Medicinal Plants for Skin Disorders: Phytochemistry and Pharmacological Insights
Abstract
1. Introduction
2. Methodology of Literature Selection
3. Medicinal Plants
3.1. Bergenia crassifolia
3.2. Black Elderberry (Sambucus nigra)
3.3. Broadleaf Plantain (Plantago major)
3.4. Canadian Goldenrod (Solidago canadensis)
3.5. Coltsfoot (Tussilago farfara)
3.6. Common Blackberry (Rubus vulgaris)
3.7. Common Dandelion (Taraxacum officinale)
3.8. Common Flax (Linum usitatissimum)
3.9. European Cranberrybush (Viburnum opulus)
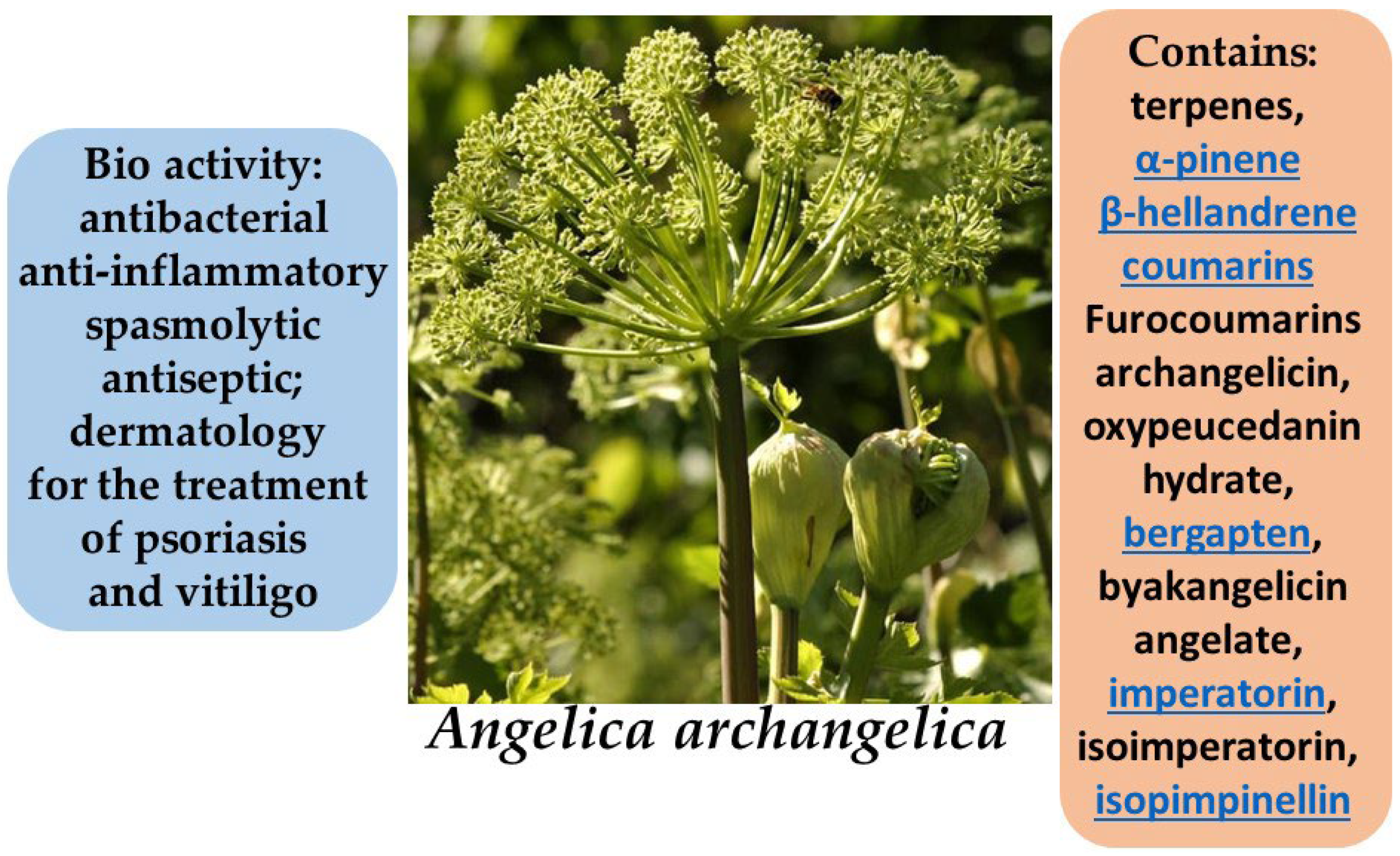
3.10. Garden Angelica (Angelica archangelica)
3.11. Greater Burdock (Arctium lappa)
3.12. Inula helenium L.
3.13. Marshmallow (Althaea officinalis)
3.14. Narrow-leaved Lavender (Lavandula angustifolia)
3.15. Red Clover (Trifolium pratense L.)
3.16. Virginian Witch Hazel (Hamamelis virginiana)
3.17. Smooth Brome (Bromus inermis Leyss.)
3.18. Turmeric (Curcuma longa)
3.19. White Willow (Salix alba)
3.20. White Wormwood (Artemisia terrae-albae)
4. Perspectives for Investigations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mias, C.; Mengeaud, V.; Bessou-Touya, S.; Duplan, H. Recent advances in understanding inflammatory acne: Deciphering the relationship between Cutibacterium acnes and Th17 inflammatory pathway. J. Eur. Acad. Dermatol. Venereol. 2023, 37 (Suppl. S7), 3–11. [Google Scholar] [CrossRef]
- Courtney, A.; Su, J.C. The psychology of atopic dermatitis. J. Clin. Med. 2024, 13, 1602. [Google Scholar] [CrossRef]
- Coondoo, A.; Phiske, M.; Verma, S.; Lahiri, K. Side-effects of topical steroids: A long overdue revisit. Indian Dermatol. Online, J. 2014, 5, 416–425. [Google Scholar] [CrossRef]
- Michalak, M. Plant extracts as skin care and therapeutic agents. Int. J. Mol. Sci. 2023, 24, 15444. [Google Scholar] [CrossRef]
- Olisova, O.Y.; Snarskaya, E.S.; Gladko, V.V.; Burova, E.P. Russian traditional medicine in dermatology. Clin. Dermatol. 2018, 36, 325–337. [Google Scholar] [CrossRef]
- Shedoeva, A.; Leavesley, D.; Upton, Z.; Fan, C. Wound healing and the use of medicinal plants. Evid. Based Complement. Altern. Med. 2019, 2019, 2684108. [Google Scholar] [CrossRef]
- Choi, H.Y.; Lee, Y.J.; Kim, C.M.; Lee, Y.-M. Revolutionizing Cosmetic Ingredients: Harnessing the Power of Antioxidants, Probiotics, Plant Extracts, and Peptides in Personal and Skin Care Products. Cosmetics 2024, 11, 157. [Google Scholar] [CrossRef]
- Cedillo-Cortezano, M.; Martinez-Cuevas, L.R.; López, J.A.M.; Barrera López, I.L.; Escutia-Perez, S.; Petricevich, V.L. Use of medicinal plants in the process of wound healing: A literature review. Pharmaceuticals 2024, 17, 303. [Google Scholar] [CrossRef]
- Iosageanu, A.; Mihai, E.; Seciu-Grama, A.M.; Utoiu, E.; Gaspar-Pintiliescu, A.; Gatea, F.; Craciunescu, O. In Vitro Wound-Healing Potential of Phenolic and Polysaccharide Extracts of Aloe vera Gel. J. Funct. Biomater. 2024, 15, 266. [Google Scholar] [CrossRef]
- Nisar, A.; Jagtap, S.; Vyavahare, S.; Deshpande, M.; Harsulkar, A.; Ranjekar, P.; Prakash, O. Phytochemicals in the treatment of inflammation-associated diseases: The journey from preclinical trials to clinical practice. Front. Pharmacol. 2023, 14, 1177050. [Google Scholar] [CrossRef]
- Gupta, B.; Malviya, R.; Mishra, P.S.; Uniyal, P. Plant-derived molecules as potent anti-skin cancer agents. A comprehensive review. Int. J. Mol. Sci. 2025, 26, 2278. [Google Scholar] [CrossRef]
- Koul, B.; Kumar, A.; Yadav, D.; Jin, J.O. Bergenia genus: Traditional uses, phytochemistry and pharmacology. Molecules 2020, 25, 5555. [Google Scholar] [CrossRef] [PubMed]
- Ajebli, M.; Eddouks, M. The promising role of plant tannins as bioactive antidiabetic agents. Curr. Med. Chem. 2019, 26, 4852–4884. [Google Scholar] [CrossRef]
- Kovaleva, T.Y.; Ermakova, V.A.; Dorovskih, E.A.; Trashchenkova, D.A.; Bokov, D.O.; Shilova, I.V.; Samylina, I.A. Phenolic Compounds and Biological Activity of Badan (Bergenia crassifolia (L.) Fritsch) Leaves Growing in Russia. Syst. Rev. Pharm. 2020, 11, 368. [Google Scholar] [CrossRef]
- Árok, R.; Végh, K.; Alberti, Á.; Kéry, Á. Phytochemical comparison and analysis of Bergenia crassifolia L. (Fritsch.) and Bergenia cordifolia Sternb. Eur. Chem. Bull. 2012, 1, 31–34. [Google Scholar]
- Stelmakh, S.; Ochirov, O.; Grigor’eva, M.; Tykheev, A.; Lebedeva, S.; Okladnikova, V.; Zhamsaranova, S. Wound-healing activity of polyhexamethyleneguanidine hydrochloride hydrogel and extract of Bergenia crassifolia on thermal burn simulation. Mong. J. Chem. 2022, 23, 51–62. [Google Scholar] [CrossRef]
- Liu, Y.; An, Z.; He, L.Y. The traditional uses, phytochemistry, pharmacology and toxicology of Bergenia purpurascens—A review. Heliyon 2023, 9, e22249. [Google Scholar] [CrossRef]
- Kushwaha, N.; Singh, A. Bergenia ciliata-phytochemistry and pharmacology: A review. Biomed. Mater. Devices 2024, 2, 891–904. [Google Scholar] [CrossRef]
- Mehta, S.; Kadian, V.; Dalal, S.; Dalal, P.; Kumar, S.; Garg, M.; Rao, R. A fresh look on bergenin: Vision of its novel drug delivery systems and pharmacological activities. Future Pharmacol. 2022, 2, 64–91. [Google Scholar] [CrossRef]
- Schmitzer, V.; Veberic, R.; Stampar, F. European elderberry (Plantago major L.) and American Elderberry (Sambucus canadensis L.): Botanical, chemical and health properties of flowers, berries and their products. In Berries: Properties, Consumption and Nutrition; Nova Science Publishers: New York, NY, USA, 2012; pp. 127–148. [Google Scholar]
- Svanberg, I.; de Vahl, E.; Ingvarsdóttir Olsen, N.; Ståhlberg, S. From Supernatural to Ornamental: Black Elder (Sambucus nigra L., Family Adoxaceae) in Sweden. Plants 2024, 13, 3068. [Google Scholar] [CrossRef]
- Ulbricht, C.; Basch, E.; Cheung, L.; Goldberg, H.; Hammerness, P.; Isaac, R.; Wortley, J. An evidence-based systematic review of elderberry and elderflower (Sambucus nigra) by the Natural Standard Research Collaboration. J. Diet. Suppl. 2014, 11, 80–120. [Google Scholar] [CrossRef]
- Knudsen, B.F.; Kaack, K.V. A review of human health and disease claims for elderberry (Sambucus nigra) fruit. In Proceedings of the I International Symposium on Elderberry, Warsaw, Poland, 9–14 June 2013. [Google Scholar] [CrossRef]
- Zwolińska, D. Rational phytotherapy as an alternative treatment for acute respiratory tract infections. Paediatr. Fam. Med. 2022, 18, 139. [Google Scholar] [CrossRef]
- Skowrońska, W.; Granica, S.; Czerwińska, M.E.; Osińska, E.; Bazylko, A. Sambucus nigra L. leaves inhibit TNF-α secretion by LPS-stimulated human neutrophils and strongly scavenge reactive oxygen species. J. Ethnopharmacol. 2022, 290, 115116. [Google Scholar] [CrossRef] [PubMed]
