Volatile Essential Oils from Different Tree Species Influence Scent Impression and Physiological Response
Abstract
1. Introduction
2. Results and Discussion
2.1. Analysis of Volatile Essential Oil Compounds
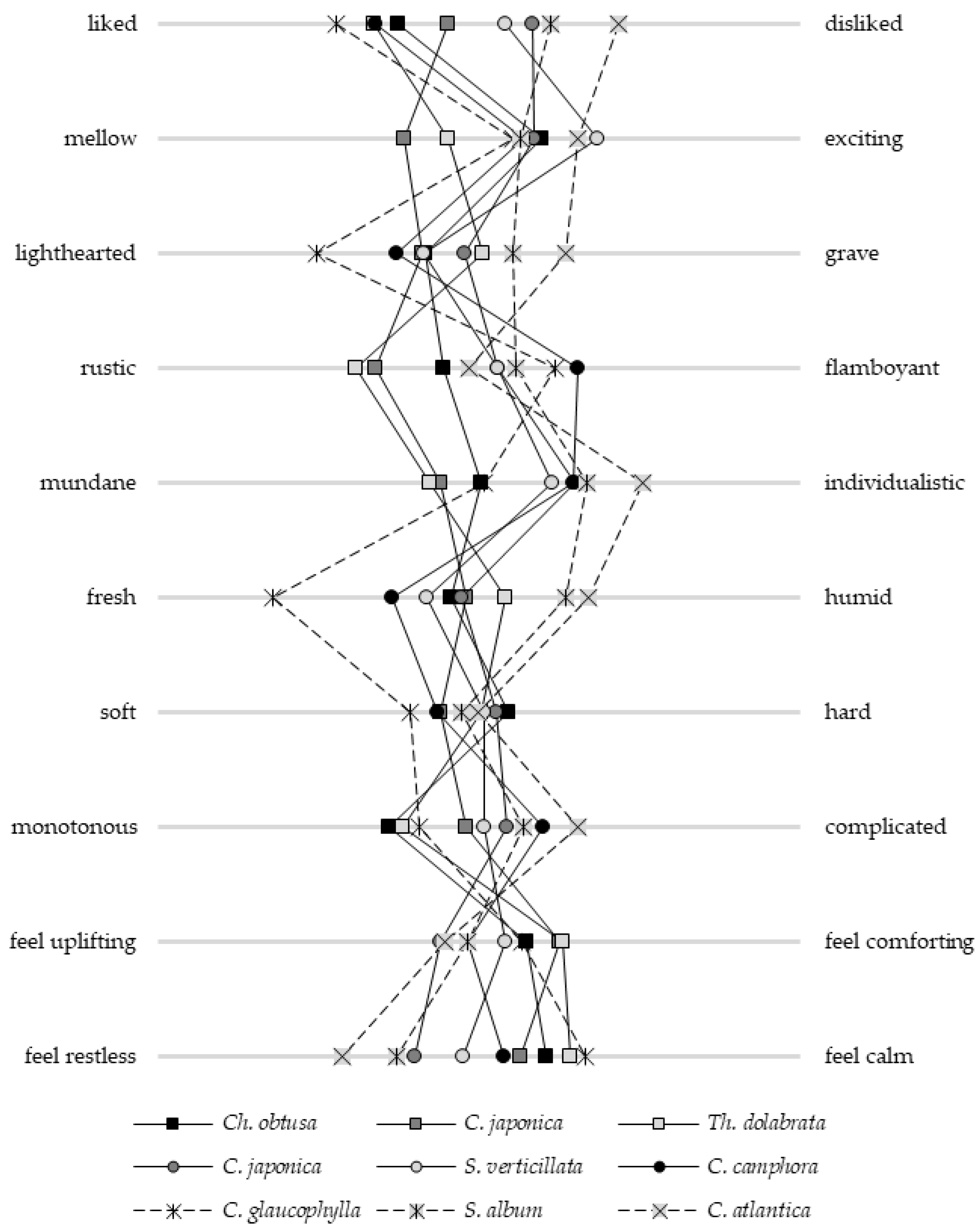
2.2. Subjective Assessments
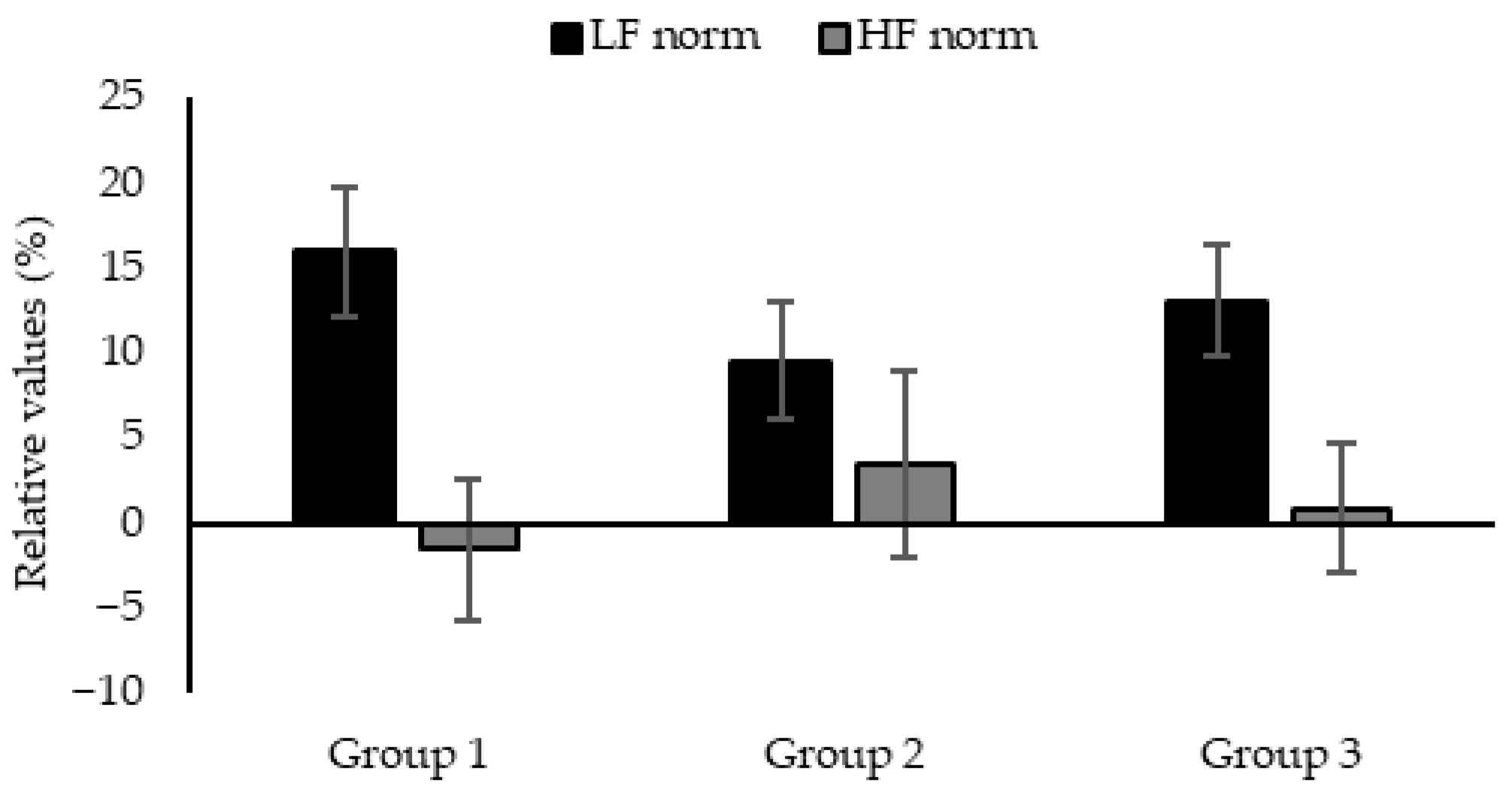
2.3. Physiological Assessments: Heart Rate Variability Analysis
2.4. Limitations
3. Materials and Methods
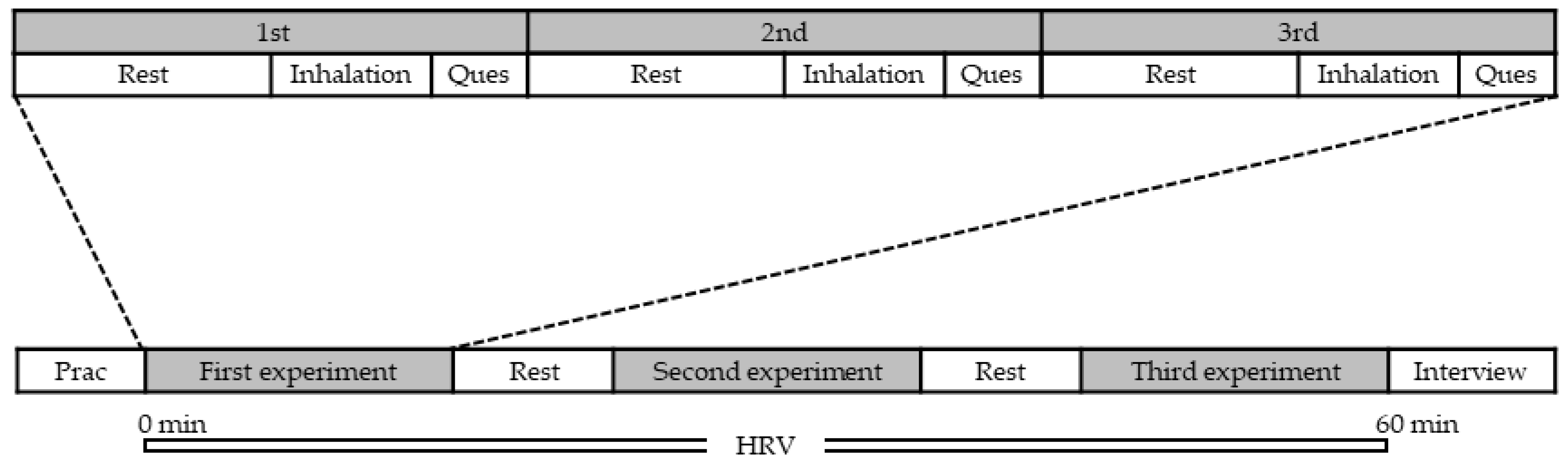
3.1. Participants and Experimental Procedure
3.2. Experimental Materials
3.3. Analysis of Volatile Essential Oil Compounds
3.4. Subjective and Physiological Assessments of the Participants
3.4.1. Subjective Assessments
3.4.2. Physiological Assessments: Heart Rate Variability Analysis
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lorena, R.L.V. Effects of essential oils on central nervous system: Focus on mental health. Phytother. Res. 2020, 35, 657–679. [Google Scholar] [CrossRef]
- Li, H.; Kong, Y.; Hu, W.; Zhang, S.; Wang, W.; Yang, M.; Luo, Y. Litsea cubeba essential oil: Component analysis, anti-Candida albicans activity and mechanism based on molecular docking. J. Oleo Sci. 2022, 71, 1221–1228. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, Y.; Chi, P.; Liu, H.; Jing, Z.; Cao, H.; Du, Y.; Zhao, Y.; Qin, X.; Zhang, W.; et al. Essential oils: Chemical constituents, potential neuropharmacological effects and aromatherapy- a review. Pharmacol. Res. Mod. Chin. Med. 2023, 6, 100210. [Google Scholar] [CrossRef]
- Nath, P.C.; Dey, P.; Paul, T.; Shil, S.; Sarkar, S.; Rustagi, S.; Bhattacharya, D.; Vora, K.; Roy, R. Essential oils and their critical implications in human use. Biocatal. Agric. Biotechnol. 2024, 60, 103258. [Google Scholar] [CrossRef]
- Kaya, O.; Bozkurt, A.; Karakus, S.; Daler, S.; Yilmaz, T.; Turan, M. Essential oils in post-harvest disease management: Metabolic impact on Narince (Vitis vinifera L. cv.) grapes against Botrytis cinerea. Physiol. Mol. Plant Pathol. 2024, 132, 102318. [Google Scholar] [CrossRef]
- Vora, L.K.; Gholap, A.D.; Hatvate, N.T.; Naren, P.; Khan, S.; Chavda, V.P.; Balar, P.C.; Gandhi, J.; Khatri, D.K. Essential oils for clinical aromatherapy: A comprehensive review. J. Ethnopharmacol. 2024, 330, 118180. [Google Scholar] [CrossRef]
- Itoh, T.; Choi, P.G.; Masuda, Y.; Shin, W.; Arai, J.; Takeda, N. Discrimination ability and concentration measurement accuracy effective components in aroma essential oils using gas sensor arrays with machine learning. Appl. Sci. 2024, 14, 8859. [Google Scholar] [CrossRef]
