Bioactive Properties and Phenolic Profile of Bioaccessible and Bioavailable Fractions of Red Radish Microgreens After In Vitro Digestion
Abstract
1. Introduction
2. Results and Discussion
2.1. Composition of Freeze-Dried Radish Microgreens
2.2. Bioaccessibility and Bioavailability of Phenolic Compounds from Radish Microgreens
| Rt (min) | λmax (nm) | [M − H]−/[M + H]+ (m/z) | Tentative Identification | Undigested Microgreens | Gastric Bioaccessible Fraction | Small Intestine Bioavailable Fraction | Large intestine Bioavailable Fraction | Ref. |
|---|---|---|---|---|---|---|---|---|
| 2.76–2.79 | 319 | 315 | Protocatechuic acid hexoside | 0.00 a | 28.66 ± 0.64 d | 17.83 ± 0.20 c | 8.87 ± 0.91 b | [36] |
| 3.22 | 279 | 447 | Rhamnosyl-ellagic acid | 38.18 ± 0.38 b | 0.00 a | 0.00 a | 0.00 a | [37] |
| 3.90 | 243 | 300 | p-Hydroxybenzoyl hexoside | 0.00 a | 8.67 ± 0.24 b | 0.00 a | 0.00 a | [36] |
| Total hydroxybenzoic acids | 38.18 ± 0.38 c | 37.33 ± 0.71 c | 17.83 ± 0.20 b | 8.87 ± 0.91 a | ||||
| 3.43 | 325 | 353 | Caffeoylquinic acid | 4.10 ± 0.17 b | 0.00 a | 0.00 a | 0.00 a | [38] |
| 3.49–3.56 | 315 | 547 | Caffeoylsinapoyl hexoside | 30.20 ± 0.49 c | 7.62 ± 0.86 b | 0.00 a | 0.00 a | [39] |
| 3.65 | 329 | 355 | Feruloyl hexoside | 43.14 ± 0.44 b | 0.00 a | 0.00 a | 0.00 a | [40] |
| 3.99 | 313 | 431 | Sinapoyl hexoside | 5.94 ± 0.81 b | 0.00 a | 0.00 a | 0.00 a | [38] |
| 4.63–4.65 | 314 | 163 | p-Coumaric acid | 0.00 a | 189.66 ± 5.97 c | 193.41 ± 9.82 c | 41.88 ± 3.36 b | [40] |
| 4.91–5.18 | 324 | 309 | Feruloylmalic acid | 0.00 a | 380.80 ± 8.58 c | 338.24 ± 30.11 c | 136.90 ± 10.03 b | [41] |
| 5.08–5.36 | 309 | 753 | Disinapoylgentiobiose | 80.83 ± 3.30 d | 68.39 ± 2.54 c | 18.45 ± 3.35 a | 26.38 ± 1.48 b | [37] |
| 5.39 | 309 | 723 | Sinapoylferuloylgentiobiose | 11.94 ± 1.96 b | 0.00 a | 0.00 a | 0.00 a | [41] |
| 6.45 | 312 | 537 | p-Coumaroyl dihydromonotropein | 4.50 ± 0.17 b | 0.00 a | 0.00 a | 0.00 a | [42] |
| 7.16–7.46 | 319 | 959 | Trisinapoylgentiobiose | 7.41 ± 0.07 c | 5.72 ± 0.16 b | 0.00 a | 0.00 a | [41] |
| 7.44 | 313 | 339 | Sinapoylmalic acid | 0.00 a | 4.61 ± 0.25 b | 0.00 a | 0.00 a | [41] |
| 9.02–9.33 | 312 | 515 | Caffeoylquinic acid hexoside | 0.00 a | 0.00 a | 7.10 ± 0.59 c | 4.67 ± 0.64 b | [42] |
| Total hydroxycinnamic acids | 188.06 ± 5.30 a | 656.80 ± 18.13 c | 557.20 ± 39.89 b | 209.83 ± 13.18 a | ||||
| 3.71–3.83 | 342 | 447 | Kaempferol-7-glucoside | 9.40 ± 0.07 b | 24.36 ± 1.62 c | 0.00 a | 0.00 a | [41] |
| 4.05–4.20 | 343 | 755 | Kaempferol-3-O-glucosyl-rhamnosyl-glucoside | 5.93 ± 0.10 c | 0.00 a | 0.00 a | 2.58 ± 0.17 b | [43] |
| 4.14 | 328 | 725 | Kaempferol pentoside-rutinoside | 6.44 ± 0.68 b | 0.00 a | 0.00 a | 0.00 a | [44] |
| 4.08–4.38 | 345 | 593 | Kaempferol 3-O-p-coumaroyl glucoside | 83.12 ± 1.85 c | 73.89 ± 4.17 b | 32.27 ± 2.94 a | 28.39 ± 1.04 a | [45] |
| 4.27–4.37 | 338 | 563 | Kaempferol-3-O-arabinoside-7-O-rhamnoside | 52.05 ± 0.47 d | 44.24 ± 1.08 c | 17.35 ± 1.57 b | 13.81 ± 1.24 a | [45] |
| 4.50–4.60 | 331 | 887 | Kaempferol derivative | 25.38 ± 0.39 c | 0.00 a | 0.00 a | 9.30 ± 0.29 b | [45] |
| 4.58–4.81 | 340 | 577 | Kaempferol dirhamnoside | 114.80 ± 1.79 d | 103.17 ± 2.01 c | 32.63 ± 3.03 a | 41.14 ± 0.52 b | [44] |
| 4.88–4.99 | 331 | 901 | Kaempferol 3-O-(p-coumaroyl)dirhamnosyl hexoside | 4.39 ± 0.38 b | 0.00 a | 0.00 a | 23.01 ± 1.84 c | [46] |
| 5.15 | 324 | 947 | Kaempferol 3-O-feruloyldiglucoside-7-O-glucoside | 4.13 ± 0.53 b | 0.00 a | 0.00 a | 0.00 a | [47] |
| 5.32 | 342 | 447 | Kaempferol 7-O-glucoside | 0.00 a | 0.00 a | 0.00 a | 20.29 ± 1.15 b | [41] |
| 5.65 | 343 | 417 | Kaempferol 7-O-pentoside | 0.00 a | 0.00 a | 0.00 a | 7.30 ± 0.30 b | [44] |
| 6.20 | 341 | 431 | Kaempferol 3-O-rhamnoside | 0.00 a | 0.00 a | 0.00 a | 20.58 ± 0.86 b | [44] |