- Mota, A.H.; Andrade, J.M.; Ntungwe, E.N.; Pereira, P.; Cebola, M.J.; Bernardo-Gil, M.G.; Reis, C.P. Green extraction of Sambucus nigra L. for potential application in skin nanocarriers. Green Mater. 2020, 8, 181–193. [Google Scholar] [CrossRef]
- Lin, P.; Hwang, E.; Ngo, H.T.; Seo, S.A.; Yi, T.H. Sambucus nigra L. ameliorates UVB-induced photoaging and inflammatory response in human skin keratinocytes. Cytotechnology 2019, 71, 1003–1017. [Google Scholar] [CrossRef]
- Bejenaru, C.; Radu, A.; Mogoşanu, G.D.; Bejenaru, L.E.; Biţă, A.; Segneanu, A.E. Plantaginaceae Juss. Family. In Natural Products and Medicinal Properties of Carpathian (Romanian) Plants; CRC Press: Boca Raton, FL, USA, 2024; pp. 309–318. [Google Scholar]
- Upadhyay, S.; Bhandari, S.; Sharma, A.; Singh, B.R.; Taj, G. Plantago lanceolata L. In Medicinal and Aromatic Plants of India; Springer Nature Switzerland: Cham, Switzerland, 2024; Volume 3, pp. 287–301. [Google Scholar] [CrossRef]
- Cardoso, F.C.; Bortolozo, B.B.; Morari, J.; e Silva, G.T.D.S.; Rosa, P.C.; Araujo, E.P.; Lima, M.H. Effect of Plantago major on wound healing in hyperglycemic mice. J. Biosci. Med. 2025, 13, 320–333. [Google Scholar] [CrossRef]
- Michielini, J.P.; Yi, X.; Brown, L.M.; Gao, S.M.; Orians, C.; Crone, E.E. Novel host plant use by a specialist insect depends on geographic variation in both the host and herbivore species. Oecologia 2024, 204, 95–105. [Google Scholar] [CrossRef]
- Gasiewska, E.; Varga, S.; de Graaf, B.H.; Sánchez Vilas, J. Effects of water limitation on the production of key secondary metabolites with medicinal properties in Plantago lanceolata and Tanacetum parthenium. All Life 2025, 18, 2467653. [Google Scholar] [CrossRef]
- Gupta, S.; Singh, R.; Sharma, A.; Rather, G.A.; Lattoo, S.K.; Dhar, M.K. Comparative transcriptome mining for terpenoid biosynthetic pathway genes in wild and cultivated species of Plantago. Protoplasma 2022, 259, 439–452. [Google Scholar] [CrossRef]
- Samuelsen, A.B. The traditional uses, chemical constituents and biological activities of Plantago major L. A review. J. Ethnopharmacol. 2000, 71, 1–21. [Google Scholar] [CrossRef]
- Noureddine, B.; Elachouri, M.; Bussmann, R.W.; Khojimatov, O.K. Plantago afra L., Plantago akkensis subsp. ounifensis (Batt.) Maire, Plantago albicans L., Plantago amplexicaulis Cav., Plantago ciliata Desf., Plantago coronopus L., Plantago lanceolata L., Plantago major L., Plantago ovata Forssk. Plantaginaceae. In Ethnobotany of Northern Africa and Levant; Springer International Publishing: Cham, Switzerland, 2024; pp. 1–24. [Google Scholar] [CrossRef]
- Tian, Z.; Cheng, J.; Xu, J.; Feng, D.; Zhong, J.; Yuan, X.; Qiang, S. Cytogeography of naturalized Solidago canadensis populations in Europe. Plants 2023, 12, 1113. [Google Scholar] [CrossRef] [PubMed]
- Nkuimi Wandjou, J.G.; Quassinti, L.; Gudžinskas, Z.; Nagy, D.U.; Cianfaglione, K.; Bramucci, M.; Maggi, F. Chemical composition and antiproliferative effect of essential oils of four Solidago species (S. canadensis, S. gigantea, S. virgaurea and S.× niederederi). Chem. Biodivers. 2020, 17, e2000685. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, W.; Shao, H.; Tang, S. Selected aspects of invasive Solidago canadensis with an emphasis on its allelopathic abilities: A review. Chem. Biodivers. 2022, 19, e202200728. [Google Scholar] [CrossRef]
- Likhanov, A.; Oliinyk, M.; Pashkevych, N.; Churilov, A.; Kozyr, M. The role of flavonoids in invasion strategy of Solidago canadensis L. Plants 2021, 10, 1748. [Google Scholar] [CrossRef]
- Fedina, L.A.; Kuprin, A.V.; Ogorodnikov, E.M. Tussilago farfara (Asteraceae) in the South of the Far East of Russia. Russ. J. Biol. Invasions 2020, 11, 88–91. [Google Scholar] [CrossRef]
- Stoyanova, M.A.; Perifanova-Nemska, M.N. Biologically active compounds from Tussilago farfara L. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1031, 012103. [Google Scholar] [CrossRef]
- Chen, S.; Dong, L.; Quan, H.; Zhou, X.; Ma, J.; Xia, W.; Fu, X. A review of the ethnobotanical value, phytochemistry, pharmacology, toxicity and quality control of Tussilago farfara L. (coltsfoot). J. Ethnopharmacol. 2021, 267, 113478. [Google Scholar] [CrossRef]
- Liu, Y.F.; Yang, X.W.; Wu, B. Chemical constituents of the flower buds of Tussilago farfara. J. Chin. Pharm. Sci. 2007, 16, 288. [Google Scholar]
- Chanaj-Kaczmarek, J.; Wojcińska, M.; Matlawska, I. Phenolics in the Tussilago farfara leaves. Herba Pol. 2013, 59. [Google Scholar] [CrossRef][Green Version]
- Das, R.; Kemisetti, D. A comprehensive review Tussilago farfara Linn.: Taxonomical, morphological classification and its pharmacological activities. Res. Rev. Plant Sci. 2024, 4, 88. [Google Scholar]
- Sõukand, R.; Hrynevich, Y.; Vasilyeva, I.; Prakofjewa, J.; Vnukovich, Y.; Paciupa, J.; Kalle, R. Multi-functionality of the few: Current and past uses of wild plants for food and healing in Liubań region, Belarus. J. Ethnobiol. Ethnomed. 2017, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Manghwar, H.; Hu, W. Study on supergenus Rubus, L.: Edible, medicinal, and phylogenetic characterization. Plants 2022, 11, 1211. [Google Scholar] [CrossRef] [PubMed]
- Bolatkyzy, N.; Nurmakhanova, A.; Berganayeva, G.; Dyusebaeva, M. Study of the chemical composition of the Rubus vulgaris plant. Chem. J. Kaz. 2024, 1, 77–88. [Google Scholar] [CrossRef]
- Graham, J.; Woodhead, M. Rubus. In Wild Crop Relatives: Genomic and Breeding Resources: Temperate Fruits; Springer: Berlin/Heidelberg, Germany, 2010; pp. 179–196. [Google Scholar] [CrossRef]
- Surya, M.I.; Suhartati, S.; Ismaini, L.; Lusini, Y.; Anggraeni, D.; Normasiwi, S.; Bakar Sidiq, M.A. Fruit nutrients of five species of wild raspberries (Rubus spp.) from Indonesian mountain’s forests. J. Trop. Life Sci. 2018, 8, 75–80. [Google Scholar] [CrossRef]
- Gudej, J.; Tomczyk, M. Determination of flavonoids, tannins and ellagic acid in leaves from Rubus, L. species. Arch. Pharm. Res. 2004, 27, 1114–1119. [Google Scholar] [CrossRef]
- Muniyandi, K.; George, E.; Sathyanarayanan, S.; George, B.P.; Abrahamse, H.; Thamburaj, S.; Thangaraj, P. Phenolics, tannins, flavonoids and anthocyanins contents influenced antioxidant and anticancer activities of Rubus fruits from Western Ghats, India. Food Sci. Hum. Wellness 2019, 8, 73–81. [Google Scholar] [CrossRef]
- Zafrilla, P.; Ferreres, F.; Tomás-Barberán, F.A. Effect of processing and storage on the antioxidant ellagic acid derivatives and flavonoids of red raspberry (Rubus idaeus) jams. J. Agric. Food Chem. 2001, 49, 3651–3655. [Google Scholar] [CrossRef]
- Gil-Martínez, L.; Mut-Salud, N.; Ruiz-García, J.A.; Falcón-Piñeiro, A.; Maijó-Ferré, M.; Baños, A.; Gómez-Caravaca, A.M. Phytochemicals determination, and antioxidant, antimicrobial, anti-inflammatory and anticancer activities of blackberry fruits. Foods 2023, 12, 1505. [Google Scholar] [CrossRef]
