Protective Effect of Tomato By-Product in Refined Sunflower Oil with Different Lipid Profiles
Abstract
1. Introduction
2. Results
2.1. Tomato By-Product Composition and Recovery of Bioactive Molecules
2.2. Physical–Chemical Analyses
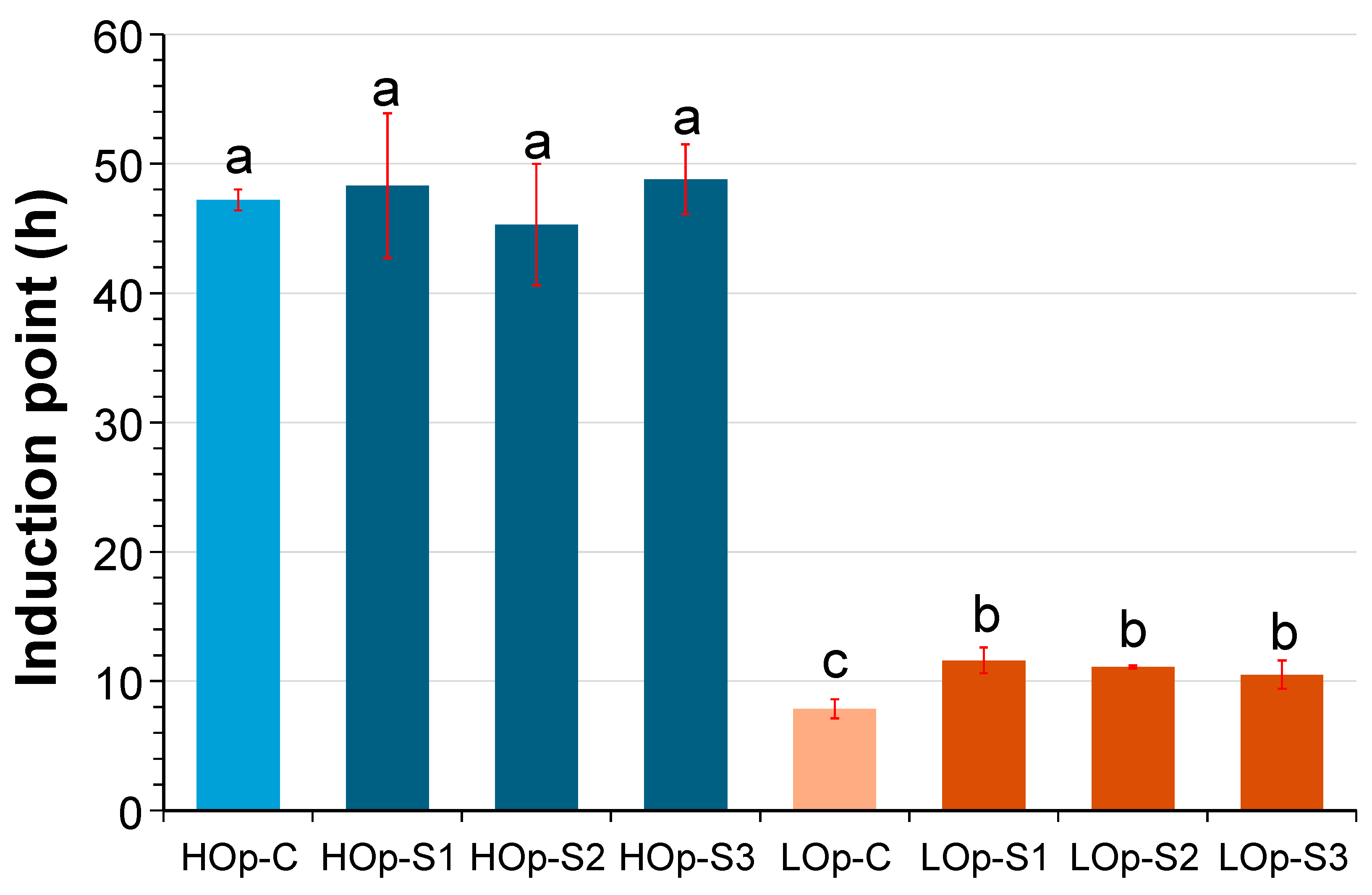
2.3. Thermooxidative Stability
3. Discussion
4. Materials and Methods
4.1. Tomato By-Product Batches, Sample Conditioning and Preparation
4.2. Recovery of Bioactive Molecules: Vegetable Oil and Pressurization Treatment
4.3. Physical–Chemical Analyses of Vegetable Oil
4.4. Thermal Oxidative Stability
4.5. Data Processing and Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| BHT | Butylated hydroxytoluene |
| GAE | Gallic acid equivalents |
| LO | Low oleic oil |
| HO | High oleic oil |
| SO | Sunflower oil |
| HPP | High-pressure processing |
| SAFA | Saturated fatty acid |
| MUFA | Monounsaturated fatty acid |
| PUFA | Polyunsaturated fatty acid |
Appendix A
| Determination | Method | S1 [43] | S2 [24] | S3 |
|---|---|---|---|---|
| Moisture (%) | ISO 712:2009 [44] | 65.15 | 84.95 | 76.57 |
| Crude protein (%) | ISO 20483:2013 [45] | 7.25 | 3.33 | 3.27 |
| Crude fat (%) | AACC, 2010 [46] | 6.32 | 1.65 | 3.45 |
| Ashes (%) | ISO 2171:2007 [47] | 1.15 | 0.86 | 1.00 |
| Dietary fiber (%) | AOAC 93.19:2000 [48] | 20.13 | 9.21 | 15.71 |
| Lycopene (µg/g DW) | Silva et al. [49] | 582.0 ± 12.7 | 1174.3 ± 43.1 | 776.7 ± 25.5 |
| Total polyphenols (µg GAE/g DW) | Sánchez-Rangel et al. [50] | 290.2 ± 4.6 | 601.0 ± 15.0 | 473.8 ± 57.6 |
| Determination | Analytical Method |
|---|---|
| Free fatty acids (acid value) | ISO 660:2020 (g oleic acid/100 g oil) [51] |
| Composition of fatty acids by gas chromatography | Preparation by ISO 12966-2:2017 [52] and analysis by ISO 12966-4:2015 [53]. |
| Tocopherols by HPLC | ISO 9936:2016 (mg/kg) [54] |
| Color | Color Space CIE L*a*b*. Device: DigiEye (VeriVide Ltd., Leicester, UK) equipped with a Nikon D90 SLR camera (Nikon Co. Ltd., Tokyo, Japan). Illuminant: Artificial Daylight F18 T8/D65 (VeriVide Ltd., Leicester, UK). Associate software: DigiEye v2.80 (VeriVide Ltd., Leicester, UK) |
References
- WPCT. World Production Estimate of Tomatoes for Processing; World Processing Tomato Council: Avignon, France, 2025. [Google Scholar]
- Casa, M.; Miccio, M.; Feo, G.D.; Paulillo, A.; Chirone, R.; Paulillo, D.; Lettieri, P.; Chirone, R. A Brief Overview on Valorization of Industrial Tomato By-Products Using the Biorefinery Cascade Approach. Detritus 2021, 15, 31–39. [Google Scholar] [CrossRef]
- Eslami, E.; Carpentieri, S.; Pataro, G.; Ferrari, G. A Comprehensive Overview of Tomato Processing By-Product Valorization by Conventional Methods versus Emerging Technologies. Foods 2023, 12, 166. [Google Scholar] [CrossRef] [PubMed]
