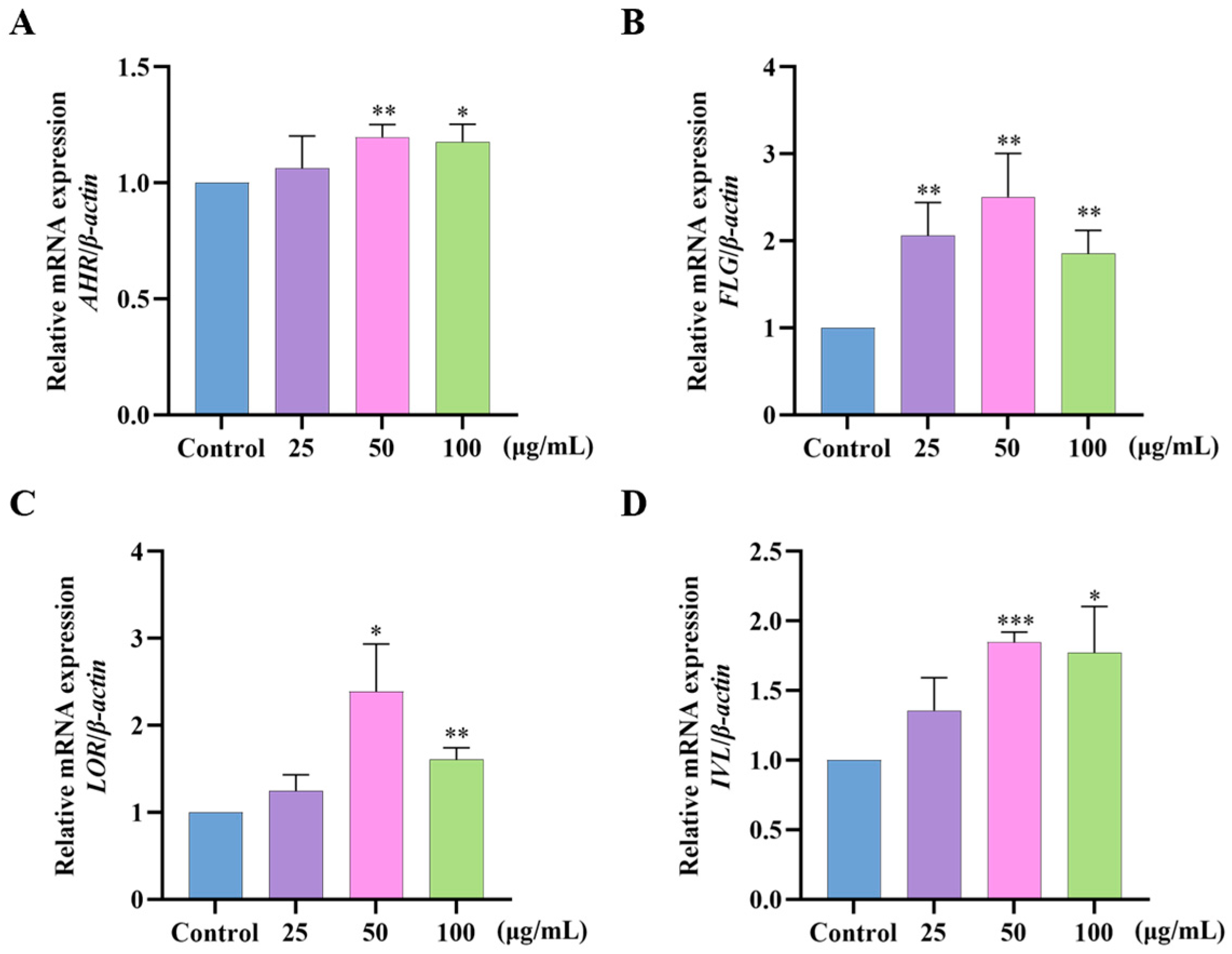
2.7. Effects of GF on AHR/FLG/LOR/IVL Gene Expression in HaCaT Cells
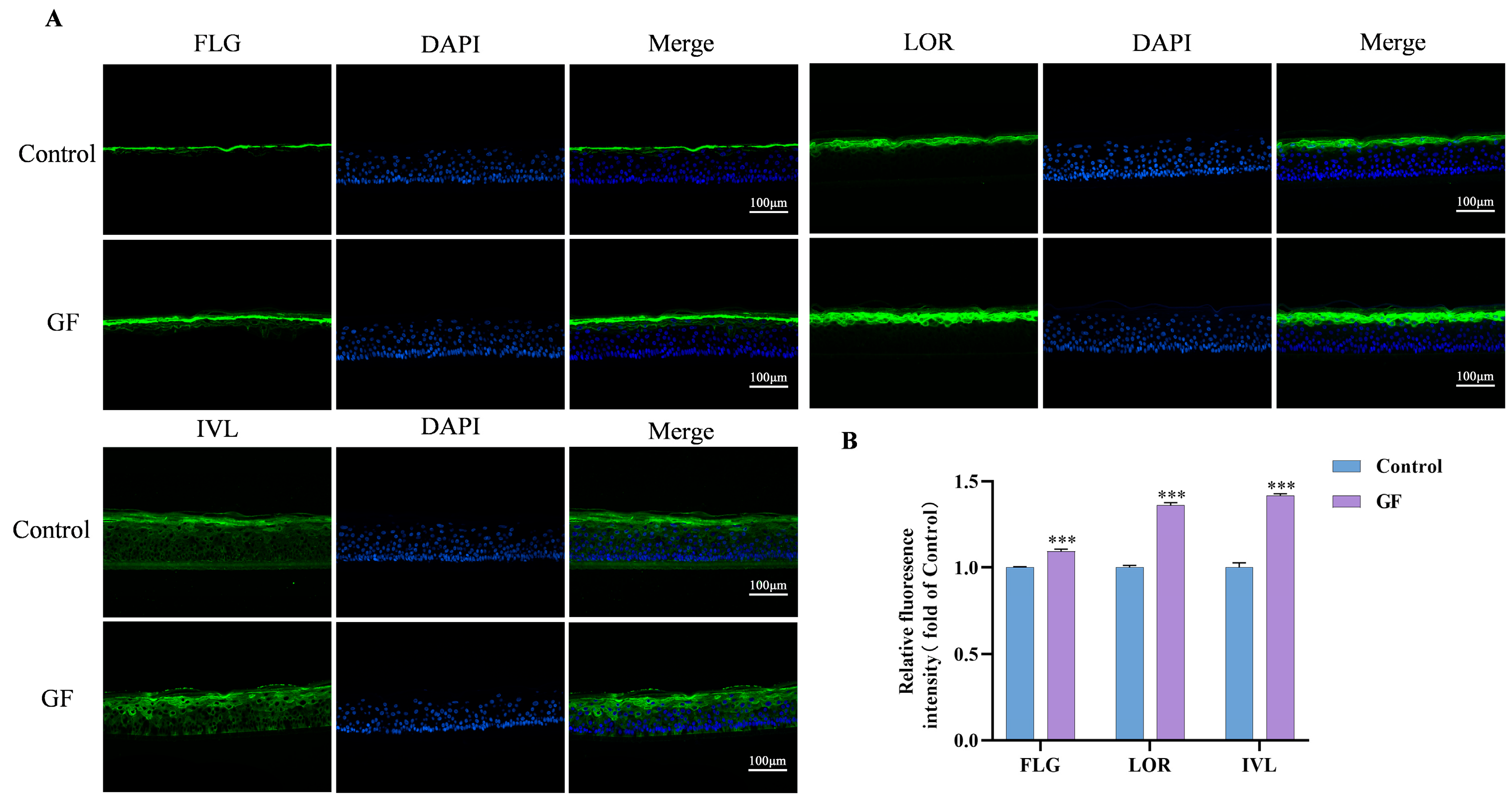
2.8. Effects of GF on FLG/LOR/IVL Expression in 3D Epidermal Skin Models
2.9. Participants’ Characteristics
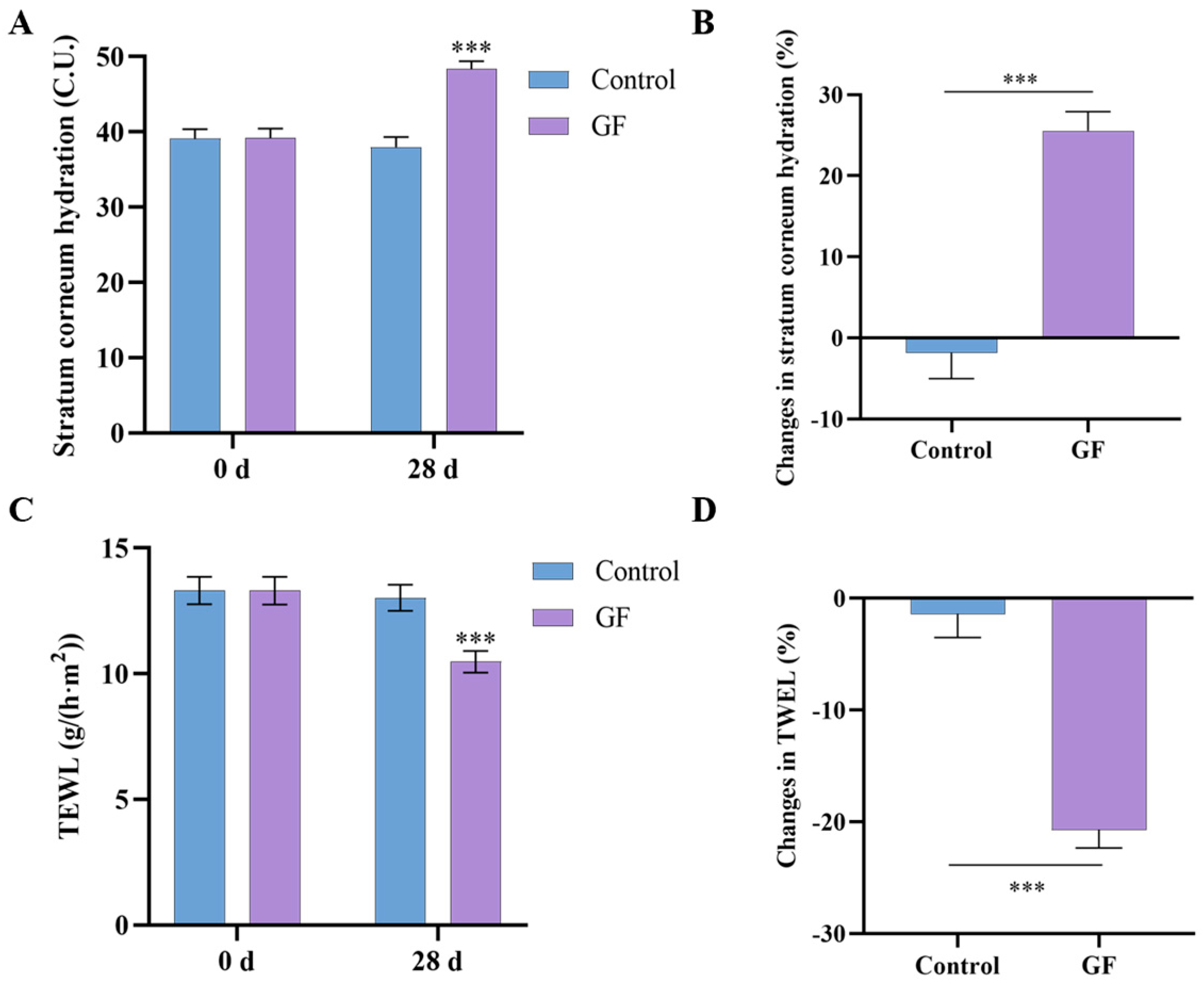
2.10. Effects of GF on Skin Hydration and TEWL
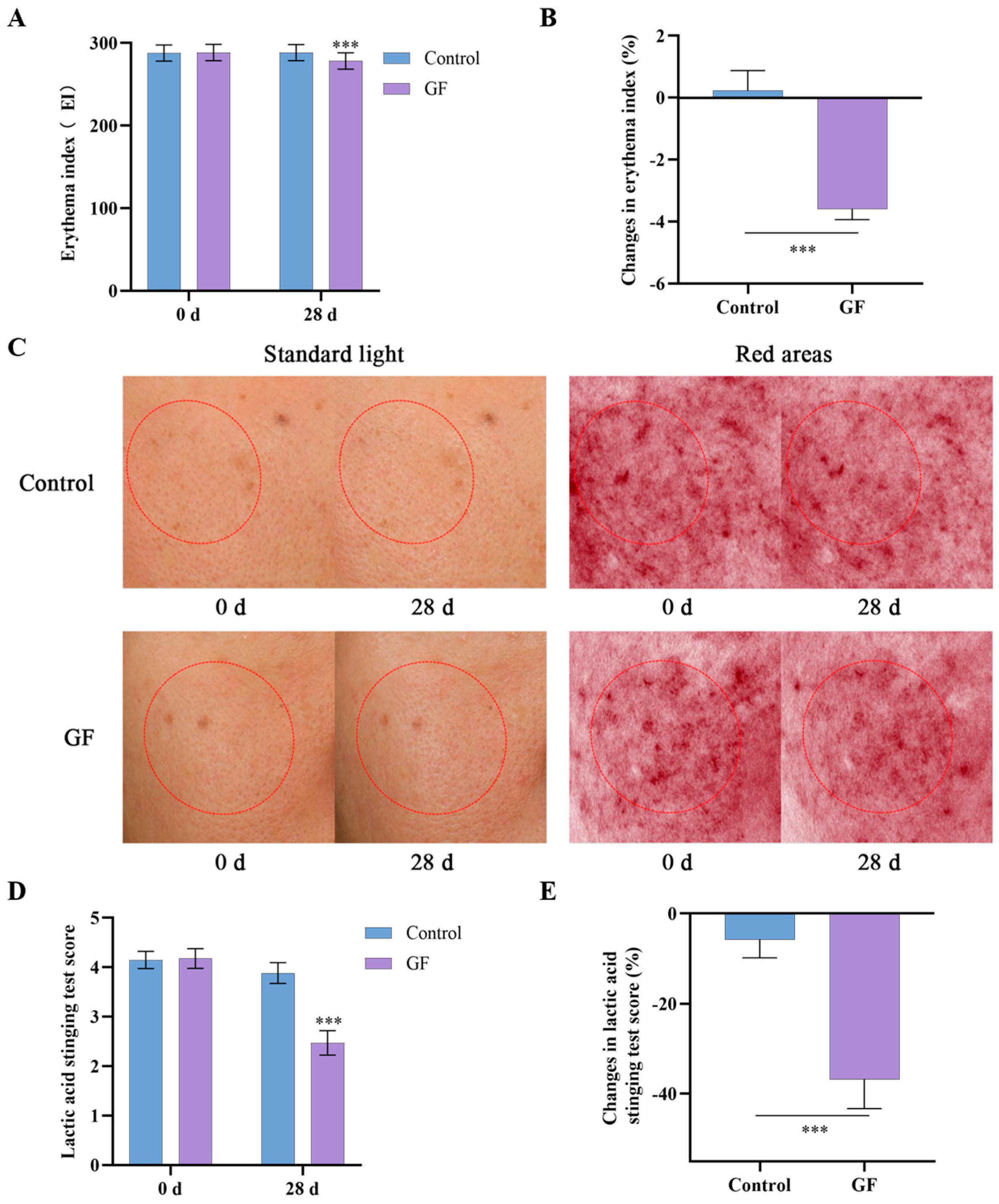
2.11. Effects of GF on Skin Redness and Tolerability
3. Discussion
4. Materials and Methods
4.1. Preparation and Analysis of GF
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Cell Scratch Assay
4.5. Detection of Reactive Oxygen Species (ROS) Production
4.6. Proteomics Assay
4.7. Molecular Docking
4.8. MD Simulation
4.9. RT-qPCR
4.10. Immunofluorescence Staining
4.11. Volunteer Tests
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GF | Gardeniae Fructus |
| AHR | Aryl hydrocarbon receptor |
| FLG | Filaggrin |
| LOR | Loricrin |
| IVL | Involucrin |
| HPLC | High-performance liquid chromatography |
| TEWL | Transepidermal water loss |
| E.I. | Erythema index |
| LAST | Lactic acid stinging test |
| HaCaT | Human keratinocytes |
| ROS | Reactive oxygen species |
| SLS | Sodium lauryl sulfate |
| DEPs | Differentially expressed proteins |
| MD | Molecular dynamics |
| RMSD | Root-mean-square deviation |
| RMSF | Root-mean-square fluctuation |
| Rg | Radius of gyration |
References
- Harris-Tryon, T.A.; Grice, E.A. Microbiota and maintenance of skin barrier function. Science 2022, 376, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Orsmond, A.; Bereza-Malcolm, L.; Lynch, T.; March, L.; Xue, M. Skin Barrier Dysregulation in Psoriasis. Int. J. Mol. Sci. 2021, 22, 10841. [Google Scholar] [CrossRef]