- Süntar, I.; Koca, U.; Keleş, H.; Akkol, E.K. Wound healing activity of Rubus sanctus Schreber (Rosaceae): Preclinical study in animal models. Evid. Based Complement. Alternat. Med. 2011, 2011, 816156. [Google Scholar] [CrossRef]
- Pozdnyakova, Y.; Sailau, A.; Solyanov, D.; Aitisheva, L.; Tatina, Y.; Britko, V. Diversity of early flowering plants of the Ulytau mountains (Central Kazakhstan). Biosyst. Divers. 2023, 31, 261–268. [Google Scholar] [CrossRef]
- Martinez, M.; Poirrier, P.; Chamy, R.; Prüfer, D.; Schulze-Gronover, C.; Jorquera, L.; Ruiz, G. Taraxacum officinale and related species—An ethnopharmacological review and its potential as a commercial medicinal plant. J. Ethnopharmacol. 2015, 169, 244–262. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.A.; Goldstone, F.; Greenham, J. Flavonoids, cinnamic acids and coumarins from the different tissues and medicinal preparations of Taraxacum officinale. Phytochemistry 1996, 42, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Su, R.; Qiao, J.; Zhao, Z.; Wang, X. Flavonoids extraction from Taraxacum officinale (dandelion): Optimisation using response surface methodology and antioxidant activity. J. Chem. 2014, 2014, 956278. [Google Scholar] [CrossRef]
- Wu, J. Antibacterial activity of Taraxacum officinale against foodborne pathogens. Pak. J. Zool. 2021, 54, 1–8. [Google Scholar] [CrossRef]
- Díaz, K.; Espinoza, L.; Madrid, A.; Pizarro, L.; Chamy, R. Isolation and identification of compounds from bioactive extracts of Taraxacum officinale Weber ex F.H. Wigg. (dandelion) as a potential source of antibacterial agents. Evid. Based Complement. Alternat. Med. 2018, 2018, 2706417. [Google Scholar] [CrossRef]
- Di Napoli, A.; Zucchetti, P. A comprehensive review of the benefits of Taraxacum officinale on human health. Bull. Natl. Res. Cent. 2021, 45, 110. [Google Scholar] [CrossRef]
- Sweeney, B.; Vora, M.; Ulbricht, C.; Basch, E. Evidence-based systematic review of dandelion (Taraxacum officinale) by Natural Standard Research Collaboration. J. Herb. Pharmacother. 2005, 5, 79–93. [Google Scholar] [CrossRef]
- Grudzinskaya, L.M.; Gemejiyeva, N.G.; Karzhaubekova, Z.Z. The Kazakhstan medicinal flora survey in a leading families volume. Bull. Karaganda Univ. Biol. Med. Geogr. Ser. 2020, 100, 39–51. [Google Scholar] [CrossRef]
- Zajac, T.; Oleksy, A.; Klimek-Kopyra, A.; Kulig, B. Biological determinants of plant and crop productivity of flax (Linum usitatissimum L.). Acta Agrobot. 2012, 65, 3–14. [Google Scholar] [CrossRef][Green Version]
- Jhala, A.J.; Hall, L.M. Flax (Linum usitatissimum L.): Current uses and future applications. Aust. J. Basic Appl. Sci. 2010, 4, 4304–4312. [Google Scholar]
- Hussain, S.; Anjum, F.M.; Butt, M.S.; Sheikh, M.A. Chemical composition and functional properties of flaxseed (Linum usitatissimum) flour. Sarhad J. Agric. 2008, 24, 649–653. [Google Scholar]
- Hussien, Z.G.; Aziz, R.A. Chemical composition and antibacterial activity of Linum usitatissimum L. (Flaxseed). Syst. Rev. Pharm. 2021, 12, 145–147. [Google Scholar]
- Herchi, W.; Bahashwan, S.; Sebei, K.; Saleh, H.B.; Kallel, H.; Boukhchina, S. Effects of germination on chemical composition and antioxidant activity of flaxseed (Linum usitatissimum L.) oil. Grasas Aceites 2015, 66, e057. [Google Scholar] [CrossRef]
- Sharil, A.T.M.; Ezzat, M.B.; Widya, L.; Nurhakim, M.H.A.; Hikmah, A.R.N.; Zafira, Z.N.; Haris, M.S. Systematic review of flaxseed (Linum usitatissimum L.) extract and formulation in wound healing. J. Pharm. Pharmacogn. Res. 2022, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gürbüz, İ.L.H.A.N.; Özkan, A.M.G.; Akaydin, G.; Salihoğlu, E.C.E.; Günbatan, T.U.Ğ.B.A.; Demirci, F.; Yeşilada, E. Folk medicine in Düzce province (Turkey). Turk. J. Bot. 2019, 43, 769–784. [Google Scholar] [CrossRef]
- Kajszczak, D.; Zakłos-Szyda, M.; Podsędek, A. Viburnum opulus L.—A review of phytochemistry and biological effects. Nutrients 2020, 12, 3398. [Google Scholar] [CrossRef]
- Kollmann, J.; Grubb, P.J. Viburnum lantana L. and Viburnum opulus L. (V. lobatum Lam., Opulus vulgaris Borkh.). J. Ecol. 2002, 90, 1044–1070. [Google Scholar] [CrossRef]
- Vijaytha, V.; Anupama, R.V.; Haridas, M. Phytochemical profiling, and antioxidant, antibacterial, and anti-inflammatory properties of Viburnum coriaceum Blume. Future J. Pharm. Sci. 2020, 6, 84. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Vergara, C.V.; Kitic, D.; Kostic, M.; Armstrong, L.; Cho, W.C. Genus Viburnum: Therapeutic potentialities and agro-food-pharma applications. Oxid. Med. Cell. Longev. 2021, 2021, 3095514. [Google Scholar] [CrossRef]
- Tkacheva, N.; Eliseeva, T. Kalina (Viburnum). J. Healthy Nutr. Diet. 2018, 3, 43–52. [Google Scholar] [CrossRef]
- Akat, F.; Almaghrebi, E. Exploring the Therapeutic Potential of Viburnum opulus L.: A Comprehensive Review of Its Medicinal Properties and Health Benefits. In Traditional Medicine in North East Africa; Bentham Science Publishers: Sharjah, United Arab Emirates, 2025; pp. 147–171. [Google Scholar] [CrossRef]
- Moldovan, B.; David, L.; Vulcu, A.; Olenic, L.; Perde-Schrepler, M.; Fischer-Fodor, E.; Filip, G.A. In vitro and in vivo anti-inflammatory properties of green synthesized silver nanoparticles using Viburnum opulus L. fruits extract. Mater. Sci. Eng. C 2017, 79, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Khvorost, O.; Shpychak, O.; Skrebtsova, K. Prospects for using the fruits of Viburnum opulus to obtain medicines of various directions of action. Ann. Mechnikov’s Inst. 2024, 4, 11–15. [Google Scholar] [CrossRef]
- Aćimović, M.; Rat, M.; Pezo, L.; Lončar, B.; Pezo, M.; Miljković, A.; Lazarević, J. Biological and chemical diversity of Angelica archangelica L.—Case study of essential oil and its biological activity. Agronomy 2022, 12, 1570. [Google Scholar] [CrossRef]
- Maurya, A.; Verma, S.C.; Gupta, V.; Shankar, M.B. Angelica archangelica L.—A phytochemical and pharmacological review. Asian J. Res. Chem. 2017, 10, 852–856. [Google Scholar] [CrossRef]
- Forycka, A.; Buchwald, W. Variability of composition of essential oil and coumarin compounds of Angelica archangelica L. Herba Pol. 2019, 65, 4. [Google Scholar] [CrossRef]
- Nurzyńska-Wierdak, R. Chemical diversity, yield, and quality of aromatic plants. Agronomy 2023, 13, 1614. [Google Scholar] [CrossRef]