- Souza da Costa, B.; García, M.O.; Muro, G.S.; Motilva, M.-J. A Comparative Evaluation of the Phenol and Lycopene Content of Tomato By-Products Subjected to Different Drying Methods. LWT 2023, 179, 114644. [Google Scholar] [CrossRef]
- Kaur, D.; Wani, A.A.; Oberoi, D.P.S.; Sogi, D.S. Effect of Extraction Conditions on Lycopene Extractions from Tomato Processing Waste Skin Using Response Surface Methodology. Food Chem. 2008, 108, 711–718. [Google Scholar] [CrossRef]
- Ciaccheri, L.; Tuccio, L.; Mencaglia, A.A.; Sikorska-Zimny, K.; Hallmann, E.; Kowalski, A.; Mignani, A.G.; Kaniszewski, S.; Agati, G. Prediction Models for Assessing Lycopene in Open-Field Cultivated Tomatoes by Means of a Portable Reflectance Sensor: Cultivar and Growing-Season Effects. J. Agric. Food Chem. 2018, 66, 4748–4757. [Google Scholar] [CrossRef]
- Garcia, E.; Barrett, D.M. Assessing Lycopene Content in California Processing Tomatoes. J. Food Process. Preserv. 2006, 30, 56–70. [Google Scholar] [CrossRef][Green Version]
- Usman, I.; Hussain, M.; Imran, A.; Afzaal, M.; Saeed, F.; Javed, M.; Arzal, A.; Ashfaq, I.; Al Jbawi, E.; Saewan, S.A. Traditional and Innovative Approaches for the Extraction of Bioactive Compounds. Int. J. Food Prop. 2022, 25, 1215–1233. [Google Scholar] [CrossRef]
- Kyriakoudi, A.; Klimantakis, K.; Kalaitzis, P.; Biliaderis, C.G.; Mourtzinos, I. Enrichment of Sunflower Oil with Tomato Carotenoids and Its Encapsulation in Ca-Alginate Beads: Preparation, Characterization and Chemical Stability upon in Vitro Digestion. Food Hydrocoll. 2024, 151, 109855. [Google Scholar] [CrossRef]
- Zuorro, A. Enhanced Lycopene Extraction from Tomato Peels by Optimized Mixed-Polarity Solvent Mixtures. Molecules 2020, 25, 2038. [Google Scholar] [CrossRef]
- Nour, V.; Corbu, A.R.; Rotaru, P.; Karageorgou, I.; Lalas, S. Effect of Carotenoids, Extracted from Dry Tomato Waste, on the Stability and Characteristics of Various Vegetable Oils. Grasas Aceites 2018, 69, e238. [Google Scholar] [CrossRef]
- Méndez-Carmona, J.Y.; Ascacio-Valdes, J.A.; Alvarez-Perez, O.B.; Hernández-Almanza, A.Y.; Ramírez-Guzman, N.; Sepúlveda, L.; Aguilar-González, M.A.; Ventura-Sobrevilla, J.M.; Aguilar, C.N. Tomato Waste as a Bioresource for Lycopene Extraction Using Emerging Technologies. Food Biosci. 2022, 49, 101966. [Google Scholar] [CrossRef]
- Hoyos, C.G.; Guerra, A.S.; Pérez, S.A.; Velásquez-Cock, J.; Villegas, M.; Gañán, P.; Gallego, R.Z. An Edible Oil Enriched with Lycopene from Pink Guava (Psidium guajava L.) Using Different Mechanical Treatments. Molecules 2022, 27, 1038. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, N.; Ghavami, M.; Rashidi, L.; Gharachorloo, M.; Nateghi, L. Effects of Adding Green Tea Extract on the Oxidative Stability and Shelf Life of Sunflower Oil during Storage. Food Chem. X 2024, 21, 101168. [Google Scholar] [CrossRef]