- Nguyen, H.L.T.; Trujillo-Paez, J.V.; Umehara, Y.; Yue, H.; Peng, G.; Kiatsurayanon, C.; Chieosilapatham, P.; Song, P.; Okumura, K.; Ogawa, H.; et al. Role of Antimicrobial Peptides in Skin Barrier Repair in Individuals with Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 7607. [Google Scholar] [CrossRef]
- Beck, L.A.; Cork, M.J.; Amagai, M.; De Benedetto, A.; Kabashima, K.; Hamilton, J.D.; Rossi, A.B. Type 2 Inflammation Contributes to Skin Barrier Dysfunction in Atopic Dermatitis. JID Innov. 2022, 2, 100131. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Li, Z.; Su, J. Air pollution and skin diseases: A comprehensive evaluation of the associated mechanism. Ecotoxicol. Environ. Saf. 2024, 278, 116429. [Google Scholar] [CrossRef]
- Rosa, D.F.; Sarandy, M.M.; Novaes, R.D.; da Matta, S.L.P.; Gonçalves, R.V. Effect of a high-fat diet and alcohol on cutaneous repair: A systematic review of murine experimental models. PLoS ONE 2017, 12, e0176240. [Google Scholar] [CrossRef] [PubMed]
- Farkas, A.; Kemény, L.; Széll, M.; Dobozy, A.; Bata-Csörgo, Z. Ethanol and acetone stimulate the proliferation of HaCaT keratinocytes: The possible role of alcohol in exacerbating psoriasis. Arch. Dermatol. Res. 2003, 295, 56–62. [Google Scholar] [CrossRef]
- Choi, E.H.; Demerjian, M.; Crumrine, D.; Brown, B.E.; Mauro, T.; Elias, P.M.; Feingold, K.R. Glucocorticoid blockade reverses psychological stress-induced abnormalities in epidermal structure and function. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R1657–R1662. [Google Scholar] [CrossRef]
- Jee, M.H.; Mraz, V.; Geisler, C.; Bonefeld, C.M. γδ T cells and inflammatory skin diseases. Immunol. Rev. 2020, 298, 61–73. [Google Scholar] [CrossRef]