| Total flavonols | 305.64 ± 5.36 d | 245.66 ± 4.23 c | 82.25 ± 7.40 a | 143.39 ± 4.70 b | ||||
| 4.3 | 507 | 595+ | Cyanidin 3-(glucosyl)rhamnoside | 2.21 ± 0.05 b | 0.00 a | 0.00 a | 0.00 a | [48] |
| 4.54 | 509 | 919 | Cyanidin 3-(coumaroyl)sophoroside-5-glucoside | 3.82 ± 0.03 b | 0.00 a | 0.00 a | 0.00 a | [37] |
| 4.58–4.91 | 507 | 579+ | Pelargonidin 3-rutinoside | 17.88 ± 0.08 d | 12.82 ± 0.30 c | 4.81 ± 0.36 b | 3.74 ± 0.59 a | [48] |
| 4.66–5.00 | 507 | 1019+ | Pelargonidin-3-(feruloyl)diglucoside-5-(malonyl) glucoside | 5.96 ± 0.08 d | 4.34 ± 0.12 c | 2.47 ± 0.10 b | 1.17 ± 0.11 a | [48] |
| Total anthocyanins | 29.87 ± 0.12 d | 17.15 ± 0.42 c | 7.28 ± 0.35 b | 4.91 ± 0.55 a | ||||
| Total phenolic compounds | 561.75 ± 7.13 b | 956.94 ± 29.52 d | 664.56 ± 46.96 c | 367.00 ± 19.77 a | ||||
| Total proanthocyanidins | 185.90 ± 5.04 c | 36.41 ± 1.01 b | 28.27 ± 0.19 a | 28.16 ± 1.33 a | ||||
2.3. Effect of In Vitro Digestion of Radish Microgreens on Antioxidant Activity
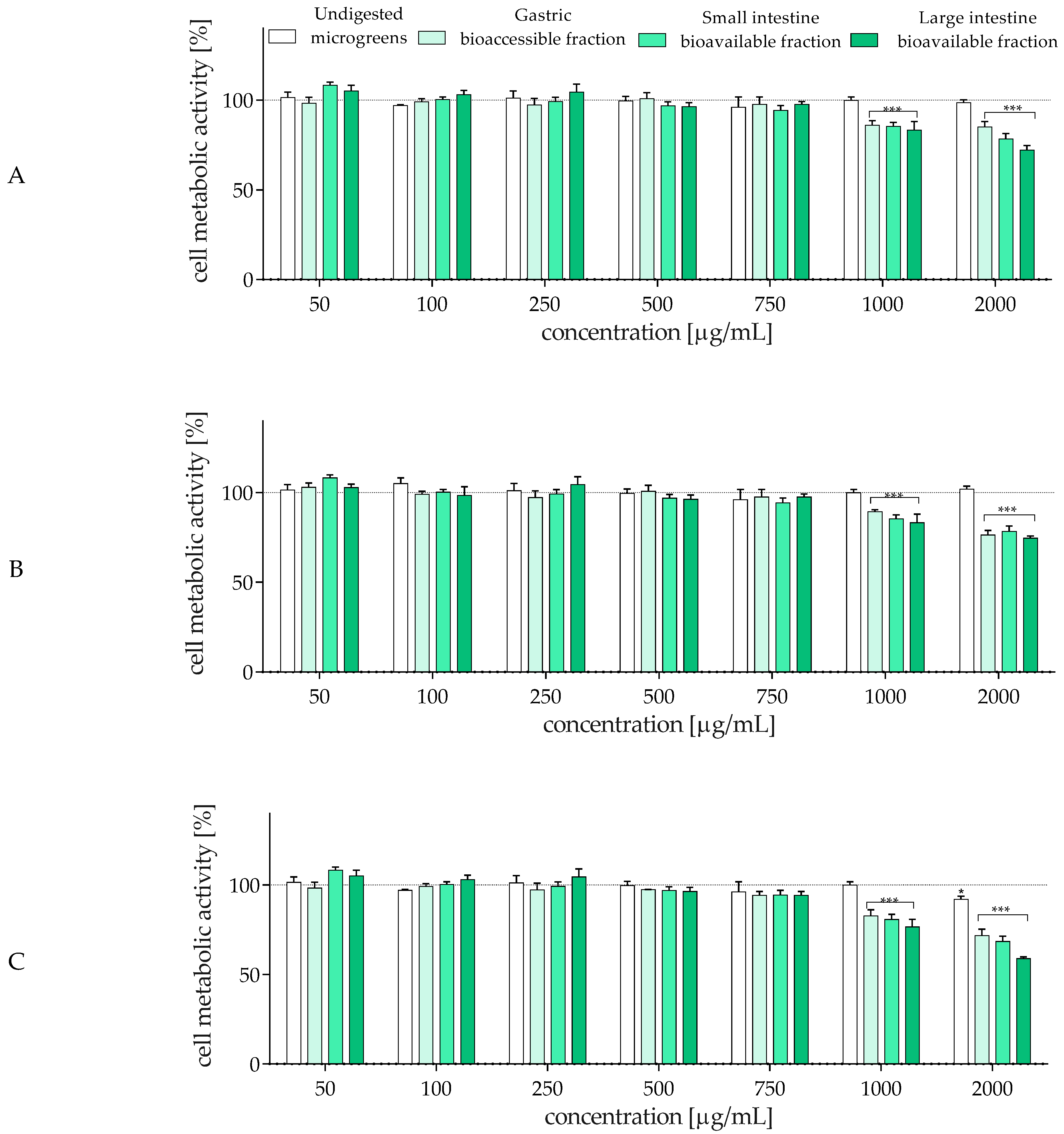
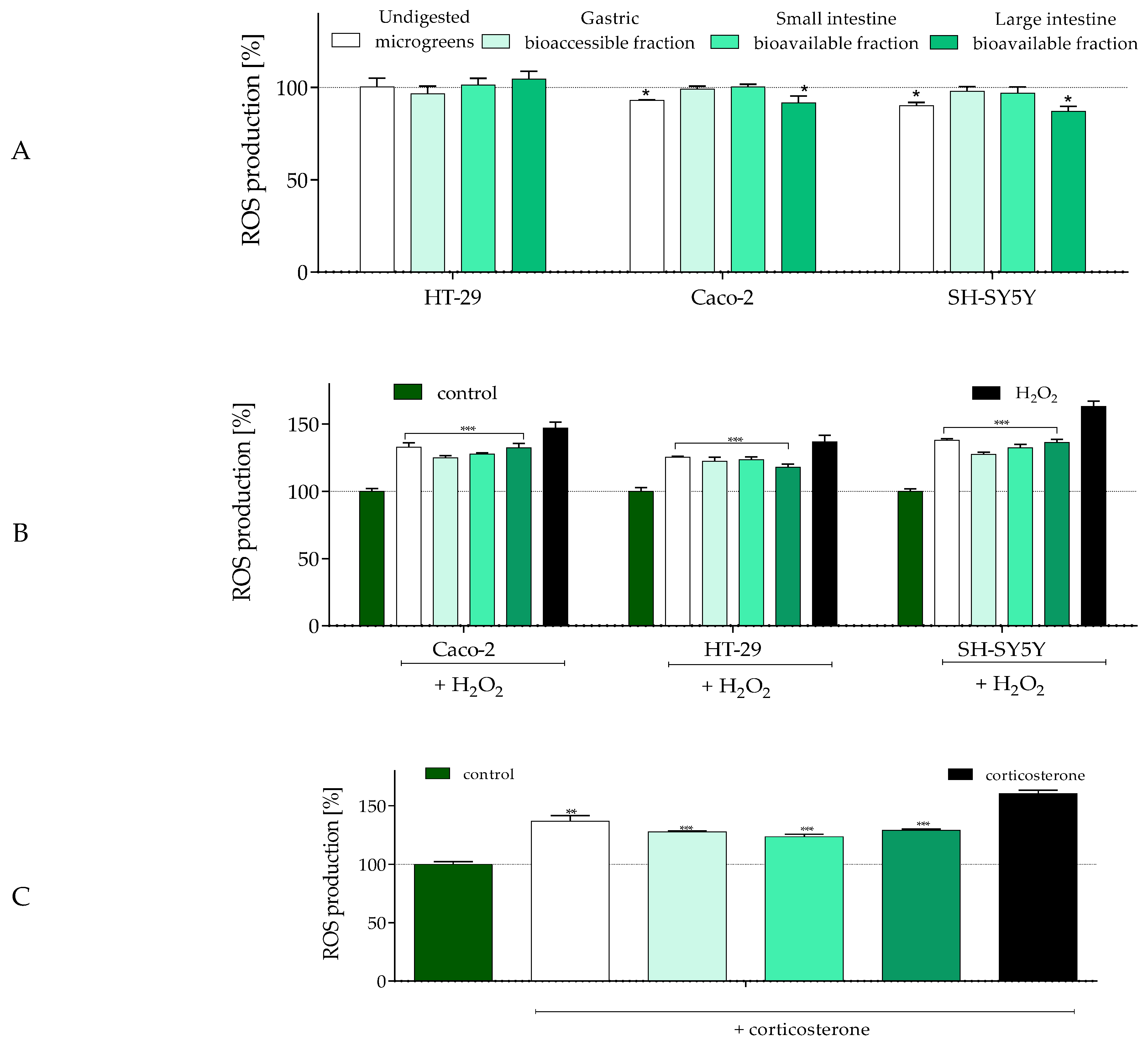
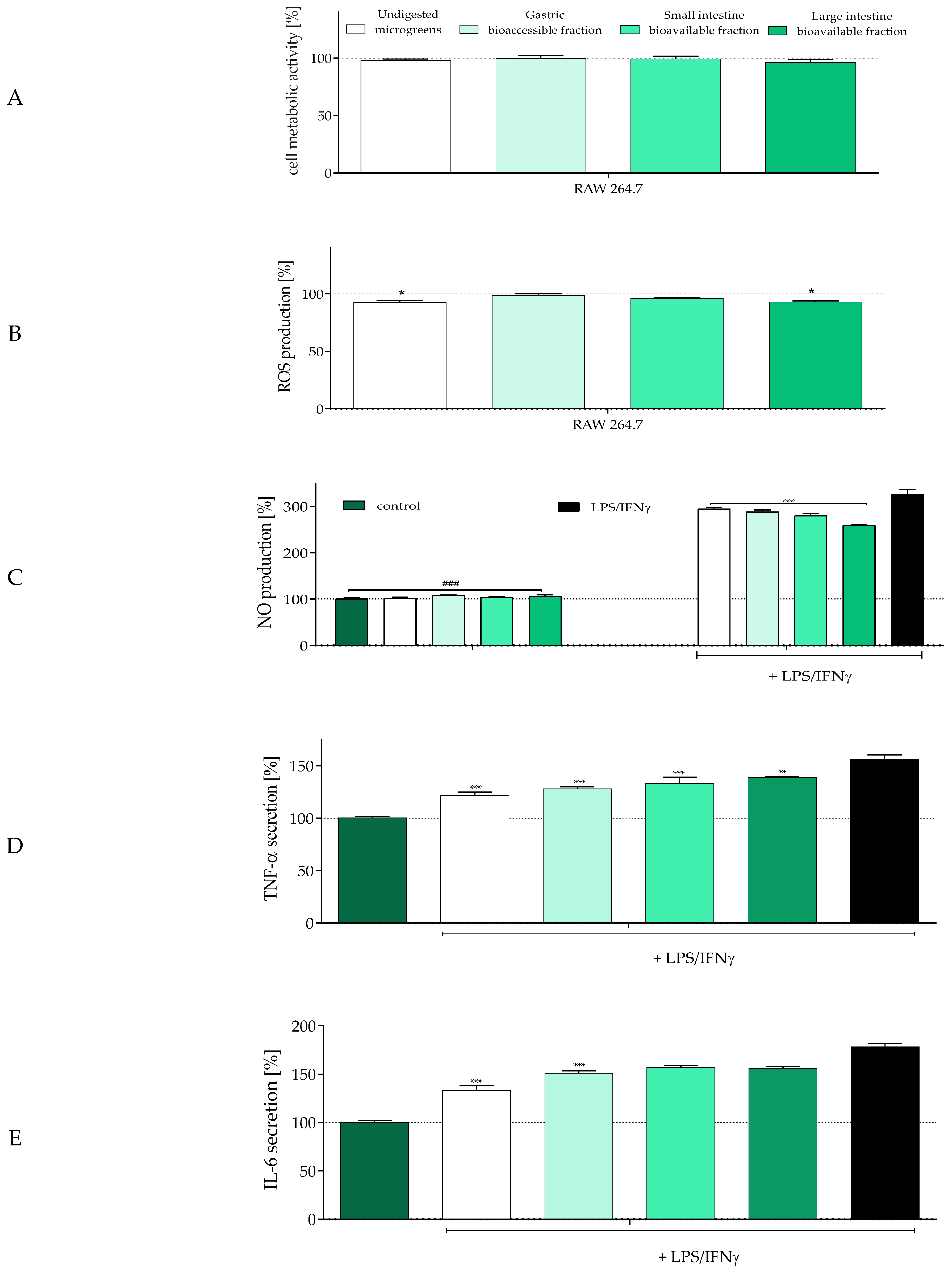
2.4. The Influence of In Vitro Digestion of Radish Microgreens on Biological Activity of Gut–Immune–Brain Axis Cells
3. Materials and Methods
3.1. Standards and Reagents
3.2. Plant Material
3.3. Preparation of Crude Extract of Phenolic Compounds
3.4. Purification of Phenolic Compounds on Sep-Pak C18 Cartridges
3.5. In Vitro Simulated Gastrointestinal Digestion
- Oral phase: 200 mg of freeze-dried radish microgreens was mixed with 3.0 mL of SSF, 0.375 mL of deionized water, and 0.125 mL of 0.03 M CaCl2(H2O)2. The samples were placed in a water bath for 3 min to equilibrate the mixture temperature to 37 °C, and then 7.5 mg of α-amylase was added. The total volume of the mixture was 3.5 mL. The mixture was incubated in a water bath with stirring (stirring speed 200, amplitude 4) at 37 °C for 2 min (Water bath shaker type 357, ELPIN-PLUS s.c., Lubawa, Poland).
- Gastric phase: The oral bolus was supplemented with 1.88 mL of SGF, 12.5 µL of 0.03 M CaCl2(H2O)2 solution, and 0.164 mL of deionized water. After mixing, the pH of the mixture was adjusted to 3.0 with 1 M HCl, and 0.4 mL of pepsin solution prepared in SGF fluid (16 mg of pepsin per sample) was added. The total volume of the mixture was 6 mL. The samples were incubated in a water bath with stirring at 37 °C for 120 min. After gastric digestion, a portion of the samples was immediately acidified to pH 2 using 1 M HCl and centrifuged for 10 min at 5500 rpm to obtain a gastric bioaccessible fraction. Phenolic compounds present in this fraction were purified on a Sep-Pak C18 cartridge.
- Small intestinal phase: Other portions of the gastric bolus were supplemented with 2.0 mL of SIF, 0.1 mL of 0.03 M CaCl2(H2O)2 solution, 0.35 mL of deionized water, and 0.5 mL of aqueous bile salt solution (0.25 g of bile salts per sample). After mixing, the pH of the mixture was adjusted to 7.0 with 1 M NaOH, and 40 mg of pancreatin per sample was added. The mixture was then transferred to the dialysis membrane, which was closed from the top and placed in a beaker containing 50 mL of PBS buffer at pH 7. The samples were incubated in a water bath with stirring at 37 °C for 120 min. After intestinal digestion, the OUT fraction with bioavailable compounds was acidified to pH 2 using 1 M HCl and purified on a Sep-Pak C18 cartridge. The residue inside the membrane (IN fraction) was then subjected to enzymatic hydrolysis using Pronase E and Viscozyme L.
3.6. Enzymatic Hydrolysis of Samples After In Vitro Digestion
3.7. Identification and Content of Individual Phenolic Compounds
3.8. Proximate Analysis
3.9. Extraction and Determination of Chlorophyll and Carotenoid Pigments
3.10. Determination of Total Phenolics and Total Proanthocyanidins
