Effective Ciprofloxacin Removal from Deionized and Salt Water by Sulfonated Pentablock Copolymer (NexarTM)
Abstract
1. Introduction
2. Results
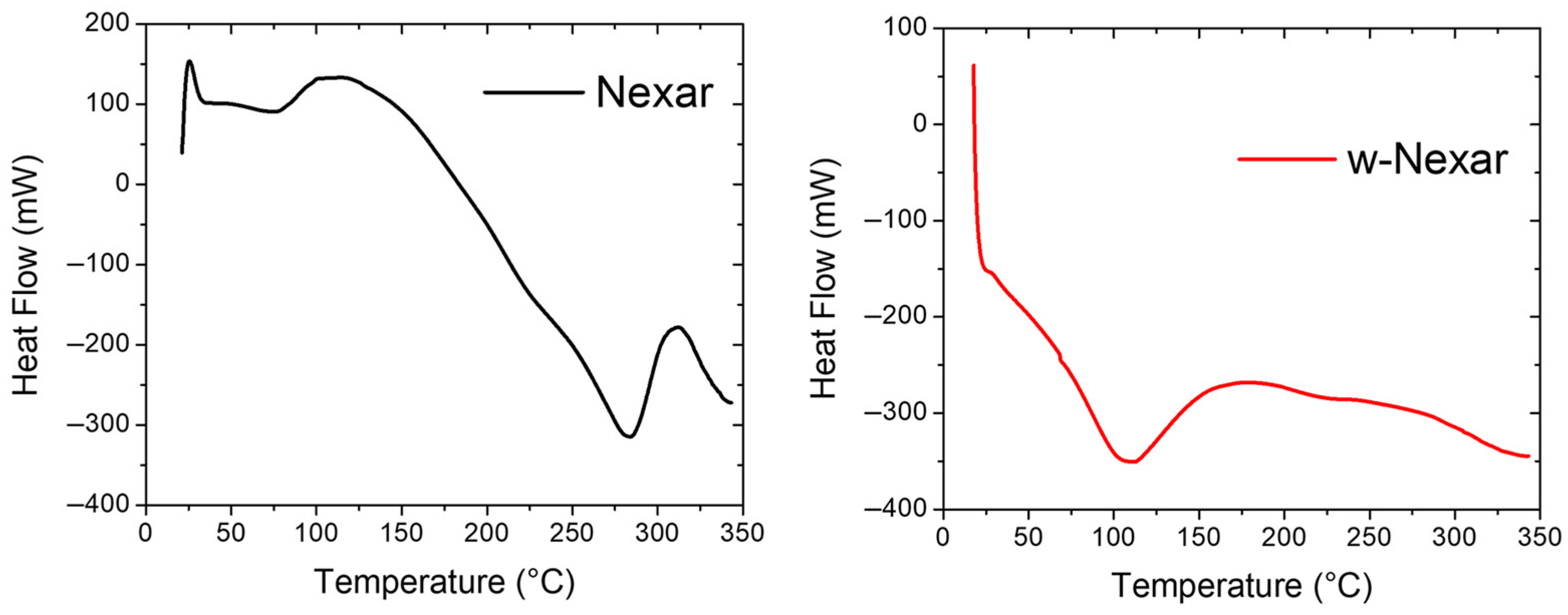
2.1. Characterization of Membranes
2.2. Ciprofloxacin Adsorption Experiments
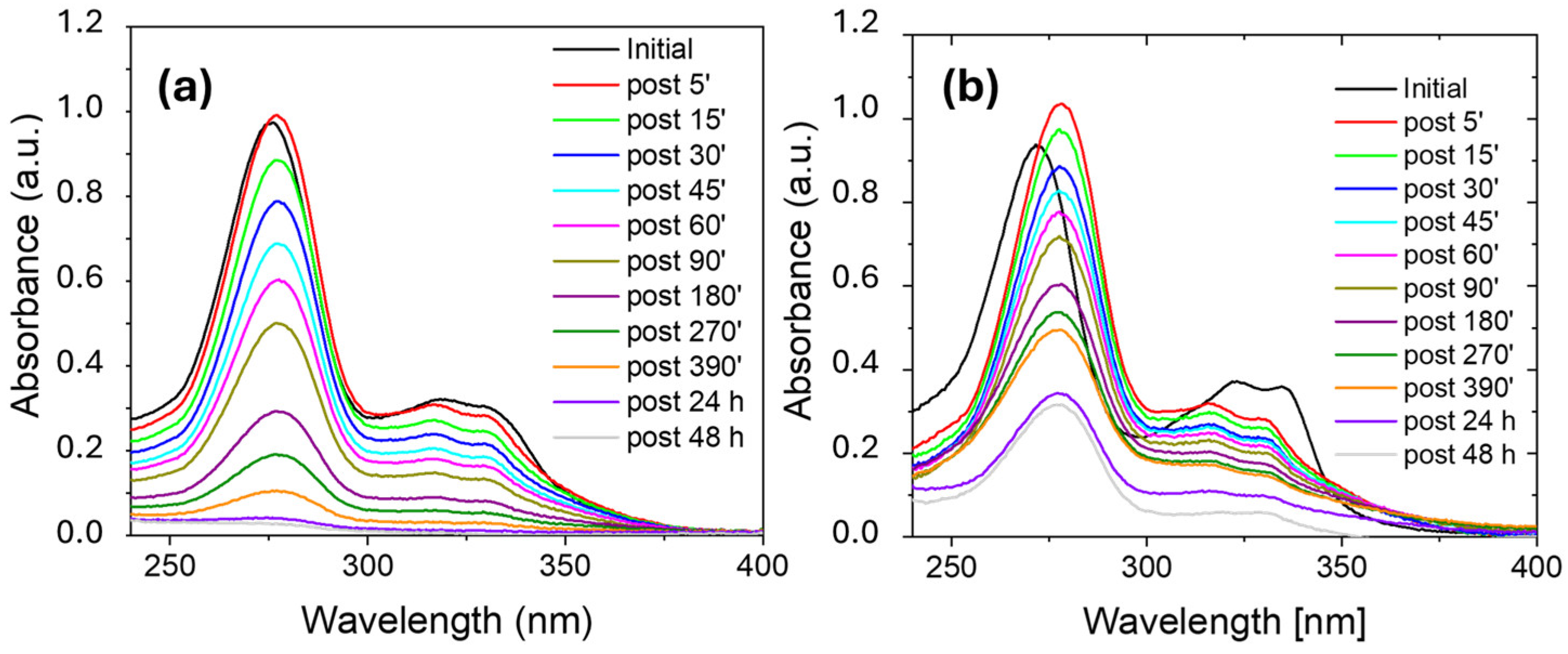
2.2.1. Adsorption Processes in MilliQ Water or Salt Water
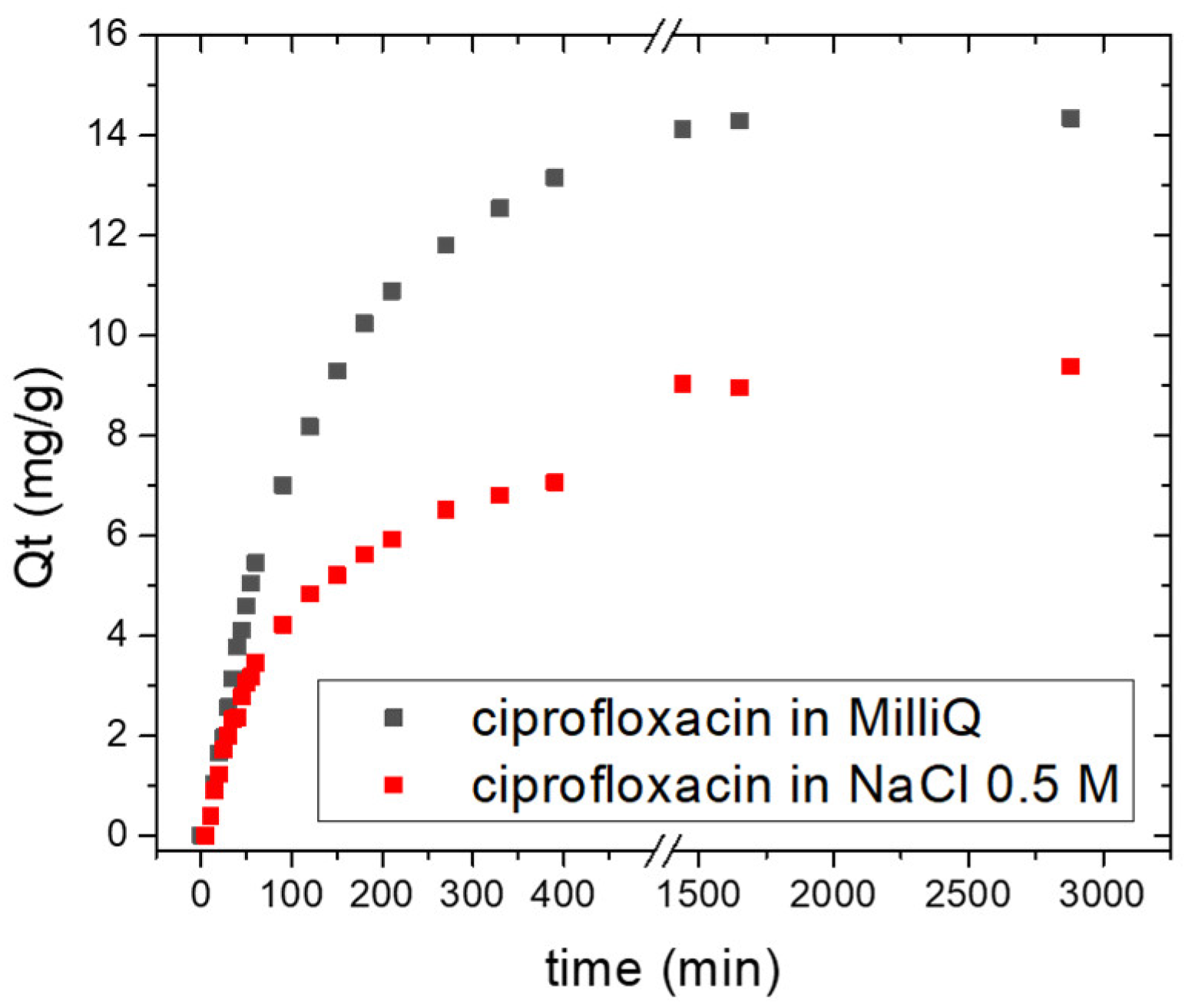
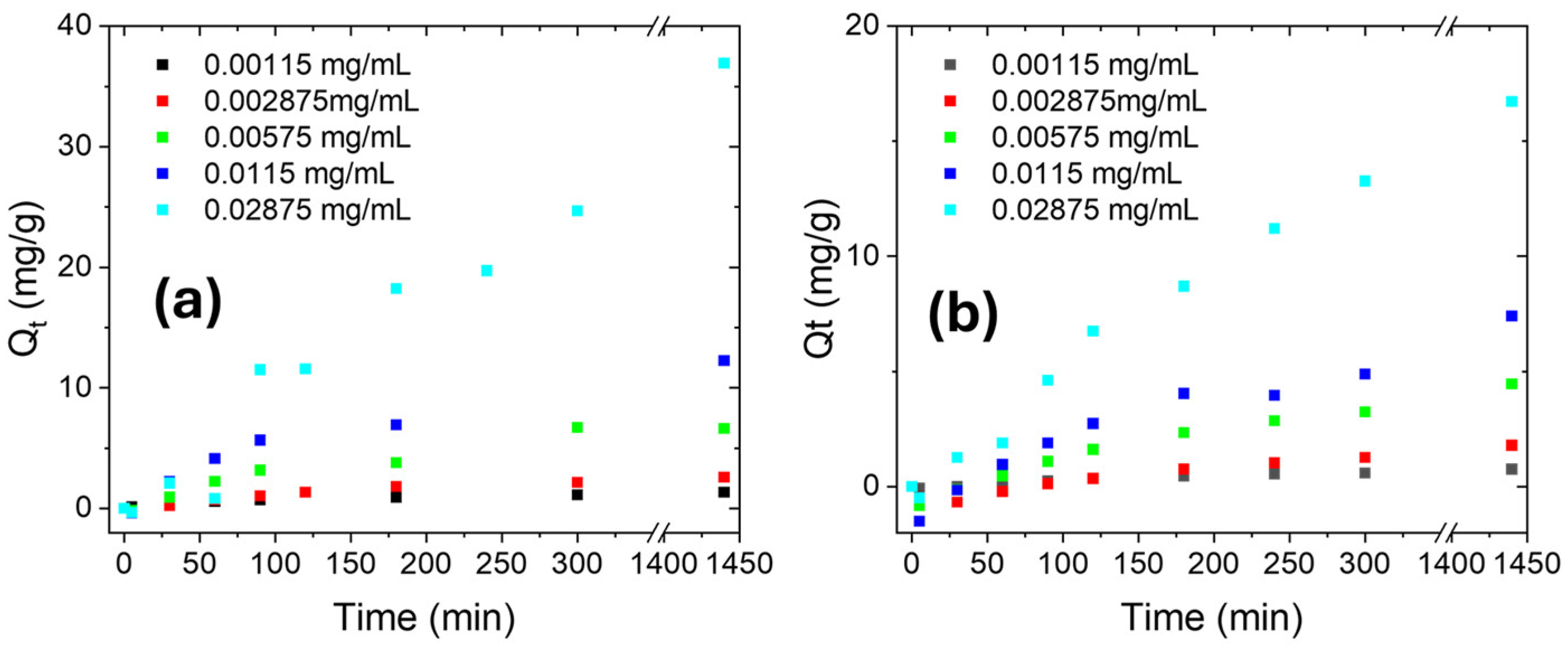
2.2.2. Kinetic Studies
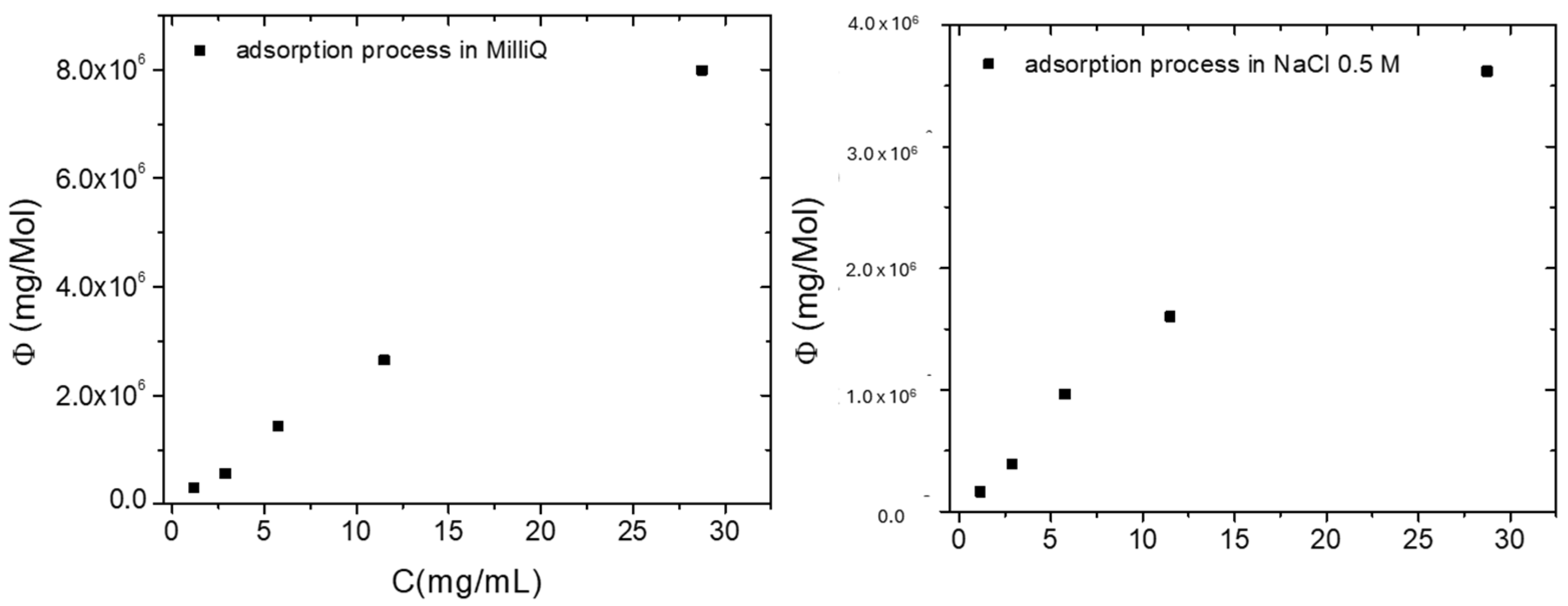
2.2.3. Isotherm Studies
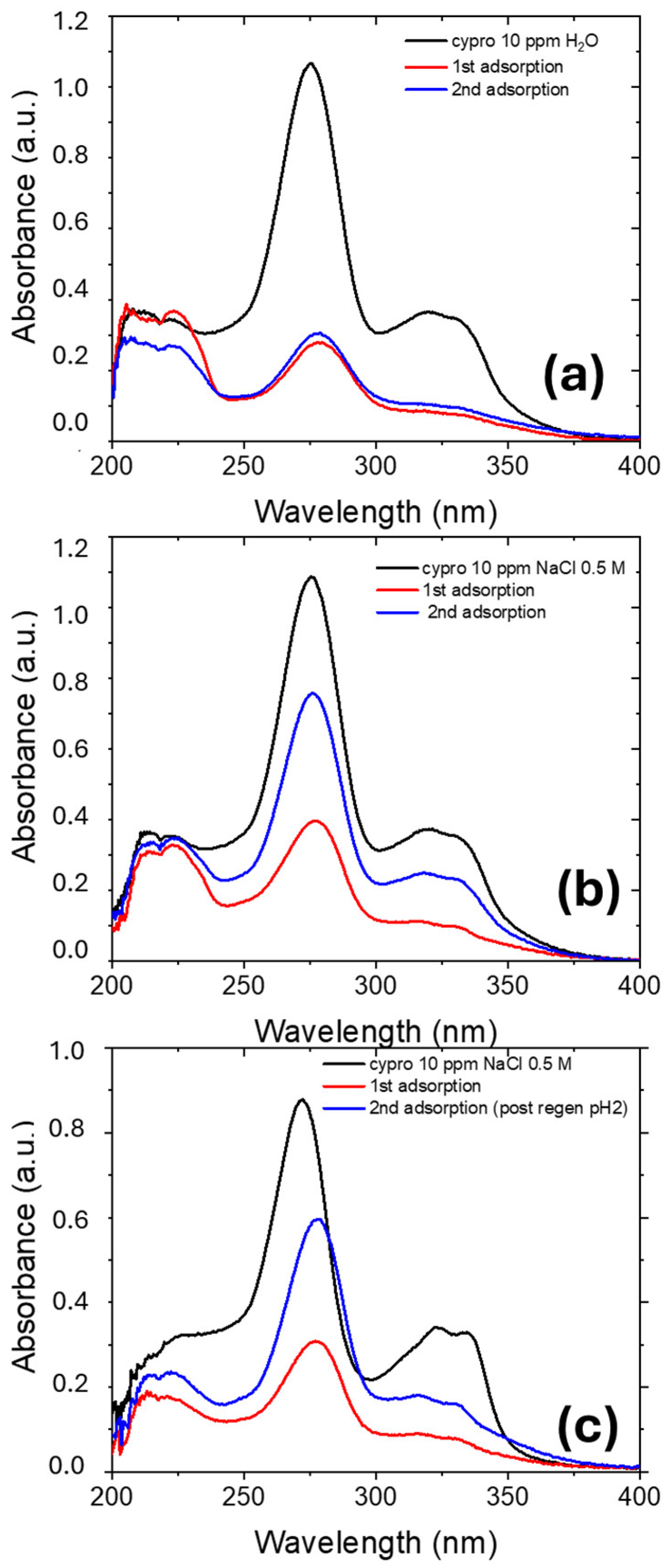
2.2.4. Regeneration of Nexar Membrane
3. Materials and Methods
3.1. Materials
3.2. Water Uptake Measurement
3.3. Characterization Techniques
3.4. Adsorption Experiments
3.5. Membranes Regeneration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mithuna, R.; Tharanyalakshmi, R.; Jain, I.; Singhal, S.; Sikarwar, D.; Das, S.; Ranjitha, J.; Ghosh, D.; Rahman, M.M.; Das, B. Emergence of antibiotic resistance due to the excessive use of antibiotics in medicines and feed additives: A global scenario with emphasis on the Indian perspective. Emerg. Contam. 2024, 10, 100389. [Google Scholar] [CrossRef]
- Chattopadhyay, M.K. Use of antibiotics as feed additives: A burning question. Front. Microbiol. 2014, 5, 334. [Google Scholar] [CrossRef]
- Sivagami, K.; Vignesh, V.J.; Srinivasan, R.; Divyapriya, G.; Nambi, I.M. Antibiotic usage, residues and resistance genes from food animals to human and environment: An Indian scenario. J. Environ. Chem. Eng. 2020, 8, 102221. [Google Scholar] [CrossRef]
- Grenni, P.; Ancona, V.; Barra Caracciolo, A. Ecological effects of antibiotics on natural ecosystems: A review. Microchem. J. 2018, 136, 25–39. [Google Scholar] [CrossRef]
- Sabri, N.; van Holst, S.; Schmitt, H.; van der Zaan, B.; Gerritsen, H.; Rijnaarts, H.; Langenhoff, A. Fate of antibiotics and antibiotic resistance genes during conventional and additional treatment technologies in wastewater treatment plants. Sci. Total Environ. 2020, 741, 140199. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Song, G.; Lim, W. A review of the toxicity in fish exposed to antibiotics. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 237, 108840. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.; Siedlewicz, G.; Pazdro, K. The Toxic Effects of Antibiotics on Freshwater and Marine Photosynthetic Microorganisms: State of the Art. Plants 2021, 10, 591. [Google Scholar] [CrossRef]
- MacGowan, A.; Macnaughton, E. Antibiotic resistance. Medicine 2017, 45, 622–628. [Google Scholar] [CrossRef]
- Karungamye, P.; Rugaika, A.; Mtei, K.; Machunda, R. The pharmaceutical disposal practices and environmental contamination: A review in East African countries. HydroResearch 2022, 5, 99–107. [Google Scholar] [CrossRef]
- Ata, R.; Tore, G.Y.; Shah, M.P. Emerging technologies for treatment of antibiotic residues from wastewater influent/effluent for sustainable environment: A case study with NFC-doped titania immobilized on polystyrene as an efficient technology. Curr. Res. Green Sustain. Chem. 2021, 4, 100065. [Google Scholar] [CrossRef]