- Chen, X.; Wen, J.; Wu, W.; Peng, Q.; Cui, X.; He, L. A review of factors influencing sensitive skin: An emphasis on built environment characteristics. Front. Public Health 2023, 11, 1269314. [Google Scholar] [CrossRef]
- Chu, V.; Ong, P.Y. Constant vigilance! Managing threats to the skin barrier. Ann. Allergy Asthma Immunol. 2024, 132, 678–685. [Google Scholar] [CrossRef]
- Zong, K.; Xu, K.; Zhang, X.; Wang, P.; Wang, Z.; Yang, S.; Li, H.; Ke, H.; He, S.; Hu, Y.; et al. Explorating the mechanism of Huangqin Tang against skin lipid accumulation through network pharmacology and experimental validation. J. Ethnopharmacol. 2023, 313, 116581. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Qin, S.; Han, J.; Meng, J.; Liang, A. A review of the ethnopharmacology, phytochemistry, pharmacology and toxicology of Fructus Gardeniae (Zhi-zi). J. Ethnopharmacol. 2022, 289, 114984. [Google Scholar] [CrossRef] [PubMed]
- Zong, K.; Li, X.; Zhou, F.; Dong, J.; Huang, Q.; Wu, J. Photoprotective Effect and Potential Mechanisms of Gardeniae Fructus Extract in UVB-Irradiated HaCaT Cells. Cosmetics 2025, 12, 72. [Google Scholar] [CrossRef]
- Kim, S.H.; Shin, M.R.; Lee, A.R.; Seo, B.I.; Park, H.J.; Roh, S.S. Improvement of Inflammation through Antioxidant Pathway of Gardeniae Fructus 50% EtOH Extract (GE) from Acute Reflux Esophagitis Rats. BioMed Res. Int. 2020, 2020, 4826176. [Google Scholar] [CrossRef]
- Fang, C.J.; Rong, X.J.; Jiang, W.W.; Chen, X.Y.; Liu, Y.L. Geniposide promotes wound healing of skin ulcers in diabetic rats through PI3K/Akt pathway. Heliyon 2023, 9, e21331. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, Y.; Yin, N.; Zhao, M.; Sun, X.; Zhang, Y.; Wang, Z. Geniposide alleviates imiquimod-induced psoriasis-like skin lesions in mice via inhibition of angiogenesis. Int. Immunopharmacol. 2024, 132, 111923. [Google Scholar] [CrossRef]