- Boukroufa, M.; Boutekedjiret, C.; Petigny, L.; Rakotomanomana, N.; Chemat, F. Bio-refinery of orange peels waste: A new concept based on integrated green and solvent free extraction process using ultrasound and microwave techniques to obtain essential oil, polyphenols and pectin. Ultrason. Sonochem. 2015, 24, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Li, X.; Zhao, P.; Zhang, X.; Qiao, O.; Huang, L.; Guo, L.; Gao, Q. A review of chemical constituents and health-promoting effects of citrus peels. Food Chem. 2021, 365, 130585. [Google Scholar] [CrossRef]
- Herzyk, F.; Piłakowska-Pietras, D.; Korzeniowska, M. Supercritical extraction techniques for obtaining biologically active substances from a variety of plant byproducts. Foods 2024, 13, 1713. [Google Scholar] [CrossRef]
- Zaccardelli, M.; Roscigno, G.; Pane, C.; Celano, G.; Di Matteo, M.; Mainente, M.; Vuotto, A.; Mencherini, T.; Esposito, T.; Vitti, A.; et al. Essential oils and quality composts sourced by recycling vegetable residues from the aromatic plant supply chain. Ind. Crops Prod. 2021, 162, 113255. [Google Scholar] [CrossRef]
- Evergetis, E.; Haroutounian, S.A. Essential oils land footprint: A sustainability meta-analysis of essential oils biopesticides. Front. Biosci. 2022, 27, 327. [Google Scholar] [CrossRef]
- Stashenko, E.; Martinez, J.R. Essential oils and the circular bioeconomy. In Essential Oils-Recent Advances, New Perspectives and Applications; Viskelis, J., Ed.; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Annual Report on Forest and Forestry in Japan. Available online: https://www.rinya.maff.go.jp/j/kikaku/hakusyo/r5hakusyo/index.html (accessed on 20 May 2025).
- Ioannou, E.; Koutsaviti, A.; Tzakou, O.; Roussis, V. The genus Pinus: A comparative study on the needle essential oil composition of 46 pine species. Phytochem. Rev. 2014, 13, 741–768. [Google Scholar] [CrossRef]
- Mediavilla, I.; Guillamón, E.; Ruiz, A.; Esteban, L.S. Essential oils from residual foliage of forest tree and shrub species: Yield and antioxidant capacity. Molecules 2021, 26, 3257. [Google Scholar] [CrossRef] [PubMed]
- Esteban, L.S.; Mediavilla, I.; Xavier, V.; Amaral, J.S.; Pires, T.C.S.P.; Calhelha, R.C.; López, C.; Barros, L. Yield, Chemical composition and bioactivity of essential oils from common Juniper (Juniperus communis L.) from different spanish origins. Molecules 2023, 28, 4448. [Google Scholar] [CrossRef]