- Dubey, K.K.; Indu; Sharma, M. Reprogramming of antibiotics to combat antimicrobial resistance. Arch. Pharm. 2020, 353, 2000168. [Google Scholar] [CrossRef]
- García-Alonso, J.A.; Sulbarán-Rangel, B.C.; Bandala, E.R.; del Real-Olvera, J. Adsorption and kinetic studies of the removal of ciprofloxacin from aqueous solutions by diatomaceous earth. Desalination Water Treat. 2019, 162, 331–340. [Google Scholar] [CrossRef]
- Rosas-Ramírez, J.R.; Orozco-Hernández, J.M.; Elizalde-Velázquez, G.A.; Raldúa, D.; Islas-Flores, H.; Gómez-Oliván, L.M. Teratogenic effects induced by paracetamol, ciprofloxacin, and their mixture on Danio rerio embryos: Oxidative stress implications. Sci. Total Environ. 2022, 806, 150541. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Ma, S.; Li, F.; Zheng, L.; Tomberlin, J.K.; Yu, Z.; Zhang, J.; Yu, C.; Fan, M.; Cai, M. Characteristics and mechanisms of ciprofloxacin degradation by black soldier fly larvae combined with associated intestinal microorganisms. Sci. Total Environ. 2022, 811, 151371. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Li, Y.; Han, S.; Ma, J. Adsorptive removal of antibiotics from aqueous solution using carbon materials. Chemosphere 2016, 153, 365–385. [Google Scholar] [CrossRef]
- Samal, K.; Mahapatra, S.; Hibzur Ali, M. Pharmaceutical wastewater as Emerging Contaminants (EC): Treatment technologies, impact on environment and human health. Energy Nexus 2022, 6, 100076. [Google Scholar] [CrossRef]
- Wang, J.; Chu, L.; Wojnárovits, L.; Takács, E. Occurrence and fate of antibiotics, antibiotic resistant genes (ARGs) and antibiotic resistant bacteria (ARB) in municipal wastewater treatment plant: An overview. Sci. Total Environ. 2020, 744, 140997. [Google Scholar] [CrossRef]
- Cardoza, L.A.; Knapp, C.W.; Larive, C.K.; Belden, J.B.; Lydy, M.; Graham, D.W. Factors Affecting the Fate of Ciprofloxacin in Aquatic Field Systems. Water Air Soil Pollut. 2005, 161, 383–398. [Google Scholar] [CrossRef]
- Shi, W.; Yan, Y.; Yan, X. Microwave-assisted synthesis of nano-scale BiVO4 photocatalysts and their excellent visible-light-driven photocatalytic activity for the degradation of ciprofloxacin. Chem. Eng. J. 2013, 215–216, 740–746. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Zhao, C.; Zhu, Y.; Sun, Z.; Fan, H.S.; Hu, X.; Zheng, H. Ciprofloxacin removal by ultrasound-enhanced carbon nanotubes/permanganate process: In situ generation of free reactive manganese species via electron transfer. Water Res. 2021, 202, 117393. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Adsorptive removal of antibiotics from water and wastewater: Progress and challenges. Sci. Total Environ. 2015, 532, 112–126. [Google Scholar] [CrossRef]
- Yao, B.; Luo, Z.; Yang, J.; Zhi, D.; Zhou, Y. FeIIFeIII layered double hydroxide modified carbon felt cathode for removal of ciprofloxacin in electro-Fenton process. Environ. Res. 2021, 197, 111144. [Google Scholar] [CrossRef]