- Giani Tagliabue, S.; Faber, S.C.; Motta, S.; Denison, M.S.; Bonati, L. Modeling the binding of diverse ligands within the Ah receptor ligand binding domain. Sci. Rep. 2019, 9, 10693. [Google Scholar] [CrossRef]
- Furue, M.; Hashimoto-Hachiya, A.; Tsuji, G. Antioxidative Phytochemicals Accelerate Epidermal Terminal Differentiation via the AHR-OVOL1 Pathway: Implications for Atopic Dermatitis. Acta Derm. Venereol. 2018, 98, 918–923. [Google Scholar] [CrossRef]
- Bock, K.W. Aryl hydrocarbon receptor (AHR)-mediated inflammation and resolution: Non-genomic and genomic signaling. Biochem. Pharmacol. 2020, 182, 114220. [Google Scholar] [CrossRef]
- Luo, Z.; Yang, L.; Zhu, T.; Fan, F.; Wang, X.; Liu, Y.; Zhan, H.; Luo, D.; Guo, J. Aucubin ameliorates atherosclerosis by modulating tryptophan metabolism and inhibiting endothelial-mesenchymal transitions via gut microbiota regulation. Phytomedicine 2024, 135, 156122. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, J.; Chandan, N.; Lio, P.; Shi, V. The Skin Barrier and Moisturization: Function, Disruption, and Mechanisms of Repair. Skin Pharmacol. Phys. 2023, 36, 174–185. [Google Scholar] [CrossRef]
- Le Lamer, M.; Pellerin, L.; Reynier, M.; Cau, L.; Pendaries, V.; Leprince, C.; Méchin, M.C.; Serre, G.; Paul, C.; Simon, M. Defects of corneocyte structural proteins and epidermal barrier in atopic dermatitis. Biol. Chem. 2015, 396, 1163–1179. [Google Scholar] [CrossRef]
- Fan, Y.; Gu, R.; Zhang, R.; Wang, M.; Xu, H.; Wang, M.; Long, C. Protective effects of extracts from Acer truncatum leaves on SLS-induced HaCaT cells. Front. Pharmacol. 2023, 14, 1068849. [Google Scholar] [CrossRef]