- Mamache, W.; Benslama, A.; Benchikh, F.; Benabdellah, H.; Lassas, S.; Amira, H.; Amira, S. Phytochemical screening, antioxidant, anti-ulcer, anti-inflammatory and analgesic activity of the aqueous extract of Angelica archangelica. Turk. J. Agric. Food Sci. Technol. 2022, 10, 334–340. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Deepa, P.; Kim, M.; Park, S.J.; Kim, S. A review of the composition of the essential oils and biological activities of Angelica species. Sci. Pharm. 2017, 85, 33. [Google Scholar] [CrossRef]
- Mishra, A.K.; Paliwal, S.K. A deep insight into chemistry and pharmacology of genus Angelica: An up-to-date systematic review. Nat. Prod. J. 2025, 15, e190324228133. [Google Scholar] [CrossRef]
- Topal, M.; Ozturk Sarıkaya, S.B.; Topal, F. Determination of Angelica archangelica’s antioxidant capacity and mineral content. ChemistrySelect 2021, 6, 7976–7980. [Google Scholar] [CrossRef]
- Kaur, A.; Bhatti, R. Understanding the phytochemistry and molecular insights to the pharmacology of Angelica archangelica L. (garden angelica) and its bioactive components. Phytother. Res. 2021, 35, 5961–5979. [Google Scholar] [CrossRef]
- Mir, S.A.; Dar, L.A.; Ali, T.; Kareem, O.; Rashid, R.; Khan, N.A.; Bader, G.N. Arctium lappa: A review on its phytochemistry and pharmacology. In Edible Plants in Health and Diseases: Volume II: Phytochemical and Pharmacological Properties; Springer Nature: Singapore, 2022; pp. 327–348. [Google Scholar] [CrossRef]
- Abdikerim, M.; Azimbaeva, G.; Izteleu, B.; Smailova, K. Comprehensive study of the main biologically active compounds in Arctium lappa plants growing in Kazakhstan. Pak. J. Bot. 2024, 56, 611–620. [Google Scholar] [CrossRef]
- Su, Y.; Fu, J.; Xie, H.; Huang, Z.; Li, Y.; Luo, Y.; Liu, Y. SSR markers development and their application in genetic diversity of burdock (Arctium lappa L.) germplasm. BMC Plant Biol. 2025, 25, 196. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Kakade, M.; Cherian, S.; Alagarasu, K.; Parashar, D. Arctigenin from Arctium lappa L. inhibits chikungunya virus by affecting its entry and replication. Phytomedicine 2024, 128, 155491. [Google Scholar] [CrossRef] [PubMed]
- Balkrishna, A.; Rana, M.; Mishra, S.; Rajput, S.K.; Dhanasekaran, M. Arctigenin: Harnessing nature’s power as an anti-inflammatory agent. Curr. Res. Complement. Altern. Med. 2024, 8, 255. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, L.; Liu, K. In vitro anti-inflammatory effects of arctigenin, a lignan from Arctium lappa L., through inhibition of the iNOS pathway. J. Ethnopharmacol. 2009, 122, 457–462. [Google Scholar] [CrossRef]
- Zhao, N.; Wang, L.; Cock, I.E. Arctium lappa L. root extracts inhibit the growth of bacterial triggers of selected autoimmune inflammatory diseases and potentiate the activity of conventional antibiotics. Pharmacogn. Commun. 2021, 11, 195–204. [Google Scholar] [CrossRef]
- Knipping, K.; van Esch, E.C.; Wijering, S.C.; van der Heide, S.; Dubois, A.E.; Garssen, J. In vitro and in vivo anti-allergic effects of Arctium lappa L. Exp. Biol. Med. 2008, 233, 1469–1477. [Google Scholar] [CrossRef]
- Sorokopudov, V.; Kabanov, A.; Bamatov, I. Features of the introduction of representatives of the genus Inula, L. BIO Web Conf. 2021, 32, 01001. [Google Scholar] [CrossRef]
- Verkhozina, A.V.; Anisimov, A.V.; Beshko, N.Y.; Biryukov, R.Y.; Bondareva, V.V.; Chernykh, D.V.; Dorofeev, N.V.; Dorofeyev, V.I.; Ebel, A.L.; Efremo, A.N.; et al. Findings to the flora of Russia and adjacent countries: New national and regional vascular plant records, 4. Bot. Pacifica 2022, 11, 129–157. [Google Scholar] [CrossRef]
- Dao, T.T.; Song, K.; Kim, J.Y.; Kim, Y.S. Igalan from Inula helenium L. suppresses the atopic-dermatitis-like response in stimulated HaCaT keratinocytes via JAK/STAT3 signaling. Inflamm. Res. 2020, 69, 309–319. [Google Scholar] [CrossRef]
- Buza, V.; Matei, M.C.; Ștefănuț, L.C. Inula helenium: A literature review on ethnomedical uses, bioactive compounds and pharmacological activities. Lucr. Științ. Ser. Med. Vet. 2020, 63, 53–59. [Google Scholar]
- Halagali, P.; Tippavajhala, V.K.; Rathnanand, M.; Sharma, H.; Pathak, R. Inulin as a Natural Ingredient in Cosmetics and Personal Care Products. In Inulin for Pharmaceutical Applications: A Versatile Biopolymer; Springer Nature: Singapore, 2025; pp. 137–146. [Google Scholar] [CrossRef]
- Kenny, C.-R.; Stojakowska, A.; Furey, A.; Lucey, B. From Monographs to Chromatograms: The Antimicrobial Potential of Inula helenium L. (Elecampane) Naturalised in Ireland. Molecules 2022, 27, 1406. [Google Scholar] [CrossRef] [PubMed]
- Petkova, N.; Ivanov, I.; Vrancheva, R.; Denev, P.; Pavlov, A. Ultrasound and microwave-assisted extraction of elecampane (Inula helenium) roots. Nat. Prod. Commun. 2017, 12, 1934578X1701200207. [Google Scholar] [CrossRef]
- Paulsen, E. Contact sensitization from Compositae-containing herbal remedies and cosmetics. Contact Dermat. 2002, 47, 189–198. [Google Scholar] [CrossRef]
- Dyakova, N.A.; Gaponov, S.P.; Slivkin, A.I.; Belenova, A.S.; Karlov, P.M.; Lavrov, S.V. Elaboration of an express technique for inulin extraction from the roots of elecampane (Inula helenium L.). IOP Conf. Ser. Earth Environ. Sci. 2021, 640, 052021. [Google Scholar] [CrossRef]
- Kianitalaei, A.; Feyzabadi, Z.; Hamedi, S.; Qaraaty, M.J.J.A.P.E.R. Althaea officinalis in traditional medicine and modern phytotherapy. J. Adv. Pharm. Educ. Res. 2019, 9 (Suppl. S2), 155. [Google Scholar]
- Xue, T.; Ruan, K.; Tang, Z.; Duan, J.; Xu, H. Isolation, structural properties, and bioactivities of polysaccharides from Althaea officinalis Linn.: A review. Int. J. Biol. Macromol. 2023, 242, 125098. [Google Scholar] [CrossRef]
- Naseri, V.; Chavoshzadeh, Z.; Mizani, A.; Daneshfard, B.; Ghaffari, F.; Abbas-Mohammadi, M.; Naseri, M. Effect of topical marshmallow (Althaea officinalis) on atopic dermatitis in children: A pilot double-blind active-controlled clinical trial of an in-silico-analyzed phytomedicine. Phytother. Res. 2021, 35, 1389–1398. [Google Scholar] [CrossRef]
- Farhat, C.; Younes, H.; Alyamani, O.A.; Mrad, M.; Hourani, N.; Khalifeh, H.; Hage-Sleiman, R. Chemical characterization and in vitro biological evaluation of aqueous extract of Althaea officinalis L. flower grown in Lebanon. J. Herb. Med. 2022, 34, 100575. [Google Scholar] [CrossRef]
- Khalighi, N.; Jabbari-Azad, F.; Barzegar-Amini, M.; Tavakkol-Afshari, J.; Layegh, P.; Salari, R. Impact of Althaea officinalis extract in patients with atopic eczema: A double-blind randomized controlled trial. Clin. Phytosci. 2021, 7, 73. [Google Scholar] [CrossRef]
- Doe, J.; Smith, A. High-Value Phytochemicals and Nutraceutical–Pharmaceutical Prospects of Althaea officinalis L. (Marshmallow): A Review. J. Pharm. Biomed. Anal. 2025, 234, 103601. [Google Scholar] [CrossRef]