- Fernández-Martínez, J.M.; Pérez-Vich, B.; Velasco, L.; Domínguez, J. Breeding for Specialty Oil Types in Sunflower. HELIA 2007, 30, 75–84. [Google Scholar] [CrossRef]
- Zambelli, A. Current Status of High Oleic Seed Oils in Food Processing. J. Am. Oil Chem. Soc. 2021, 98, 129–137. [Google Scholar] [CrossRef]
- Tonin, P. Les Productions Françaises d’oléagineux de Spécialité: Des Démarches En Filière Pour Créer de La Valeur Dans Nos Territoires. OCL 2018, 25, D203. [Google Scholar] [CrossRef]
- Kunthakudee, N.; Chutvirasakul, B.; Ramakul, P. Green Extraction of Lycopene from Tomato Peel Waste Using Vegetable Oil. AIP Conf. Proc. 2023, 2669, 030014. [Google Scholar] [CrossRef]
- Benakmoum, A.; Abbeddou, S.; Ammouche, A.; Kefalas, P.; Gerasopoulos, D. Valorisation of Low Quality Edible Oil with Tomato Peel Waste. Food Chem. 2008, 110, 684–690. [Google Scholar] [CrossRef]
- Córdoba, M.G.; Pérez-Nevado, F.; Aranda, E.; Ciruelos, A.; Martínez-Mediero, J. Comparative Study of the Pigment Content of Different Crop Cycle Tomato Varieties for Industry. Acta Hortic. 2003, 613, 407–409. [Google Scholar] [CrossRef]
- Kaboré, K.; Konaté, K.; Sanou, A.; Dakuyo, R.; Sama, H.; Santara, B.; Compaoré, E.W.R.; Dicko, M.H. Tomato By-Products, a Source of Nutrients for the Prevention and Reduction of Malnutrition. Nutrients 2022, 14, 2871. [Google Scholar] [CrossRef]
- Xi, J. Effect of High Pressure Processing on the Extraction of Lycopene in Tomato Paste Waste. Chem. Eng. Technol. 2006, 29, 736–739. [Google Scholar] [CrossRef]
- Jun, X. Application of High Hydrostatic Pressure Processing of Food to Extracting Lycopene from Tomato Paste Waste. High Press. Res. 2006, 26, 33–41. [Google Scholar] [CrossRef]
- Fernandez-Pan, I.; Horvitz, S.; Ibañez, F.C.; Arroqui, C.; Beriain, M.J.; Virseda, P. Extra-Virgin Olive Oil Enriched with Lycopene: From Industrial Tomato by-Products to Consumer. Food Sci. Nutr. 2024, 12, 5815–5823. [Google Scholar] [CrossRef]
- Barba, F.J.; Esteve, M.J.; Frigola, A. Impact of High-Pressure Processing on Vitamin E (α-, γ-, and δ-Tocopherol), Vitamin D (Cholecalciferol and Ergocalciferol), and Fatty Acid Profiles in Liquid Foods. J. Agric. Food Chem. 2012, 60, 3763–3768. [Google Scholar] [CrossRef]
- Tauber, S.; Fehn, S.; Schmidt, M.; Schwarzenbolz, U.; Böhm, V. Characterization and Application of a Microemulsion as Model System for Lipophilic Phytochemicals in High-Pressure Processing. Appl. Res. 2024, 3, e202400016. [Google Scholar] [CrossRef]
- Zhong, Y.; Yuan, X.; Feng, Q.; Wang, Q.; Pan, H.; Qiao, Z.; Wang, T.; Zhuang, Y. Application of Polyphenols as Natural Antioxidants in Edible Oils: Current Status, Antioxidant Mechanism, and Advanced Technology. Food Res. Int. 2025, 208, 116234. [Google Scholar] [CrossRef]