- Girardi, C.; Greve, J.; Lamshöft, M.; Fetzer, I.; Miltner, A.; Schäffer, A.; Kästner, M. Biodegradation of ciprofloxacin in water and soil and its effects on the microbial communities. J. Hazard. Mater. 2011, 198, 22–30. [Google Scholar] [CrossRef]
- Gadipelly, C.; Pérez-González, A.; Yadav, G.D.; Ortiz, I.; Ibáñez, R.; Rathod, V.K.; Marathe, K.V. Pharmaceutical Industry Wastewater: Review of the Technologies for Water Treatment and Reuse. J. Ind. Eng. Chem. 2014, 53, 11571–11592. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.Á.; Prados-Joya, G.; Ocampo-Pérez, R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef]
- Jones, E.R.; van Vliet, M.T.H.; Qadir, M.; Bierkens, M.F.P. Country-level and gridded estimates of wastewater production, collection, treatment and reuse. Earth Syst. Sci. Data 2021, 13, 237–254. [Google Scholar] [CrossRef]
- Fouda-Mbanga, B.G.; Onotu, O.; Tywabi-Ngeva, Z. Advantages of the reuse of spent adsorbents and potential applications in environmental remediation: A review. Green Anal. Chem. 2024, 11, 100156. [Google Scholar] [CrossRef]
- Nageeb, M. Adsorption Technique for the Removal of Organic Pollutants from Water and Wastewater. In Organic Pollutants-Monitoring, Risk and Treatment; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef]
- Zhang, Z.; Luo, X.; Liu, Y.; Zhou, P.; Ma, G.; Lei, Z.; Lei, L. A low cost and highly efficient adsorbent (activated carbon) prepared from waste potato residue. J. Taiwan Inst. Chem. Eng. 2015, 49, 206–211. [Google Scholar] [CrossRef]
- Snyder, S.A.; Adham, S.; Redding, A.M.; Cannon, F.S.; DeCarolis, J.; Oppenheimer, J.; Wert, E.C.; Yoon, Y. Role of membranes and activated carbon in the removal of endocrine disruptors and pharmaceuticals. Desalination 2007, 202, 156–181. [Google Scholar] [CrossRef]
- Al-Buriahi, A.K.; Al-shaibani, M.M.; Mohamed, R.M.S.R.; Al-Gheethi, A.A.; Sharma, A.; Ismail, N. Ciprofloxacin removal from non-clinical environment: A critical review of current methods and future trend prospects. J. Water Process Eng. 2022, 47, 102725. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Ahmadi, S.; Ghosh, S.; Othmani, A.; Osagie, C.; Meskini, M.; AlKafaas, S.S.; Malloum, A.; Khanday, W.A.; Jacob, A.O.; et al. Recent advances on sustainable adsorbents for the remediation of noxious pollutants from water and wastewater: A critical review. Arab. J. Chem. 2023, 16, 105303. [Google Scholar] [CrossRef]
- Qalyoubi, L.; Al-Othman, A.; Al-Asheh, S.; Shirvanimoghaddam, K.; Mahmoodi, R.; Naebe, M. Textile-based biochar for the removal of ciprofloxacin antibiotics from water. Emergent Mater. 2024, 7, 577–588. [Google Scholar] [CrossRef]