- Rodrigues, T.; Lima, A.; Wortham, T.; Arruda, F.; Janeiro, A.; Baptista, J.; Lima, E. Essential oil composition and anti-cholinesterase properties of Cryptomeria japonica foliage harvested in São Miguel Island (Azores) in Two Different Seasons. Plants 2024, 13, 3277. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, N.; Pei, S.; Yao, L. Odor perception of aromatherapy essential oils with different chemical types: Influence of gender and two cultural characteristics. Front. Psychol. 2022, 13, 998612. [Google Scholar] [CrossRef]
- Pei, S.; Qu, Y.; Lu, J.; Yao, L.; Zhang, N. Influence of subject’s characteristics and cognitive factors on the perception of odors from aromatic plants and physiological response. Build. Environ. 2025, 271, 112629. [Google Scholar] [CrossRef]
- Kim, C.; Song, C. Physiological and psychological relaxation effects of Fir essential oil on university students. Int. J. Environ. Res. Public Health 2022, 19, 5063. [Google Scholar] [CrossRef]
- Thangaleela, S.; Sivamaruthi, B.S.; Kesika, P.; Bharathi, M.; Kunaviktikul, W.; Klunklin, A.; Chanthapoon, C.; Chaiyasut, C. Essential Oils, Phytoncides, Aromachology, and Aromatherapy—A Review. Appl. Sci. 2022, 12, 4495. [Google Scholar] [CrossRef]
- Gong, X.; Yang, Y.; Xu, T.; Yao, D.; Lin, S.; Chang, W. Assessing the anxiolytic and relaxation effects of Cinnamomum camphora essential oil in university students: A comparative study of EEG, physiological measures, and psychological responses. Front. Psychol. 2024, 15, 1423870. [Google Scholar] [CrossRef]
- Jirovetz, L.; Buchbauer, G.; Denkova, Z.; Stoyanova, A.; Murgov, I.; Gearon, V.; Birkbeck, S.; Schmidt, E.; Geissler, M. Comparative study on the antimicrobial activities of different sandalwood essential oils of various origin. Flavour Fragr. J. 2006, 21, 465–468. [Google Scholar] [CrossRef]
- Brophy, J.J.; Goldsack, R.J.; Forster, P.I.; Copeland, L.M.; O’Sullivan, W.; Rozefelds, A.C. Chemistry of the Australian gymnosperms. part IX. The leaf oils of the Australian members of the Genus Callitris (Cupressaceae). J. Essent. Oil Res. 2011, 19, 57–71. [Google Scholar] [CrossRef]
- Cheng, W.W.; Lin, C.T.; Chu, F.H.; Chang, S.T.; Wang, S.Y. Neuropharmacological activities of phytoncide released from Cryptomeria japonica. J. Wood Sci. 2009, 55, 27–31. [Google Scholar] [CrossRef]