- Wang, C.; Shang, H.; Zhang, S.; Wang, X.; Liu, D.; Shen, M.; Li, N.; Jiang, Y.; Wei, K.; Zhu, R. Hexavalent chromium disrupts the skin barrier by targeting ROS-mediated mitochondrial pathway apoptosis in keratinocytes. Chem. Biol. Interact. 2023, 379, 110523. [Google Scholar] [CrossRef]
- Dong, J.; Li, B.; Xu, P.; Kong, L.; Zhang, W.; Wu, D.; Tian, Y.; Wang, X. Skin barrier repair efficacy and safety evaluation of an extract from Gardenia jasminoides J.Ellis. Nat. Prod. Res. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Mosa, F.E.S.; Alqahtani, M.A.; El-Ghiaty, M.A.; Barakat, K.; El-Kadi, A.O.S. Identifying novel aryl hydrocarbon receptor (AhR) modulators from clinically approved drugs: In silico screening and In vitro validation. Arch. Biochem. Biophys. 2024, 754, 109958. [Google Scholar] [CrossRef]
- Alexander, H.; Brown, S.; Danby, S.; Flohr, C. Research Techniques Made Simple: Transepidermal Water Loss Measurement as a Research Tool. J. Investig. Dermatol. 2018, 138, 2295–2300.e2291. [Google Scholar] [CrossRef]
- Denda, M.; Nakanishi, S. Do epidermal keratinocytes have sensory and information processing systems? Exp. Dermatol. 2022, 31, 459–474. [Google Scholar] [CrossRef] [PubMed]
- Talagas, M.; Lebonvallet, N.; Berthod, F.; Misery, L. Lifting the veil on the keratinocyte contribution to cutaneous nociception. Protein Cell 2020, 11, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.R.U.; Augustine, R.; Zahid, A.A.; Ahmed, R.; Tariq, M.; Hasan, A. Reduced Graphene Oxide Incorporated GelMA Hydrogel Promotes Angiogenesis For Wound Healing Applications. Int. J. Nanomed. 2019, 14, 9603–9617. [Google Scholar] [CrossRef]