- Jara-Cobos, L.; Abad-Delgado, D.; Ponce-Montalvo, J.; Menendez, M.; Peñafiel, M.E. Removal of ciprofloxacin from an aqueous medium by adsorption on natural and hydrolyzed bentonites. Front. Environ. Sci. 2023, 11, 1239754. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Ayati, A.; Davoodi, R.; Tanhaei, B.; Karimi, F.; Malekmohammadi, S.; Orooji, Y.; Fu, L.; Sillanpää, M. Recent advances in using of chitosan-based adsorbents for removal of pharmaceutical contaminants: A review. J. Clean. Prod. 2021, 291, 125880. [Google Scholar] [CrossRef]
- Gabet-Giraud, V.; Miège, C.; Choubert, J.; Ruel, S.M.; Coquery, M. Occurrence and removal of estrogens and beta blockers by various processes in wastewater treatment plants. Sci. Total Environ. 2010, 408, 4257–4269. [Google Scholar] [CrossRef]
- Adam, M.R.; Othman, M.H.D.; Kurniawan, T.A.; Puteh, M.H.; Ismail, A.; Khongnakorn, W.; Rahman, M.A.; Jaafar, J. Advances in adsorptive membrane technology for water treatment and resource recovery applications: A critical review. J. Environ. Chem. Eng. 2022, 10, 107633. [Google Scholar] [CrossRef]
- Filice, S.; D’Angelo, D.; Libertino, S.; Nicotera, I.; Kosma, V.; Privitera, V.; Scalese, S. Graphene oxide and titania hybrid Nafion membranes for efficient removal of methyl orange dye from water. Carbon 2015, 82, 489–499. [Google Scholar] [CrossRef]
- D’Angelo, D.; Filice, S.; Scarangella, A.; Iannazzo, D.; Compagnini, G.; Scalese, S. Bi2O3/Nexar® polymer nanocomposite membranes for azo dyes removal by UV–vis or visible light irradiation. Catal. Today 2019, 321–322, 158–163. [Google Scholar] [CrossRef]
- Filice, S.; D’Angelo, D.; Scarangella, A.; Iannazzo, D.; Compagnini, G.; Scalese, S. Highly effective and reusable sulfonated pentablock copolymer nanocomposites for water purification applications. RSC Adv. 2017, 7, 45521–45534. [Google Scholar] [CrossRef]
- Qalyoubi, L.; Al-Othman, A.; Al-Asheh, S. Removal of ciprofloxacin antibiotic pollutants from wastewater using nano-composite adsorptive membranes. Environ. Res. 2022, 215, 114182. [Google Scholar] [CrossRef]
- Filice, S.; Mazurkiewicz-Pawlicka, M.; Malolepszy, A.; Stobinski, L.; Kwiatkowski, R.; Boczkowska, A.; Gradon, L.; Scalese, S. Sulfonated Pentablock Copolymer Membranes and Graphene Oxide Addition for Efficient Removal of Metal Ions from Water. Nanomaterials 2020, 10, 1157. [Google Scholar] [CrossRef]
- Filice, S.; Scuderi, V.; Scalese, S. Sulfonated Pentablock Copolymer (NexarTM) for Water Remediation and Other Applications. Polymers 2024, 16, 2009. [Google Scholar] [CrossRef]
- Filice, S.; Sciuto, E.L.; Scalese, S.; Faro, G.; Libertino, S.; Corso, D.; Timpanaro, R.M.; Laganà, P.; Coniglio, M.A. Innovative Antibiofilm Smart Surface against Legionella for Water Systems. Microorganisms 2022, 10, 870. [Google Scholar] [CrossRef]
- Sciuto, E.L.; Filice, S.; Coniglio, M.A.; Faro, G.; Gradon, L.; Galati, C.; Spinella, N.; Libertino, S.; Scalese, S. Antimicrobial s-PBC Coatings for Innovative Multifunctional Water Filters. Molecules 2020, 25, 5196. [Google Scholar] [CrossRef] [PubMed]
- Sciuto, E.L.; Laganà, P.; Filice, S.; Scalese, S.; Libertino, S.; Corso, D.; Faro, G.; Coniglio, M.A. Environmental Management of Legionella in Domestic Water Systems: Consolidated and Innovative Approaches for Disinfection Methods and Risk Assessment. Microorganisms 2021, 9, 577. [Google Scholar] [CrossRef] [PubMed]