- Bonaterra, G.A.; Bronischewski, K.; Hunold, P.; Schwarzbach, H.; Heinrich, E.U.; Fink, C.; Kinscherf, R. Anti-inflammatory and anti-oxidative effects of Phytohustil® and root extract of Althaea officinalis L. on macrophages in vitro. Front. Pharmacol. 2020, 11, 290. [Google Scholar] [CrossRef] [PubMed]
- Bonaterra, G.A.; Schmitt, J.; Schneider, K.; Schwarzbach, H.; Aziz-Kalbhenn, H.; Kelber, O.; Kinscherf, R. Phytohustil® and root extract of Althaea officinalis L. exert anti-inflammatory and anti-oxidative properties and improve the migratory capacity of endothelial cells in vitro. Front. Pharmacol. 2022, 13, 948248. [Google Scholar] [CrossRef] [PubMed]
- Crișan, I.; Ona, A.; Vârban, D.; Muntean, L.; Vârban, R.; Stoie, A.; Morea, A. Current trends for lavender (Lavandula angustifolia Mill.) crops and products with emphasis on essential oil quality. Plants 2023, 12, 357. [Google Scholar] [CrossRef]
- Yegorova, N.A.; Mitrofanova, I.V.; Brailko, V.A.; Grebennikova, O.A.; Paliy, A.E.; Stavtseva, I.V. Morphogenetic, physiological, and biochemical features of Lavandula angustifolia at long-term micropropagation in vitro. Russ. J. Plant Physiol. 2019, 66, 326–334. [Google Scholar] [CrossRef]
- Jianu, C.; Pop, G.; Gruia, A.T.; Horhat, F.G. Chemical composition and antimicrobial activity of essential oils of lavender (Lavandula angustifolia) and lavandin (Lavandula × intermedia) grown in Western Romania. Int. J. Agric. Biol. 2013, 15, 772–776. [Google Scholar]
- TienVinh, D.; Hoa, M.T.; Khai, P.C.; Van Minh, T. Micropropagation of lavender (Lavandula angustifolia). Seeds 2017, 4, 7–11. [Google Scholar]
- Adaszyńska, M.; Swarcewicz, M.; Dobrowolska, A. Chemical and mineral composition in varieties of lavender (Lavandula angustifolia L.). Prog. Plant Prot. 2011, 51, 15–20. [Google Scholar]
- Cardia, G.F.E.; Silva-Filho, S.E.; Silva, E.L.; Uchida, N.S.; Cavalcante, H.A.O.; Cassarotti, L.L.; Cuman, R.K.N. Effect of lavender (Lavandula angustifolia) essential oil on acute inflammatory response. Evid. Based Complement. Alternat. Med. 2018, 2018, 1413940. [Google Scholar] [CrossRef]
- Sharma, L.; Chandra, M.; Puneeta, A. Health benefits of lavender (Lavandula angustifolia). Int. J. Physiol. Nutr. Phys. Educ. 2020, 4, 1274–1277. [Google Scholar]
- Andrei, F.; Ersilia, A.; Tulcan, C.; Dragomirescu, A. Chemical composition and the potential of Lavandula angustifolia L. oil as a skin depigmentant. Rec. Nat. Prod. 2018, 12, 340. [Google Scholar] [CrossRef]
- Galea, C.; Cocoș, D.I.; Feier, F.; Moales, D. The use of essential oils in the development of dermato-cosmetic products. Med. Mater. 2023, 3, 31–36. [Google Scholar] [CrossRef]
- Vlaisavljević, S.; Kaurinović, B.; Popović, M.; Vasiljević, S. Profile of phenolic compounds in Trifolium pratense L. extracts at different growth stages and their biological activities. Int. J. Food Prop. 2017, 20, 3090–3101. [Google Scholar] [CrossRef]
- McKenna, P.; Cannon, N.; Conway, J.; Dooley, J.; Davies, W.P. Red clover (Trifolium pratense) in conservation agriculture: A compelling case for increased adoption. Int. J. Agric. Sustain. 2018, 16, 342–366. [Google Scholar] [CrossRef]
- Antonescu, A.I.; Miere, F.; Fritea, L.; Ganea, M.; Zdrinca, M.; Dobjanschi, L.; Cavalu, S. Perspectives on the combined effects of Ocimum basilicum and Trifolium pratense extracts in terms of phytochemical profile and pharmacological effects. Plants 2021, 10, 1390. [Google Scholar] [CrossRef]
- Gligor, D.; Kazlauskaite, J.A.; Bernatoniene, J.; Ivanauskas, L. Multidirectional Effects of Trifolium pratense L. (Red Clover) Extracts: Antioxidant, Estrogenic and Metabolic Activities. Molecules 2023, 28, 5178. [Google Scholar] [CrossRef]
- Dlamini, Z.; Jancsó, M.; Székely, Á.; Kolozsvári, I.; Túri, N.; Bakti, B.; Zalai, M.; Kun, Á. Assessing Yield, Biomass Production, and Forage Quality of Red Clover (Trifolium pratense L.) in Agroforestry System: One-Year Study in Szarvas, Hungary. Agronomy 2024, 14, 1921. [Google Scholar] [CrossRef]
- Manzoureh, R.; Farahpour, M.R. Topical administration of hydroethanolic extract of Trifolium pratense (red clover) accelerates wound healing by apoptosis and re-epithelialization. Biotech. Histochem. 2021, 96, 276–286. [Google Scholar] [CrossRef]
- Antonescu, A.I.M.; Antonescu, A.; Miere, F.G.; Fritea, L.; Teodorescu, A.G.; Vicas, L.; Cavalu, S. Novel topical formulations based on O. basilicum and T. pratense: Antioxidant, antimicrobial, and anti-inflammatory effect. Pharmacophore 2022, 13, 80–90. [Google Scholar] [CrossRef]
- Wen, J.; Shi, S. A phylogenetic and biogeographic study of Hamamelis (Hamamelidaceae), an eastern Asian and eastern North American disjunct genus. Biochem. Syst. Ecol. 1999, 27, 55–66. [Google Scholar] [CrossRef]
- Li, J.; Bogle, A.L.; Klein, A.S.; Donoghue, M.J. Phylogeny and biogeography of Hamamelis (Hamamelidaceae). Harv. Pap. Bot. 2000, 5, 171–178. [Google Scholar]
- Abbas, T.F.; Abbas, M.F.; Lafta, A.J. Antibacterial activity and medical properties of Witch Hazel Hamamelis virginiana. Ann. Trop. Med. Public Health 2020, 23, 46. [Google Scholar] [CrossRef]
- Natella, F.; Guantario, B.; Ambra, R.; Ranaldi, G.; Intorre, F.; Burki, C.; Canali, R. Human metabolites of Hamaforton™ (Hamamelis virginiana L. Extract) modulate fibroblast extracellular matrix components in response to UV-A irradiation. Front. Pharmacol. 2021, 12, 747638. [Google Scholar] [CrossRef] [PubMed]
- Piazza, S.; Martinelli, G.; Vrhovsek, U.; Masuero, D.; Fumagalli, M.; Magnavacca, A.; Sangiovanni, E. Anti-inflammatory and anti-acne effects of Hamamelis virginiana bark in human keratinocytes. Antioxidants 2022, 11, 1119. [Google Scholar] [CrossRef] [PubMed]
- Trüeb, R.M. Efficacy, tolerability, and superiority of propylene glycol-free, North American witch-hazel (Hamamelis virginiana)-based solution of 5% minoxidil sulfate for the treatment of female androgenetic alopecia. Int. J. Trichol. 2023, 15, 108–112. [Google Scholar] [CrossRef]
- Arct, J.; Pytkowska, K.; Dzierzgowski, S.; Neofitna, M. Oczar wirginijski (Hamamelis virginiana) w kosmetyce. Pol. J. Cosmetol. 2018, 21, 139–144. [Google Scholar]
- Turan, Ç.; Öner, Ü. Lip mesotherapy with dexpanthenol as a novel approach to prevent isotretinoin-associated cheilitis. Dermatol. Pract. Concept. 2023, 13, e2023012. [Google Scholar] [CrossRef]
- Otfinowski, R.K.N.C.C.P.M.; Kenkel, N.C.; Catling, P.M. The biology of Canadian weeds. 134 Bromus inermis Leyss. Can. J. Plant Sci. 2007, 87, 183–198. [Google Scholar] [CrossRef]
- Vasylenko, N.; Averchev, O.; Lavrenko, S.; Avercheva, N.; Lavrenko, N. Growth, Development and Productivity of Bromus inermis Depending on the Elements of Growing Technology in Non-Irradiated Conditions. 2020. Available online: https://www.agrolifejournal.usamv.ro/index.php/agrolife/article/view/714 (accessed on 15 December 2020).