3.11. Extraction and Analysis of Vitamin C
3.12. In Vitro Antioxidant Activity Assays
3.13. Cell Culture and Treatment
3.14. Metabolic Activity
3.15. Oxidative Stress Parameters
3.16. Nitric Oxide (NO) Production and Secretion of TNFα and IL6
3.17. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, A.; Panwar, A.; Kumar, S.; Kumar, V.; Dhiman, A.; Sharma, V.; Sharma, S. Brassicaceae microgreens: Pioneering sustainable solutions for functional benefits in modern diets and food security. S. Afr. J. Bot. 2025, 182, 93–111. [Google Scholar] [CrossRef]
- Seth, T.; Mishra, G.P.; Chattopadhyay, A.; Roy, P.D.; Devi, M.; Sahu, A.; Sarangi, S.K.; Mhatre, C.S.; Lyngdoh, Y.A.; Chandra, V.; et al. Microgreens: Functional food for nutrition and dietary diversification. Plants 2025, 14, 526. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Singh, J.; Kaur, S.; Gunjal, M.; Kaur, J.; Nanda, V.; Ullah, R.; Ercisli, S.; Rasane, P. Emergence of microgreens as a valuable food, current understanding of their market and consumer perception: A review. Food Chem. X 2024, 23, 101527. [Google Scholar] [CrossRef] [PubMed]
- Tallei, T.E.; Kapantow, N.H.; Niode, N.J.; Sailah, I.; Savitri, M.; Lahay, M.Y.; Barasarathi, J. The therapeutic potential of red radish microgreens in modulating inflammation and cancer pathways. CyTA-J. Food 2025, 23, 2467410. [Google Scholar] [CrossRef]
- Vučetić, A.; Šovljanski, O.; Pezo, L.; Gligorijević, N.; Kostić, S.; Vulić, J.; Čanadanović-Brunet, J. A comprehensive antioxidant and nutritional profiling of Brassicaceae microgreens. Antioxidants 2025, 14, 191. [Google Scholar] [CrossRef] [PubMed]
- Kaimuangpak, K.; Lehtonen, M.; Rautio, J.; Weerapreeyakul, N. Unraveled cancer cell survival-associated amino acid metabolism of HepG2 cells altered by Thai rat-tailed radish microgreen extract examined by untargeted LC-MS/MS analysis. Food Chem. 2025, 474, 143206. [Google Scholar] [CrossRef] [PubMed]
- Truzzi, F.; Whittaker, A.; Roncuzzi, C.; Saltari, A.; Levesque, M.P.; Dinelli, G. Microgreens: Functional food with antiproliferative cancer properties influenced by light. Foods 2021, 10, 1690. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, B.; López-García, G.; Máñez, V.; Alegría, A.; Borberá, R.; Cilla, A. Antiproliferative effect of bioaccessible fractions of four Brassicaceae microgreens on human colon cancer cells linked to their phytochemical composition. Antioxidants 2020, 9, 368. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.M.; Aly, T.A.; Khattab, M.S.; Abdel-Rahim, E.A.; Al-Farga, A. Pathological and biochemical evaluation of radish microgreen on diabetes and aflatoxicosis in rats. Environ. Sci. Pollut. Res. 2023, 30, 98389–98399. [Google Scholar] [CrossRef] [PubMed]
- Dhanalakshmi, G. In vitro and In silico study of anti diabetic activity on Raphanus sativus microgreen and mature leaf. B R Nahata Smriti Sansthan Int. J. Phram. Sci. Clin. Res. 2022, 2, 120–126. [Google Scholar] [CrossRef]
- Aly, T.A.; Fayed Attia Koutb, F.M.; Fayed, S.A.; Ahmed, A.M.; ELRahim, E.A. Biochemical and histopathological evaluation of radish microgreen and clover etiolated sprouts against diabetic mellitus rats. Eur. J. Pharm. Med. Res. 2020, 7, 126–134. [Google Scholar]
- Wojdyło, A.; Nowicka, P.; Tkacz, K.; Turkiewicz, I.P. Sprouts vs. microgreens as novel functional foods: Variation of nutritional and phytochemical profiles and their in vitro bioactive properties. Molecules 2020, 25, 4648. [Google Scholar] [CrossRef] [PubMed]
- Yim, S.; Jeong, S.U.; Yoon, J.H.; Lee, H.B.; Park, H.Y.; Lee, E. Seasonal variation in bioactive compounds and anti-adipogenic effects of radish greens in 3T3-L1 adipocytes. Mol. Cell. Toxicol. 2025, 1–11. [Google Scholar] [CrossRef]