- Filice, S.; Scuderi, V.; Libertino, S.; Zimbone, M.; Galati, C.; Spinella, N.; Gradon, L.; Falqui, L.; Scalese, S. Sulfonated Pentablock Copolymer Coating of Polypropylene Filters for Dye and Metal Ions Effective Removal by Integrated Adsorption and Filtration Process. Int. J. Mol. Sci. 2022, 23, 11777. [Google Scholar] [CrossRef] [PubMed]
- Filice, S.; Scuderi, V.; Zimbone, M.; Libertino, S.; La Piana, L.; Farina, R.A.; Scalese, S. Sulfonated Pentablock Copolymer with Graphene Oxide for Co2+ Ions Removal: Efficiency, Interaction Mechanisms and Secondary Reaction Products. Coatings 2023, 13, 1715. [Google Scholar] [CrossRef]
- Nasef, M.M.; Saidi, H. Surface studies of radiation grafted sulfonic acid membranes: XPS and SEM analysis. Appl. Surf. Sci. 2006, 252, 3073–3084. [Google Scholar] [CrossRef]
- Yu, Y.; Hearon, K.; Wilson, T.S.; Maitland, D.J. The effect of moisture absorption on the physical properties of polyurethane shape memory polymer foams. Smart Mater. Struct. 2011, 20, 085010. [Google Scholar] [CrossRef]
- Voogt, B.; Huinink, H.P.; Erich, S.J.F.; Scheerder, J.; Venema, P.; Keddie, J.L.; Adan, O.C.G. Film Formation of High Tg Latex Using Hydroplasticization: Explanations from NMR Relaxometry. Langmuir 2019, 35, 12418–12427. [Google Scholar] [CrossRef]
- Hallinan, D.T.; Minelli, M.; Oparaji, O.; Sardano, A.; Iyiola, O.; Garcia, A.R.; Burnett, D.J. Effect of Polystyrene Synthesis Method on Water Sorption and Glass Transition. Membranes 2022, 12, 1059. [Google Scholar] [CrossRef]
- Filice, S.; Urzì, G.; Milazzo, R.G.; Privitera, S.M.S.; Lombardo, S.A.; Compagnini, G.; Scalese, S. Applicability of a New Sulfonated Pentablock Copolymer Membrane and Modified Gas Diffusion Layers for Low-Cost Water Splitting Processes. Energies 2019, 12, 2064. [Google Scholar] [CrossRef]
- Tozar, T.; Boni, M.; Staicu, A.; Pascu, M.L. Optical Characterization of Ciprofloxacin Photolytic Degradation by UV-Pulsed Laser Radiation. Molecules 2021, 26, 2324. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Pandey, S.; Mewada, A.; Patil, V.; Khade, M.; Goshi, E.; Sharon, M. Antibiotic Conjugated Fluorescent Carbon Dots as a Theranostic Agent for Controlled Drug Release, Bioimaging, and Enhanced Antimicrobial Activity. J. Drug Deliv. 2014, 2014, 282193. [Google Scholar] [CrossRef] [PubMed]
- Sharifpour, N.; Moghaddam, F.M.; Mardani, G.; Malakootian, M. Evaluation of the activated carbon coated with multiwalled carbon nanotubes in removal of ciprofloxacin from aqueous solutions. Appl. Water Sci. 2020, 10, 140. [Google Scholar] [CrossRef]
- Dzeranov, A.; Bondarenko, L.; Dzhardimalieva, G.; Kelbysheva, E.; Zmeev, D.; Patsaeva, S.; Tropskaya, N.; Jorobekova, S.J.; Kydralieva, K. Enhanced Interaction of Ciprofloxacin with Humic Substances and Magnetite-Silica-Nanoparticles in Multicomponent System: Spectrophotometric and Electrokinetic Studies. J. Biomed. Photonics Eng. 2024, 10, 020301. [Google Scholar] [CrossRef]