- Yang, D.; Fu, M.; Zhao, Q.; Wang, Y.; Li, T.; Feng, B.; Li, E.; Nishijima, Y.; Sun, Z.; Hu, Z. α-ionone promotes keratinocyte functions and accelerates epidermal barrier recovery. Ann. Transl. Med. 2023, 11, 297. [Google Scholar] [CrossRef]
- Piipponen, M.; Li, D.; Landén, N.X. The Immune Functions of Keratinocytes in Skin Wound Healing. Int. J. Mol. Sci. 2020, 21, 8790. [Google Scholar] [CrossRef]
- Leśniak, W. Epigenetic Regulation of Epidermal Differentiation. Epigenomes 2021, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Vukmanovic-Stejic, M. Skin barrier immunity and ageing. Immunology 2020, 160, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Ku, J.M.; Lee, S.H.; Shim, H.J.; Park, D.S.; Sung, J.W.; Shin, Y.C.; Ko, S.-G. Skin Improvement Effects of Gardeniae fructus Extract in HaCaT Keratinocytes, B16F10 Melanocytes, and CCD-986sk Fibroblast Cells. Cosmetics 2019, 6, 48. [Google Scholar] [CrossRef]
- Sondermann, N.C.; Faßbender, S.; Hartung, F.; Hätälä, A.M.; Rolfes, K.M.; Vogel, C.F.A.; Haarmann-Stemmann, T. Functions of the aryl hydrocarbon receptor (AHR) beyond the canonical AHR/ARNT signaling pathway. Biochem. Pharmacol. 2023, 208, 115371. [Google Scholar] [CrossRef]
- Ishitsuka, Y.; Roop, D.R. Loricrin: Past, Present, and Future. Int. J. Mol. Sci. 2020, 21, 2271. [Google Scholar] [CrossRef]
- Paik, S.J.; Kim, D.J.; Jung, S.K. Preventive Effect of Pharmaceutical Phytochemicals Targeting the Src Family of Protein Tyrosine Kinases and Aryl Hydrocarbon Receptor on Environmental Stress-Induced Skin Disease. Int. J. Mol. Sci. 2023, 24, 5953. [Google Scholar] [CrossRef]
- Shin, M.R.; Lee, J.A.; Kim, M.; Lee, S.; Oh, M.; Moon, J.; Nam, J.W.; Choi, H.; Mun, Y.J.; Roh, S.S. Gardeniae Fructus Attenuates Thioacetamide-Induced Liver Fibrosis in Mice via Both AMPK/SIRT1/NF-κB Pathway and Nrf2 Signaling. Antioxidants 2021, 10, 1837. [Google Scholar] [CrossRef]