- Kehili, M.; Choura, S.; Zammel, A.; Allouche, N.; Sayadi, S. Oxidative Stability of Refined Olive and Sunflower Oils Supplemented with Lycopene-Rich Oleoresin from Tomato Peels Industrial by-Product, during Accelerated Shelf-Life Storage. Food Chem. 2018, 246, 295–304. [Google Scholar] [CrossRef]
- Gheonea (Dima), I.; Aprodu, I.; Enachi, E.; Horincar, G.; Bolea, C.A.; Bahrim, G.E.; Râpeanu, G.; Stănciuc, N. Investigations on Thermostability of Carotenoids from Tomato Peels in Oils Using a Kinetic Approach. J. Food Process. Preserv. 2020, 44, e14303. [Google Scholar] [CrossRef]
- Kaur, D.; Sogi, D.S.; Wani, A.A. Oxidative Stability of Soybean Triacylglycerol Using Carotenoids and Y-Tocopherol. Int. J. Food Prop. 2015, 18, 2605–2613. [Google Scholar] [CrossRef]
- Henry, L.K.; Catignani, G.L.; Schwartz, S.J. The Influence of Carotenoids and Tocopherols on the Stability of Safflower Seed Oil during Heat-Catalyzed Oxidation. J. Am. Oil Chem. Soc. 1998, 75, 1399–1402. [Google Scholar] [CrossRef]
- Zeb, A.; Murkovic, M. Pro-Oxidant Effects of β-Carotene during Thermal Oxidation of Edible Oils. J. Am. Oil Chem. Soc. 2013, 90, 881–889. [Google Scholar] [CrossRef]
- Lavecchia, R.; Zuorro, A. Thermal Stability of Tomato Lycopene in Vegetable Oils. Chem. Technol. Indian J. 2006, 1, 80–87. [Google Scholar]
- Roman, O.; Heyd, B.; Broyart, B.; Castillo, R.; Maillard, M.-N. Oxidative Reactivity of Unsaturated Fatty Acids from Sunflower, High Oleic Sunflower and Rapeseed Oils Subjected to Heat Treatment, under Controlled Conditions. LWT 2013, 52, 49–59. [Google Scholar] [CrossRef]
- Liu, C.; Wang, W.; Zhang, H.; Luo, S.; Wang, X.; Wang, L.; Yu, D. Effects of α-Tocopherol, β-Carotene and Epigallocatechin Gallate on the Oxidative Stability of Sunflower Oil. J. Oleo Sci. 2023, 72, 521–531. [Google Scholar] [CrossRef]
- Zuorro, A.; Lavecchia, R.; González, E.; Kafarov, V. Kinetics of Lycopene Degradation in Sunflower and Grape Seed Oils. Orient. J. Chem. 2018, 34, 2229–2235. [Google Scholar] [CrossRef]
- Hackett, M.M.; Lee, J.H.; Francis, D.; Schwartz, S.J. Thermal Stability and Isomerization of Lycopene in Tomato Oleoresins from Different Varieties. J. Food Sci. 2004, 69, 536–541. [Google Scholar] [CrossRef]
- Varas Condori, M.A.; Pascual Chagman, G.J.; Barriga-Sanchez, M.; Villegas Vilchez, L.F.; Ursetta, S.; Guevara Pérez, A.; Hidalgo, A. Effect of Tomato (Solanum lycopersicum L.) Lycopene-Rich Extract on the Kinetics of Rancidity and Shelf-Life of Linseed (Linum usitatissimum L.) Oil. Food Chem. 2020, 302, 125327. [Google Scholar] [CrossRef]
- Salas, J.J.; Bootello, M.A.; Piispa, E.; Hornyák, L.; Venegas-Calerón, M.; Martínez-Force, E.; Garcés, R. The Effect of Enzymatic Interesterification on the High Oleic-High Stearic Sunflower Oil Fractionation and the Physico-Chemical Properties of Stearins. LWT 2023, 184, 115042. [Google Scholar] [CrossRef]