- Ohira, T.; Park, B.J.; Kurosumi, Y.; Miyazaki, Y. Evaluation of dried-wood odors: Comparison between analytical and sensory data on odors from dried sugi (Cryptomeria japonica) wood. J. Wood Sci. 2009, 55, 144–148. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, T.; Liao, X.; Zhou, Y.; Chen, S.; Chen, J.; Xiong, W. Extraction of camphor tree essential oil by steam distillation and supercritical CO2 extraction. Molecules 2022, 27, 5385. [Google Scholar] [CrossRef]
- Kostić, E.; Kitić, D.; Vujović, M.; Marković, M.; Pavlović, A.; Stojanović, G. A chemometric approach to the headspace sampled volatiles of selected Salvia species from Southeastern Serbia. Bot. Serbica 2022, 46, 285–294. [Google Scholar] [CrossRef]
- Matsubara, E.; Matsui, N.; Kambara, K. Utilization of essential oils mainly from Cupressaceae trees in the work environment creates a psychophysiological stress-relieving effect. Wood Sci. Technol. 2023, 57, 1197–1214. [Google Scholar] [CrossRef]
- Kimura, A.; Sugiyama, H.; Sasaki, S.; Yatagai, M. Psychological and physiological effects in humans induced by the visual and olfactory stimulations of an interior environment made of Hiba (Thujopsis dolabrata) wood. Mokuzai Gakkaishi 2011, 57, 150–159. [Google Scholar] [CrossRef]
- Bakó, E.; Böszörményi, A.; Vargáné Szabó, B.; Engh, M.A.; Hegyi, P.; Ványolós, A.; Csupor, D. Chemometric analysis of monoterpenes and sesquiterpenes of conifers. Front. Plant Sci. 2024, 15, 1392539. [Google Scholar] [CrossRef]
- Dietz, C.; Cook, D.; Huismann, M.; Wilson, C.; Ford, R. The multisensory perception of hop essential oil: A review. J. Inst. Brew. 2020, 126, 320–342. [Google Scholar] [CrossRef]
- Wang, B.; Meng, Q.; Xiao, L.; Li, R.; Peng, C.; Liao, X.; Yan, J.; Liu, H.; Xie, G.; Ho, C.T.; et al. Characterization of aroma compound of Pu-erh ripen tea using solvent assisted flavor evaporation coupled with gas chromatography-mass spectrometry and gas chromatography-olfactometry. Food Sci. Hum. Wellness 2022, 11, 618–626. [Google Scholar] [CrossRef]
- Schreiner, L.; Bauer, J.; Ortner, E.; Buettner, A. Structure-odor activity studies on derivatives of aromatic and oxygenated monoterpenoids synthesized by modifying p-cymene. J. Nat. Prod. 2020, 83, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, L.; Ortner, E.; Buettner, A. Nosy confirmation: Reconstitution of the characteristic odor of softwood via quantitative analysis and human sensory evaluation. Anal. Bioanal. Chem. 2020, 412, 1137–1149. [Google Scholar] [CrossRef] [PubMed]