- Rizvi, A.; Kumar, S.; Satyanarayana, G.N.V.; Ansari, N.G.; Saxena, S. Evaluation of growing conditions, organoleptic properties, antimicrobial activities and biochemical characterization of three culinary microgreens. Food Humanit. 2025, 4, 100547. [Google Scholar] [CrossRef]
- Zhong, Y.; Xie, Y.; Zhang, D.; Li, G.; Yu, J. Integrated metabolomic and transcriptomic analysis of metabolic diversity and biosynthesis of glucosinolates and flavonoids in various cultivars of radish microgreens. Food Biosci. 2024, 59, 104055. [Google Scholar] [CrossRef]
- Bié, J.; Sepodes, B.; Fernandes, P.C.B.; Ribeiro, M.H.L. Polyphenols in health and disease: Gut microbiota, bioaccessibility, and bioavailability. Compounds 2023, 3, 40–72. [Google Scholar] [CrossRef]
- Eseberri, I.; Trepiana, J.; Léniz, A.; Gómez-García, I.; Carr-Ugarte, H.; González, M.; Portillo, M.P. Variability in the beneficial effects of phenolic compounds: A Review. Nutrients 2022, 14, 1925. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ochoa, Á.; Cádiz-Gurrea, M.d.l.L.; Fernández-Moreno, P.; Rojas-García, A.; Arráez-Román, D.; Segura-Carretero, A. Recent analytical approaches for the study of bioavailability and metabolism of bioactive phenolic compounds. Molecules 2022, 27, 777. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Sui, Y.; Shi, J.; Cai, S.; Xiong, T.; Cai, F.; Zhou, L.; Mei, X. In Vitro gastrointestinal digestion of various sweet potato leaves: Polyphenol profiles, bioaccessibility and bioavailability elucidation. Antioxidants 2024, 13, 520. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Tan, Y.; McClements, D.J. Applications of the INFOGEST in vitro digestion model to foods: A review. Annu. Rev. Food Sci. Technol. 2023, 14, 135–156. [Google Scholar] [CrossRef] [PubMed]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of in vitro digestion on composition, bioaccessibility and antioxidant activity of food polyphenols—A non-systematic review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef] [PubMed]
- Šola, I.; Vujčić Bok, V.; Popović, M.; Gagić, S. Phytochemical composition and functional properties of Brassicaceae microgreens: Impact of in vitro digestion. Int. J. Mol. Sci. 2024, 25, 11831. [Google Scholar] [CrossRef] [PubMed]
- Tomas, M.; Zhang, L.; Zengin, G.; Rocchetti, G.; Capanoglu, E.; Lucini, L. Metabolomic insight into the profile, in vitro bioaccessibility and bioactive properties of polyphenols and glucosinolates from four Brassicaceae microgreens. Food Res. Int. 2021, 140, 110039. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, B.; López-García, G.; Máñez, V.; Alegría, A.; Barberá, R.; Cilla, A. Evaluation of the bioaccessibility of antioxidant bioactive compounds and minerals of four genotypes of Brassicaceae microgreens. Foods 2019, 8, 250. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.O.; Bowtell, J.L.; O’Leary, M.F. Microvegetables and their potential health relevance: A systematic review of in vitro and in vivo evidence. J. Food Biochem. 2025, 1, 9953644. [Google Scholar] [CrossRef]
- Kajszczak, D.; Sosnowska, D.; Bonikowski, R.; Szymczak, K.; Frąszczak, B.; Pielech-Przybylska, K.; Podsędek, A. Comparative nutrient study of Raphanus sativus L. sprouts microgreens, and roots. Agronomy 2025, 15, 1216. [Google Scholar] [CrossRef]
- Sęczyk, Ł.; Sugier, D.; Świeca, M.; Gawlik-Dziki, U. The effect of in vitro digestion, food matrix, and hydrothermal treatment on the potential bioaccessibility of selected phenolic compounds. Food Chem. 2021, 344, 128581. [Google Scholar] [CrossRef] [PubMed]
- Tarko, T.; Duda-Chodak, A. Influence of food matrix on the bioaccessibility of fruit polyphenolic compounds. J. Agric. Food Chem. 2020, 68, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Lone, J.K.; Pandey, R. Microgreens on the rise: Expanding our horizons from farm to fork. Heliyon 2024, 10, e25870. [Google Scholar] [CrossRef] [PubMed]