- Mackiewicz-Walec, E.; Żarczyński, P.J.; Krzebietke, S.J.; Żarczyńska, K. Smooth Brome (Bromus inermis L.)—A versatile grass: A review. Agriculture 2024, 14, 854. [Google Scholar] [CrossRef]
- Mosse, I.B.; Sedlyar, N.I.; Babenko, A.S.; Mosse, K.A.; Kilchevsky, A.V. Determination of Epigenetic Markers of Human Psychoemotional Status; JINR: Dubna, Russia, 2021; p. 48. [Google Scholar]
- Mueller, R.S.; Bettenay, S.V.; Tideman, L. Aero-allergens in canine atopic dermatitis in southeastern Australia based on 1000 intradermal skin tests. Aust. Vet. J. 2000, 78, 392–399. [Google Scholar] [CrossRef]
- Ahmed, K.M.; Gupta, B.M.; Gupta, R. Curcuma longa (medicinal plant) research: A scientometric assessment of global publications output during 1997–2016. Pharmacogn. J. 2018, 10, 998–1006. [Google Scholar] [CrossRef]
- Ayer, D.K. Breeding for quality improvement in turmeric (Curcuma longa L.): A review. Adv. Plants Agric. Res. 2017, 6, 201–204. [Google Scholar] [CrossRef]
- Hossain, M.A.; Ishimine, Y. Growth, yield and quality of turmeric (Curcuma longa L.) cultivated on dark-red soil, gray soil and red soil in Okinawa, Japan. Plant Prod. Sci. 2005, 8, 482–486. [Google Scholar] [CrossRef]
- Li, S.; Yuan, W.; Deng, G.; Wang, P.; Yang, P.; Aggarwal, B. Chemical composition and product quality control of turmeric (Curcuma longa L.). Pharm. Crop. 2011, 2, 28–54. [Google Scholar] [CrossRef]
- Albaqami, J.J.; Hamdi, H.; Narayanankutty, A.; Visakh, N.U.; Sasidharan, A.; Kuttithodi, A.M.; Pathrose, B. Chemical composition and biological activities of the leaf essential oils of Curcuma longa, Curcuma aromatica and Curcuma angustifolia. Antibiotics 2022, 11, 1547. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Setzer, W.N. Chemical composition and biological activities of essential oils of Curcuma species. Nutrients 2018, 10, 1196. [Google Scholar] [CrossRef]
- Gounder, D.K.; Lingamallu, J. Comparison of chemical composition and antioxidant potential of volatile oil from fresh, dried and cured turmeric (Curcuma longa) rhizomes. Ind. Crops Prod. 2012, 38, 124–131. [Google Scholar] [CrossRef]
- Gopinath, H.; Karthikeyan, K. Turmeric: A condiment, cosmetic and cure. Indian J. Dermatol. Venereol. Leprol. 2018, 84, 16. [Google Scholar] [CrossRef]
- Zaman, S.U.; Akhtar, N. Effect of turmeric (Curcuma longa Zingiberaceae) extract cream on human skin sebum secretion. Trop. J. Pharm. Res. 2013, 12, 665–669. [Google Scholar] [CrossRef]
- Asada, K.; Ohara, T.; Muroyama, K.; Yamamoto, Y.; Murosaki, S. Effects of hot water extract of Curcuma longa on human epidermal keratinocytes in vitro and skin conditions in healthy participants: A randomized, double-blind, placebo-controlled trial. J. Cosmet. Dermatol. 2019, 18, 1866–1874. [Google Scholar] [CrossRef] [PubMed]
- Kaur, C.D.; Saraf, S. Topical vesicular formulations of Curcuma longa extract on recuperating the ultraviolet radiation–damaged skin. J. Cosmet. Dermatol. 2011, 10, 260–265. [Google Scholar] [CrossRef]
- Dickmann, D.I.; Kuzovkina, J. Poplars and willows of the world, with emphasis on silviculturally important species. In Poplars and Willows: Trees for Society and the Environment; The Food and Agriculture Organization of the United Nations:: Rome, Italy; CABI: Wallingford, UK, 2014; pp. 8–91. [Google Scholar] [CrossRef]
- Javed, B.; Farooq, F.; Ibrahim, M.; Abbas, H.A.B.; Jawwad, H.; Zehra, S.S.; Nawaz, K. Antibacterial and antifungal activity of methanolic extracts of Salix alba L. against various disease causing pathogens. Braz. J. Biol. 2021, 83, e243332. [Google Scholar] [CrossRef]
- Andrei, M. Multiple natural approaches of Salix alba. Arch. Microbiol. Immunol. 2024, 8, 410–427. [Google Scholar] [CrossRef]
- Zheng, L.; Jacquier, J.C.; Harbourne, N. Preparation of polyphenol-rich herbal beverages from white willow (Salix alba) bark with potential Alzheimer’s disease inhibitory activity in silico. Beverages 2024, 10, 75. [Google Scholar] [CrossRef]