- Ghadiriasli, R.; Mahmoud, M.A.A.; Wagenstaller, M.; van de Kuilen, J.W.; Buettner, A. Chemo-sensory characterization of aroma active compounds of native oak wood in relation to their geographical origins. Food Res. Int. 2021, 150, 110776. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, E.; Matsui, N.; Ohira, T. Evaluation of the psychophysiological effects of the Cupressaceae family wood odor. Wood Sci. Technol. 2019, 54, 269–286. [Google Scholar] [CrossRef]
- Yang, J.; Sarathy, R.; Walsh, S.M. Do review valence and review volume impact consumer’s purchase decisions as assumed? Nankai Bus. Rev. Int. 2016, 7, 231–257. [Google Scholar] [CrossRef]
- Roseman, M.G.; Joung, H.W.; Choi, E.K.; Kim, H.S. The effects of restaurant nutrition menu labelling on college student’s healthy eating behaviours. Public Health Nutr. 2016, 20, 797–804. [Google Scholar] [CrossRef]
- Chaniaud, N.; Métayer, N.; Megalakaki, O.; Loup-Escande, E. Effect of prior health knowledge on the usability of two home medical devices: Usability study. JMIR Mhealth Uhealth 2020, 8, e17983. [Google Scholar] [CrossRef]
- Herz, R.S. I know what I like: Understanding odor preferences. In The Smell Culture Reader; Drobnick, J., Ed.; Berg Oxford: New York, NY, USA, 2006; pp. 190–203. [Google Scholar]
- Wiesmann, M.; Yousry, I.; Heuberger, E.; Nolte, A.; Ilmberger, J.; Kobal, G.; Yousry, T.A.; Kettenmann, B.; Naidich, T.P. Functional magnetic resonance imaging of human olfaction. Neuroimaging Clin. N. Am. 2001, 11, 237–250. [Google Scholar] [CrossRef]
- Marciani, L.; Pfeiffer, J.C.; Hort, J.; Head, K.; Bush, D.; Taylor, A.J.; Spiller, R.C.; Francis, S.; Gowland, P.A. Improved methods of fMRI studies of combined taste and aroma stimuli. J. Neurosci. Methods 2006, 158, 186–194. [Google Scholar] [CrossRef]
- Xiao, W.; Lv, Q.; Gao, X.; Sun, Z.; Yan, X.; Wei, Y. Different brain activation in response to repeated odors of pleasantness and unpleasantness. Chemosens. Percept. 2020, 13, 84–91. [Google Scholar] [CrossRef]