- Bafumo, R.F.; Alloggia, F.P.; Ramirez, D.A.; Maza, M.A.; Fontana, A.; Moreno, D.A.; Camargo, A.B. Optimal brassicacea family microgreens from a phytochemical and sensory perspective. Food Res. Int. 2024, 193, 114812. [Google Scholar] [CrossRef] [PubMed]
- Ntsoane, M.L.; Manhivi, V.E.; Shoko, T.; Seke, F.; Sultanbawa, Y.; Sivakumar, D. Brassica microgreens cabbage (Brassica oleracea), radish (Raphanus sativus) and rocket (Eruca vesicaria L.) Cav: Application of red-light emitting diodes lighting during postharvest storage and in vitro digestion on bioactive compounds and antioxidant activity. Int. J. Food Sci. Technol. 2024, 59, 1432–1442. [Google Scholar] [CrossRef]
- García-Tenesaca, M.M.; Llugany, M.; Boada, R.; Sánchez-Martín, M.J.; Valiente, M. Phytochemical profile, bioactive properties, and Se speciation of Se-biofortified red radish (Raphanus sativus), green pea (Pisum sativum), and alfalfa (Medicago sativa) microgreens. J. Agric. Food Chem. 2024, 72, 4947–4957. [Google Scholar] [CrossRef] [PubMed]
- Tilahun, S.; Baek, M.W.; An, K.-S.; Choi, H.R.; Lee, J.H.; Hong, J.S.; Jeong, C.S. Radish microgreens produced without substrate in a vertical multi-layered growing unit are rich in nutritional metabolites. Front. Plant Sci. 2023, 14, 1236055. [Google Scholar] [CrossRef] [PubMed]
- Salvadó, J.M.; Casanova, E.; Fernández-Iglesias, A.; Arola, L.; Bladé, C. Roles of proanthocyanidin rich extracts in obesity. Food Funct. 2015, 6, 1053–1071. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardized static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Moon, J.K.; Hur, S.J.; Lee, J. Structural changes in mulberry (Morus microphylla. Buckl) and chokeberry (Aronia melanocarpa) anthocyanins during simulated in vitro human digestion. Food Chem. 2020, 318, 126449. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xiao, Z.; Lin, L.Z.; Lester, G.E.; Wang, Q.; Harnly, J.M.; Chen, P. Profiling polyphenols in five Brassica species microgreens by UHPLC-PDA-ESI/HRMSn. J. Agric. Food Chem. 2013, 61, 10960–10970. [Google Scholar] [CrossRef] [PubMed]
- Senica, M.; Stampar, F.; Veberic, R.; Mikulic-Petkovsek, M. Fruit seeds of the Rosaceae family: A waste, new life, or a danger to human health? J. Agric. Food Chem. 2017, 65, 10621–10629. [Google Scholar] [CrossRef] [PubMed]
- Hammouda, H.; Chérif, J.K.; Trabelsi-Ayadi, M.; Baron, A.; Guyot, S. Detailed polyphenol and tannin composition and its variability in Tunisian dates (Phoenix dactylifera L.) at different maturity stages. J. Agric. Food Chem. 2013, 61, 3252–3263. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, A.V.; Dabić, D.C.; Momirovic, N.M.; Dojčinović, B.P.; Milojković-Opsenica, D.M.; Tešić, Z.L.; Natić, M.M. Chemical composition of two different extracts of berries harvested in Serbia. J. Agric. Food Chem. 2013, 61, 4188–4194. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.Z.; Sun, J.; Chen, P.; Harnly, J.A. LC-PDA-ESI/MSn identification of new anthocyanins in purple Bordeaux radish (Raphanus sativus L. variety). J. Agric. Food Chem. 2011, 59, 6616–6627. [Google Scholar] [CrossRef] [PubMed]
- Bujor, O.C.; Le Bourvellec, C.; Volf, I.; Popa, V.I.; Dufour, C. Seasonal variations of the phenolic constituents in bilberry (Vaccinium myrtillus L.) leaves, stems and fruits, and their antioxidant activity. Food Chem. 2016, 213, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Koley, T.K.; Khan, Z.; Oulkar, D.; Singh, B.K.; Maurya, A.; Singh, B.; Banerjee, K. High resolution LC-MS characterization of phenolic compounds and the evaluation of antioxidant properties of a tropical purple radish genotype. Arab. J. Chem. 2020, 13, 1355–1366. [Google Scholar] [CrossRef]
- Lachowicz, S.; Kapusta, I.; Świeca, M.; Stinco, C.M.; Meléndez-Martínez, A.J.; Bieniek, A. In vitro biological activities of fruits and leaves of Elaeagnus multiflora Thunb. and their isoprenoids and polyphenolics profile. Antioxidants 2020, 9, 436. [Google Scholar] [CrossRef] [PubMed]