- Velasco, J.; García-González, A.; Zamora, R.; Hidalgo, F.J.; Ruiz-Méndez, M.V. Olive Pomace Oil Improves the Oxidative Stability and Nutritional Value of Oil-Based Cakes with Anise Essence, a Traditional Confectionery Product in Spain. LWT 2023, 184, 115081. [Google Scholar] [CrossRef]
- Gandul-Rojas, B.; Gallardo-Guerrero, L. Pigment Changes during Preservation of Green Table Olive Specialities Treated with Alkali and without Fermentation: Effect of Thermal Treatments and Storage Conditions. Food Res. Int. 2018, 108, 57–67. [Google Scholar] [CrossRef]
- Vazquez Roncero, A.; Janer del Valle, C.; Janer del Valle, M.L. Determinacion de Los Polifenoles Totales Del Aceite de Oliva. Grasas Aceites 1973, 24, 350–357. [Google Scholar]
- Fernandez-Pan, I.; Ibañez, F.C.; Virseda, P.; Arozarena, I.; Beriain, M.J. Stabilization and Valorization of Tomato Byproduct: A Case Study for the Bakery Industry. J. Food Sci. 2023, 88, 4483–4494. [Google Scholar] [CrossRef] [PubMed]
- ISO 712-1:2029; Determination of Moisture Content—Reference Method; Cereals, Pulses And by-Products. ISO: Geneva, Switzerland, 2009.
- ISO 20483:2013; Determination of the Nitrogen Content and Calculation of the Crude Protein Content—Kjeldahl Method; CEREALS and Pulses. ISO: Geneva, Switzerland, 2013.
- AACC 30-25.01; Crude Fat in Wheat, Corn, and Soy Flour, Feeds, and Mixed Feeds. Cereals & Grains Association: St. Paul, MN, USA, 2010.
- ISO 2171:2007; Determination of Ash Yield by Incineration; Cereals, Pulses and by-Products. ISO: Geneva, Switzerland, 2007.
- AOAC 991.43; Total, Soluble and Insoluble Dietary Fiber in Foods. Association of Official Analytical Chemists: Rockville, ML, USA, 2000.
- Silva, Y.P.A.; Borba, B.C.; Pereira, V.A.; Reis, M.G.; Caliari, M.; Brooks, M.S.-L.; Ferreira, T.A.P.C. Characterization of Tomato Processing By-Product for Use as a Potential Functional Food Ingredient: Nutritional Composition, Antioxidant Activity and Bioactive Compounds. Int. J. Food Sci. Nutr. 2019, 70, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu Assay Revisited: Improvement of Its Specificity for Total Phenolic Content Determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- ISO 660:2020; Determination of Acid Value and Acidity; Animal and Vegetable Fats and Oils. ISO: Geneve, Switzerland, 2020.
- ISO 12966-2:2017; Gas Chromatography of Fatty Acid Methyl Esters. Part 2: Preparation of Methyl Esters of Fatty Acids; Animal and Vegetable Fats and Oils. ISO: Geneva, Switzerland, 2017.
- ISO 12966-4:2015; Gas Chromatography of Fatty Acid Methyl Esters. Part 4: Determination by Capillary Gas Chromatography; Animal and Vegetable Fats and Oils. ISO: Geneva, Switzerland, 2015.
- ISO 9936:2016; Determination of Tocopherol and Tocotrienol Contents by High-Performance Liquid Chromatography; Animal and Vegetable Fats and Oils. ISO: Geneva, Switzerland, 2016.