- Sulaiman, G.M.; Hussien, N.N.; Marzoog, T.R.; Awad, H.A. Phenolic content, antioxidant, antimicrobial and cytotoxic activities of ethanolic extract of Salix alba. Am. J. Biochem. Biotechnol. 2013, 9, 41–46. [Google Scholar] [CrossRef]
- Maloshtan, L.M.; Pidgaina, V.V. Pharmacological activity cream of extract Salix alba and zinc for allergic contact dermatitis. Farmatsevtychnyi Zhurnal 2022, 6, 68–74. [Google Scholar] [CrossRef]
- Maistro, E.L.; Terrazzas, P.M.; Perazzo, F.F.; Gaivão, I.O.N.D.M.; Sawaya, A.C.H.F.; Rosa, P.C.P. Salix alba (white willow) medicinal plant presents genotoxic effects in human cultured leukocytes. J. Toxicol. Environ. Health A 2019, 82, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- Grubov, V.I. (Ed.) Plants of Central Asia—Plant Collection from China and Mongolia Vol. 14A: Compositae (Anthemideae); CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar] [CrossRef]
- Rakhimova, N.K.; Rakhimova, T.; Sadinov, J.S. Current state of Anabasis salsa pasture varieties in Karakalpak Ustyurt (Uzbekistan) due to Aral Sea drying. Plant Sci. Today 2022, 9, 25–30. [Google Scholar] [CrossRef]
- Berganayeva, G.; Kudaibergenova, B.; Litvinenko, Y.; Nazarova, I.; Sydykbayeva, S.; Vassilina, G.; Dyusebaeva, M. Medicinal plants of the flora of Kazakhstan used in the treatment of skin diseases. Molecules 2023, 28, 4192. [Google Scholar] [CrossRef]
- Ydyrys, A.; Zhamanbayeva, G.; Zhaparkulova, N.; Aralbaeva, A.; Askerbay, G.; Kenzheyeva, Z.; Murzakhmetova, M. The systematic assessment of the membrane-stabilizing and antioxidant activities of several Kazakhstani plants in the Asteraceae family. Plants 2023, 13, 96. [Google Scholar] [CrossRef]
- Hosseini, S.H.; Hosseini, S.V.; Mohammadi, M.; Ahmadyousefi, M. Asteraceae family: Phytochemical composition, pharmacological effects and traditional uses. Res. Ethnobiol. Conserv. 2024, 1, 63–86. [Google Scholar]
- Dyusebaeva, M.A.; Berillo, D.A.; Berganayeva, A.E.; Berganayeva, G.E.; Ibragimova, N.A.; Jumabayeva, S.M.; Vassilina, G.K. Antimicrobial activity of silver nanoparticles stabilized by liposoluble extract of Artemisia terrae-albae. Processes 2023, 11, 3041. [Google Scholar] [CrossRef]
- Ryabushkina, N.; Gemedjieva, N.; Kobaisy, M.; Cantrell, C.L. Brief review of Kazakhstan flora and use of its wild species. Asian Australas. J. Plant Sci. Biotechnol. 2008, 2, 64–71. [Google Scholar]
- Turuspekov, Y.; Genievskaya, Y.; Baibulatova, A.; Zatybekov, A.; Kotuhov, Y.; Ishmuratova, M.; Abugalieva, S. Phylogenetic taxonomy of Artemisia, L. species from Kazakhstan based on matK analyses. Proc. Latv. Acad. Sci. 2018, 72, 29. [Google Scholar] [CrossRef]
- Sagyndykova, M.; Imanbayeva, A.; Gassanova, G.; Ishmuratova, M. Current Status and Resources of Alhagi pseudalhagi (Fabaceae) in the Atyrau Region, Western Kazakhstan. Diversity 2024, 16, 219. [Google Scholar] [CrossRef]



















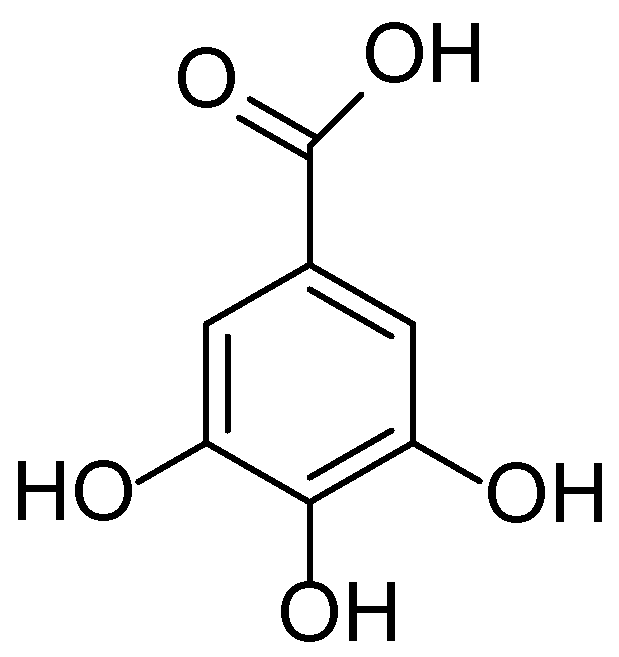




| No. | Plant Species | Key Bioactive Compounds | Pharmacological Activities | Dermatological Applications | References |
|---|---|---|---|---|---|
| 1.1 | Bergenia crassifolia | Arbutin, tannins, flavonoids | Anti-inflammatory, depigmenting, antimicrobial | Burns, hyperpigmentation, inflammation | [13,14,15,16,17,18,19] |
| 1.2 | Sambucus nigra | Flavonoids, anthocyanins, organic acids | Antioxidant, anti-inflammatory, antibacterial | Inflammation, anti-photoaging | [22,23,24,25,27] |
| 1.3 | Plantago major | Aucubin, flavonoids, phenolic acids | Anti-inflammatory, wound-healing, antimicrobial | Wounds, burns, eczema | [31,32,33,34,35] |
| 1.4 | Solidago canadensis | Flavonoids, saponins, essential oils | Anti-inflammatory, antioxidant, antimicrobial | Inflammation, eczema | [36,37,38,39] |
| 1.5 | Tussilago farfara | Flavonoids, phenolic acids, sesquiterpenes | Anti-inflammatory, antioxidant, antimicrobial | Skin irritations, acne, wounds | [42,43,44,45,46] |