- Farid, M.M.; Ibrahim, F.M.; Ragheb, A.Y.; Mohammed, R.S.; Hegazi, N.M.; Shabrawy, M.O.; El Kawashty, S.A.; Marzouk, M.M. Comprehensive phytochemical characterization of Raphanus raphanistrum L.: In vitro antioxidant and antihyperglycemic evaluation. Sci. Afr. 2022, 16, e01154. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, P.; Lin, L.; Harnly, J.M.; Yu, L.L.; Li, Z. Tentative identification, quantitation, and principal component analysis of green pu-erh, green, and white teas using UPLC/DAD/MS. Food Chem. 2011, 126, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Jiang, J.; Ran, L.; Lu, C.; Wei, C.; Wang, Y. Analysis of flavonoids and hydroxycinnamic acid derivatives in rapeseeds (Brassica napus L. var. napus) by HPLC-PDA–ESI (−)-MSn/HRMS. J. Agric. Food Chem. 2014, 62, 2935–2945. [Google Scholar] [CrossRef]
- Wu, X.; Prior, R.L. Identification and characterization of anthocyanins by High-Performance Liquid Chromatography−Electrospray Ionization−Tandem Mass Spectrometry in common foods in the United States: Vegetables, nuts, and grains. J. Agric. Food Chem. 2005, 53, 3101–3113. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Zhang, Y.; Chen, Q.; Fu, X.; Huang, Q.; Zhang, B.; Dong, H.; Li, C. Exploring the synergistic benefits of insoluble dietary fiber and bound phenolics: Unveiling the role of bound phenolics in enhancing bioactivities of insoluble dietary fiber. Trends Food Sci. Technol. 2024, 149, 104554. [Google Scholar] [CrossRef]
- Amna, D.; Islam, M.R.; Farooq, A.; Munawar, I. Unveiling the functional implications and complex interplay between bound phenolic compounds and phenolics in food: A comprehensive review. Agrobiol. Rec. 2023, 13, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, G.; Gregorio, R.P.; Lorenzo, J.M.; Barba, F.J.; Oliveira, P.G.; Prieto, M.A.; Simal-Gandara, J.; Mosele, J.I.; Matilva, M.-J.; Tomas, M.; et al. Functional implications of bound phenolic compounds and phenolics–Food interaction: A review. Comp. Rev. Food Sci. Food Saf. 2022, 21, 811–842. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Li, S.Y.; Ge, S.H.; Lin, S.L. Review of distribution, extraction methods, and health benefits of bound phenolics in food plants. J. Agric. Food Chem. 2020, 68, 3330–3343. [Google Scholar] [CrossRef] [PubMed]
- Pająk, P.; Socha, R.; Gałkowska, D.; Rożnowski, J.; Fortuna, T. Phenolic profile and antioxidant activity in selected seeds and sprouts. Food Chem. 2014, 143, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Goyeneche, R.; Roura, S.; Ponce, A.; Vega-Gálvez, A.; Quispe-Fuentes, I.; Uribe, E.; Di Scala, K. Chemical characterization and antioxidant capacity of red radish (Raphanus sativus L.) leaves and roots. J. Funct. Foods 2015, 16, 256–264. [Google Scholar] [CrossRef]
- Papillo, V.A.; Vitaglione, P.; Graziani, G.; Gokmen, V.; Fogliano, V. Release of antioxidant capacity from five plant foods during a multistep enzymatic digestion protocol. J. Agric. Food Chem. 2014, 62, 4119–4126. [Google Scholar] [CrossRef] [PubMed]
- Valdés, L.; Cuervo, A.; Salazar, N.; Ruas-Madiedo, P.; Gueimonde, M.; González, S. The relationship between phenolic compounds from diet and microbiota: Impact on human health. Food Funct. 2015, 6, 2424–2439. [Google Scholar] [CrossRef] [PubMed]
- Mannino, G.; Chinigò, G.; Serio, G.; Genova, T.; Gentile, C.; Munaron, L.; Bertea, C.M. Proanthocyanidins and where to find them: A meta-analytic approach to investigate their chemistry, biosynthesis, distribution, and effect on human health. Antioxidants 2021, 10, 1229. [Google Scholar] [CrossRef] [PubMed]