- Asikin, Y.; Kawahira, S.; Goki, M.; Hirose, N.; Kyoda, S.; Wada, K. Extended aroma extract dilution analysis profile of Shiikuwasha (Citrus depressa Hayata) pulp essential oil. J. Food Drug Anal. 2018, 26, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Zheng, Q.; Hu, P.; Huang, X.; Yang, M.; Ren, G.; Du, Q.; Luo, J.; Zhang, K.; Li, J.; et al. Sedative and hypnotic effects of compound Anshen essential oil inhalation for insomnia. BMC Complement. Altern. Med. 2019, 19, 306. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Niazi, A.; Payandar, F.M.; Jamali, J.; Arefadib, N. The effect of aromatherapy with Citrus Aurantium essential oil on depression, stress and anxiety in pregnant women; a randomized controlled clinical trial. Taiwan. J. Obstet. Gynecol. 2025, 64, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Kontaris, I.; East, B.S.; Wilson, D.A. Behavioral and neurobiological convergence of odor, mood and emotion: A review. Front. Behav. Neurosci. 2020, 14, 35. [Google Scholar] [CrossRef]
- Herz, R.S.; Larsson, M.; Trujillo, R.; Casola, M.C.; Ahmed, F.K.; Lipe, S.; Brashear, M.E. A three-factor benefits framework for understanding consumer preference for scented household products: Psychological interactions and implications for future development. Cogn. Res. Princ. Implic. 2022, 7, 28. [Google Scholar] [CrossRef]
- Bensafi, M.; Rouby, C.; Farget, V.; Bertrand, M.; Vigouroux, M.; Holley, A. Autonomic nervous system responses to odours: The role of pleasantness and arousal. Chem. Senses 2002, 27, 703–709. [Google Scholar] [CrossRef]
- Matsubara, E.; Ohira, T. Inhalation of Japanese cedar (Cryptomeria japonica) wood odor causes psychological relaxation after monotonous work among female participants. Biomed. Res. 2018, 39, 241–249. [Google Scholar] [CrossRef]
- Berntson, G.G.; Cacioppo, J.T.; Quigley, K.S.; Fabro, V.T. Autonomic space and psychophysiological response. Psychophysiology 1994, 31, 44–61. [Google Scholar] [CrossRef]
- Gianaros, P.J.; Quigley, K.S. Autonomic origins of a nonsignal stimulus-elicited bradycardia and its habituation in humans. Psychophysiology 2003, 38, 540–547. [Google Scholar] [CrossRef]
- Du, B.; Schwartz-Narbonne, H.; Tandoc, M.; Heffernan, E.M.; Mack, M.L.; Siegel, J.A. The impact of emissions from an essential oil diffuser on cognitive performance. Indoor Air 2022, 32, e12919. [Google Scholar] [CrossRef]
- Zhang, X.; He, X.; Zhang, R.; Wang, L.; Kong, H.; Wang, K.; Vieira, C.L.Z.; Koutrakis, P.; Huang, S.; Xiong, J.; et al. Emissions of volatile organic compounds from reed diffusers in indoor environments. Phys. Sci. 2024, 5, 102142. [Google Scholar] [CrossRef]