- Fernández-Gallego, N.; Sánchez-Madrid, F.; Cibrian, D. Role of AHR Ligands in Skin Homeostasis and Cutaneous Inflammation. Cells 2021, 10, 3176. [Google Scholar] [CrossRef]
- Mayrovitz, H.N. Transepidermal water loss and stratum corneum hydration in forearm versus hand palm. Skin Res. Technol. 2023, 29, e13218. [Google Scholar] [CrossRef] [PubMed]
- Schleusener, J.; Salazar, A.; von Hagen, J.; Lademann, J.; Darvin, M.E. Retaining Skin Barrier Function Properties of the Stratum Corneum with Components of the Natural Moisturizing Factor-A Randomized, Placebo-Controlled Double-Blind In Vivo Study. Molecules 2021, 26, 1649. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.J.; Kim, Y.J.; Myeong, S.; Huh, G.; Kim, W.S. Clinical Evaluation of Conditioned Media of Human Umbilical Cord Blood Mesenchymal Stem Cells for Improvement of Symptoms of Sensitive Skin: Prospective, Single Blinded, Split-face Study. Ann. Dermatol. 2023, 35, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Manav, V.; Karaali, M.G.; Erdem, O.; Koku Aksu, A.E. Association between biophysical properties and anxiety in patients with sensitive skin. Skin Res. Technol. 2022, 28, 556–563. [Google Scholar] [CrossRef]
- Liu, L.; Jiao, Y.; Yang, M.; Wu, L.; Long, G.; Hu, W. Network Pharmacology, Molecular Docking and Molecular Dynamics to Explore the Potential Immunomodulatory Mechanisms of Deer Antler. Int. J. Mol. Sci. 2023, 24, 10370. [Google Scholar] [CrossRef]
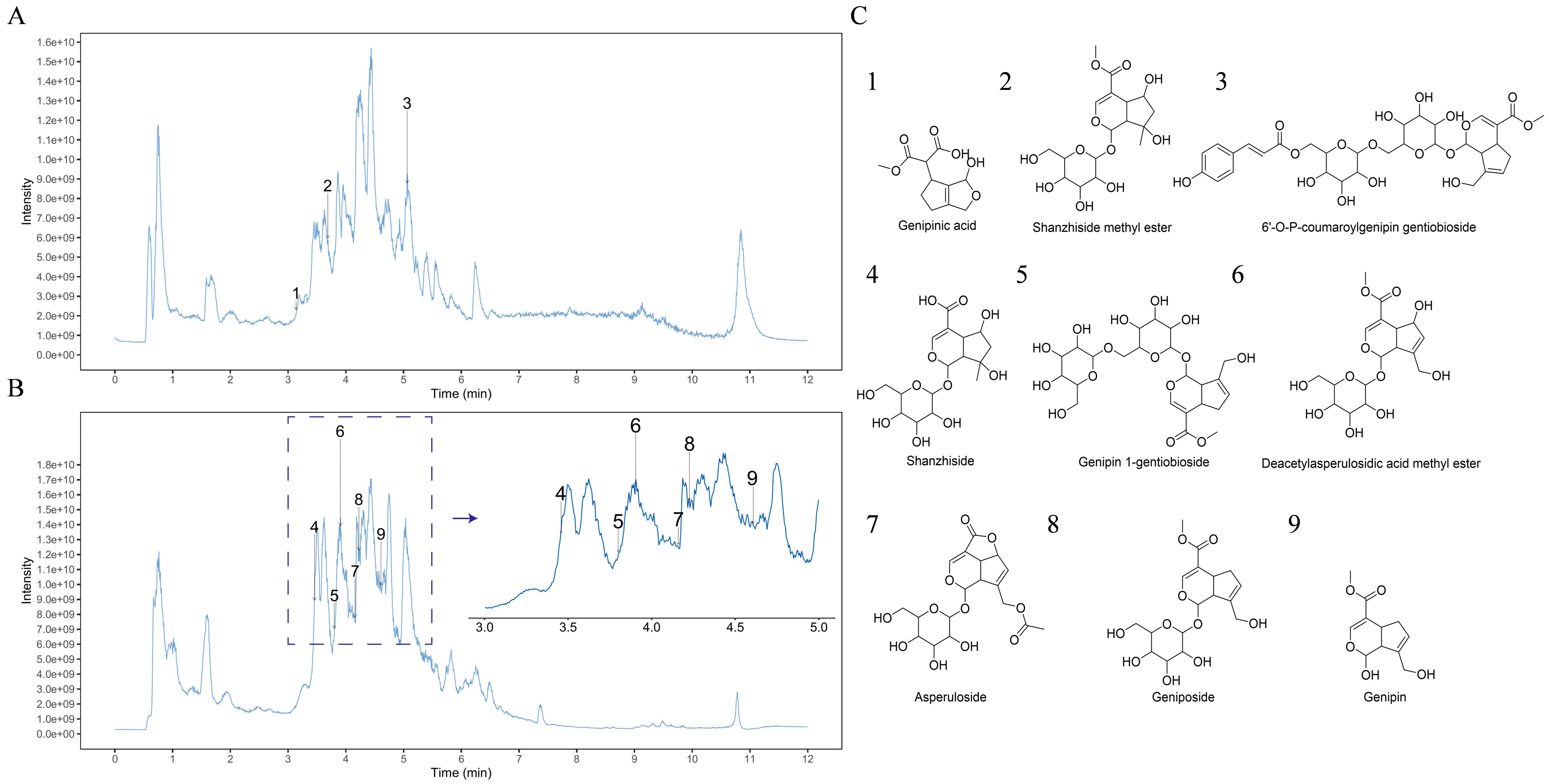
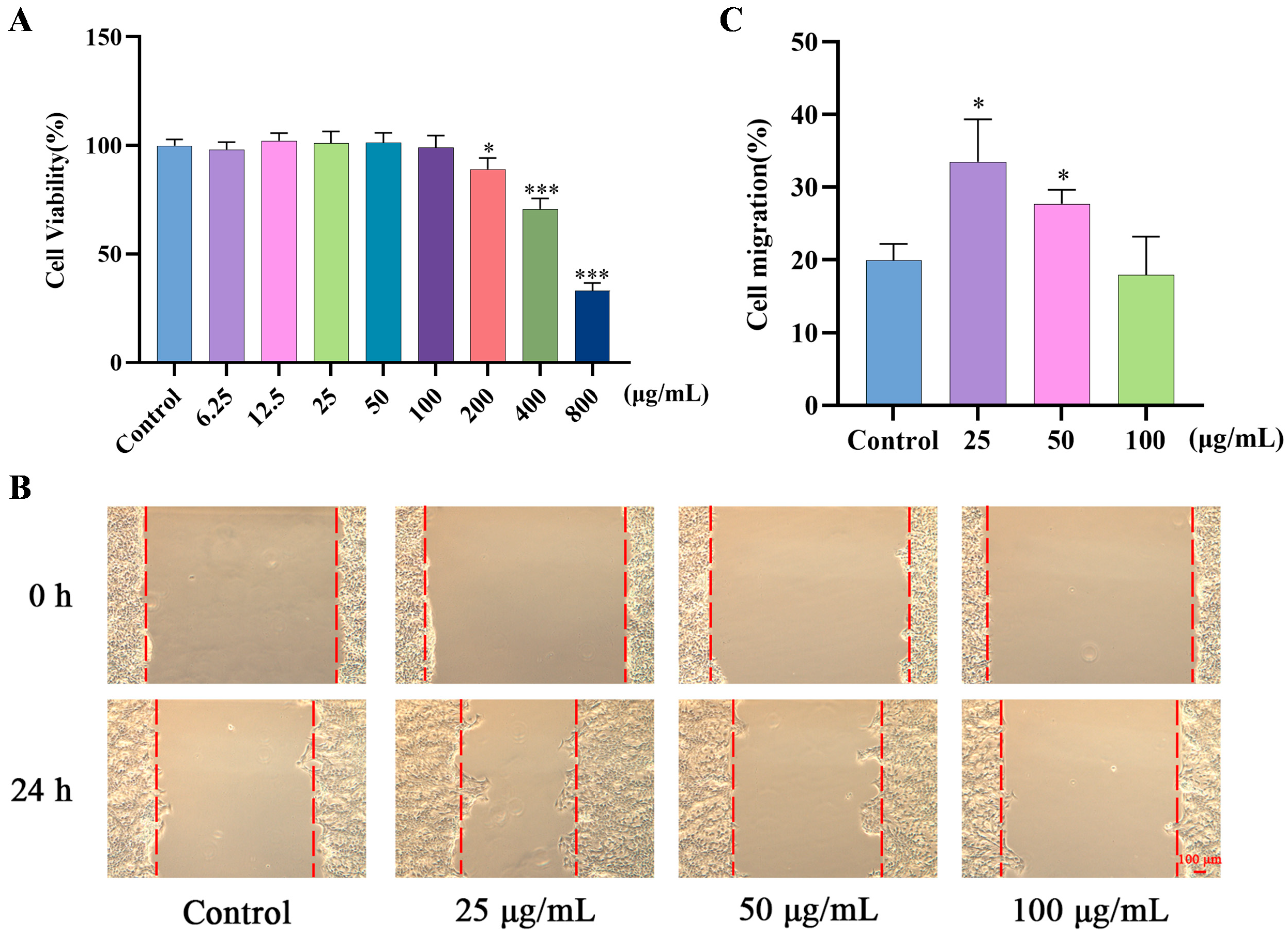
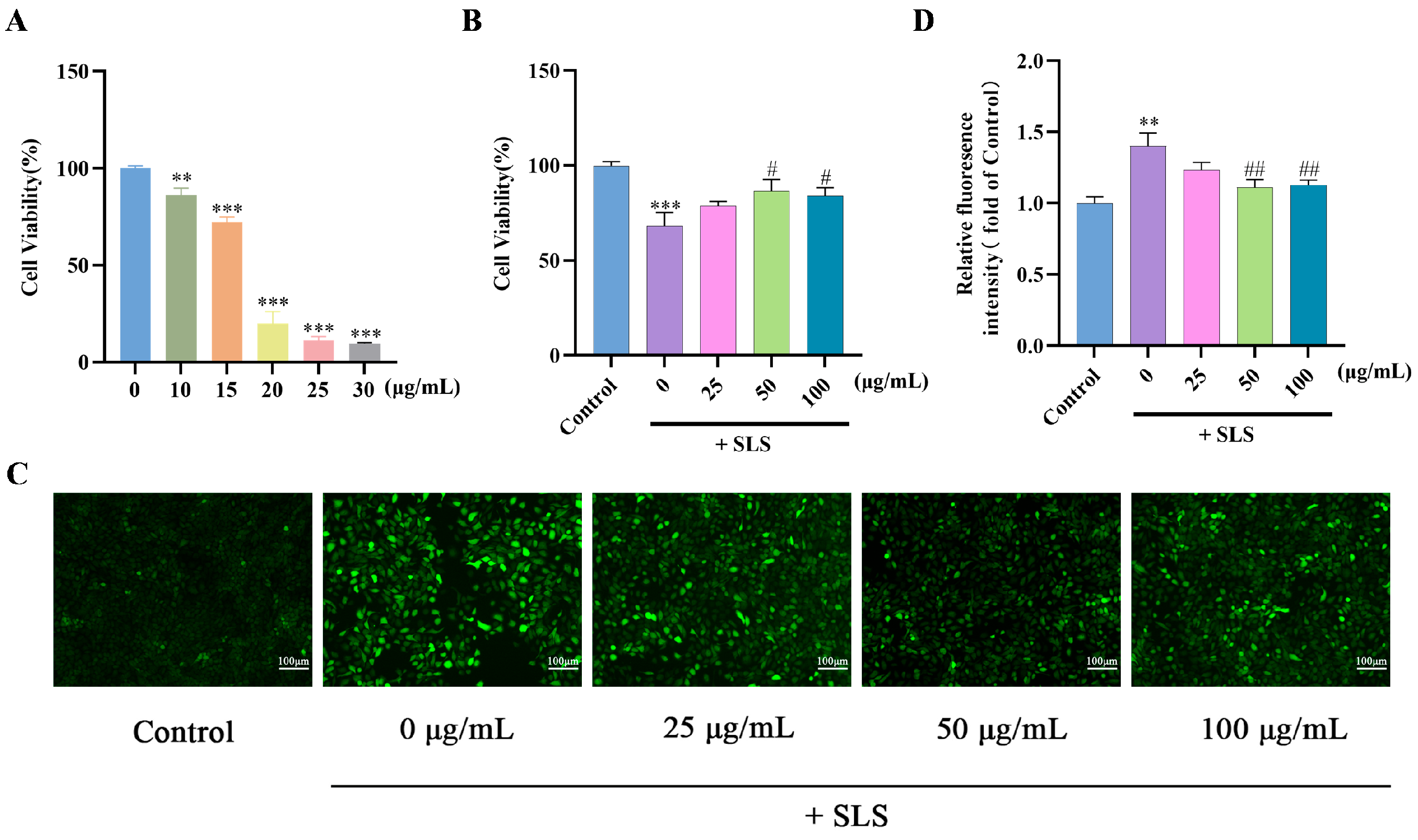
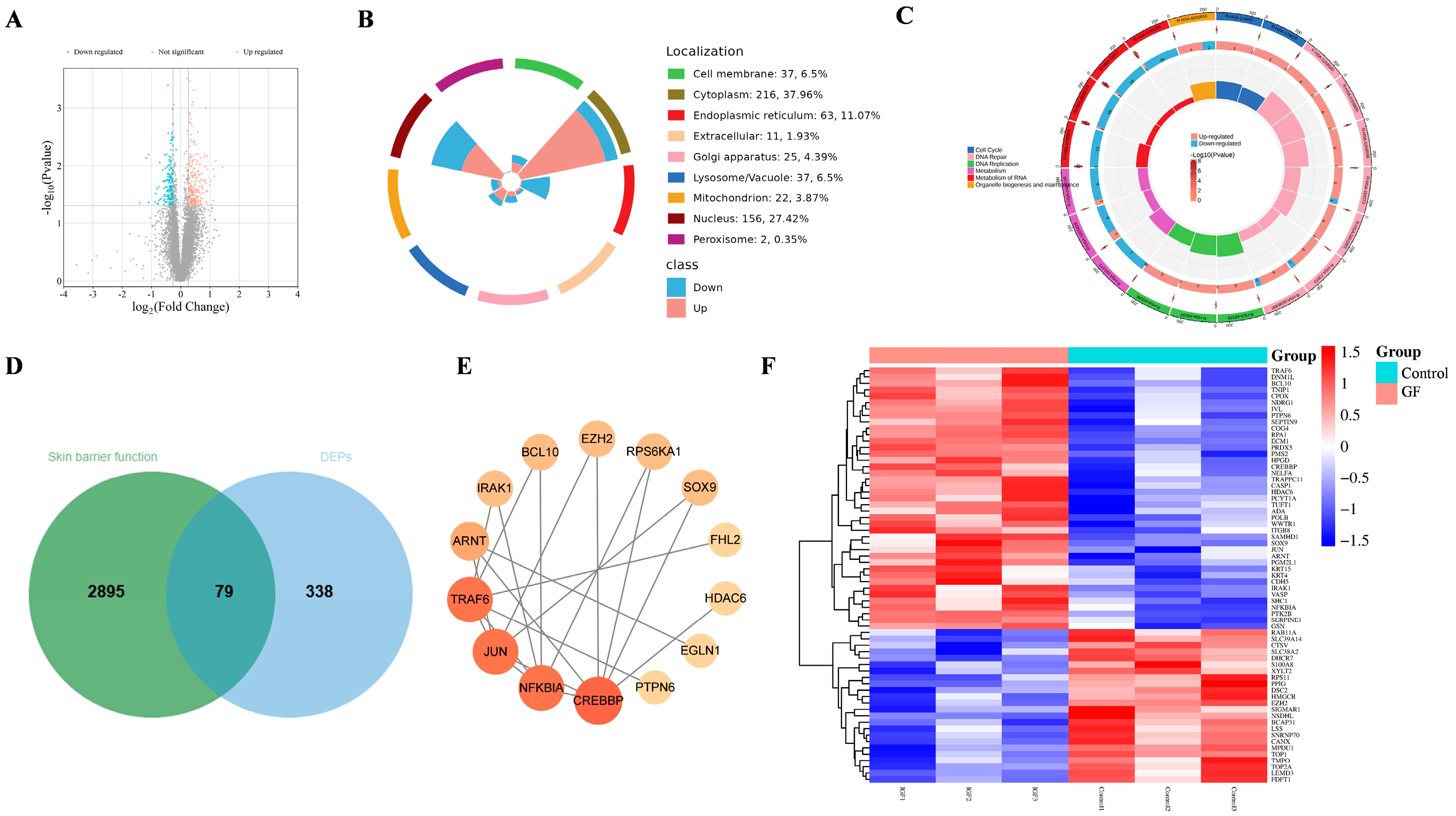
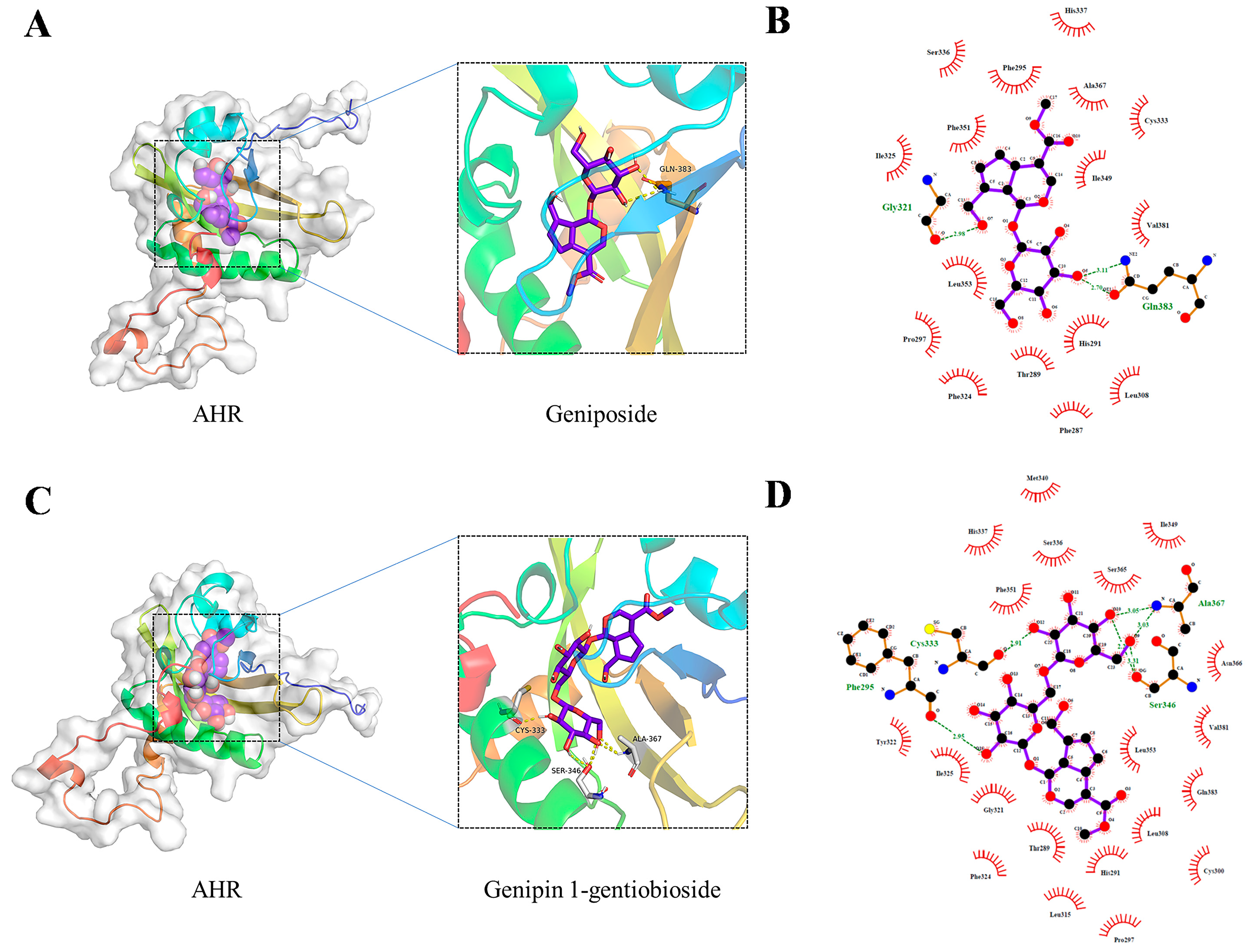