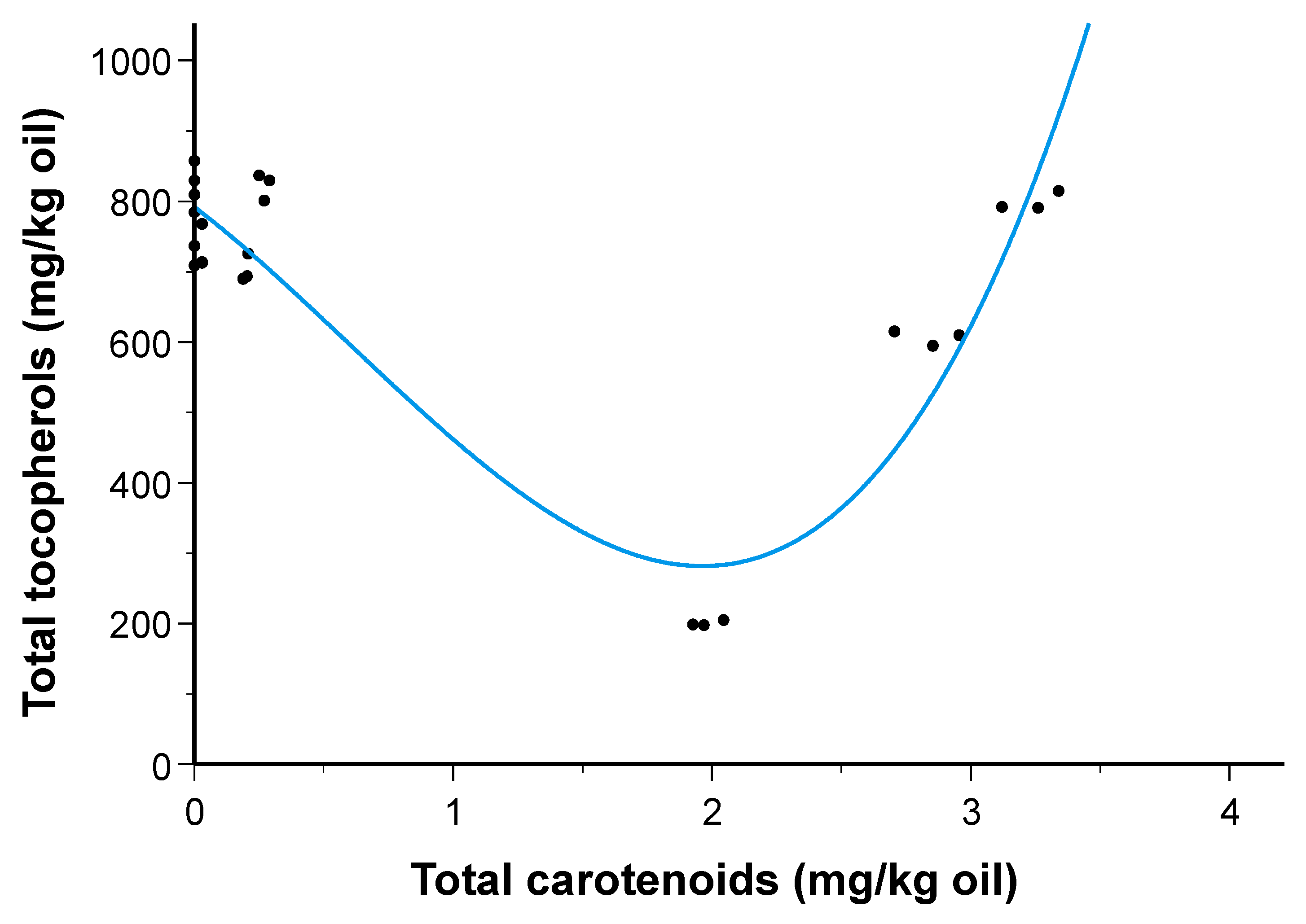
| Carotenoid | HOp-C | HOp-S1 | HOp-S2 | HOp-S3 | LOp-C | LOp-S1 | LOp-S2 | LOp-S3 |
|---|---|---|---|---|---|---|---|---|
| cis-lycopene | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 0.01 ± 0.01 c | 0.09 ± 0.00 b | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 0.13 ± 0.03 a | 0.14 ± 0.02 a |
| trans-lycopene | 0.00 ± 0.00 e | 0.03 ± 0.01 e | 0.18 ± 0.02 de | 1.80 ± 0.07 c | 0.00 ± 0.00 e | 0.27 ± 0.03 d | 2.96 ± 0.16 a | 2.56 ± 0.14 b |
| trans-β-carotene | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.01 ± 0.01 d | 0.09 ± 0.00 c | 0.00 ± 0.00 d | 0.01 ± 0.01 d | 0.15 ± 0.02 a | 0.13 ± 0.01 b |
| trans-lutein | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.01 ± 0.01 a | 0.01 ± 0.01 a |
| Parameter | HOp-C | HOp-S1 | HOp-S2 | HOp-S3 | LOp-C | LOp-S1 | LOp-S2 | LOp-S3 |
|---|---|---|---|---|---|---|---|---|
| L* | 39.35 ± 1.45 bc | 39.75 ± 1.31 bc | 37.66 ± 0.19 c | 31.92 ± 1.19 d | 43.32 ± 0.07 a | 41.31 ± 0.51 ab | 34.39 ± 0.37 d | 34.24 ± 1.49 d |
| a* | −1.83 ± 0.07 d | 5.18 ± 0.06 c | 15.03 ± 0.11 b | 26.65 ± 3.14 a | −1.73 ± 0.04 d | 3.78 ± 0.11 c | 16.1 ± 0.19 b | 28.51 ± 1.51 a |
| b* | 10.73 ± 0.13 d | 18.98 ± 0.60 c | 22.82 ± 0.17 b | 38.47 ± 2.41 a | 12.31 ± 0.10 d | 19.64 ± 0.22 c | 24.1 ± 0.19 b | 37.6 ± 1.85 a |
| Tocopherol | HOu-C | HOp-C | HOp-S1 | HOp-S2 | HOp-S3 | LOu-C | LOp-C | LOp-S1 | LOp-S2 | LOp-S3 |
|---|---|---|---|---|---|---|---|---|---|---|
| α | 736.4 ± 23.6 abc | 712.7 ± 34.9 bcd | 701.4 ± 29.2 bcd | 676.7 ± 16.8 d | 162.0 ± 3.7 f | 804.0 ± 24.7 a | 747.0 ± 24.9 ab | 778.3 ± 22.5 ab | 759.3 ± 11.0 abc | 578.7 ± 12.0 e |
| β | 27.4 ± 1.6 abc | 27.0 ± 2.7 abc | 26.7 ± 2.1 abc | 24.4 ± 4.1 c | 29.7 ± 0.6 abc | 27.4 ± 1.6 abc | 26.8 ± 1.5 abc | 30.3 ± 2.4 ab | 32.3 ± 1.8 a | 24.7 ± 2.1 bc |
| γ | 4.0 ± 0.1 cd | 4.0 ± 1.0 cd | 3.7 ± 0.6 cd | 2.0 ± 1.0 d | 9.0 ± 0.1 a | 1.3 ± 0.2 e | 5.0 ± 2.2 bc | 8.0 ± 2.5 ab | 8.3 ± 0.5 ab | 3.4 ± 0.6 cd |
| total | 767.7 ± 25.1 abc | 743.7 ± 38.5 abc | 731.7 ± 31.5 bc | 700.4 ± 21.3 c | 200.4 ± 4.2 e | 831.4 ± 26.8 a | 778.8 ± 29.1 ab | 816.5 ± 19.9 a | 799.8 ± 12.7 ab | 606.7 ± 10.5 d |