- Hase, H.; Mitsuda, M. Effects of room temperature and relative humidity conditions of olfactory threshold, odor intensity and hedonics. J. Hum. Liv. Environ. 2012, 19, 35–43. [Google Scholar] [CrossRef]

| Wood | Foliage | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compounds | RI | Ch. obtusa | C. japonica | Th. dolabrata | S. album | C. atlantica | C. japonica | S. verticillata | C. camphora | C. glaucophylla |
| tricyclene | 921 | 5.9 | – | – | 15.9 | – | 3.1 | 14.1 | – | 3.1 |
| α-thujene | 924 | – | – | – | – | – | 1.3 | – | – | – |
| α-pinene | 932 | 34.0 | 39.7 | 10.9 | 24.2 | 23.3 | 6.7 | 24.3 | 4.2 | 34.6 |
| camphene | 946 | 27.7 | 8.9 | – | 11.3 | 5.0 | 13.1 | 21.0 | 3.9 | 11.6 |
| β-pinene | 980 | 0.6 | – | – | – | – | 0.2 | 0.4 | 0.5 | 0.9 |
| myrcene | 988 | – | – | – | – | – | 5.0 | 4.8 | 13.6 | 1.0 |
| 3-carene | 1008 | 12.0 | – | 4.6 | – | – | 24.0 | 12.2 | 10.0 | 8.7 |
| p-cymene | 1020 | 4.6 | 7.0 | 12.4 | 33.3 | 3.7 | 10.5 | 3.8 | 8.6 | – |
| limonene | 1024 | 4.5 | 16.9 | – | 15.4 | 8.5 | 9.5 | 7.3 | 20.8 | 29.5 |
| cis-β-ocimene | 1032 | – | – | – | – | – | – | – | 7.3 | – |
| trans-β-ocimene | 1044 | – | – | – | – | – | – | – | 8.4 | – |
| γ-terpinene | 1054 | 4.1 | – | 4.3 | – | – | 14.4 | 4.3 | 4.3 | 2.7 |
| terpinolene | 1086 | 6.6 | – | 11.5 | – | 2.6 | 11.0 | 7.1 | 11.1 | 6.1 |
| p-cymenene | 1089 | – | – | – | – | – | 1.1 | 0.6 | 1.5 | 1.0 |
| linalool | 1095 | – | – | – | – | – | – | – | 2.5 | – |
| allo-ocimene | 1128 | – | – | – | – | – | – | – | 1.7 | – |
| 4-acetyl-1-methylcyclohexene | 1131 | – | – | – | – | 37.2 | – | – | – | – |
| camphor | 1141 | – | – | – | – | – | – | – | 1.5 | – |
| bornyl acetate | 1287 | – | – | – | – | – | – | – | – | 0.9 |
| carvacrol | 1298 | – | – | 27.7 | – | – | – | – | – | – |
| α-cedrene | 1409 | – | 4.1 | – | – | – | – | – | – | – |
| β-cedrene | 1419 | – | – | – | – | 2.5 | – | – | – | – |
| thujopsene | 1429 | – | 5.0 | 28.7 | – | – | – | – | – | – |
| α-himachalene | 1449 | – | – | – | – | 7.7 | – | – | – | – |
| γ-himachalene | 1481 | – | – | – | – | 2.3 | – | – | – | – |
| α-cuprenene | 1505 | – | – | – | – | 7.3 | – | – | – | – |
| Monoterpene hydrocarbons | 100.0 | 74.7 | 43.6 | 100.0 | 44.2 | 100.0 | 100.0 | 96.0 | 99.1 | |
| Oxygenated monoterpene | 0.0 | 0.0 | 27.7 | 0.0 | 38.1 | 0.0 | 0.0 | 4.0 | 0.9 | |
| Sesquiterpene hydrocarbons | 0.0 | 25.3 | 28.7 | 0.0 | 17.7 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Item | Factor 1 | Factor 2 | Factor 3 | Communality |