- Ghosalya, M.K.; Talebi, P.; Singh, H.; Klyushin, A.; Kokkonen, E.; Mansouri, M.A.; Huttula, M.; Cao, W.; Urpelainen, S. Solar light driven atomic and electronic transformations in a plasmonic Ni@NiO/NiCO3 photocatalyst revealed by ambient pressure X-ray photoelectron spectroscopy. Catal. Sci. Technol. 2024, 14, 3029–3040. [Google Scholar] [CrossRef]
- Plazinski, W.; Rudzinski, W.; Plazinska, A. Theoretical models of sorption kinetics including a surface reaction mechanism: A review. Adv. Colloid Interface Sci. 2009, 152, 2–13. [Google Scholar] [CrossRef]
- Vafakhah, S.; Bahrololoom, M.E.; Saeedikhani, M. Adsorption Kinetics of Cupric Ions on Mixture of Modified Corn Stalk and Modified Tomato Waste. J. Water Resour. Prot. 2016, 08, 1238–1250. [Google Scholar] [CrossRef]
- Largitte, L.; Pasquier, R. A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chem. Eng. Res. Des. 2016, 109, 495–504. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of Adsorption on Carbon from Solutions. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Zhu, B.; Gu, T. Surfactant adsorption at solid-liquid interfaces. Adv. Colloid Interface Sci. 1991, 37, 1–32. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Gupta, A. Arsenic adsorption onto iron oxide-coated cement (IOCC): Regression analysis of equilibrium data with several isotherm models and their optimization. Chem. Eng. J. 2006, 122, 93–106. [Google Scholar] [CrossRef]
- Hans, S.; Stadie, N.P. Langmuir’s Theory of Adsorption: A Centennial Review. Langmuir ACS J. Surf. Colloids 2019, 35, 5409–5426. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef]
- Sips, R. On the structure of a catalyst surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Redlich, O.; Peterson, D.L. A useful adsorption isotherm. J. Phys. Chem. 1959, 63, 1024–1026. [Google Scholar] [CrossRef]
- De Vargas Brião, G.; Hashim, M.A.; Chu, K.H. The Sips isotherm equation: Often used and sometimes misused. Sep. Sci. Technol. 2023, 58, 884–892. [Google Scholar] [CrossRef]
- Foo, K.; Hameed, B. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Giles, C.H.; Smith, D.; Huitson, A. A general treatment and classification of the solute adsorption isotherm. I. Theoretical. J. Colloid Interface Sci. 1974, 47, 755–765. [Google Scholar] [CrossRef]
- Saraydın, D.; Işıkver, Y.; Karadağ, E. An evaluation on S-type adsorption isotherm in the model of crosslinked polyhydroxamates/oxazine dyes/water interactions. Adsorption 2022, 28, 249–260. [Google Scholar] [CrossRef]
- Avcı, A.; İnci, İ.; Baylan, N. A Comparative Adsorption Study with Various Adsorbents for the Removal of Ciprofloxacin Hydrochloride from Water. Water Air Soil Pollut. 2019, 230, 250. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, X.; Feng, P.; Chai, H.; Huang, Y. ZIF-67 derived hollow cobalt sulfide as superior adsorbent for effective adsorption removal of ciprofloxacin antibiotics Chem. Eng. J. 2018, 344, 95–104. [Google Scholar]