| (a) Saturated fatty acids | ||||||||||
| Acid | HOu-C | HOp-C | HOp-S1 | HOp-S2 | HOp-S3 | LOu-C | LOp-C | LOp-S1 | LOp-S2 | LOp-S3 |
| C14:0 | 0.04 ± 0.01 b | 0.04 ± 0.01 b | 0.04 ± 0.01 b | 0.04 ± 0.01 b | 0.04 ± 0.01 b | 0.08 ± 0.01 a | 0.08 ± 0.01 a | 0.08 ± 0.01 a | 0.08 ± 0.01 a | 0.07 ± 0.01 a |
| C16:0 | 3.59 ± 0.02 b | 3.59 ± 0.23 b | 3.60 ± 0.22 b | 3.59 ± 0.22 b | 3.60 ± 0.02 b | 6.32 ± 0.32 a | 6.24 ± 0.02 a | 6.32 ± 0.32 a | 6.32 ± 0.32 a | 6.25 ± 0.02 a |
| C17:0 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.04 ± 0.02 | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.04 ± 0.02 | 0.03 ± 0.01 |
| C18:0 | 2.80 ± 0.13 b | 2.80 ± 0.02 b | 2.81 ± 0.01 b | 2.80 ± 0.14 b | 2.80 ± 0.13 b | 3.34 ± 0.13 a | 3.40 ± 0.01 a | 3.33 ± 0.13 a | 3.33 ± 0.13 a | 3.40 ± 0.01 a |
| C20:0 | 0.25 ± 0.03 | 0.25 ± 0.03 | 0.25 ± 0.01 | 0.26 ± 0.01 | 0.26 ± 0.01 | 0.25 ± 0.03 | 0.24 ± 0.04 | 0.24 ± 0.03 | 0.24 ± 0.03 | 0.24 ± 0.03 |
| C22:0 | 0.86 ± 0.01 a | 0.87 ± 0.01 a | 0.87 ± 0.01 a | 0.88 ± 0.01 a | 0.87 ± 0.01 a | 0.69 ± 0.05 b | 0.71 ± 0.02 b | 0.72 ± 0.04 b | 0.72 ± 0.04 b | 0.71 ± 0.01 b |
| C24:0 | 0.31 ± 0.01 | 0.31 ± 0.01 | 0.31 ± 0.08 | 0.31 ± 0.07 | 0.31 ± 0.08 | 0.25 ± 0.05 | 0.25 ± 0.01 | 0.26 ± 0.05 | 0.25 ± 0.05 | 0.25 ± 0.01 |
| Ʃ SAFA | 7.88 ± 0.14 b | 7.89 ± 0.26 b | 7.90 ± 0.31 b | 7.91 ± 0.21 b | 7.90 ± 0.11 b | 10.93 ± 0.31 a | 10.94 ± 0.05 a | 10.96 ± 0.32 a | 10.97 ± 0.15 a | 10.94 ± 0.07 a |
| (b) Monounsaturated fatty acids | ||||||||||
| C16:1 | 0.14 ± 0.01 | 0.14 ± 0.01 | 0.15 ± 0.01 | 0.14 ± 0.01 | 0.15 ± 0.01 | 0.13 ± 0.02 | 0.12 ± 0.01 | 0.13 ± 0.01 | 0.13 ± 0.02 | 0.12 ± 0.01 |
| C17:1 | 0.15 ± 0.13 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.04 ± 0.01 |
| cis-C18:1 | 85.95 ± 0.01 a | 85.95 ± 0.01 a | 85.91 ± 0.01 a | 85.95 ± 0.02 a | 86.01 ± 0.08 a | 29.55 ± 0.83 b | 29.59 ± 0.05 b | 29.57 ± 0.82 b | 29.57 ± 0.84 b | 30.32 ± 0.13 b |
| trans-C18:1 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.02 | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.04 ± 0.02 | 0.02 ± 0.01 |
| C20:1 | 0.25 ± 0.02 a | 0.26 ± 0.02 a | 0.26 ± 0.02 a | 0.26 ± 0.03 a | 0.25 ± 0.03 a | 0.16 ± 0.01 b | 0.15 ± 0.01 b | 0.16 ± 0.01 b | 0.15 ± 0.01 b | 0.15 ± 0.01 b |