| Ingredient | Docking Score (Kcal/Mol) |
|---|---|
| geniposide | −7.6 |
| genipin-1-gentiobioside | −5.1 |
| Gene | Forward Sequence (5′-3′) | Reverse Sequence (5′-3′) |
|---|---|---|
| β-actin | TGGCACCCAGCACAATGAA | CTAAGTCATAGTCCGCCTAGAAGCA |
| AHR | CAAATCCTTCCAAGCGGCATA | CGCTGAGCCTAAGAACTGAAAG |
| FLG | TTCGGCAAATCCTGAAGAATCC | ACTGTGCTTTCTGTGCTTGTG |
| LOR | GGAGATCAGTGCTCCTCACA | AGCAGAACTAGATGCAGCCG |
| IVL | GGGTATTGACTGGAGGAGGAACA | AGCCTTACTGTGAGTCTGGTTGA |
| Ingredient | Placebo Gel | GF Gel |
|---|---|---|
| WATER | 94.20% | 92.60% |
| PENTYLENE GLYCOL | 3% | 3% |
| GF | / | 2% |
| GLYCERIN | 2% | 2% |
| ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER | 0.35% | 0.35% |
| ARGININE | 0.25% | 0.25% |
| HYDROXYACETOPHENONE | 0.20% | 0.20% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zong, K.; Zhou, F.; Xu, K.; Dong, J.; Huang, Q.; Wu, J. Gardeniae Fructus Enhances Skin Barrier Function via AHR-Mediated FLG/LOR/IVL Expression. Molecules 2025, 30, 3764. https://doi.org/10.3390/molecules30183764
Zong K, Zhou F, Xu K, Dong J, Huang Q, Wu J. Gardeniae Fructus Enhances Skin Barrier Function via AHR-Mediated FLG/LOR/IVL Expression. Molecules. 2025; 30(18):3764. https://doi.org/10.3390/molecules30183764
Chicago/Turabian StyleZong, Kaile, Fangni Zhou, Kewei Xu, Junzi Dong, Qing Huang, and Jianxin Wu. 2025. "Gardeniae Fructus Enhances Skin Barrier Function via AHR-Mediated FLG/LOR/IVL Expression" Molecules 30, no. 18: 3764. https://doi.org/10.3390/molecules30183764
APA StyleZong, K., Zhou, F., Xu, K., Dong, J., Huang, Q., & Wu, J. (2025). Gardeniae Fructus Enhances Skin Barrier Function via AHR-Mediated FLG/LOR/IVL Expression. Molecules, 30(18), 3764. https://doi.org/10.3390/molecules30183764