- Niwano, Y.; Kohzaki, H.; Shirato, M.; Shishido, S.; Nakamura, K. Metabolic fate of orally ingested proanthocyanidins through the digestive tract. Antioxidants 2023, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Hubrecht, R.C.; Carter, E. The 3Rs and humane experimental technique: Implementing change. Animals 2019, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Lian, P.; Braber, S.; Varasteh, S.; Wichers, H.J.; Folkerst, G. Hypoxia and heat stress affect epithelial integrity in a Caco-2/HT-29 co-culture. Sci. Rep. 2021, 11, 13186. [Google Scholar] [CrossRef] [PubMed]
- Loh, J.S.; Mak, W.Q.; Tan, L.K.S.; Ng, C.X.; Chan, H.H.; Yeow, S.H.; Foo, J.B.; Ong, Y.S.; How, C.W.; Khaw, K.Y. Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct. Target. Ther. 2024, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Masanetz, R.K.; Winkler, J.; Winner, B.; Günther, C.; Süß, P. The gut–immune–brain axis: An important route for neuropsychiatric morbidity in inflammatory bowel disease. Int. J. Mol. Sci. 2022, 23, 11111. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Tellez, L.; Perkins, M.H.; Perez, I.O.; Qu, T.; Ferreira, J.; Ferreira, T.L.; Quinn, D.; Liu, Z.-W.; Gao, X.-B.; et al. A neural circuit for gut-induced reward. Cell 2018, 175, 665–678.e23. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, Z.; Lu, X.; Zhong, H.; He, R.; Feng, Z.; Guan, R. Protective mechanism of quercetin nanoliposomes on hydrogen peroxide-induced oxidative damage in 3D Caco-2 cell model. J. Funct. Foods 2025, 24, 106650. [Google Scholar] [CrossRef]
- Xu, B.; Lang, L.M.; Li, S.Z.; Guo, J.R.; Wang, J.F.; Wang, D.; Zhang, L.P.; Yang, H.M.; Lian, S. Cortisol excessmediated mitochondrial damage induced hippocampal neuronal apoptosis in mice following cold exposure. Cells 2019, 8, 612. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Sun, Z.; Wang, F.; Xu, C.; Geng, M.; Chen, H.; Duan, D. Shuyusan-containing serum protects SH-SY5Y cells against corticosterone-induced impairment. Neural Regen. Res. 2013, 8, 2060–2068. [Google Scholar] [CrossRef] [PubMed]
- Enayati, A.; Soghi, A.; Butler, A.E.; Rizzo, M.; Sahebkar, A. The effect of curcumin on the gut-brain axis: Therapeutic implications. J. Neurogastroenterol. Motil. 2023, 29, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Facchin, B.M.; Dos Reis, G.O.; Vieira, G.N.; Mohor, E.T.B.; da Rosa, J.S.; Kretzer, I.F.; Demarchi, I.G.; Dalmarco, E.M. Inflammatory biomarkers on an LPS-induced RAW 264.7 cell model: A systematic review and meta-analysis. Inflamm. Res. 2022, 71, 741–758. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Wang, L.; Liu, Y.; Zhang, Q.; Li, Y.; Wu, Z. Release of phenolics compounds from Rubus idaeus L. dried fruits and seeds during simulated in vitro digestion and their bio-activities. J. Funct. Foods 2018, 46, 57–65. [Google Scholar] [CrossRef]
- Kajszczak, D.; Kowalska-Baron, A.; Sosnowska, D.; Podsędek, A. In vitro inhibitory effects of Viburnum opulus bark and flower extracts on digestion of potato starch and carbohydrate hydrolases activity. Molecules 2022, 27, 3118. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, L.; Narváez, A.; Izzo, L.; Graziani, G.; Ritieni, A. In vitro bioaccessibility and antioxidant activity of coffee silverskin polyphenolic extract and characterization of bioactive compounds using UHPLC-Q-Orbitrap HRMS. Molecules 2020, 25, 2132. [Google Scholar] [CrossRef] [PubMed]
- Kajszczak, D.; Sosnowska, D.; Frąszczak, B.; Podsędek, A. Composition, anti-diabetic, and antioxidant potential of Raphanus sativus leaves. Molecules 2024, 29, 5689. [Google Scholar] [CrossRef] [PubMed]
- Nollet, L.M. Physical characterization and nutrient analysis. In Handbook of Food Analysis, 2nd ed.; Dekker, M., Ed.; CRC Press: Boca Raton, FL, USA, 2004; pp. 61–67, 77–78, 173–176. [Google Scholar]