| Ʃ MUFA | 86.48 ± 0.13 a | 86.40 ± 0.02 a | 86.36 ± 0.02 a | 86.39 ± 0.04 a | 86.46 ± 0.08 a | 29.89 ± 0.83 b | 29.89 ± 0.05 b | 29.90 ± 0.83 b | 29.90 ± 0.85 b | 30.64 ± 0.11 b |
| (c) Polyunsaturated fatty acids | ||||||||||
| cis-C18:2 | 5.68 ± 0.01 b | 5.67 ± 0.01 b | 5.69 ± 0.02 b | 5.65 ± 0.01 b | 5.59 ± 0.07 b | 59.12 ± 1.57 a | 59.13 ± 0.05 a | 59.08 ± 1.55 a | 59.08 ± 1.57 a | 58.37 ± 0.17 a |
| trans-C18:2 | 0.08 ± 0.01 b | 0.08 ± 0.01 b | 0.08 ± 0.01 b | 0.08 ± 0.01 b | 0.08 ± 0.01 b | 0.13 ± 0.04 a | 0.08 ± 0.01 b | 0.12 ± 0.03 ab | 0.12 ± 0.03 ab | 0.09 ± 0.01 b |
| cis-C18:3 | 0.08 ± 0.01 b | 0.08 ± 0.01 b | 0.08 ± 0.01 b | 0.08 ± 0.01 b | 0.08 ± 0.01 b | 0.10 ± 0.02 a | 0.08 ± 0.01 b | 0.09 ± 0.02 ab | 0.09 ± 0.02 ab | 0.08 ± 0.01 b |
| trans-C18:3 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 |
| Ʃ PUFA | 5.85 ± 0.01 b | 5.85 ± 0.02 b | 5.87 ± 0.03 b | 5.83 ± 0.00 b | 5.76 ± 0.08 b | 59.49 ± 1.57 a | 59.37 ± 0.05 a | 59.44 ± 1.57 a | 59.43 ± 1.48 a | 58.62 ± 0.16 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Pan, I.; Horvitz, S.; Ibañez, F.C.; Vírseda, P.; Beriain, M.J. Protective Effect of Tomato By-Product in Refined Sunflower Oil with Different Lipid Profiles. Molecules 2025, 30, 2968. https://doi.org/10.3390/molecules30142968
Fernández-Pan I, Horvitz S, Ibañez FC, Vírseda P, Beriain MJ. Protective Effect of Tomato By-Product in Refined Sunflower Oil with Different Lipid Profiles. Molecules. 2025; 30(14):2968. https://doi.org/10.3390/molecules30142968
Chicago/Turabian StyleFernández-Pan, Idoya, Sandra Horvitz, Francisco C. Ibañez, Paloma Vírseda, and María José Beriain. 2025. "Protective Effect of Tomato By-Product in Refined Sunflower Oil with Different Lipid Profiles" Molecules 30, no. 14: 2968. https://doi.org/10.3390/molecules30142968
APA StyleFernández-Pan, I., Horvitz, S., Ibañez, F. C., Vírseda, P., & Beriain, M. J. (2025). Protective Effect of Tomato By-Product in Refined Sunflower Oil with Different Lipid Profiles. Molecules, 30(14), 2968. https://doi.org/10.3390/molecules30142968