- Lin, C.; Lee, C. Adsorption of ciprofloxacin in water using Fe3O4 nanoparticles formed at low temperature and high reactant concentrations in a rotating packed bed with co-precipitation. Mater. Chem. Phys. 2020, 240, 122049. [Google Scholar] [CrossRef]
- Peñafiel, M.E.; Matesanz, J.M.; Vanegas, E.; Bermejo, D.; Mosteo, R.; Ormad, M.P. Comparative adsorption of ciprofloxacin on sugarcane bagasse from Ecuador and on commercial powdered activated carbon. Sci. Total Environ. 2021, 750, 141498. [Google Scholar] [CrossRef]
- Atugoda, T.; Wijesekara, H.; Werellagama, D.R.I.B.; Jinadasa, K.B.S.N.; Bolan, N.S.; Vithanage, M. Adsorptive interaction of antibiotic ciprofloxacin on polyethylene microplastics: Implications for vector transport in water. Environ. Technol. Innov. 2020, 19, 100971. [Google Scholar] [CrossRef]
- Kini, V.; Mondal, D.; Sundarabal, N.; Nag, P.; Sadani, K. Recent advances in electrochemical sensing and remediation technologies for ciprofloxacin. Environ. Sci. Pollut. Res. Int. 2025, 32, 2210–2237. [Google Scholar] [CrossRef]
- Dresp, S.; Dionigi, F.; Klingenhof, M.; Strasser, P. Direct Electrolytic Splitting of Seawater: Opportunities and Challenges. ACS Energy Lett. 2019, 4, 933–942. [Google Scholar] [CrossRef]
- Chang, J.; Yang, Y. Advancements in Seawater Electrolysis: Progressing from Fundamental Research to Applied Electrolyzer Application. Renewables 2023, 1, 415–454. [Google Scholar] [CrossRef]
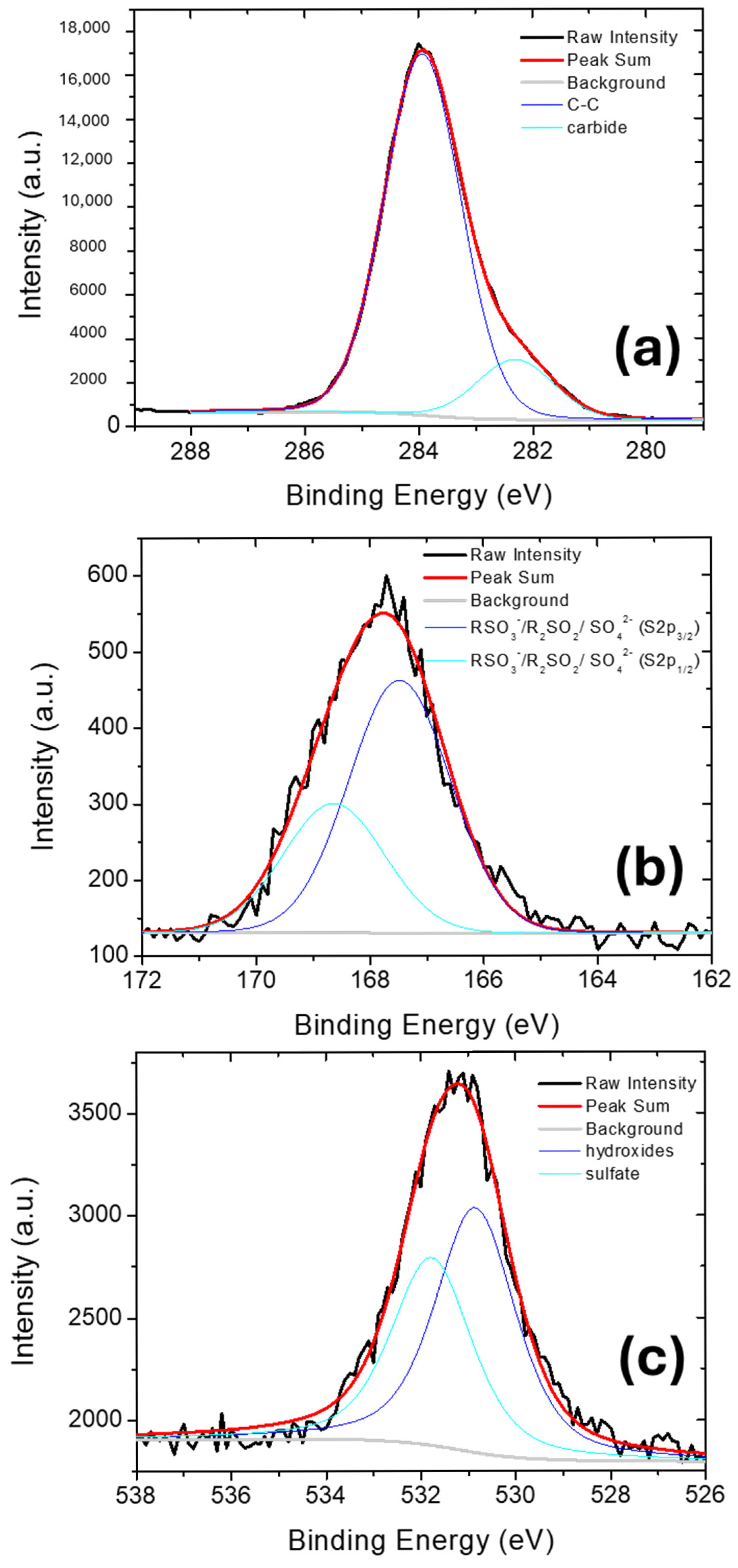

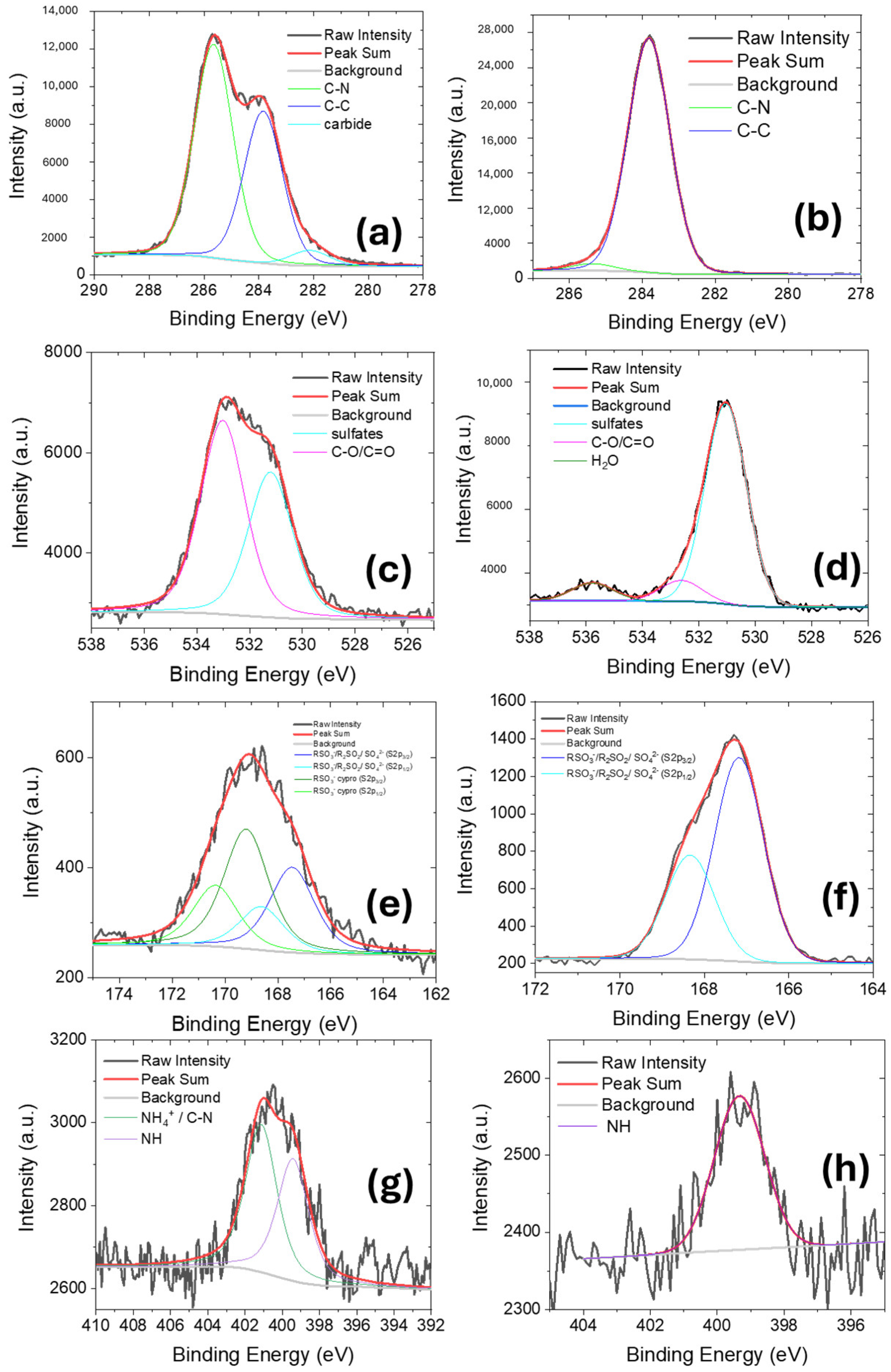
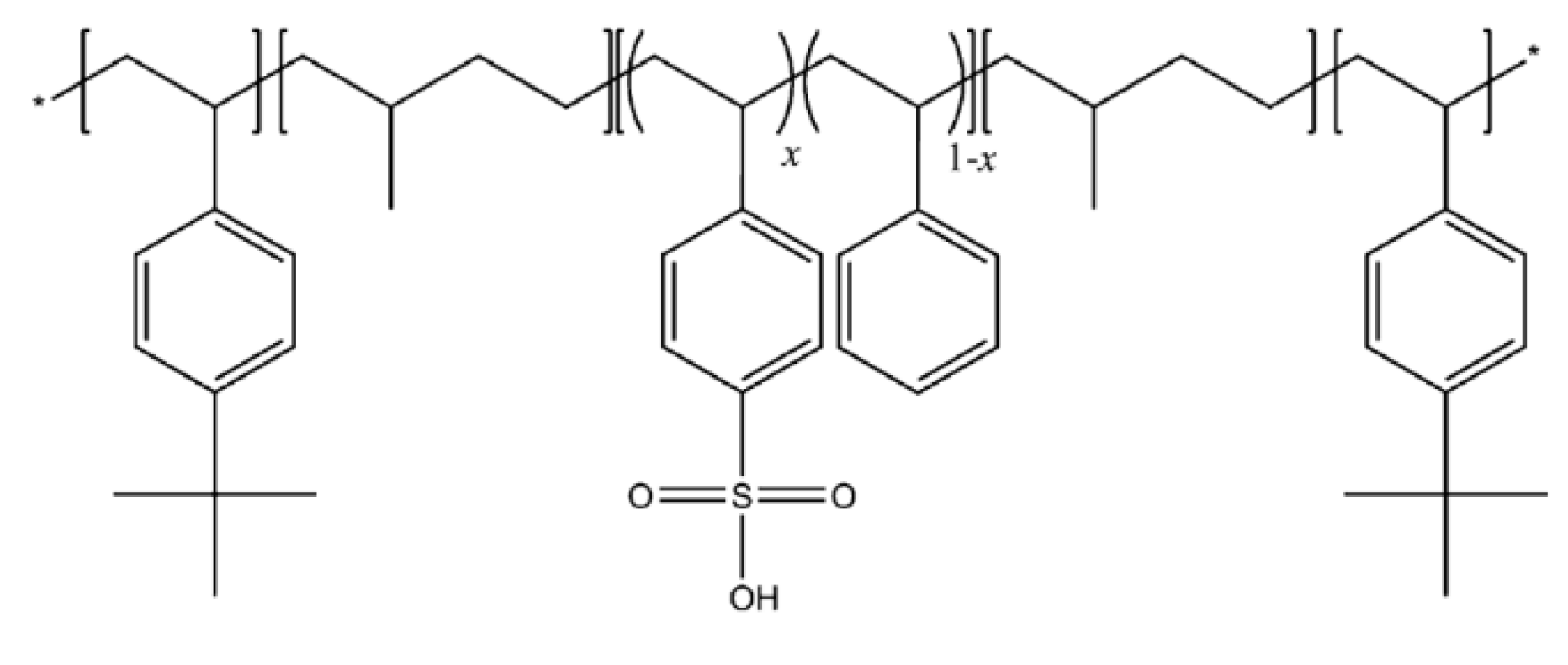
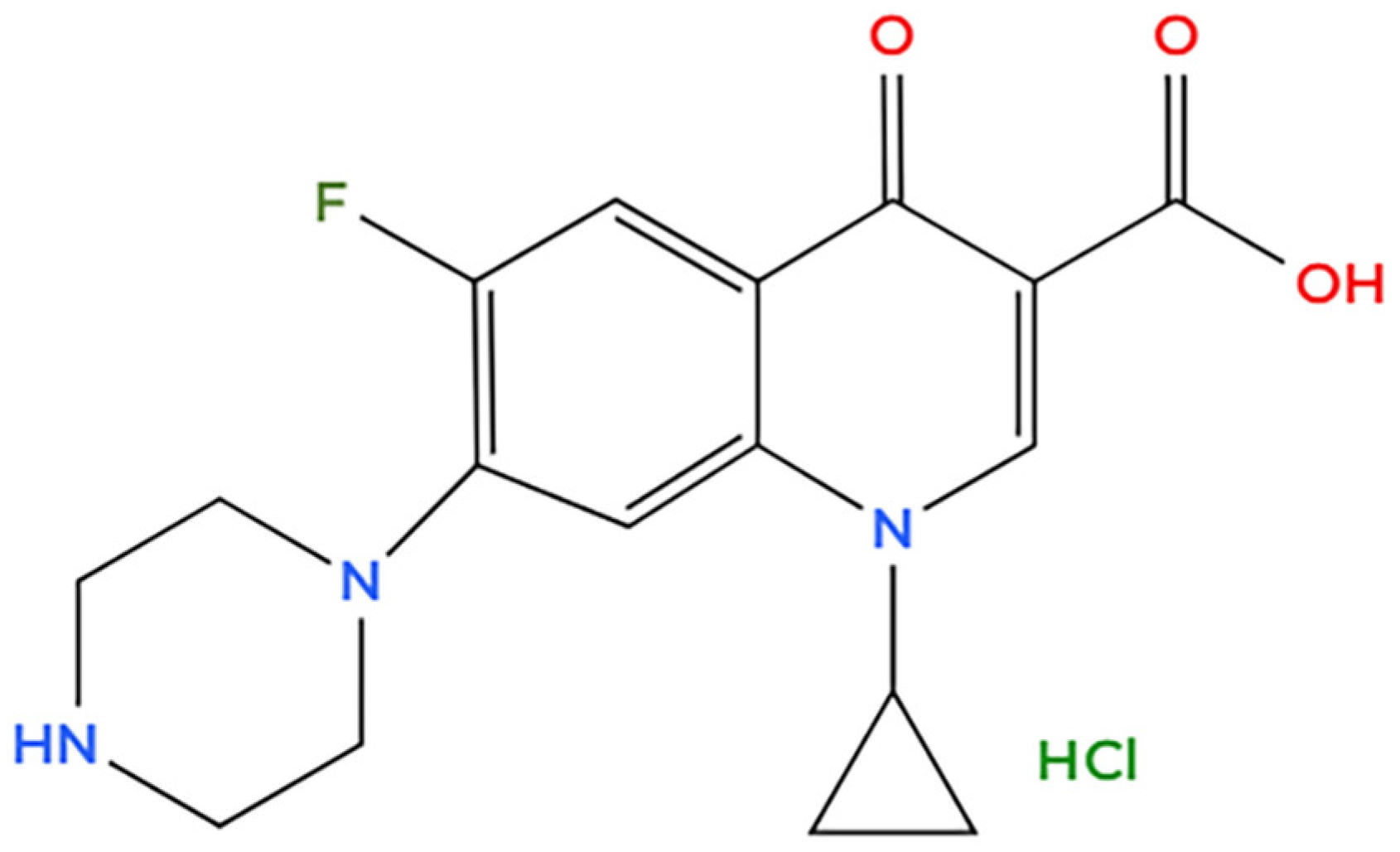
| Peak | Species | Binding Energy (eV) | Relative Amount (%) |
|---|---|---|---|
| C1s | Carbide | 282.3 | 14.3 |
| C-C | 283.9 | 85.7 | |
| S2p | RSO3−/R2SO2/SO42− (S2p3/2) | 167.5 | 66.2 |
| RSO3−/R2SO2/SO42− (S2p1/2) | 168.6 | 33.8 | |
| O1s | hydroxides | 530.8 | 56.6 |
| sulfates | 531.8 | 43.4 |
| Peak | Species | Nexar | Nexar Post Adsorption in MilliQ | Nexar Post Adsorption in Salt Water | |||
|---|---|---|---|---|---|---|---|
| Binding Energy (eV) | Relative Amount (%) | Binding Energy (eV) | Relative Amount (%) | Binding Energy (eV) | Relative Amount (%) | ||
| C1s | Carbide | 282.3 | 14.3 | 282.1 | 4.0 | -- | -- |
| C-C | 283.9 | 85.7 | 283.8 | 39.9 | 283.8 | 97.0 | |
| C-N/C-O | -- | -- | 285.6 | 56.1 | 285.4 | 3.0 | |
| O1s | hydroxides | 530.8 | 56.6 | -- | -- | -- | -- |
| sulfates | 531.8 | 43.4 | 531.2 | 42.8 | 531.0 | 84 | |
| C-O/C=O | -- | -- | 533.0 | 57.2 | 532.6 | 9 | |
| Gas phase H2O | -- | -- | -- | -- | 535.8 | 7 | |
| S2p | RSO3−/R2SO2/SO42− (S2p3/2) | 167.5 | 66.2 | 166.9 | 21.2 | 167.2 | 66.2 |
| RSO3−/R2SO2/SO42− (S2p1/2) | 168.6 | 33.8 | 168.1 | 10.2 | 168.3 | 33.8 | |
| RSO3− cypro (S2p3/2) | -- | -- | 168.9 | 45.0 | -- | -- | |
| RSO3− cypro (S2p1/2) | -- | -- | 170.1 | 23.0 | -- | -- | |
| N1s | NH (organic) | -- | -- | 399.4 | 45.3 | 399.3 | 100 |