- Podsędek, A.; Frąszczak, B.; Kajszczak, D.; Sosnowska, D. Evaluation of bioactive compounds and antioxidant activity of green and red kale (Brassica oleracea L var. acephala) microgreens under white, red, and blue LED combinations. Agronomy 2024, 14, 2454. [Google Scholar] [CrossRef]
- De Menezes, E.W.; Grande, F.; Giuntini, E.B.; Lopes, T.D.V.C.; Dan, M.C.T.; do Prado, S.B.R.; Lajolo, F.M. Impact of dietary fiber energy on the calculation of food total energy value in the Brazilian food composition database. Food Chem. 2016, 193, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Costache, M.A.; Campeanu, G.; Neata, G. Studies concerning the extraction of chlorophyll and total carotenoids from vegetables. Rom. Biotechnol. Lett. 2012, 17, 7702–7708. [Google Scholar]
- Rösch, D.; Bergmann, M.; Knorr, D.; Kroh, L.W. Structure–antioxidant efficiency relationships of phenolic compounds and their contribution to the antioxidant activity of sea buckthorn juice. J. Agric. Food Chem. 2003, 51, 4233–4239. [Google Scholar] [CrossRef] [PubMed]
- Aubert, C.; Bony, P.; Chalot, G.; Landry, P.; Lurol, S. Effects of storage temperature, storage duration, and subsequent ripening on the physicochemical characteristics, volatile compounds, and phytochemicals of western red nectarine (Prunus persica L. Batsch). J. Agric. Food Chem. 2014, 62, 4707–4724. [Google Scholar] [CrossRef] [PubMed]
- Grzelczyk, J.; Budryn, G.; Szwajgier, D.; Baranowska-Wójcik, E.; Zakłos-Szyda, M. Evaluation of the effect of roasting and digestion on biological activity of compounds of coffee extracts-in vitro assessment of the bioavailability, cytoprotective properties and modulation of inflammatory response. Food Chem. 2024, 460, 140648. [Google Scholar] [CrossRef] [PubMed]
| Components | Content (g/100 g) | Components | Content (mg/100 g) |
|---|---|---|---|
| Moisture | 3.81 ± 0.10 | Chlorophyll a | 324.30 ± 13.09 |
| Total protein | 21.05 ± 0.34 | Chlorophyll b | 144.58 ± 6.67 |
| Crude fat | 5.44 ± 0.32 | Total chlorophylls | 468.87 ± 17.19 |
| Available carbohydrates | 26.17 ± 1.96 | Total carotenoids | 51.95 ± 4.43 |
| Ash | 15.16 ± 0.07 | Total phenolics | 562.27 ± 17.35 |
| Total dietary fiber | 28.37 ± 1.57 | Total proanthocyanidins | 185.90 ± 5.04 |
| Insoluble dietary fiber | 25.79 ± 1.49 | Vitamin C | 7.94 ± 0.33 |
| Sample | ABTS | FRAP | SARSA |
|---|---|---|---|
| Undigested microgreens | 18.78 ± 0.30 a | 18.25 ± 0.66 a | 121.81± 6.75 a |
| Gastric biaccessible fraction | 51.42 ± 4.54 b | 41.68 ± 2.39 c | 194.82 ± 13.44 c |
| Small intestine bioavailable fraction | 21.80 ± 1.90 a | 25.70 ± 0.63 b | 156.67 ± 5.82 b |
| Large intestine bioavailable fraction | 20.62 ± 1.71 a | 23.38 ± 2.03 b | 142.84 ± 16.43 a b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sosnowska, D.; Zakłos-Szyda, M.; Kajszczak, D.; Podsędek, A. Bioactive Properties and Phenolic Profile of Bioaccessible and Bioavailable Fractions of Red Radish Microgreens After In Vitro Digestion. Molecules 2025, 30, 2976. https://doi.org/10.3390/molecules30142976
Sosnowska D, Zakłos-Szyda M, Kajszczak D, Podsędek A. Bioactive Properties and Phenolic Profile of Bioaccessible and Bioavailable Fractions of Red Radish Microgreens After In Vitro Digestion. Molecules. 2025; 30(14):2976. https://doi.org/10.3390/molecules30142976
Chicago/Turabian StyleSosnowska, Dorota, Małgorzata Zakłos-Szyda, Dominika Kajszczak, and Anna Podsędek. 2025. "Bioactive Properties and Phenolic Profile of Bioaccessible and Bioavailable Fractions of Red Radish Microgreens After In Vitro Digestion" Molecules 30, no. 14: 2976. https://doi.org/10.3390/molecules30142976
APA StyleSosnowska, D., Zakłos-Szyda, M., Kajszczak, D., & Podsędek, A. (2025). Bioactive Properties and Phenolic Profile of Bioaccessible and Bioavailable Fractions of Red Radish Microgreens After In Vitro Digestion. Molecules, 30(14), 2976. https://doi.org/10.3390/molecules30142976