| 1.6 | Rubus vulgaris | Anthocyanins, ellagitannins, phenolic acids | Antioxidant, anti-inflammatory, antimicrobial | Eczema, psoriasis, acne | [51,52,53,54] |
| 1.7 | Taraxacum officinale | Sesquiterpenes, flavonoids, phenolic acids | Anti-inflammatory, antioxidant, antimicrobial | Eczema, acne, inflammation | [60,61,62,63,64] |
| 1.8 | Linum usitatissimum | Lignans, phenolic acids, fatty acids | Anti-inflammatory, antioxidant, wound-healing | Burns, eczema, dermatitis | [68,69,70,71] |
| 1.9 | Viburnum opulus | Flavonoids, anthocyanins, tannins | Antioxidant, anti-inflammatory, antimicrobial | Inflammation, eczema, dermatitis | [74,75,76,78,79] |
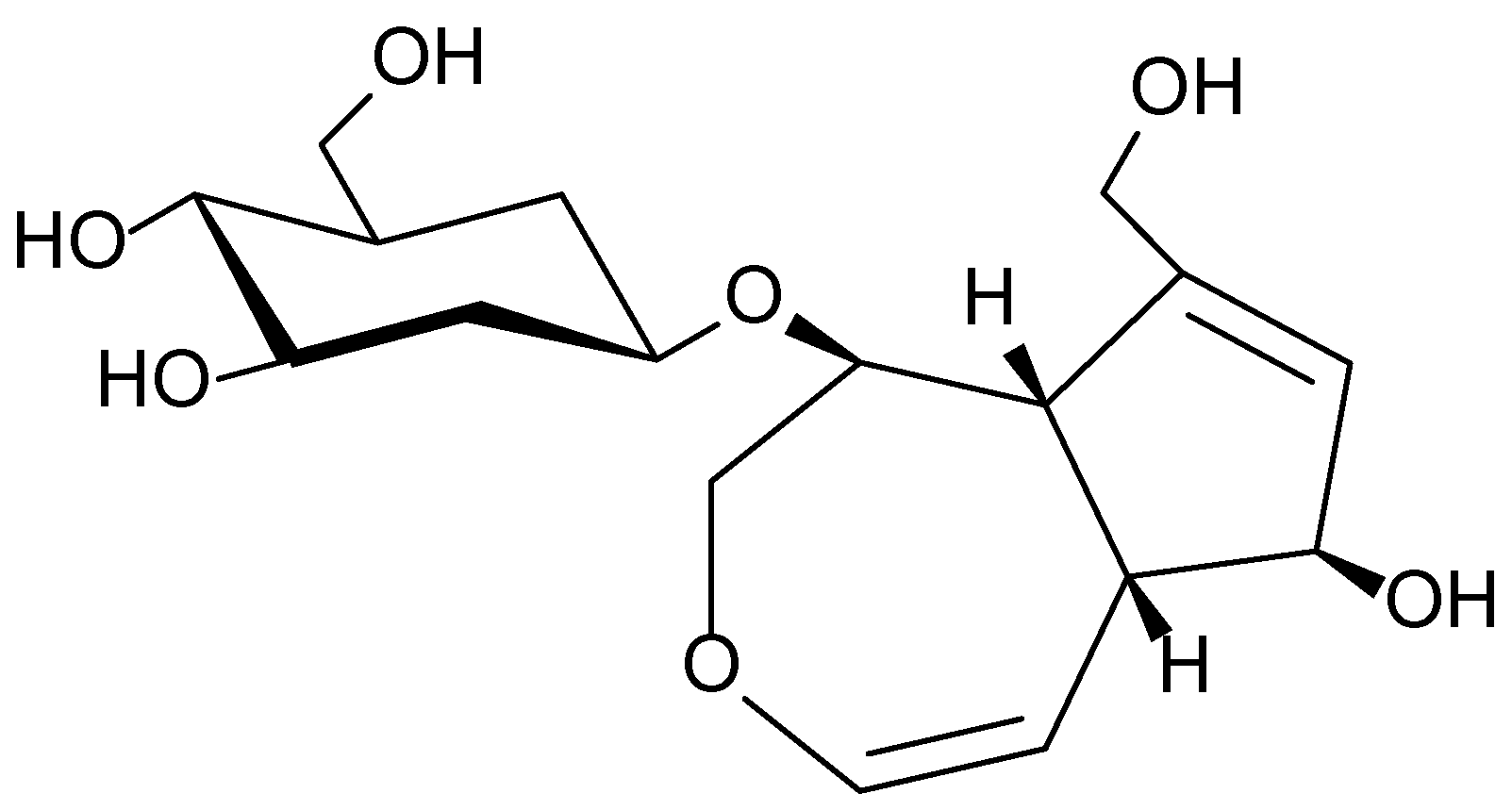
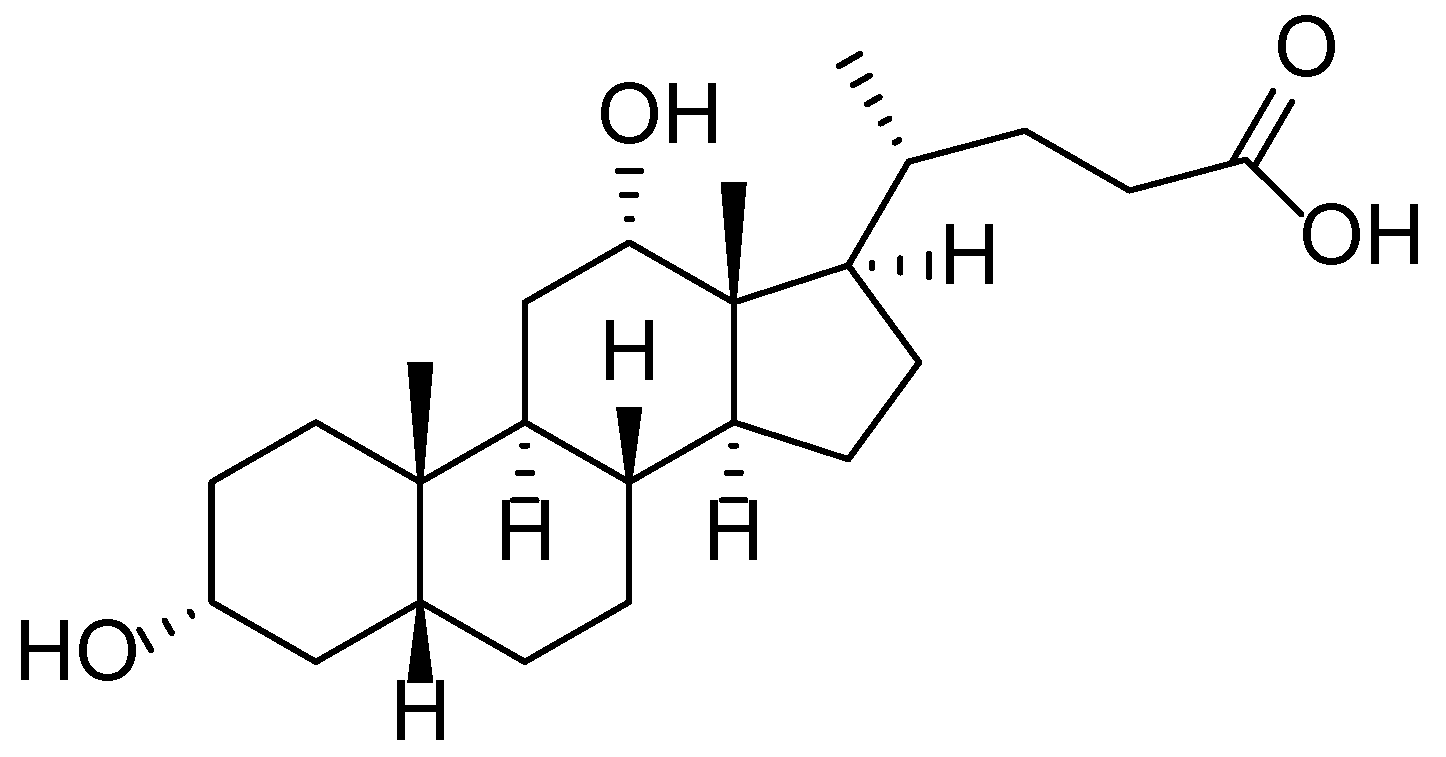
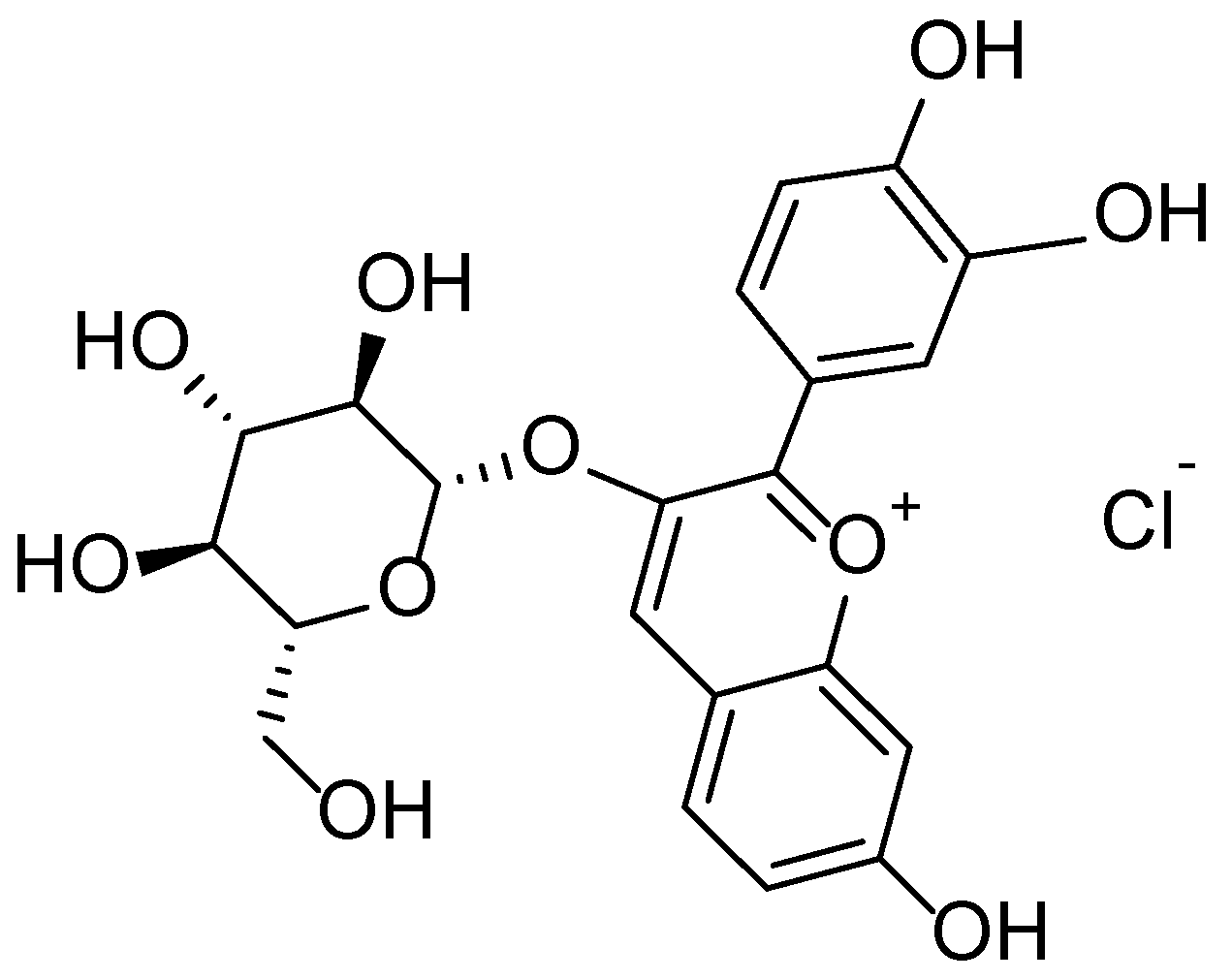
| 1.10 | Angelica archangelica | Furanocoumarins, sesquiterpene lactones | Anti-inflammatory, antioxidant, antibacterial | Psoriasis, inflammation, vitiligo | [82,83,84,85,86,87,88] |
| 1.11 | Arctium lappa | Lignans, phenolic acids, inulin | Anti-inflammatory, antioxidant, antimicrobial | Dermatitis, acne, wounds | [92,93,94,95,96] |
| 1.12 | Inula helenium | Inulin, sesquiterpene lactones, flavonoids | Anti-inflammatory, antioxidant, wound-healing | Eczema, dermatitis, psoriasis, wounds | [99,100,102,103,104,105] |
| 1.13 | Althaea officinalis | Polysaccharides, flavonoids, tannins | Anti-inflammatory, emollient, antimicrobial | Eczema, psoriasis, inflammation | [108,109,110,111,112,113] |
| 1.14 | Lavandula angustifolia | Terpenoids, coumarins, essential oils | Anti-inflammatory, antibacterial, antioxidant | Acne, eczema, inflammation | [119,120,121] |
| 1.15 | Trifolium pratense | Isoflavones, flavonoids, phenolic acids | Anti-inflammatory, antioxidant, antimicrobial | Aging skin, eczema, wounds | [125,126,127,128,129] |
| 1.16 | Hamamelis virginiana | Tannins, flavonoids, gallic acid | Astringent, anti-inflammatory, antioxidant | Oily skin, acne, inflammation | [132,133,136,137] |
| 1.17 | Bromus inermis | Phenolic acids, coumarins, essential oils | Anti-inflammatory, antioxidant, antimicrobial | Acne, dermatitis, inflammation | [141,142] |
| 1.18 | Curcuma longa | Curcuminoids, sesquiterpenoids, essential oils | Anti-inflammatory, antioxidant, antimicrobial | Acne, eczema, inflammation, anti-photoaging | [145,148,149,150,151,152,153] |
| 1.19 | Salix alba | Salicin, flavonoids, phenolic acids | Anti-inflammatory, antioxidant, antimicrobial | Acne, dermatitis, inflammation | [156,157,158,159,160] |
| 1.20 | Artemisia terrae-albae | Sesquiterpenes, flavonoids, phenolic acids | Anti-inflammatory, antioxidant, antimicrobial | Dermatitis, eczema, psoriasis, inflammation | [164,165,166,167,168,169] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolatkyzy, N.; Shepilov, D.; Turmanov, R.; Berillo, D.; Vassilina, T.; Ibragimova, N.; Berganayeva, G.; Dyusebaeva, M. Medicinal Plants for Skin Disorders: Phytochemistry and Pharmacological Insights. Molecules 2025, 30, 3281. https://doi.org/10.3390/molecules30153281
Bolatkyzy N, Shepilov D, Turmanov R, Berillo D, Vassilina T, Ibragimova N, Berganayeva G, Dyusebaeva M. Medicinal Plants for Skin Disorders: Phytochemistry and Pharmacological Insights. Molecules. 2025; 30(15):3281. https://doi.org/10.3390/molecules30153281
Chicago/Turabian StyleBolatkyzy, Nazerke, Daniil Shepilov, Rakhymzhan Turmanov, Dmitriy Berillo, Tursunay Vassilina, Nailya Ibragimova, Gulzat Berganayeva, and Moldyr Dyusebaeva. 2025. "Medicinal Plants for Skin Disorders: Phytochemistry and Pharmacological Insights" Molecules 30, no. 15: 3281. https://doi.org/10.3390/molecules30153281
APA StyleBolatkyzy, N., Shepilov, D., Turmanov, R., Berillo, D., Vassilina, T., Ibragimova, N., Berganayeva, G., & Dyusebaeva, M. (2025). Medicinal Plants for Skin Disorders: Phytochemistry and Pharmacological Insights. Molecules, 30(15), 3281. https://doi.org/10.3390/molecules30153281