| NH4+/C-N (organic) | -- | -- | 401.1 | 54.7 | -- | -- | |
| Ciprofloxacin Solution | R2 | k1 (min−1) | Qe (mg/g) | Qe 48h(mg/g) |
|---|---|---|---|---|
| MilliQ | 0.995 | −0.0064 ± 0.0001 | 13.95 | 14.30 |
| NaCl 0.5 M | 0.959 | −0.0024 ± 1.1314 | 8.62 | 9.40 |
| Process Time | Model | Ciprofloxacin in MilliQ | Ciprofloxacin in NaCl 0.5 M | ||||
|---|---|---|---|---|---|---|---|
| R2 | k1 (min−1) | Qe (mg/g) | R2 | k1 (min−1) | Qe (mg/g) | ||
| 0–60 min | PFO | 0.998 | −0.0092 | 15.39 | 0.994 | −0.0047 ± 0.0001 | 9.34 |
| R2 | k2 (min−1) | Qe (mg/g) | R2 | k2 (min−1) | Qe (mg/g) | ||
| 60–390 min | PSO | 0.999 | 0.0004 | 17.80 | 0.999 | 0.0011 | 8.94 |
| Ciprofloxacin Initial Concentration (ppm) | Qe (mg/g) in MilliQ | Qe (mg/g) in NaCl 0.5 M |
|---|---|---|
| 1.15 | 1.34 | 0.75 |
| 2.87 | 2.60 | 1.78 |
| 5.75 | 6.62 | 4.46 |
| 11.50 | 12.25 | 7.40 |
| 28.75 | 36.93 | 16.73 |
| Ciprofloxacin Solutions | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| Qmax (mg/g) | KL (L/mg) | R2 | 1/n | KF (L1/n mg(1−1/n)/g) | R2 | |
| MilliQ | 61 | −59.84 | 0.978 | 1.0440 | 1.0247 | 0.989 |
| NaCl 0.5 M | −244 | 244.65 | 0.997 | 0.9737 | 0.6825 | 0.991 |
| Sample | Element Peak Area Ratio | |||
|---|---|---|---|---|
| O/C | S/C | N/C | Na/C | |
| Nexar | 0.17 | 0.03 | -- | -- |
| A | 0.99 | 0.08 | 0.10 | - |
| B | 0.41 | 0.06 | 0.06 | 0.03 |
| C | 0.31 | 0.06 | 0.01 | 0.09 |
| D | 0.42 | 0.04 | 0.01 | 0.04 |
| Adsorbents | Adsorption Capacity (mg/g) | References |
|---|---|---|
| Nexar (MilliQ) | 14.30 | This work |
| Nexar (NaCl 0.5 M) | 9.40 | This work |
| MWCNTs | 1.74 | [74] |
| Fe3O4 NPs | 24 | [76] |
| Hollow Co3S4 NPs | 427.3 | [75] |
| Powdered activated carbon | 13.6 | [77] |
| Montmorillonite | 0.60 | [74] |
| Modified Montmorillonite | 1.67 | [74] |
| Alumina | 1.15 | [74] |
| PES/ZrP | 8.9 | [41] |
| Polyethylene microplastic | 2–3 | [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filice, S.; Crispi, S.; Scuderi, V.; Iannazzo, D.; Celesti, C.; Scalese, S. Effective Ciprofloxacin Removal from Deionized and Salt Water by Sulfonated Pentablock Copolymer (NexarTM). Molecules 2025, 30, 3275. https://doi.org/10.3390/molecules30153275
Filice S, Crispi S, Scuderi V, Iannazzo D, Celesti C, Scalese S. Effective Ciprofloxacin Removal from Deionized and Salt Water by Sulfonated Pentablock Copolymer (NexarTM). Molecules. 2025; 30(15):3275. https://doi.org/10.3390/molecules30153275
Chicago/Turabian StyleFilice, Simona, Simona Crispi, Viviana Scuderi, Daniela Iannazzo, Consuelo Celesti, and Silvia Scalese. 2025. "Effective Ciprofloxacin Removal from Deionized and Salt Water by Sulfonated Pentablock Copolymer (NexarTM)" Molecules 30, no. 15: 3275. https://doi.org/10.3390/molecules30153275
APA StyleFilice, S., Crispi, S., Scuderi, V., Iannazzo, D., Celesti, C., & Scalese, S. (2025). Effective Ciprofloxacin Removal from Deionized and Salt Water by Sulfonated Pentablock Copolymer (NexarTM). Molecules, 30(15), 3275. https://doi.org/10.3390/molecules30153275