|---|---|---|---|---|
| feel restless–feel calm | −0.97 | −0.19 | −0.16 | 1.00 |
| liked–disliked | 0.70 | 0.36 | 0.12 | 0.69 |
| feel uplifting–feel comforting | −0.63 | 0.07 | −0.29 | 0.54 |
| fresh–humid | 0.10 | 0.86 | −0.08 | 0.76 |
| lighthearted–grave | 0.10 | 0.83 | 0.09 | 0.71 |
| mundane–individualistic | 0.24 | 0.24 | 0.88 | 0.90 |
| rustic–flamboyant | 0.12 | −0.23 | 0.62 | 0.53 |
| mellow–exciting | 0.47 | −0.11 | 0.49 | 0.53 |
| monotonous–complicated | 0.18 | 0.40 | 0.47 | 0.51 |
| Eigenvalues | 2.17 | 1.89 | 1.76 | |
| % of variance | 24.06 | 21.01 | 19.55 | |
| % of cumulative variance | 24.06 | 45.07 | 64.62 |
| Age of Participants | Male | Female | Total |
| 20s | 3 | 5 | 8 |
| 30s | 8 | 5 | 13 |
| 40s | 7 | 1 | 8 |
| 50s | 4 | 2 | 6 |
| Total | 22 | 13 | 35 |
| Basic Information on the Use of Aromas | Agree/Usually | Neutral/Sometimes | Disagree/Rarely |
| Interest | 32 | 2 | 3 |
| Preference | 28 | 5 | 4 |
| Frequency | 9 | 20 | 8 |
| Essential Oils | Producing Area | Amount Added (µL) |
|---|---|---|
| Wood | ||
| Chamaecyparis obtusa | Wakayama, Japan | 10 |
| Cryptomeria japonica | Oita, Japan | 20 |
| Thujopsis dolabrata | Aomori, Japan | 5 |
| Santalum album | Australia | 10 |
| Cedrus atlantica | Morocco | 10 |
| Foliage | ||
| Cryptomeria japonica | Gifu, Japan | 5 |
| Sciadopitys verticillata | Nara, Wakayama, Japan | 5 |
| Cinnamomum camphora | Kagoshima, Japan | 5 |
| Callitris glaucophylla | Australia | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsubara, E.; Matsui, N. Volatile Essential Oils from Different Tree Species Influence Scent Impression and Physiological Response. Molecules 2025, 30, 3288. https://doi.org/10.3390/molecules30153288
Matsubara E, Matsui N. Volatile Essential Oils from Different Tree Species Influence Scent Impression and Physiological Response. Molecules. 2025; 30(15):3288. https://doi.org/10.3390/molecules30153288
Chicago/Turabian StyleMatsubara, Eri, and Naoyuki Matsui. 2025. "Volatile Essential Oils from Different Tree Species Influence Scent Impression and Physiological Response" Molecules 30, no. 15: 3288. https://doi.org/10.3390/molecules30153288
APA StyleMatsubara, E., & Matsui, N. (2025). Volatile Essential Oils from Different Tree Species Influence Scent Impression and Physiological Response. Molecules, 30(15), 3288. https://doi.org/10.3390/molecules30153288






